Abstract
Purpose
Increased body mass index (BMI) has been associated with poor outcomes in women with breast cancer. We evaluated the association between BMI and pathological complete response (pCR) in the I-SPY 2 trial.
Methods
978 patientsenrolled in the I-SPY 2 trial 3/2010–11/2016 and had a recorded baseline BMI prior to treatment were included in the analysis. Tumor subtypes were defined by hormone receptor and HER2 status. Pretreatment BMI was categorized as obese (BMI≥30 kg/m2), overweight (25≤BMI < 30 kg/m2), and normal/underweight (< 25 kg/m2). pCR was defined as elimination of detectable invasive cancer in the breast and lymph nodes (ypT0/Tis and ypN0) at the time of surgery. Logistic regression analysis was used to determine associations between BMI and pCR. Event-free survival (EFS) and overall survival (OS) between different BMI categories were examined using Cox proportional hazards regression.
Results
The median age in the study population was 49 years. pCR rates were 32.8% in normal/underweight, 31.4% in overweight, and 32.5% in obese patients. In univariable analysis, there was no significant difference in pCR with BMI. In multivariable analysis adjusted for race/ethnicity, age, menopausal status, breast cancer subtype, and clinical stage, there was no significant difference in pCR after neoadjuvant chemotherapy for obese compared with normal/underweight patients (OR = 1.1, 95% CI: 0.68–1.63, p = 0.83), and for overweight compared with normal/underweight (OR = 1, 95% CI: 0.64–1.47, p = 0.88). We tested for potential interaction between BMI and breast cancer subtype; however, the interaction was not significant in the multivariable model (p = 0.09). Multivariate Cox regression showed there was no difference in EFS (p = 0.81) or OS (p = 0.52) between obese, overweight, and normal/underweight breast cancer patients with a median follow-up time of 3.8 years.
Conclusions
We found no difference in pCR rates by BMI with actual body weight based neoadjuvant chemotherapy in this biologically high-risk breast cancer population in the I-SPY2 trial.
Keywords: Obesity, Body Mass Index, Breast Cancer, Neoadjuvant Chemotherapy, Pathological Complete Response
Introduction
Observational studies have shown increased body mass index (BMI) is a risk factor for developing breast cancer, especially hormone receptor positive breast cancers [1, 2]. Obesity and being overweight are also associated with advanced stage of breast cancer at diagnosis and have been independently associated with poor breast cancer outcomes [3–5]. Pathological complete response (pCR) is a surrogate of long-term outcomes of locally advanced breast cancer such as event-free survival (EFS) and overall survival (OS) [6, 7]. Studies investigating the relationship between BMI and pCR after neoadjuvant chemotherapy in breast cancer have demonstrated mixed results, with some revealing an association of increased BMI with poorer pCR rates after neoadjuvant chemotherapy [8–11], while others did not reveal any significant association [12–14]. Most were retrospective studies, some using data from more than a decade ago [8]. Chemotherapy regimens varied substantially from study to study, as did chemotherapy dosage. Since oncology clinical practices may cap chemotherapy dosage to a maximum body-surface area (BSA) of 2.0m2 to avoid increased toxicity [11, 15], it is not clear if the observed worse pCR rate in obese breast cancer patients is related to chemotherapy underdosing rather than BMI itself [8, 11]; and it is also unclear whether the observed worse pCR in obese patients has any correlation with breast cancer biological subtypes.
The I-SPY 2 (Investigation of Serial studies to Predict Your Therapeutic Response with Imaging and Molecular AnaLysis 2, NCT01042379) trial is an ongoing, multicenter, adaptive, phase II clinical trial platform that includes multiple experimental arms to evaluate new agents combined with standard neoadjuvant chemotherapy for the treatment of breast cancers with a high risk of recurrence, in comparison to standard chemotherapy regimen in a common control arm [16]. The trial uses pCR as primary end point. Importantly, chemotherapy dosage is not capped, but is given based on actual body weight [17]. The I-SPY 2 trial platform provides the advantage of eliminating some of the above-mentioned confounding factors while studying the association of BMI and neoadjuvant chemotherapy outcomes of breast cancer.
The purpose of this study was to examine the association of BMI with pCR, EFS, and OS in women with high-risk early stage breast cancer enrolled in the I-SPY 2 trial.
Methods
Study population and data collection
Women aged 18 years or older with a diagnosis of clinical stage II or III breast cancer, with a tumor diameter of at least 2.5cm by clinical examination and at least 2cm as assessed by imaging were eligible to participate in the I-SPY 2 trial. Exclusion criteria were an Eastern Cooperative Oncology Group performance status score greater than 1, and prior chemotherapy for this cancer. Patients with hormone receptor positive tumors and low risk MammaPrint® scores were also excluded given the lack of benefit from systemic chemotherapy [18].
In this trial, participants were randomized to different neoadjuvant treatment regimens based on biomarker status, determined by Bayesian probabilities of pCR within a specific biomarker subtype with the experimental regimen. The biomarker status was based on hormone receptors (HR), human epidermal growth factor receptor 2 (HER2) and a 70-gene assay of MammaPrint® at baseline. All participants received weekly intravenous paclitaxel (12 doses of 80mg per square meter of BSA) alone (control arm), or in combination with the assigned experimental regimen (experiment arm), followed by four doses of intravenous doxorubicin (60mg per square meter of BSA) and cyclophosphamide (600mg per square meter of BSA) every two to three weeks, with myeloid growth factor support if needed. Patients with HER2 + cancer also received trastuzumab for the first 12 weeks, given with a loading dose of 4mg per kilogram of body weight (week 1), followed by a maintenance dose of 2mg per kilogram every 3 weeks (weeks 4, 7, and 10). After receiving accelerated approval from the FDA [19], Pertuzumab was added to standard therapy for HER2 + patients, given with a loading dose of 840mg (week 1), followed by a maintenance dose of 420mg every 3 weeks (weeks 4, 7, and 10). All chemotherapy drugs were dosed based on actual body weight. Patients then underwent surgery which included axillary lymph node sampling. Radiation and adjuvant endocrine therapy after surgery were recommended in accordance with standard guidelines.
All participants provided written informed consent before undergoing screening for the study, and a second consent was obtained before treatment was initiated if the individual was eligible after random assignment to open-label treatment arms. All participating sites of this trial received approval from an institutional review board.
Measures
The primary outcomes in this analysis were pCR [20], defined as elimination of detectable invasive cancer in the breast and lymph nodes (ypT0/Tis and ypN0) at the time of surgery; RCB (residual tumor burden) if pCR was not achieved [21]; and EFS and OS. The primary exposure of interest for this analysis was pretreatment BMI, categorized as obese (BMI≥30 kg/m2), overweight (25≤BMI < 30 kg/m2), and normal/underweight (< 25 kg/m2) based on World Health Organization criteria.
Demographic and clinical covariates included in multivariate analysis and defined a priori were age at screening (years); race/ethnicity (Non-Hispanic White vs. Non-Hispanic Black/African American vs. Latinx vs. other); breast cancer subtype including HR+/HER2+, HR+/HER2−, HR−/HER2 + and triple negative (HR−/HER2−); menopausal status (pre- vs. peri- vs. post-menopausal); and advanced vs. early tumor stage (stage III vs. I or II).
Statistical analysis
Chi-squared and Anova were used to evaluate the association between BMI category and patient characteristics as appropriate. Logistic regression analysis was used to estimate associations between BMI and pCR, and linear regression to estimate the association between BMI and RCB; and Cox proportional hazards regression to estimate the associations between BMI and EFS, and between BMI and OS. These models were adjusted for the covariates listed above; because of limited degrees of freedom due to the total number of events, we excluded race/ethnicity from the covariates in the survival analyses. We report odds ratios (OR), linear coefficients, hazard ratios (HR), and respective 95% confidence intervals (CI). OR > 1 indicate greater odds of having pCR; hazard ratios > 1 indicate greater hazard of dying or having a major event. Analyses were run in SAS 9.4. All statistical tests were two-sided, and P values less than 0.05 were considered statistically significant.
Results
Patient characteristics
In total, 977 patients with a recorded baseline BMI were included in this study. Of these, 35.6% (N = 348) were normal/underweight, 31.6% (N = 309) overweight, and 32.8% (N = 320) obese (Table 1). The mean age was 48.7 ± 10.6 years. Overweight (mean age 49.9 years) and obese (mean age 49.7 years) patients were significantly older than normal/underweight patients (mean age 46.8; p < 0.0001). There were more Non-Hispanic Black / African American and Hispanic participants among those who were obese compared to normal/underweight and overweight. BMI category was not significantly associated with menopausal status, cancer stage, or cancer hormonal subtype.
Table 1.
Patient characteristics according to BMI category.
| Patient Characteristics | BMI < 25 (N = 348) | 25 ≤BMI < 30 (N=309) | BMI ≥30 (N=320) | Total (N=977) | P |
|---|---|---|---|---|---|
| # of pts (%) | # of pts (%) | # of pts (%) | # of pts (%) | ||
| Age at Initial Treatment | |||||
| Mean (STD) | 46.8 (10.6) | 49.9 (10.7) | 49.7 (10.1) | 48.7 (10.6) | <.0001 |
| Race/Ethnicity | <.0001 | ||||
| African American / Black | 21 (6) | 26 (8.4) | 71 (22.2) | 118 (12.1) | |
| American Indian / Native or Hawaiian / Pacific Islander | 4 (1.2) | 2 (0.7) | 5 (1.6) | 11 (1.1) | |
| Asian | 35 (10.1) | 27 (8.7) | 9 (2.8) | 71 (7.3) | |
| Hispanic | 23 (6.6) | 43 (13.9) | 49 (15.3) | 115 (11.8) | |
| Non-Hispanic White | 265 (76.2) | 211 (68.3) | 186 (58.1) | 662 (67.8) | |
| Menopausal Status | 0.14 | ||||
| Pre- | 193 (64.3) | 147 (57.0) | 135 (54.4) | 475 (58.9) | |
| Peri- | 12 (4.0) | 9 (3.5) | 13 (5.2) | 34 (4.2) | |
| Post- | 95 (31.7) | 102 (39.5) | 100 (40.3) | 297 (36.9) | |
| Missing | 48 | 51 | 72 | 171 | |
| Hormonal and HER2 Status | 0.53 | ||||
| HR+/HER2+ | 63 (18.1) | 44 (14.2) | 48 (15.0) | 155 (15.9) | |
| HR+/HER2- | 130 (37.4) | 130 (42.1) | 118 (36.9) | 378 (38.7) | |
| HR-/HER2+ | 28 (8.1) | 31 (10.0) | 29 (9.1) | 88 (9.0) | |
| Age at Initial Treatment | |||||
| HR-/HER2- | 127 (36.5) | 104 (33.7) | 125 (39.1) | 356 (36.4) | |
| Cancer Stage | 0.14 | ||||
| I | 8 (2.7) | 9 (3.5) | 4 (1.6) | 21 (2.6) | |
| II | 222 (75.5) | 173 (66.8) | 183 (70.9) | 578 (71.3) | |
| III | 64 (21.8) | 77 (29.7) | 71 (27.5) | 212 (26.1) | |
| Missing | 54 | 50 | 62 | 166 | |
Relationship between BMI and Pathological Response
The overall pCR rate after neoadjuvant chemotherapy was 32.2%. pCR rates were 32.8% in normal/underweight, 31.4% in overweight, and 32.5% in obese patients, with no significant difference in the unadjusted or adjusted analysis (obese vs. normal/underweight, unadjusted OR = 0.99, 95% CI 0.71–1.37, adjusted OR = 1.05, 95% CI 0.68–1.63; overweight vs. normal/underweight, unadjusted OR = 0.94, 95% CI 0.68–1.30, adjusted OR = 0.97, 95% CI 0.64–1.47, Table 2). We ran an additional sensitivity analysis with continuous BMI as predictor, and this association was not significant, either.
Table 2:
Odds Ratios (OR) of pathological complete response (pCR) by BMI categories and adjusted variables
| # of pts | pCR | OR (95% CI) | P | OR (95% CI) | P | |
|---|---|---|---|---|---|---|
| (N) | N (%) | Unadjusted | Adjusted* | |||
| BMI | ||||||
| Normal / Underweight | 348 | 114 (32.8) | 1 (Ref.) | 1 (Ref.) | ||
| Overweight | 309 | 97 (31.4) | 0.94 (0.68–1.30) | 0.71 | 0.97 (0.64– 1.47) | 0.88 |
| Obese | 320 | 104 (32.5) | 0.99 (0.71–1.37) | 0.94 | 1.05 (0.68– 1.63) | 0.83 |
| BMI (continuous, per additonal unit) | 0.99 (0.97–1.01) | 0.50 | 0.99 (0.96– 1.02) | 0.64 | ||
| Model stratified by cancer status (unadjusted odds ratios for continuous BMI, per additional unit) | ||||||
| Subtype stratum | ||||||
| HR+/HER2− | 378 | 64 (16.9) | 1.01 (0.96–1.05) | 0.81 | ||
| HR+/HER2+ | 155 | 57 (36.8) | 0.97 (0.91–1.03) | 0.32 | ||
| HR−/HER2+ | 88 | 55 (62.5) | 0.93 (0.86–1.00) | 0.06 | ||
| HR−/HER2− | 356 | 139 (39.0) | 1.00 (0.97–1.04) | 0.99 | ||
Adjusted for age at screening, hormonal cancer subtype, race, stage, and menopausal status
Although an interaction between BMI and hormonal breast cancer subtype was not significant, we ran the unadjusted logistic regression models (predictor: continuous BMI) stratified by cancer hormonal subtype because cancer outcomes typically differ by hormonal subtypes. The association of BMI with PCR status was not significant in any of these models (Table 2). We did notice a trend towards decreased pCR rates with increasing BMI in the HR−/HER2 + subgroup (N = 88, Table 3) which did not reach statistical significance. The pCR rate in this hormonal subtype group was 75% in normal/underweight, 64.5% in overweight, and 48.3% in obese patients (overall p = 0.11). Using linear regression to compare Residual Cancer Burden (RCB) by BMI, RCB index was not associated with BMI category in either the unadjusted or the adjusted model, or for any cancer hormonal subtype after stratification (Table 4).
Table 3.
pCR rate of different BMI categories by breast cancer subtypes.
| Breast Cancer Subtype | pCR | Normal/Underweight N (%) | Overweight N (%) | Obese N (%) | P |
|---|---|---|---|---|---|
| HR+/HER2+ | No | 39 (61.9) | 27 (61.4) | 32 (66.7) | 0.83 |
| Yes | 24 (38.1) | 17 (38.6) | 16 (33.3) | ||
| HR+/HER2- | No | 105 (80.8) | 116 (89.2) | 93 (78.8) | 0.06 |
| Yes | 25 (19.2) | 14 (10.8) | 25 (21.2) | ||
| HR-/HER2+ | No | 7 (25.0) | 11 (35.5) | 15 (51.7) | 0.11 |
| Yes | 21 (75.0) | 20 (64.5) | 14 (48.3) | ||
| HR-/HER2- | No | 83 (65.4) | 58 (55.8) | 76 (60.8) | 0.33 |
| Yes | 44 (34.7) | 46 (44.2) | 49 (39.2) |
(HR: hormone receptor, HER2: human epidermal growth factor receptor 2, pCR: pathological complete response)
Table 4.
Association of BMI categories and continuous RCB index in patients who did not achieve pCR.
| Coefficient (95% CI) | P | Coefficient (95% CI) | P | |
|---|---|---|---|---|
| Unadjusted | Adjusted* | |||
| BMI | 0.95 | 0.56 | ||
| Normal / Underweight | 0 (Ref.) | 0 (Ref.) | ||
| Overweight | 0.02 (−0.20–0.24) | 0.86 | −0.14 (−0.38–0.11) | 0.28 |
| Obese | 0.03 (−0.18–0.25) | 0.75 | −0.06 (−0.32–0.20) | 0.67 |
| BMI (continuous, per additonal unit) | 0.00 (−0.01–0.02) | 0.54 | 0.00 (−0.02–0.02) | 0.84 |
| Model stratified by cancer status (coefficients for continuous BMI, per additional unit) | ||||
| Subtype stratum | ||||
| HR+/HER2− | 0.00 (−0.02–0.02) | 0.87 | ||
| HR+/HER2+ | 0.01 (−0.03–0.05) | 0.61 | ||
| HR−/HER2+ | 0.03 (−0.01–0.07) | 0.13 | ||
| HR−/HER2− | 0.00 (−0.02–0.03) | 0.80 | ||
Adjusted for age at screening, hormonal cancer subtype, race, stage, and menopausal status
Relationship of BMI with EFS and OS
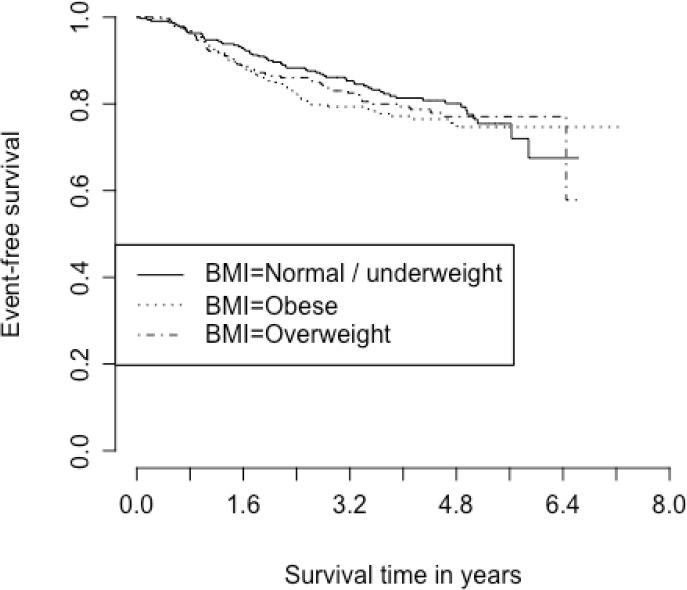
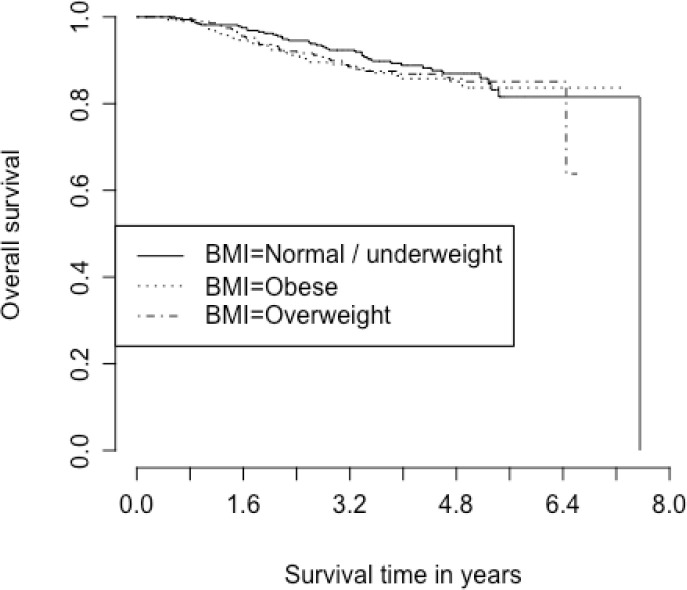
With a median follow-up time of 3.8 years, estimated OS at 5 years was 85.3% (95% CI 82.3–87.8%; 111 deaths out of 895 participants with known survival status) and estimated EFS at 5 years was 76.5% (95% CI 73.1–79.5%; 182 events out of 895 participants with known event status). BMI was not associated with EFS or OS in this study population (Tables 5 and 6). Due to limited events, we were unable to stratify these models by cancer hormonal subtype. Kaplan Meier curves for EFS and OS in different BMI categories are shown in Figs. 1 and 2.
Table 5.
Association of BMI with overall survival (hazard ratios, HR)
| BMI | Adjusted HR* | 95% CI | P |
|---|---|---|---|
| Normal / Underweight | 1 (Ref.) | ||
| Overweight | 1.13 | 0.69–1.87 | 0.63 |
| Obese | 0.82 | 0.46–1.44 | 0.48 |
Adjusted for age, hormonal cancer subtype, stage, and menopausal status
Table 6:
Association of BMI with Event-free Survival
| BMI | Adjusted HR* | 95% CI | P |
|---|---|---|---|
| Normal / Underweight | 1 (Ref.) | ||
| Overweight | 0.99 | 0.67–1.49 | 0.98 |
| Obese | 0.88 | 0.57–1.35 | 0.56 |
Adjusted for age, hormonal cancer subtype, stage, and menopausal status
Figure 1.
Kaplan Meier curve for event-free survival based on BMI category
Figure 2.
Kaplan Meier curve for overall survival based on BMI category
Discussion
In this clinical trial using actual body weight-based chemotherapy, higher baseline BMI was not associated with decreasing pCR rate after neoadjuvant chemotherapy in biologically high-risk early stage breast cancer patients, nor was it associated with worse EFS or OS. The overall pCR rate was 32.2% in our study, which was modest in comparison of other studies [22, 11]. The I-SPY 2 trial used standard chemotherapy regimen +/− HER2 targeted therapy depending on the HER2 status. It should also be noted, however, that this clinical trial also included patients with HR + breast cancer which have historically demonstrated lower response rates to chemotherapy [16]. This may explain why the overall pCR rate was modest after including the HR+/HER2− population, as HER2 + patients had a considerably higher pCR rate of 68% in our study [20].
Although several prospective studies and meta-analyses have reported that increased body weight was associated with poorer breast cancer outcomes such as OS and EFS, especially in postmenopausal women [23, 15, 24], it has been a challenge to clarify the underlying cause. In part, this has been attributed to possible interactions between BMI and comorbidities such as diabetes, coronary artery disease, cerebral artery disease, and socioeconomic status [25–28].
Neoadjuvant chemotherapy has recently become the standard of care for biologically high-risk breast cancers. Achieving pCR at the time of surgery is a surrogate marker for better long-term breast cancer outcomes [6, 29]. The Collaborative Trials in Neoadjuvant Breast Cancer (CTNeoBC) results indicated a long-term benefit for patients achieving pCR, as pCR was positively associated with overall EFS (hazard ratio 0.48, 95% CI 0.43–0.54) and overall OS (hazard ratio 0.36, 95% CI 0.31–0.42) [7]. Monitoring pCR rates among overweight and obese breast cancer patients who received neoadjuvant chemotherapy may help us understand why higher BMI is associated with poorer breast cancer outcomes.
Litton et al did the first large retrospective study in this regard, finding that patients with higher BMI were more likely to present with high-risk tumor characteristics and were less likely to achieve pCR after neoadjuvant chemotherapy; and that higher BMI was associated with worse OS [8]. Elsamany and colleagues performed a similar retrospective analysis in Saudi Arabian and Egyptian populations, and Fontanella et al did a pooled analysis of four clinical trials in Germany, both studies showed high BMI was associated with worse pCR rate [10, 11]. However, similar studies by Erbes et al and Kogawa et al did not reveal any statistically significant association between increased BMI and worse pCR [12, 14]. We previously performed a meta-analysis with total of 18,702 patients, with pooled univariable analysis demonstrating increased BMI was associated with worse pCR rate in overweight and obese patients [30]. Yet this meta-analysis has limitations given most included studies were retrospective in nature, multivariable analysis and subgroup analysis based on different subtypes of breast cancer were not able to be performed due to lack of standardization of patient characteristics; there were significant variations of chemotherapy regimens, and inclusion of non-weight based chemotherapy dosing [30].
Using the I-SPY 2 trial data to investigate the association of increased BMI with pCR outcome has several advantages. First, this is a currently active clinical trial platform using standard concurrent treatment regimens for each subtype of breast cancer, with a focus on treating high risk, biologically active breast cancer. Second, the I-SPY 2 trial uses standard treatment protocols and chemotherapy is given based on actual body weight. Lastly, it is one of the largest multicenter randomized clinical trials focusing on neoadjuvant therapy for breast cancer. These advantages may eliminate the potential biases originating from the variation of chemotherapy regimens and the underdosing of chemotherapy agents in patients with elevated BMI. In this strictly designed clinical trial, we did not identify any statistically significant evidence that higher BMI was associated with decreasing pCR rate in the high-risk early stage breast cancer group with various hormonal subtypes; nor within each hormonal subtype group after stratification. This result was different from most of the retrospective studies discussed above.
Our study reinforced the potential importance of dosing chemotherapy based on actual body weight. Some clinicians may reduce chemotherapy dosage in overweight and obese patients because of the fear of overdosing and excessive toxicity with higher chemotherapy dosage, although randomized clinical trials have demonstrated that this practice contributes to worse outcomes and guidelines recommend against this practice [31–33]. In the I-SPY 2 trial, chemotherapy dosing is strictly based on actual body weight, even if patients’ BSA is above 2.0m2. In Litton’s study, the chemotherapy dose of each patient was not documented and not able to be verified [8]. In Fontanella’s study, more than half of the study population had chemotherapy dosage capped at 2.0m2 [11]. It is possible that the poorer breast cancer outcomes in overweight and obese patients from these studies was attributable to chemotherapy underdosing rather than the influence of BMI on the chemotherapy effectiveness in these patients.
Our study has several limitations. Although the I-SPY 2 trial is a prospective study, the correlation of BMI to pCR is not the predetermined primary end point of this trial. While our analysis included almost 1000 women, dividing the study population by tumor subtype, ethnicity and BMI limited our statistical power; especially in the subgroup analysis of BMI in different breast cancer subtypes and its impact on breast cancer outcomes. As there were too few deaths/recurrences in patients who achieved pCR (RCB = 0), we were not able to run a meaningful survival analysis to determine whether BMI has an impact on OS and EFS regardless of patients’ pCR status. Longer follow up is needed to understand the overall impact on OS and EFS.
Conclusion
We observed no difference in pCR rates by baseline BMI in this biologically high-risk breast cancer population receiving actual body weight-based neoadjuvant chemotherapy. These findings suggest the importance of treating overweight and obese patients with chemotherapy dosage based on actual weight. Longer follow up and further work, however, is needed to understand the role of body mass and breast cancer outcomes across all breast cancer subtypes.
Acknowledgements:
We would like to thank the ISPY2 clinical trial team for their enrollment of subjects to this study, and the ongoing commitment to care of breast cancer patients.
Funding:
This study was conducted by volunteer members of ISPY2 clinical trial research team. The clinical trial itself is funded by NIH/NCI PO1 CA210961-01A1 for DY receives research support.
Abbreviations
- BMI
Increased body mass index
- pCR
Pathological complete response
- EFS
Event-free survival
- OS
Overall survival
- BSA
Body-surface area
- I-SPY 2
Investigation of Serial studies to Predict Your Therapeutic Response with Imaging and Molecular AnaLysis 2
- HR
Hormone receptors
- HER2
Human epidermal growth factor receptor 2
- RCB
Residual cancer burden
- OR
Odds ratios
- CI
Confidence intervals
Footnotes
Declarations
Disclosures: DP, CY, and DY receive research funding from Quantum Leap Collaborative. The remaining authors have no disclosures.
Ethics consideration: All participants provided written informed consent before undergoing screening and a second consent was obtained before treatment was initiated if the individual was eligible after random assignment to open-label treatment arms. All participating sites of this trial received approval from an institutional review board.
Contributor Information
Haiyun Wang, Cancer Care Associates.
Douglas Yee, University of Minnesota Department of Medicine: University of Minnesota Twin Cities Department of Medicine.
David Potter, University of Minnesota Department of Medicine: University of Minnesota Twin Cities Department of Medicine.
Patricia Jewett, University of Minnesota Department of Medicine: University of Minnesota Twin Cities Department of Medicine.
Christina Yau, University of California San Francisco.
Heather Beckwith, University of Minnesota Department of Medicine: University of Minnesota Twin Cities Department of Medicine.
Allison Watson, Sanford Health.
Nicholas O’Grady, University of California San Francisco.
Amy Wilson, Quantumleap.
Susie Brain, University of California San Francisco.
Paula Pohlmann, MD Anderson Nellie B Connally Breast Center: The University of Texas MD Anderson Cancer Center Nellie B Connally Breast Center.
Anne Blaes, University of Minnesota Medical Center.
Data:
Data is available upon request.
References
- 1.Pischon T, Nimptsch K (2016) Obesity and Risk of Cancer: An Introductory Overview. Recent Results Cancer Res 208:1–15. 10.1007/978-3-319-42542-9_1 [DOI] [PubMed] [Google Scholar]
- 2.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A (2014) Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev 36:114–136. 10.1093/epirev/mxt010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawood S, Broglio K, Gonzalez-Angulo AM, Kau SW, Islam R, Hortobagyi GN, Cristofanilli M (2008) Prognostic value of body mass index in locally advanced breast cancer. Clin Cancer Res 14(6):1718–1725. 10.1158/1078-0432.ccr-07-1479 [DOI] [PubMed] [Google Scholar]
- 4.Kroenke CH, Chen WY, Rosner B, Holmes MD (2005) Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol 23(7):1370–1378. 10.1200/jco.2005.01.079 [DOI] [PubMed] [Google Scholar]
- 5.Protani M, Coory M, Martin JH (2010) Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 123(3):627–635. 10.1007/s10549-010-0990-0 [DOI] [PubMed] [Google Scholar]
- 6.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N (2008) Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26(5):778–785. 10.1200/jco.2007.15.0235 [DOI] [PubMed] [Google Scholar]
- 7.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172. 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 8.Litton JK, Gonzalez-Angulo AM, Warneke CL, Buzdar AU, Kau SW, Bondy M, Mahabir S, Hortobagyi GN, Brewster AM (2008) Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol 26(25):4072–4077. 10.1200/JCO.2007.14.4527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Chen CM, Zhou Y, Zhou RJ, Yu KD, Shao ZM (2012) Obesity or overweight is associated with worse pathological response to neoadjuvant chemotherapy among Chinese women with breast cancer. PLoS ONE 7(7):e41380. 10.1371/journal.pone.0041380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsamany S, Alzahrani A, Abozeed WN, Rasmy A, Farooq MU, Elbiomy MA, Rawah E, Alsaleh K, Abdel-Aziz NM (2015) Mammographic breast density: Predictive value for pathological response to neoadjuvant chemotherapy in breast cancer patients. Breast 24(5):576–581. 10.1016/j.breast.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 11.Fontanella C, Lederer B, Gade S, Vanoppen M, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Gerber B, Hanusch C, Hilfrich J, Huober J, Schneeweiss A, Paepke S, Jackisch C, Mehta K, Nekljudova V, Untch M, Neven P, von Minckwitz G, Loibl S (2015) Impact of body mass index on neoadjuvant treatment outcome: a pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast Cancer Res Treat 150(1):127–139. 10.1007/s10549-015-3287-5 [DOI] [PubMed] [Google Scholar]
- 12.Erbes T, Stickeler E, Rücker G, Buroh S, Asberger J, Dany N, Thornton S, Iborra S, Hirschfeld M, Gitsch G, Mayer S (2016) BMI and Pathologic Complete Response to Neoadjuvant Chemotherapy in Breast Cancer: A Study and Meta-Analysis. Clin Breast Cancer 16(4):e119–132. 10.1016/j.clbc.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 13.Eralp Y, Smith TL, Altundağ K, Kau SW, Litton J, Valero V, Buzdar A, Hortobagyi GN, Arun B (2009) Clinical features associated with a favorable outcome following neoadjuvant chemotherapy in women with localized breast cancer aged 35 years or younger. J Cancer Res Clin Oncol 135(1):141–148. 10.1007/s00432-008-0428-9 [DOI] [PubMed] [Google Scholar]
- 14.Kogawa T, Fujii T, Fouad TM, Liu DD, Harano K, Masuda H, Iwase T, Barnett C, Park YS, Lim B, Tripathy D, Litton JK, Ueno NT (2018) Impact of change in body mass index during neoadjuvant chemotherapy and survival among breast cancer subtypes. Breast Cancer Res Treat 171(2):501–511. 10.1007/s10549-018-4853-4 [DOI] [PubMed] [Google Scholar]
- 15.Arce-Salinas C, Aguilar-Ponce JL, Villarreal-Garza C, Lara-Medina FU, Olvera-Caraza D, Alvarado Miranda A, Flores-Diaz D, Mohar A (2014) Overweight and obesity as poor prognostic factors in locally advanced breast cancer patients. Breast Cancer Res Treat 146(1):183–188. 10.1007/s10549-014-2977-8 [DOI] [PubMed] [Google Scholar]
- 16.Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ (2009) I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther 86(1):97–100. 10.1038/clpt.2009.68 [DOI] [PubMed] [Google Scholar]
- 17.Rugo HS, Olopade OI, DeMichele A, Yau C, van ‘t Veer LJ, Buxton MB, Hogarth M, Hylton NM, Paoloni M, Perlmutter J, Symmans WF, Yee D, Chien AJ, Wallace AM, Kaplan HG, Boughey JC, Haddad TC, Albain KS, Liu MC, Isaacs C, Khan QJ, Lang JE, Viscusi RK, Pusztai L, Moulder SL, Chui SY, Kemmer KA, Elias AD, Edmiston KK, Euhus DM, Haley BB, Nanda R, Northfelt DW, Tripathy D, Wood WC, Ewing C, Schwab R, Lyandres J, Davis SE, Hirst GL, Sanil A, Berry DA, Esserman LJ, Investigators I-S (2016) Adaptive Randomization of Veliparib-Carboplatin Treatment in Breast Cancer. N Engl J Med 375(1):23–34. 10.1056/NEJMoa1513749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga JY, Brain E, Causeret S, DeLorenzi M, Glas AM, Golfinopoulos V, Goulioti T, Knox S, Matos E, Meulemans B, Neijenhuis PA, Nitz U, Passalacqua R, Ravdin P, Rubio IT, Saghatchian M, Smilde TJ, Sotiriou C, Stork L, Straehle C, Thomas G, Thompson AM, van der Hoeven JM, Vuylsteke P, Bernards R, Tryfonidis K, Rutgers E, Piccart M, Investigators M (2016) 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med 375(8):717–729. 10.1056/NEJMoa1602253 [DOI] [PubMed] [Google Scholar]
- 19.von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N, Clark E, Knott A, Lang I, Levy C, Yardley DA, Bines J, Gelber RD, Piccart M, Baselga J (2017) Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med 377(2):122–131. 10.1056/NEJMoa1703643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yee D, DeMichele AM, Yau C, Isaacs C, Symmans WF, Albain KS, Chen YY, Krings G, Wei S, Harada S, Datnow B, Fadare O, Klein M, Pambuccian S, Chen B, Adamson K, Sams S, Mhawech-Fauceglia P, Magliocco A, Feldman M, Rendi M, Sattar H, Zeck J, Ocal IT, Tawfik O, LeBeau LG, Sahoo S, Vinh T, Chien AJ, Forero-Torres A, Stringer-Reasor E, Wallace AM, Pusztai L, Boughey JC, Ellis ED, Elias AD, Lu J, Lang JE, Han HS, Clark AS, Nanda R, Northfelt DW, Khan QJ, Viscusi RK, Euhus DM, Edmiston KK, Chui SY, Kemmer K, Park JW, Liu MC, Olopade O, Leyland-Jones B, Tripathy D, Moulder SL, Rugo HS, Schwab R, Lo S, Helsten T, Beckwith H, Haugen P, Hylton NM, Van’t Veer LJ, Perlmutter J, Melisko ME, Wilson A, Peterson G, Asare AL, Buxton MB, Paoloni M, Clennell JL, Hirst GL, Singhrao R, Steeg K, Matthews JB, Asare SM, Sanil A, Berry SM, Esserman LJ, Berry DA (2020) Association of Event-Free and Distant Recurrence-Free Survival With Individual-Level Pathologic Complete Response in Neoadjuvant Treatment of Stages 2 and 3 Breast Cancer: Three-Year Follow-up Analysis for the I-SPY2 Adaptively Randomized Clinical Trial. JAMA Oncol 6(9):1355–1362. 10.1001/jamaoncol.2020.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Symmans WF, Yau C, Chen YY, Balassanian R, Klein ME, Pusztai L, Nanda R, Parker BA, Datnow B, Krings G, Wei S, Feldman MD, Duan X, Chen B, Sattar H, Khazai L, Zeck JC, Sams S, Mhawech-Fauceglia P, Rendi M, Sahoo S, Ocal IT, Fan F, LeBeau LG, Vinh T, Troxell ML, Chien AJ, Wallace AM, Forero-Torres A, Ellis E, Albain KS, Murthy RK, Boughey JC, Liu MC, Haley BB, Elias AD, Clark AS, Kemmer K, Isaacs C, Lang JE, Han HS, Edmiston K, Viscusi RK, Northfelt DW, Khan QJ, Leyland-Jones B, Venters SJ, Shad S, Matthews JB, Asare SM, Buxton M, Asare AL, Rugo HS, Schwab RB, Helsten T, Hylton NM, van ‘t Veer L, Perlmutter J, DeMichele AM, Yee D, Berry DA, Esserman LJ (2021) Assessment of Residual Cancer Burden and Event-Free Survival in Neoadjuvant Treatment for High-risk Breast Cancer: An Analysis of Data From the I-SPY2 Randomized Clinical Trial. JAMA Oncol 7(11):1654–1663. 10.1001/jamaoncol.2021.3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warner ET, Ballman KV, Strand C, Boughey JC, Buzdar AU, Carey LA, Sikov WM, Partridge AH (2016) Impact of race, ethnicity, and BMI on achievement of pathologic complete response following neoadjuvant chemotherapy for breast cancer: a pooled analysis of four prospective Alliance clinical trials (A151426). Breast Cancer Res Treat 159(1):109–118. 10.1007/s10549-016-3918-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T (2014) Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 25(10):1901–1914. 10.1093/annonc/mdu042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borugian MJ, Sheps SB, Kim-Sing C, Olivotto IA, Van Patten C, Dunn BP, Coldman AJ, Potter JD, Gallagher RP, Hislop TG (2003) Waist-to-hip ratio and breast cancer mortality. Am J Epidemiol 158(10):963–968. 10.1093/aje/kwg236 [DOI] [PubMed] [Google Scholar]
- 25.Kang C, LeRoith D, Gallagher EJ (2018) Diabetes, Obesity, and Breast Cancer. Endocrinology 159(11):3801–3812. 10.1210/en.2018-00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moghani Lankarani M, Assari S (2017) Diabetes, hypertension, obesity, and long-term risk of renal disease mortality: Racial and socioeconomic differences. J Diabetes Investig 8(4):590–599. 10.1111/jdi.12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moustsen IR, Friberg AS, Larsen SB, Duun-Henriksen AK, Tjønneland A, Kjaer SK, Brasso K, Johansen C, Dalton SO (2019) The association between education and risk of major cardiovascular events among prostate cancer patients: a study from the Diet, Cancer and Health study. Acta Oncol 58(5):715–721. 10.1080/0284186x.2019.1572924 [DOI] [PubMed] [Google Scholar]
- 28.den Hartigh LJ (2019) Conjugated Linoleic Acid Effects on Cancer, Obesity, and Atherosclerosis: A Review of Pre-Clinical and Human Trials with Current Perspectives. Nutrients 11(2). 10.3390/nu11020370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B (2001) Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 3096–102. 10.1093/oxfordjournals.jncimonographs.a003469 [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Zhang S, Yee D, Basu S, Beckwith H, Potter D, Blaes A (2021) Impact of body mass index on pathological complete response following neoadjuvant chemotherapy in operable breast cancer: a meta-analysis. Breast Cancer. 10.1007/s12282-020-01194-w [DOI] [PubMed] [Google Scholar]
- 31.Rosner GL, Hargis JB, Hollis DR, Budman DR, Weiss RB, Henderson IC, Schilsky RL (1996) Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: results from cancer and leukemia group B study 8541. J Clin Oncol 14(11):3000–3008. 10.1200/jco.1996.14.11.3000 [DOI] [PubMed] [Google Scholar]
- 32.Griggs JJ, Sorbero ME, Lyman GH (2005) Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med 165(11):1267–1273. 10.1001/archinte.165.11.1267 [DOI] [PubMed] [Google Scholar]
- 33.Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ, Hryniuk WM, Morrison VA, Pini TM, Runowicz CD, Rosner GL, Shayne M, Sparreboom A, Sucheston LE, Lyman GH (2012) Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 30(13):1553–1561. 10.1200/jco.2011.39.9436 [DOI] [PubMed] [Google Scholar]
- 34.Oldenhove G, Boucquey E, Taquin A, Acolty V, Bonetti L, Ryffel B, Le Bert M, Englebert K, Boon L, Moser M (2018) PD-1 Is Involved in the Dysregulation of Type 2 Innate Lymphoid Cells in a Murine Model of Obesity. Cell Rep 25(8):2053–2060e2054. 10.1016/j.celrep.2018.10.091 [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, Grossenbacher SK, Withers SS, Rebhun RB, Hartigan-O’Connor DJ, Mendez-Lagares G, Tarantal AF, Isseroff RR, Griffith TS, Schalper KA, Merleev A, Saha A, Maverakis E, Kelly K, Aljumaily R, Ibrahimi S, Mukherjee S, Machiorlatti M, Vesely SK, Longo DL, Blazar BR, Canter RJ, Murphy WJ, Monjazeb AM (2019) Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med 25(1):141–151. 10.1038/s41591-018-0221-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naik A, Monjazeb AM, Decock J (2019) The Obesity Paradox in Cancer, Tumor Immunology, and Immunotherapy: Potential Therapeutic Implications in Triple Negative Breast Cancer. Front Immunol 10:1940. 10.3389/fimmu.2019.01940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, Lane S, Lee DY, Kaper M, McKean M, Beckermann KE, Rubinstein SM, Rooney I, Musib L, Budha N, Hsu J, Nowicki TS, Avila A, Haas T, Puligandla M, Lee S, Fang S, Wargo JA, Gershenwald JE, Lee JE, Hwu P, Chapman PB, Sosman JA, Schadendorf D, Grob JJ, Flaherty KT, Walker D, Yan Y, McKenna E, Legos JJ, Carlino MS, Ribas A, Kirkwood JM, Long GV, Johnson DB, Menzies AM, Davies MA (2018) Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol 19(3):310–322. 10.1016/s1470-2045(18)30078–0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request.