Abstract
Objectives
Plasma P-tau181 is an increasingly established diagnostic marker for Alzheimer’s disease (AD). Further validation in prospective cohorts is still needed, as well as the study of confounding factors that could influence its blood level.
Methods
This study is ancillary to the prospective multicentre Biomarker of AmyLoid pepTide and AlZheimer’s diseAse Risk cohort that enrolled participants with mild cognitive impairment (MCI) who were examined for conversion to dementia for up to 3 years. Plasma Ptau-181 was measured using the ultrasensitive Quanterix HD-X assay.
Results
Among 476 MCI participants, 67% were amyloid positive (Aβ+) at baseline and 30% developed dementia. Plasma P-tau181 was higher in the Aβ+ population (3.9 (SD 1.4) vs 2.6 (SD 1.4) pg/mL) and in MCI that converted to dementia (3.8 (SD 1.5) vs 2.9 (SD 1.4) pg/mL). The addition of plasma P-tau181 to a logistic regression model combining age, sex, APOEε4 status and Mini Mental State Examination improved predictive performance (areas under the curve 0.691–0.744 for conversion and 0.786–0.849 for Aβ+). The Kaplan-Meier curve of conversion to dementia, according to the tertiles of plasma P-tau181, revealed a significant predictive value (Log rank p<0.0001) with an HR of 3.8 (95% CI 2.5 to 5.8). In addition, patients with plasma P-Tau(181) ≤2.32 pg/mL had a conversion rate of less than 20% over a 3-year period. Using a linear regression approach, chronic kidney disease, creatinine and estimated glomerular filtration rate were independently associated with plasma P-tau181 concentrations.
Conclusions
Plasma P-tau181 effectively detects Aβ+ status and conversion to dementia, confirming the value of this blood biomarker for the management of AD. However, renal function significantly modifies its levels and may thus induce diagnostic errors if not taken into account.
Keywords: dementia, alzheimer's disease, biochemistry
WHAT IS ALREADY KNOWN ON THIS TOPIC
The clinical use of plasma phosphorylated tau 181 (P-tau181) for Alzheimer’s disease is being considered but further validation and study of confounding factors are still needed.
WHAT THIS STUDY ADDS
In our large prospective cohort, P-tau181 predicts brain amyloidopathy and conversion to dementia in patients with mild cognitive impairment, but renal function significantly alters plasma levels and thus may induce diagnostic errors if not taken into account.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our study suggests that measurement of creatinine or estimation of glomerular filtration rate, which are easy and standardisable ways to provide information on renal function, will contribute to optimal interpretation of plasma P-tau181 results in routine clinical practice.
Introduction
Alzheimer’s disease (AD) accounts for 60%–70% of dementia and is thus a major public health problem and socioeconomic burden that is only increasing with the ageing of the population. For a long time, diagnostic efforts have been minimal, due to the lack of preventive measures or curative treatment. However, in recent years, it has been demonstrated that modifiable risk factors account for 40% of AD.1 Furthermore, many potential treatments are in the final stages of study.2 Early diagnosis will be the key to further understanding of AD and successfully treating it.
The development of biological biomarkers, linked to amyloid and tau pathology, has greatly contributed to the understanding of the presymptomatic and postsymptomatic AD ‘continuum’, in which, thanks to genetic forms, it has been shown that the disease was present from a biological point of view decades before its clinical appearance.3 This opens up therapeutic perspectives and allows us to use these biomarkers in early diagnosis and even in risk assessment. Their relevance has already been proven in cerebrospinal fluid (CSF) justifying their implementation in clinical routine.4 5 Furthermore, the blood is now amenable to diagnostic assays, thanks to ultrasensitive techniques, including mass spectrometry.
The first analytes used in blood were amyloid peptides. Their levels in serum can detect amyloid positive (Aβ+) patients, as defined by amyloid PET or CSF analysis, and predict evolution of patients within the AD continuum, including conversion of patients, with mild cognitive impairment (MCI), to dementia.6 While the detection of total tau in blood was not so discriminating,7 detection of its phosphorylated forms at position threonine 181 was a real breakthrough.8 P-tau181 concentration is predictive of amyloid or tau PET findings and is significantly higher in AD and MCI compared with subjects without cognitive impairment or compared with other causes of dementia.8–10 Its prognostic value has also been established in some longitudinal studies11–13 and its overall performance allow to consider a clinical application. To reach this milestone, we will need additional prospective data, in vitro diagnostic certified kits and sufficient information on preanalytical stability.14 15 It will equally be paramount to identify any confounding factors that may alter clinical performance in routine use.14
In this work, we used the prospective multicentre Biomarker of AmyLoid pepTide and AlZheimer’s diseAse Risk (BALTAZAR) cohort16 to confirm the ability of plasma P-tau181 to detect brain amyloidopathy and also to predict the conversion of MCI patients to dementia stage. As plasma biomarkers are soon to be implemented in routine practice, we also investigated potential confounding factors that must be considered to interpret the results adequately.
Materials and methods
Study population
The BALTAZAR study is a multicentre prospective cohort study (ClinicalTrials.gov Identifier #NCT01315639) that enrolled patients with MCI or AD, according to a previously described protocol.16 All participants had clinical, neuropsychological, brain MRI and biological assessments (see below). Right and left hippocampal volume was obtain for each participant using automatic segmentation of the hippocampus. The hippocampal volume was normalised using the following calculation: hippocampal volume/total brain volume×mean total brain volume. CSF samples were collected only in accepting participants. APOE was genotyped in a single centralised laboratory. MCI subjects were selected according to the Petersen’ criteria17 and then they were dichotomised into amnestic (aMCI) and non-amnestic (naMCI) phenotypes, based on the presence of memory impairment on the free and cued selective reminding test related to age, sex and educational level. The characteristic of the aMCI/naMCI population is fully described elsewhere.16 Patients had visits every 6 months for 3 years. MCI participants were reassessed for conversion to dementia at each visit by the clinician.6 The progression from MCI to dementia was defined by evaluating the following parameters: (1) decline in cognitive function (measured by changes from the baseline in scores of the Mini Mental State Examination (MMSE)), (2) disability in activities of daily living (ADL) (instrumental ADL (IADL) >1) and (3) clinical dementia rating sum of boxes (>1). The conversions were reviewed by an adjudication committee.
In this study, we analysed 476 available baseline plasma samples from patients with MCI diagnosis (365 aMCI and 111 naMCI).
Biological biomarker measurements
To minimise preanalytical and analytical problems, identical collection tubes were used across centres to collect plasma (EDTA BD Vacutainer K2E, ref 367 525, Becton Dickinson, USA) and for CSF (10 mL polypropylene tube, ref 62.610.201, Sarstedt, Germany). Blood and CSF samples were collected on the same day. All aliquots were stored in the same low-binding Eppendorf LoBind microtubes (Eppendorf, ref 022431064, Hamburg, Germany). Baseline blood samples were used to measure fasting glycaemia, cholesterol (total, high-density lipoproteins (HDL), low-density lipoprotein (LDL)), prealbumin, albumin, creatinine.16 Estimated glomerular filtration rate (eGFR), based on creatinine, age and sex, was computed using the chronic kidney disease (CKD)-Epidemiology Collaboration (CKD-EPI) equation18. CSF biomarkers were measured in a single centralised laboratory using commercially available Innotest assays for tau and phosphorylated tau at position T181 (P-tau181) or Euroimmun for amyloid peptides Aβ1–42 and Aβ1–40. Positive amyloid status (Aβ+) was defined, as previously, when the CSF Aβ1–42/Aβ1–40 ratio was below 0.1.19
Plasma P-tau181 was determined using a commercial P-tau181 assay kit (Quanterix, USA) based on ultrasensitive Simoa technology20 on an HD-X analytical platform. All samples were fourfold diluted with the provided dilution buffer to minimise matrix effects. After dilution, the lowest limit of detection was 0.019 pg/mL and the limit of quantification was of 0.085 pg/mL. Quality controls with low (QC 1 with mean concentration of 3.82 pg/mL) or high (QC 2–52.4 pg/mL) P-tau181 known concentration were provided in the kits. Inter-assay variation for QC 1 and QC 2 was low, with coefficient of variation (CV) of 7% and 5%, respectively. We also used two serum pools (average P-tau181 of 4.47 pg/mL and 2.81 pg/mL) as internal QCs run at the beginning and end of each sample plate. These had low inter-assay CV of 3% and 6%, respectively.
Statistical analyses
General characteristics were analysed in the whole MCI sample, according to MCI subtype (aMCI and naMCI), conversion to dementia and to plasma P-tau181 tertile. Categorical variables are presented as percentages and counts (% (N)); continuous variables, as mean and SD (M (SD)), or median (25–75th percentile), and comparisons were assessed by χ2 tests, t-tests, Mann-Whitney U test and analysis of variance (ANOVA, Kruskal-Wallis test). The relationship between conversion and plasma P-tau181 was assessed using regression models with age, sex and baseline presence of APOE ε4 allele as covariables.
Kaplan-Meier curves were drawn for conversion according to plasma P-tau181tertile and overall differences between tertiles was calculated by log rank test. We also examined how plasma P-tau181 improved dementia risk prediction using logistic regression with age, sex, APOE ε4 and MMSE score at baseline and by calculating continuous net reclassification improvement (NRI).21 Receiving operator characteristic (ROC) curves, using conversion as a dependent variable, were also used to compute for different factors. The corresponding areas under the curve (AUCs) were compared using the Delong method.22 Logistic regression model (enter model), Kaplan Meier and ROC curves were generated with MedCalc (V.20.111) software. In all analyses, the two-sided α-level of 0.05 was used for significance testing.
Results
Characteristics of the MCI participants at baseline
Of the 539 MCI participants enrolled in the BALTAZAR study, 63 were excluded due to missing data or absence of plasma P-tau181 biomarkers. In this study, we analysed, 476 MCI participants (mean age 77.7 (SD 5.5) years, 61.4% women) with 365 aMCI (77%) and 111 naMCI (23%) at baseline (table 1, online supplemental table 1). Average MMSE score was 26.4 (SD 2.5) and 39.8% (n=185) were APOE ε4 carriers. During the clinical follow-up period of 6 to 36 months, 30% (n=144) of the MCI participants developed dementia, on average 14.6 (SD 8.2) months after the baseline visit and in 95% of the cases, they converted to clinically probable AD.6
Table 1.
Characteristics in the whole MCI population and between MCI participants who converted, or not, to dementia within 3 years
| All MCI | MCI non-converters | MCI converters | P value | P$ | |
| N=476 | N=332 | N=144 | |||
| Patient characteristics | |||||
| Age (years) | 77.7 (5.5) | 77.3 (5.4) | 78.5 (5.7) | 0.048 | 0.003 |
| Women (%) | 292 (61.4) | 205 (61.7) | 60.4 | 0.78 | 0.67 |
| BMI (kg/m2) | 25 (3.8) | 25.1 (3.8) | 24.8 (3.8) | 0.39 | 0.95 |
| MMSE (/30) | 26.4 (2.5) | 26.7 (2.5) | 25.6 (2.5) | <0.0001 | 0.0002 |
| 1 or 2 APOE4 alleles | 185 (39.8) | 105 (31.6) | 80 (55.5) | <0.0001 | <0.0001 |
| Hippocampal volume (R+L) (cm3) | 4.55 (1.12) | 4.79 (1.05) | 4.01 (1.09) | <0.0001 | <0.0001 |
| CSF biomarkers* | |||||
| Aβ1–40 (pg/mL) | 7434 (2241) | 7458 (2298) | 7389 (2143) | 0.83 | 0.64 |
| Aβ1–42 (pg/mL) | 766 (384) | 857 (398) | 593 (288) | <0.0001 | <0.0001 |
| Aβ1–42/Aβ1–40 | 0.104 (0.045) | 0.116 (0.045) | 0.082 (0.036) | <0.0001 | <0.0001 |
| Tau (pg/mL) | 578 (254) | 376.1 (185.2) | 547 (223.4) | <0.0001 | <0.0001 |
| p-tau181 (pg/mL) | 76.7 (30.2) | 58.58 (24.47) | 80.3 (33.58) | <0.0001 | <0.0001 |
| Blood biomarkers | |||||
| Fasting glycaemia (mmol/L) | 5.37 (1.19) | 5.34 (1.21) | 5.45 (1.14) | 0.34 | 0.20 |
| Triglycerides (mmol/L) | 1.21 (0.59) | 1.2 (0.58) | 1.24 (0.6) | 0.54 | 0.82 |
| Cholesterol total (mmol/L) | 5.5 (1.16) | 5.48 (1.2) | 5.53 (1.07) | 0.67 | 0.91 |
| Cholesterol HDL (mmol/L) | 1.74 (0.52) | 1.75 (0.53) | 1.72 (0.48) | 0.66 | 0.98 |
| Cholesterol LDL (mmol/L) | 3.2 (1) | 3.19 (1.01) | 3.24 (0.98) | 0.65 | 0.90 |
| Prealbumin (mg/dl) | 27.6 (5.4) | 28.1 (6.2) | 27.7 (5.8) | 0.53 | 0.52 |
| Albumin (g/L) | 40.3 (3.9) | 40.5 (3.5) | 39.8 (4.5) | 0.09 | 0.11 |
| Creatinine (μmol/L) | 78.2 (21.3) | 79.4 (22.2) | 73.7 (13.6) | 0.10 | 0.82 |
| eGFR (mL/min/1.73 m2) | 76.9 (14.7) | 77.0 (14.8) | 76.7 (14.7) | 0.85 | 0.80 |
| Plasma P-tau181(pg/mL) | 3.19 (1.49) | 2.9 (1.4) | 3.8 (1.5) | <0.0001 | <0.0001 |
P: Comparison between the three groups, by ANOVA or χ2; P$: comparison between the three groups by linear regression adjusted for age, sex and the presence of the APOE ε4 allele; % (number) were used to describe categorical variable, mean±SD for continuous variables.
*CSF biomarkers were available in 140 and 74 MCI non-converters and converters, respectively.
ANOVA, analysis of variance; APOE, apolipoprotein E; BMI, body mass index; CSF, cerebrospinal fluid; eGFR, estimated glomerular filtration rate; MCI, mild cognitive impairment; MMSE, Mini Mental State Examination; R+L, right+left.
jnnp-2022-330540supp001.pdf (97.3KB, pdf)
Comparison between MCI converters and non-converters: P-tau predicts conversion to AD
At baseline, the MCI converters to dementia were older, had a lower MMSE score, were more often APOE ε4 carriers and had more severe hippocampal atrophy (table 1). As lumbar puncture was optional in our cohort, CSF values were available in 214 subjects. CSF Aβ1–40, Aβ1–42, tau and p-tau181 values were highly differential between MCI that converted or not.
We next turned our attention to the efficacy of markers in the blood, as these samples were available from all participants. We have previously described differential levels of plasma amyloid biomarkers in the BALTAZAR cohort.6 Here we found plasma P-tau181 increased significantly, on average by 30%, between MCI non-converters at 2.9 (SD 1.4) pg/mL and converters at 3.8 (SD 1.5) pg/mL (p<0.0001). Plasma P-tau remained significant after adjustment for age, sex and APOE ε4 status (table 1). No difference was observed between MCI converters and non-converters for metabolic or renal function blood biomarkers: fasting blood glucose (glycaemia), triglycerides, cholesterol (total, HDL, LDL), prealbumin, albumin and creatinine or eGFR.
Comparison between Aβ+ and Aβ− patients: tau predicts amyloid status
The amyloid status, Aβ+ corresponding to the (A+) ATN classification,3 was defined based on the CSF Aβ1–42/Aβ1–40 ratio.23 Almost half of the MCI participants had a lumbar puncture and 117 of them were Aβ+ and 97 were Aβ−. At baseline, Aβ+ patients were older, had a lower MMSE score and were more often APOE ε4 carriers. However, unlike MCI converters, Aβ+ patients did not have a lower hippocampal volume (table 2). CSF Aβ1–42, Tau and p-tau181 values were also highly differential between Aβ+ and Aβ− patients. Plasma P-tau181 was significantly higher on average by 50% in Aβ+ than in Aβ− (3.9 (SD 1.4) vs 2.6 (SD 1.4) pg/mL, p<0.0001). This difference remained significant after adjustment for age, sex and APOE ε4 status (table 2).
Table 2.
Characteristics in the whole population and between Aβ− and + patients
| All | Aβ− | Aβ+ | P value | P value$ | |
| N=214 | N=97 | N=117 | |||
| Patient characteristics | |||||
| Age (years) | 77.4 (5.6) | 76.6 (5.1) | 78 (5.9) | 0.073 | 0.0122 |
| Women (%) | 127 (59.3) | 53 (54.6) | 74 (64.9) | 0.20 | 0.36 |
| BMI (kg/m2) | 24.7 (3.7) | 25.4 (3.7) | 24.2 (3.6) | 0.024 | 0.26 |
| MMSE (/30) | 26.4 (2.4) | 27.1 (2) | 25.8 (2.5) | <0.0001 | 0.0007 |
| One or 2 APOE4 alleles | 78 (36.4) | 15 (15.5) | 63 (57.3) | <0.0001 | <0.0001 |
| Hippocampal volume (R+L) (cm3) | 4.56 (1.09) | 4.64 (1.23) | 4.5 (0.96) | 0.41 | 0.91 |
| CSF biomarkers | |||||
| Aβ1–40 (pg/mL) | 7434 (2241) | 7421 (1896) | 7446 (2499) | 0.93 | 0.62 |
| Aβ1–42 (pg/mL) | 766 (385) | 1095 (287) | 494 (196) | <0.0001 | <0.0001 |
| Aβ1–42/Aβ1–40 | 0.104 (0.045) | 0.149 (0.022) | 0.068 (0.018) | <0.0001 | <0.0001 |
| Tau (pg/mL) | 433 (213) | 320 (133) | 533 (222) | <0.0001 | <0.0001 |
| p-tau181 (pg/mL) | 66.4 (30.2) | 51.3 (15.1) | 79 (33.8) | <0.0001 | <0.0001 |
| Blood biomarkers | |||||
| Fasting glycaemia (mmol/L) | 5.4 (1.1) | 5.4 (1.1) | 5.4 (1.2) | 0.99 | 0.71 |
| Triglycerides (mmol/L) | 1.17 (0.6) | 1.18 (0.48) | 1.15 (0.69) | 0.70 | 0.62 |
| Cholesterol total (mmol/L) | 5.5 (1.2) | 5.5 (1.3) | 5.5 (1.1) | 0.78 | 0.29 |
| Cholesterol LDL (mmol/L) | 1.7 (0.5) | 1.8 (0.5) | 1.7 (0.5) | 0.58 | 0.81 |
| Cholesterol HDL (mmol/L) | 3.2 (1) | 3.3 (1.1) | 3.2 (0.9) | 0.92 | 0.21 |
| Prealbumin (mg/dL) | 28.4 (6.4) | 28.5 (7.6) | 28.3 (5.1) | 0.77 | 0.77 |
| Albumin (g/L) | 40.1 (4.3) | 39.9 (3.6) | 40.2 (4.9) | 0.60 | 0.38 |
| Creatinine (μmol/L) | 79.0 (23.0) | 80.1 (24.9) | 78.2 (21.5) | 0.54 | 0.76 |
| eGFR (mL/min/1.73 m2) | 76.9 (14.7) | 76.9 (15.5) | 77.5 (15.5) | 0.62 | 0.45 |
| Plasma P-tau181(pg/mL) | 3.3 (1.5) | 2.6 (1.4) | 3.9 (1.4) | <0.0001 | <0.0001 |
P: Comparison between the three groups, by ANOVA or χ2); P$: comparison between the three groups by linear regression adjusted for age, sex and the presence of the APOE ε4 allele; % (number) were used to describe categorical variables, mean±SD for continuous variables. HDL, high-density lipoproteins; LDL, low-density lipoproteins.
ANOVA, analysis of variance; APOE, apolipoprotein E; Aβ+, amyloid positive; BMI, body mass index; CSF, cerebrospinal fluid; eGFR, estimated glomerular filtration rate; MCI, mild cognitive impairment; MMSE, Mini Mental State Examination; R+L, right+left.
P-tau181 improves predictive power of age, sex, APOEε4 status and MMSE for MCI conversion and amyloid status detection
Using a logistic regression approach with conversion as a dependent variable and age, sex, APOEε4 status and MMSE as independent variables, it was possible to predict conversion (p<0.0001) with an AUC of the model fit of 0.691 (95% CI 0.655 to 0.741). The addition of plasma P-tau181 resulted in a significant increase of the AUC to 0.744 (95% CI 0.702 to 0.784) (online supplemental table 3). The added value of plasma P-tau181 was further documented by computing the NRI of the two models. This revealed a 12.8% improvement in patient classification between MCI converters and non-converters due to plasma P-tau181. Since blood biomarkers are intended to replace CSF biomarkers, we compared the respective values of plasma and CSF P-tau181 for amyloid status detection in the subcohort where patients had undergone a lumbar puncture. The addition in the model of plasma or CSF P-tau181 resulted in a significant increase of the AUC from 0.786 (95% CI 0.723 to 0.84) for age, sex, APOEε4 status and MMSE to 0.849 (95% CI 0.792 to 0.895) and 0.857 (95% CI 0.801 to 0.902), respectively (P(difference)=0.00075 and 0.0002). AUCs obtained by the addition of plasma or CSF P-tau181 for conversion or amyloid status detection (0.750 and 0.752, respectively) were not different (P(difference)=0.81). Importantly, when creatinine or eGFR were associated with P-tau181 using logistic regression, they did not give in better performance models.
Association of plasma P-tau181 with other biomarkers and cohort characteristics
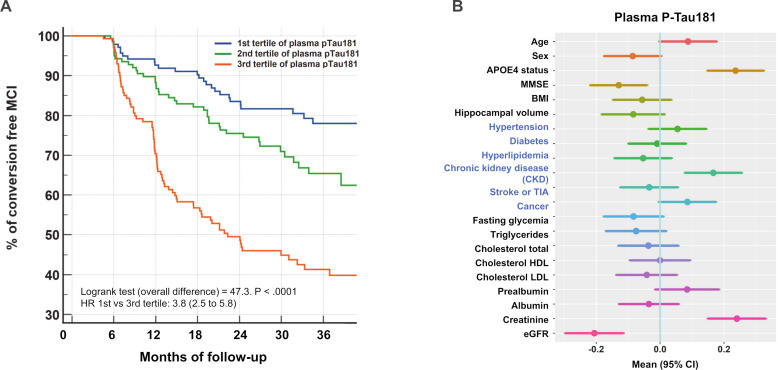
The relationships and correlations between plasma P-tau181 concentration and the other biomarkers and cohort characteristics were analysed after splitting the population by tertile (table 3). Age, body mass index (BMI) and APO ε4 were significantly different between tertile. Patient conversion rate was also clearly correlated with plasma P-tau181, with values of 16.4%, 26.1% and 47.8% in the first, second and third tertile, respectively. Distribution of Aβ+ patients was also greatly increased along with tertile, a relationship that was confirmed by the high correlation observed between plasma P-tau181 and CSF Aβ1–42/Aβ1–40 use to define the Aβ+ status (correlation coefficient=−0.4428, p<0.0001). eGFR decreased (p=0.017) while creatinine very significantly increased (p<0.0001) in the higher plasma P-tau181 tertiles. All these results remained significant after adjustment for age, sex and APOE ε4 status (table 3). The relationship between plasma P-tau181 and MCI conversion was further documented by plotting the Kaplan-Meier curve of conversion to dementia according to the tertiles (figure 1A). A very significant overall difference was observed (Log rank p<0.0001) and the HR between the first and the third tertile was 3.8 (95% CI 2.5 to 5.8).
Table 3.
Characteristics in the different P-tau181 tertiles
| First tertile | Second tertile | Third tertile | P value | P value$ | |
| N=158 | N=157 | N=161 | |||
| Plasma P-tau181 | |||||
| Age (years) | 76.9 (5.4) | 77.9 (5.1) | 78.3 (5.9) | 0.06 | 0.0009 |
| Women (%) | 101 (63.9) | 96 (61.1) | 95 (59.0) | 0.66 | 0.32 |
| BMI (kg/m2) | 25.8 (3.7) | 24.9 (3.9) | 24.4 (3.7) | 0.004 | 0.01 |
| MMSE (/30) | 26.7 (2.4) | 26.4 (2.5) | 26.1 (2.7) | 0.16 | 0.19 |
| 1 or 2 APOE4 alleles (%) | 38 (17.7) | 63 (40.1) | 84 (52.2) | <0.0001 | <0.0001 |
| Hippocampal volume (R+L) (cm3) | 4.75 (1.13) | 4.38 (1.16) | 4.5 (1.04) | 0.02 | 0.28 |
| Aβ+ status (%) | 13 (19.1) | 40 (62.5) | 64 (78.0) | <0.0001 | <0.0001 |
| Conversion MCI (%) | 26 (16.4) | 41 (26.1) | 77 (47.8) | <0.0001 | <0.0001 |
| Blood biomarkers | |||||
| Fasting glycaemia (mmol/L) | 5.46 (1.28) | 5.4 (1.1) | 5.26 (1.18) | 0.32 | 0.12 |
| Triglycerides (mmol/L) | 1.2 (0.6) | 1.3 (0.7) | 1.2 (0.5) | 0.26 | 0.23 |
| Cholesterol (mmol/L) | 5.52 (1.19) | 5.48 (1.23) | 5.5 (1.06) | 0.94 | 0.82 |
| Cholesterol HDL (mmol/L) | 1.72 (0.47) | 1.76 (0.56) | 1.75 (0.52) | 0.85 | 0.29 |
| Cholesterol LDL (mmol/L) | 3.25 (1.03) | 3.15 (1.07) | 3.21 (0.9) | 0.69 | 0.59 |
| Prealbumin (mg/dL) | 27.5 (6.2) | 28 (6.7) | 28.4 (5.4) | 0.47 | 0.17 |
| Albumin (g/L) | 40.4 (3.7) | 40.2 (4.8) | 40.3 (3.1) | 0.87 | 0.90 |
| Creatinine (μmol/L) | 73.5 (16.5) | 78.6 (19) | 82.1 (26.1) | 0.002 | 0.0005 |
| eGFR (mL/min/1.73 m2) | 80.3 (13.5) | 76 (14.1) | 74.4 (15.9) | 0.02 | 0.01 |
| Plasma P-tau181(pg/mL) | 1.75 (0.38) | 2.93 (0.36) | 4.86 (1.19) | NA | NA |
P: Comparison between the three groups, by ANOVA or χ2); P$: comparison between the three groups with linear regression adjusted for age, sex and the presence of the APOE ε4 allele; % (number) were used to describe categorical variables, mean±SD for continuous variables.
ANOVA, analysis of variance; APOE, apolipoprotein E; BMI, body mass index; eGFR, estimated glomerular filtration rate; MCI, mild cognitive impairment; MMSE, Mini Mental State Examination; NA, not available; R+L, right+left.
Figure 1.
(A): Kaplan-Meier curve of conversion to dementia according to the tertiles of plasma P-tau181 in MCI subjects. (B): Associations between multiple factors and plasma P-tau181 concentrations. Forest plots of associations between demographic, comorbidities (in blue) and biological variables and plasma P-tau181, using linear regression. Means and 95% CIs are provided. Z-scores are used to compare the factors between them. APOE, apolipoprotein E; BMI, body mass index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MCI, mild cognitive impairment; MMSE, Mini Mental State Examination; TIA, transient ischaemic attack; HDL, high-density lipoproteins; LDL, low-density lipoproteins.
Impact of comorbidities and covariates on P-tau181 concentration and diagnostic performance
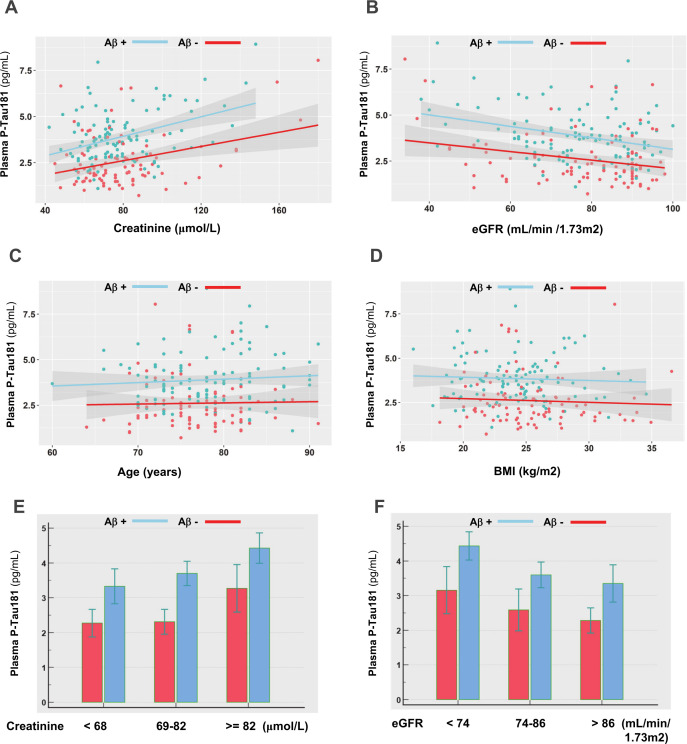
The relationship between plasma P-tau181 concentration and comorbidities, demographic factors and biological information collected at baseline in the BALTAZAR cohort was investigated using a linear regression approach. We identified APOE status, creatinine and eGFR as strongly connected to plasma P-tau181 (figure 1B). To a lesser degree, age and BMI also affected plasma P-tau181levels. Among comorbidities we tested, chronic kidney disease (CKD) appeared strongly linked to P-tau181 levels. All these results remained very similar after adjustment for age, sex and APOE ε4 status. To further evaluate the impact of these covariates, we plotted the correlation between creatinine, eGFR, BMI and age in the population stratified by amyloid status (figure 2A–D). The correlation in the BALTAZAR subpopulation with lumbar puncture (n=214) remained significant only for eGFR and creatinine (online supplemental table 2). We observed higher values of plasma P-tau181 in the Aβ+ population. P-tau181 levels were also correlated with low and high values of eGFR and creatinine. To confirm this observation, we stratified the population based on tertile of creatinine or eGFR levels (figure 2E,F, table 4). The ANOVA confirmed that the mean level of plasma P-tau181 was globally differential among both creatinine or eGFR tertiles (p<0.001). Finally, to evaluate the impact on P-tau181 on the detection of amyloid status, we computed the ROC curves and determined best cutpoints and corresponding performance for Aβ+ (table 4).
Figure 2.
(A–D): Correlation between plasma P-tau181 and creatinine, eGFR, age and BMI in the amyloid negative and positive populations. (E, F): Levels of plasma P-tau181 in the amyloid negative and positive populations, by tertiles of creatinine or eGFR. Concentrations of plasma P-tau181 were significantly different between amyloid negative and positive patients in all cases, as tested using a Mann-Whitney U test (p<0.05). Aβ+, amyloid positive; BMI, body mass index; eGFR, estimated glomerular filtration rate (unit: mL/min/1.73 m2).
Table 4.
Performance of plasma P-tau181 for Aβ+ detection with regard to renal function (creatinine and eGFR)
| Population with CSF biomarkers available | Total | Creatinine <68 First tertile |
Creatinine 69–82 Second tertile |
Creatinine ≥82 Third tertile |
| Creatinine (μmol/L) | 74 (71–77) | 61 (57–64) | 73 (71–80) | 95 (84–111) |
| Plasma P-tau 181(pg/mL) | 3.0 (2.8–3.4) | 2.3 (1.8–3.3) | 2.9 (2.5–3.9) | 3.7 (2.7–5.2) |
| Plasma P-tau 181 in Aβ− | 2.2 (1.9–2.4) | 1.9 (1.5–2.4) | 2.3 (1.6–2.7) | 2.9 (1.9–4.0) |
| Plasma P-tau 181 in Aβ+ | 3.7 (2.8–4.5) | 3.1 (2.2–4.3) | 3.6 (2.9–4.0) | 4.2 (3.6–5.4) |
| AUC Plasma P-tau 181 Aβ+ | 0.783 | 0.763 | 0.872 | 0.733 |
| Plasma P-tau 181 cutpoints (pg/mL) | 2.77 | 2.31 | 2.77 | 3.52 |
| Sensitivity (%) | 80.9 | 75.0 | 89.5 | 80.0 |
| Specificity (%) | 69.6 | 73.0 | 80.0 | 70.0 |
| Population with CSF biomarkers available | Total |
eGFR <74
first tertile |
eGFR 74–86
second tertile |
eGFR >86
third tertile |
| eGFR (mL/min/1.73 m2) | 79 (68–89) | 64 (54–69) | 79 (76–84) | 91 (89–93) |
| Plasma P-tau 181(pg/mL) | 3.0 (2.1–3.9) | 3.5 (2.4–4.2) | 3.1 (2.1–3.9) | 2.7 (1.8–3.9) |
| Plasma P-tau 181 in Aβ− | 2.2 (1.7–3.0) | 2.7 (1.7–3.4) | 2.0 (1.7–2.6) | 2.0 (1.6–2.9) |
| Plasma P-tau 181 in Aβ+ | 3.7 (2.8–4.5) | 3.8 (3.1–5.4) | 3.8 (2.8–4.5) | 3.3 (2.7–4.2) |
| AUC Plasma P-tau 181 Aβ+ | 0.783 | 0.763 | 0.872 | 0.733 |
| Plasma P-tau 181 cutpoints (pg/mL) | 2.77 | 2.81 | 2.71 | 2.44 |
| Sensitivity (%) | 80.9 | 86.4 | 84.2 | 78.8 |
| Specificity (%) | 69.6 | 60.0 | 77.8 | 68.6 |
Values of creatinine and plasma P-tau181 are express as median (25–75th percentile). Cutpoints correspond to the best Youden index on the ROC curves.
AUC, area under the curve; Aβ+, amyloid positive; CSF, cerebrospinal fluid; eGFR, estimated glomerular filtration rate; ROC, receiving operator characteristic.
Discussion
Here, we present results from a large-scale multicentre prospective longitudinal cohort of clinically defined MCI participants, referred to memory centre, with a follow-up of 3 years. Our principal finding is that patients who convert to dementia have 30% higher levels of plasma P-tau181 independently of age, sex or APOE ε4. Importantly, 48% of MCI participants among the highest tertile of plasma P-tau181 (>3.61) converted to dementia and thus had a fourfold higher risk. In addition, patients in the first P-tau(181) tertile (ie, with a value ≤2.32 pg/mL) have a conversion rate of 19.8% over a 3-year period. It is likely that combining P-tau(181) with other blood biomarkers such as plasma amyloid peptides could improve this prediction. This information is valuable for patient management and for using therapeutic strategies to prevent progression. In this MCI population, plasma P-tau181 also predicted amyloid status (based on the CSF Aβ1–42/Aβ1–40 ratio), with Aβ+ patients having 50% higher P-tau181 levels than their Aβ− counterparts.
One important element that raises the interest of using this plasma biomarker in the future is its added value above that of just using a combination of age, sex, APOEε4 status and MMSE. It is noteworthy that adding plasma P-tau181 significantly improved the detection of both Aβ+ patients and MCI converters. Even more striking is that this added value of plasma P-tau181 was equivalent to that of CSF P-tau181. This finding will impact future clinical use of the approach, as it might avoid the need for lumbar puncture. The capacity of plasma P-tau181 to detect Aβ+ patients as well as AD and MCI when compared with control and to other diseases has been described.8 9 11–13 24–27 However, the only previous other large study focusing on MCI conversion was that of Karikari et al 11 who observed that baseline concentrations of plasma P-tau181 accurately predicted future dementia and Aβ+ status (as defined by PET). As well as validating this previous study, our study has the added value of the biological data collected in the BALTAZAR cohort. These include metabolic blood biomarkers: fasting glycaemia, triglycerides, cholesterol (total, HDL, LDL), prealbumin, albumin, creatinine and eGFR, which can be used to monitor diabetes, cardiovascular risk, nutritional status or kidney function. None of these factors were differential, either in comparing MCI converters to non-converters, or when comparing Aβ+ and Aβ− patients. However, when we investigated factors influencing P-tau181 level, by comparing tertiles or through a linear regression method, we first identified age and BMI as cofounding factors. These two factors have previously been associated with P-tau181, as well as with other blood biomarkers like neurofilaments.28 Age increases both plasma and CSF values of neurodegenerative biomarkers like total tau29 yet to be determined reasons. For BMI, a likely relevant factor is the dilution of neuronal biomarkers in the blood volume.
Among the comorbidities that were associated with P-tau181, CKD was the most differential. This association with CKD has already been reported in recent studies.30 31 However, in the BALTAZAR cohort, we have access to the clinical chemistry profile realised on the same plasma sample used for P-tau181 measurement. We thus noted that P-tau181 correlates with markers of kidney function: creatinine and eGFR. Adding these parameters improved the ability of P-tau181 to detect Aβ+ patients, whereas adding age and BMI did not. Strikingly P-tau181 and creatinine stratify together irrespective of other variables. Namely, in situations with increased creatinine (≥82 µmol/L) or low eGFR (<74 mL/min/1.73 m2), indicating a moderate impaired kidney function, levels of P-tau181 were increased in both Aβ+ and Aβ− patients, as well as in MCI patients converting or not to dementia.
A major suggestion of our study is tailoring the clinical cutpoints of P-tau181 to renal function. We advocate minimising the false detection of a pathological situation in patients by always combining plasma P-tau181 with an assessment of renal function, for example, through creatinine measurement and GFR estimation. This recommendation should be confirmed for other P-tau isoforms (P-tau217, P-tau231) measured by immunoassay25 or mass spectrometry.32 We cannot exclude at this stage that altered renal function may also contribute in some way to the progression of AD.33 Indeed, this hypothesis is supported by the difference in creatine level between naMCI and aMCI population. To understand the relationship between renal function and P-tau levels, i’s clearance by the kidney will therefore have to be studied in more detail. Of note, only very small amounts of this biomarker were detected in the free form or associated with exosome in urine.34 35
The present study has some limitations. To increase the likelihood of conversion to AD we excluded participants with Lewy body, Parkinson, frontotemporal or vascular MCI disorders. Therefore, 77% of subjects had aMCI and 30% of participants developed dementia which in 95% of cases was represented by probable AD. Amyloid status was available in only a part of the population, since the BALTAZAR study focused on conversion, and it was defined using CSF biomarkers rather than with PET amyloid.
The main strengths of the study lie in the large sample size of MCI participants that are well described, the controlled preanalytical conditions, the centralised plasma P-tau181 analyses and the availability of clinical chemistry analyte measurement realised in the same sample tube.
Conclusion
This study of our well-characterised population confirms the clinical relevance of plasma P-tau181 for the detection of amyloid status, which is important for risk assessment, patient management and inclusion in clinical trials. We also demonstrate the strong predictive value of this blood biomarker for the prognosis of MCI patients, thus addressing an important medical need in memory centres. The question remains as to the use of blood biomarkers as a screening tool in patients without cognitive impairment who have risk factors and may benefit most from preventive strategies, and/or as triage tests in patients with early symptoms for whom future investigations, including imaging and spinal tap, are being considered. Finally, we identified and quantified the impact of renal function, assessed by creatinine levels and GFR estimation, on P-tau181 blood levels. These measures are an easy and standardisable way to provide essential information about kidney function and thus to optimise interpretation of results in routine clinical practice.
Footnotes
Twitter: @sylvain_lehmann
Collaborators: BALTAZAR study groupOlivier Hanon [1], Frédéric Blanc [2], Yasmina Boudali [1], Audrey Gabelle [3], Jacques Touchon [3], Marie-Laure Seux [1], Hermine Lenoir [1], Catherine Bayle [1], Stéphanie Bombois [4], Christine Delmaire [4], Xavier Delbeuck [5], Florence Moulin [1], Emmanuelle Duron [6], Florence Latour [7], Matthieu Plichart [1], Sophie Pichierri [8], Galdric Orvoën [1], Evelyne Galbrun [9], Giovanni Castelnovo [10], Lisette Volpe-Gillot [11], Florien Labourée [1], Pascaline Cassagnaud [12], Claire Paquet [13], Françoise Lala [14], Bruno Vellas [14], Julien Dumurgier [13], Anne-Sophie Rigaud [1], Christine Perret-Guillaume [15], Eliana Alonso [16], Foucaud du Boisgueheneuc [17], Laurence Hugonot-Diener [1], Adeline Rollin-Sillaire [12], Olivier Martinaud [18], Clémence Boully [1], Yann Spivac [19], Agnès Devendeville [20], Joël Belmin [21], Philippe-Henri Robert [22], Thierry Dantoine [23], Laure Caillard [1], David Wallon [24], Didier Hannequin [18], Nathalie Sastre [14], Sophie Haffen [25], Anna Kearney-Schwartz [15], Jean-Luc Novella [26], Vincent Deramecourt [12], Valérie Chauvire [27], Gabiel Abitbol [1], Nathalie Schwald [19], Caroline Hommet [28], François Sellal [29], Marie-Ange Cariot [16], Mohamed Abdellaoui [30], Sarah Benisty [31], Salim Gherabli [1], Pierre Anthony [29], Frédéric Bloch [32], Nathalie Charasz [1], Sophie Chauvelier [1], Jean-Yves Gaubert [1], Guillaume Sacco [22], Olivier Guerin [22], Jacques Boddaert [33], Marc Paccalin [17], Marie-Anne Mackowiak [12], Marie-Thérèse Rabus [9], Valérie Gissot [34], Athanase Benetos [15], Candice Picard [20], Céline Guillemaud [35], Gilles Berrut [8], Claire Gervais [22], Jaques Hugon [13], Jean-Marc Michel [29], JeanPhilippe David [19], Marion Paulin [12], Pierre-Jean Ousset [14], Pierre Vandel [36], Sylvie Pariel [21], Vincent Camus [37], Anne Chawakilian [1], Léna Kermanac’h [1], Anne-Cécile Troussiere [12], Cécile Adam [23], Diane Dupuy [20], Elena Paillaud [16], Hélène Briault [9], Isabelle Saulnier [38], Karl Mondon [37], Marie-Agnès Picat [23], Marie Laurent [16], Olivier Godefroy [20], Rezki Daheb [16], Stéphanie Libercier [29], Djamila Krabchi [1], Marie Chupin [39], Luc Buée [42], JeanSébastien Vidal [1], Edouard Chaussade [1], Sylvain Lehmann [40], Bernadette Allinquant [41], Susanna Schraen-Maschke [42]. Affiliations of the BALATAZAR group[1] Université de Paris, EA 4468, APHP, Hopital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, F-75013 Paris, France. [2] Université de Strasbourg, CHRU de Strasbourg, Memory Resource and Research Centre of Strasbourg/Colmar, French National Centre for Scientific Research (CNRS), ICube Laboratory and Fédération de Médecine Translationnelle de Strasbourg (FMTS), Team Imagerie Multimodale Intégrative en Santé (IMIS)/Neurocrypto, F-67000 Strasbourg, France. [3] Université de Montpellier, CHRU Montpellier, Memory Research and Resources center, department of Neurology, Inserm INM NeuroPEPs team, excellence center of neurodegenerative disorders, F-34000 Montpellier, France. [4] Univ. Lille, CHU Lille, Memory Resource and Research Centre of Lille, Department of Neurology, Inserm U1171, Degenerative and Vascular Cognitive Disorders, F-59000 Lille, France. [5] Univ. Lille, Inserm U1171 Degenerative and Vascular Cognitive Disorders, F-59000 Lille, France. [6] Université Paris-Saclay, APHP, Hôpital Paul Brousse, département de gériatrie, UVSQ, Inserm, CESP, Team MOODS, F-94800 Villejuif, France. [7] Centre Hospitalier de la Côte Basque, Department of Gerontology, F-64100 Bayonne, France. [8] Université de Nantes, EA 4334 Movement-Interactions-Performance, CHU Nantes, Memory Research Resource Center of Nantes, F-44000 Nantes, France. [9] Sorbonne Universités, AP-HP, Centre Hospitalier Émile-Roux, Department of Gérontology 2, F-94450 Limeil-Brévannes, France. [10] CHU de Nimes, Neurology Department, F-30000 Nimes, France. [11] Hôpital Léopold Bellan, Memory Clinic, F-75014 Paris, France. [12] Univ. Lille, CHU de Lille, Memory Resource and Research Centre of Lille, Department of Neurology, F-59000 Lille, France. [13] Université de Paris, APHP, Groupe Hospitalier Saint Louis-LariboisièreFernand Widal, Memory Resource and Research Centre of Paris Nord-Ile de France, F-75010 Paris, France. [14] Université de Toulouse III, CHU La Grave-Casselardit, Memory Resource and Research Centre of Midi-Pyrénées, F-31300 Toulouse, France. [15] Université de Lorraine, CHRU de Nancy, Memory Resource and Research Centre of Lorraine, F-54000 Nancy, France. [16] AP-HP, HEGP, Service de Gériatrie, F-75015, Paris, France. [17] CHU de Poitiers, Memory Resource and Research Centre of Poitiers, F-86000 Poitiers, France. [18] CHU Charles Nicolle, Memory Resource and Research Centre of HauteNormandie, F-76000 Rouen, France. [19] APHP, Centre Hospitalier Émile-Roux, Department of Gérontology 1, F-94450 Limeil-Brévannes, France. [20] CHU d’Amiens-Picardie, Memory Resource and Research Centre of Amiens Picardie, F-80000 Amiens, France. [21] APHP, Hôpitaux Universitaires Pitie-Salpêtrière-Charles Foix, Service de Gériatrie Ambulatoire, F-75013 Paris, France. [22] CHU de Nice, Memory Research Resource Center of PACA Est, F-06100 Nice, France. [23] CHU de Limoges, Memory Research Resource Center of Limoges, F-87000 Limoges, France. [24] Université de Rouen Normandie, CHU Charles Nicolle, Memory Resource and Research Centre of Haute-Normandie, Inserm U1079, IRIB, F-76000 Rouen, France. [25] CHU de Besançon, Memory Resource and Research Centre of BesançonFranche-Comté, F-25000 Besançon, France. [26] Université de Reims Champagne Ardenne, EA 3797, CHU de Reims, Memory Resource and Research Centre of Champagne Ardenne, F-51100 Reims, France. [27] CHU d’Angers, Memory Resource and Research Centre of Angers, F-49000 Angers, France. [28] CHRU de Tours, Memory Resource and Research Centre of Tours, F-37000 Tours, France. [29] Université de Strasbourg, CHRU de Strasbourg, Memory Resource and Research Centre of Strasbourg/Colmar, Inserm U-118, F-67000 Strasbourg, France. [30] Univ Paris Est Creteil, EA 4391 Excitabilité Nerveuse et Thérapeutique, CHU Henri Mondor, Department of Neurology, F-94000 Créteil, France. [31] Fondation ophtalmologique Adolphe de Rothschild, Department of Neurology, F-75019 Paris, France. [32] CHU d’Amiens-Picardie, Department of Gerontology, F-80000 Amiens, France. [33] Sorbonne Universités, APHP, Hôpitaux Universitaires Pitie-Salpêtrière-Charles Foix, Memory Resource and Research Centre, Centre des Maladies Cognitives et Comportementales IM2A, Inserm UMR 8256, F-75013 Paris, France. [34] Université François-Rabelais de Tours, CHRU de Tours, Memory Resource and Research Centre of Tours, Inserm CIC 1415, F-37000 Tours, France. [35] Sorbonne Universités, APHP, Hôpitaux Universitaires Pitie-Salpêtrière-Charles Foix, Memory Resource and Research Centre, Centre des Maladies Cognitives et Comportementales IM2A, F-75013 Paris, France. [36] Université Bourgogne Franche-Comté, EA 481 Neuroscience, IFR 133, CHU de Besançon, Memory Resource and Research Centre of BesançonFranche-Comté, F-25000 Besançon, France. [37] Université François-Rabelais de Tours, CHRU de Tours, Memory Resource and Research Centre of Tours, Inserm U930 Imagerie et Cerveau, F-37000 Tours, France. [38] Université de Limoges, EA 6310 HAVAE, CHU de Limoges, Memory Research Resource Center of Limoges, F-87000 Limoges, France. [39] Université Paris-Saclay, Neurospin, CEA, cati-neuroimaging.com, CATI Multicenter Neuroimaging Platform, F-91190 Gif-sur-Yvette, France. [40] LBPC-PPC, Université de Montpellier, INM INSERM, IRMB CHU de Montpellier, Montpellier, France. [41] Université de Paris, Inserm UMR-S 894, F-75014 Paris, France. [42] Univ. Lille, Inserm, CHU Lille, U1172-LilNCog, LiCEND, LabEx DISTALZ, F-59000 Lille, France.
Contributors: SL, J-SV and OH take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: OH, SB, AG, SS-M and SL. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: SL. Critical revision of the manuscript for important intellectual content: All authors.Statistical analysis: SL and J-SV. Obtained funding: OH, SB, AG, SS-M and SL. All authors had full access to the data and contributed to revision and editing of the manuscript. SL is responsible for the overall content as the guarantor.
Funding: The French ministry of Health (Programme Hospitalier de Recherche Clinique), Grant/Award Numbers:PHRC2009/01-04,PHRC-13-0404; The Foundation Plan Alzheimer; Fondation pour la Recherche Médicale (FRM); The Gerontopôle d’Ile de France (GEROND'IF). None of the funding bodies had any role in study design, in the collection, analysis, and interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data and informed consent form are available upon request after publication (APHP, Paris). Requests will be considered by each study investigator based on the information provided by the requester regarding the study and analysis plan. If the use is appropriate, a data sharing agreement will be put in place before distributing a fully de-identified version of the dataset, including the data dictionary used for analysis with individual participant data.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by The study was approved by the Paris EthicsCommittee (CPP Ile de France IV Saint Louis Hospital).ClinicalTrials.gov Identifier#NCT01315639 Participants gave informed consent to participate in the study before taking part.
References
- 1. Lee M, Whitsel E, Avery C, et al. Variation in population attributable fraction of dementia associated with potentially modifiable risk factors by race and ethnicity in the US. JAMA Netw Open 2022;5:e2219672. 10.1001/jamanetworkopen.2022.19672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Piton M, Hirtz C, Desmetz C, et al. Alzheimer's disease: advances in drug development. J Alzheimers Dis 2018;65:3–13. 10.3233/JAD-180145 [DOI] [PubMed] [Google Scholar]
- 3. Jack CR, Bennett DA, Blennow K, et al. NIA‐AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer's & Dementia 2018;14:535–62. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dumurgier J, Schraen S, Gabelle A, et al. Cerebrospinal fluid amyloid-β 42/40 ratio in clinical setting of memory centers: a multicentric study. Alzheimers Res Ther 2015;7:30. 10.1186/s13195-015-0114-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gabelle A, Dumurgier J, Vercruysse O, et al. Impact of the 2008-2012 French Alzheimer plan on the use of cerebrospinal fluid biomarkers in research memory center: the PLM study. J Alzheimers Dis 2013;34:297–305. 10.3233/JAD-121549 [DOI] [PubMed] [Google Scholar]
- 6. Hanon O, Vidal Jean‐Sébastien, Lehmann S, et al. Plasma amyloid beta predicts conversion to dementia in subjects with mild cognitive impairment: the BALTAZAR study. Alzheimer's & Dementia 2022. (published Online First: 2022/02/22). 10.1002/alz.12613 [DOI] [PubMed] [Google Scholar]
- 7. Zetterberg H, Wilson D, Andreasson U, et al. Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther 2013;5:9. 10.1186/alzrt163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mielke MM, Hagen CE, Xu J, et al. Plasma phospho‐tau181 increases with Alzheimer’s disease clinical severity and is associated with tau‐ and amyloid‐positron emission tomography. Alzheimer's & Dementia 2018;14:989–97. 10.1016/j.jalz.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. The Lancet Neurology 2020;19:422–33. 10.1016/S1474-4422(20)30071-5 [DOI] [PubMed] [Google Scholar]
- 10. McGrath ER, Beiser AS, O'Donnell A, et al. Blood phosphorylated tau 181 as a biomarker for amyloid burden on brain PET in cognitively healthy adults. J Alzheimers Dis 2022;87:1517–26. 10.3233/JAD-215639 [DOI] [PubMed] [Google Scholar]
- 11. Karikari TK, Benedet AL, Ashton NJ, et al. Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer’s Disease Neuroimaging Initiative. Mol Psychiatry 2021;26:429–42. 10.1038/s41380-020-00923-z [DOI] [PubMed] [Google Scholar]
- 12. Clark C, Lewczuk P, Kornhuber J, et al. Plasma neurofilament light and phosphorylated tau 181 as biomarkers of Alzheimer’s disease pathology and clinical disease progression. Alzheimers Res Ther 2021;13:65. 10.1186/s13195-021-00805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen S-D, Huang Y-Y, Shen X-N, et al. Longitudinal plasma phosphorylated tau 181 tracks disease progression in Alzheimer’s disease. Transl Psychiatry 2021;11:356. 10.1038/s41398-021-01476-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer’s Association Appropriate Use Recommendations for Blood Biomarkers in Alzheimer’s Disease. Alzheimer's & Dementia 2022;18. 10.1002/alz.070020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. The Lancet Neurology 2022;21:66–77. 10.1016/S1474-4422(21)00361-6 [DOI] [PubMed] [Google Scholar]
- 16. Hanon O, Vidal Jean‐Sébastien, Lehmann S, et al. Plasma amyloid levels within the Alzheimer’s process and correlations with central biomarkers. Alzheimer's & Dementia 2018;14:858–68. 10.1016/j.jalz.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 17. Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–8. 10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lehmann S, Delaby C, Boursier G, et al. Relevance of Aβ42/40 ratio for detection of Alzheimer disease pathology in clinical routine: the PLMR scale. Front Aging Neurosci 2018;10:138. 10.3389/fnagi.2018.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rissin DM, Walt DR. Digital concentration readout of single enzyme molecules using femtoliter arrays and Poisson statistics. Nano Lett 2006;6:520–3. 10.1021/nl060227d [DOI] [PubMed] [Google Scholar]
- 21. Pencina MJ, D'Agostino RB, D'Agostino RB, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–72. 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 22. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 23. Delaby C, Alcolea D, Hirtz C, et al. Blood amyloid and tau biomarkers as predictors of cerebrospinal fluid profiles. J Neural Transm 2022;129:231–7. 10.1007/s00702-022-02474-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thijssen EH, La Joie R, Strom A, et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. The Lancet Neurology 2021;20:739–52. 10.1016/S1474-4422(21)00214-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bayoumy S, Verberk IMW, den Dulk B, et al. Clinical and analytical comparison of six Simoa assays for plasma p-tau isoforms p-Tau181, P-tau217, and P-tau231. Alzheimers Res Ther 2021;13:198. 10.1186/s13195-021-00939-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med 2020;26:387–97. 10.1038/s41591-020-0762-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benussi A, Karikari TK, Ashton N, et al. Diagnostic and prognostic value of serum NfL and p-Tau 181 in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry 2020;91:960–7. 10.1136/jnnp-2020-323487 [DOI] [PubMed] [Google Scholar]
- 28. Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. The Lancet Neurology 2022;21:246–57. 10.1016/S1474-4422(22)00009-6 [DOI] [PubMed] [Google Scholar]
- 29. Blomberg M, Jensen M, Basun H, et al. Cerebrospinal fluid tau levels increase with age in healthy individuals. Dement Geriatr Cogn Disord 2001;12:127–32. 10.1159/000051246 [DOI] [PubMed] [Google Scholar]
- 30. Syrjanen JA, Campbell MR, Algeciras‐Schimnich A, et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimer's & Dementia 2022;18:1128–40. 10.1002/alz.12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mielke MM, Dage JL, Frank RD, et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nat Med 2022;28:1398–405. 10.1038/s41591-022-01822-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Janelidze S, Bali D, Ashton NJ, et al. Head-To-Head comparison of 10 plasma phospho-tau assays in prodromal Alzheimer's disease. Brain 2022. doi: 10.1093/brain/awac333. [Epub ahead of print: 10 Sep 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh-Manoux A, Oumarou-Ibrahim A, Machado-Fragua MD, et al. Association between kidney function and incidence of dementia: 10-year follow-up of the Whitehall II cohort study. Age Ageing 2022;51. 10.1093/ageing/afab259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chan H-N, Xu D, Ho S-L, et al. Ultra-Sensitive detection of protein biomarkers for diagnosis of Alzheimer's disease. Chem Sci 2017;8:4012–8. 10.1039/C6SC05615F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun R, Wang H, Shi Y, et al. A Pilot Study of Urinary Exosomes in Alzheimer’s Disease. Neurodegener Dis 2020;19:184–91. 10.1159/000505851 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2022-330540supp001.pdf (97.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data and informed consent form are available upon request after publication (APHP, Paris). Requests will be considered by each study investigator based on the information provided by the requester regarding the study and analysis plan. If the use is appropriate, a data sharing agreement will be put in place before distributing a fully de-identified version of the dataset, including the data dictionary used for analysis with individual participant data.