Abstract
Background
Body composition data have been limited to adults with disease or older age. The prognostic impact in otherwise asymptomatic adults is unclear.
Purpose
To use artificial intelligence–based body composition metrics from routine abdominal CT scans in asymptomatic adults to clarify the association between obesity, liver steatosis, myopenia, and myosteatosis and the risk of mortality.
Materials and Methods
In this retrospective single-center study, consecutive adult outpatients undergoing routine colorectal cancer screening from April 2004 to December 2016 were included. Using a U-Net algorithm, the following body composition metrics were extracted from low-dose, noncontrast, supine multidetector abdominal CT scans: total muscle area, muscle density, subcutaneous and visceral fat area, and volumetric liver density. Abnormal body composition was defined by the presence of liver steatosis, obesity, muscle fatty infiltration (myosteatosis), and/or low muscle mass (myopenia). The incidence of death and major adverse cardiovascular events were recorded during a median follow-up of 8.8 years. Multivariable analyses were performed accounting for age, sex, smoking status, myosteatosis, liver steatosis, myopenia, type 2 diabetes, obesity, visceral fat, and history of cardiovascular events.
Results
Overall, 8982 consecutive outpatients (mean age, 57 years ± 8 [SD]; 5008 female, 3974 male) were included. Abnormal body composition was found in 86% (434 of 507) of patients who died during follow-up. Myosteatosis was found in 278 of 507 patients (55%) who died (15.5% absolute risk at 10 years). Myosteatosis, obesity, liver steatosis, and myopenia were associated with increased mortality risk (hazard ratio [HR]: 4.33 [95% CI: 3.63, 5.16], 1.27 [95% CI: 1.06, 1.53], 1.86 [95% CI: 1.56, 2.21], and 1.75 [95% CI: 1.43, 2.14], respectively). In 8303 patients (excluding 679 patients without complete data), after multivariable adjustment, myosteatosis remained associated with increased mortality risk (HR, 1.89 [95% CI: 1.52, 2.35]; P < .001).
Conclusion
Artificial intelligence–based profiling of body composition from routine abdominal CT scans identified myosteatosis as a key predictor of mortality risk in asymptomatic adults.
© RSNA, 2023
Supplemental material is available for this article.
See also the editorial by Tong and Magudia in this issue.
Summary
Artificial intelligence–based profiling of body composition from routine abdominal CT scans identified myosteatosis as a key predictor of mortality risk in asymptomatic adults.
Key Results
■ In this retrospective study of artificial intelligence–based body composition metrics from abdominal CT images in asymptomatic adults (n = 8982) undergoing routine colorectal cancer screening, myosteatosis was found in 55% (278 of 507) of patients who died (median follow-up, 8.8 years).
■ Myosteatosis, obesity, liver steatosis, and myopenia were associated with increased mortality risk (hazard ratio [HR], 4.33, 1.27, 1.86, and 1.75, respectively).
■ After multivariable adjustment, myosteatosis remained associated with increased mortality risk (HR, 1.89; P < .001).
Introduction
The world is witnessing unprecedented levels of food supply and a dramatic increase in sedentary lifestyles, particularly in developed or developing countries (1). This situation fosters the storage of excess energy as lipids in adipose (fatty) tissue, which eventually leads to obesity (1). Body mass index (BMI) is a metric used to assess the severity of obesity (1). A high BMI is associated with an increased risk for adverse events (1). However, large-scale population studies report that patients with similar BMI values can have strikingly different comorbidities and levels of health risk (2). During the past decade, imaging reports based on CT or MRI have shown that BMI poorly reflects body composition (3–5). In fact, muscle mass and/or fat accumulation between abdominal organs (“visceral” fat) or in nonadipose tissue, such as in the liver (liver steatosis) and in the muscles (myosteatosis), are better associated with the level of health risk than BMI (3–11). The typically silent accumulation of fat in ectopic locations is now considered to be one of the major risk factors of mortality, independent of obesity (12).
At the present time, a great deal of medical interest is centered on liver steatosis, especially when resulting from caloric imbalance (ie, nonalcoholic fatty liver disease) (13,14). Likewise, the prognostic implication of low muscle mass (referred to as sarcopenia [8] or myopenia [9]) has drawn exponential attention (8,10,15). Of note, the literature is somewhat inconsistent on the terminology. “Sarcopenia” usually relies on functionality measurements (8,15), while “myopenia” exclusively refers to low muscle mass (9). By contrast, myosteatosis (which can coexist with sarcopenia and/or myopenia) receives low consideration from the medical community and policy makers. A plausible explanation is that CT or MRI, necessary for myosteatosis evaluation, are typically not performed in asymptomatic adults. Thus, most data on myosteatosis originate from diagnostic or follow-up imaging in patients with disease (16–19). In the latter context, determining the mortality associated with myosteatosis is difficult. A few studies in older adults (65–70 years) consistently reported an association between ectopic fat accumulation and the risk for adverse events (20–25). However, none of these studies controlled simultaneously for visceral fat, liver steatosis, myopenia, and myosteatosis, although all these parameters potentially increase the risk for major adverse cardiovascular events (MACEs) and death.
Thus, our aim was to use artificial intelligence–based body composition metrics from routine abdominal CT scans in asymptomatic adults to clarify the association between obesity, liver steatosis, myopenia, and myosteatosis and the risk of mortality.
Materials and Methods
Cohort
This retrospective single-center study included 9223 consecutive adults who were asymptomatic outpatients, aged 50–70 years, and who underwent low-dose unenhanced abdominal CT for colorectal cancer screening as part of routine health maintenance from April 2004 to December 2016. The study was approved by the institutional review board of the University of Wisconsin School of Medicine and Public Health. The requirement for informed consent was waived for this retrospective, Health Insurance Portability and Accountability Act–compliant study. The incidence of death and MACEs were recorded during a median follow-up of 8.8 years. Of note, patients from this large cohort have been previously reported in a prior article (26) that dealt with the investigation of multiple CT-based biomarkers (eg, muscle density, visceral fat, aortic calcification, vertebral density), whereas in this article we delve specifically into myosteatosis and its interaction with other lipid depots such as visceral fat and liver steatosis. Exclusion criteria included (a) missing data for key body composition metrics (ie, BMI, muscle area, liver density, or muscle density) and (b) evident muscle artifacts on CT images (Fig 1). Of note, smoking status data and visceral fat measurements were missing for 581 and 111 patients, respectively (n = 679 total, when accounting for overlaps). Hence, univariable analyses are based on 8982 patients, while multivariable analyses (Cox regressions and random forest) are based on 8303 patients (Fig 1).
Figure 1:
Flowchart shows patient inclusion and exclusion. Patients with missing body composition data (body mass index [BMI], liver density, skeletal muscle index [SMI], or muscle density) or with evident muscle artifacts at CT were excluded before the univariable analysis. Multivariable analysis was performed on the cohort with complete data available, including smoking status, medical history of cardiovascular events, and visceral fat measurements. (* = data were missing in 679 patients, when accounting for overlaps.)
CT Protocol
The low-dose, noncontrast, supine multidetector abdominal CT acquisitions used for this investigation were all performed at 120 kVp using a single system (GE Healthcare) with modulated milliamperes to achieve a noise index of 50, typically resulting in an effective dose of 2–3 mSv. Acquisition parameters were 1.25-mm collimation and a 1-mm reconstruction interval.
Definitions
Myopenia was considered according to sex-specific cutoffs of the skeletal muscle index (SMI). SMI values were computed by dividing muscle area (in square centimeters) by the patient's height squared (in square meters). Myopenia was defined by an SMI less than 39 cm2/m2 in female patients and less than 50 cm2/m2 in male patients (27). Of note, we did not use the term sarcopenia due to the absence of a functional muscle measurement (8). Myosteatosis was considered for lowest quartile (Q1) values of muscle density (corresponding to ≤18.9 HU and ≤28.1 HU for female and male patients, respectively). Liver steatosis was defined according to a previously described liver density cutoff of 57 HU, corresponding to a 5% MRI-derived proton density fat fraction (PDFF) equivalent (28,29). A previously validated formula was used to estimate PDFF values from CT-derived densities as follows: PDFF (%) = –0.58 × CT attenuation (Hounsfield units) + 38.2 (29). Abnormal body composition was defined by the presence of at least one of the following conditions at inclusion: liver steatosis, obesity, muscle fatty infiltration (myosteatosis), or low muscle mass (myopenia). MACEs were defined as cerebrovascular disease, myocardial infarction, heart failure, or aortic aneurysm occurring during follow-up. Adverse events were defined as at least one MACE or death.
Explanatory Variables
Body composition metrics were extracted using a specific artificial intelligence methodology (modified three-dimensional U-Net) for anatomic tissue segmentation and quantification (Fig S1), which was previously described elsewhere (30,31). In brief, automated data from the CT scan at the third lumbar level consisted of total muscle area (in square centimeters) and muscle density (in Hounsfield units) measured on the whole muscle area, and data from the first lumbar level consisted of subcutaneous and visceral fat area (in square centimeters) and volumetric liver density (in Hounsfield units). Those metrics were used to establish the presence of myopenia, myosteatosis, and liver steatosis in the study cohort. Clinical data that included age, sex, smoking status, type 2 diabetes, obesity, and history of cardiovascular events were retrieved from electronic health records.
Statistical Analysis
Data are displayed as means ± SDs, unless otherwise specified. To compare continuous variables across subgroups, P values were computed with the two-sided Student t test for normally distributed variables and the Bonferroni post hoc correction for multiple testing. To compare the statistical differences between categorical values across groups, we used the χ2 test. For the time-to-event survival analysis, we generated Kaplan-Meier curves by splitting predictor variables into quartiles (Q1 vs Q2, Q3, Q4) for muscle density or by category for the other body composition parameters. We used Cox proportional hazards models (days after the time of imaging as the time scale) to derive risk predictions and hazard ratios (HRs) adjusted for confounders, including age, sex, smoking status, myosteatosis, liver steatosis, myopenia, type 2 diabetes, obesity, visceral fat, and history of cardiovascular events. Events that occurred at or before study inclusion were considered part of the patient's medical history. Patients lost to follow-up were censored in time-to-event analyses. In addition, we implemented a multistate Markov model (32,33) based on the stratified risks of transitions to pathologic stages. In this model, different transitions are possible (eg, from inclusion to death, from inclusion to a MACE to death, from inclusion to MACEs to loss to follow-up). Hence, individuals can be in successive types of intermediate states (eg, experiencing one MACE or multiple MACEs during follow-up) until they reach an absorbing state (in our case, death or loss to follow-up). Finally, we exploited a random forest survival model (34) using overall mortality as the outcome and accounting for right-censoring (see Appendix S1). From the model, we extracted variable importance values (using the built-in permutation-based importance calculation) (34) and partial dependence curves using the R (The R Foundation) package survex (33). In statistical analyses, P < .05 was considered indicative of a statistically significant difference. For sample size calculation, we hypothesized that individuals with myosteatosis might have at least a 1.5- to two-fold increased mortality risk at any given time during the study compared with individuals without myosteatosis. For α = .05 (type I error rate), β = .20 (type II error rate), and a prevalence of myosteatosis of 25% (per quartile definition), the total number of death events needed to test our primary hypothesis ranged from 87 to 255. All individuals (n = 8982) meeting inclusion criteria were included in the study and 507 patients died during follow-up; thus, statistical power requirements were met to test our primary hypothesis. Statistical analyses were performed by an author (M.N.) using SPSS (version 24; IBM), GraphPad Prism (version 8; GraphPad Software), and R (version 4.0; The R Foundation ). The statistical code is available at https://github.com/maxnach92/Radiology_Myosteatosis.
Results
Characteristics of the Cohort
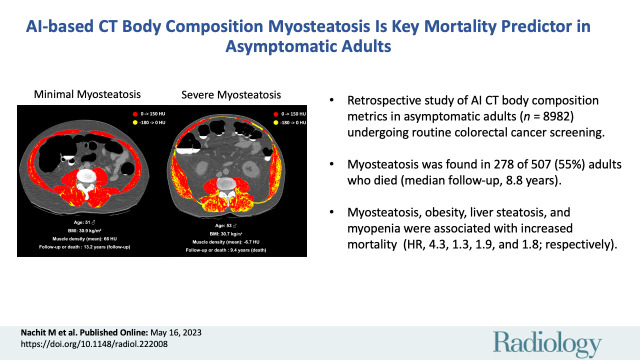
Of 9223 healthy asymptomatic adult outpatients, 226 were excluded due to missing data and 15 due to evident muscle measure artifacts at CT (mean density less than –29 HU and/or SMI <10 cm2/m2). After exclusions, 8982 consecutive adult outpatients (mean age, 57 years ± 8 [SD]; 5008 female, 3974 male) were included (Fig 1) and followed up for a median duration of 8.8 years. From a body composition standpoint, liver steatosis was the most frequent pathologic feature (49%; 4395 of 8982 patients), followed by obesity (33%; 2994 of 8982 patients), myosteatosis (25%; 2245 of 8982 patients), and myopenia (18%; 1589 of 8982 patients). Patient characteristics for the total cohort and stratified according to abnormal body composition feature are displayed in Table 1. Overall, 20% (1836 of 8982) of patients experienced at least one adverse event (cardiovascular event or death) and 6% (507 of 8982) of patients died during the follow-up period. At inclusion, body composition was abnormal in 80% (1467 of 1836) and 86% (434 of 507) of patients who experienced at least one adverse event or who died during the follow-up period, respectively (Fig 2). Myosteatosis, illustrated in Figure 3, was associated with the highest risk of experiencing an adverse event (Table 1). When compared with patients with no myosteatosis, those with myosteatosis were older (55.4 years ± 6.4 vs 61.8 years ± 9.2, P < .001) and more often had obesity (26% [1779 of 6737] vs 54% [1215 of 2245], P < .001) and type 2 diabetes (2% [111 of 6737] vs 5% [115 of 2245], P < .001). Although unambiguous with a density-based color scale (Fig 3), myosteatosis was less evident on raw CT images (Fig S2).
Table 1:
Patient Characteristics Stratified according to Abnormal Body Composition Feature
Figure 2:

Venn diagrams show the number of patients according to abnormal body composition feature (myosteatosis, liver steatosis, myopenia, obesity) at baseline and their intersection with the number of patients (A) who experienced at least one adverse event (ie, cardiovascular event or death; n = 1836) and (B) who died during follow-up (n = 507). Adverse events mostly occurred in patients with abnormal body composition.
Figure 3:
(A) Unenhanced axial abdominal CT image with a Hounsfield unit–based color scale of skeletal muscles in a 51-year-old man with obesity, smoking history, no type 2 diabetes, and no history of cardiovascular events at inclusion shows mild fatty infiltration in the muscles (myosteatosis, yellow), with most voxels in the positive range of Hounsfield units (red). The patient was lost to follow-up after 13.2 years. (B) Unenhanced axial abdominal CT image with a Hounsfield unit–based color scale of skeletal muscles in a 53-year-old man with obesity, smoking history, no type 2 diabetes, and no history of cardiovascular events at inclusion shows severe fatty infiltration in the muscles (myosteatosis, yellow), mostly distributed in the paravertebral (ie, erector spinae and multifidus) and oblique muscle groups. The patient died after 9.4 years of follow-up. BMI = body mass index.
Time-to-Event Analysis Based on Obesity, Liver Steatosis, Myopenia, or Myosteatosis
All parameters increased the risk for adverse events and overall mortality (P < .05) (Fig 4). Having myosteatosis at inclusion (2245 of 8982 patients, hence 25% prevalence per quartile definition) was associated with an HR of 4.33 (95% CI: 3.63, 5.16; P < .001) (Fig 4D). Other parameters included obesity (HR, 1.27 [95% CI: 1.06, 1.53]; P = .008), liver steatosis (HR, 1.86 [95% CI: 1.56, 2.21]; P < .001), and myopenia (HR, 1.75 [95% CI: 1.43, 2.14]; P < .001) (Fig 4A–4C). Thus, based on nonoverlapping 95% CIs, myosteatosis had the highest impact on the mortality rate when compared with other parameters. Individuals with myosteatosis at inclusion had a mortality rate of 1.72 per 100 person-years. Based on Kaplan-Meier analysis, the absolute mortality risk at 10 years conferred by myosteatosis was 15.5% compared with the risk conferred by obesity (7.6%), liver steatosis (8.5%), or myopenia (9.7%). Myosteatosis, liver steatosis, and, even more so, the association of myosteatosis and liver steatosis at baseline increased the risk for de novo type 2 diabetes. In this case, the association was mainly driven by liver steatosis (Fig S3). The total number of events according to muscle status is shown in Table S1.
Figure 4:
Kaplan-Meier curves of survival probability according to body composition parameter, including (A) obesity, (B) liver steatosis, (C) myopenia, and (D) myosteatosis, show an association between myosteatosis and high mortality risk. Statistics were computed with the Mantel-Cox log-rank test. HR = hazard ratio.
Multivariable Cox Regression Analysis
From a body composition perspective, myosteatosis, myopenia, and liver steatosis—but not visceral fat area or BMI—were independent predictors of overall mortality risk when age, sex, smoking status, type 2 diabetes, obesity, and history of cardiovascular were taken into account (Table 2). When variables were taken as continuous values (ie, muscle density, liver density, and SMI for myosteatosis, liver steatosis, and myopenia, respectively), muscle density remained significantly associated with an increased mortality risk (HR, 1.42 [95% CI: 1.29, 1.57]; P < .001) (Table S2).
Table 2:
Multivariable Cox Regression Analysis for Overall Death Risk

Multistate Markov Model and Risk of Death Based on Body Composition
After multivariable adjustment in a multistate Markov model (Fig 5), myosteatosis remained the only body composition parameter associated with the risk of death following one or two MACEs (HR, 1.61 [95% CI: 1.09, 2.37; P = .02] or 1.91 [95% CI: 1.07, 3.42; P = .03], respectively) (Table 3). Of note, myosteatosis was also associated with the risk of experiencing a first MACE (HR, 1.13 [95% CI: 1.00, 1.28]; P = .058) (Table S3).
Figure 5:
Schematic shows possible transition states of the multistate Markov model, starting at inclusion (ie, CT scan acquisition) and leading to intermediate states (ie, major adverse cardiovascular events [MACEs], indicated by gray circles) and/or to an absorbing state (ie, death or loss to follow-up, indicated by red circles). Transitions numbered 2, 4, 6, 8, or 9 lead to an absorbing state and transitions numbered 1, 3, 5, or 7 lead to an intermediate state. CV = cardiovascular.
Table 3:
Multistate Markov Model for Mortality Risk Accounting for MACEs
Random Forest Survival Model and Risk of Death Based on Body Composition
When tested on unseen data (see Appendix S1), the performance of the random forest model was very good (Harrell C index, 0.79) and was maintained when fully stratified for sex (Harrell C index, 0.78 and 0.77 in male and female patients, respectively). In line with our previous results, muscle density was the most important body composition predictor, especially in male patients (importance of muscle density [0.039] vs BMI [0.013], SMI [0.002], or liver density [0.0013]) (Fig S4). Interestingly, BMI, a parameter with little influence in the Cox regression models, was the second most important body composition predictor of mortality, with an importance value of 0.015 and 0.013 in male and female patients, respectively (Fig S4). Partial dependence curves (33) show that age, muscle density, and, to a lesser extent, BMI were the most impactful parameters modulating mortality risk during follow-up (Fig 6).
Figure 6:
Partial dependence curves extracted from the random forest survival model show the survival function value of each body composition parameter according to (A) sex as a covariate and (B) male and (C) female sex. These results highlight the importance of muscle density in modulating mortality risk (see Appendix S1), independent from the quartile-based stratification; thus, myosteatosis is the most impactful body composition predictor of mortality. BMI = body mass index, HU = Hounsfield unit.
Discussion
To date, most of the medical interest on body composition and its impact on health is directed toward (visceral) obesity, liver steatosis, and muscle wasting, while myosteatosis is overlooked. Notwithstanding, myosteatosis robustly associates with prognosis in patients with chronic diseases (19,35–37), especially cancer (16,17) and chronic liver and kidney diseases (18,37–40), including in transplantation (41–43). More recently, myosteatosis was deemed to predict the risk of severe SARS-CoV-2 infection (44) and its long-term complications (45). Yet, myosteatosis is still absent from the agendas of policy makers and remains largely ignored by the medical community. Herein, we found that myosteatosis (considered as the lowest sex-specific quartile of muscle density, hence 25% prevalence in the study cohort) substantially increased the mortality risk of asymptomatic adults (hazard ratio [HR], 4.33 [95% CI: 3.63, 5.16]). Remarkably, after multivariable adjustment, myosteatosis was associated with a mortality risk comparable to that of smoking or type 2 diabetes (HR, 1.89 [95% CI: 1.52, 2.35] vs 1.91 [95% CI: 1.57, 2.33] or 1.56 [95% CI: 1.08, 2.25], respectively). We used complementary statistical approaches to challenge these findings, by accounting for incident major adverse cardiovascular events (MACEs) using a multistate Markov model and a random forest model, which is a machine learning algorithm naturally able to handle nonlinear predictors (eg, body mass index). In either case, the strong association between myosteatosis and mortality risk was maintained. After multivariable adjustment, myosteatosis remained associated with an increased mortality risk following one or two MACEs, (HR, 1.61 [95% CI: 1.09, 2.37; P = .02] and 1.91 [95% CI: 1.07, 3.42; P = .03], respectively), often occurring several years (approximately 6–9 years) after the initial CT assessment. This raises the question of whether myosteatosis could be a key indicator of resilience, reflecting an individual capacity to cope with future adverse events.
Arguably, the present data add substance and robustness to the relationship between myosteatosis and the level of health risk, extending its scope beyond older adults (20–23). We found that, in the general population, having myosteatosis is of no less importance than having obesity, liver steatosis, or type 2 diabetes. While tremendous research efforts are underway for the latter conditions, whether to screen for (and if so, by whom) and how to manage myosteatosis in otherwiseasymptomatic adults is not yet settled. Besides, how myosteatosis influences prognosis remains largely unknown. Previous data indicate that the relationship between myosteatosis and adverse outcomes is independent of lifestyle (24). This comes as no surprise as dysmetabolic features (eg, obesity, liver steatosis, type 2 diabetes) are a direct reflection of lifestyle, while self-reported dietary habits and physical activity at a given time point may not accurately recapitulate the reality. Hence, when metabolic parameters are considered, it seems coherent that lifestyle parameters cannot entirely explain the relationship between myosteatosis and health risk. Of note, the thresholds of muscle density (in Hounsfield units) corresponding to the lowest sex-specific quartiles in our cohort are somewhat lower than myosteatosis cutoffs reported in other (almost exclusively with pathology) populations (19,35,46). These discrepancies might be explained by several factors, such as the statistical approach used to compute those cutoffs (ie, outcome-based and, rarely, sex-specific in most of the literature vs distribution-based in our study) and the need for semiautomated segmentation with predetermined Hounsfield unit ranges (often –30 to 150 HU [19,35,46]) in the literature versus the fully-automated pipeline implemented in the present study.
Our study had several strengths compared with other studies (16–18,20–23,37,42,43), including our cohort being representative of the general population. We dissected the relationship between body composition and health risk using cutting-edge artificial intelligence tools while controlling for multiple locations of lipid depots (ie, visceral area, liver, and skeletal muscles). Also, data were systematically presented in a sex-stratified fashion and myosteatosis was consistently ranked as the most important body composition predictor of mortality risk using a comprehensive set of statistical approaches. To the best of our knowledge, it is anticipated that the UK Biobank (with approximately 40000 MRI studies, expected to reach 100000 MRI studies within the next few years [47]) and All of Us, a National Institutes of Health research program, will provide comparable data. In fact, the first study reporting on myosteatosis using data from the UK Biobank confirmed its prognostic value (24), but the available follow-up data are limited given that the imaging portion started recently (approximately 3 years of follow-up from the imaging assessment).
Our study had several limitations. First, we included mild steatosis (MRI-derived PDFF equivalent of 5%) in our binary threshold consideration. At near-normal levels, CT-based estimation of liver fat is less reliable (26). Second, physical activity, or lack thereof, and dietary habits were not systematically recorded; however, this information is of interest because lifestyle parameters modulate lipid distribution and prognosis (19). Third, we did not evaluate muscle strength, a functional parameter independently associated with poor outcomes (48).
In conclusion, artificial intelligence–based profiling of body composition from routine abdominal CT scans identified myosteatosis (ie, fat accumulation within muscles) as a key predictor of mortality risk in asymptomatic adults. Taken together, we hope that this report will contribute to making the medical community aware of the importance of myosteatosis, a silent pathologic condition that is not exclusively associated with coexisting disease. Mechanistic studies are needed to determine whether therapeutic reduction of muscle fat and/or modulation of plausible noxious pathways induced by myosteatosis could be novel strategies to promote healthy aging. We believe that our study and others will help build momentum to pursue (and extend) research efforts in this field, raising the question of whether muscle “health” should be actively investigated and managed using CT-based densities (or the MRI-derived proton density fat fraction) as a readout, with dedicated nutritional, pharmaceutical, and/or physical activity supports as possible interventions.
Supported in part by the Intramural Research Program of the National Institutes of Health (NIH) Clinical Center. This study used the high-performance computing capabilities of the NIH Biowulf cluster. M.N. supported by a Fund for Research Training in Industry and Agriculture (FRIA) PhD fellowship (Fund for Scientific Research [FNRS], Belgium) (grant 31618719). I.A.L. supported by FNRS (grants PDR T.0141.19, CDR J.0119.22, PDR THEMA P.C006.22).
Disclosures of conflicts of interest: M.N. Patents planned, issued, or pending (PCT/EP2022/065769 Ref WO/2022/258788). Y.H. No relevant relationships. R.M.S. Cooperative research and development agreement with PingAn; patent licenses, software licenses, or royalties from iCAD, Philips, ScanMed, PingAn, and Translation Holdings. I.A.L. Associate editor for Clinical Science and Liver International; patents planned, issued, or pending (PCT/EP2022/065769 Ref WO/2022/258788). P.J.P. Consulting fees from Bracco, Nanox, and GE Healthcare.
Abbreviations:
- BMI
- body mass index
- HR
- hazard ratio
- MACE
- major adverse cardiovascular event
- PDFF
- proton density fat fraction
- SMI
- skeletal muscle index
References
- 1. Blüher M . Obesity: global epidemiology and pathogenesis . Nat Rev Endocrinol 2019. ; 15 ( 5 ): 288 – 298 . [DOI] [PubMed] [Google Scholar]
- 2. González-Muniesa P , Mártinez-González MA , Hu FB , et al . Obesity . Nat Rev Dis Primers 2017. ; 3 ( 1 ): 17034 . [DOI] [PubMed] [Google Scholar]
- 3. Prado CMM , Lieffers JR , McCargar LJ , et al . Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study . Lancet Oncol 2008. ; 9 ( 7 ): 629 – 635 . [DOI] [PubMed] [Google Scholar]
- 4. Ali R , Baracos VE , Sawyer MB , et al . Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens . Cancer Med 2016. ; 5 ( 4 ): 607 – 616 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin L , Birdsell L , Macdonald N , et al . Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index . J Clin Oncol 2013. ; 31 ( 12 ): 1539 – 1547 . [DOI] [PubMed] [Google Scholar]
- 6. Ferrara D , Montecucco F , Dallegri F , Carbone F . Impact of different ectopic fat depots on cardiovascular and metabolic diseases . J Cell Physiol 2019. ; 234 ( 12 ): 21630 – 21641 . [DOI] [PubMed] [Google Scholar]
- 7. Neeland IJ , Ross R , Després JP , et al . Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement . Lancet Diabetes Endocrinol 2019. ; 7 ( 9 ): 715 – 725 . [DOI] [PubMed] [Google Scholar]
- 8. Cruz-Jentoft AJ , Bahat G , Bauer J , et al . Sarcopenia: revised European consensus on definition and diagnosis . Age Ageing 2019. ; 48 ( 1 ): 16 – 31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fearon K , Evans WJ , Anker SD . Myopenia-a new universal term for muscle wasting . J Cachexia Sarcopenia Muscle 2011. ; 2 ( 1 ): 1 – 3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prado CM , Purcell SA , Alish C , et al . Implications of low muscle mass across the continuum of care: a narrative review . Ann Med 2018. ; 50 ( 8 ): 675 – 693 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stefan N , Häring HU , Hu FB , Schulze MB . Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications . Lancet Diabetes Endocrinol 2013. ; 1 ( 2 ): 152 – 162 . [DOI] [PubMed] [Google Scholar]
- 12. Gastaldelli A , Gaggini M . Ectopic fat: a target for cardiometabolic risk management . Expert Rev Cardiovasc Ther 2016. ; 14 ( 12 ): 1301 – 1303 . [DOI] [PubMed] [Google Scholar]
- 13. Sanyal AJ . Past, present and future perspectives in nonalcoholic fatty liver disease . Nat Rev Gastroenterol Hepatol 2019. ; 16 ( 6 ): 377 – 386 . [DOI] [PubMed] [Google Scholar]
- 14. Fraile JM , Palliyil S , Barelle C , Porter AJ , Kovaleva M . Non-Alcoholic Steatohepatitis (NASH) - A Review of a Crowded Clinical Landscape, Driven by a Complex Disease . Drug Des Devel Ther 2021. ; 15 : 3997 – 4009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cruz-Jentoft AJ , Sayer AA . Sarcopenia . Lancet 2019. ; 393 ( 10191 ): 2636 – 2646 . [DOI] [PubMed] [Google Scholar]
- 16. Kazemi-Bajestani SMR , Mazurak VC , Baracos V . Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes . Semin Cell Dev Biol 2016. ; 54 : 2 – 10 . [DOI] [PubMed] [Google Scholar]
- 17. Kroenke CH , Prado CM , Meyerhardt JA , et al . Muscle radiodensity and mortality in patients with colorectal cancer . Cancer 2018. ; 124 ( 14 ): 3008 – 3015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montano-Loza AJ , Angulo P , Meza-Junco J , et al . Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis . J Cachexia Sarcopenia Muscle 2016. ; 7 ( 2 ): 126 – 135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Correa-de-Araujo R , Addison O , Miljkovic I , et al . Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging . Front Physiol 2020. ; 11 : 963 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miljkovic I , Kuipers AL , Cauley JA , et al . Greater Skeletal Muscle Fat Infiltration Is Associated With Higher All-Cause and Cardiovascular Mortality in Older Men . J Gerontol A Biol Sci Med Sci 2015. ; 70 ( 9 ): 1133 – 1140 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larsen B , Bellettiere J , Allison M , et al . Muscle area and density and risk of all-cause mortality: The Multi-Ethnic Study of Atherosclerosis . Metabolism 2020. ; 111 : 154321 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lenchik L , Barnard R , Boutin RD , et al . Automated Muscle Measurement on Chest CT Predicts All-Cause Mortality in Older Adults From the National Lung Screening Trial . J Gerontol A Biol Sci Med Sci 2021. ; 76 ( 2 ): 277 – 285 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ballin M , Nordström P , Niklasson J , Nordström A . Associations of Visceral Adipose Tissue and Skeletal Muscle Density With Incident Stroke, Myocardial Infarction, and All-Cause Mortality in Community-Dwelling 70-Year-Old Individuals: A Prospective Cohort Study . J Am Heart Assoc 2021. ; 10 ( 9 ): e020065 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Linge J , Petersson M , Forsgren MF , Sanyal AJ , Dahlqvist Leinhard O . Adverse muscle composition predicts all-cause mortality in the UK Biobank imaging study . J Cachexia Sarcopenia Muscle 2021. ; 12 ( 6 ): 1513 – 1526 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kiefer LS , Fabian J , Rospleszcz S , et al . Assessment of the degree of abdominal myosteatosis by magnetic resonance imaging in subjects with diabetes, prediabetes and healthy controls from the general population . Eur J Radiol 2018. ; 105 : 261 – 268 . [DOI] [PubMed] [Google Scholar]
- 26. Pickhardt PJ , Graffy PM , Zea R , et al . Automated CT biomarkers for opportunistic prediction of future cardiovascular events and mortality in an asymptomatic screening population: a retrospective cohort study . Lancet Digit Health 2020. ; 2 ( 4 ): e192 – e200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carey EJ , Lai JC , Wang CW , et al . A multicenter study to define sarcopenia in patients with end-stage liver disease . Liver Transpl 2017. ; 23 ( 5 ): 625 – 633 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pickhardt PJ , Hahn L , Muñoz del Rio A , Park SH , Reeder SB , Said A . Natural history of hepatic steatosis: observed outcomes for subsequent liver and cardiovascular complications . AJR Am J Roentgenol 2014. ; 202 ( 4 ): 752 – 758 . [DOI] [PubMed] [Google Scholar]
- 29. Pickhardt PJ , Graffy PM , Reeder SB , Hernando D , Li K . Quantification of Liver Fat Content With Unenhanced MDCT: Phantom and Clinical Correlation With MRI Proton Density Fat Fraction . AJR Am J Roentgenol 2018. ; 211 ( 3 ): W151 – W157 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Graffy PM , Liu J , Pickhardt PJ , Burns JE , Yao J , Summers RM . Deep learning-based muscle segmentation and quantification at abdominal CT: application to a longitudinal adult screening cohort for sarcopenia assessment . Br J Radiol 2019. ; 92 ( 1100 ): 20190327 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Graffy PM , Sandfort V , Summers RM , Pickhardt PJ . Automated Liver Fat Quantification at Nonenhanced Abdominal CT for Population-based Steatosis Assessment . Radiology 2019. ; 293 ( 2 ): 334 – 342 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Putter H , de Wreede LC , Fiocco M , Geskus RB , Bonneville EF , Manevski D . mstate: Data Preparation, Estimation and Prediction in Multi-State Models . https://CRAN.R-project.org/package=mstate. Published November 8, 2021. Accessed December 7, 2022 . [Google Scholar]
- 33. Spytek M , Krzyziński M , Baniecki H , Biecek P . survex: Explainable Machine Learning in Survival Analysis . https://CRAN.R-project.org/package=survex. Published December 1, 2022. Accessed December 7, 2022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ishwaran H , Kogalur UB . randomForestSRC: Fast Unified Random Forests for Survival, Regression, and Classification (RF-SRC) . https://CRAN.R-project.org/package=randomForestSRC. Published July 6, 2022. Accessed December 7, 2022 . [Google Scholar]
- 35. Ahn H , Kim DW , Ko Y , et al . Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: A new paradigm beyond sarcopenia . Ageing Res Rev 2021. ; 70 : 101398 . [DOI] [PubMed] [Google Scholar]
- 36. Keddar M , Muylle T , Carrie E , et al . Non-invasive Quantification of Fat Deposits in Skeletal Muscle Predicts Cardiovascular Outcome in Kidney Failure . Front Physiol 2020. ; 11 : 130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilkinson TJ , Gould DW , Nixon DGD , Watson EL , Smith AC . Quality over quantity? Association of skeletal muscle myosteatosis and myofibrosis on physical function in chronic kidney disease . Nephrol Dial Transplant 2019. ; 34 ( 8 ): 1344 – 1353 . [DOI] [PubMed] [Google Scholar]
- 38. Nachit M , De Rudder M , Thissen JP , et al . Myosteatosis rather than sarcopenia associates with non-alcoholic steatohepatitis in non-alcoholic fatty liver disease preclinical models . J Cachexia Sarcopenia Muscle 2021. ; 12 ( 1 ): 144 – 158 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nachit M , Kwanten WJ , Thissen JP , et al . Muscle fat content is strongly associated with NASH: A longitudinal study in patients with morbid obesity . J Hepatol 2021. ; 75 ( 2 ): 292 – 301 . [DOI] [PubMed] [Google Scholar]
- 40. Nachit M , Lanthier N , Rodriguez J , et al . A dynamic association between myosteatosis and liver stiffness: Results from a prospective interventional study in obese patients . JHEP Rep 2021. ; 3 ( 4 ): 100323 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ebadi M , Montano-Loza AJ . Clinical relevance of skeletal muscle abnormalities in patients with cirrhosis . Dig Liver Dis 2019. ; 51 ( 11 ): 1493 – 1499 . [DOI] [PubMed] [Google Scholar]
- 42. Czigany Z , Kramp W , Bednarsch J , et al . Myosteatosis to predict inferior perioperative outcome in patients undergoing orthotopic liver transplantation . Am J Transplant 2020. ; 20 ( 2 ): 493 – 503 . [DOI] [PubMed] [Google Scholar]
- 43. Morel A , Ouamri Y , Canouï-Poitrine F , et al . Myosteatosis as an independent risk factor for mortality after kidney allograft transplantation: a retrospective cohort study . J Cachexia Sarcopenia Muscle 2022. ; 13 ( 1 ): 386 – 396 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yi X , Liu H , Zhu L , et al . Myosteatosis predicting risk of transition to severe COVID-19 infection . Clin Nutr 2022. ; 41 ( 12 ): 3007 – 3015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Lorenzo R , Palmisano A , Esposito A , et al . Myosteatosis significantly predicts persistent dyspnea and mobility problems in COVID-19 survivors . Front Nutr 2022. ; 9 : 846901 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ebadi M , Tsien C , Bhanji RA , et al . Myosteatosis in Cirrhosis: A Review of Diagnosis, Pathophysiological Mechanisms and Potential Interventions . Cells 2022. ; 11 ( 7 ): 1216 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Littlejohns TJ , Holliday J , Gibson LM , et al . The UK Biobank imaging enhancement of 100,000 participants: rationale, data collection, management and future directions . Nat Commun 2020. ; 11 ( 1 ): 2624 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Celis-Morales CA , Welsh P , Lyall DM , et al . Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants . BMJ 2018. ; 361 : k1651 . [DOI] [PMC free article] [PubMed] [Google Scholar]



![Flowchart shows patient inclusion and exclusion. Patients with missing body composition data (body mass index [BMI], liver density, skeletal muscle index [SMI], or muscle density) or with evident muscle artifacts at CT were excluded before the univariable analysis. Multivariable analysis was performed on the cohort with complete data available, including smoking status, medical history of cardiovascular events, and visceral fat measurements. (* = data were missing in 679 patients, when accounting for overlaps.)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/fbf2/10315523/d64ac6d3299c/radiol.222008.fig1.jpg)



![Schematic shows possible transition states of the multistate Markov model, starting at inclusion (ie, CT scan acquisition) and leading to intermediate states (ie, major adverse cardiovascular events [MACEs], indicated by gray circles) and/or to an absorbing state (ie, death or loss to follow-up, indicated by red circles). Transitions numbered 2, 4, 6, 8, or 9 lead to an absorbing state and transitions numbered 1, 3, 5, or 7 lead to an intermediate state. CV = cardiovascular.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/fbf2/10315523/46a4fe20ec3d/radiol.222008.fig5.jpg)

