Abstract
Background
Basic resource needs related to transportation, housing, food, and medications are important social determinants of health and modifiable indicators of poverty, but their role in modifying the risk of frailty and health‐related quality of life (HRQoL) remains unknown. The goal of our study was to examine the prevalence of unmet basic needs and their association with frailty and HRQoL in a cohort of older adults with cancer.
Methods
The CARE registry prospectively enrolls older adults (≥60 years) with cancer. Assessments of transportation, housing, and material hardship were added to the CARE tool in 8/2020. The 44‐item CARE Frailty Index was used to define frailty, and subdomains of physical and mental HRQoL were assessed using the PROMIS® 10‐global. Multivariable analysis examined the association between unmet needs with frailty and HRQoL subdomains, adjusting for covariates.
Results
The cohort included 494 participants. Median age of 69 years, 63.6% were male and 20.2% were Non‐Hispanic (NH) Black. Unmet basic needs were reported in 17.8% (transportation 11.5%, housing 2.8%, and material hardship 7.5%). Those with unmet needs were more often NH Black (33.0% vs. 17.8%, p = 0.006) and less educated (<high school: 19.5% vs. 9.7%, p = 0.023). Compared to those without unmet needs, unmet needs were associated with higher odds of frailty (adjusted odds ratio [aOR] 3.3, 95% CI 1.8–5.9), low physical (aOR = 2.1, 95% CI 1.2–3.8) and low mental (aOR = 2.5, 95% CI 1.4–4.4) HRQoL.
Conclusions
Unmet basic needs represent a novel exposure that is independently associated with frailty and low HRQoL and warrants the development of targeted interventions.
Keywords: aging, basic resource needs, cancer, concrete resource needs, frailty, geriatric oncology
1. INTRODUCTION
Despite advances in cancer prevention, detection, and management over the past several decades, disparities in cancer outcomes persist across the cancer continuum. 1 Nonbiological correlates of health outcomes, commonly known as social determinants of heath, are associated with cancer outcomes, 2 and increasingly recognized as important targets for reducing health disparities. 3 Unmet basic resource needs related to transportation, housing, food, and medications are important and modifiable indicators of poverty. 4 Prior work among adults with chronic cardiometabolic diseases demonstrates that targeting basic unmet needs can lead to tangible improvements in health outcomes. 5 Although poverty is associated with inferior health outcomes among patients with cancer, most studies have relied on area‐level measures of poverty (e.g., at the county level) to approximate individual‐level poverty. 6 , 7 , 8 Examination of “current poverty” (≥20% of population living in poverty) and/or “persistent poverty” (≥20% of population living in poverty for three consecutive decades) has yielded consistent and strong associations with increased mortality and inferior cancer outcomes. Nevertheless, both the concrete driver(s) of these poverty‐related findings and the role of individual‐level factors in these associations remain unknown.
There is a dearth of information regarding the prevalence of unmet basic resource needs among older adults with cancer, which is crucial as older adults represent the majority of new cancer diagnoses and cancer deaths. 9 The management of cancer in older adults is often complicated by the coexistence of age‐related impairments and comorbid conditions. Frailty is a recognized state of increased vulnerability and is prevalent in older adults with cancer. 10 , 11 Frailty is associated with increased chemotherapy toxicities, hospitalizations, long‐term care placement and reduced health‐related quality of life (HRQoL), and inferior survival. 12 , 13 , 14 , 15 Socioeconomic status are known to contribute to frailty in the general population, but the relationship of frailty with poverty among older adults with cancer remains uncertain. 16 , 17 , 18 The role of unmet basic resource needs, self‐identified unmet resource needs related to transportation, housing, food, utilities, and medications/medical care, in modifying the risk of frailty among older adults with cancer remains unknown.
We address these knowledge gaps by examining the prevalence of unmet basic resource needs and its association with frailty and HRQoL in a cohort of older adults with cancer.
2. METHODS
2.1. Study population
The Cancer and Aging Resilience Evaluation (CARE) study at the University of Alabama at Birmingham (UAB) is a registry of older adults (≥60 years) seen at UAB Hospital and Clinics for their cancer care; enrollment began in 2017, and a brief social determinants of health section was added to the core tool in August 2020. 19 We included adults 60 years or older, given the uncertainty of the appropriate chronologic age cutoff for “older” adults and among CARE participants, there is a similar prevalence of age‐related impairments and frailty among those 60–65 years and those 65–75 years and > 75 years. 20 For this study, we included the subset of participants recruited between August 2020 and April 2022 that completed the social determinants of health section. This study was approved by the Institutional Review Board at University of Alabama at Birmingham (IRB‐300000092) and performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments.
2.2. Study measures
2.2.1. Basic resource needs
Social determinants of health measures embedded within the CARE tool assessed basic needs as outlined here: (1) Health‐related transportation insecurity was measured using two previously published items: 21 How much trouble is it for you to get transportation to your doctor? [dichotomized as some trouble/a lot of trouble versus a little trouble/no trouble]; Have you ever missed a doctor's appointment because of transportation issues? [yes/no]. Patients were classified as having unmet transportation needs if they identified trouble in either of these questions. (2) Housing insecurity was assessed using two items adapted from the Protocol for Responding to and Assessing Patients Assets, Risks and Experiences (PRAPARE) instrument developed by the National Association of Community Health Centers. 22 The first inquired about the participant's current housing situation [I have housing; I do not have housing (staying with others, in a hotel, in a shelter, living outside on the street, on a beach, in a car, or in a park)] and the second asked whether participants were worried about losing their housing [yes/no]. 23 Patients were classified as having unmet housing needs if they responded I do not have housing or had concerns about losing their housing. (3) Material hardship (security of food, utilities, and medications/medical care) was assessed using a single item adapted from the PRAPARE instrument: In the past year, have you or any family members you live with been unable to get any of the following when it was really needed? [a list of yes/no items was provided: food, utilities, medicine or any healthcare (medical, dental, mental health, vision), phone, clothing, child care, or other]. 23 Patients were classified as having unmet material needs if they answered yes to any of the items. Patients were classified as having overall unmet basic resource needs if they had any unmet needs in one or more of the categories above (transportation, housing or material hardship).
2.2.2. Frailty
Using the principles of deficit accumulation and following the procedures outlined by Searle et al., 24 we constructed the CARE Frailty Index. 25 Based on 44 identified health deficits from the CARE geriatric assessment tool, the CARE Frailty Index was calculated as the proportion of deficits for each patient (range 0–1). Participants were required to have nonmissing data for at least 30 items in order to compute a valid frailty score and were categorized as robust (0–0.2), prefrail (0.2–0.35), and frail (>0.35), as previously described. 24 Our team has previously shown that the CARE frailty index predicts functional decline, severe chemotherapy toxicities, and survival among older adults; 25 similarly constructed frailty indices have shown comparable results. 12 , 13 , 14 , 15 , 26 See Table S1 for a full list of the CARE frailty index items.
2.2.3. Health‐related quality of life
The CARE tool assesses HRQoL using the National Institutes of Health Patient‐Reported Outcomes Measurement Information System® (PROMIS®) Global Health 10‐item short‐form. The PROMIS Global Health 10‐item scale includes separate scoring for physical and mental health subscales. 27 , 28 PROMIS measures have been tested in large samples of adults in the United States and item responses are converted to t‐scores with a standardized mean score of 50 and a standard deviation of 10. 30 The minimal clinically relevant difference for PROMIS ranges from 2 to 6 points, and a score of ≤40 (1 standard deviation) is considered impaired for the subscales. 29 Low physical and mental subdomains of HRQoL were defined as a t‐score of ≤40 (1 standard deviation).
2.2.4. Covariates
Patients self‐reported information regarding race, ethnicity, education, marital status, and employment. Urban–rural status was obtained via patient‐reported ZIP code merged with Rural–Urban Commuting Area (RUCA) code data. Categorization B from the University of Washington School of Medicine was used to define urban, micropolitan, and rural status. 30 , 31 Urban and micropolitan were combined into one urban group due to similarity in outcomes between the two as discussed in a prior study. 32 Information regarding cancer stage, cancer type, and date of diagnosis were abstracted from the electronic medical record.
2.3. Statistical analyses
Distribution‐appropriate bivariate statistical tests, namely chi‐squared test/Fisher's exact test for categorical variables, were used to compare patient characteristics and frailty categories between those with and without unmet basic resource needs. Logistic regression models were used to evaluate the association between unmet basic resource needs with frailty and physical and mental domains of HRQoL. An additional logistic regression model was used to assess predictors of unmet basic resource needs. Multivariable models were adjusted for potential confounders including age, sex, race/ethnicity, education, marital status, employment status, urban–rural status, cancer type, and cancer stage. All hypothesis testing was two‐sided and the level of significance was set at 0.05. All statistical analyses were conducted using SAS statistical software version 9.4 (SAS Institute Inc.).
3. RESULTS
3.1. Patients
Between August 2020 and April 2022, a total of 494 older adults completed the CARE tool and the social determinants of health survey (see Figure S1 for consort diagram). Most participants were age 60–69 years (56.2%), male (63.6%), and non‐Hispanic White (75.1%) (Table 1). Most participants had a high school education or some college (52.9%), were married (57.6%), and were retired (62.1%). Most participants resided in urban (90.7%) versus rural areas (9.3%). Finally, participants had either colorectal cancer (33.1%), pancreatic (18.0%), hepatobiliary (12.0%), or other cancers (36.9%). The majority had advanced stage disease (stage III: 30.6%; stage IV: 44.5%). Overall, 29.0% of the cohort was frail (see Table S1 for prevalence of individual frailty item impairments); impaired physical HRQoL was observed in 36.2% and impaired mental HRQoL in 39.1%. Median time from cancer diagnosis to CARE tool completion was 35 days.
TABLE 1.
Participant characteristics.
| Variable | Total | Unmet need, 88 (17.8%) | No unmet needs, 406 (82.2%) | p‐value |
|---|---|---|---|---|
| Age group | 0.630 | |||
| 60–64 | 147 (29.9) | 30 (34.1) | 117 (29.0) | |
| 65–69 | 129 (26.3) | 26 (29.6) | 103 (25.6) | |
| 70–74 | 102 (20.8) | 14 (15.9) | 88 (21.8) | |
| 75–79 | 57 (11.6) | 9 (10.2) | 48 (11.9) | |
| 80+ | 56 (11.4) | 9 (10.2) | 47 (11.7) | |
| Sex, male | 314 (63.6) | 52 (59.1) | 262 (64.5) | 0.336 |
| Race/Ethnicity | 0.021 | |||
| Non‐Hispanic White | 364 (75.1) | 56 (64.4) | 308 (77.4) | |
| Non‐Hispanic Black | 98 (20.2) | 27 (31.0) | 71 (17.8) | |
| Other | 23 (4.7) | 4 (4.6) | 19 (4.8) | |
| Education | 0.023 | |||
| <High school | 56 (11.4) | 17 (19.5) | 39 (9.7) | |
| High school | 148 (30.2) | 31 (35.6) | 117 (29.0) | |
| Some college | 111 (22.7) | 18 (20.7) | 93 (23.1) | |
| Associate's/Bachelor's degree | 101 (20.6) | 13 (14.9) | 88 (21.8) | |
| Advanced degree | 74 (15.1) | 8 (9.2) | 66 (16.4) | |
| Employment status | <0.001 | |||
| Retired | 303 (62.1) | 50 (56.8) | 253 (63.3) | |
| Disabled | 67 (13.7) | 23 (26.1) | 44 (11.0) | |
| Part‐time (<32 h/week) | 12 (2.5) | 3 (3.4) | 9 (2.3) | |
| Full‐time (≥32 h/week) | 62 (12.7) | 3 (3.4) | 59 (14.8) | |
| Other | 44 (9.0) | 9 (10.2) | 35 (8.8) | |
| Marital status | <0.001 | |||
| Single | 46 (9.4) | 18 (20.9) | 28 (6.9) | |
| Widowed/Divorced/Separated | 162 (33.1) | 37 (43.0) | 125 (30.9) | |
| Married | 282 (57.6) | 31 (36.1) | 251 (62.1) | |
| Urban–rural residence | 0.465 | |||
| Urban | 448 (90.7) | 78 (88.6) | 370 (91.1) | |
| Rural | 46 (9.3) | 10 (11.4) | 36 (8.9) | |
| Cancer type | <0.001 | |||
| Colorectal | 162 (33.1) | 25 (28.7) | 137 (34.0) | |
| Pancreatic | 88 (18.0) | 16 (18.4) | 72 (17.9) | |
| Hepatobiliary | 59 (12.0) | 22 (25.3) | 37 (9.2) | |
| Other | 181 (36.9) | 24 (27.6) | 157 (39.0) | |
| Cancer stage | 0.675 | |||
| 0–II | 117 (24.9) | 23 (26.4) | 94 (24.5) | |
| III | 144 (30.6) | 29 (33.3) | 115 (30.0) | |
| IV | 209 (44.5) | 35 (40.2) | 174 (45.4) | |
| Frailty | 142 (29.0) | 47 (54.0) | 95 (23.6) | <0.001 |
| Impaired physical HRQoL | 167 (36.2) | 48 (57.1) | 119 (31.5) | <0.001 |
| Impaired mental HRQoL | 189 (39.1) | 53 (61.6) | 136 (34.2) | <0.001 |
Note: Bold values indicate significance at < 0.05.
3.2. Basic unmet needs
Overall, 17.8% of patients were classified as having a basic unmet need; 14.2% had an unmet need in one category, 3.2% in two categories and 0.4% in three categories (see Figure 1). Transportation insecurity was the most prevalent unmet need at 11.5%, followed by material hardship at 7.5% (subcategories including food: 4.3%, utilities: 4.1%, medicine/health care: 5.9%, phone: 4.1%, clothing: 3.9%, childcare: 1.8%) and housing insecurity at 2.8%.
FIGURE 1.
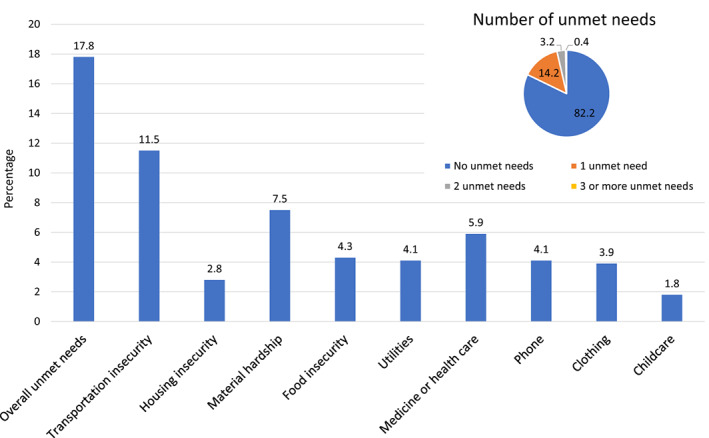
Prevalence of basic unmet needs.
3.3. Patients with and without basic unmet needs
When compared with those without any basic unmet need, there was an over‐representation of non‐Hispanic Black (31% vs. 17.8%, p = 0.021), lower education (high school: 35.6% vs. 29.0%, p = 0.023), those who were disabled (26.1% vs. 11.0%, p < 0.001), and those who were unmarried (single: 20.9% vs. 6.9%; widowed/divorced/separated: 43.0% vs. 30.9%, p < 0.001) among patients with any basic unmet need (Table 1). In addition, there was also a higher proportion of participants with pancreatic or hepatobiliary cancers (pancreatic: 18.4% vs. 17.9%; hepatobiliary: 25.3% vs. 9.2%, p < 0.001) among those with basic unmet needs. However, there was no difference by cancer stage (p = 0.675). Finally, those with any unmet need were more likely to be frail (54.0% vs. 23.6%, p < 0.001) and have impaired physical (57.1% vs. 31.5%, p < 0.001) and mental (61.6% vs. 34.2%, p < 0.001) HRQoL. Predictors of having any basic unmet need included non‐Hispanic Black relative to non‐Hispanic White race (odds ratio [OR]: 2.1, 95% CI: 1.2, 3.5), being single (OR: 5.2, 95% CI: 2.6, 10.5) or widowed/divorced relative to married (OR: 2.4, 95% CI: 1.4, 4.0), and having less than a high school education relative to an advanced degree (OR: 3.60, 95% CI: 1.42, 9.10), and having hepatobiliary relative to colorectal cancer (OR: 3.3, 95% CI: 1.7, 6.4) (Table 2).
TABLE 2.
Unadjusted odds ratios of demographics and clinical variables to basic unmet needs.
| Demographics | Unadjusted odds (95% CI) |
|---|---|
| Age group | |
| 60–64 | REF |
| 65–69 | 0.98 (0.55, 1.77) |
| 70–74 | 0.62 (0.31, 1.24) |
| 75–79 | 0.73 (0.32, 1.66) |
| 80+ | 0.75 (0.33, 1.69) |
| Sex | |
| Female | REF |
| Male | 0.79 (0.50, 1.27) |
| Race | |
| Non‐Hispanic White | REF |
| Non‐Hispanic Black | 2.09 (1.24, 3.54) |
| Other | 1.16 (0.38, 3.53) |
| Educational level | |
| Less than high school | 3.60 (1.42, 9.10) |
| High school graduate | 2.19 (0.95, 5.03) |
| Some college | 1.60 (0.66, 3.89) |
| Associate/Bachelors | 1.22 (0.48, 3.11) |
| Advanced Degree | REF |
| Marital status | |
| Married | REF |
| Single | 5.21 (2.59, 10.48) |
| Widowed/Divorced | 2.40 (1.42, 4.04) |
| Urban–Rural status | |
| Urban | REF |
| Rural | 1.32 (0.63, 2.77) |
| Clinical | |
| Cancer type | |
| Colorectal | REF |
| Pancreatic | 1.22 (0.61, 2.43) |
| Hepatobiliary | 3.26 (1.65, 6.42) |
| Other | 0.84 (0.46, 1.53) |
| Cancer stage | |
| I/II | REF |
| III | 1.03 (0.56, 1.90) |
| IV | 0.82 (0.46, 1.47) |
Note: Bold values indicate significance at < 0.05.
Abbreviation: CI, confidence interval.
3.4. Multivariable analysis
Having any basic unmet need was associated with 3.3‐fold higher adjusted odds of frailty compared to not having a basic unmet need (adjusted OR [aOR] 3.3, 95% CI: 1.8, 5.9) after adjustment for age, sex, race/ethnicity, education, employment status, marital status, urban–rural status, cancer type, and cancer stage (Figure 2 and Table S2). Additionally, having any basic unmet need was associated with 2.1‐fold higher odds of physical (aOR 2.1, 95% CI: 1.2, 3.8) and 2.5‐fold higher odds of mental (aOR 2.5, 95% CI: 1.4, 4.4) impaired HRQoL after adjusting for these variables (see Figure 2 and Table S3).
FIGURE 2.
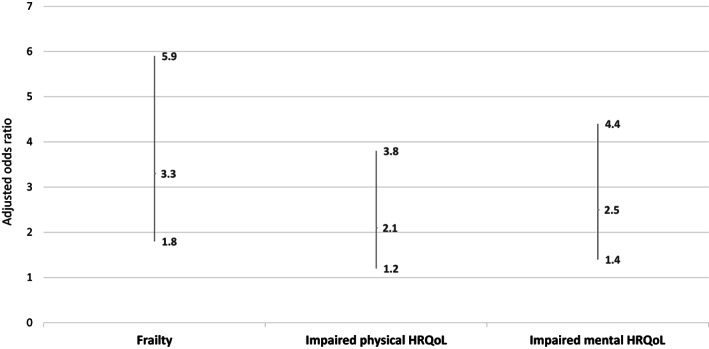
Multivariable logistic regression of the association between basic unmet needs with frailty and reduced physical and mental health‐related quality of life.
4. DISCUSSION
Here, we use a unique prospectively assembled registry of older adults with cancer to examine the association between basic unmet resource needs and frailty and HRQoL, and reveal that unmet needs were associated with a 3.3‐fold higher adjusted odds of frailty and more than a twofold higher adjusted odds of impaired physical and mental HRQoL. Our findings are consistent with the notion that social determinants of health are associated with frailty and HRQoL among older adults with cancer. The most prevalent unmet need was transportation insecurity, with many reporting missing appointments due to transportation difficulties. In addition, several participants reported material insecurities including food insecurity or difficulty obtaining medicine or health care.
The prevalence of unmet basic needs in our study is lower than expected compared with prior studies. In one of the largest intervention studies to date by Berkowitz et al, 34.6% of adults within a primary care network in the Boston metropolitan area screened positive for an unmet need. 5 In families of pediatric cancer survivors, household material hardship, defined as insecurity of food, housing, or energy, had a similar prevalence of 32%. 33 More specifically, the prevalence of food insecurity in our study was lower than anticipated based on the literature. Prior estimates range widely from 8% to 55% of patients with cancer experiencing food insecurity depending on the setting, with those having the highest prevalence from low‐income and underserved urban communities. 34 , 35 Previous studies have found similar associations with food insecurity, including non‐Hispanic Black race and lower education. 36 While the prevalence of transportation barriers was the highest among the examined basic needs in our study, the prevalence of transportation issues was also lower than the literature suggests. 37 Similarly, the prevalence of housing insecurity varies by population, but is often higher than the 3% we found. 38 The lower prevalence of unmet needs in our study of older adults may be explained in part by a trend toward increased basic unmet needs among younger patients in these prior studies. 34 , 38 , 39 In addition, most studies of individual social determinants (transportation, housing, and food insecurity) are focused on the social determinants as their primary outcome and thus can utilize longer, multi‐item questionnaires to determine insecurity; on the contrary, our study integrated an abbreviated survey into a more global registry survey, which is likely less sensitive. 35 Furthermore, although our study population is from the US Deep South, an area notable for health disparities, this sample is from a single large academic medical center and not representative of the entire region nor directly comparable to many of the prior studies from urban areas of the northeastern United States. 4 , 5 , 33 Of note, UAB Hospital and Clinics provide care for all patients irrespective of insurance and/or immigration status, and prior research from the CARE Registry demonstrated a mix of 51.5% Medicare, 2.7% Medicaid, 3.6% uninsured/self‐pay, and 42.3% private insurance. 40
Although socioeconomic conditions are known to contribute to the incidence of frailty, this study is among the first to examine the association of basic unmet needs, recognized as concrete, remediable indicators of poverty, with frailty among older adults with cancer. 16 , 17 , 18 While we cannot demonstrate causality within the context of our cross‐sectional study, several plausible mechanisms exist to suggest that unmet needs may lead to increased frailty. First, unmet basic needs are likely associated with less access to medical care and reduced preventative care, thus resulting over time in increased rates of frailty. 4 , 41 Second, insecurity of basic needs may contribute to higher rates of mental distress and stress, and in turn, this chronic stress may result in increased frailty. 42 , 43 Lastly, the mental distress and stress related to unmet needs may increase risk behaviors related to frailty (e.g., smoking and alcohol consumption). 44 , 45
Interventions related to social determinants of health have been effective in improving health outcomes across health conditions. 46 More specifically, interventions to address unmet basic resource needs such as food, housing, or medications have improved clinical outcomes. 5 For example, addressing unmet basic needs among 1700 study participants resulted in improvements in blood pressure and cholesterol levels. 5 Similar multicomponent interventions, that include social determinants of health, have demonstrated effectiveness in improving diabetes outcomes and HRQoL. 47 The existing strategies vary substantially in terms of involved workforce (professional vs. lay), setting (community vs. clinic or hospital based), and length of interaction (episodic vs. longitudinal). 4 , 5 While further work is necessary to determine the most applicable strategy to address the unmet needs of older adults doing so may prove useful in improving cancer outcomes.
One of the populations identified with the most unmet needs were Black participants. Racial disparities in cancer outcomes are well‐recognized, yet the underlying causes remain an area of ongoing focus. The illustrated differences in unmet basic needs by race, may in part, explain some outcome disparities. Black participants within the CARE registry report a higher prevalence of financial distress, which is intrinsically related to the basic unmet needs. 40 However, while racial disparities in frailty among older adults with cancer have been demonstrated, unmet needs nevertheless remained significantly associated with increased frailty even after controlling for race. 48 The growing evidence suggest that some combination of unmet needs, financial strain, and low socioeconomics likely contribute to racial disparities in health outcomes; thus, identifying the appropriate target amenable to intervention among these domains is a promising avenue toward reducing racial disparities and promoting health equity.
Our study is not without limitations. As our analyses are cross‐sectional in nature, no causality or directionality can be drawn between associations. Our study population was from a single center in the Southeastern United States and may not be representative of other older adult populations. Our study relies on patient‐reported measures of health, including for the report of unmet needs and the geriatric assessment information, and lacks additional objective assessment to collaborate these reports. However, the use of patient‐reported outcomes measures has expanded dramatically over the last decade and has been shown to be frequently more accurate than provider or caregiver reports. 49 , 50 And although the CARE Registry has a high enrollment proportion (approximately 80%), 19 there remains some potential for selection bias.
Assessing social determinants of health within oncology care identifies critical and potentially remediable basic unmet needs that may be important drivers of poor outcomes among older adults with cancer. Investigating interventions to address basic unmet needs will be critical to improving HRQoL and reducing the adverse outcomes associated with frailty in vulnerable older adults with cancer. Although high‐quality interventional studies on these unmet needs are lacking, assessing and addressing these basic unmet needs in oncology practice in the short term makes intuitive sense, and may improve the outcomes of our most vulnerable cancer populations.
AUTHOR CONTRIBUTIONS
Grant R Williams: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Mackenzie E Fowler: Conceptualization (equal); data curation (equal); formal analysis (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Smith Giri: Conceptualization (equal); methodology (equal); writing – review and editing (equal). Chen Dai: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Christian Harmon: Data curation (equal); writing – review and editing (equal). Mustafa AL‐Obaidi: Data curation (equal); writing – review and editing (equal). Coryn Stephenson: Investigation (supporting); writing – review and editing (equal). Kira Bona: Conceptualization (equal); writing – review and editing (equal). Wendy Landier: Conceptualization (equal); writing – review and editing (equal). Smita Bhatia: Conceptualization (equal); methodology (equal); supervision (equal); writing – review and editing (equal). Julie A. Wolfson: Conceptualization (equal); methodology (equal); writing – review and editing (equal).
FUNDING INFORMATION
Supported in part by the National Cancer Institute of the National Institutes of Health (K08CA234225) and the Doris Duke Charitable Foundation CARES program at UAB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Previously presented at the American Society of Clinical Oncology (ASCO) 2022 annual meeting.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
ETHICS APPROVAL
Approved by the IRB at UAB (IRB‐300000092) and patients all consented.
Supporting information
Figure S1.
Table S1.
Table S2.
Table S3.
Williams GR, Fowler M, Giri S, et al. Association of unmet basic resource needs with frailty and quality of life among older adults with cancer—Results from the CARE registry. Cancer Med. 2023;12:13846‐13855. doi: 10.1002/cam4.6038
DATA AVAILABILITY STATEMENT
Study data are available upon request to the corresponding author.
REFERENCES
- 1. Cronin KA, Lake AJ, Scott S, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2018;124(13):2785‐2800. doi: 10.1002/cncr.31551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel MI, Lopez AM, Blackstock W, et al. Cancer disparities and health equity: a policy Statement from the American Society of Clinical Oncology. J Clin Oncol. 2020;38(29):3439‐3448. doi: 10.1200/JCO.20.00642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Braveman P, Gottlieb L. The social determinants of health: it's time to consider the causes of the causes. Public Health Rep. 2014;129(Suppl 2):19‐31. doi: 10.1177/00333549141291S206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berkowitz SA, Hulberg AC, Hong C, et al. Addressing basic resource needs to improve primary care quality: a community collaboration programme. BMJ Qual Saf. 2016;25(3):164‐172. doi: 10.1136/bmjqs-2015-004521 [DOI] [PubMed] [Google Scholar]
- 5. Berkowitz SA, Hulberg AC, Standish S, Reznor G, Atlas SJ. Addressing unmet basic resource needs as part of chronic cardiometabolic disease management. JAMA Intern Med. 2017;177(2):244‐252. doi: 10.1001/jamainternmed.2016.7691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boscoe FP, Johnson CJ, Sherman RL, Stinchcomb DG, Lin G, Henry KA. The relationship between area poverty rate and site‐specific cancer incidence in the United States. Cancer. 2014;120(14):2191‐2198. doi: 10.1002/cncr.28632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moss JL, Pinto CN, Srinivasan S, Cronin KA, Croyle RT. Persistent poverty and cancer mortality rates: an analysis of county‐level poverty designations. Cancer Epidemiol Biomarkers Prev. 2020;29(10):1949‐1954. doi: 10.1158/1055-9965.EPI-20-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78‐93. doi: 10.3322/canjclin.54.2.78 [DOI] [PubMed] [Google Scholar]
- 9. Garner WB, Smith BD, Ludmir EB, et al. Predicting future cancer incidence by age, race, ethnicity, and sex. J Geriatr Oncol. 2022;14:101393. doi: 10.1016/j.jgo.2022.10.008 [DOI] [PubMed] [Google Scholar]
- 10. Ethun CG, Bilen MA, Jani AB, Maithel SK, Ogan K, Master VA. Frailty and cancer: implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin. 2017;67(5):362‐377. doi: 10.3322/caac.21406 [DOI] [PubMed] [Google Scholar]
- 11. Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26(6):1091‐1101. doi: 10.1093/annonc/mdu540 [DOI] [PubMed] [Google Scholar]
- 12. Cohen HJ, Smith D, Sun CL, et al. Frailty as determined by a comprehensive geriatric assessment‐derived deficit‐accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016;122(24):3865‐3872. doi: 10.1002/cncr.30269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams GR, Dunham L, Chang Y, et al. Geriatric assessment predicts hospitalization frequency and long‐term care use in older adult cancer survivors. J Oncol Pract. 2019;15(5):e399‐e409. doi: 10.1200/JOP.18.00368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williams GR, Deal AM, Sanoff HK, et al. Frailty and health‐related quality of life in older women with breast cancer. Support Care Cancer. 2019;27(7):2693‐2698. doi: 10.1007/s00520-018-4558-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guerard EJ, Deal AM, Chang Y, et al. Frailty index developed from a cancer‐specific geriatric assessment and the association with mortality among older adults with cancer. J Natl Compr Cancer Netw. 2017;15(7):894‐902. doi: 10.6004/jnccn.2017.0122 [DOI] [PubMed] [Google Scholar]
- 16. Hayajneh AA, Rababa M. The Association of Frailty with poverty in older adults: a systematic review. Dement Geriatr Cogn Disord. 2021;50(5):407‐413. doi: 10.1159/000520486 [DOI] [PubMed] [Google Scholar]
- 17. Stolz E, Mayerl H, Waxenegger A, Freidl W. Explaining the impact of poverty on old‐age frailty in Europe: material, psychosocial and behavioural factors. Eur J Pub Health. 2017;27(6):1003‐1009. doi: 10.1093/eurpub/ckx079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watts PN, Blane D, Netuveli G. Minimum income for healthy living and frailty in adults over 65 years old in the English longitudinal study of ageing: a population‐based cohort study. BMJ Open. 2019;9(2):e025334. doi: 10.1136/bmjopen-2018-025334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williams GR, Kenzik KM, Parman M, et al. Integrating geriatric assessment into routine gastrointestinal (GI) consultation: the cancer and aging resilience evaluation (CARE). J Geriatr Oncol. 2020;11(2):270‐273. doi: 10.1016/j.jgo.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giri S, Al‐Obaidi M, Weaver A, et al. Association between chronologic age and geriatric assessment‐identified impairments: findings from the CARE registry. J Natl Compr Canc Netw. 2021;19:1‐6. doi: 10.6004/jnccn.2020.7679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Locatelli SM, Sharp LK, Syed ST, Bhansari S, Gerber BS. Measuring health‐related transportation barriers in urban settings. J Appl Meas. 2017;18(2):178‐193. [PMC free article] [PubMed] [Google Scholar]
- 22. PRAPARE: Protocol for Responding to and Assessing Patients' Assets, Risks, and Experiences. National Association of Community Health Centers. Accessed 4/14/2022. https://prapare.org/
- 23. Kusnoor SV, Koonce TY, Hurley ST, et al. Collection of social determinants of health in the community clinic setting: a cross‐sectional study. BMC Public Health. 2018;18(1):550. doi: 10.1186/s12889-018-5453-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giri S, Al‐Obaidi M, Harmon C, et al. Patient‐reported geriatric assessment‐based frailty index among older adults with gastrointestinal malignancies. J Am Geriatr Soc. 2022;71:136‐144. doi: 10.1111/jgs.18054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giri S, Mir N, Al‐Obaidi M, et al. Use of single‐item self‐rated health measure to identify frailty and geriatric assessment‐identified impairments among older adults with cancer. Oncologist. 2022;27(1):e45‐e52. doi: 10.1093/oncolo/oyab020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient‐reported outcomes measurement information system (PROMIS) global items. Qual Life Res Int J Qual Life Asp Treat Care Rehab. 2009;18(7):873‐880. doi: 10.1007/s11136-009-9496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pergolotti M, Deal AM, Williams GR, et al. Activities, function, and health‐related quality of life (HRQOL) of older adults with cancer. Journal of Geriatric Oncology. 2017;8(4):249‐254. doi: 10.1016/j.jgo.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six patient‐reported outcomes measurement information system‐cancer scales in advanced‐stage cancer patients. Multicenter study research support, N.I.H., extramural. J Clin Epidemiol. 2011;64(5):507‐516. doi: 10.1016/j.jclinepi.2010.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Medicine UoWSo . RUCA Data: Using RUCA Data. Accessed March 21, 2022. https://depts.washington.edu/uwruca/ruca‐uses.php
- 31. Medicine UoWSo . RUCA Data: Code Definitions. Accessed March 21, 2022. https://depts.washington.edu/uwruca/ruca‐codes.php
- 32. Fowler ME, Kenzik K, Al‐Obaidi M, et al. Rural‐urban disparities in geriatric assessment (GA) impairments and mortality among older adults with cancer: results from the cancer and aging resilience evaluation (CARE) registry. J Clin Oncol. 2022;40(16):12012. doi: 10.1200/JCO.2022.40.16_suppl.12012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bilodeau M, Ma C, Al‐Sayegh H, Wolfe J, Bona K. Household material hardship in families of children post‐chemotherapy. Pediatr Blood Cancer. 2018;65(1):e26743. doi: 10.1002/pbc.26743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gany F, Lee T, Ramirez J, et al. Do our patients have enough to eat?: food insecurity among urban low‐income cancer patients. J Health Care Poor Underserved. 2014;25(3):1153‐1168. doi: 10.1353/hpu.2014.0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel KG, Borno HT, Seligman HK. Food insecurity screening: a missing piece in cancer management. Cancer. 2019;125(20):3494‐3501. doi: 10.1002/cncr.32291 [DOI] [PubMed] [Google Scholar]
- 36. Simmons LA, Modesitt SC, Brody AC, Leggin AB. Food insecurity among cancer patients in Kentucky: a pilot study. J Oncol Pract. 2006;2(6):274‐279. doi: 10.1200/JOP.2006.2.6.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang C, Yabroff KR, Deng L, et al. Self‐reported transportation barriers to health care among US cancer survivors. JAMA Oncol. 2022;8(5):775‐778. doi: 10.1001/jamaoncol.2022.0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fan Q, Keene DE, Banegas MP, et al. Housing insecurity among patients with cancer. J Natl Cancer Inst. 2022;114:1584‐1592. doi: 10.1093/jnci/djac136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trego ML, Baba ZM, DiSantis KI, Longacre ML. Food insecurity among adult cancer survivors in the United States. J Cancer Surviv. 2019;13(4):641‐652. doi: 10.1007/s11764-019-00783-9 [DOI] [PubMed] [Google Scholar]
- 40. Giri S, Clark D, Al‐Obaidi M, et al. Financial distress among older adults with cancer. JCO Oncol Pract. 2021;17(6):e764‐e773. doi: 10.1200/OP.20.00601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martin P, Liaw W, Bazemore A, Jetty A, Petterson S, Kushel M. Adults with housing insecurity have worse access to primary and preventive care. J Am Board Fam Med. 2019;32(4):521‐530. doi: 10.3122/jabfm.2019.04.180374 [DOI] [PubMed] [Google Scholar]
- 42. Ni Mhaolain AM, Fan CW, Romero‐Ortuno R, et al. Frailty, depression, and anxiety in later life. Int Psychogeriatr. 2012;24(8):1265‐1274. doi: 10.1017/S1041610211002110 [DOI] [PubMed] [Google Scholar]
- 43. Haapanen MJ, Perala MM, Salonen MK, et al. Early life stress and frailty in old age: the Helsinki birth cohort study. BMC Geriatr. 2018;18(1):179. doi: 10.1186/s12877-018-0873-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. DeClercq V, Duhamel TA, Theou O, Kehler S. Association between lifestyle behaviors and frailty in Atlantic Canadian males and females. Arch Gerontol Geriatr. 2020;91:104207. doi: 10.1016/j.archger.2020.104207 [DOI] [PubMed] [Google Scholar]
- 45. Lafortune L, Martin S, Kelly S, et al. Behavioural risk factors in mid‐life associated with successful ageing, disability, dementia and frailty in later life: a rapid systematic review. PLoS One. 2016;11(2):e0144405. doi: 10.1371/journal.pone.0144405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taylor LA, Tan AX, Coyle CE, et al. Leveraging the social determinants of health: what works? PLoS One. 2016;11(8):e0160217. doi: 10.1371/journal.pone.0160217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ali MK, Singh K, Kondal D, et al. Effectiveness of a multicomponent quality improvement strategy to improve achievement of diabetes care goals: a randomized, controlled trial. Ann Intern Med. 2016;165(6):399‐408. doi: 10.7326/M15-2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Williams GR, Al‐Obaidi M, Harmon C, et al. Racial disparities in frailty and geriatric assessment impairments in older adults with cancer in the deep south: results from the CARE registry. Cancer. 2022;128(12):2313‐2319. doi: 10.1002/cncr.34178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chang EM, Gillespie EF, Shaverdian N. Truthfulness in patient‐reported outcomes: factors affecting patients' responses and impact on data quality. Patient Relat Outcome Meas. 2019;10:171‐186. doi: 10.2147/PROM.S178344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Atkinson TM, Ryan SJ, Bennett AV, et al. The association between clinician‐based common terminology criteria for adverse events (CTCAE) and patient‐reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24(8):3669‐3676. doi: 10.1007/s00520-016-3297-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Table S1.
Table S2.
Table S3.
Data Availability Statement
Study data are available upon request to the corresponding author.


