Abstract
Temporomandibular joint osteoarthritis (TMJ OA) is a progressive degenerative disease of the temporomandibular joint (TMJ). The unclear etiology and mechanisms of TMJ OA bring great difficulties to early diagnosis and effective treatment, causing enormous burdens to patients’ life and social economics. In this narrative review, we summarized the main pathological changes of TMJ OA, including inflammatory responses, degeneration of extracellular matrix (ECM), abnormal cell biological behaviors (apoptosis, autophagy, and differentiation) in TMJ tissue, and aberrant angiogenesis. All pathological features are closely linked to each other, forming a vicious cycle in the process of TMJ OA, which results in prolonged disease duration and makes it difficult to cure. Various molecules and signaling pathways are involved in TMJ OA pathogenesis, including nuclear factor kappa-B (NF-κB), mitogen-activated protein kinases (MAPKs), extracellular regulated protein kinases (ERKs) and transforming growth factor (TGF)-β signaling pathways et al. One molecule or pathway can contribute to several pathological changes, and the crosstalk between different molecules and pathways can further lead to a complicated condition TMJ OA. TMJ OA has miscellaneous etiology, complex clinical status, depressed treatment results, and poor prognosis. Therefore, novel in-vivo and in-vitro models, novel medicine, materials, and approaches for therapeutic procedures might be helpful for further investigation of TMJ OA. Furthermore, the role of genetic factors in TMJ OA needs to be elucidated to establish more reasonable and effective clinical strategies for diagnosing and treating TMJ OA.
Keywords: Temporomandibular joint osteoarthritis, Inflammation, Extracellular matrix, Biological behaviors, Angiogenesis, Molecular pathways
Introduction
Temporomandibular joint osteoarthritis (TMJ OA) is a progressive degenerative disease in the temporomandibular joint (TMJ) tissue. Pain, clicking in the temporomandibular region, and abnormal mandibular movements are considered to be the main symptoms of TMJ OA. TMJ OA is the most common subtype of temporomandibular disorders (TMD), affecting about 30% of the population in the Chinese mainland. Furthermore, 14.56% of TMD patients show definite imaging lesions on the articular surface and bone tissue. Aging, gender, and mechanical stress are the most common factors of TMJ OA.1 Other factors, including trauma, body postures, oral parafunctional habits, and psychological stress, are also associated with the etiology of TMJ OA.2 Currently, the main therapeutic principle for TMJ OA is conservative treatment, including joint protection, physical therapy (physical movement exercise and laser therapy), occlusal splints, nonsteroidal anti-inflammatory drugs (NSAIDs), and glucosamine sulfate. Unfortunately, due to the ambiguity of etiology and pathogenesis, current conservative therapeutic approaches for TMJ OA seem ineffective for curing this long-lasting disease, Moreover, TMJ OA may finally lead to limitation of jaw movement, and even orofacial deformity in adolescences, which burdens patients’ lives and social economy.
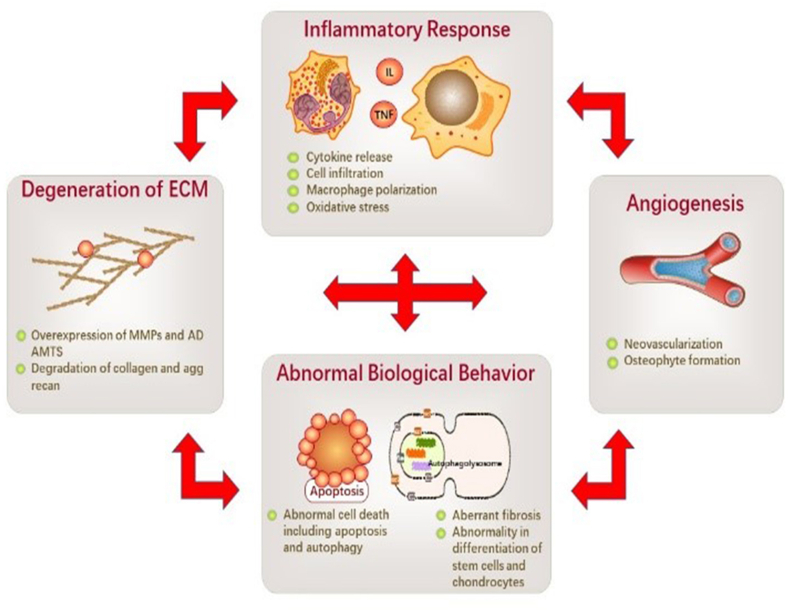
Inflammatory responses, degeneration of extracellular matrix (ECM), abnormal cell biological behaviors, and angiogenesis in TMJ tissue are the main pathological changes of TMJ OA.3 Inflammatory responses, including cytokines release, phagocyte chemotaxis, and macrophage polarization, can lead to the breakdown of the homeostasis of TMJ tissue, resulting in the imbalance of the anabolic and catabolic activities leading to ECM degeneration. Inflammatory factors and catabolic product accumulation can further cause hypoxia and oxidative stress to cells in TMJ tissue, leading to abnormal cell biological behaviors and abnormal angiogenesis. Cell and vascular abnormality can cause a more severe inflammatory and degenerative status, forming a vicious circle and a protracted course of TMJ OA (Fig. 1).
Fig. 1.
Potential pathological changes and the vicious cycle of TMJ OA. The pathological changes of TMJ OA typically include inflammatory response, ECM degeneration, abnormal cell biological behaviors, and aberrant angiogenesis. Inflammatory responses may occur in cartilage, disc, synovium, and all other TMJ tissue, characterized by cytokine release, cell infiltration, M1 macrophage polarization, and excessive oxidative stress. ECM degeneration is mainly caused by overexpression of MMPs and ADAMTS, leading to the degradation of collagens and aggrecan. Various cells in TMJ tissue may display excessive apoptosis, suppressed autophagy, and abnormal differentiation and activation. Neovascularization also occurs and primarily develops into osteophytes. One pathological change can be the causation or the result of other pathological changes, forming a vicious cycle in TMJ OA pathogenesis. Thus, it may prolong the disease process making it hard to cure.
Accordingly, we make a comprehensive review of the present studies conducted on pathological and corresponding molecular mechanisms of TMJ OA, aiming to present a promising aspect of utilizing the mechanisms for targeting the suppression of TMJ OA.
Pathological changes in temporomandibular joint osteoarthritis
Inflammatory responses
Inflammation is believed to be one of the most crucial factors in the pathological changes in TMJ OA.4 It has been elucidated that several cytokines, including interleukin (IL) -1β, IL-6, IL-8, IL-17, and tumor necrosis factor (TNF) -α, are elevated in TMJ synovial fluid of TMJ OA patients.4, 5, 6 Among them, IL-1β plays a pivotal role in exacerbating the pathological process of TMJ OA by promoting the release of matrix-degrading enzymes, inhibiting the differentiation of mesenchymal stem cells and mediating the crosstalk between several pro-inflammatory molecules and pathways.5,7, 8, 9 The expression of cyclooxygenase (COX) -2 and prostaglandin (PG) E2 is increased in fibroblast-like synoviocytes (FLS) derived from TMJ stimulated with IL-1β, resulting in the activation of ptgs-2/PGE2 pathway and inhibiting the proteoglycan production.10,11 Cell migration-inducible chemokines, including monocyte chemoattractant protein (MCP-1, or CCL2), macrophage inflammatory protein-3a (MIP-3a, or CCL20), stromal cell-derived factor 1 (SDF-1, or CXCL12) and intracellular adhesion molecule 1 (ICAM-1), are highly expressed in human TMJ FLS.12,13 IL-1β can interact with high mobility group box protein 1 (HMGB1), amplifying the production of cytokines and chemokines and aggravating inflammation.14 Furthermore, Toll-like receptor (TLR)-4 can interact with IL-1β and activate MyD88, leading to NF-κB pathway activation.15,16 Ge et al. demonstrated that NF-κB and Wnt5a signaling pathways were involved in the inflammatory responses when TMJ condylar chondrocytes (CC) were stimulated with IL-1β. Moreover, NF-κB was required to activate Wnt5a when stimulated by IL-1β.9
Epigenetics refers to changes in gene expression that are stable between cell divisions or sometimes generations but do not involve changes in the DNA sequences. The contents of epigenetics include DNA methylation, acetylation, and deacetylation of histone and non-coding RNAs et al. Epigenetics is associated with many different human diseases and is also involved in TMJ OA.17 Histone deacetylase (HDAC) is a type of protein that promotes the deacetylation of histones, leading to higher condensation of chromatins and inhibition of gene translation.17,18 Liao et al. have demonstrated that HDAC10 upregulation can contribute to inflammatory activation of TMJ synovium-derived mesenchymal stem cells (SMSCs) mediated by IL-1β, intervening in the cartilage repair of TMJ OA.17 BRD4 is a double bromodomain protein of the BET family binding to the acetylated histone to regulate gene expression. Inhibition of BRD4 can attenuate TREM1-mediated inflammatory responses in TMJ OA.18 Non-coding RNAs (ncRNAs) are derived from many sequences generating non-protein-coding RNAs in the human genome and have vital functions in translation, RNA splicing, and regulation of gene expression.20 MicroRNAs, circRNAs, and long non-coding RNAs (lncRNAs) are all involved in inflammatory responses of TMJ OA, leading to the release of pro-inflammatory cytokines and matrix-degrading enzymes. LncRNAs, including AK094629 and HOTAIR, can lead to the aggravation of inflammation, while AK094629 can interact with the MAPK pathway.20,21 Increased expression of miR-1246 in extracellular vesicles (EVs) derived from M1 macrophages (M1-EVs) has a pro-inflammatory effect on TMJ OA via activation of the Wnt/β-catenin pathway.22 Hsa_circ_0000448 is also involved in the progression of TMJ OA by promoting the upregulation of TNF-α through the ceRNA mechanism.23
Other molecules and pathways also participate in the inflammatory responses of TMJ OA. For example, connexin 43 (Cx43) is localized at the cell surface and is capable of forming hemichannels, which plays a role in mechanotransduction and is widely expressed in chondrocytes and osteocytes. It is reported that fluid flow shear stress (FFSS) on ATDC5 cells leads to increased expression of Cx43, followed by enhanced synthesis and release of PGE2.24 Recently, leptin, a non-glycosylated polypeptide hormone synthesized predominantly by white adipose tissue, has been increasingly proven to be a cytokine-like hormone functioning as an immune response modulator. Xiong et al. showed that leptin could upregulate IL-6 production via JAK/STAT3, p38 MAPK, and PI3K/Akt (protein kinase B) pathways when binding to Ob-Rb in TMJ FLS.25 In addition, considering that the incidence of TMJ OA in females is higher than in males, estrogen is believed to be involved in TMJ OA. It has been demonstrated that estrogen could interact with cadherin-11, a cell adhesion molecule, to promote M1-like macrophage activation and aggravate the acute inflammation in TMJ tissue.26 Estradiol can also activate the NF-κB pathway, causing the induction of upregulating pro-inflammatory cytokines such as IL-1β, IL-6, and COX2.27 Moreover, SDF-1, Dkk-1, and p38 MAPK are also involved in generating and processing TMJ OA.28, 29, 30
Several molecules suppress inflammatory responses in TMJ OA through different molecular pathways. In complete Freund's adjuvant-induced (CFA) and disc-perforation-induced TMJ OA model, IL-37 was able to shift M1 macrophage into the M2 phenotype and inhibited the M1-polarized phenotype through the NLRP3 (NOD-like receptor pyrin domain-containing 3) signaling pathway.31,32 The interaction between the tumor necrosis factor receptor OX40 and its ligand OX40L (TNFSF4) can lead to the stimulation of T-cells. After IL-1β and TNF-α established TMJ CC inflammatory model, miR-145-5p was upregulated, suppressing the progression of TMJ OA by targeting ox40l to regulate T-cell-mediated immune response.33 In contrast to estrogen, progesterone (P4) displays a protective effect on TMJ OA. P4 replacement attenuates TMJ OA and diminishes the DNA-binding effect of NF-κB in a dose-dependent manner in ovariectomized TMJ OA rats. The in-vitro experiment also demonstrated that P4 could partially reverse the transcription of cytokines in TMJ synoviocytes induced by IL-1β and TNF-α.34 Bmal1, a circadian gene, downregulates IL-6 levels by decreasing extracellular signal-regulated kinase (ERK) phosphorylation in TMJ OA cartilage.35 Other molecules, including 15-deoxy-Δ12,14-prostaglandin J2 and p21, also play a protective role in TMJ OA by inhibiting the expression and release of pro-inflammatory cytokines IL-1β, IL-6, IL-12, IL-18, chemokines, including cytokine-induced neutrophil chemoattractant 1 (CINC-1) and matrix-degrading enzymes MMP9, MMP13.36,37
Detailed information about inflammatory responses and corresponding molecular mechanisms in TMJ OA is displayed in Table 1.
Table 1.
Molecules and pathways involved in inflammatory responses in TMJ OA.
| Main Factors | Pathways | Effect on Inflammation | References |
|---|---|---|---|
| CRP | – | Aggravation | 4 |
| IL-17a | NF-κB, PI3K/Akt | Aggravation | 6 |
| IL-1β |
|
Aggravation | 7, 8, 9,13 |
| COX2 | – | Aggravation | 10 |
| PGE2 | – | Aggravation | 11 |
| MIP-3 | MAPK, NF-κB | Aggravation | 12 |
| HMGB1 | – | Aggravation | 14 |
| TLR4/MyD88 | NF-κB p38 MAPK | Aggravation | 15,16 |
| HDAC(10) | NF-κB | Aggravation | 17,18 |
| BRD4 | – | Aggravation | 19 |
| LncRNA HOTAIR | – | Aggravation | 20 |
| LncRNA AK094629 | MAPK | Aggravation | 21 |
| miR-1246 | Wnt/β-catenin | Aggravation | 22 |
| hsa_circ_0000448 | – | Aggravation | 23 |
| Connexin 43 | – | Aggravation | 24 |
| Leptin | JAK/STAT3, PI3K/Akt, p38 MAPK | Aggravation | 25 |
| Estrogen | NF-κB | Aggravation | 26,27 |
| SDF-1 | – | Aggravation | 28 |
| Dkk-1 | Wnt/β-catenin NF-κB |
Aggravation | 29 |
| p38 MAPK | p38 MAPK | Aggravation | 30 |
| IL-37 | ERK, JNK, NF-κB, p38 | Inhibition | 31,32 |
| miR-146-5p | – | Inhibition | 33 |
| Progesterone (P4) | NF-κB | Inhibition | 34 |
| Bmal1 | MAPK/ERK | Inhibition | 35 |
| 15d-PGJ2 | – | Inhibition | 36 |
| p21 | – | Inhibition | 37 |
Abbreviations: CRP: C-reaction protein; IL: interleukin; NF-κB: nuclear factor-kappa B; PI3K: phosphoinositide-3 kinase; Akt: protein kinase B; iNOS: inducible nitric oxide synthase; MMP: matrix metalloproteinase; Pin1: peptidyl-prolyl cis-trans isomerase NIMA-interacting 1; HMGB: high mobility group box; TLR: toll-like receptor; SDF-1: stromal cell-derived factor 1; MAPK: mitogen-activated protein kinases; JAK: Janus kinase; STAT3: signal transducer and activator of transcription 3; ERK: Extracellular Signal-Regulated Kinase; JNK: c-Jun N-terminal kinase.
Extracellular matrix degradation
ECM, produced by proximal cells and tissues, contains a wide variety of molecules forming a well-organized network, which is the main component of the microenvironment and acts as extrinsic regulators for various physiological and pathological processes.38 The ECM of TMJ cartilage is mainly composed of collagen type I, collagen type II, and proteoglycans. It protects against elastic and shear forces and regulates chondrocytes’ metabolism and biological behaviors.3,39 ECM degeneration is closely related to inflammatory response, excessive mechanical loading, aging, estrogen level, and osteoclastogenesis in osteoarthritis.2,39,40 It starts from the expression of matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) in the cells.3 MMP and ADAMTS belong to the subfamily of zinc-dependent proteinases, which play a significant role in repairing and remodeling ECM in both physiological and pathological conditions.38,40,41 IL-1β-induced overexpression of MMP13, ADAMTS4, and ADAMTS5 could lead to cartilage damage in IL-1RA−/− mice.39 In the TMJ OA mouse model, the expression of IL-6 and MMP12 was increased. Overexpression of IL-6 could also lead to the increased expression of ADAMTS4 and ADAMTS5, forming a positive feedback loop and resulting in chronic cartilage degeneration in TMJ.2 Another study found that MMP3, one of the predominant proteinases in degrading ECM, was closely linked to heavy masticatory loading, while MMP8 might be protective and related to estrogen levels and aging.40 Furthermore, ERK signaling may have a crucial role in controlling the expression of MMPs.42
Several intra- or extracellular molecules and epigenetics regulate the ECM degeneration process. In unilateral anterior crossbite (UAC) TMJ OA model, Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted (RANTES) and Stromal cell-derived factor 1 (SDF-1) was significantly upregulated. RANTES and SDF-1 are linked with C–C chemokine receptor type 1 (CCR1) and C-X-C chemokine receptor type 4 (CXCR4), respectively.43 SDF-1 combined with CXCR4 in chondrocytes shifts TGF-β receptor type I (TβRI) to activin receptor-like kinase 1 (ALK1) from activin receptor-like kinase 5 (ALK5).44 Both molecules can promote cartilage degeneration and subchondral bone resorption, suggesting a potential link between inflammatory responses and ECM degeneration. Periostin, a matricellular protein belonging to the fasciclin family, is upregulated by excessive mechanical loading. The increased expression of periostin induces the expression of ADAMTS5 via the NF-κB pathway, leading to ECM degeneration and condylar resorption.45 Osteopontin (OPN), a matricellular protein regulating tissue repair and remodeling, is upregulated in the TMJ OA rat model and activates the NF-κB signaling pathway to stimulate the expression of MMPs.46 HMGB2 participates in TMJ OA and is necessary for TMJ cartilage in sensing pressure loading. Increased level of HMGB2 is associated with cartilage degeneration induced by excessive mechanical load.47 In addition, miR-21-5p also plays a vital role in matrix degradation by targeting growth differentiation factor 5 (Gdf5). Knock-out (KO) of miR-21-5p or Gdf5 stimulation significantly decreases the expression of MMP13 and reverses the degeneration of condylar cartilage.48
Estrogen has also been suggested to have a crucial role in ECM degeneration in TMJ OA. The pathogenic effect of estrogen on the degeneration of ECM primarily depends on estrogen receptors (ER) α and β, which are widely expressed in females, especially in TMJ fibrocartilage. When 17β-estradiol activates its receptor Erα, MMP9 is upregulated via the ERK signaling pathway, and NF-κB and ELK-1, direct targets of ERK, contribute to estrogen/Erα/ERK-induced MMP9 upregulation.49 Another study has revealed that in combination with Erβ, estrogen could increase the expression of HIF (hypoxia-inducible factor) -2α and down-streaming cytokines, MMP13 and COLX, leading to aggravation of ECM degeneration.50
The Notch signaling pathway comprises a family of four type-1 transmembrane receptors (Notch 1–4). In the canonical Notch signaling pathway, Notch binds to Notch ligands and activates the release and translocation of the Notch1 intracellular domain (NICD) to the nucleus.51 The Notch cascade is involved in regulating the behaviors of chondrocytes.3 In the TMJ OA model, IL-1β stimulates the overexpression of Notch1 and NICD and increases the expression of IL-6 and TNF-α. In contrast, inhibition of Notch1 could suppress cartilage degeneration by balancing MMP and TIMP (tissue inhibitors of matrix metalloproteinases) -1 via the NF-κB signaling pathway.51
Transforming growth factor β (TGF-β) is a multifunctional cytokine and structurally belongs to the TGF-β superfamily, including four subtypes (TGF-β1, β2, β3, β1β2). Studies have shown that TGF-β is one of the most abundant cytokines in bone matrix and is responsible for regulating the remodeling of bone tissues.52 The role of the TGF-β signaling pathway is controversial in TMJ OA. It seems that increased expression of TGF-β1 is unnecessary for chondrocytes with completed differentiation. Overexpression of TGF-β1 involved p-Smad2 signaling and HtrA1 production, resulting in MMP13 overproduction and breakdown of cartilage ECM.53 In contrast, another study found that TGF-β1-induced Smad activation could inhibit the expression of MMPs. The crosstalk between TGF-β/Smad3 and S1P/S1P3 signaling pathways could downregulate the expression of MMP9 and MMP13 and induce chondrocyte migration via Rac1, RhoA, and Cdc42.52
Various molecules and pathways inhibit ECM degeneration in TMJ OA. LOXL2, a subtype of the lysyl oxidase (LOX) protein family, is a copper-dependent amine oxidase that remodels ECM and establishes the cross-link between collagens and elastin. Studies have shown that LOXL2 is upregulated in TMJ OA models and may be a protective factor for promoting ECM anabolism and inhibiting catabolism via NF-κB, ERK, and Integrin/FAK pathways.54,55 Lubricin is a large proteoglycan encoded by gene proteoglycan 4 (Prg4) and is pivotal for lubricating the articular surface and maintaining articular integrity. Deletion of Prg4 led to OA-like degeneration of condylar in condyle.56 Similarly, type V collagen is essential in establishing and maintaining the ECM. Moreover, the knock-out (KO) of type V collagen could induce the degeneration and malformation of ECM of condylar cartilage.57 Deletion of Axin1 contributes to overexpression of MMP13 and ADAMTS5 via Wnt/β-catenin and fibroblast growth factor (FGF)/ERK1/2 pathways, thus leading to TMJ cartilage degeneration.58 Furthermore, Fgfr3 conditional KO (cKO) cartilage expresses higher Runx2, Mmp13, Col10, and Adamts5 via Indian Hedgehog (Ihh) signaling pathway.59 In addition, miR-29b attenuates ECM degeneration by rescuing the decrease of the percentage areas of proteoglycan and type II collagen.60 (Table 2).
Table 2.
Molecules and pathways involved in degeneration of ECM in TMJ OA.
| Main Factors | Pathways | Effect on ECM Degeneration | References |
|---|---|---|---|
| IL-1β, MMP13 | – | Aggravation | 39 |
| MMP12 | – | Aggravation | 2 |
| MMP3/8 | – | Aggravation | 40 |
| MMP1/3/13 | ERK | Aggravation | 42 |
| RANTES | – | Aggravation | 43 |
| SDF-1 | – | Aggravation | 43,44 |
| Periostin | NF-κB | Aggravation | 45 |
| Osteopontin | NF-κB | Aggravation | 46 |
| HMGB2 | Wnt/β-catenin | Aggravation | 47 |
| miR-21-5p | – | Aggravation | 48 |
| Estrogen | ERK, NF-κB | Aggravation | 49,50 |
| Notch1 | NF-κB, Notch | Aggravation | 51 |
| Smad3 |
|
Inhibition | 52 |
| TGF-β1 | TGF-β1/Smad2 | Aggravation | 53 |
| LOXL2 | NF-κB, ERK, Integrin/FAK | Inhibition | 54,55 |
| Lubricin | – | Inhibition | 56 |
| COLV | Wnt/β-catenin | Inhibition | 57 |
| Axin1 |
|
Inhibition | 58 |
| FGFR3 | Ihh | Inhibition | 59 |
| miR-29b | – | Inhibition | 60 |
Abbreviations: IL: interleukin; MMP: matrix metalloproteinase; ADAMTS: a disintegrin and metalloproteinase with thrombospondin motifs; NF-κB: nuclear factor-kappa B; ERK: Extracellular Signal-Regulated Kinase; RANTES: regulated upon activation, normal T cell expressed and presumably secreted; SDF-1: stromal cell-derived factor 1; TβR1: TGF-beta type I receptor; HMGB: high mobility group box; COL: collagen; S1P: sphingosine-1-phosphate; TGF-β: transforming growth factor beta; FAK: focal adhesion kinase; FGF: fibroblast growth factor; FGFR3: fibroblast growth factor receptor type 3.
Abnormal cell biological behaviors
Accumulating evidence has shown that the progression of TMJ OA is closely related to tabnormal cell proliferation, differentiation, death, and cell metabolism in cartilage and subchondral bone. These abnormalities in cell biological behaviors may have deteriorative effect on the hemostasis of the TMJ environment, leading to aggravated inflammation, degradation of ECM, and remodeling of subchondral bone in TMJ.
Apoptosis
Apoptosis is an active and well-organized cell death under physiological or pathological conditions. Apoptosis-related genes regulate the process of apoptosis, called “programmed cell death” or type I cell death. It is characterized by decreased cell volume, the loss of intercellular connections, and the attachment and detachment of cells from surrounding cells. Apoptosis can be divided into endogenous and exogenous apoptosis, referring to the mitochondrial-related apoptosis pathway and the apoptosis process induced by death receptors respectively, Various factors can induce apoptosis, including trauma, aging, DNA damage, inflammation, and endoplasmic reticulum stress (ERS).61,62 It is considered that the increase of apoptosis is one of the essential factors in the progression of TMJ OA. Apoptosis provides space for neovascularization and is the source of abnormal cartilage mineralization, leading to the destruction of cartilage and subchondral bone of TMJ.3
NcRNAs have a crucial role in regulating apoptosis in TMJ OA. In the lipopolysaccharide (LPS)-induced inflammatory model, lncRNA PVT1 was found to exacerbate the expression of TNF-α by sponging the microRNA, miR-211-3p.63 In the synovial fluid of TMJ OA patients, lncRNA HOTAIR is significantly upregulated, and HOTAIR upregulation contributes IL-1β induced MMP1, MMP3, and MMP9 expression and chondrocyte apoptosis.20 Analogously, circRNA GCN1L1 could promote synoviocyte proliferation, catabolism of cartilage ECM and chondrocyte apoptosis by sponging miR-330-3p and targeting the TNF-α gene.64 In addition, miR-15b impedes chondrocyte proliferation by targeting IGF1 and IGF1R and, in turn, promotes chondrocyte apoptosis by targeting BCL2.65 In Complete Freund's Adjuvant (CFA)-induced rat TMJ OA model and IL-1β-induced in-vitro model, miR-101-3p was detected to be downregulated, while miR-101-3p could enhance cell apoptosis induced by IL-1β targeting the gene ubiquitin-conjugating enzyme 2D1 (UBE2D1) and Frizzled class receptor 4 (FZD4).66
Calcium is crucial in chondrocyte apoptosis, and calcium influx could be elevated under mechanical loading.3 Excessive calcium loading leads to misfolded protein accumulation in the endoplasmic reticulum lumen, termed ER stress (ERS). The mechanistic target of rapamycin kinase (mTOR) is a highly conserved protein kinase with two different functional complexes, mTORC1, and mTORC2. In a study, UAC and FFSS induced calcium overload in chondrocytes in-vivo and in-vitro, which activates the mTORC1/EIF2AK3/CASP12/DDIT3 signaling pathway and leads to the enhancement of chondrocyte apoptosis.67
The role of the canonical Wnt/β-catenin pathway in TMJ OA is controversial. It has been demonstrated that excessive compressive force could reduce endogenous β-catenin and result in OA-like lesions.3 In contrast, Hui et al. found that in β-catenin conditional activation mice (β-cat(ex3)Agc1CreER), condylar cartilage defect was present, and the expression of proliferating cell nuclear antigen (PCNA) and Ki67 was significantly reduced, with cell proliferation decreased, and cell apoptosis increased in TMJ tissue.68
The Ihh signaling pathway is also a vital regulator in chondrocyte apoptosis. In a bite-raising TMJ OA model, Ihh, Smoothened (Smo), and Gli zinc finger transcription factor 1 (Gli1) were found to be activated, and the expression of MMP13 and cysteinyl aspartate specific proteinase-3 (caspase-3) were upregulated, leading to hypertrophy and apoptosis of chondrocytes in TMJ OA.69
As mentioned above, p21 and Smad3, which are proven to participate in the inhibition of inflammatory response and degeneration of ECM, respectively, also play a role in inhibiting chondrocyte apoptosis.37,52 N6-methyladenosine (m6A), a type of internal modification in messenger RNA (mRNA), also participates in apoptosis in TMJ OA. Methyltransferase-like 3 (Mettl3) can catalyze Bcl2 mRNA and inhibit chondrocyte apoptosis induced by TNF-α via m6A/Ythdf1/Bcl2 signal axis.70 These studies indicate that cell apoptosis may be closely related to the inflammatory response and degeneration of ECM in TMJ (Table 3).
Table 3.
Molecules and pathways involved in apoptosis in TMJ OA.
| Main Factors | Pathways | Effect on Apoptosis | References |
|---|---|---|---|
| LncRNA PVT1 | – | Aggravation | 63 |
| LncRNA HOTAIR | – | Aggravation | 20 |
| CircRNA GCN1L1 | – | Aggravation | 64 |
| miR-15b | – | Aggravation | 65 |
| miR-101a-3p | – | Aggravation | 66 |
| mTORC1 | – | Aggravation | 67 |
| β-catenin | Wnt/β-catenin | Aggravation | 68 |
| Ihh | Ihh | Aggravation | 69 |
| p21 | – | Inhibition | 37 |
| Smad3 |
|
Inhibition | 52 |
| Mettl3 | m6A/Ythdf1/Bcl2 | Inhibition | 70 |
Abbreviations: mTORC: mammalian target of rapamycin complex; EIF2AK3: ukaryotic translation initiation factor 2 alpha kinase 3; TGF-β: transforming growth factor beta; S1P: sphingosine-1-phosphate; mettl3: m6A regulators like methyltransferase-like 3; Ythdf1: YT521-B homology (YTH) domain-containing proteins 1; m6A: N6-methyladenosine.
Autophagy
Autophagy is another form of cell death known as programmed non-apoptotic cell death, which is essential for cleaning up senescent cells, promoting wound healing and cell renewal. In autophagy, cells degrade their internal damaged organelles and macromolecules through lysosomes, activate autophagy-related genes, and realize the turnover and circulation of intracellular substances. Classical autophagy includes endogenous and exogenous stimulation (DNA damage, cell senescence, and inflammatory environment et al.), autophagy induction, autophagosome formation, and autophagy lysosome formation. Abnormality in chondrocyte autophagy has been proven to be one of the significant factors and characteristics of OA. Multiple pathways, including PI3K/AKT/mTOR, MEK/ERK, and SIRT1/AMPK pathways, are involved in the regulation of autophagy in the pathogenesis of OA.61
Currently, there are few studies on autophagy in TMJ OA. Nevertheless, autophagy is considered a vital survival mechanism for chondrocytes in TMJ OA.3 Current studies on autophagy in TMJ OA primarily focus on mTOR. According to the study by Liu et al. the expression of mTOR was upregulated in response to IL-1β, and the ratio of LC3-II/LC3-I was decreased, leading to lowered autophagosome formation.71 Similarly, another study showed that in the TMJ OA model induced by estrogen deficiency and high bite force, the expression of Beclin-1 and LC3 were significantly downregulated while mTOR was upregulated and autophagic activity was lowered.72 Yang et al. revealed that calcium overload induced by excessive mechanical loading promotes ERS, switching autophagy to apoptosis via the p-ERN1/p-PRKAA1/2 (AMPK)/mTORC1 signaling axis.67 Fibroblast growth factor receptor 1 (FGFR1) is a tyrosine kinase receptor belonging to the FGF family. Fgfr1 deficiency promotes autophagy activity and attenuates TMJ OA progression.73 In addition, Mettl3 could inhibit autophagy through m6A/Ythdf1/Bcl2 signal axis.70 (Table 4).
Table 4.
Molecules and pathways involved in autophagy in TMJ OA.
| Main Factors | Pathways | Effect on Autophagy | References |
|---|---|---|---|
| Mettl3 | m6A/Ythdf1/Bcl2 | Inhibition | 70 |
| mTOR | – | Inhibition | 71 |
| mTOR, estrogen | – | Inhibition | 72 |
| mTORC1 | – | Inhibition | 67 |
| FGFR1 | – | Inhibition | 73 |
Abbreviations: mettl3: m6A regulators like methyltransferase-like 3; Ythdf1: YT521-B homology (YTH) domain-containing proteins 1; m6A: N6-methyladenosine; mTOR: mammalian target of rapamycin; mTORC: mammalian target of rapamycin complex; FGFR1: fibroblast growth factor receptor type 1.
Other cell biological behaviors
Abnormal biological behaviors of cells in TMJ OA also include alteration of osteoclast and osteoblast activity, abnormal differentiation of stem cells and chondrocytes, enhanced fibrogenesis by FLS and increased sensitivity to the stimulations.
One common pathological change TMJ OA brings is abnormal subchondral bone remodeling, which is also one of the first processes and signs. In the early stage, subchondral bone primarily displays bone loss, while cartilage calcification and stiffness of the osteochondral interface occur in the later stage.3 Subchondral bone remodeling is predominantly characterized by the elevated osteoclast activity and decreased osteoblast activity. Several molecules are related to osteoclast activation. Netrin-1, an axon-guiding protein, is upregulated in the synovial fluid of TMJ OA patients and monoiodoacetic acid (MIA)-induced TMJ OA mice and in combination with its receptor, Unc5b, the osteoclast formation is stimulated.74 Wnt5a/receptor tyrosine kinase-like orphan receptor 2 (Ror2) signaling derived from TMJ bone marrow mesenchymal stem cells (BMSCs) is enhanced by UAC, with increased RANKL and CXCL12 expression and osteoclast precursors migration and differentiation via JNK and Ca2+/nuclear factor of activated T-cells (NFAT) pathway.75 Overactivation of the TGF-β/TβRI signaling pathway is detected in TMJ OA cartilage and subchondral bone, leading to a disturbed bone marrow microenvironment and abnormal osteoclast activation.76 VEGF could initiate TMJ OA by promoting osteoclast differentiation and subchondral bone resorption.77 In the incisor malocclusion model, semaphorin 4D (Sema 4D) and Plexin B1, a pair of osteoblast differentiation suppressors, are increased at week 3, accompanied by the increased osteoclast activity and decreased osteoblast activity, leading to bone remodeling in TMJ.78
In contrast, inhibition of abnormal cell biological behaviors can attenuate the severity of TMJ OA and promote cartilage regeneration and bone repair. Two microRNAs, miR-26b and miR-29b play a role in osteoblast activation and osteoclast inhibition, respectively, suppressing abnormal subchondral bone remodeling.60,79 FGF1 and epidermal growth factor (EGF) activate the MAPK/ERK signaling pathway to suppress the expression of myofibroblast markers, α-smooth muscle actin (α-SMA), and type I collagen, and they can cooperate with inflammatory cytokines, including IL-1β and TNF-α, to inhibit fibrosis in TMJ OA.80 DNA methyltransferase (DNMT) 3b is a member of DNMTs controlling DNA methylation. Dnmt3b gain-of-function in TMJ stem and progenitor cells express more type II collagen and less type X collagen and show enhanced premature differentiation via β-catenin activation, thus alleviating cartilage degradation and progression in TMJ OA.81
The inhibition of abnormal chondrogenesis and chondrocyte differentiation appears pivotal in promoting correct bone formation and endochondral ossification. Calcium-sensing receptor (CaSR), a member of the class C G protein-coupled receptor (GPCP-C), is widely expressed in maturing and hypertrophic chondrocytes in growth plates and is an essential promoter of chondrocyte terminal differentiation. UAC and FFSS-induced Ca2+ overload and CaSR localization in ER accelerate chondrocyte terminal differentiation and exacerbated matrix degradation in TMJ cartilage.82 In contrast, parathyroid hormone-related peptide receptor 1 (PTH1R) activation rescues UAC and FFSS-promoted chondrocyte terminal differentiation and OA-like lesions by inhibiting the Ihh signaling pathway.83 Moreover, type VI collagen is one of the primary components in TMJ cartilage. In Col6α2 KO cartilage, reduced bone volume and abnormal chondrocyte differentiation are detected, and type VI collagen possibly regulates endochondral ossification and chondrocyte differentiation via pSmad1/5/8 signaling.84 Similarly, in Prg4-null TMJ, ectopic cartilage develops, and ectopic mineralization occurs, leading to irreversible OA-like changes in TMJ, suggesting that Prg4 is crucial for maintaining TMJ integrity and functions.85 (Table 5).
Table 5.
Molecules and pathways involved in other biological behaviors in TMJ OA.
| Main Factors | Pathways | Effects | References |
|---|---|---|---|
| Netrin-1 | – | Osteoclast activation | 74 |
| Wnt5a/Ror2 | JNK, Ca2+/NFAT | Osteoclast activation | 75 |
| TGF-β/TβRI | TGFβ/Smad2,3 | Osteoclast activation | 76 |
| VEGF | – | Osteoclast activation | 77 |
| Sema 4D, Plexin B1 | – | Osteoclast activation Osteoblast inhibition | 78 |
| miR-29b | Wnt5a | Osteoclast inhibition | 60 |
| miR-26b | Wnt/β-catenin | Osteoblast activation | 79 |
| FGF1, EGF + IL-1β, TNF-α | MAPK/ERK | FLS activity inhibition | 80 |
| Dnmt3b | Wnt/β-catenin | Stem/progenitor cells differentiation regulation | 81 |
| CaSR | – | Chondrocyte terminal differentiation acceleration | 82 |
| PTH1R | Ihh | Chondrocyte terminal differentiation inhibition | 83 |
| COL VI | – | Chondrocyte differentiation regulation | 84 |
| Lubricin (Prg4) | Ihh, BMP | Ectopic chondrogenesis inhibition | 85 |
Abbreviations: Ror2: eceptor tyrosine kinase-like orphan receptor 2; JNK: c-Jun N-terminal kinase; NFAT: Nuclear factor of activated T cells; TGF-β: transforming growth factor beta; TβR1: TGF-beta type I receptor; COL: collagen; VEGF: vascular endothelial growth factor; MMP: matrix metalloproteinase; VEGF: vascular endothelial growth factor; FGF: fibroblast growth factor; EGF: epidermal growth factor; Dnmt3b: DNA methylation 3b; CaSR: calcium-sensing receptor; PTH1R: parathyroid hormone type 1 receptor.
Angiogenesis
In addition to inflammatory responses, degeneration of ECM, and abnormal biological behaviors, angiogenesis has been considered to be a crucial pathological change in TMJ OA. In mature, healthy articular cartilage, cells typically produce angiogenic inhibitors, while patients with TMJ OA exhibit higher-density vascularization, which has been detected in histopathological studies.86,87 Vascularization in OA has been observed to promote the formation of osteophytes. After osteophyte is formed, vascularization sustains, leading to the formation of osteophyte and destruction of articular cartilage.3,87 Neovascularization is accompanied by sympathetic and sensory nerve formation, which may contribute to pain in TMJ OA.87 In addition, angiogenesis in OA promotes the transportation of inflammatory factors and degradation and premature mineralization of ECM in cartilage.3
VEGF, a vascular permeability factor and selective endothelial mitogen, has a crucial role in physiological and pathological process of angiogenesis and accumulates in the chondrocytes of mandibular condyles with OA.88,89 Several pathways are involved in the upregulation of VEGF. One of the most studied signaling pathways is the hypoxia-inducible factor (HIF)-1α/VEGF/Notch pathway. In a study using chronic sleep deprivation (CSD)-induced TMJ OA model, HIF-1α/VEGF/Notch pathway was activated bidirectionally by hypoxia, mediating angiogenesis in TMJ OA.90 Dickkopf-related protein 1 (DKK-1), a secreted protein inhibiting Wnt/β-catenin signaling, is upregulated in concordance with VEGF and could promote nuclear translocation of HIF-1α.86 HMGB1 directs the nuclear localization of HIF-1α and promotes the expression of VEGF via JNK and ERK signaling pathways.91
ERK signaling pathway is another essential pathway mediating angiogenesis in TMJ OA. The inflammatory cytokine IL-6 could activate estrogen-related receptor γ (ERRγ) via ERK1/2 signaling to promote the synthesis of VEGF, leading to the degradation of ECM and angiogenesis.87 The crosstalk between ERK and Notch pathway may be crucial in regulating neovascularization in TMJ OA. As mentioned above, HMGB1 could activate HIF-1α/VEGF via the ERK pathway.91 Delta-like ligand 4 (Dll4), an essential component of the Notch pathway functioning mainly in stem cell self-renewal and vascularization, was upregulated in the CSD TMJ OA rat model. It was demonstrated that when VEGF binds to its receptor VEGFR2, ERK1/2 is activated, increasing Dll4 expression.89 In addition, IL-1β could promote VEGF synthesis through the NF-κB signaling pathway,92 while estrogen promotes VEGF synthesis via ERβ/HIF-2α pathway.50
In addition, another vascularization regulator, angioprotein (Ang), is also involved in bone remodeling of TMJ. Ang-1, one subtype of Ang, is overexpressed by the activation of IL-1β via p38 MAPK signaling. The expression of Ang-1 and Ang-2 was increased in a rabbit model treated with biochemical stimulation by experimental forward mandibular positioning (FMP), leading to angiogenesis and remodeling of the mandibular condylar.93
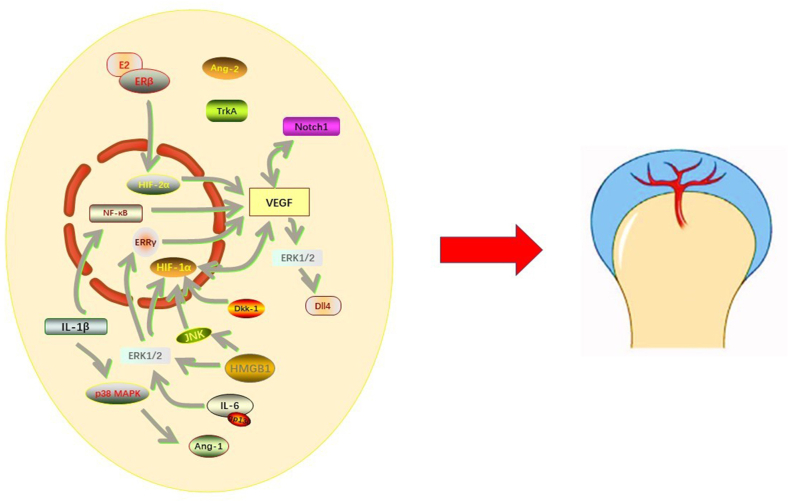
The crosstalk in angiogenesis and corresponding molecular mechanisms of TMJ OA are exhibited in Fig. 2.
Fig. 2.
Molecules and pathways Involved in angiogenesis in TMJ OA. VEGF, Ang-1, and Ang-2 are the main pro-angiogenic factors in the pathogenesis of TMJ OA. Among them, VEGF plays a pivotal role in neovascularization. Several signaling pathways, including IL-1β/NF-κB, IL-6/ERK1/2/ERRγ, HIF-1α, Notch1, E2/ERβ/HIF-2α are involved in this process. Excessive neovascularization leads to pain in the TMJ region, osteophyte formation, and more severe inflammatory responses in TMJ.
Discussion and future directions
The etiology and pathophysiology of TMJ OA are unclear, and there is still no consensus about the precise pathological changes, biomarkers, and pathways in TMJ OA. Nevertheless, emerging studies have suggested that inflammation, ECM degeneration, abnormal biological behaviors, and angiogenesis participate in TMJ OA. Various signaling pathways are involved in the pathogenesis of TMJ OA. Among them, the NF-κB signaling pathway is essential in mediating inflammatory response and degradation of ECM, which has been demonstrated in many studies. The Wnt/β-catenin pathway is a “double-edged sword” under different conditions in TMJ OA. The overexpression of β-catenin leads to enhanced chondrocyte apoptosis and OA-like defect, while in a murine model with loss-of-function of β-catenin, reduced cell proliferation and impaired cartilage occurred.57,68 MAPK and ERK signaling are involved in inflammation and cartilage degeneration and play a critical role in angiogenesis. The Ihh signaling mainly participates in cell behaviors, including apoptosis, ectopic chondrogenesis, chondrocyte terminal differentiation, and crosstalk with PTH1R and BMP signaling pathways. Notch signaling is a pivotal pathway in neovascularization and ECM degeneration in TMJ OA. Other pathways, including TGF-β/Smad, JNK, and JAK/STAT3 et al. also have a role in TMJ OA development. Notably, estrogen may be one of the primary reasons why the incidence of TMJ OA in females is higher than in males. A higher estrogen level is closely related to inflammatory cytokine release, M1 macrophage polarization, degeneration of ECM, and decreased cell autophagy via NF-κB, ERK, and mTOR signaling.26,49,72 However, a previous study showed that a lack of estrogen reduces subchondral bone volume.50 Therefore, considering the complex crosstalk between various pathological changes, molecules, and signaling pathways in TMJ OA, further studies should notice the complicated networks in TMJ OA pathogenesis.
Current in vivo animal models of TMJ OA are established in various methods, including modifying mechanical loading (UAC, bite-raising, prolonged mouth-opening), injection of inflammatory mediators, including MIA, CFA, and IL-1β, surgeries, and gene editing.3 Different animals, such as mice, SD/Wistar rats, New Zealand white rabbits, and pigs, are used to establish animal models of TMJ OA.3,81,83 For in-vitro cell models of TMJ OA, FFSS and injection of IL-1β or TNF-α are commonly used.3 Different cell types are chosen to establish cell TMJ OA models, such as ATDC5 cells and rat, rabbit, or pig chondrocytes.35,55,57 Despite various selections in TMJ OA models, it is still difficult to reproduce or intimate the complicated clinical cases of TMJ OA. Moreover, obtaining human fibrocartilage of TMJ seems problematic due to technical and ethical reasons. Further studies must focus on improving the methods of establishing TMJ OA in-vivo and in-vitro models and applying newly developed techniques such as organoid and 3D printing.94
Studies have shown that targeting the molecules and pathways mentioned above might be potential novel treatments for TMJ OA (Table 6). Recent studies have demonstrated that extracellular vesicles (EVs) or exosomes derived from stem cells are vital structures in cell therapy95 which contain nucleotides and signaling proteins to modulate cell biological behaviors. Wang et al. found that EVs derived from BMSCs could promote cartilage reconstruction via HIPPO and autotaxin/Yes-associated protein (YAP) signaling axis in TMJ OA.96 Two studies focusing on miRNAs in EVs and exosomes showed that miR-100-5p and miR-27b-3p displayed anti-inflammatory effects on TMJ OA. MiR-100-5p in exosomes inhibits inflammatory cytokine release and ECM degradation by targeting mTOR,95 while miR-27b-3p could promote M2 macrophage differentiation and inhibit M1 polarization by targeting colony-stimulating factor 1 (CSF-1).97 Low-intensity pulsed ultrasound (LIPUS), which uses a low frequency (1–3 MHz) and intensity (less than 100 mW/cm2), could attenuate inflammatory responses and degeneration of ECM by targeting HIF-1α, HIF-2α, and Zn2+ exporter (ZNT)-9.98,99 Other chemical ingredients, including curcumin, glucocorticoids, and etanercept, have their roles in protecting TMJ cartilage and subchondral bone in TMJ OA by attenuating pain, oxidative stress, inflammatory response, and ECM degradation or by enhancing autophagy and chondrogenesis. Unfortunately, current therapy for TMJ OA hardly sets an eye on condyle repair and reconstruction.71,100 Various medical treatments have been proven effective in treating TMJ OA in animal and cell models but few clinical trials were constructed. Therefore, it is essential to conduct further studies focusing on novel medicine, materials, and approaches that can promote the regeneration of TMJ in humans; specifically, more clinical trials are needed on the agenda.
Table 6.
Potential therapeutic procedures towards molecules involved in TMJ OA.
| Therapeutic Procedures | Main Factors Affected | Pathways | Effects | References |
|---|---|---|---|---|
| Exosome | miR-100-5p | – | Attenuating inflammation and ECM degeneration | 95 |
| BMSC EVs | ATX, YAP | HIPPO | Inducing cartilage reconstruction | 96 |
| Exosome | miR-27b-3p | – | Inhibiting M1 polarization | 97 |
| LIPUS | ZNT-9 | – | Attenuating ECM degeneration | 98 |
| LIPUS | HIF-1α, HIF-2α | – | Attenuating inflammation and ECM degeneration | 99 |
| Curcumin | Nrf2, ARE | Nrf2/ARE | Attenuating inflammation and oxidative stress | 100 |
| Hyaluronic acid (HA) | NLRP3 | – | Attenuating inflammation | 101 |
| Yohimbine | Ikkβ, p65 | NF-κB | Attenuating inflammation | 102 |
| Methylprednisolone | TNF-α, CRP | – | Attenuating inflammation | 103 |
| Glycyrrhizin | HMGB1 | NF-κB/Akt | Attenuating inflammation | 104 |
| RAGE/TLR4 | ||||
| Genistein | p65 | NF-κB | Attenuating inflammation | 105 |
| Resveratrol | COX2, p65 | NF-κB | Attenuating inflammation and apoptosis | 106 |
| Etanercept | HIF-2α, EF5 | – | Attenuating pain and hypoxia | 107 |
| Rapamycin | IL-1β, mTOR, LC3 | Wnt/β-catenin | Enhancing autophagy | 71 |
| Rebamipide | RANKL | NF-κB, MAPK | Inhibiting oxidative stress and osteoclast | 108 |
| Strontium ranelate | β-catenin | Wnt/β-catenin | Promoting chondrogenesis | 109 |
Abbreviations: mTOR: mammalian target of rapamycin; BMSC: bone marrow mesenchymal stem cell; EV: extracellular vesicles; ATX: autotaxin; CSF: colony-stimulating factor; ZNT: zinc transporter; MTF: metal responsive transcription factor; HIF: hypoxia-inducible factor; NF-κB: nuclear factor-kappa B; HMGB: high mobility group box protein; Akt: protein kinase B; RAGE: receptor for advanced glycation end products.
Besides, it has been widely accepted that the cause of TMJ OA is closely related to the combination of physical and psychological factors. However, the susceptibility of TMJ OA is not similar between different individuals. Though it has been suggested that TMD is not a type of hereditary disease, the role of gene mutation and genetic polymorphism in TMJ OA is still unclear.110 Several studies have revealed that genotype plays a vital role in the risk of developing TMD, including TNFA-308 (rs1800629) polymorphism, catechol-O-methyltransferase (COMT) (rs9332377) polymorphism, and an 86-bp variable number tandem repeat (VNTR) in IL-1Ra gene.111, 112, 113 Studies on genetic factors of TMJ OA can undoubtedly contribute to predicting susceptibility, making a precise diagnosis, and tailoring therapeutic procedures for patients.114
Conclusion
TMJ OA is a complicated disease exhibiting various pathological changes, including inflammatory response, degeneration of ECM, abnormal cell biological behaviors, and aberrant angiogenesis in TMJ tissue. One pathological change is closely linked to others, thus forming a vicious circle in TMJ OA. Many molecules and signaling pathways are involved in the pathogenesis of different sections in TMJ OA and may serve as potential targets to help find effective therapeutic procedures for TMJ OA. Though in-vivo and in-vitro models of TMJ OA have been successfully established in various ways, it is urgent to develop models closer to human TMJ OA. New medicine, materials, and approaches for treating TMJ OA and the role of genetic factors in TMJ OA still need further investigation.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
This article is funded by Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-038A).
Contributor Information
Zhang Xu, Email: zhangxu@tmu.edu.cn.
Li Chang-yi, Email: lichangyi@tmu.edu.cn.
References
- 1.Cui C., Zheng L., Fan Y., et al. Parathyroid hormone ameliorates temporomandibular joint osteoarthritic-like changes related to age. Cell Prolif. 2020;53 doi: 10.1111/cpr.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamashita-Futani Y., Jokaji R., Ooi K., et al. Metalloelastase-12 is involved in the temporomandibular joint inflammatory response as well as cartilage degradation by aggrecanases in STR/Ort mice. Biomed Rep. 2021;14:51. doi: 10.3892/br.2021.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B., Guan G., Mei L., et al. Pathological mechanism of chondrocytes and the surrounding environment during osteoarthritis of temporomandibular joint. J Cell Mol Med. 2021;25:4902–4911. doi: 10.1111/jcmm.16514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Y., Zhou M., Jian Z., et al. C-reactive protein knockout attenuates temporomandibular joint inflammation in rats. J Immunol Res. 2022;2022 doi: 10.1155/2022/8613986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shrivastava M., Battaglino R., Ye L. A comprehensive review on biomarkers associated with painful temporomandibular disorders. Int J Oral Sci. 2021;13:23. doi: 10.1038/s41368-021-00129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hattori T., Ogura N., Akutsu M., et al. Gene expression profiling of IL-17A-treated synovial fibroblasts from the human temporomandibular joint. Mediat Inflamm. 2015;2015 doi: 10.1155/2015/436067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W., Sun Y., He Y., et al. IL-1β impedes the chondrogenic differentiation of synovial fluid mesenchymal stem cells in the human temporomandibular joint. Int J Mol Med. 2017;39:317–326. doi: 10.3892/ijmm.2016.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan F., Xie J.L., Liu K.Y., et al. Xanthan gum protects temporomandibular chondrocytes from IL-1β through Pin1/NF-κB signaling pathway. Mol Med Rep. 2020;22:1129–1136. doi: 10.3892/mmr.2020.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge X.P., Gan Y.H., Zhang C.G., et al. Requirement of the NF-κB pathway for induction of Wnt-5A by interleukin-1β in condylar chondrocytes of the temporomandibular joint: functional crosstalk between the Wnt-5A and NF-κB signaling pathways. Osteoarthritis Cartilage. 2011;19:111–117. doi: 10.1016/j.joca.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Kawashima M., Ogura N., Akutsu M., et al. The anti-inflammatory effect of cyclooxygenase inhibitors in fibroblast-like synoviocytes from the human temporomandibular joint results from the suppression of PGE2 production. J Oral Pathol Med. 2013;42:499–506. doi: 10.1111/jop.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nazet U., Feulner L., Muschter D., et al. Mechanical stress induce PG-E2 in murine synovial fibroblasts originating from the temporomandibular joint. Cells. 2021;10:298. doi: 10.3390/cells10020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akutsu M., Ogura N., Ito K., et al. Effects of interleukin-1β and tumor necrosis factor-α on macrophage inflammatory protein-3α production in synovial fibroblast-like cells from human temporomandibular joints. J Oral Pathol Med. 2013;42:491–498. doi: 10.1111/jop.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibi M., Horie S., Kyakumoto S., et al. Cell-cell interactions between monocytes/macrophages and synoviocyte-like cells promote inflammatory cell infiltration mediated by augmentation of MCP-1 production in temporomandibular joint. Biosci Rep. 2018;38 doi: 10.1042/BSR20171217. BSR20171217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C., Cai H., Meng Q., et al. IL-1β mediating high mobility group box protein-1 expression in condylar chondrocyte during temporomandibular joint inflammation. J Oral Pathol Med. 2016;45:539–545. doi: 10.1111/jop.12401. [DOI] [PubMed] [Google Scholar]
- 15.Liu X., Cai H.X., Cao P.Y., et al. TLR4 contributes to the damage of cartilage and subchondral bone in discectomy-induced TMJOA mice. J Cell Mol Med. 2020;24:11489–11499. doi: 10.1111/jcmm.15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin X., Xie J., Sun S., et al. Toll-like receptor 4 (TLR4) stimulates synovial injury of temporomandibular joint in rats through the activation of p38 mitogen-activated protein kinase (MAPK) signaling pathway. Med Sci Mon Int Med J Exp Clin Res. 2018;24:4405–4412. doi: 10.12659/MSM.908526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao W., Sun J., Liu W., et al. HDAC10 upregulation contributes to interleukin 1β-mediated inflammatory activation of synovium-derived mesenchymal stem cells in temporomandibular joint. J Cell Physiol. 2019;234:12646–12662. doi: 10.1002/jcp.27873. [DOI] [PubMed] [Google Scholar]
- 18.Liao W.T., Sun J.D., Wang Y., et al. Histone deacetylase inhibitors attenuated interleukin-1β-induced chondrogenesis inhibition in synovium-derived mesenchymal stem cells of the temporomandibular joint. Bone Joint Res. 2022;11:40–48. doi: 10.1302/2046-3758.111.BJR-2021-0059.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Z., Yang R., Zhang L., et al. BRD4 inhibition alleviates mechanical stress-induced TMJ OA-like pathological changes and attenuates TREM1-mediated inflammatory response. Clin Epigenet. 2021;13:10. doi: 10.1186/s13148-021-01008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C., Wang P., Jiang P., et al. Upregulation of lncRNA HOTAIR contributes to IL-1β-induced MMP overexpression and chondrocytes apoptosis in temporomandibular joint osteoarthritis. Gene. 2016;586:248–253. doi: 10.1016/j.gene.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Jia J., Sun J., Liao W., et al. Knockdown of long non-coding RNA AK094629 attenuates the interleukin-1β induced expression of interleukin-6 in synovium-derived mesenchymal stem cells from the temporomandibular joint. Mol Med Rep. 2020;22:1195–1204. doi: 10.3892/mmr.2020.11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng S., Yan Y., Li R., et al. Extracellular vesicles from M1-polarized macrophages promote inflammation in the temporomandibular joint via miR-1246 activation of the Wnt/β-catenin pathway. Ann N Y Acad Sci. 2021;1503:48–59. doi: 10.1111/nyas.14590. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y., Zhu H., Bu L., et al. Expression profile of circular RNA s in TMJ osteoarthritis synovial tissues and potential functions of hsa_circ_0000448 with specific back-spliced junction. Am J Transl Res. 2019;11:5357–5374. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J., Zhang H.Y., Zhang M., et al. Connexin43 hemichannels mediate small molecule exchange between chondrocytes and matrix in biomechanically-stimulated temporomandibular joint cartilage. Osteoarthritis Cartilage. 2014;22:822–830. doi: 10.1016/j.joca.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong H., Li W., Li J., et al. Elevated leptin levels in temporomandibular joint osteoarthritis promote proinflammatory cytokine IL-6 expression in synovial fibroblasts. J Oral Pathol Med. 2019;48:251–259. doi: 10.1111/jop.12819. [DOI] [PubMed] [Google Scholar]
- 26.Kou X.X., Li C.S., He D.Q., et al. Estradiol promotes M1-like macrophage activation through cadherin-11 to aggravate temporomandibular joint inflammation in rats. J Immunol. 2015;194:2810–2818. doi: 10.4049/jimmunol.1303188. [DOI] [PubMed] [Google Scholar]
- 27.Xue X.T., Zhang T., Cui S.J., et al. Sexual dimorphism of estrogen-sensitized synoviocytes contributes to gender difference in temporomandibular joint osteoarthritis. Oral Dis. 2018;24:1503–1513. doi: 10.1111/odi.12905. [DOI] [PubMed] [Google Scholar]
- 28.Qi D., Sun S., Han L., et al. Stromal cell-derived factor-1 regulates the secretion of interleukin-1β in the temporomandibular joint of rats with synovial inflammation. J Oral Pathol Med. 2020;49:933–939. doi: 10.1111/jop.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Sousa L.M., Dos Santos Alves J.M., da Silva Martins C., et al. Immunoexpression of canonical Wnt and NF-κB signaling pathways in the temporomandibular joint of arthritic rats. Inflamm Res. 2019;68:889–900. doi: 10.1007/s00011-019-01274-4. [DOI] [PubMed] [Google Scholar]
- 30.Jiang L., Lin X., Ji P. Effect of p38 mitogen activated protein kinase inhibitor on temporomandibular joint synovitis induced by occlusal alteration. J Oral Maxillofac Surg. 2016;74:1131–1139. doi: 10.1016/j.joms.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Luo P., Peng S., Yan Y., et al. IL-37 inhibits M1-like macrophage activation to ameliorate temporomandibular joint inflammation through the NLRP3 pathway. Rheumatology. 2020;59:3070–3080. doi: 10.1093/rheumatology/keaa192. [DOI] [PubMed] [Google Scholar]
- 32.Luo P., Feng C., Jiang C., et al. IL-37b alleviates inflammation in the temporomandibular joint cartilage via IL-1R8 pathway. Cell Prolif. 2019;52 doi: 10.1111/cpr.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu D., Wei W., Hefeng Y., et al. Upregulated ox40l can be inhibited by miR-146a-5p in condylar chondrocytes induced by IL-1β and TNF-α: a possible regulatory mechanism in osteoarthritis. Int Arch Allergy Immunol. 2021;182:408–416. doi: 10.1159/000512291. [DOI] [PubMed] [Google Scholar]
- 34.Xue X.T., Kou X.X., Li C.S., et al. Progesterone attenuates temporomandibular joint inflammation through inhibition of NF-κB pathway in ovariectomized rats. Sci Rep. 2017;7 doi: 10.1038/s41598-017-15285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen G., Zhao H., Ma S., et al. Circadian rhythm protein Bmal1 modulates cartilage gene expression in temporomandibular joint osteoarthritis via the MAPK/ERK pathway. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.527744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva Quinteiro M., Henrique Napimoga M., Gomes Macedo C., et al. 15-deoxy-Δ12,14-prostaglandin J2 reduces albumin-induced arthritis in temporomandibular joint of rats. Eur J Pharmacol. 2014;740:58–65. doi: 10.1016/j.ejphar.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Khurel-Ochir T., Izawa T., Iwasa A., et al. The immunoregulatory role of p21 in the development of the temporomandibular joint-osteoarthritis. Clin Exp Dent Res. 2021;7:313–322. doi: 10.1002/cre2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simões G., Pereira T., Caseiro A. Matrix metaloproteinases in vascular pathology. Microvasc Res. 2022;143 doi: 10.1016/j.mvr.2022.104398. [DOI] [PubMed] [Google Scholar]
- 39.Tabeian H., Betti B.F., Dos Santos Cirqueira C., et al. IL-1β damages fibrocartilage and upregulates MMP-13 expression in fibrochondrocytes in the condyle of the temporomandibular joint. Int J Mol Sci. 2019;20:2260. doi: 10.3390/ijms20092260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu J., Mursu E., Typpö M., et al. MMP-3 and MMP-8 in rat mandibular condylar cartilage associated with dietary loading, estrogen level, and aging. Arch Oral Biol. 2019;97:238–244. doi: 10.1016/j.archoralbio.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 41.Łukaszewicz-Zając M., Dulewicz M., Mroczko B. A disintegrin and metalloproteinase (ADAM) family: their significance in malignant tumors of the central nervous system (CNS) Int J Mol Sci. 2021;22 doi: 10.3390/ijms221910378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma C., Wu G., Wang Z., et al. Effects of chronic sleep deprivation on the extracellular signal-regulated kinase pathway in the temporomandibular joint of rats. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu L., Zhang X., Zhang M., et al. RANTES and SDF-1 are keys in cell-based therapy of TMJ osteoarthritis. J Dent Res. 2015;94:1601–1609. doi: 10.1177/0022034515604621. [DOI] [PubMed] [Google Scholar]
- 44.Qin H.J., Xu T., Wu H.T., et al. SDF-1/CXCR4 axis coordinates crosstalk between subchondral bone and articular cartilage in osteoarthritis pathogenesis. Bone. 2019;125:140–150. doi: 10.1016/j.bone.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Fan B., Liu X., Chen X., et al. Periostin mediates condylar resorption via the NF-κB-ADAMTS5 pathway. Inflammation. 2020;43:455–465. doi: 10.1007/s10753-019-01129-4. [DOI] [PubMed] [Google Scholar]
- 46.Ding F., Wang J., Zhu G., et al. Osteopontin stimulates matrix metalloproteinase expression through the nuclear factor-κB signaling pathway in rat temporomandibular joint and condylar chondrocytes. Am J Transl Res. 2017;9:316–329. [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Y., Lu H., Deng L., et al. HMGB2 is associated with pressure loading in chondrocytes of temporomandibular joint: in vitro and in vivo study. Cytokine. 2020;126 doi: 10.1016/j.cyto.2019.154875. [DOI] [PubMed] [Google Scholar]
- 48.Zhang A., Ma S., Yuan L., et al. Knockout of miR-21-5p alleviates cartilage matrix degradation by targeting Gdf5 in temporomandibular joint osteoarthritis. Bone Joint Res. 2020;9:689–700. doi: 10.1302/2046-3758.910.BJR-2020-0140.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmad N., Chen S., Wang W., et al. 17β-estradiol induces MMP-9 and MMP-13 in TMJ fibrochondrocytes via estrogen receptor α. J Dent Res. 2018;97:1023–1030. doi: 10.1177/0022034518767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye T., He F., Lu L., et al. The effect of oestrogen on mandibular condylar cartilage via hypoxia-inducible factor-2α during osteoarthritis development. Bone. 2020;130 doi: 10.1016/j.bone.2019.115123. [DOI] [PubMed] [Google Scholar]
- 51.Ying W., Yuan F., He P., et al. Inhibition of Notch1 protects against IL-1β-induced inflammation and cartilage destruction in temporomandibular chondrocytes. Mol Med Rep. 2017;15:4391–4397. doi: 10.3892/mmr.2017.6511. [DOI] [PubMed] [Google Scholar]
- 52.Mori H., Izawa T., Tanaka E. Smad3 deficiency leads to mandibular condyle degradation via the sphingosine 1-phosphate (S1P)/S1P3 signaling axis. Am J Pathol. 2015;185:2742–2756. doi: 10.1016/j.ajpath.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 53.Long E., Motwani R., Reece D., et al. The role of TGF-ß1 in osteoarthritis of the temporomandibular joint in two genetic mouse models. Arch Oral Biol. 2016;67:68–73. doi: 10.1016/j.archoralbio.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Alshenibr W., Tashkandi M.M., Alsaqer S.F., et al. Anabolic role of lysyl oxidase like-2 in cartilage of knee and temporomandibular joints with osteoarthritis. Arthritis Res Ther. 2017;19:179. doi: 10.1186/s13075-017-1388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C., Zhu M., Wang H., et al. LOXL2 attenuates osteoarthritis through inactivating Integrin/FAK signaling. Sci Rep. 2021;11 doi: 10.1038/s41598-021-96348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hill A., Duran J., Purcell P. Lubricin protects the temporomandibular joint surfaces from degeneration. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chandrasekaran P., Kwok B., Han B., et al. Type V collagen regulates the structure and biomechanics of TMJ condylar cartilage: a fibrous-hyaline hybrid. Matrix Biol. 2021;102:1–19. doi: 10.1016/j.matbio.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou Y., Shu B., Xie R., et al. Deletion of Axin1 in condylar chondrocytes leads to osteoarthritis-like phenotype in temporomandibular joint via activation of β-catenin and FGF signaling. J Cell Physiol. 2019;234:1720–1729. doi: 10.1002/jcp.27043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou S., Xie Y., Li W., et al. Conditional deletion of fgfr3 in chondrocytes leads to osteoarthritis-like defects in temporomandibular joint of adult mice. Sci Rep. 2016;6 doi: 10.1038/srep24039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun J.L., Yan J.F., Yu S.B., et al. MicroRNA-29b promotes subchondral bone loss in TMJ osteoarthritis. J Dent Res. 2020;99:1469–1477. doi: 10.1177/0022034520937617. [DOI] [PubMed] [Google Scholar]
- 61.Kong H., Wang X.Q., Zhang X.A. Exercise for Osteoarthritis: a literature review of pathology and mechanism. Front Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.854026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang J., Hu S., Bian Y., et al. Targeting cell death: pyroptosis, ferroptosis, apoptosis and necroptosis in osteoarthritis. Front Cell Dev Biol. 2022;9 doi: 10.3389/fcell.2021.789948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu K., Meng Z., Xian X.M., et al. LncRNA PVT1 induces chondrocyte apoptosis through upregulation of TNF-α in synoviocytes by sponging miR-211-3p. Mol Cell Probes. 2020;52 doi: 10.1016/j.mcp.2020.101560. [DOI] [PubMed] [Google Scholar]
- 64.Zhu H., Hu Y., Wang C., et al. CircGCN1L1 promotes synoviocyte proliferation and chondrocyte apoptosis by targeting miR-330-3p and TNF-α in TMJ osteoarthritis. Cell Death Dis. 2020;11:284. doi: 10.1038/s41419-020-2447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao P., Feng Y., Deng M., et al. MiR-15b is a key regulator of proliferation and apoptosis of chondrocytes from patients with condylar hyperplasia by targeting IGF1, IGF1R and BCL2. Osteoarthritis Cartilage. 2019;27:336–346. doi: 10.1016/j.joca.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Mao D., Wu M., Wei J., et al. MicroRNA-101a-3p could be involved in the pathogenesis of temporomandibular joint osteoarthritis by mediating UBE2D1 and FZD4. J Oral Pathol Med. 2021;50:236–243. doi: 10.1111/jop.13131. [DOI] [PubMed] [Google Scholar]
- 67.Yang H., Wen Y., Zhang M., et al. MTORC1 coordinates the autophagy and apoptosis signaling in articular chondrocytes in osteoarthritic temporomandibular joint. Autophagy. 2020;16:271–288. doi: 10.1080/15548627.2019.1606647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hui T., Zhou Y., Wang T., et al. Activation of β-catenin signaling in aggrecan-expressing cells in temporomandibular joint causes osteoarthritis-like defects. Int J Oral Sci. 2018;10:13. doi: 10.1038/s41368-018-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Long H.Q., Tian P.F., Guan Y.X., et al. Expression of Ihh signaling pathway in condylar cartilage after bite-raising in adult rats. J Mol Histol. 2019;50:459–470. doi: 10.1007/s10735-019-09840-0. [DOI] [PubMed] [Google Scholar]
- 70.He Y., Wang W., Xu X., et al. Mettl3 inhibits the apoptosis and autophagy of chondrocytes in inflammation through mediating Bcl2 stability via Ythdf1-mediated m6A modification. Bone. 2022;154 doi: 10.1016/j.bone.2021.116182. [DOI] [PubMed] [Google Scholar]
- 71.Liu W., Luo H., Wang R., et al. Rapamycin-induced autophagy promotes the chondrogenic differentiation of synovium-derived mesenchymal stem cells in the temporomandibular joint in response to IL-1β. BioMed Res Int. 2020;2020 doi: 10.1155/2020/4035306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J., Zhang S., Qi W.J., et al. Mechanism and potential contributing factors to temporomandibular joint osteoarthritis. Oral Dis. 2023;29:1060–1069. doi: 10.1111/odi.14061. [DOI] [PubMed] [Google Scholar]
- 73.Wang Z., Huang J., Zhou S., et al. Loss of Fgfr1 in chondrocytes inhibits osteoarthritis by promoting autophagic activity in temporomandibular joint. J Biol Chem. 2018;293:8761–8774. doi: 10.1074/jbc.RA118.002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiao M., Hu Z., Jiang H., et al. The expression of Netrin-1 in the MIA-induced osteoarthritic temporomandibular joint in mice. Sci Rep. 2021;11 doi: 10.1038/s41598-021-95251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang T., Zhang J., Cao Y., et al. Wnt5a/Ror2 mediates temporomandibular joint subchondral bone remodeling. J Dent Res. 2015;94:803–812. doi: 10.1177/0022034515576051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng L., Pi C., Zhang J., et al. Aberrant activation of latent transforming growth factor-β initiates the onset of temporomandibular joint osteoarthritis. Bone Res. 2018;6:26. doi: 10.1038/s41413-018-0027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shen P., Jiao Z., Zheng J.S., et al. Injecting vascular endothelial growth factor into the temporomandibular joint induces osteoarthritis in mice. Sci Rep. 2015;5 doi: 10.1038/srep16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu L., Huang J., Zhang X., et al. Changes of temporomandibular joint and semaphorin 4D/Plexin-B1 expression in a mouse model of incisor malocclusion. J Oral Facial Pain Headache. 2014;28:68–79. doi: 10.11607/jop.1082. [DOI] [PubMed] [Google Scholar]
- 79.Yang J., Xu Y., Xue X., et al. MicroRNA-26b regulates BMSC osteogenic differentiation of TMJ subchondral bone through β-catenin in osteoarthritis. Bone. 2022;162 doi: 10.1016/j.bone.2022.116448. [DOI] [PubMed] [Google Scholar]
- 80.Matsumoto S., Yokota S., Chosa N., et al. Receptor tyrosine kinase ligands and inflammatory cytokines cooperatively suppress the fibrogenic activity in temporomandibular-joint-derived fibroblast-like synoviocytes via mitogen-activated protein kinase kinase/extracellular signal-regulated kinase. Exp Ther Med. 2020;20:1967–1974. doi: 10.3892/etm.2020.8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou Y., Chen M., O'Keefe R.J., et al. Epigenetic and therapeutic implications of dnmt3b in temporomandibular joint osteoarthritis. Am J Transl Res. 2019;11:1736–1747. [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang M., Yang H., Wan X., et al. Prevention of injury-induced osteoarthritis in rodent temporomandibular joint by targeting chondrocyte CaSR. J Bone Miner Res. 2019;34:726–738. doi: 10.1002/jbmr.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang H., Zhang M., Liu Q., et al. Inhibition of Ihh reverses temporomandibular joint osteoarthritis via a PTH1R signaling dependent mechanism. Int J Mol Sci. 2019;20:3797. doi: 10.3390/ijms20153797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Komori T., Ji Y., Pham H., et al. Type VI collagen regulates endochondral ossification in the temporomandibular joint. JBMR Plus. 2022;6 doi: 10.1002/jbm4.10617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bechtold T.E., Saunders C., Decker R.S., et al. Osteophyte formation and matrix mineralization in a TMJ osteoarthritis mouse model are associated with ectopic hedgehog signaling. Matrix Biol. 2016;52–54:339–354. doi: 10.1016/j.matbio.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang S.J., Li W., Li Y.J., et al. Dickkopf-related protein 1 induces angiogenesis by upregulating vascular endothelial growth factor in the synovial fibroblasts of patients with temporomandibular joint disorders. Mol Med Rep. 2015;12:4959–4966. doi: 10.3892/mmr.2015.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao H., Liu S., Ma C., et al. Estrogen-related receptor γ induces angiogenesis and extracellular matrix degradation of temporomandibular joint osteoarthritis in rats. Front Pharmacol. 2019;10:1290. doi: 10.3389/fphar.2019.01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ke J., Liu Y., Long X., et al. Up-regulation of vascular endothelial growth factor in synovial fibroblasts from human temporomandibular joint by hypoxia. J Oral Pathol Med. 2007;36:290–296. doi: 10.1111/j.1600-0714.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 89.Dong Y., Wu G., Zhu T., et al. VEGF promotes cartilage angiogenesis by phospho-ERK1/2 activation of Dll4 signaling in temporomandibular joint osteoarthritis caused by chronic sleep disturbance in Wistar rats. Oncotarget. 2017;8:17849–17861. doi: 10.18632/oncotarget.14874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y., Zhao B., Zhu Y., et al. HIF-1-VEGF-Notch mediates angiogenesis in temporomandibular joint osteoarthritis. Am J Transl Res. 2019;11:2969–2982. [PMC free article] [PubMed] [Google Scholar]
- 91.Feng Y., Ke J., Cao P., et al. HMGB1-induced angiogenesis in perforated disc cells of human temporomandibular joint. J Cell Mol Med. 2018;22:1283–1291. doi: 10.1111/jcmm.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu J., Cai H., Meng Q., et al. IL-1β-regulating angiogenic factors expression in perforated temporomandibular disk cells via NF-κB pathway. J Oral Pathol Med. 2016;45:605–612. doi: 10.1111/jop.12420. [DOI] [PubMed] [Google Scholar]
- 93.Jing Z., Gu Z., Feng J. Forward mandibular positioning enhances the expression of Ang-1 and Ang-2 in rabbit condylar chondrocytes. Mol Med Rep. 2013;8:1094–1098. doi: 10.3892/mmr.2013.1620. [DOI] [PubMed] [Google Scholar]
- 94.Lu K., Ma F., Yi D., et al. Molecular signaling in temporomandibular joint osteoarthritis. J Orthop Translat. 2021;32:21–27. doi: 10.1016/j.jot.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luo P., Jiang C., Ji P., et al. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res Ther. 2019;10:216. doi: 10.1186/s13287-019-1341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y., Zhao M., Li W., et al. BMSC-derived small extracellular vesicles induce cartilage reconstruction of temporomandibular joint osteoarthritis via autotaxin-YAP signaling axis. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.656153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y., Zhang Z., Wang B., et al. Inflammation-stimulated MSC-derived small extracellular vesicle miR-27b-3p regulates macrophages by targeting CSF-1 to promote temporomandibular joint condylar regeneration. Small. 2022;18 doi: 10.1002/smll.202107354. [DOI] [PubMed] [Google Scholar]
- 98.He D., Wang J., Li Y., et al. Low-intensity pulsed ultrasound promotes aggrecan expression via ZNT-9 in temporomandibular joint chondrocytes. Gene. 2021;768 doi: 10.1016/j.gene.2020.145318. [DOI] [PubMed] [Google Scholar]
- 99.Yang T., Liang C., Chen L., et al. Low-intensity pulsed ultrasound alleviates hypoxia-induced chondrocyte damage in temporomandibular disorders by modulating the hypoxia-inducible factor pathway. Front Pharmacol. 2020;11:689. doi: 10.3389/fphar.2020.00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jiang C., Luo P., Li X., et al. Nrf2/ARE is a key pathway for curcumin-mediated protection of TMJ chondrocytes from oxidative stress and inflammation. Cell Stress Chaperones. 2020;25:395–406. doi: 10.1007/s12192-020-01079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jia M., Lv Y., Xu Y., et al. A comparative analysis of NLRP3-related inflammatory mediators in synovial fluid in temporomandibular joint osteoarthritis and internal derangement. BMC Muscoskel Disord. 2021;22:229. doi: 10.1186/s12891-021-04092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ou F., Huang Y., Sun J., et al. Yohimbine ameliorates temporomandibular joint chondrocyte inflammation with suppression of NF-κB pathway. Inflammation. 2021;44:80–90. doi: 10.1007/s10753-020-01310-0. [DOI] [PubMed] [Google Scholar]
- 103.Fredriksson L., Alstergren P., Kopp S. Tumor necrosis factor-alpha in temporomandibular joint synovial fluid predicts treatment effects on pain by intra-articular glucocorticoid treatment. Mediat Inflamm. 2006;2006 doi: 10.1155/MI/2006/59425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu Z., Xiao M., Cai H., et al. Glycyrrhizin regulates rat TMJOA progression by inhibiting the HMGB1-RAGE/TLR4-NF-κB/AKT pathway. J Cell Mol Med. 2022;26:925–936. doi: 10.1111/jcmm.17149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yuan J., Ding W., Wu N., et al. Protective effect of genistein on condylar cartilage through downregulating NF-κB expression in experimentally created osteoarthritis rats. BioMed Res Int. 2019;2019 doi: 10.1155/2019/2629791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li W., Hu S., Chen X., et al. The antioxidant resveratrol protects against chondrocyte apoptosis by regulating the COX-2/NF-κB pathway in created temporomandibular osteoarthritis. BioMed Res Int. 2021;2021 doi: 10.1155/2021/9978651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sperry M.M., Yu Y.H., Kartha S., et al. Intra-articular etanercept attenuates pain and hypoxia from TMJ loading in the rat. J Orthop Res. 2020;38:1316–1326. doi: 10.1002/jor.24581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Izawa T., Mori H., Shinohara T., et al. Rebamipide attenuates mandibular condylar degeneration in a murine model of TMJ-OA by mediating a chondroprotective effect and by downregulating RANKL-mediated osteoclastogenesis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu H., Liu Y., Yang X., et al. Strontium ranelate promotes chondrogenesis through inhibition of the Wnt/β-catenin pathway. Stem Cell Res Ther. 2021;12:296. doi: 10.1186/s13287-021-02372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aneiros-Guerrero A., Lendinez A.M., Palomares A.R., et al. Genetic polymorphisms in folate pathway enzymes, DRD4 and GSTM1 are related to temporomandibular disorder. BMC Med Genet. 2011;12:75. doi: 10.1186/1471-2350-12-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Furquim B.D., Flamengui L.M., Repeke C.E., et al. Influence of TNF-α-308 G/A gene polymorphism on temporomandibular disorder. Am J Orthod Dentofacial Orthop. 2016;149:692–698. doi: 10.1016/j.ajodo.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 112.Tumer M.K., Nursal A.F., Tekcan A., et al. The IL-1Ra gene variable number tandem repeat variant is associated with susceptibility to temporomandibular disorders in Turkish population. J Clin Lab Anal. 2018;32 doi: 10.1002/jcla.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Souza Tesch R., Ladeira Bonato L., Quinelato V., et al. Evaluation of genetic risk related to catechol-O-methyltransferase (COMT) and β2-adrenergic receptor (ADRB2) activity in different diagnostic subgroups of temporomandibular disorder in Brazilian patients. Int J Oral Maxillofac Surg. 2020;49:237–243. doi: 10.1016/j.ijom.2019.06.027. [DOI] [PubMed] [Google Scholar]
- 114.Sangani D., Suzuki A., VonVille H., et al. Gene mutations associated with temporomandibular joint disorders: a systematic review. OAli. 2015;2:e1583. doi: 10.4236/oalib.1101583. [DOI] [PMC free article] [PubMed] [Google Scholar]