Abstract
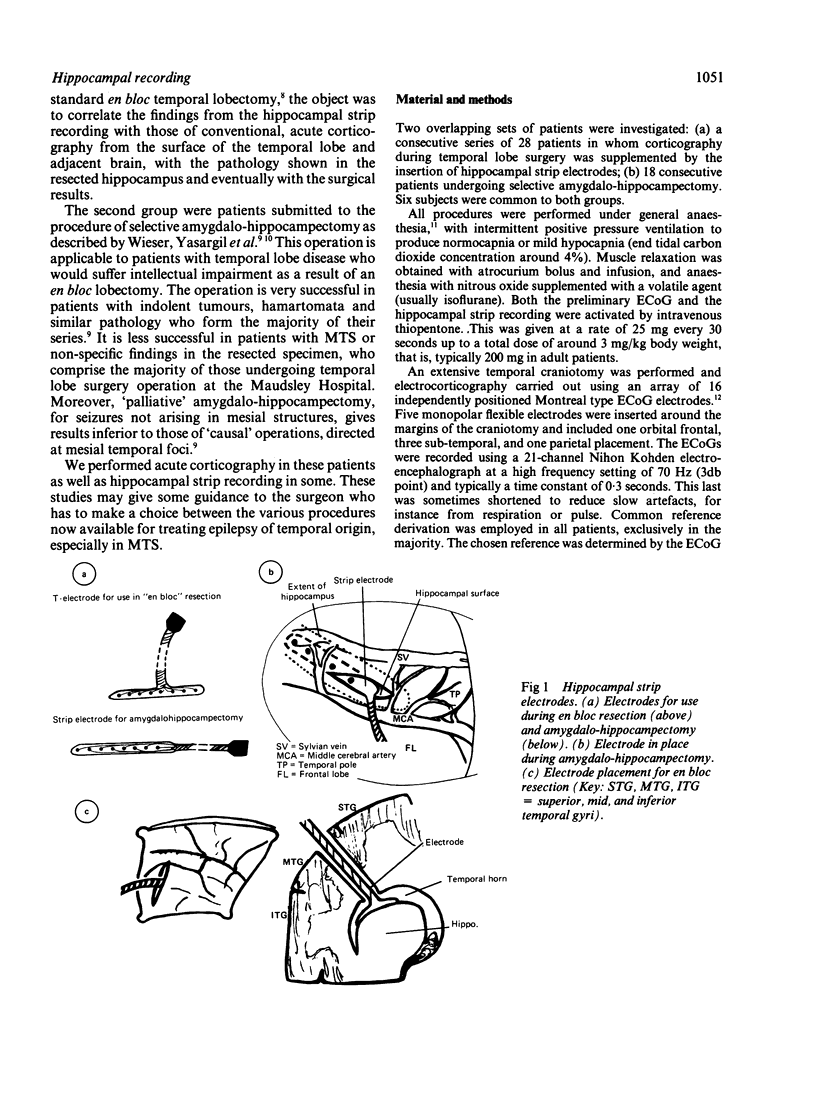
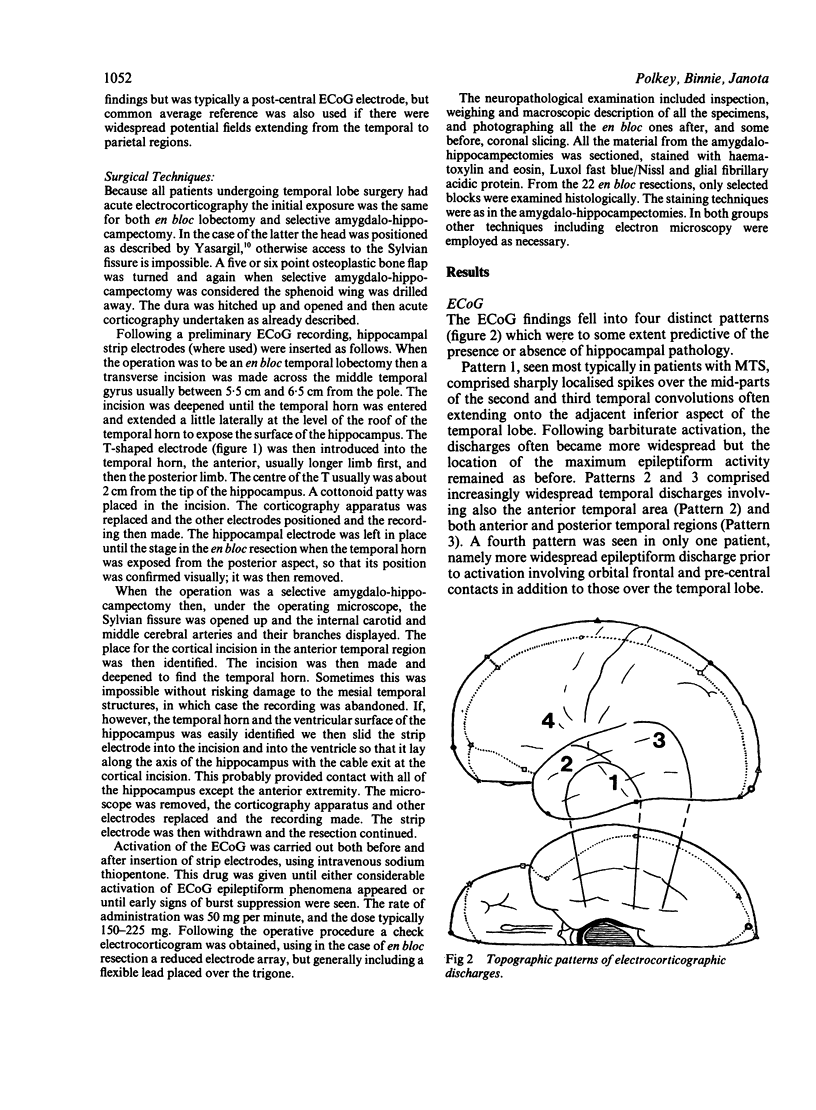
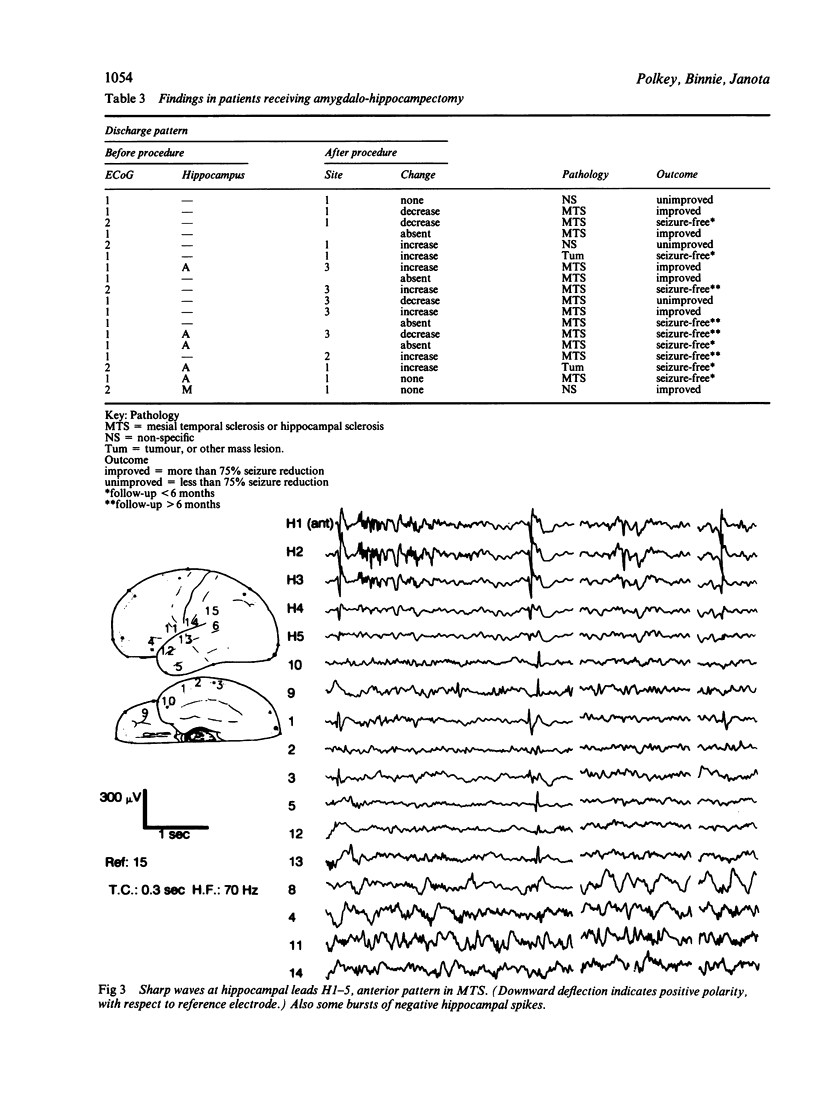
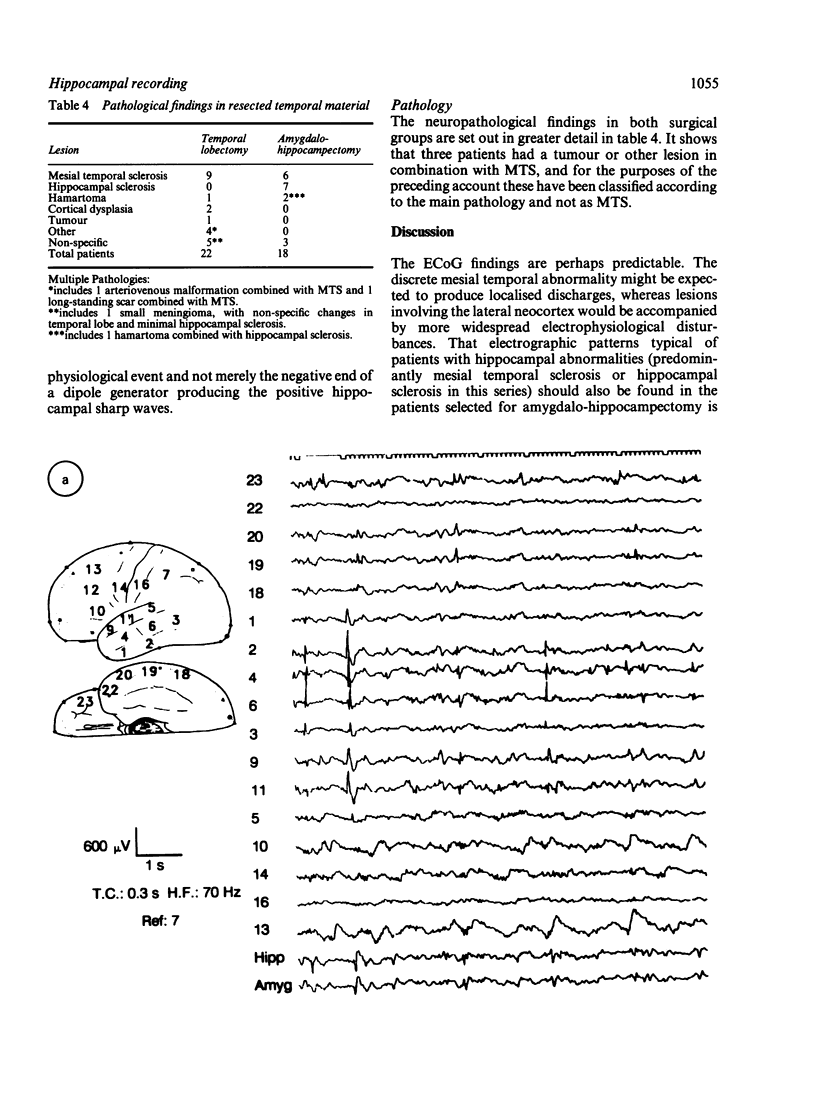
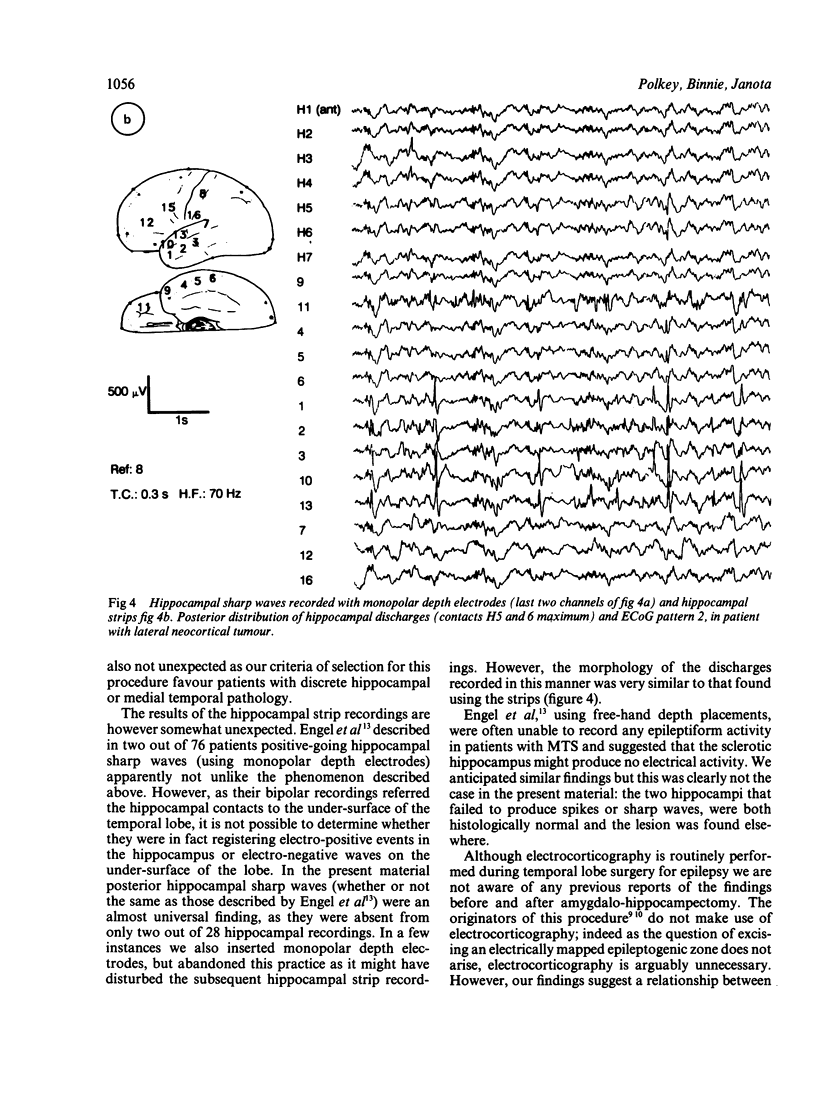
An electrocorticographic (ECoG) study is reported of patients undergoing surgery for epilepsy of temporal lobe origin. During 22 en bloc resections and six out of a total of 18 amygdalo-hippocampectomies, the activity of the hippocampus was also recorded by a multipolar strip electrode placed along its axis on the ventricular surface. Patients with mesial temporal pathology, chiefly mesial temporal sclerosis, made up the majority of those selected for amygdalo-hippocampectomy. They showed a characteristic ECoG pattern, with spikes localised to the mid part of the second and third convolutions and inferior aspect of the temporal lobe. Typically, this was associated with hippocampal discharges showing an anterior maximum. Pathology involving lateral temporal neocortex and non-specific findings were associated with more widespread temporal spikes and a maximum discharge amplitude over the mid and posterior parts of the hippocampus. It is suggested that intraoperative recording of the ECoG and hippocampal activity may provide a guide to the choice between en bloc resection and amygdalo-hippocampectomy.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brant-Zawadzki M., Davis P. L., Crooks L. E., Mills C. M., Norman D., Newton T. H., Sheldon P., Kaufman L. NMR demonstration of cerebral abnormalities: comparison with CT. AJR Am J Roentgenol. 1983 May;140(5):847–854. doi: 10.2214/ajr.140.5.847. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr, Driver M. V., Falconer M. A. Electrophysiological correlates of pathology and surgical results in temporal lobe epilepsy. Brain. 1975 Mar;98(1):129–156. doi: 10.1093/brain/98.1.129. [DOI] [PubMed] [Google Scholar]
- McLachlan R. S., Nicholson R. L., Black S., Carr T., Blume W. T. Nuclear magnetic resonance imaging, a new approach to the investigation of refractory temporal lobe epilepsy. Epilepsia. 1985 Nov-Dec;26(6):555–562. doi: 10.1111/j.1528-1157.1985.tb05691.x. [DOI] [PubMed] [Google Scholar]
- Spencer D. D., Spencer S. S., Mattson R. H., Williamson P. D., Novelly R. A. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery. 1984 Nov;15(5):667–671. doi: 10.1227/00006123-198411000-00005. [DOI] [PubMed] [Google Scholar]
- Sperling M. R., Wilson G., Engel J., Jr, Babb T. L., Phelps M., Bradley W. Magnetic resonance imaging in intractable partial epilepsy: correlative studies. Ann Neurol. 1986 Jul;20(1):57–62. doi: 10.1002/ana.410200110. [DOI] [PubMed] [Google Scholar]
- Yaşargil M. G., Teddy P. J., Roth P. Selective amygdalo-hippocampectomy. Operative anatomy and surgical technique. Adv Tech Stand Neurosurg. 1985;12:93–123. doi: 10.1007/978-3-7091-7008-3_2. [DOI] [PubMed] [Google Scholar]


