Abstract
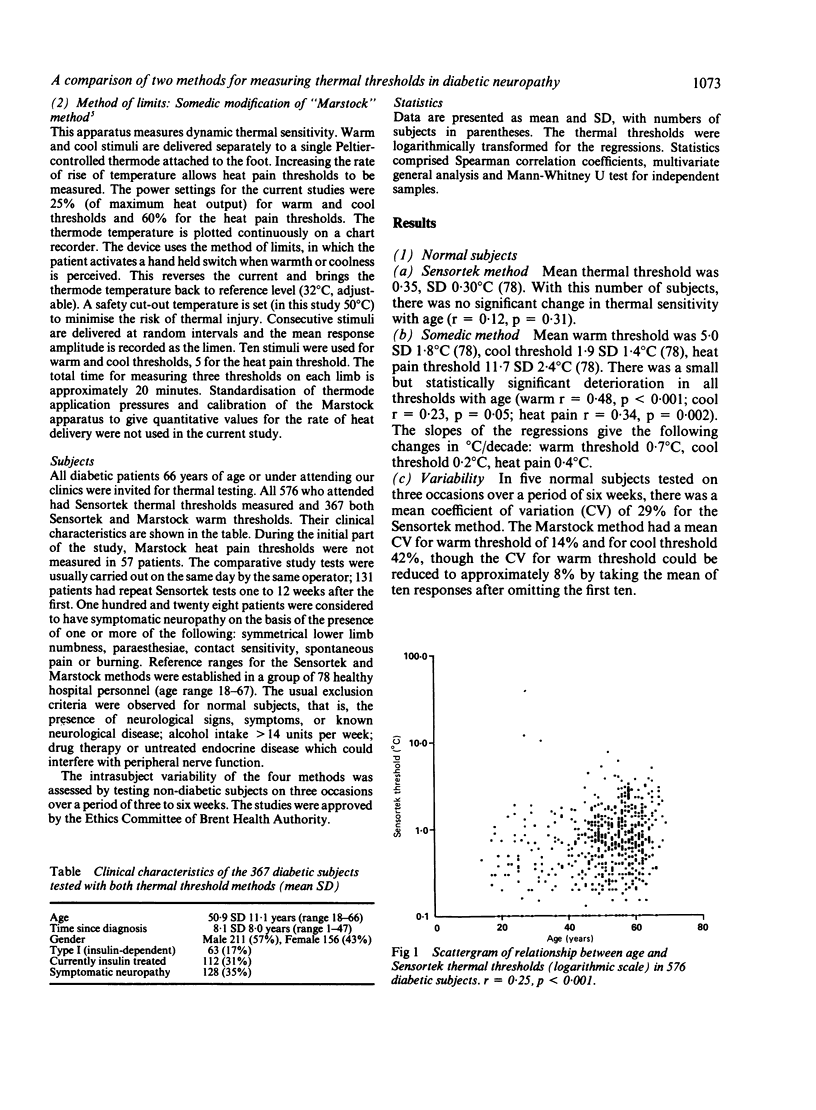
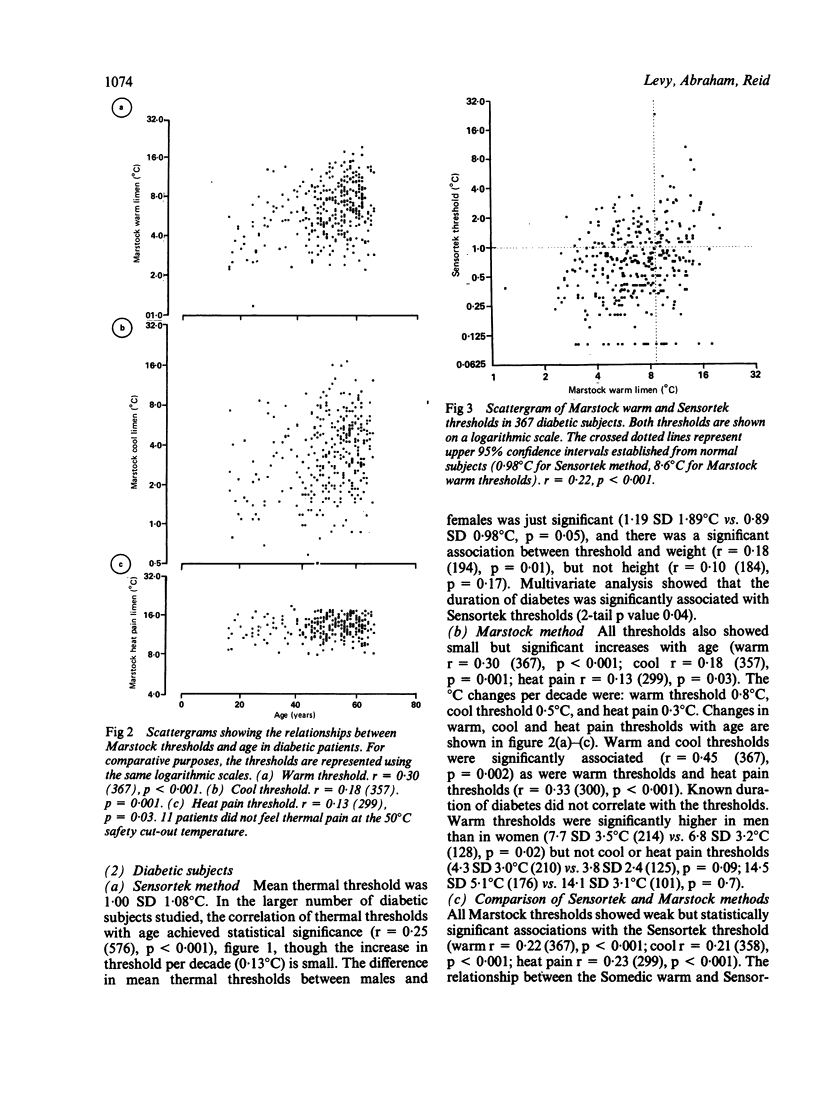
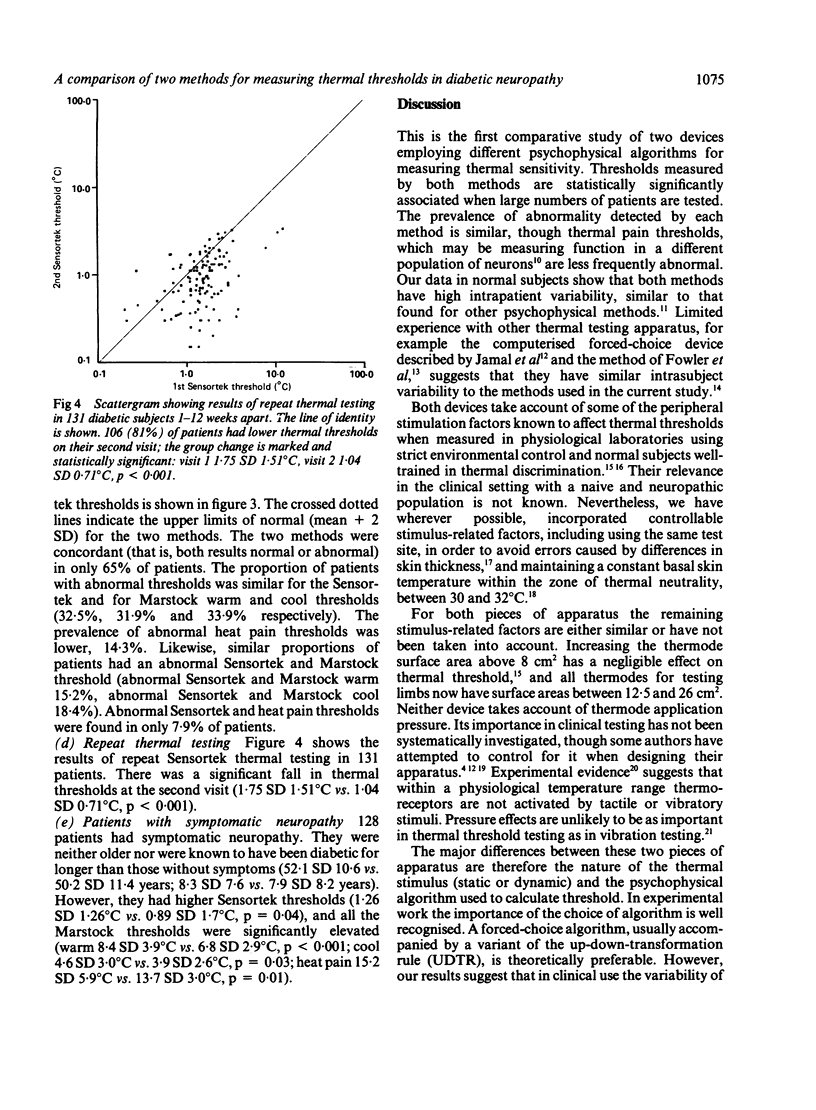
Thermal thresholds can be measured psychophysically using either the method of limits or a forced-choice method. We have compared the two methods in 367 diabetic patients, 128 with symptomatic neuropathy. The Sensortek method was chosen for the forced-choice device, the Somedic modification of the Marstock method for a method of limits. Cooling and heat pain thresholds were also measured using the Marstock method. Somedic thermal thresholds increase with age in normal subjects, but not to a clinically significant degree. In diabetics Marstock warm threshold increased by 0.8 degrees C/decade, Sensortek by 0.1 degrees C/decade. Both methods had a high coefficient of variation in normal subjects (Sensortek 29%, Marstock warm 14%, cool 42%). The prevalence of abnormal thresholds was similar for both methods (28-32%), though Marstock heat pain thresholds were less frequently abnormal (18%). Only 15-18% of patients had abnormal results in both tests. Sensortek thresholds were significantly lower on repeat testing, and all thresholds were higher in symptomatic patients. Both methods are suitable for clinical thermal testing, though the method of limits is quicker. In screening studies the choice of a suitable apparatus need not be determined by the psychophysical basis of the test.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arezzo J. C., Schaumburg H. H., Laudadio C. Thermal sensitivity tester. Device for quantitative assessment of thermal sense in diabetic neuropathy. Diabetes. 1986 May;35(5):590–592. doi: 10.2337/diab.35.5.590. [DOI] [PubMed] [Google Scholar]
- Bertelsmann F. W., Heimans J. J., Weber E. J., van der Veen E. A., Schouten J. A. Thermal discrimination thresholds in normal subjects and in patients with diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1985 Jul;48(7):686–690. doi: 10.1136/jnnp.48.7.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S., Till S., Sönksen P., Smith S. Use of a biothesiometer to measure individual vibration thresholds and their variation in 519 non-diabetic subjects. Br Med J (Clin Res Ed) 1984 Jun 16;288(6433):1793–1795. doi: 10.1136/bmj.288.6433.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman L. J., Cummins K. L., Reaven G. M., Ceranski J., Greenfield M. S., Doberne L. Studies of diabetic polyneuropathy using conduction velocity distribution (DCV) analysis. Neurology. 1983 Jun;33(6):773–779. doi: 10.1212/wnl.33.6.773. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Zimmerman I. R., O'Brien P. C., Ness A., Caskey P. E., Karnes J., Bushek W. Introduction of automated systems to evaluate touch-pressure, vibration, and thermal cutaneous sensation in man. Ann Neurol. 1978 Dec;4(6):502–510. doi: 10.1002/ana.410040605. [DOI] [PubMed] [Google Scholar]
- Fagius J., Wahren L. K. Variability of sensory threshold determination in clinical use. J Neurol Sci. 1981 Jul;51(1):11–27. doi: 10.1016/0022-510x(81)90056-3. [DOI] [PubMed] [Google Scholar]
- Fowler C. J., Carroll M. B., Burns D., Howe N., Robinson K. A portable system for measuring cutaneous thresholds for warming and cooling. J Neurol Neurosurg Psychiatry. 1987 Sep;50(9):1211–1215. doi: 10.1136/jnnp.50.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler C. J., Sitzoglou K., Ali Z., Halonen P. The conduction velocities of peripheral nerve fibres conveying sensations of warming and cooling. J Neurol Neurosurg Psychiatry. 1988 Sep;51(9):1164–1170. doi: 10.1136/jnnp.51.9.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhstorfer H., Lindblom U., Schmidt W. C. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry. 1976 Nov;39(11):1071–1075. doi: 10.1136/jnnp.39.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. M., Lindblom U. Standardised method of determining vibratory perception thresholds for diagnosis and screening in neurological investigation. J Neurol Neurosurg Psychiatry. 1979 Sep;42(9):793–803. doi: 10.1136/jnnp.42.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy R. J., Clark C. A., Malcolm P. N., Watkins P. J. Evaluation of thermal and vibration sensation in diabetic neuropathy. Diabetologia. 1985 Mar;28(3):131–137. doi: 10.1007/BF00273859. [DOI] [PubMed] [Google Scholar]
- Jamal G. A., Hansen S., Weir A. I., Ballantyne J. P. An improved automated method for the measurement of thermal thresholds. 1. Normal subjects. J Neurol Neurosurg Psychiatry. 1985 Apr;48(4):354–360. doi: 10.1136/jnnp.48.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal G. A., Weir A. I., Ballantyne J. P., Hansen S. Thermal discrimination thresholds in normal subjects and in patients with diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1986 Mar;49(3):335–336. doi: 10.1136/jnnp.49.3.335-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenshalo D. R., Scott H. A., Jr Temporal course of thermal adaptation. Science. 1966 Mar 4;151(3714):1095–1096. doi: 10.1126/science.151.3714.1095. [DOI] [PubMed] [Google Scholar]
- Konietzny F., Hensel H. Letters and notes: Warm fiber activity in human skin nerves. Pflugers Arch. 1975 Sep 9;359(3):265–267. doi: 10.1007/BF00587384. [DOI] [PubMed] [Google Scholar]
- LELE P. P. Relationship between cutaneous thermal thresholds, skin temperature and cross-sectional area of the stimulus. J Physiol. 1954 Nov 29;126(2):191–205. doi: 10.1113/jphysiol.1954.sp005203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. M., Abraham R. R., Abraham R. M. Small- and large-fiber involvement in early diabetic neuropathy: a study with the medial plantar response and sensory thresholds. Diabetes Care. 1987 Jul-Aug;10(4):441–447. doi: 10.2337/diacare.10.4.441. [DOI] [PubMed] [Google Scholar]
- Morley G. K., Mooradian A. D., Levine A. S., Morley J. E. Mechanism of pain in diabetic peripheral neuropathy. Effect of glucose on pain perception in humans. Am J Med. 1984 Jul;77(1):79–82. doi: 10.1016/0002-9343(84)90439-x. [DOI] [PubMed] [Google Scholar]
- Parkhouse N., Le Quesne P. M. Quantitative objective assessment of peripheral nociceptive C fibre function. J Neurol Neurosurg Psychiatry. 1988 Jan;51(1):28–34. doi: 10.1136/jnnp.51.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekuler R., Nash D., Armstrong R. Sensitive, objective procedure for evaluating response to light touch. Neurology. 1973 Dec;23(12):1282–1291. doi: 10.1212/wnl.23.12.1282. [DOI] [PubMed] [Google Scholar]
- Smith S. A. Reduced sinus arrhythmia in diabetic autonomic neuropathy: diagnostic value of an age-related normal range. Br Med J (Clin Res Ed) 1982 Dec 4;285(6355):1599–1601. doi: 10.1136/bmj.285.6355.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosenko J. M., Kato M., Soto R. A., Gadia M. T., Ayyar D. R. Specific assessments of warm and cool sensitivities in adult diabetic patients. Diabetes Care. 1988 Jun;11(6):481–483. doi: 10.2337/diacare.11.6.481. [DOI] [PubMed] [Google Scholar]
- Stoll A. M. Thermal properties of human skin related to nondestructive measurement of epidermal thickness. J Invest Dermatol. 1977 Sep;69(3):328–332. doi: 10.1111/1523-1747.ep12507865. [DOI] [PubMed] [Google Scholar]
- Ziegler D., Mayer P., Wiefels K., Gries F. A. Assessment of small and large fiber function in long-term type 1 (insulin-dependent) diabetic patients with and without painful neuropathy. Pain. 1988 Jul;34(1):1–10. doi: 10.1016/0304-3959(88)90175-3. [DOI] [PubMed] [Google Scholar]


