Abstract
Although there is a plethora of cancer associated-factors that can ultimately culminate in death (cachexia, organ impairment, metastases, opportunistic infections, etc.), the focal element of every terminal malignancy is the failure of our natural defences to control unlimited cell proliferation. The reasons why our defences apparently lack efficiency is a complex question, potentially indicating that, under Darwinian terms, solutions other than preventing cancer progression are also important contributors. In analogy with host-parasite systems, we propose to call this latter option ‘tolerance’ to cancer. Here, we argue that the ubiquity of oncogenic processes among metazoans is at least partially attributable to both the limitations of resistance mechanisms and to the evolution of tolerance to cancer. Deciphering the ecological contexts of alternative responses to the cancer burden is not a semantic question, but rather a focal point in understanding the evolutionary ecology of host-tumour relationships, the evolution of our defences, as well as why and when certain cancers are likely to be detrimental for survival.
Key words: Cancer, Resistance, Tolerance
Introduction
When infected by a pathogen, hosts have evolved a series of defensive responses that can be broadly categorized into three strategies: (i) avoidance; defined as behaviour to reduce contact with pathogens; (ii) resistance; defined as the ability of a host to limit or inhibit pathogen replication, thus reducing infection severity and parasite burden and (iii) tolerance; defined as the ability of an infected host to limit the impact of infection on host fitness (Hart, 1990; Råberg et al., 2007, 2009; Medzhitov et al., 2012). When relying on resistance a host can employ a multitude of, often costly, behavioural, morphological, physiological defence mechanisms to limit and eliminate the negative effects of the pathogens (Lochmiller and Deerenberg, 2000; Råberg et al., 2009). Unlike resistance, host tolerance does not limit infection, but reduces or compensates parasite-induced damages through reduced immuno-pathology, increased wound repair mechanisms and greater resilience to tissue injuries (Råberg et al., 2009). Tolerance thus offsets and/or reduces the pathogen's impact at significantly lower fitness costs compared with responses involving resistance (Råberg et al., 2009). Both resistance and tolerance have been shown to be genetically controlled, be heritable and often evolve independently (Mazé-Guilmo et al., 2014; Lough et al., 2015).
Resistance and tolerance may result in a similar fitness benefit for the host. However, their outcomes give rise to two different evolutionary scenarios. Assuming that resistance carries a cost (see above), when a gene conferring pathogen resistance spreads through a population, the prevalence of the pathogen declines, reducing the fitness advantage of carrying the resistance gene and therefore ultimately resulting in a negative feedback loop (Roy and Kirchner, 2000; Råberg et al., 2009; Hayward et al., 2014). The combination of the fitness cost and a possible co-evolutionary relationship between pathogen and host will prevent resistant genes from becoming fixed within a host population (Roy and Kirchner, 2000). Tolerance, however, does not directly affect pathogen survival and its effect on pathogen prevalence (Roy and Kirchner, 2000; Råberg et al., 2009). This strategy results in prolonged period of pathogen infection ultimately resulting in an increase in pathogen prevalence within the host population. It, therefore, creates a positive feedback loop that increases the chance of ‘tolerance genes’ becoming fixed in the host population (Roy and Kirchner, 2000; Lough et al., 2015). Therefore, the two host defence strategies may have opposing outcomes; host resistance has been considered to promote host heterogeneity, while tolerance has been proposed to purge the heterogeneity in the host population (Hayward et al., 2014). However, if the efficiency and the cost of the two strategies are different, or if a trade-off exists between resistance and tolerance, then host genetic variance can be maintained even under tolerance (Best et al., 2008; Carval and Ferriere, 2010; Hayward et al., 2014).
As proposed by Dillman and Schneider (2015), resistance and disease tolerance should be applicable to any challenges to the host that is to say not only to infections but also to cancer. Despite the importance of oncogenic processes in animal evolutionary ecology (Thomas et al., 2017), the concept of tolerance to cancer has until now received little attention. We propose that the ubiquity of oncogenic processes among metazoans is not only attributable to limitations in resistance mechanisms but also to tolerance to cancer. Before discussing this topic, it is important to first define cancer, a family of potentially lethal pathologies in which normal cells lose their typical cooperative behaviour, proliferate, spread and hence become malignant. The majority of deaths directly caused by cancer can be attributed to metastases (i.e. disseminated tumour spread), not to the oncogenic phenomena occurring along the continuum of precancerous lesions to locally confined tumours (i.e. in situ neoplasia). Even if they do not always lead to metastases, the presence of oncogenic processes has been demonstrated in both human and animal populations (Madsen et al., 2017; Thomas et al., 2018b). Invasive cancers are therefore the final stage of a series of oncogenic phenomena that are widespread among metazoan species. The dynamic interaction between the malignant cells and the host determines why certain malignancies evolve into invasive cancers while others do not. Although the majority of invasive cancers occur in old age, the development of precancerous lesions, dysplasia and hyperplasia in youth can however still impair fitness, particularly when their control requires the activation of host defences (i.e. trade-offs) (Jacqueline et al., 2017; Thomas et al., 2018a, 2018b).
Secondly, it is also important to point out that while the dynamics of ‘typical’ host-pathogen interactions (that occur across generations) can be easily translated to transmissible tumours that also affect several generations, applying the classical co-evolutionary theory to non-transmissible cancers is not that straightforward. Non-transmissible cancers do not experience any epidemiological feedback because these malignant cell lines arise and go extinct within the same host, i.e. the only evolutionary pressure on non-transmissible cancers is the intrinsic defences of a single host genotype and any external anti-neoplastic therapeutic modality. We therefore explore the concept of tolerance being a potential host response to both transmissible and non-transmissible cancers.
The trade-offs of resisting non-transmissible and transmissible cancers
Non-transmissible premalignant lesions and malignant transformation occur throughout the life of organism, and although defence mechanisms are effective in suppressing tumour growth to a certain extent (DeGregori, 2011), cancer cells (similar to pathogens (Janeway et al., 2001; Finlay and McFadden, 2006)) may eventually evolve to circumvent and/or escape immune control (e.g. the process of immunoediting: Elimination, Equilibrium, Escape, (Dunn et al., 2004)) and ultimately cause the demise of the organism. Our defences against malignant cells could in theory be more efficient, but they are also traded against other fitness-related functions (Jacqueline et al., 2017). Depending on several intrinsic (e.g. lifespan, metabolic rate) and extrinsic factors (e.g. resource availability), the organism can optimize its response to cancer by resisting and suppressing malignant cell growth or to tolerate neoplasia and allocate resources to other processes that directly and/or indirectly maximize reproduction. The high cost of immune system functioning leads to trade-offs with other life history traits and demands (Norris and Evans, 2000; van der Most et al., 2011) and determines the best host strategy to achieve maximal fitness. Accordingly, over the last decades, immuno-ecological studies have illustrated various trade-offs between host responses, i.e. immune activation and investment in other anabolic activities (Sheldon and Verhulst, 1996; Sadd and Schmid-Hempel, 2009). For example, under natural conditions, the immune system of multicellular organisms is regularly challenged by both micro- and macro-parasites, as well as by malignant cells, and hence it is required to act at both fronts. While producing a Th1 response is usually protective against cancer and intracellular pathogens (Knutson and Disis, 2005), Th2 activation is both linked to negative prognosis in some cancers (Lippitz, 2013) but also to conferring protection against macro-parasites. Jacqueline et al. (2017) suggested that trade-offs may exist because the cytokines that instruct immune cells to differentiate into the Th1 pathway tend to inhibit Th2 effectors (Kidd, 2003).
In Tasmanian devils, where a transmissible cancer epidemic, the devil facial tumour disease (DFTD), has decimated devil numbers, there is some evidence for the recent evolution of resistance mechanisms. Indeed, in areas where the cancer has been present for more than four generations, changes in allele frequencies of key immune genes related to cancer and immune function have been observed (Epstein et al., 2016; Hubert et al., 2018). Furthermore, genome comparison of healthy devils and animals with regressed tumours identified three genomic regions and proposed a polygenic basis to DFTD (Margres et al., 2018). In addition, natural immune responses (DFTD antibodies) have resulted in tumour regressions and recovery after infection (Pye et al., 2016) and differences in expression of immune profiles (Ujvari et al., 2016b) have had significant effects on susceptibility to acquire DFTD. In areas where devil populations have been exposed to DFTD through multiple generations, infected individuals have doubled their survival time from 6–9 months to 18–24 months after acquiring DFTD (Hamede et al., 2012; Wells et al., 2017). Although a longer lifespan may also result from the devils developing a higher tolerance to tumours, the genetic studies indicate that devil populations are adapting to the cancer epidemic via shifting the frequency of alleles (Epstein et al., 2016; Hubert et al., 2018) and potentially via phenotypic plasticity (Ujvari et al., 2016b) that provide resistance to DFTD. Nevertheless, the efficiency remains limited given the high-DFTD prevalence in the wild.
Similarly, another naturally occurring transmissible cancer cell line, the canine transmissible venereal tumour (CTVT), has caused host adaptations over the last 6000–10 000 years (Murgia et al., 2006; Siddle and Kaufman, 2013; Murchison et al., 2014; Decker et al., 2015; Siddle and Kaufman, 2015; Ostrander et al., 2016). CTVT is a sexually transmitted naturally occurring tumour in dogs that has most likely originated in a post-domestication canid (Murchison et al., 2014; Decker et al., 2015). The malignant cell growth is generally localized on the external genitalia and the cancer cells are transmitted during coitus. Metastases and the death of the host have only been observed in young and immunosuppressed adult dogs (Yang, 1988; Das and Das, 2000; Mukaratirwa and Gruys, 2003). While the transmissible cancer cells might have killed their ancestral host during the early stages of emergence and development, at present the tumour can regress after a short progressive growth phase in healthy dogs (Yang, 1988). Regression occurs via a step-wise process: initiated by a strong inflammatory response followed by immune cell infiltration (T, NK and B cells), cell cycle arrest and ultimately ending in the loss of tumour cells and cell migration (Frampton et al., 2018). CTVT can be cured by a single dose of vincristine or radiation (Gonzalez et al., 2000), and thus Frampton et al. (2018) proposed that ‘CTVT is particularly susceptible to changes that break tolerance to this cancer’. CTVT represents the final stages of an evolutionary tug-of war between selfish malignant cell lines and host genomes resembling to that observed in host-parasite interactions (sensu ‘Red-Queen’ dynamics; Van Valen, 1973; Schmid-Hempel, 2011). Over the last 5–10 millennia both the host and the contagious cancer cells had to continuously adapt to avoid the extinction of either and/or both (Ujvari et al., 2016a). Adaptive immune responses can now be observed in dogs, and the CTVT genome also bears evidence to such ongoing arms-race (Decker et al., 2015), i.e. somatic mutations in the CTVT genome in all aspects of somatic cell participation in immune surveillance, genome integrity maintenance and the regulation of cell apoptosis. The aetiology of CTVT in combination with host adaptive immune responses carries the signature of thousands of years of co-evolution between the dogs and their parasitic malignant cell line (Decker et al., 2015; Ostrander et al., 2016).
Strong immune responses also cause costs in terms of oxidative stress levels since the production of reactive oxygen species is a defence mechanism used by immune cells to limit pathogen progression (Klasing, 2007; Hasselquist and Nilsson, 2012). A tolerant strategy at the organism level would thus prevent the establishment of systemic levels of oxidative stress that would impair reproduction. Similarly, a tolerant strategy might be adopted at the organ level to protect organs essential for reproduction and/or short-term survival (i.e. ovaries, testes, brain etc.) from developing oxidative stress levels that would impair the normal functioning of these tissues. Inversely, organisms might use a resistant strategy to prevent the development of neoplasia in important tissues and especially small ones like the pancreas since the establishment and growth of any tumours would impair the normal functioning of these tissues (as opposed to the lungs, where one lobe can still function with a non-invasive tumour) (Thomas et al., 2016).
Strong immune responses can also lead to autoimmune diseases and tumours may in return exploit mechanisms selected to prevent these autoimmune disorders, precluding the development of an adequate antitumour response. In this process, called immune tolerance, tumours themselves have the ability to thwart the development of effective immune responses against their antigens (Mapara and Sykes, 2004). Both infectious pathogens and cancer cells may take advantage of immune tolerance to escape immune recognition and elimination (de Lafaille and Lafaille, 2009). Treg cells inhibit anti-tumour NK cells and hence immune tolerance may actually lead to cancer progression (Ghiringhelli et al., 2006). For example, increased Treg levels (measured by the expression of the Treg marker FOXP3) were found to be important to tumour establishment in various human cancers (Wieczorek et al., 2009). Additionally, accumulation of metabolic enzymes, such as IDO and arginase, and high expression of tolerance-inducing ligands like FasL, PD-1, CTLA-4 and B7 suppress T cell proliferation and activation in the tumour microenvironment (Maher et al., 2002; Becker et al., 2013). Regulating host immune responses and initiating host immune tolerance could be beneficial for both non-transmissible and transmissible cancers. The fitness of a non-transmissible malignant cell is determined by its own proliferation. Thus, cell properties, such as initiating host immune tolerance, that maximize proliferation in local tissues will be selected. The fitness of transmissible cancer cells is also defined by their ability to be transmitted from one host to another, suggesting that Darwinian forces will also tend to maximize the transmission related traits, e.g. evoking tolerance by the host that would allow more time to jump host (Ujvari et al., 2016a).
Over the lifetime of an individual, natural selection has also mainly favoured the evolution of resistance to cancer that acts before or during reproductive age (Crespi and Summers, 2005; DeGregori, 2011). Natural selection is indeed unlikely to favour efficient anticancer defences that emerge or progress after the reproductive life (Jacqueline et al., 2017). Thus, following the attenuation of immune and tumour suppressor mechanisms (e.g. the progressive decline of the immune system with age, also termed immunosenescence), it is therefore expected that malignant progressions are facilitated with advancing age, but see Matheu et al. (2007). Certain cancers may also have a very slow progression rate (e.g. for prostate cancer, malignant cells often grow so slowly that they never break free of the gland, spread to distant sites and pose a serious risk to health and longevity (Nelson et al., 2003)), so that even if they emerge during the reproductive period they are unlikely to significantly impact host fitness (except in species for which a long post-reproductive lifespan improves the likelihood that the grandchildren reach the age of sexual maturity (Engelhardt et al., 2019)). For such cancers, natural selection would not be expected to favour mechanisms that prevent the progression, even during the reproductive period (Hochberg et al., 2013). Intermediate situations can also theoretically exist, when for instance only a partial (and thus low cost) defence, that does not involve complete tumour elimination, is enough to contain the disease until the end of the reproductive period. For instance, Thomas et al. (2018a) recently argued that to delay malignant progression, our immune system may have been shaped by selection to act in the same way that adaptive therapy (Gatenby et al., 2009), i.e. to kill only a few malignant cells to control tumour burden by maintaining competition between immune-sensitive cells and resistant ones (i.e. a natural adaptive therapy).
Apart from evolutionary trade-offs, another factor that is likely to influence the evolution of cancer resistance is the body size of the species (Caulin and Maley, 2011). Peto's paradox demonstrates that, despite having larger bodies with more potential cells to develop cancer, the risk for cancer does not scale with size in larger species. Within a species, the opposite is true – shorter individuals tend to have cancer at a lower rate than taller individuals (Nunney, 2018). Since large-bodied species have intrinsically higher resistance to cancer (due to duplications of tumour-suppressor genes, as well as due to other genomic and anatomical adaptations that prevent the initiation of cancer, e.g. (Abegglen et al., 2015; Tollis et al., 2019), it is also likely that both tolerance and resistance strategies may be influenced by body mass.
All these limitations in our resistance mechanisms are likely to be relevant in explaining the ubiquity of oncogenic processes. Apart from strategies primarily based on malignant cell eradication, the theory also predicts the existence of alternative options, such as tolerating malignant cells, to alleviate the fitness costs associated with cancer progression.
Several theories can explain the evolution of tolerance to cancer
It is empirically known that certain individuals, for genetic and/or environmental reasons, cope better than others with their cancer, sensu they expressed lower pathological consequences associated with the presence of tumours (Sanchez-Ortiz et al., 2006; Reyes-Gibby et al., 2007; Matic et al., 2017; Zhang et al., 2017). The same phenomenon occurs in animals, i.e. in Tasmanian devil females have been found to be more tolerant to DFTD (measured as the decrease in body condition index per unit of tumour volume) than males (Ruiz-Aravena et al., 2018). Furthermore, although no sex-specific differences have been observed in the frequency of biting injuries, and DFTD equally effecting both sexes (Hamede et al., 2013), recent studies have shown tumour regression in more females than in males (six females vs one male in (Margres et al., 2018); and five females vs one male in (Wright et al., 2017)). The sex-specific differences might be related to their different energetic and physiological demands at the critical time of infection (mating season, (Hamede et al., 2009)) and disease progression. Females are able to maintain body condition, particularly early in DFTD progression when tumour volume is low, due to their plasticity in response to infection, including reallocation of resources (e.g. manipulating litter size) across reproductive stages such as pregnancy, carrying pouch young and late lactation. Males, on the other hand, suffer the greatest energetic costs during the intensive mating season, where they can lose up to 25% of their body weight and undergo sharp endocrine stress. Given the low chances of surviving to the next mating season (devils are annual breeders), males could optimize their fitness by allocating most resources to the current mating season at the expense of fighting DFTD infection. The differences in tolerance to infection in the devil/DFTD system suggest that, given the premises of phenotypic variation in response to infection (Ujvari et al., 2016b; Ruiz-Aravena et al., 2018) and changes in host genome (Epstein et al., 2016; Hubert et al., 2018) and the tumour (Murchison et al., 2012; Hamede et al., 2015), long-term coevolution should select for an endemic transmissible cancer in a highly tolerant host. However, the topic of tolerance to cancer is still in its infancy, and hence lacks clear definition. Here, under the umbrella of tolerance to cancer we propose to include all processes that, alternatively to resistance, permit equivalent fitness outcomes.
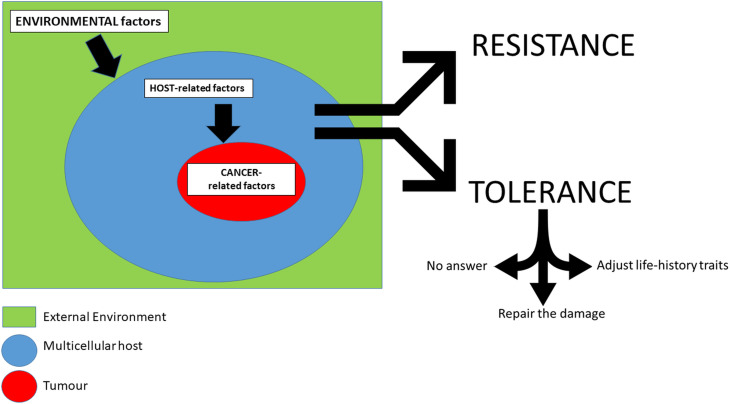
The fact that certain individuals tolerate the presence of tumours better than others in Darwinian terms might be mediated through several mechanisms (Fig. 1). All things being equal, a first explanation could be simply related to the probability of metastasis. Cancer is usually not detrimental/lethal until it metastasizes. The distribution pattern of metastatic cancer will undoubtedly influence disease trajectory (i.e. how well the cancer is tolerated). In this regard, various factors such as tissue of origin, oncogenic driver mutations as well as anatomical features (e.g. proximity to lymphatic vessels) may impact the ability of cancer to disperse and to establish metastatic niches in other tissues/organs (Obenauf and Massagué, 2015). Since metastasis is partially a stochastic process, and due to the variance in the dissemination pattern of metastatic cancer cells, disease trajectories can vary. Individuals may exhibit differences in their ability to tolerate cancer simply based on the chance distribution of metastatic cancer cells to vital organs (van Niekerk et al., 2016).
Fig. 1.
Factors influencing the balance of resistance/tolerance to cancer. Environmental factors: Level of resources, competition, predation, impact of parasites and ecological stochasticity. Host related factors: 1. Fixed traits: ecological status, gender etc. 2. Variable traits: age, size, energy reserves. Cancer related factors: 1. Cancer traits (proliferation rate, invasiveness, ‘bad luck’/metastasis). 2. Affected organ (key and accessory organs).
The co-evolutionary nature of host-tumour interactions in the context of transmissible cancers, in contrast to non-transmissible ones, is theoretically expected to interfere with the evolution of tolerance responses. The coevolutionary arms races between hosts and classical parasites are indeed likely to favour adaptations of parasites that lead to tolerance as opposed to resistance, because the selection pressure on the parasite to overcome tolerance is not as great as it is to overcome resistance. Therefore, the potential for the evolutionary arms race and hence for the evolution of parasite driven (i.e., cancer cell-driven) tolerance mechanisms may appear restricted for non-transmissible cancers. The reason why it seems paradoxically not to be the case suggests that the evolution of tolerance is mostly host-driven, and/or that it occurs mostly in the case of those cancers for which tolerant responses from the hosts will be efficient at limiting the fitness costs due to malignant progression (i.e. resistance vs. tolerance is adjusted by the host in a plastic way depending on cancer types).
Here, we also propose three additional non-exclusive strategies. First, individuals might not develop any answer in response to the emergence of neoplasia if the cost is too high. A second option might favour the repair of the damage caused by the tumour to keep the body functioning. Finally, with the last strategy, individuals affected by cancer might adjust their life-history strategy (by breeding at a younger age or by investing more in reproduction) to compensate for a potential decrease of fitness caused by the development of neoplasia. Both transmissible and non-transmissible cancers may trigger the evolution of these host responses.
The first option should mostly occur in species having fast life-history traits, and also when cancer resistance is costly and/or when cancer progression is slow. In these situations, due to the high costs associated with the activation of both innate and adaptive immune responses, it might be a priori more profitable in Darwinian terms to do nothing against the malignant progression. This strategy should often be associated with the reallocation of resources to reproduction and thus an adjustment of life-history traits (see below for examples). Under the second option, similar to host-parasite interactions, host responses could manifest in repairing the damage caused by malignant cells, thus tolerating the cancer while maintaining the functioning of the body. Mechanisms related to the detoxification of host-derived and tumour-produced toxic compounds would also fall under the umbrella of this strategy. This second scenario is likely to occur in species displaying slow life history traits and when tumours grow slowly. Among the possible evidence of the second option, meningioma is a kind of brain malignancies that mostly grows on the surface of the brain, pushing it away instead of growing from within. Meningiomas are slow-growing tumours with low potential to spread, and evidence suggests that the brain is capable of adjusting to their presence, up to the time point when further adaptation is not possible anymore (Jurić et al., 2013). In this case, brain plasticity includes adaptation and autoregulation of the circulation, thus ensuring stable blood flow. As a consequence, meningiomas may grow quite large before they cause symptoms (d’Avella et al., 1999; Tuna et al., 1999; Pawełczyk et al., 2012; Mumoli et al., 2013). Additional examples of giant tumours also exist (e.g. esophagus and cardia (Tsuzuki et al., 1971)), potentially implying tissue adaptability to keep the body functional.
The last option to mediate a tolerant strategy in response to cancer development is the potential adjustment of life-history traits. An abundant literature on host-parasite relationships has now shown that life-history traits in host species can undergo plastic responses to parasitism to compensate for the negative costs exerted by parasites on host fitness (Wieczorek et al., 2009, Becker et al., 2013). Parasitized individuals may, for instance, increase their reproductive activities before dying or being castrated by parasites (Minchella and Loverde, 1981; Sorci et al., 1996; Polak and Starmer, 1998; Adamo, 1999). If they are unable to resist infection by other means (e.g. inducible defences, immunological resistance, long-distance migration etc.) selection should indeed favour life-history adjustments if they partly compensate the parasite-induced losses by reproducing earlier (Forbes, 1993). Among recent examples, Vézilier et al. (2015) demonstrated that female mosquitoes, when parasitized by Plasmodium falciparum, compensate their reduced lifespan and loss of fecundity by beginning to lay their eggs two days earlier. In the context of cancer, there is little evidence of such adjustments, at the individual or population levels. For instance, Arnal et al. (2017) found that females in Drosophila harbouring early stages of a gut cancer reach the peak of oviposition earlier than healthy females, before dying sooner too. Another example of altered life-history strategies in response to exposure to cancer involves the Tasmanian devils (Sarcophilus harrisii) and their transmissible cancer, the DFTD. Following the appearance of this cancer, the vast majority of females only survive to produce one litter (Jones et al., 2008), but precocial breeding by one-year-old females has been observed at several study sites. Thus, although we cannot exclude that it simply results from a reduced competition for resources, devil populations may have responded to the cancer-induced mortality by rapidly transitioning from an iteroparous (multiple reproductive cycles) to a semelparous (single breeding) reproductive strategy (Jones et al., 2008). In humans, BRCA1 and BRCA2 mutations are inherited and predispose women to breast and ovarian cancer, but even though carriers of these mutations have reduced survival, they also have enhanced fertility (Easton et al., 1995; Smith et al., 2011). While this result may indicate antagonistic pleiotropy (i.e. when one gene controls more than one trait, at least one of these traits is beneficial to the organism's fitness and at least one is detrimental to fitness), it is also compatible with the hypothesis of adaptive adjustments of life history traits in individuals harbouring inherited oncogenic mutations (Arnal et al., 2016).
Ecological and evolutionary implications of tolerance to cancer
Transmissible cancers can interfere with the evolution of host resistance. For instance, in Tasmanian devils, the probability of achieving earlier maturity (either due to decreased competition resulting in increased resource availability, or due to direct selection for precocial breeding) may have delayed the evolution of resistance to DFTD, by permitting the transmission of genes of DFTD sensitive individuals to the next generation. In addition, the lack of development of resistance to DFTD across populations continues to facilitate the spread of this transmissible cancer. Since Tasmanian devils need to reach a critical body mass to become sexually mature, and hence Tasmanian devils cannot breed at an age younger than one year, it could be expected that their populations are now at the critical point when selection will favour resistance to this cancer. Similarly, when cancer is not-transmissible but is due to inherited oncogenic mutations, the adjustments of life history traits can also prevent natural selection from purging bad genes in the population. In the human example previously mentioned, the higher fertility of women bearing BRCA1 and BRCA2 is likely to favour the transmission of these oncogenic mutations to the next generations. This suggests that the existence of life-history trait adjustments could influence the persistence of oncogenic mutations in certain populations (Arnal et al., 2016).
Wildlife species are currently exposed to an increasing number of mutagenic substances and environmental conditions resulting from human activity (i.e. chemical pollutants, light pollution, non-natural food etc.), and hence cancer rates are likely to increase in many species (Giraudeau et al., 2018). However, at the moment it is unclear whether animals affected by malignant growths will respond by adapting resistance or tolerance strategies, and whether one can predict these responses (for example by comparing their life history traits, or their level of exposure to malignant transformations). In any case, animals naturally or artificially exposed to environmental oncogenic factors might, sometimes rapidly, evolve specific adaptations to cope with pollutants and their adverse effects on fitness (Vittecoq et al., 2018). Studying these species could be important in identifying when and why tolerance will be the favoured option by selection.
Medical and societal implications of tolerance to cancer
Similarly to therapeutic approaches that aim to prevent malignant progression, natural tumour suppressor responses will likely favour the evolution of mechanisms allowing cancer cells to circumvent them (i.e. the mutual antagonism between hosts and malignant cells can generate strong natural selection), while solutions that do not prevent progression do not trigger this selection. Similar to host-parasite systems, tolerance or resistance is therefore expected to influence the evolutionary trajectory of oncogenesis in radically different directions. Even when the proliferation rate looks similar (e.g. a lower level of resistance after the reproductive period can result in the same proliferation rate than under a tolerance scenario), the tumours will be qualitatively different. From a medical perspective this is important to consider because when cancer is diagnosed, the tumours have often already been present for years, and have been exposed to and have responded to our natural immune and tumour-suppressor responses. If they respond differently to therapies, knowing which scenario shaped the tumour development prior to detection is important for the prediction of treatment outcomes.
A good example of the crucial role of these strategies (i.e. tolerance vs resistance) on neoplasia development and aggressiveness is the fact that both these strategies can theoretically impact the level of intratumoural heterogeneity, an important clinical determinant of patient outcomes. Intratumoural heterogeneity can be the underlying factor of incomplete treatment responses, acquired and/or innate resistance and disease relapse in response to chemotherapy and targeted agents (Maley, 2007; Meacham and Morrison, 2013; Mengelbier et al., 2015; Pribluda et al., 2015; Roerink et al., 2018). Giraudeau et al. (2019) recently proposed that some organs might have developed stronger immune defences than others with a better ability to counteract the emergence and development of neoplasia, leading to higher levels of intratumoural heterogeneity. In other words, only aggressive neoplasms with higher levels of intratumoural heterogeneity should succeed in emerging and developing in organs strongly protected by immune defences. In this context, we can thus predict that a resistant strategy at the individual or at the organ level would select for aggressive tumours with high levels of intratumoural heterogeneity, while a tolerant strategy would lead to the faster development of less aggressive tumours.
More research is needed to understand when and why, resistance, partial resistance (e.g. natural adaptive therapy) and/or tolerance are the options retained by multicellular organisms during their lifetime. We also need to better understand their consequences for individuals reaching the end of the reproductive period. Indeed, although these options can sometimes be equivalent from an evolutionary perspective, they can have a dramatic impact on current treatment approaches and life aspirations that prioritize maximizing survival, rather than fitness. Because of a mismatch between what evolution would have selected for and personal life expectations of individuals, it is therefore important to identify the strategies a given organism follows to deal with malignancies. Therapies could be developed to mimic and/or enhance these natural defence approaches (being selection or tolerance) to enhance organismal survival.
Acknowledgement
This work is supported by an ARC Linkage (LP170101105), ARC DE170101116, Deakin SEBE_RGS_2019, an ANR TRANSCAN (ANR-18-CE35-0009), the Rotary Club Les Sables d'Olonne and a CNRS ‘International Associated Laboratory Grant'.
References
- Abegglen LM, Caulin AF, Chan A, Lee K, Robinson R, Campbell MS, Kiso WK, Schmitt DL, Waddell PJ, Bhaskara S, Jensen ST, Maley CC and Schiffman JD (2015) Potential mechanisms for cancer resistance in elephants and comparative cellular response to DNA damage in humans. JAMA 314, 1850–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo SA (1999) Evidence for adaptive changes in egg laying in crickets exposed to bacteria and parasites. Animal Behaviour 57, 117–124. [DOI] [PubMed] [Google Scholar]
- Arnal A, Tissot T, Ujvari B, Nunney L, Solary E, Laplane L, Bonhomme F, Vittecoq M, Tasiemski A and Renaud F (2016) The guardians of inherited oncogenic vulnerabilities. Evolution 70, 1–6. [DOI] [PubMed] [Google Scholar]
- Arnal A, Jacqueline C, Ujvari B, Leger L, Moreno C, Faugere D, Tasiemski A, Boidin-Wichlacz C, Misse D and Renaud F (2017) Cancer brings forward oviposition in the fly Drosophila melanogaster. Ecology and Evolution 7, 272–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JC, Andersen MH, Schrama D and Straten PT (2013) Immune-suppressive properties of the tumor microenvironment. Cancer Immunology Immunotherapy 62, 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best A, White A and Boots M (2008) Maintenance of host variation in tolerance to pathogens and parasites. Proceedings of the National Academy of Sciences 105, 20786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carval D and Ferriere R (2010) A unified model for the coevolution of resistance, tolerance, and virulence. Evolution 64, 2988–3009. [DOI] [PubMed] [Google Scholar]
- Caulin AF and Maley CC (2011). Peto's Paradox: evolution's prescription for cancer prevention. Trends in Ecology and Evolution 26, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi B. and Summers K (2005) Evolutionary biology of cancer. Trends in Ecology and Evolution 20, 545–552. [DOI] [PubMed] [Google Scholar]
- Das U and Das A (2000) Review of canine transmissible venereal sarcoma. Veterinary Research Communications 24, 545–556. [DOI] [PubMed] [Google Scholar]
- d'Avella D, Salpietro FM, Alafaci C and Tomasello F (1999) Giant olfactory meningiomas: the pterional approach and its relevance for minimizing surgical morbidity. Skull Base Surgery 9, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker B, Davis BW, Rimbault M, Long AH, Karlins E, Jagannathan V, Reiman R, Parker HG, Drögemüller C, Corneveaux JJ, Chapman ES, Trent JM, Leeb T, Huentelman MJ, Wayne RK, Karyadi DM and Ostrander EA (2015) Comparison against 186 canid whole-genome sequences reveals survival strategies of an ancient clonally transmissible canine tumor. Genome Research 25, 1646–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGregori J (2011) Evolved tumor suppression: why are we so good at not getting cancer? Cancer Research 71, 3739–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lafaille MAC and Lafaille JJ (2009) Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 30, 626–635. [DOI] [PubMed] [Google Scholar]
- Dillman AR and Schneider DS (2015) Defining resistance and tolerance to cancer. Cell Reports 13, 884–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Old LJ and Schreiber RD (2004) The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21, 137–148. [DOI] [PubMed] [Google Scholar]
- Easton DF, Ford D and Bishop DT (1995) Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. American Journal of Human Genetics 56, 265. [PMC free article] [PubMed] [Google Scholar]
- Engelhardt SC, Bergeron P, Gagnon A, Dillon L and Pelletier F (2019) Using geographic distance as a potential proxy for help in the assessment of the grandmother hypothesis. Current Biology 29, 651–656.e653. [DOI] [PubMed] [Google Scholar]
- Epstein BM, Jones M, Hamede R, Hendricks S, McCallum H, Murchison EP, Schönfeld B, Wiench C, Hohenlohe P and Storfer A (2016) Rapid evolutionary response to a transmissible cancer in Tasmanian devils. Nature Communications 7, 12684–12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BB and McFadden G (2006) Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 124, 767–782. [DOI] [PubMed] [Google Scholar]
- Forbes MR (1993) Parasitism and host reproductive effort. Oikos 67, 444–450. [Google Scholar]
- Frampton D, Schwenzer H, Marino G, Butcher LM, Pollara G, Kriston-Vizi J, Venturini C, Austin R, de Castro KF, Ketteler R, Chain B, Goldstein RA, Weiss RA, Beck S and Fassati A (2018) Molecular signatures of regression of the Canine Transmissible Venereal Tumor. Cancer Cell 33, 620–633.e626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby RA, Silva AS, Gillies RJ and Frieden BR (2009) Adaptive therapy. Cancer Research 69, 4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Ménard C, Martin F and Zitvogel L (2006) The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunological Reviews 214, 229–238. [DOI] [PubMed] [Google Scholar]
- Giraudeau M, Sepp T, Ujvari B, Ewald PW and Thomas F (2018) Human activities might influence oncogenic processes in wild animal populations. Nature Ecology and Evolution 2, 1065–1070. [DOI] [PubMed] [Google Scholar]
- Giraudeau M, Sepp T, Ujvari B, Renaud F, Tasiemski A, Roche B, Capp JP and Thomas F (2019) Differences in mutational processes and intra-tumour heterogeneity between organs: the local selective filter hypothesis. Evolution, Medicine, and Public Health 2019, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez CM, Griffey SM, Naydan DK, Flores E, Cepeda R, Cattaneo G and Madewell BR (2000) Canine transmissible venereal tumour: a morphological and immunohistochemical study of 11 tumours in growth phase and during regression after chemotherapy. Journal of Comparative Pathology 122, 241–248. [DOI] [PubMed] [Google Scholar]
- Hamede RK, Bashford J, McCallum H and Jones M (2009) Contact networks in a wild Tasmanian devil (Sarcophilus harrisii) population: using social network analysis to reveal seasonal variability in social behaviour and its implications for transmission of devil facial tumour disease. Ecology Letters 12, 1147–1157. [DOI] [PubMed] [Google Scholar]
- Hamede R, Lachish S, Belov K, Woods G, Kreiss A, Pearse AM, Lazenby B, Jones M and McCallum H (2012) Reduced effect of Tasmanian devil facial tumor disease at the disease front. Conservation Biology 26, 124–134. [DOI] [PubMed] [Google Scholar]
- Hamede RK, McCallum H and Jones M (2013) Biting injuries and transmission of Tasmanian devil facial tumour disease. Journal of Animal Ecology 82, 182–190. [DOI] [PubMed] [Google Scholar]
- Hamede RK, Pearse AM, Swift K, Barmuta LA, Murchison EP and Jones ME (2015) Transmissible cancer in Tasmanian devils: localized lineage replacement and host population response. Proceedings of the Royal Society of London B: Biological Sciences 282, 20151468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL (1990) Behavioral adaptations to pathogens and parasites: five strategies. Neuroscience and Biobehavioral Reviews 14: 273–294. [DOI] [PubMed] [Google Scholar]
- Hasselquist D and Nilsson JA (2012) Physiological mechanisms mediating costs of immune responses: what can we learn from studies of birds? Animal Behaviour 83, 1303–1312. [Google Scholar]
- Hayward AD, Garnier R, Watt KA, Pilkington JG, Grenfell BT, Matthews JB, Pemberton JM, Nussey DH and Graham AL (2014) Heritable, heterogeneous, and costly resistance of sheep against nematodes and potential feedbacks to epidemiological dynamics. The American Naturalist 184, S58–S76. [DOI] [PubMed] [Google Scholar]
- Hochberg ME, Thomas F, Assenat E and Hibner U (2013) Preventive evolutionary medicine of cancers. Evolutionary Applications 6, 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert JN, Zerjal T and Hospital F (2018) Cancer-and behavior-related genes are targeted by selection in the Tasmanian devil (Sarcophilus harrisii). PloS One 13, e0201838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacqueline C, Biro PA, Beckmann C, Moller AP, Renaud F, Sorci G, Tasiemski A, Ujvari B and Thomas F (2017) Cancer: a disease at the crossroads of trade-offs. Evolutionary Applications 10, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Travers P and Walport M (2001) Pathogens have evolved various means of evading or subverting normal host defenses. In Janeway CAJ, Travers P and Walport M (eds), Immunology: The Immune System in Health and Disease. New York, USA: Garland Science, pp. 1–10. [Google Scholar]
- Jones ME, Cockburn A, Hamede R, Hawkins C, Hesterman H, Lachish S, Mann D, McCallum H and Pemberton D (2008) Life-history change in disease-ravaged Tasmanian devil populations. Proceedings of the National Academy of Sciences 105, 10023–10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurić S, Jančuljak D, Tomić S and Štimac D (2013) Brain tissue adaptability to slow-growing tumors: case report of clivus meningioma. Collegium Antropologicum 37, 1011–1014. [PubMed] [Google Scholar]
- Kidd P (2003) Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Alternative Medicine Review 8, 223–246. [PubMed] [Google Scholar]
- Klasing KC (2007) Nutrition and the immune system. British Poultry Science 48, 525–537. [DOI] [PubMed] [Google Scholar]
- Knutson KL and Disis ML (2005) Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunotherapy 54, 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippitz BE 2013. Cytokine patterns in patients with cancer: a systematic review. The Lancet Oncology 14, e218–e228. [DOI] [PubMed] [Google Scholar]
- Lochmiller RL and Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88, 87–98. [Google Scholar]
- Lough GI, Kyriazakis I, Bergmann S, Lengeling A and Doeschl-Wilson A (2015) Health trajectories reveal the dynamic contributions of host genetic resistance and tolerance to infection outcome. Proceedings of the Royal Society of London B: Biological Sciences. 282, 20152151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen T, Arnal A, Vittecoq M, Bernex F, Abadie J, Labrut S, Garcia D, Faugère D, Lemberger K, Beckmann C, Roche B, Thomas F and Ujvari B (2017) Chapter 2: Cancer prevalence and etiology in wild and captive animals. In Ujvari B, Roche B and Thomas F (eds). Ecology and Evolution of Cancer. London: Academic Press, pp. 11–46. [Google Scholar]
- Maher S, Toomey D, Condron C and Bouchier-Hayes D (2002) Activation-induced cell death: the controversial role of Fas and Fas ligand in immune privilege and tumour counterattack. Immunology and Cell Biology 80, 131–137. [DOI] [PubMed] [Google Scholar]
- Maley CC (2007) Multistage carcinogenesis in Barrett's esophagus. Cancer Letters 245, 22–32. [DOI] [PubMed] [Google Scholar]
- Mapara MY and Sykes M (2004) Tolerance and cancer: mechanisms of tumor evasion and strategies for breaking tolerance. Journal of Clinical Oncology 22, 1136–1151. [DOI] [PubMed] [Google Scholar]
- Margres MJ, Ruiz-Aravena M, Hamede R, Jones ME, Lawrance MF, Hendricks SA, Patton A, Davis BW, Ostrander EA and McCallum H (2018) The genomic basis of tumor regression in Tasmanian devils (Sarcophilus harrisii). Genome Biology and Evolution 10, 3012–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C, Flores JM, Viña J, Blasco MA and Serrano M (2007) Delayed ageing through damage protection by the Arf/p53 pathway. Nature 448, 375. [DOI] [PubMed] [Google Scholar]
- Matic M, Lm Jongen J, Elens L, Wildt S, Tibboel D, Ae Sillevis Smitt P and Hn van Schaik R (2017) Advanced cancer pain: the search for genetic factors correlated with interindividual variability in opioid requirement. Pharmacogenomics 18, DOI: 10.2217/pgs-2017-2060. [DOI] [PubMed] [Google Scholar]
- Mazé-Guilmo E, Loot G, Páez DJ, Lefèvre T and Blanchet S (2014) Heritable variation in host tolerance and resistance inferred from a wild host-parasite system. Proceedings of the Royal Society of London B: Biological Sciences 281, 20132567–20132567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham CE and Morrison SJ (2013) Tumour heterogeneity and cancer cell plasticity. Nature 501, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Schneider DS and Soares MP (2012) Disease tolerance as a defense strategy. Science 335, 936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengelbier LH, Karlsson J, Lindgren D, Valind A, Lilljebjörn H, Jansson C, Bexell D, Braekeveldt N, Ameur A and Jonson T (2015) Intratumoral genome diversity parallels progression and predicts outcome in pediatric cancer. Nature Communications 6, 6125. [DOI] [PubMed] [Google Scholar]
- Minchella DJ and Loverde PT (1981) A cost of increased early reproductive effort in the snail Biomphalaria glabrata. The American Naturalist 118, 876–881. [Google Scholar]
- Mukaratirwa S and Gruys E (2003) Canine transmissible venereal tumour: cytogenetic origin, immunophenotype, and immunobiology. A review. Veterinary Quarterly 25, 101–111. [DOI] [PubMed] [Google Scholar]
- Mumoli N, Pulerà F, Vitale J and Camaiti A (2013) Frontal lobe syndrome caused by a giant meningioma presenting as depression and bipolar disorder. Singapore Medical Journal 54, e158–e159. [DOI] [PubMed] [Google Scholar]
- Murchison EP, Schulz-Trieglaff OB, Ning Z, Alexandrov LB, Bauer MJ, Fu B, Hims M, Ding Z, Ivakhno S and Stewart C (2012) Genome sequencing and analysis of the Tasmanian devil and its transmissible cancer. Cell 148, 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison EP, Wedge DC, Alexandrov LB, Fu B, Martincorena I, Ning Z, Tubio JMC, Werner EI, Allen J, De Nardi AB, Donelan EM, Marino G, Fassati A, Campbell PJ, Yang F, Burt A, Weiss RA and Stratton MR (2014) Transmissible dog cancer genome reveals the origin and history of an ancient cell lineage. Science 343, 437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia CL, Pritchard K, Kim SY, Fassati A and Weiss RA (2006) Clonal origin and evolution of a transmissible cancer. Cell 126, 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WG, De Marzo AM and Isaacs WB (2003) Prostate cancer. New England Journal of Medicine 349, 366–381. [DOI] [PubMed] [Google Scholar]
- Norris K and Evans MR (2000) Ecological immunology: life history trade-offs and immune defense in birds. Behavioral Ecology 11, 19–26. [Google Scholar]
- Nunney L (2018) Size matters: height, cell number and a person's risk of cancer. Proceedings of the Royal Society B: Biological Sciences 285, 20181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenauf AC and Massagué J (2015) Surviving at a distance: organ specific metastasis. Trends in Cancer 1, 76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander EA, Davis BW and Ostrander GK (2016) Transmissible tumors: breaking the cancer paradigm. Trends in Genetics 32, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawełczyk A, Łojek E, Rabe-Jabłotiska J, Pawełczyk T, Godlewski B and Radek M (2012) Depression or apathy? A diagnostic trap: a huge right frontal lobe meningioma diagnosed and treated as mild atypical depression episode – a case study. Psychiatria Polska 46, 903–913. [PubMed] [Google Scholar]
- Polak M and Starmer WT (1998) Parasite–induced risk of mortality elevates reproductive effort in male Drosophila. Proceedings of the Royal Society of London B: Biological Sciences 265, 2197–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribluda A, Cecile C and Jackson EL (2015) Intratumoral heterogeneity: from diversity comes resistance. Clinical Cancer Research 21, 2916–2923. [DOI] [PubMed] [Google Scholar]
- Pye RJ, Pemberton D, Tovar C, Tubio JMC, Dun KA, Fox S, Darby J, Hayes D, Knowles GA, Kreiss A, Siddle HVT, Swift K, Lyons AB, Murchison EP and Woods GM (2016) A second transmissible cancer in Tasmanian devils. Proceedings of the National Academy of Sciences 113: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L, Sim D and Read AF (2007) Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318, 812. [DOI] [PubMed] [Google Scholar]
- Råberg L, Graham AL and Read AF (2009) Decomposing health: tolerance and resistance to parasites in animals. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences 364, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Gibby CC, Shete S, Rakvåg T, Bhat SV, Skorpen F, Bruera E, Kaasa S and Klepstad P (2007) Exploring joint effects of genes and the clinical efficacy of morphine for cancer pain: OPRM1 and COMT gene. Pain 130, 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerink SF, Sasaki N, Lee-Six H, Young MD, Alexandrov LB, Behjati S, Mitchell TJ, Grossmann S, Lightfoot H and Egan DA (2018) Intra-tumour diversification in colorectal cancer at the single-cell level. Nature 556, 457. [DOI] [PubMed] [Google Scholar]
- Roy BA and Kirchner JW (2000) Evolutionary dynamics of pathogen resistance and tolerance. Evolution 54, 51–63. [DOI] [PubMed] [Google Scholar]
- Ruiz-Aravena M, Jones M, Carver S, Estay S, Espejo C, Storfer A and Hamede RK (2018) Sex bias in ability to cope with cancer: Tasmanian devils and facial tumour disease. Proceedings of the Royal Society B: Biological Sciences 285, 20182239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadd BM and Schmid-Hempel P (2009) Principles of ecological immunology. Evolutionary Applications 2, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ortiz RF, Troncoso P, Babaian RJ, Lloreta J, Johnston DA and Pettaway CA (2006) African-American men with nonpalpable prostate cancer exhibit greater tumor volume than matched white men. Cancer: Interdisciplinary International Journal of the American Cancer Society 107, 75–82. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P (2011) Evolutionary Parasitology: The Integrated Study of Infections, Immunology, Ecology, and Genetics. Oxford [England]; New York: Oxford University Press. [Google Scholar]
- Sheldon BC and Verhulst S (1996). Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends in Ecology and Evolution 11, 317–321. [DOI] [PubMed] [Google Scholar]
- Siddle HV and Kaufman J (2013) A tale of two tumours: comparison of the immune escape strategies of contagious cancers. Molecular Immunology 55, 190–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddle HV and Kaufman J (2015) Immunology of naturally transmissible tumours. Immunology 144, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Hanson HA, Mineau GP and Buys SS (2011) Effects of BRCA1 and BRCA2 mutations on female fertility. Proceedings of the Royal Society of London B: Biological Sciences 279, 1389–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorci G, Clobert J and Michalakis Y (1996) Cost of reproduction and cost of parasitism in the common lizard, Lacerta vivipara. Oikos 76, 121–130. [Google Scholar]
- Thomas F, Nesse R, Gatenby R, Gidoin C, Renaud F and Roche B (2016) Evolutionary ecology of organs: a missing link in cancer development? Trends in Cancer 2, 409–415. [DOI] [PubMed] [Google Scholar]
- Thomas F, Jacqueline C, Tissot T, Henard M, Blanchet S, Loot G, Dawson E, Mery F, Renaud F, Montagne J, Beckmann C, Biro PA, Hamede R and Ujvari B (2017) The importance of cancer cells for animal evolutionary ecology. Nature Ecology and Evolution 1, 1592–1595. [DOI] [PubMed] [Google Scholar]
- Thomas F, Donnadieu E, Charriere GM, Jacqueline C, Tasiemski A, Pujol P, Renaud F, Roche B, Hamede R, Brown J, Gatenby R and Ujvari B (2018a) Is adaptive therapy natural? PLOS Biology 16, e2007066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas F, Vavre F, Tissot T, Vittecoq M, Giraudeau M, Bernex F, Misse D, Renaud F, Raven N, Beckmann C, Hamede R, Biro PA and Ujvari B (2018b) Cancer is not (only) a senescence problem. Trends in Cancer 4, 169–172. [DOI] [PubMed] [Google Scholar]
- Tollis M, Robbins J, Webb AE, Kuderna LFK, Caulin AF, Garcia JD, Bèrubè M, Pourmand N, Marques-Bonet T, O'Connell MJ, Palsbøll PJ and Maley CC (2019) Return to the sea, get huge, beat cancer: an analysis of cetacean genomes including an assembly for the humpback whale (Megaptera novaeangliae). Molecular Biology and Evolution 36, 1746–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki T, Kakegawa T, Arimori M, Ueda M, Watanabe H, Okamoto T and Akakura I (1971) Giant leiomyoma of the esophagus and cardia weighing more than 1000 grams. Chest 60, 396–399. [DOI] [PubMed] [Google Scholar]
- Tuna M, Grocer AI, Gezercan Y, Vural A, Ildan F, Haciyakupoglu S and Karadayi A (1999) Huge meningiomas: a review of 93 cases. Skull Base Surgery 9, 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujvari B, Gatenby RA and Thomas F (2016a) The evolutionary ecology of transmissible cancers. Infection, Genetics and Evolution 39, 293–303. [DOI] [PubMed] [Google Scholar]
- Ujvari B, Hamede R, Peck S, Pemberton D, Jones M, Belov K and Madsen T (2016b) Immunoglobulin dynamics and cancer prevalence in Tasmanian devils (Sarcophilus harrisii). Scientific Reports 6, 25093–25093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Most PJ, de Jong B, Parmentier HK and Verhulst S (2011) Trade-off between growth and immune function: a meta-analysis of selection experiments. Functional Ecology 25, 74–80. [Google Scholar]
- van Niekerk G, Loos B, Nell T and Engelbrecht AM (2016) Cancer tolerance, resistance, pathogenicity and virulence: deconstructing the disease state. Future Oncology 12, 1369–1380. [DOI] [PubMed] [Google Scholar]
- Van Valen L (1973) A new evolutionary law. Evolutionary Theory 1, 1–30. [Google Scholar]
- Vézilier J, Nicot A, Gandon S and Rivero A (2015) Plasmodium infection brings forward mosquito oviposition. Biology Letters 11, 20140840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittecoq M, Giraudeau M, Sepp T, Marcogliese DJ, Klaassen M, Renaud F, Ujvari B and Thomas F (2018) Turning natural adaptations to oncogenic factors into an ally in the war against cancer. Evolutionary Applications 11, 836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells K, Hamede RK, Kerlin DH, Storfer A, Hohenlohe PA, Jones ME and McCallum HI (2017) Infection of the fittest: devil facial tumour disease has greatest effect on individuals with highest reproductive output. Ecology Letters 20, 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek G, Asemissen A, Model F, Turbachova I, Floess S, Liebenberg V, Baron U, Stauch D, Kotsch K and Pratschke J (2009) Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Research 69, 599–608. [DOI] [PubMed] [Google Scholar]
- Wright B, Willet C, Hamede R, Jones M, Belov K and Wade C (2017) Variants in the host genome may inhibit tumour growth in devil facial tumours: evidence from genome-wide association. Scientific Reports 7, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. J. 1988. Immunobiology of a spontaneously regressive tumor, the canine transmissible venereal sarcoma (review). Anticancer Research 8, 93–95. [PubMed] [Google Scholar]
- Zhang W, Edwards A, Flemington EK and Zhang K (2017) Racial disparities in patient survival and tumor mutation burden, and the association between tumor mutation burden and cancer incidence rate. Scientific Reports 7, 13639. [DOI] [PMC free article] [PubMed] [Google Scholar]