Abstract
Current systematic review and meta-analysis demonstrate the prevalence reports of filariasis in animals in Iran along with human cases. Studies were screened, relevant papers were selected and the random-effect model was used by forest plot with 95% confidence interval (CI). Of 17 records of human case-reports, particularly from Khuzestan province (5 cases), Dirofilaria repens was the most detected parasite (10 cases) with higher involvement of the right eye (7 cases) than other organs. Eleven animal species were reported to be parasitised by filarioids in Iran. The prevalence of Dirofilaria immitis in canids was 14.69% (95% CI: 10.33–19.67), with highest rates (20.92%; 95% CI: 13.84–29.03) in free-ranging dogs. Male (10.07%; 95% CI: 5.10–16.47) and more than 1-year old (20.77%; 95% CI: 8.66–36.42) dogs were more likely to be found infected. The frequency of other filarioids of zoonotic interest was: Acanthocheilonema reconditum in dogs 2.15% (95% CI: 0.71–4.33), Dipetalonema evansi in camels 10.16% (95% CI: 4.73–17.34), Onchocerca cervicalis in horses 3.63% (95% CI: 1.44–6.75%) and Onchocerca fasciata 16.57% (95% CI: 10.12–24.24%) in camels. Still, our knowledge on parasitic filariae in Iran is limited and more investigation is needed in both human and animal populations.
Key words: Diversity, Filarioids, Iran, meta-analysis, systematic review
Introduction
Filarial nematodes (Spirurida, Onchocercidae) are parasitic helminths, which produce motile microfilariae (mfs), as first-stage larva (L1) in their vertebrate definitive hosts, which subsequently develop into the third-stage larvae (L3) in blood-feeding arthropods as their intermediate hosts and biological vectors (Orihel and Eberhard, 1998; Otranto and Deplazes, 2019). Adult filarial worms dwell in host's blood vessels, body cavities, lymphatic ducts and/or connective tissues (Chatterjee and Nutman, 2015). Parasitic filarioids have been isolated from most vertebrate species, except fish, but only those species found in mammals have shown to represent a zoonotic threat to human populations (Anderson, 2000). Nonetheless, humans are mainly affected by Wuchereria bancrofti and, to a lesser extent, Brugia malayi and Brugia timori causing the lymphatic filariasis in about 68 million people in 73 countries worldwide, as well as onchocerciasis due to Onchocerca volvulus, the causative agent of river blindness, which affects 40 million people mostly in Africa (Taylor et al., 2010; WHO, 2015; Tekle et al., 2016). The global health burden for both human filarial infections is approximately 3.3 million disability-adjusted life-years (DALYs) (Kwarteng et al., 2016). While the species above are typically anthroponotic and mainly spread in developing countries, filarioids of the genus Dirofilaria, particularly Dirofilaria immitis (D. immitis) and D. repens, cause human cases worldwide such as in the USA (Orihel and Eberhard, 1998; Theis, 2005) and Europe (Simón et al., 2012), highlighting their role as an emerging zoonosis for humans (Simón et al., 2012).
In recent years, the number of human cases associated with various filarial nematodes, particularly Onchocerca and Dirofilaria spp. have been increased in Iran (Jamshidi et al., 2008; Tavakolizadeh and Mobedi, 2009; Ashrafi et al., 2010; Mowlavi et al., 2014; Maraghi et al., 2016; Mirahmadi et al., 2017; Tabatabaei et al., 2017), due to environment changes and changing agricultural practices, leading to dwelling in the vicinity of arthropod vectors and increased contact between pet and wildlife animal species (Otranto et al., 2013; Otranto and Deplazes, 2019). This implicates the need for more focused studies regarding the prevalence, diversity and bioecological behaviour of such vector-borne diseases, particularly among different animal species (e.g. canids and herbivores) in the country.
Altogether, our better understanding of parasitic filarioids would be beneficial to alert the public and to avert cases of zoonotic human filariasis. In addition, several original prevalence studies on animal filariasis (Oryan et al., 2008; Alborzi et al., 2010; Akhtardanesh et al., 2011; Sazmand et al., 2013; Khodabakhsh et al., 2016; Sazmand et al., 2016; Zarei et al., 2016; Solgi et al., 2018; Anvari et al., 2019) along with various human case-reports in Iran (Negahban et al., 2007; Mowlavi et al., 2014; Maraghi et al., 2015; Mirahmadi et al., 2017; Nabie et al., 2017) highlights the importance of a review of the literature to better elucidate the filariae fauna in the country. Therefore, the current systematic review with meta-analysis was designed to assess the human filariasis case reports as well as prevalence and diversity of animal filarial nematodes in Iran.
Methods
This systematic review was accomplished on the basis of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (Moher et al., 2009).
Information sources and search
The systematic searching procedure was performed without time limitation until 1 December 2019. All articles on the prevalence of filarial nematodes in animals in Iran were retrieved via major English (Pubmed, Scopus, Web of Science and Google Scholar) and Persian databases (Scientific information database (SID), Magiran, Iran Medex, Iran Doc) using medical subject heading (MeSH) terms comprising: (‘Prevalence’, OR ‘epidemiology’), AND (‘Dirofilaria’, ‘Onchocerca’, ‘Acanthocheilonema’, ‘Dipetalonema’, ‘Cercopithifilaria’ ‘Litomosoides’, ‘Stephanofilaria’, ‘Suifilaria’, ‘Parafilaria’, ‘Brugia’, ‘Mansonella’, ‘Setaria’, ‘Elaephora’, ‘(Eulimdana) Pelecitus’, ‘Chandlerella’, ‘Cardiofilaria’) AND (‘Iran’). Also, keywords required for searching infection in human individuals was: ‘Filariasis’, ‘Filarial’, ‘Infection’, ‘Human cases’, ‘Case-report’, ‘Iran’. All searches were conducted in both English and Persian languages.
Eligibility criteria, study selection and data collection
Animal prevalence studies based on blood microscopy, necropsy, serology, Knott's test and/or histopathologic methods were eligible to undergo meta-analysis, whereas human case-reports regarding filarial infections were only included in the systematic review section. Those papers without full-text accessibility were excluded and any contradiction in the study selection process was resolved by discussion and consensus. The whole searching and extraction procedures were done by an expert researcher, and then double-checked by other colleagues.
Statistical analysis
Point estimates and their 95% confidence intervals of pooled prevalence of all included studies were calculated. Forest plots were used to visualize the heterogeneity among the included studies. The heterogeneity index among the included studies was determined using I2 and Cochrane Q tests to show the variation in study outcomes between individual studies (Higgins et al., 2003). The subgroup analysis was conducted according to year, host, location, gender, age and diagnostic method. Egger test was used to check for the presence of publication bias. This bias distorts the results and, when present, published studies are no longer a representative sample of the available evidence (Egger et al., 1997). P value less than 0.05 was considered statistically significant. All analytical functions were applied by Stata/s.e. software version 12.0 (StataCorp, College Station, TX 77845, USA).
Results
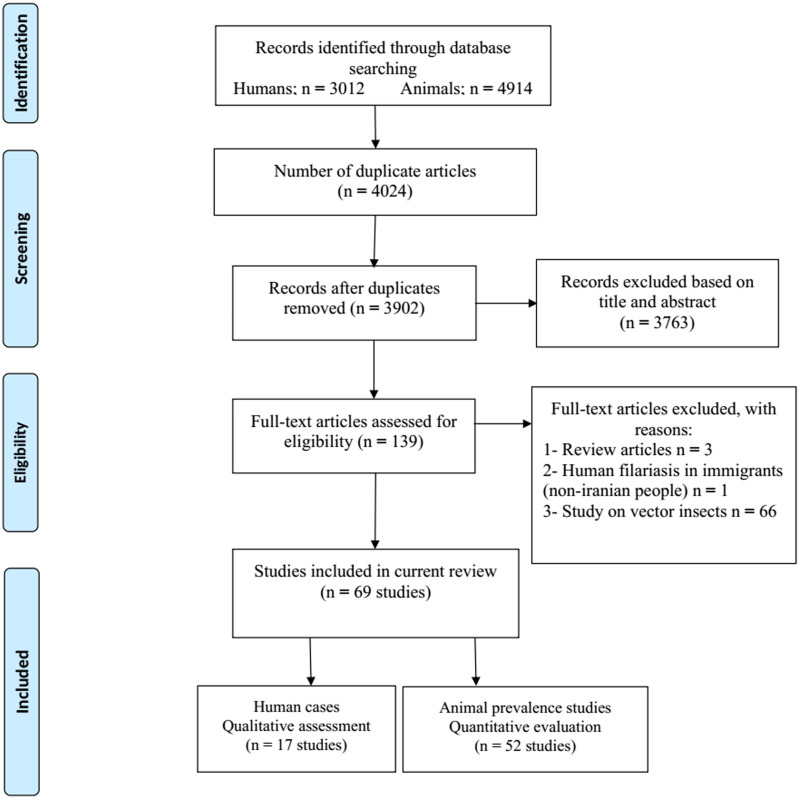
We analysed 3012 papers from database searching, while 2561 were irrelevant (based on their title/abstract, were review papers and/or study on a non-Iranian immigrant), 450 were excluded for duplication, and one article was omitted due to lack of proper diagnosis. Finally, 17 entries relevant to case reports of human filariasis in Iran met our inclusion criteria (Fig. 1). According to Table 1, the cases were reported from 9 provinces of Iran, mostly caused by D. repens. The routine diagnosis was based on microscopic identification of the worm and/or examination of histopathologic sections; only three studies used polymerase chain reaction (PCR) and sequencing methods for molecular evaluation. The right eye was the most parasitised organ (7 cases), followed by left eye (2 cases), as well as single cases in the lower eyelid, cheek, forehead, spermatic cord, breast, chest, forearm and thigh. Cases were reported more frequently in men than women (64.7% vs 35.29%), and among adults than children (82.35% vs 17.64).
Fig. 1.
PRISMA flow diagram describing included/excluded studies up to 1 December 2019 (Moher et al., 2009)
Table 1.
Characteristics of human filariasis case report studies in Iran up to 1 December 2019
| Reference | Sex/ age/ city or province/ year of report/ ref | Symptoms/signs | Genus and species of parasite | diagnostic methods | Outcome |
|---|---|---|---|---|---|
| Subcutaneous Dirofilariasis | |||||
| Ashrafi et al. (2010) | F/ 39 y/Rasht, Guilan/ 2010/ |
|
Dirofilaria repens |
|
Cure |
| Subcutaneous Dirofilariasis | |||||
| Negahban et al. (2007) | M/ 40 y/ Shiraz, Fars/ 2007 |
|
Dirofilaria repens |
|
Cure |
| Subcutaneous Dirofilariasis | |||||
| Athari (2003) | M/ 22 y/ Chalus, Mazandaran/ 2003/ |
|
Dirofilaria repens |
|
Cure |
| Subcutaneous Dirofilariasis | |||||
| Maraghi et al. (2006) | M/ 34 y/ Ahvaz, Khuzestan/ 2006/ |
|
Dirofilaria repens |
|
Cure |
| Subcutaneous Dirofilariasis | |||||
| Maraghi et al. (2006) | M/ 37 y/ Ahvaz, Khuzestan/ 2006/ |
|
Dirofilaria repens |
|
Cure |
| Ocular Dirofilariasis | |||||
| Jamshidi et al. (2008) | M/ 49 y/ Tehran, Tehran/ 2010/ |
|
Dirofilaria repens |
|
NR |
| Ocular Dirofilariasis | |||||
| Mirahmadi et al. (2017) | M/ 2 y/ Chabahar, Sistan & Baluchistan/ 2017/ |
|
Dirofilaria immitis |
|
Cure |
| Ocular Dirofilariasis | |||||
| Rouhani and Athari (2003) | M/ 20 y/ Mazandaran/ 2003/ |
|
Dirofilaria, but the species could not be identified | Microscopically; the presence of longitudinal cuticular ridges, a thick muscle cell layer, the presence of internal organs, consisting of intestine and reproductive organs and the presence of nuclei per histological section in the lateral cord, led to identify the specimen as a Dirofilaria, without species identification. | NR |
| Ocular onchocerciasis | |||||
| Mowlavi et al. (2014) | M/ 20 y/ Qom, Qom/ 2013/ |
|
Onchocerca lupi |
|
Cure |
| Orbital Dirofilariasis | |||||
| Tavakolizadeh and Mobedi (2009) | F/ 24 y/ Tehran, Tehran/ 2009 |
|
Dirofilaria repens |
|
Cure |
| Ophthalmic Dirofilariasis | |||||
| Maraghi et al. (2016) | F/ 54 y/ Abadan, Khuzestan/ 2016/ |
|
Dirofilaria repens |
|
NR |
| Subconjunctival Dirofilariasis | |||||
| Tabatabaei et al. (2017) | M/ 59 y/ Tabriz, East Azerbaijan/ 2017/ |
|
Dirofilaria immitis |
|
Cured, except for faint temporal conjunctival scar. |
| Subconjunctival Dirofilariasis | |||||
| Maraghi et al. (2006) | M/ 35 y/ Ahvaz, Khuzestan/ 2006/ |
|
Dirofilaria repens |
|
Cure |
| Subconjunctival Setariasis | |||||
| Nabie et al. (2017) | F/ 15 y/ Tabriz, East Azerbaijan/ 2017/ |
|
Setaria equina |
|
Cure |
| Periocular Dirofilariasis | |||||
| Jamshidi et al. (2008) | F/ 27 y/ Bandar Abbas, Hormozgan/ 2008 |
|
The worm was diagnosed as, likelihood, Dirofilaria immitis |
|
Cure |
| Dirofilaria in Hydrocele | |||||
| Salahi-Moghadam and Banihashemi (2016) | M/ 5 y/ Bandar Abbas, Hormozgan/ 2016/ |
|
Dirofilaria immitis |
|
Cure |
| Breast Dirofilariasis | |||||
| Maraghi et al. (2015) | F/ 40 y/ Abadan, Khuzestan/ 2015/ |
|
Dirofilaria repens |
|
Cure |
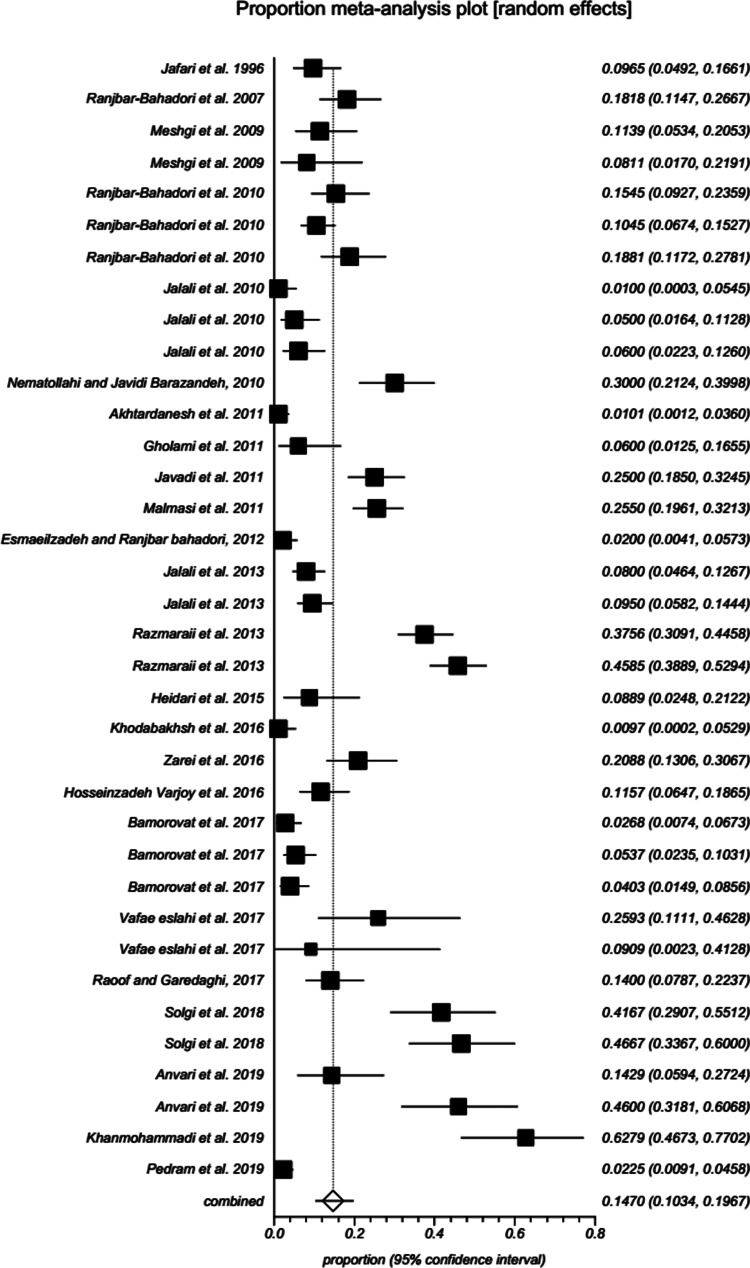
Information related to Filarioidea spp identified in various animal hosts (camel, cattle, small ruminants, dog, wild canids (jackals and red fox), horse, donkey, rodent, cat and pigeon) in Iran was illustrated as Supplementary Table 1. The systematic searching for original prevalence studies in animal hosts yielded 52 eligible papers based on our inclusion/exclusion criteria (Fig. 1). According to random-effects model meta-analysis, the pooled prevalence of D. immitis in canids of Iran was 14.69% (95% CI: 10.33–19.67) (Table 2; Supplementary Table 2; Fig. 2). Subgroup analysis was performed with summarized details in Table 3. Year-based analysis demonstrated a higher prevalence trend of D. immitis in published literature beyond 2011 [16.25% (95% CI: 10.23–23.35)] than those before 2011 [11.58% (95% CI: 7.37–16.60)] (P < 0.001). The higher prevalence was detected in free-ranging dogs (20.92%; 95% CI: 13.84–29.03), followed by wild canids (jackals and red fox) (10.72%; 95% CI: 6.59–15.70) and owned dogs (6.61%; 95% CI: 3.46–10.68) (P < 0.001). Geographical distribution showed the highest and lowest prevalence rates in western [25.29% (95% CI: 14.44–37.99)] and southern [6.10% (95% CI: 3.40–9.51)] parts of Iran, respectively (P < 0.001). Based on gender of dogs in Iran, D. immitis was more prevalent in males (10.07%; 95% CI: 5.10–16.47) than females (9.23%; 95% CI: 3.68–16.97) (P = 0.131). The heartworm was more prevalent in animals equal or more than 1-year old (20.77%; 95% CI: 8.66–36.42) than those younger (8.40%; 95% CI: 0.01– 32.00) (P = 0.006). According to the diagnostic method, the pooled prevalence of D. immitis in canids of Iran was as follows: microscopic 13.86% (95% CI: 9.46– 18.94), PCR 16.09% (95% CI: 3.51–35.38) and serology 16.95% (95% CI: 4.98–34.08) (P = 0.01).
Table 2.
Prevalence, publication bias, and heterogeneity of Dirofilaria immitis, Dipetalonema spp, Onchocera spp, Setaria spp and Parafilaria multipapillosa in animal hosts in Iran up to 1 December 2019
| Species | Host | Prevalence, % (95% CI) | Cochran Q | df | I2 (%) | P value | Egger bias | P value |
|---|---|---|---|---|---|---|---|---|
| D. immitis | Canids | 14.69 (10.33–19.67) | 661.2 | 35 | 94.7% | P < 0.001 | 3.0 | 0.266 |
| D. evansi | Camel | 10.16 (4.73–17.34) | 339.4 | 10 | 97.1% | P < 0.001 | 9.0 | 0.010 |
| D. reconditum | Dog | 2.15 (0.71–4.33) | 6.3 | 3 | 52.7% | P = 0.095 | 1.9 | 0.050 |
| O. cervicalis | Horse, Donkey | 3.63 (1.44–6.75) | 0.1 | 1 | – | P = 0.658 | – | – |
| O. fasciata | Camel | 16.57 (10.12–24.24) | 60.8 | 6 | 90.1 | P < 0.001 | 5.8 | 0.009 |
| Setaria equina | Horse, Donkey | 12.15 (0.04–40.70) | 16.8 | 1 | – | P < 0.001 | – | – |
| Setaria spp | Cattle | 45.47 (14.45–78.61) | 2677.7 | 5 | 99.8 | P < 0.001 | −50.4 | 0.016 |
| P. multipapillosa | Horse, Donkey | 5.85 (3.75–8.37) | 5.9 | 3 | 49.7% | P = 0.113 | −2.0 | 0.164 |
Fig. 2.
Forest plot of the prevalence of D. immitis in canids of Iran up to 1 December 2019. A square is appointed to each individual study with a horizontal line as confidence intervals and the area of each square is proportional to the study's weight in the meta-analysis. Also, a diamond is assigned to the meta-analysed measure of effect. A vertical line representing no effect is also plotted. If the confidence intervals for individual studies overlap with this line, it demonstrates that at the given level of confidence their effect sizes do not differ from no effect for the individual study.
Table 3.
Prevalence of Dirofilaria immitis in canines according to year, host, location, gender, age and diagnostic method in Iran up to 1 December 2019
| Subgroup variable | Prevalence (95% CI) | I2 (%) | Heterogeneity (Q) | P value | Interaction test (X2) | P value |
|---|---|---|---|---|---|---|
| Year | ||||||
| <2011 | 11.58 (7.37–16.60) | 84% | 62.5 | P < 0.001 | 177.1 | P < 0.001 |
| ⩾2011 | 16.25 (10.23–23.35) | 96% | 596.4 | P < 0.001 | ||
| Host | ||||||
| Wild Canines | 10.72 (6.59–15.70) | 0% | 0.2 | P = 0.963 | 123.9 | P < 0.001 |
| Owned dogs | 6.61 (3.46–10.68) | 88.1% | 84.2 | P < 0.001 | ||
| Stray dogs | 20.92 (13.84–29.03) | 95.6% | 453.7 | P < 0.001 | ||
| Location | ||||||
| North | 13.59 (7.29–21.46) | 88.3% | 59.6 | P < 0.001 | 123.1 | P < 0.001 |
| South | 6.10 (3.40–9.51) | 65.1% | 11.4 | P = 0.021 | ||
| East | 9.03 (3.15–17.52) | 92% | 74.5 | P < 0.001 | ||
| West | 25.29 (14.44–37.99) | 95.4% | 173.1 | P < 0.001 | ||
| Centre | 20.60 (5.93–41.13) | 96.7% | 121.1 | P < 0.001 | ||
| Gender | ||||||
| Male | 10.07 (5.10–16.47) | 87.9% | 99.4 | P < 0.001 | 89.1 | P = 0.131 |
| Female | 9.23 (3.68–16.97) | 87.3% | 86.9 | P < 0.001 | ||
| Age | ||||||
| <1 Year old | 8.40 (0.01– 32.00) | 77% | 13.0 | P = 0.004 | 10.8 | P = 0.006 |
| ⩾1 Year old | 20.77 (8.66–36.42) | 90.1% | 40.5 | P < 0.001 | ||
| Diagnostic method | ||||||
| Serological | 16.95 (4.98–34.08) | 96.6% | 146.9 | P < 0.001 | 110.0 | P = 0.010 |
| Microscopic | 13.86 (9.46– 18.94) | 92% | 286.0 | P < 0.001 | ||
| PCR | 16.09 (3.51–35.38) | 97.7% | 215.8 | P < 0.001 | ||
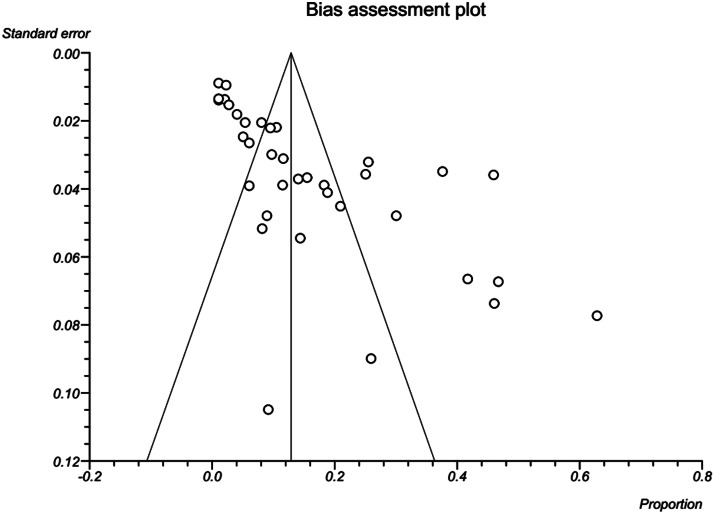
Results revealed a strong significant heterogeneity (Q = 661.2, df = 35, I2 = 94.7%, P < 0.001) among the selected studies. Subgroup analysis revealed that there were statistically significant differences between the overall prevalence of D. immitis in canids of Iran and year (X2 = 177.1, P < 0.001), host (X2 = 123.9, P < 0.001), location (X2 = 123.1, P < 0.001), age (X2 = 10.8, P = 0.006) and diagnostic method (X2 = 110.0, P = 0.010). Publication bias was checked by Egger's regression test, showed that it may not have a substantial impact on total prevalence estimate (Egger; bias: 3.0, P = 0.266) (Fig. 3).
Fig. 3.
A bias assessment plot from Egger for the prevalence of D. immitis in canids of Iran up to 1 December 2019. In the absence of publication bias, it assumes that studies with high precision will be plotted near the average, and studies with low precision will be spread evenly on both sides of the average, creating a roughly funnel-shaped distribution. Deviation from this shape can indicate publication bias.
Based on the random-effects model meta-analysis, the pooled prevalence, publication bias and heterogeneity of other filarioids in Iran are depicted in Table 2. Furthermore, Table 4 demonstrates the subgroup analysis of Dipetalonema evansi (D. evansi) and Onchocerca fasciata (O. fasciata) in camels of Iran (Supplementary Figs 1–7).
Table 4.
Subgroup analysis of Dipetalonema evansi and Onchocerca fasciata in camels of Iran up to 1 December 2019
| Subgroup variable | Prevalence (95% CI) | I2 (%) | Heterogeneity (Q) | P value | Interaction test (X2) | P value |
|---|---|---|---|---|---|---|
| Dipetalonema evansi | ||||||
| Gender | ||||||
| Male | 10.62 (3.13–21.82) | 92.2% | 25.7 | P < 0.001 | 2.9 | P = 0.084 |
| Female | 8.01 (5.00–11.64) | 0% | 0.2 | P = 0.903 | ||
| Diagnostic method | ||||||
| Microscopic examination | 9.94 (2.63–21.21) | 97.1% | 172.4 | P < 0.001 | 13.2 | P < 0.001 |
| Modified Knott test | 7.24 (0.15–23.46) | 98.4% | 127.1 | P < 0.001 | ||
| Onchocerca fasciata | ||||||
| Gender | ||||||
| Male | 11.52 (4.56–21.12) | 80.9% | 10.4 | P = 0.005 | 4.4 | P = 0.035 |
| Female | 18.54 (8.77–30.91) | 84.2% | 12.6 | P = 0.001 | ||
| Diagnostic method | ||||||
| Microscopic examination | 18.56 (9.87–29.23) | 84.2% | 12.6 | P = 0.001 | 6.1 | P = 0.013 |
| Histopathologic methods | 15.28 (6.54–26.84) | 92.7% | 41.1 | P < 0.001 | ||
Discussion
Nowadays, the complex interaction between humans and animals has subjected to revolutionary changes in several aspects including human behaviour, demographics, land use and environment changes (Otranto and Deplazes, 2019). This also leads to an unprecedented encounter between humans and infectious agents and the problem of the emergence of infectious zoonotic diseases. Regarding filariasis, this occurs in those areas of the world where insect vectors inhabit, particularly in developing countries, inflicting significant threat to human and animal health (Thompson et al., 2010). With respect to the importance of this issue and increased number of human cases in recent years, we sought to extend our knowledge on poorly-known filarial infections in the human and examined animals in a vast Middle Eastern country, Iran.
Regarding human cases, most reports were from Khuzestan province, southwestern Iran. This territory is located in the vicinity of the Persian Gulf and possesses favourable milieu such as abundant dams and lagoons as well as tropical temperatures throughout the year, required for the colonization of blood-sucking arthropod vectors (Hamidinia et al., 2016). The eyes or the conjunctiva are a frequent site of choice for filarioids (Otranto and Eberhard, 2011), as we also found most Iranian cases in right and left eyes, respectively. Dirofilariasis due to Dirofilaria spp. were the most abundant filarial infection in humans in Iran. Despite remarkable seroprevalence of antibodies to Dirofilaria in human societies of endemic areas (Simon et al., 1991; Espinoza et al., 1993; Vieira et al., 1998; Tasić-Otašević et al., 2014), human dirofilariasis is underdiagnosed, though the substantial increase in cases has been recorded worldwide mainly as subcutaneous/ocular form (Simón et al., 2012). In the current review, it was realized that D. repens was the most isolated species, commonly from subcutaneous tissue and right eye. Iranian cases with D. immitis had an eye infection as well as an interesting case removed from the spermatic cord in relation to testicular hydrocele (Salahi-Moghadam and Banihashemi, 2016). There was only a single case of intraocular involvement (anterior chamber/vitreous body) among Iranian dirofilariasis cases (Mirahmadi et al., 2017), similar to some cases from other countries including Turkey and Brazil (Gungel et al., 2009; Otranto et al., 2011a). Cases of human pulmonary dirofilariasis, which has been predominantly reported from Japan and USA (Simón et al., 2012), were not detected in Iran, which could be due to lack of available serological assessment, though clinicians should be alerted to it (Khedri et al., 2014). In an international scale, human infections by D. repens are prevalent in Eurasia region, with the highest incidences of subcutaneous/ocular dirofilariasis occurring in Europe particularly in Russia and Italy (Simón et al., 2012). In Asia, Sri Lanka and India are predominant regarding subcutaneous/ocular dirofilariasis (Simón et al., 2017). Subcutaneous nodules due to D. repens usually emerge over a period of weeks or months, with rigid, elastic solidity (Simón et al., 2012). Approximately, 30–35% of D. repens-related infections involve ocular sites, entailing considerable consequences such as floaters, damaged vision, glaucoma, crystalline lens, the opacity of the vitreous humor and blepharedema (Pampiglione and Rivasi, 2000; Stringfellow et al., 2002). Altogether, the spread of human dirofilariasis due to D. repens may be the result of vector habitat outreach, impaired immune responses to subcutaneous parasites and inadequate diagnosis and therapy in the primary hosts, i.e. dogs (Otranto and Deplazes, 2019).
Among Iranian case reports, subconjunctival infection to Onchocerca lupi (O. lupi) in a 20-year-old male commuter inhabiting Qom province was interesting, since it was the first human case of Onchocerca infection in the country (Mowlavi et al., 2014). Mowlavi et al. mentioned that the patient with multiple parasitic nodules in his eye may have got infected by blackflies in northern Tehran or Culicoides species in Qom suburbs (Mowlavi et al., 2014). Onchocerca lupi was first described in a wolf (Canis lupus cubanensis) in Russia in 1967 (Rodonaja, 1967). Otranto et al., in 2011 confirmed the first certain case of ocular O. lupi in a human by morphological and molecular analysis in Turkey where there was no report of canine onchocerciasis before (Otranto et al., 2011b, 2012). Based on a published systematic review, there have been increasing reports of human infections with O. lupi, especially during last decade, raising the zoonotic potential of this nematode in Europe, Middle East and USA (Grácio et al., 2015). Although ocular cases are more common, O. lupi has occasionally been recovered from the spinal cord or subcutaneous nodules of infected humans (Grácio et al., 2015). Notwithstanding reported infections in dogs and cats from some European countries (Hermosilla et al., 2005; Faísca et al., 2010; Maia et al., 2015; Tudor et al., 2016; Hodžić et al., 2018) as well as USA and Canada (Labelle et al., 2011; Otranto et al., 2015b), there exist paucity of data about O. lupi prevalence in canid population of Iran and its potential blackfly (Simuliidae) or Culicoides vectors.
Setaria spp. are parasites of some herbivores, with adults causing fibrinous peritonitis and immature forms aberrantly migrating in accidental hosts (Ahmad and Srivastava, 2007). A subconjunctival setariasis due to Setaria equina (S. equina) in the left eye of a 15-year-old girl was reported from northwestern Iran (Nabie et al., 2017). So far, only few cases of human infection to adult Setaria spp. has been reported globally. Panaitescu et al., documented four human cases of subconjunctival S. labiatopapillosa infection in Romania with photophobia, swelling and tearing signs (Panaitescu et al., 1999). Another report from Romania emphasized subconjunctival infection of an old man with Setaria sp. (Ţălu et al., 2012). Despite a wide array of Culicidae mosquito vectors for setariasis around the world, comprising Aedes, Anopheles, Armigeres, Culex and Mansonia (Azari-Hamidian et al., 2019), less is understood about the possible vectors in Iran. Azari-Hamidian et al., in 2009 did the only present survey and found Anopheles maculipennis mosquitoes in northwestern Iran infected to Setaria (Azari-Hamidian et al., 2009).
Dirofilaria immitis, agent of cardiopulmonary dirofilariasis in canids, was the predominant species of filarioids among all examined animals in Iran. The highest detection rate was obtained by serology (16.95%; 95% CI = 3.51–35.38%) as convenient techniques for screening or field-based appraisal, while the lowest prevalence was determined using microscopy (13.86%; 95% CI = 9.46–18.94%). Serological methods are appropriate, especially for the diagnosis of amicrofilaremic infections (Simón et al., 2012). Geographically, both D. immitis and D. repens are sympatric in most territories (Simón et al., 2017), although the latter was only detected in a recent multiplex-PCR study in Iran with 26% prevalence in dogs (Pedram et al., 2019). Globally, several studies have reported the prevalence of canine D. immitis infection. In continental Portugal where heartworm is endemic, 4–9% prevalence was reported (Alho et al., 2018). The prevalence in Turkey, neighbouring Iran, was zero to 18% (Köse and Erdoğan, 2012). In Greece, canine D. immitis prevalence ranged between 0.7% and 25% (Angelou et al., 2019; Diakou et al., 2019). In Poland, Eastern Europe very low prevalence (<1%) was observed (Krämer et al., 2014). The highest canine heartworm prevalence was reported from Madeira Island with 40% (Genchi and Kramer, 2019). Reports from the Far East countries are rare, with 2–15% and 18% in China (Liu et al., 2013)and Thailand (Boonyapakorn et al., 2008), respectively. Moreover, 4.7–29.5% prevalence rates were observed in India (Borthakur et al., 2015). A few studies have reported D. immitis and D. repens in African countries including Algeria (Tahir et al., 2017), Tunisia (Rjeibi et al., 2017), Mozambique (Schwan and Durand, 2002) and Tanzania (Mukendi et al., 2016) with 1.4–14.5% prevalence rates. The highest prevalence of D. immitis in the Americas has been reported in US Eastern states, Caribbean Islands and some parts of Argentina and Brazil (20.4–74%) (Lee et al., 2010; Little et al., 2014; Barrett and Little, 2016; Simón et al., 2017).
Based on the findings of this current review, the highest prevalence of D. immitis was observed in stray dogs (20.92%; 95% CI = 13.84–29.03%), whereas 10.72% (95% CI = 6.59–15.7%) and 6.61% (95% CI = 3.46–10.68%) prevalence rates were reported in wild canids (jackals and red fox) and owned dogs, respectively, in Iran. An increasing trend has been shown of heartworm infection among European populations of jackals (7.7–23.3%), foxes (3.7–35%) and raccoon dogs (31.1%) (Marconcini et al., 1996; Cirovic et al., 2014; Simón et al., 2017). A recent study in Canada showed 4.8% prevalence of D. immitis in wild canids (Kotwa et al., 2019). Wild canids, directly or indirectly, possibly play a critical role in the maintenance and transmission of D. immitis (Simón et al., 2017). Distribution patterns of D. immitis in canids may be influenced by different ecosystems, such that foxes in agricultural regions of Europe were more infected than those foxes in semiarid or mountainous territories. High interactions among wildlife, pets and humans in suburban/agricultural areas could affect the transmission dynamics of dirofilariasis (Gortázar et al., 1994; Marks and Bloomfield, 1998).
Based on our findings, D. immitis prevalence was prevalent in western (25.29%; 95% CI = 14.44–37.99%) and northern (13.59%; 95% CI = 7.29–21.46%) Iran. From a historical perspective, the first observation of the heartworm in Iran dates back to 1969 when Sadighian reported necropsy documentation of stray dogs in Caspian Sea littoral, northern Iran (Sadighian, 1969). Climate and environment are important extrinsic factors for survival and development of vector mosquitoes and subsequent occurrence of dirofilariasis. Regarding the ectothermic nature of mosquitoes and their reliance on water supplies, climatic parameters including humidity/precipitation and temperature substantially impact their colonization, population density, diversity and activity (Simón et al., 2017). Also, from a parasitic standpoint, extrinsic incubation (8–20 days with 22–30°C temperatures) is influential for L3 larvae development (Simón et al., 2012). Such favourable circumstances are provided in the western and northern parts of Iran, where there exist huge water resources, irrigation systems and high precipitation rates annually. In contrast, low prevalence rates in eastern and southern parts of Iran are observed, where weak water supplies and low annual precipitation exist. An expedient exemplary of climate impact on the prevalence of dirofilariasis is represented in Grand Canary Island, where various altitudes possess different semitropical climates. Accordingly, D. immitis prevalence among canines of various zones differ significantly, from 30.4% in mild climate zone to 10% in the temperate cold climate zone (Montoya-Alonso et al., 2010). Although not significant, the prevalence in males was partly more than females in our review (10.07% vs 9.23%; P = 0.131), which is consistent with findings of other investigations (Reifur et al., 2004; Simsek et al., 2008). Also, canids over 1-year old were over 2-fold more susceptible than younger (<1-year old) ones (20.77% vs 8.40%; P = 0.006), in agreement with another study in Brazil (Reifur et al., 2004). Adult animals had probably accumulated more exposure time to insect bites, thus had a higher prevalence of infection.
Besides canine Dirofilaria infection in Iran, we only found two studies regarding feline dirofilariasis in Ardabil and Khuzestan provinces. Heartworm infection is a subclinical condition in domestic/wild felids and only a few worms reach maturity; hence, there may be a limited number of blood microfilariae, which, in turn, substantially reduces the chance of transmission (Simón et al., 2012; Penezic et al., 2014). Otranto et al. (2015a) also corroborate our findings, highlighting the lower contribution of felids in the epidemiology of heartworm disease (Otranto et al., 2015a). In the USA, 3–19% prevalence ranges have been reported regarding feline dirofilariasis. Studies in Europe have shown a 7–27% (Italy) and 33% (Canary Islands) seroprevalence rates. In Japan, 2–5.2% of cats were seropositive for dirofilariasis (Simón et al., 2012).
Other known, but less frequent filarioid nematodes found in Iran are D. evansi (syn. Deraiophoronema evansi) in camels, and Acanthocheilonema reconditum (A. reconditum) in dogs. The camel parasite was isolated from 7 provinces with a total prevalence of 10.16% (95% CI = 4.73–17.34%). Male dromedaries were more parasitised than females (10.62% vs 8.01%), consistent with Mahran study (Mahran, 2004), although it was not significant (P = 0.084). A significant association was observed between the prevalence of D. evansi in Iranian camels and diagnostic method (X2 = 13.2; P < 0.001). It seems that using a microscope examination is more appropriate for identifying D. evansi than the Knott test. One-humped camel (Camelus dromedarius) is the dominant species in Iran, with particular tolerance to the harsh desert environment and some pathogens, although D. evansi induces clinical disease (Sazmand and Joachim, 2017). Adult worms accumulate in large number in various affected organs, comprising spermatic cord, epididymis, testicles, heart and lungs. The acute disease could lead to emaciation, orchitis, heart failure, arteriosclerosis and nervous impairment (Oryan et al., 2008). Our knowledge on this parasite is limited to prevalence studies in arid, semi-arid countries of the world, including Egypt, Nigeria, Saudia Arabia, Iran and India (Pathak and Chahabra, 2010; Sazmand et al., 2013; Egbe-Nwiyi et al., 2016; El-Khabaz et al., 2019). Globally, the estimated prevalence in adult camels was 2.5–4%, while it was 47.5% in less than 1-year-old camels (Muhammad and Athar, 2000). A molecular study by PCR and sequencing methods in 2016 revealed paraphyly of D. evansi and D. gracile, which deserves further investigations (Sazmand et al., 2016). Acanthocheilonema reconditum living in canine subcutis and on muscle fascia develops mild parasitism in dogs with no major damages (Saari et al., 2019). Approximately, 2.15% (95% CI = 0.71–4.33%) of the dog population in Iran was reported to have this infection. In a multispecies survey in Romania on filarioid infections, A. reconditum DNA was only detected in a red fox (0.33%) (Ionică et al., 2017). Diagnosis is important only to differentiate their mfs from the life-threatening species, D. immitis (Otranto and Deplazes, 2019).
With respect to other filarioids infecting domestic livestock in Iran, we could only estimate the weighted prevalence of Parafilaria multipapillosa (P. multipapillosa) (referred to as Filaria haemorrhagica) in equids (5.85%; 95% CI: 3.75–8.37%), S. equina in equids (12.15%; 95% CI: 0.04–40.7%) and S. digitata in ruminants (45.47%; 95% CI: 14.45–78.61%). Several Onchocerca spp. parasitise livestock including, O. fasciata (camel; connective tissue, ligamentum nuchae) (16.57%; 95% CI: 10.12–24.24%), O. cervicalis (horse and donkey; cervical ligament) (3.63%; 95% CI: 1.44–6.57%) and O. reticulata (horse and donkey; connective tissue, flexor tendon) which are found in Iran. We found a statistically significant association between prevalence of O. fasciata in Iranian camels and gender (X2 = 4.4: P = 0.035) and diagnostic method (X2 = 6.1; P = 0.013). It seems that using microscopic examination may be more likely to detect O. fasciata than histopathologic methods. Prevalence studies are actually rare on P. multipapillosa, while there is more on P. bovicola of cattle (not found in Iran) (Bech-Nielsen et al., 1982; Solismaa et al., 2008; Borgsteede et al., 2009). Setariosis is a benign infection and even high rates of microfilaraemia could be well tolerated (Hornok et al., 2007). Survey of filarial nematodes of 188 donkeys in Egypt showed a total infection rate of 86.7% mostly in males (86.73%), regarding O. cervicalis (82.98%), O. reticulata (4.26%), S. equina (36.17%) and P. multipapillosa (5.32%) (Radwan et al., 2016). In Hungary, 18 of 195 (9.2%) horses had mfs by Knott technique for S. equina (Hornok et al., 2007). In a Finnish study, 209 skin biopsies of cattle revealed 78 (37%) positive for Onchocerca sp. mfs (Solismaa et al., 2008). Altogether, the above information obtained from current systematic review and meta-analysis represents that there is a gap in our knowledge and understanding in the field of filariasis in livestock that needs global collaboration for better realization of such arthropod-borne helminths.
Concluding remarks
As we stated in the current systematic review and meta-analysis, Dirofilaria spp. infections (D. repens cases in humans and D. immitis in canids) were the most frequently found species of all filarioid nematodes in Iran. Most cases have been reported from rainy provinces with favourable temperatures for vector hosts. Also, most cases were detected during the last two decades, which indicates increased prevalence, and/or improved diagnostics or awareness in the public and clinical understanding of Dirofilaria infections. In this context, there is an urgent need to nationwide epidemiological surveys, e.g. serodiagnosis to detect human pulmonary cases, as well as preventive therapy of, at least pet dogs. Regarding less evident, but existing filarioids, such as the interesting human case of O. lupi in Qom province and those being found in herbivores much large-scale studies in the context of the host-parasite-vector axis are recommended to be done in the future for better understanding of the epidemiology of these filarioid infections.
Acknowledgements
The authors sincerely declare their appreciation of Professor Domenico Otranto (Head of Department of Veterinary Medicine, University of Bari, Italy) for his noteworthy comments on this manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S003118202000058X.
click here to view supplementary material
References
- Ahmad R and Srivastava AK (2007) Biochemical composition and metabolic pathways of filarial worms Setaria cervi: search for new antifilarial agents. Journal of Helminthology 81, 261–280. [DOI] [PubMed] [Google Scholar]
- Akhtardanesh B, Radfar MH, Voosough D and Darijani N (2011) Seroprevalence of canine heartworm disease in Kerman, southeastern Iran. Comparative Clinical Pathology 20, 573–577. [Google Scholar]
- Alborzi A, Mosallanejad B, Najafabadi M and Nikpoor Z (2010) Infestation of heartworm (Dirofilaria immitis) in a cat in Ahvaz City: a case report. Journal of Veterinary Research 65, 255–271. [Google Scholar]
- Alho AM, Meireles J, Schnyder M, Cardoso L, Belo S, Deplazes P and de Carvalho LM (2018) Dirofilaria immitis and Angiostrongylus vasorum: the current situation of two major canine heartworms in Portugal. Veterinary Parasitology 252, 120–126. [DOI] [PubMed] [Google Scholar]
- Anderson RC (2000) Nematode Parasites of Vertebrates: Their Development and Transmission. UK: Cabi. [Google Scholar]
- Angelou A, Gelasakis AI, Verde N, Pantchev N, Schaper R, Chandrashekar R and Papadopoulos E (2019) Prevalence and risk factors for selected canine vector-borne diseases in Greece. Parasites & vectors 12, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anvari D, Saadati D, Siyadatpanah A and Gholami S (2019) Prevalence of dirofilariasis in shepherd and stray dogs in Iranshahr, southeast of Iran. Journal of Parasitic Diseases 43, 319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi K, Golchai J and Geranmayeh S (2010) Human subcutaneous dirofilariasis due to Dirofilaria (Nochtiella) repens: clinically suspected as cutaneous fascioliasis. Iranian Journal of Public Health 39, 105. [PMC free article] [PubMed] [Google Scholar]
- Athari A (2003) Zoonotic subcutaneous dirofilariasis in Iran. Archives of Iranian Medicine 6, 63–65. [Google Scholar]
- Azari-Hamidian S, Yaghoobi-Ershadi M, Javadian E, Abai M, Mobedi I, Linton YM and Harbach R (2009) Distribution and ecology of mosquitoes in a focus of dirofilariasis in northwestern Iran, with the first finding of filarial larvae in naturally infected local mosquitoes. Medical and Veterinary Entomology 23, 111–121. [DOI] [PubMed] [Google Scholar]
- Azari-Hamidian S, Norouzi B and Harbach RE (2019) A detailed review of the mosquitoes (Diptera: Culicidae) of Iran and their medical and veterinary importance. Acta Tropica 194, 106–122. [DOI] [PubMed] [Google Scholar]
- Barrett AW and Little SE (2016) Vector-Borne infections in tornado-displaced and owner-relinquished dogs in Oklahoma, USA. Vector Borne and Zoonotic Diseases 16, 428–430. [DOI] [PubMed] [Google Scholar]
- Bech-Nielsen S, Bornstein S, Christensson D, Wallgren TB, Zakrisson G and Chirico J (1982) Parafilaria bovicola (Tubangui 1934) in cattle: epizootiology-vector studies and experimental transmission of Parafilaria bovicola to cattle. American Journal of Veterinary Research 43, 948–954. [PubMed] [Google Scholar]
- Boonyapakorn C, Srikitjakarn L, Morakote N and Hoerchner F (2008) The epidemiology of Dirofilaria immitis infection in outpatient dogs at Chiang Mai University Small Animal Hospital, Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 39, 33–38. [PubMed] [Google Scholar]
- Borgsteede FHM, van Wuijckhuise L, Peutz J, Roumen T and Kock P (2009) Import of Parafilaria bovicola in the Netherlands. Veterinary Parasitology 161, 146–149. [DOI] [PubMed] [Google Scholar]
- Borthakur SK, Deka DK, Islam S, Sarma DK and Sarmah PC (2015) Prevalence and molecular epidemiological data on Dirofilaria immitis in dogs from Northeastern States of India. TheScientificWorldJournal 2015, 265385–265385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S and Nutman TB (2015) Filarial nematodes. In Jorgensen JH, Pfaller MA and Carroll KC Manual of Clinical Microbiology, 11th Edn, ASM Press (US): American Society of Microbiology, pp. 2461–2470. [Google Scholar]
- Cirovic D, Penezic A, Pavlovic I, Kulisic Z, Cosic N, Burazerovic J and Maletic V (2014) First records of Dirofilaria repens in wild canids from the region of Central Balkan. Acta Veterinaria Hungarica 62, 481–488. [DOI] [PubMed] [Google Scholar]
- Diakou A, Soubasis N, Chochlios T, Oikonomidis IL, Tselekis D, Koutinas C, Karaiosif R, Psaralexi E, Tsouloufi TK and Brellou G (2019) Canine and feline dirofilariosis in a highly enzootic area: first report of feline dirofilariosis in Greece. Parasitology Research 118, 677–682. [DOI] [PubMed] [Google Scholar]
- Egbe-Nwiyi TNC, Paul BT and Muhammed YY (2016) Haemoparasites and haematological parameters of the one humped camel (Camelus Dromedarius) slaughtered in Maiduguri Abattoir, Nigeria. Global Journal of Medical Research: Veterinary Science and Veterinary Medicine 16, 7. [Google Scholar]
- Egger M, Smith GD, Schneider M and Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khabaz KAS, Abdel-Hakeem SS and Arfa MI (2019) Protozoan and helminthes parasites endorsed by imported camels (camel dromedaries) to Egypt. Journal of Parasitic Diseases 43, 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza E, Cordero M, Muro A, Lorente F and Simón F (1993) Anti-Dirofilaria immitis IgE: seroepidemiology and seasonal variation in an exposed human population. Tropical medicine and parasitology: official organ of Deutsche Tropenmedizinische Gesellschaft and of Deutsche Gesellschaft fur Technische Zusammenarbeit (GTZ) 44, 172–176. [PubMed] [Google Scholar]
- Faísca P, Morales-Hojas R, Alves M, Gomes J, Botelho M, Melo M and Xufre A (2010) A case of canine ocular onchocercosis in Portugal. Veterinary Ophthalmology 13, 117–121. [DOI] [PubMed] [Google Scholar]
- Genchi C and Kramer LH (2019) The prevalence of Dirofilaria immitis and D. repens in the Old world. Veterinary Parasitology 280, 108995. doi: 10.1016/j.vetpar.2019.108995. [DOI] [PubMed] [Google Scholar]
- Gortázar C, Castillo J, Lucientes J, Blanco J, Arriolabengoa A and Calvete C (1994) Factors affecting Dirofilaria immitis prevalence in red foxes in northeastern Spain. Journal of Wildlife Diseases 30, 545–547. [DOI] [PubMed] [Google Scholar]
- Grácio AJS, Richter J, Komnenou AT and Grácio MA (2015) Onchocerciasis caused by Onchocerca lupi: an emerging zoonotic infection. Systematic review. Parasitology Research 114, 2401–2413. [DOI] [PubMed] [Google Scholar]
- Gungel H, Kara N, Pinarci EY, Albayrak S, Baylancicek DO and Uysal HK (2009). An uncommon case with intravitreal worm. Intravitreal Dirofilaria infection. British Journal of Ophthalmology, 93, 573–574, 697. [DOI] [PubMed] [Google Scholar]
- Hamidinia D, Maraghi S, Azimi F, Ai A and Shirian S (2016) The role of climate on prevalence or eradication of vesical schistosomiasis in Khuzestan Province of Iran. Journal of Parasitic Diseases 40, 387–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosilla C, Hetzel U, Bausch M, Grübl J and Bauer C (2005) First autochthonous case of canine ocular onchocercosis in Germany. Veterinary Record-English Edition 156, 450–451. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ and Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodžić A, Hinney B, König S, Naucke T, Duscher G and Joachim A (2018) A case of ocular infection with Onchocerca lupi in a dog from Germany. Transboundary and Emerging Diseases 65, e214–e216. [DOI] [PubMed] [Google Scholar]
- Hornok S, Genchi C, Bazzocchi C, Fok E and Farkas R (2007) Prevalence of Setaria equina microfilaraemia in horses in Hungary. Veterinary Record 161, 814–816. [PubMed] [Google Scholar]
- Ionică AM, Matei IA, D'Amico G, Ababii J, Daskalaki AA, Sándor AD, Enache DV, Gherman CM and Mihalca AD (2017) Filarioid infections in wild carnivores: a multispecies survey in Romania. Parasites & vectors 10, 332–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi A, Jamshidi M, Mobedi I and Khosroara M (2008) Periocular dirofilariasis in a young woman: a case report. The Korean journal of parasitology 46, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedri J, Radfar MH, Borji H, Azizzadeh M and Akhtardanesh B (2014) Canine Heartworm in Southeastern of Iran with review of disease distribution. Iranian journal of parasitology 9, 560. [PMC free article] [PubMed] [Google Scholar]
- Khodabakhsh M, Malmasi A, Mohebali M, Zarei Z, Kia EB and Azarm A (2016) Feline Dirofilariosis due to Dirofilaria immitis in Meshkin Shahr District, Northwestern Iran. Iranian journal of parasitology 11, 269–273. [PMC free article] [PubMed] [Google Scholar]
- Köse M and Erdoğan M (2012) Serological screening of canine heartworm (Dirofilaria immitis) infections in Turkey. Berliner und Munchener Tierarztliche Wochenschrift 125, 503–508. [PubMed] [Google Scholar]
- Kotwa JD, Jardine CM, Berke O, Pearl DL, Mercer NJ and Peregrine AS (2019) Prevalence and distribution of Dirofilaria immitis infection in wild canids in southern Ontario. Veterinary Parasitology: Regional Studies and Reports 18, 100349. [DOI] [PubMed] [Google Scholar]
- Krämer F, Schaper R, Schunack B, Połozowski A, Piekarska J, Szwedko A, Jodies R, Kowalska D, Schüpbach D and Pantchev N (2014) Serological detection of Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato and Ehrlichia canis antibodies and Dirofilaria immitis antigen in a countrywide survey in dogs in Poland. Parasitology Research 113, 3229–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwarteng A, Ahuno ST and Akoto FO (2016) Killing filarial nematode parasites: role of treatment options and host immune response. Infectious Diseases of Poverty 5, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle AL, Daniels JB, Dix M and Labelle P (2011) Onchocerca lupi causing ocular disease in two cats. Veterinary Ophthalmology 14, 105–110. [DOI] [PubMed] [Google Scholar]
- Lee AC, Montgomery SP, Theis JH, Blagburn BL and Eberhard ML (2010) Public health issues concerning the widespread distribution of canine heartworm disease. Trends in Parasitology 26, 168–173. [DOI] [PubMed] [Google Scholar]
- Little SE, Beall MJ, Bowman DD, Chandrashekar R and Stamaris J (2014) Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2010–2012. Parasites & Vectors 7, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yang N, He J, Yang M and Sun M (2013) Prevalence of Dirofilaria immitis in dogs in Shenyang, Northeastern China. Korean Journal of Parasitology 51, 375–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahran OM (2004) Some studies on blood parasites in camels (Camelus dromedarius) at Shalatin City, Red Sea Governorate. Assiut Veterinary Medical Journal 50, 172–184. [Google Scholar]
- Maia C, Annoscia G, Latrofa MS, Pereira A, Giannelli A, Pedroso L and Otranto D (2015) Onchocerca lupi nematode in cat, Portugal. Emerging Infectious Diseases 21, 2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraghi S, Rahdar M, Akbari H, Radmanesh M and Saberi A (2006) Human dirofilariasis due to Dirofilaria repens in Ahvaz-Iran: a report of three cases. Pakistan Journal of Medical Sciences 22, 211–213. [Google Scholar]
- Maraghi S, Sameri A and Jeddi Y (2015) Human Dirofilaria repens infection of the breast: a case report. Archives of Medical Laboratory Sciences 1, 42–44. doi: 10.22037/amls.v1i1.9403. [DOI] [Google Scholar]
- Maraghi S, Naini-Kashani M, Masroupour M, Sameri A and Jeddi Y (2016) Ophtalmic dirofilariasis. Archives of Medical Laboratory Sciences 2, 36–38. [Google Scholar]
- Marconcini A, Magi M, Macchioni G and Sassetti M (1996) Filariosis in foxes in Italy. Veterinary Research Communications 20, 316–319. [DOI] [PubMed] [Google Scholar]
- Marks CA and Bloomfield TE (1998) Canine heartworm (Dirofilaria immitis) detected in red foxes (Vulpes vulpes) in urban Melbourne. Veterinary Parasitology 78, 147–154. [DOI] [PubMed] [Google Scholar]
- Mirahmadi H, Maleki A, Hasanzadeh R, Ahoo MB, Mobedi I and Rostami A (2017) Ocular dirofilariasis by Dirofilaria immitis in a child in Iran: a case report and review of the literature. Parasitology International 66, 978–981. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J and Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine 151, 264–269. [DOI] [PubMed] [Google Scholar]
- Montoya-Alonso JA, Mellado I, Carretón E, Cabrera-Pedrero ED, Morchón R and Simón F (2010) Canine dirofilariosis caused by Dirofilaria immitis is a risk factor for the human population on the island of Gran Canaria, Canary Islands, Spain. Parasitology Research 107, 1265–1269. [DOI] [PubMed] [Google Scholar]
- Mowlavi G, Farzbod F, Kheirkhah A, Mobedi I, Bowman D and Naddaf S (2014) Human ocular onchocerciasis caused by Onchocerca lupi (Spirurida. Onchocercidae) in Iran. Journal of Helminthology 88, 250–255. [DOI] [PubMed] [Google Scholar]
- Muhammad G and Athar M (2000) Dipetalonemiasis, Toxoplasmosis and piroplasmosis in camels. In Gahlot TK (ed.), Selected Topics in Camelids. Chandan: Camel Publishing House, pp. 271–274. [Google Scholar]
- Mukendi JP, Kimbita E, Mbanzulu KM, Maindo PP and Misinzo G (2016) Morphological and molecular detection of canine dirofilarial species of veterinary and medical importance in Morogoro municipality, Tanzania. Veterinary Parasitology 220, 1–3. [DOI] [PubMed] [Google Scholar]
- Nabie R, Spotin A and Rouhani S (2017) Subconjunctival setariasis due to Setaria equina infection; a case report and a literature review. Parasitology International 66, 930–932. [DOI] [PubMed] [Google Scholar]
- Negahban S, Daneshbod Y, Atefi S, Daneshbod K, Sadjjadi SM, Hosseini SV, Bedayat GR and Abidi H (2007) Dirofilaria repens diagnosed by the presence of microfilariae in fine needle aspirates. Acta Cytologica 51, 567–570. [DOI] [PubMed] [Google Scholar]
- Orihel TC and Eberhard ML (1998) Zoonotic filariasis. Clinical Microbiology Reviews 11, 366–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oryan A, Valinezhad A and Bahrami S (2008) Prevalence and pathology of camel filariasis in Iran. Parasitology Research 103, 1125–1131. [DOI] [PubMed] [Google Scholar]
- Otranto D and Deplazes P (2019) Zoonotic nematodes of wild carnivores. International Journal for Parasitology: Parasites and Wildlife 9, 370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D and Eberhard ML (2011) Zoonotic helminths affecting the human eye. Parasites & vectors 4, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D, Diniz DG, Dantas-Torres F, Casiraghi M, de Almeida IN, de Almeida LN, dos Santos JN, Furtado AP, de Almeida Sobrinho EF and Bain O (2011a) Human intraocular filariasis caused by Dirofilaria sp. nematode, Brazil. Emerging Infectious Diseases 17, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D, Sakru N, Testini G, Gürlü VP, Yakar K, Lia RP, Dantas-Torres F and Bain O (2011b) First evidence of human zoonotic infection by Onchocerca lupi (Spirurida, Onchocercidae). The American journal of tropical medicine and hygiene 84, 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D, Dantas-Torres F, Cebeci Z, Yeniad B, Buyukbabani N, Boral OB, Gustinelli A, Mounir T, Mutafchiev Y and Bain O (2012) Human ocular filariasis: further evidence on the zoonotic role of Onchocerca lupi. Parasites & vectors 5, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D, Dantas-Torres F, Brianti E, Traversa D, Petrić D, Genchi C and Capelli G (2013) Vector-borne helminths of dogs and humans in Europe. Parasites & vectors 6, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D, Cantacessi C, Dantas-Torres F, Brianti E, Pfeffer M, Genchi C, Guberti V, Capelli G and Deplazes P (2015a) The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: Helminths and arthropods. Veterinary Parasitology 213, 24–37. [DOI] [PubMed] [Google Scholar]
- Otranto D, Giannelli A, Latrofa MS, Dantas-Torres F, Trumble NS, Chavkin M, Kennard G, Eberhard ML and Bowman DD (2015b) Canine infections with Onchocerca lupi nematodes, United States, 2011–2014. Emerging Infectious Diseases 21, 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampiglione S and Rivasi F (2000) Human dirofilariasis due to Dirofilaria (Nochtiella) repens: an update of world literature from 1995 to 2000. Dirofilaria 37, 82. [PubMed] [Google Scholar]
- Panaitescu D, Preda A, Bain O and Vasile-Bugarin A (1999) Four cases of human filariosis due to Setaria labiatopapillosa found in Bucharest, Romania. Roumanian Archives of Microbiology and Immunology 58, 203–207. [PubMed] [Google Scholar]
- Pathak KML and Chahabra MB (2010) Parasites and parasitic diseases of the camel in India: a review. Indian Journal of Animal Sciences 80, 699–706. [Google Scholar]
- Pedram N, Tabrizi AS, Hosseinzadeh S, Pourmontaseri M and Rakhshandehroo E (2019) Prevalence of Dirofilaria immitis and Dirofilaria repens in outdoor dogs in Tehran Province, Iran. Comparative Clinical Pathology 28, 1–5. [Google Scholar]
- Penezic A, Selakovic S, Pavlovic I and Cirovic D (2014) First findings and prevalence of adult heartworms (Dirofilaria immitis) in wild carnivores from Serbia. Parasitology Research 113, 3281–3285. [DOI] [PubMed] [Google Scholar]
- Radwan AM, Ahmed NE, Elakabawy LM, Ramadan MY and Elmadawy RS (2016) Prevalence and pathogenesis of some filarial nematodes infecting donkeys in Egypt. Veterinary world 9, 888–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifur L, Thomaz-Soccol V and Montiani-Ferreira F (2004) Epidemiological aspects of filariosis in dogs on the coast of Parana state, Brazil: with emphasis on Dirofilaria immitis. Veterinary Parasitology 122, 273–286. [DOI] [PubMed] [Google Scholar]
- Rjeibi MR, Rouatbi M, Mabrouk M, Tabib I, Rekik M and Gharbi M (2017) Molecular study of Dirofilaria immitis and Dirofilaria repens in dogs from Tunisia. Transboundary and Emerging Diseases 64, 1505–1509. [DOI] [PubMed] [Google Scholar]
- Rodonaja T (1967) A new species of Nematode, Onchocerca lupi n. sp., from Canis lupus cubanensis. Soobshchenyia Akad. Nauk Gruzinskoy SSR 45, 715–719. [Google Scholar]
- Rouhani S and Athari A (2003) Ocular dirofilariasis in Iran: a case report. Medical Journal of The Islamic Republic of Iran (MJIRI 17, 85–86. [Google Scholar]
- Saari S, Nareaho A and Nikander S (2019) Canine Parasites and Parasitic Diseases. London, UK: Academic Press. [Google Scholar]
- Sadighian A (1969) Helminth parasites of stray dogs and jackals in Shahsavar area, caspian region, Iran. Journal of Parasitology 55, 372–374. [PubMed] [Google Scholar]
- Salahi-Moghadam A and Banihashemi H (2016) Unusual location of dirofilaria immitis in A 5-year-old boy's hydrocele: a case report. Hormozgan Medical Journal 20, 210–213. [Google Scholar]
- Sazmand A and Joachim A (2017) Parasitic diseases of camels in Iran (1931–2017) – a literature review. Parasite 24, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazmand A, Tafti MH, Hekmatimoghaddam S and Moobedi I (2013) Dipetalonema evansi infection in camels of Iran's central area. Pakistan Journal of Biological Sciences 16, 647–650. [DOI] [PubMed] [Google Scholar]
- Sazmand A, Eigner B, Mirzaei M, Hekmatimoghaddam S, Harl J, Duscher GG, Fuehrer H-P and Joachim A (2016) Molecular identification and phylogenetic analysis of Dipetalonema evansi (LEWIS, 1882) in camels (Camelus dromedarius) of Iran. Parasitology Research 115, 1605–1610. [DOI] [PubMed] [Google Scholar]
- Schwan EV and Durand DT (2002) Canine filariosis caused by Dirofilaria immitis in Mozambique: a small survey based on the identification of microfilariae. Journal of the South African Veterinary Association 73, 124–126. [DOI] [PubMed] [Google Scholar]
- Simon F, Muro A, Cordero M and Martin J (1991) A seroepidemiologic survey of human dirofilariosis in Western Spain. Tropical Medicine and Parasitology 42, 106–108. [PubMed] [Google Scholar]
- Simón F, Siles-Lucas M, Morchón R, González-Miguel J, Mellado I, Carretón E and Montoya-Alonso JA (2012) Human and animal dirofilariasis: the emergence of a zoonotic mosaic. Clinical Microbiology Reviews 25, 507–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón F, González-Miguel J, Diosdado A, Gómez PJ, Morchón R and Kartashev V (2017) The complexity of zoonotic filariasis episystem and its consequences: a multidisciplinary view. BioMed Research International 2017, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek S, Utuk AE, Koroglu E and Rishniw M (2008) Serological and molecular studies on Dirofilaria immitis in dogs from Turkey. Journal of Helminthology 82, 181–186. [DOI] [PubMed] [Google Scholar]
- Solgi R, Sadjjadi SM, Mohebali M, Zarei Z, Golkar M and Raz A (2018) Development of New recombinant DgK antigen for diagnosis of Dirofilaria immitis infections in dogs using ELISA technique and Its comparison to molecular methods. Iranian Biomedical Journal 22, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solismaa M, Laaksonen S, Nylund M, Pitkanen E, Airakorpi R and Oksanen A (2008) Filarioid nematodes in cattle, sheep and horses in Finland. Acta Veterinaria Scandinavica 50, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfellow GJ, Francis IC, Coroneo MT and Walker J (2002) Orbital dirofilariasis. Clinical & Experimental Ophthalmology 30, 378–380. [DOI] [PubMed] [Google Scholar]
- Tabatabaei SA, Soleimani M, Nikmanesh B, Mahmoudzadeh R, Vahedian Z, Salabati M, Soleimani Z, Matini A and Noorbakhsh M (2017) Human subconjunctival dirofilariasis presenting as the daytime photophobia: a case report. Iranian Journal of Public Health 46, 1430. [PMC free article] [PubMed] [Google Scholar]
- Tahir D, Damene H, Davoust B and Parola P (2017) First molecular detection of Dirofilaria immitis (Spirurida: Onchocercidae) infection in dogs from Northern Algeria. Comparative Immunology, Microbiology and Infectious Diseases 51, 66–68. [DOI] [PubMed] [Google Scholar]
- Ţălu S, Ştefănuţ A, Mihalca A and Coroiu Z (2012) Subconjunctival infestation with Setaria. Helminthologia 49, 119–121. [Google Scholar]
- Tasić-Otašević SA, Gabrielli SV, Tasić AV, MiladinovićTasić NL, Kostić JT, Ignjatović AM, Dragonjić LDP, Milošević ZG, Arsić-Arsenijević VS and Cancrini GA (2014) Seroreactivity to Dirofilaria antigens in people from different areas of Serbia. BMC Infectious Diseases 14, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakolizadeh S and Mobedi I (2009) Orbital dirofilariasis in Iran: a case report. The Korean journal of parasitology 47, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, Hoerauf A and Bockarie M (2010) Lymphatic filariasis and onchocerciasis. The Lancet, 376, 1175–1185. [DOI] [PubMed] [Google Scholar]
- Tekle AH, Zouré HG, Noma M, Boussinesq M, Coffeng LE, Stolk WA and Remme JH (2016) Progress towards onchocerciasis elimination in the participating countries of the African Programme for Onchocerciasis Control: epidemiological evaluation results. Infectious Diseases of Poverty 5, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis J (2005) Public health aspects of dirofilariasis in the United States. Veterinary Parasitology 133, 157–180. [DOI] [PubMed] [Google Scholar]
- Thompson R, Lymbery A and Smith A (2010) Parasites, emerging disease and wildlife conservation. International Journal for Parasitology 40, 1163–1170. [DOI] [PubMed] [Google Scholar]
- Tudor P, Turcitu M, Mateescu C, Dantas-Torres F, Tudor N, Bărbuceanu F, Ciuca L, Burcoveanu I, Acatrinei D and Rinaldi L (2016) Zoonotic ocular onchocercosis caused by Onchocerca lupi in dogs in Romania. Parasitology Research 115, 859–862. [DOI] [PubMed] [Google Scholar]
- Vieira C, Vélez I, Montoya M, Agudelo S, Alvarez M, Genchi C and Simon F (1998) Dirofilaria immitis in Tikuna Indians and their dogs in the Colombian Amazon. Annals of Tropical Medicine and Parasitology 92, 123–125. [DOI] [PubMed] [Google Scholar]
- WHO (2015) Global programme to eliminate lymphatic filariasis: progress report, 2014. Weekly Epidemiological Record = Relevé épidémiologique hebdomadaire 90, 489–504. [PubMed] [Google Scholar]
- Zarei Z, Kia EB, Heidari Z, Mikaeili F, Mohebali M and Sharifdini M (2016) Age and sex distribution of Dirofilaria immitis among dogs in Meshkin-Shahr, northwest Iran and molecular analysis of the isolates based on COX1 gene. Veterinary Research Forum 7, 329–334. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S003118202000058X.
click here to view supplementary material