Abstract
Various treatments are found to be moderately effective in managing Demodex-related diseases except tea tree oil (TTO) and terpinen-4-ol (T4O), which showed superior miticidal and anti-inflammatory effects in numerous clinical studies. Their possible effects include lowering mite counts, relieving Demodex-related symptoms, and modulating the immune system. This review summarizes the current clinical topical and oral treatments in human demodicosis, their possible mechanisms of action, side-effects and resistance in treating this condition. TTO (especially T4O) is found to be the most effective followed by metronidazole, ivermectin and permethrin in managing the disease. This is because TTO has anti-parasitic, anti-bacterial, anti-fungal, anti-inflammatory and wound-healing effects. Furthermore, nanoTTO can even release its contents into fungus and Pseudomonas biofilms. Combinations of different treatments are occasionally needed for refractory cases, especially for individuals with underlying genetic predisposal or are immuno-compromised. Although the current treatments show efficacy in controlling the Demodex mite population and the related symptoms, further research needs to be focused on the efficacy and drug delivery technology in order to develop alternative treatments with better side-effects profiles, less toxicity, lower risk of resistance and are more cost-effective.
Key words: Crotamiton, demodicosis, ivermectin, lindane, metronidazole, permethrin, tea tree oil, terpinen-4-ol
Introduction
Human demodicosis is a transmittable parasitic dermatosis caused by the acarine mite Demodex, which are found in human pilosebaceous follicles. There are two species of Demodex in humans: Demodex folliculorum which is approximately 0.3–0.4 mm long and has a longer opisthosoma and usually resides in the hair follicles, and Demodex brevis, which is approximately 0.2–0.3 mm long, and has a shorter opisthosoma and usually resides in sebaceous and Meibomian glands (Cheng et al., 2015). Demodex is most commonly found in the hair follicles of the nasolabial folds, the nose and eyelids, but other regions such as genital regions have also been reported in humans (Aylesworth and Vance, 1982; Ugras et al., 2009). They can migrate from one follicle to another during the night as bright light can deter them from coming out of the follicles in day time (Norn, 1971; Rather and Hassan, 2014). The life cycle of the Demodex mite is approximately 14–18 days from the egg to the larval stage and eventually reaches its adult stage that lasts for 5 days. The female mite may live an additional 5 days after oviposition (Rufli and Mumcuoglu, 1981). The mating of the parasite takes place in the follicle opening and eggs are laid inside the hair follicles or sebaceous glands (Maraghi et al., 2013). The mites can survive longer at lower temperatures (68 h at 20°C vs 23 h at 37°C in vitro) and they have better motility at 16–20°C (Zhao et al., 2009).
Epidemiology study of human demodicosis
Several research studies reported that the two Demodex species which can infest humans are found in all ethnic groups without any gender preference, but it may be occupation dependent (Norn, 1971; Rufli and Mumcuoglu, 1981; Lacey et al., 2009; Ozer et al., 2012; Biernat et al., 2018). Transmission occurs by human-to-human transfer of mites through close contact (Ozer et al., 2012). The Demodex rate of infestation in healthy individuals is about 80–90% (Enginyurt et al., 2015) and the number of Demodex is positively correlated with age. Human Demodex mites are present in all age groups except in newborn infants (Akcinar et al., 2018; Zhong et al., 2019) and the prevalence is as high as 100% in elderly people (Basta-Juzbasic et al., 2002; Vargas-Arzola et al., 2012). Since Demodex passes to newborns through close physical contact from their parents after birth, it is postulated that due to low sebum production in their skins, infants and children have a much lower incidence of Demodex colonization compared to adults (Basta-Juzbasic et al., 2002).
Diseases associated with human demodicosis
Demodex infection has been reported to be associated with ocular and auricular conditions such as chalaza (Liang et al., 2014), blepharo-conjunctivitis (Liang et al., 2010), blepharitis (Liu et al., 2010; Salem et al., 2013), otitis externa and myringitis (Klemm et al., 2009). Demodex infestation is also associated with dermatological conditions such as acne vulgaris (Karincaoglu et al., 2004), pityriasis folliculorum (Patrizi et al., 1997; Hsu et al., 2009), rosacea (Ozturkcan et al., 2004), perioral dermatitis (Karincaoglu et al., 2004), neutrophilic sebaceous adenitis (Liaqat et al., 2015), sebaceous adenoma (Dhingra et al., 2009), seborrheic dermatitis (Bikowski and Del Rosso, 2009), papulo-pustular eruption (Aydogan et al., 2006), alopecia (Helou et al., 2016), androgenic alopecia (Zari et al., 2008), scalp folliculitis (Fernandez-Flores and Alija, 2009; Helou et al., 2016) and nipple infection (Yokoyama et al., 2014; Hoda and Cheng, 2019). There are also associations with systemic conditions such as oily skin complexion (Porta Guardia, 2015), ageing (Baima and Sticherling, 2002), type II and gestational diabetes (Gokce et al., 2013; Keskin Kurt et al., 2014), malignancy (Erbagci et al., 2003; Inci et al., 2012; Sonmez et al., 2013), polycystic ovarian syndrome (Benk Silfeler et al., 2015), obesity (Dokuyucu et al., 2016), sickle cell anaemia (Kaya et al., 2019), immunosuppression (Cotliar and Frankfurt, 2013; Yamaoka et al., 2014; Chovatiya and Colegio, 2016; Hitraya-Low et al., 2016; Hachfi et al., 2019), malnutrition and low socioeconomic status (Kaya et al., 2013). Other factors such as alcohol dependency (Kokaçya et al., 2016b) and contact lens use (Jalbert and Rejab, 2015; Tarkowski et al., 2015) also play a part in the pathogenesis in demodicosis infection. Interestingly, Demodex infections have also been associated with psychiatric conditions such as depression (Kokacya et al., 2015) and schizophrenia (Kokaçya et al., 2016a). This may be due to the weakening of the immune system and frequently impaired social behaviours such as a lack of hygienic self-care.
Pathogenesis of human demodicosis
The pathogenesis of demodicosis involves the direct blockage of the meibomian glands (Gao et al., 2007) and skin barrier impairment of the follicles caused by the Demodex mite's mouthpiece and claws. Digestive enzymes such as protease and lipase secreted by the Demodex mites may provoke host protease-activated receptors, and may promote the expression of the anti-microbial peptide and upregulate pro-inflammatory cytokines (Bevins and Liu, 2007). The chitin exoskeleton of the mites, crystalline biological waste products and contaminant bacterium inside the mites such as Streptococci, Staphylococci, Bacillus cereus and Bacillus oleronius may trigger the inflammatory cascade by the toll-like receptor (TLR2) innate immunity pathway (Liu et al., 2010) and may help the induction of TH9 cells via IL-4 secretion (Gazi et al., 2019). Bacillus oleronius may increase the release of the 83 and 62 kDa proteins that activate the inflammatory responses in the host (Lacey et al., 2009). Bacillus cereus is strongly associated with tissue-destructive or reactive exoenzyme productions such as different kinds of haemolysins, phospholipases and enterotoxins (Tatu et al., 2016). An adaptive immune response such as CD4 helper T cells can infiltrate the site with other immune responding cells including macrophages and Langerhans cells (Georgala et al., 2001).
Genetic predisposition is an important risk factor for human demodicosis. Patients with human leukocyte antigen phenotype HLA-Cw2 and HLA-Cw4 are more susceptible to Demodex over-proliferation due to reduction in Nk2 cells and T1 cells adaptive immunity response where those with the HLA-A2 phenotype were less susceptible (Akilov and Mumcuoglu, 2003). Other haplotypes such as HLA A3-Cw4, A3-Cw2, A3-B17, A3-B35 and B35-Cw4 also showed a positive correlation with human demodicosis (Akilov and Mumcuoglu, 2003). The carbohydrate-like Tn antigen expressed by Demodex and modulation of the secretion of pro-inflammatory mediators such as interleukin (IL)-8 and tumour necrosis factor (TNF)-α from the pilosebaceous unit may play a role in interfering with the innate immune response of the host to facilitate the invasion and population expansion of Demodex (Moran et al., 2017). Moreover, infestation of mites can elevate tear cytokine levels, especially IL-1β and IL-17, which can cause inflammation of the lid margin and ocular surface in some individuals (Kim et al., 2011a; Kim et al., 2011c).
Methodology of this systematic review
This review will address the following objectives:
To examine the strengths and limitations of the current research concerning the use of tea tree oil (TTO), terpinen-4-ol (T4O), metronidazole, ivermectin, permethrin, crotamiton, lindane and miscellaneous treatments in treating human demodicosis.
To explore and determine the most effective treatments studied in this article for human demodicosis.
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009). Strategies to identify eligible studies included a systematic search of four electronic databases including Embase, PubMed, Medline and Cochrane. The search strategy covered the time period from 1970 to 2020 June 6th; therefore, this review will examine randomized control trials completed during this period in which TTO, T4O, metronidazole, ivermectin, permethrin, crotamiton, lindane, pilocarpine and miscellaneous treatments were significant in treating human demodicosis. The following search terms were used: demodicosis, Demodex, D. folliculorum, D. brevis, TTO, T4O, metronidazole, ivermectin, permethrin, crotamiton, lindane, pilocarpine, hexachlorocyclohexane (full search strategy available upon request). This search was performed with library staff to ensure inclusion of all relevant articles and Boolean operators were used with care. All references were initially reviewed by title, abstract and screened according to the following inclusion and exclusion criteria.
Inclusion criteria
Studies met inclusion criteria if they were original research; English language; prospective and retrospective data; drugs including TTO, T4O, metronidazole, ivermectin, permethrin, crotamiton, lindane, pilocarpine and miscellaneous treatments which had pharmacological significance in treating demodicosis were included; only human studies were included.
Exclusion criteria
Exclusion criteria comprised the following: review articles, theoretical discussions, conference articles, abstract articles, comment or reply articles, erratum or corrigendum. Data regarding the use of TTO, T4O, metronidazole, ivermectin, permethrin, crotamiton, lindane, pilocarpine and miscellaneous treatments not used for human demodicosis were excluded. Demodicosis not related to humans but in animals such as canine were excluded.
There was a total of 769 articles identified from all databases. After removal of duplicates, 535 articles remained. In all, 417 articles were excluded based on title and review of abstract. When there was uncertainty regarding the inclusion of an article, this was screened by two reviewers. A total of 118 full-text articles were selected. Twenty-three articles did not meet inclusion criteria and were removed, leaving a total of 95 articles for critical review (see Fig. 1). The best available National Health and Medical Research Council (NHMRC) level of evidence (LOE) available was II (randomized control trial). The studies were rated according to NHMRC (National Health and Medical Research Council, 2009) referred in Table 1.
Fig. 1.
PRISMA flow diagram of this systematic review.
Table 1.
Hierarchy of evidence provided by the National Health and Medical Research Council in Australia
| Level of evidence (LOE) | Design |
|---|---|
| I | A systematic review of level II studies |
| II | A randomized control trial |
| III-1 | A pseudo-randomized control trial (i.e. alternate allocation or some other method) |
| III-2 | A comparative study with concurrent controls:
|
| III-3 | A comparative study without concurrent controls:
|
| IV | Case series/study with either post-test or pre-test/post-test outcomes |
Throughout this review, recommendations for specific treatments are made based on the category of LOE provided by the National Health and Medical Research Council in Australia described in Table 1 and on the highest evidence available at the time of writing. In general, recommendations of a lower Roman numeral number should be considered as having a greater LOE supporting the specific treatment.
For most of the recommendations in this article, the evidence was derived from results of a recent systematic review or meta-analysis (LOE I), randomized control clinical trials (RCTs) (LOE II), and other types of clinical trials other than RCTs (LOE III 1-3) and case series or case studies (LOE IV). There are a few randomized controlled studies and open-label trial studies published evaluating therapeutic options for human demodicosis. Recommendations for a specific treatment did not take into consideration whether the products are available in a specific country and whether it is approved for the treatment of human demodicosis by the local health governing body. Pharmaceutical products and treatment protocols in the respective countries need to be verified by the treating clinicians.
Current pharmacological management of human demodicosis
A recent systematic review and meta-analysis by Navel et al. focusing on Demodex pharmacological managements identified several systemic and topical treatments and they proposed topical TTO (5–50%) or T4O (0.1–38%) with usual lid hygiene once or twice daily for the first 3 months should be the first-line treatment (LOE I) in Demodex-related infections especially if blepharitis is present. As the adverse effects such as eyelid erythema, cutaneous eczema, itching or burning sensations in orbital and surrounding area with topical uses of TTO (5–50%) (Gao et al., 2005; Gao et al., 2007; Kheirkhah et al., 2007; Liang et al., 2010; Kim et al., 2011a; Galea et al., 2014; Yam et al., 2014; Hirsch-Hoffmann et al., 2015; Nicholls et al., 2016; Karakurt and Zeytun, 2018; Ergun et al., 2019) or T4O (0.1–38%) (Tighe et al., 2013; Murphy et al., 2018; Evren Kemer et al., 2020) were very rare and benign, and systemic reactions were never reported in those trials. In second-line or in severe cases, systemic treatments such as ivermectin or metronidazole could be added (LOE I), which may also decrease recurrence in complicated cases (Navel et al., 2019). Another systematic review by Jacob et al. about Demodex-associated inflammatory skin conditions recommended metronidazole taken orally has shown efficacy in reducing Demodex density (LOE I) but the long-term outcomes of this treatment are unknown. Additionally, topical application of permethrin daily or twice daily was shown to be efficacious across multiple human dermatological studies (LOE I) but it was associated with adverse skin irritation. Crotamiton and benzyl benzoate were recommended in the same review as moderate effective treatments in reducing mite counts (LOE I) but their effects on clinical improvement are unknown. Surprisingly, TTO or T4O treatment were not investigated or mentioned in that systematic review (Jacob et al., 2019). One of the systematic reviews by Ebbelaar et al. on topical ivermectin in the treatment of papulopustular rosacea suggested that topical ivermectin 1.0% cream daily is a new and effective treatment in papulopustular rosacea because of its anti-inflammatory and acaricidal activity against Demodex mites (LOE I). Topical ivermectin demonstrated a significant reduction in inflammatory lesions compared to topical metronidazole (LOE I). Although topical ivermectin seems to be more efficacious than topical metronidazole to manage dermatological human demodicosis, with both treatment options, about 60% of patients relapsed within 36 weeks after discontinuation of treatment (Ebbelaar et al., 2018). By evaluating the results according to the systematic reviews mentioned above, TTO or T4O appear to have superior clinical outcomes compared with other treatments commercially available in terms of their efficacy and adverse effects profile.
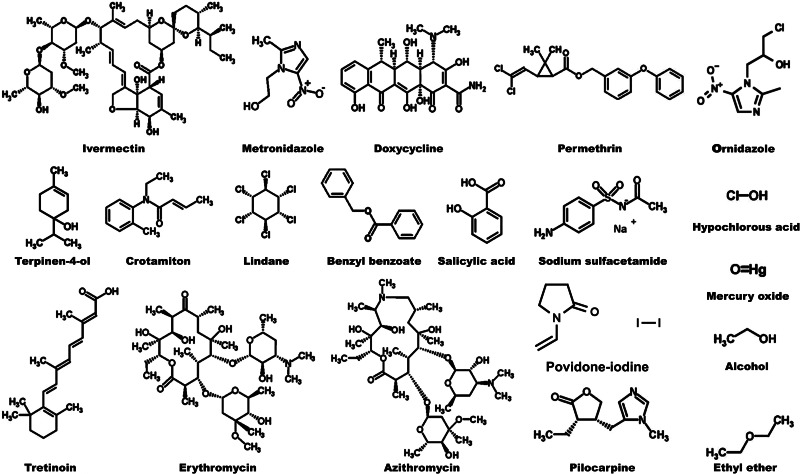
In this review, we discuss several classes of treatment in human demodicosis available in the clinical setting. TTO or T4O demonstrated complete eradication of mites and reduction of Demodex-related symptoms across many studies without any major adverse effects being reported (Gao et al., 2007; Kim et al., 2011a; Gao et al., 2012; Koo et al., 2012; Tighe et al., 2013; Galea et al., 2014; Hirsch-Hoffmann et al., 2015; Karakurt and Zeytun, 2018). Some of the treatments other than TTO or T4O may have patient compliance issues due to the side-effects or the dosing regimens (Hirsch-Hoffmann et al., 2015). So far, other than TTO and T4O, metronidazole, ivermectin, doxycycline, permethrin, crotamiton, lindane, benzyl benzoate and pilocarpine have been trialled and showed some anti-Demodex effects. The structures of the treatment molecules included in this publication are shown in Fig. 2.
Fig. 2.
The chemical structures of terpinen-4-ol, ivermectin, metronidazole, permethrin, doxycycline, crotamiton, lindane, benzyl benzoate, erythromycin, alcohol, azithromycin, ethyl ether, hypochlorous acid, mercury oxide, pilocarpine, tretinoin, salicylic acid, povidone-iodine and sodium sulfacetamide.
Tea tree oil and terpinen-4-ol
TTO is an essential oil, extracted and distillated from the leaves and terminal branches of an Australian native plant called Melaleuca alternifolia (Huynh et al., 2012). There are 15 major monoterpenes in TTO. They are T4O, γ-terpinene, α-terpinene, 1,8-cineole, p-cymene, terpinolene, α-terpineol, α-pinene, sabinene, aromadendrene, ledene, δ-cadinene, limonene, globulol and viridiflorol of the ISO 4730 T4O type TTO (ISO, 2017). Because of its acaricidal, anti-bacterial, anti-viral, anti-inflammatory, wound healing and immunomodulatory effects, TTO shows favourable results in managing human demodicosis (Lam et al., 2018). T4O is the major TTO component that may competitively block the neurotransmitter terminating enzyme acetylcholinesterase (AchE) in parasites that may mediate to the arthropodicidal effect (Mills et al., 2004); it is also a potent inhibitor of lipopolysaccharide (LPS)-induced cytokines, such as IL-1β, IL-6 and IL-10, produced by mononuclear phagocytic macrophages upon activation of TLR4 and TLR2/4; this inhibition is mediated by interfering with the NF-κB, p38 or ERK mitogen-activated protein kinase (MAPK) metabolic pathways (Nogueira et al., 2014) and thereby reducing inflammation.
The anti-demodicosis effect of TTO is still not fully understood. TTO could disrupt various membranes structures of different pathogens by its lipophilic properties (Low et al., 2017). In one of the in vitro trials, when TTO was added to suspensions containing Staphylococcus aureus cells, quick responses such as leakage of potassium ions and inhibition of cellular respiration were observed, caused by disruption of the cytoplasmic and mitochondrial membranes (Cox et al., 2000). Moreover, in S. aureus, autolysin activity of TTO was demonstrated due to the release of membrane-bound cell wall autolytic enzymes that resulted in cell death (Carson et al., 2002). TTO nanoparticles can enter various cells more efficiently through the extracellular matrix, by releasing the TTO into fungus (Souza et al., 2017) and Pseudomonas biofilms (Comin et al., 2016) resulting in the enhancement of antimicrobial effect (Rai et al., 2017), as some of the Demodex species are associated with opportunistic Pseudomonas and fungal infections (Abu-Samra and Shuaib, 2014; Vanam et al., 2018).
In a Demodex blepharitis randomized control trial by Koo et al., 50% TTO lid scrub followed by 10% TTO daily lid scrub for 4 weeks decreased mean Demodex count from 4 to 3.2 per 8 epilated lashes and the ocular surface discomfort index (OSDI) score was reduced from 34.5 to 24.1 (LOE II). Complete eradication of Demodex was demonstrated in 23.6% of patients post-treatment in the TTO intervention group. There were no major side-effects of TTO treatment reported when the patients were doing the correct scrubbing method (Koo et al., 2012).
One of the randomized controlled interventional treatment studies by Murphy et al. using TTO facewash contains 38% T4O in treating D. folliculorum blepharitis. Patients were given step-by-step instructions provided to subjects for nightly lid scrubs at home. OSDI decreased from 27.4 to 16.2 and the average count of D. folliculorum reduced from 4.9 to 1.9 per each eyelash (LOE II). No adverse effect was reported in this study (Murphy et al., 2018).
In another randomized single-blinded control trial by Karakurt and Zeytun in patients with demodectic blepharitis, twice a day 7.5% TTO eyelash shampoo was recommended to the patients. Complete eradication was attained in 4 weeks in 36% of the patients who used eyelash shampoo with TTO with the average Demodex count of 6.33 per eyelash (P < 0.001) (LOE II). Ocular symptoms and average scores also reduced significantly within the 4 weeks treatment period (P < 0.001). The results demonstrated that eyelash shampoo with TTO is three times more effective at achieving full Demodex eradication compared with the TTO-free eyelash shampoo control, significantly reducing the Demodex count, and relieving ocular symptoms in patients where full reduction cannot be attained, without adverse side-effects being reported (Karakurt and Zeytun, 2018).
One of the randomized double-blind clinical studies by Ergun et al. compared the efficacy and safety of two topical TTO-based cleansing gel formulations in the chronic blepharitis study. Patients were instructed to use the formulation twice daily for 4 weeks: cleansing gel formulation containing 3% (w/w) TTO plus calendula oil, borage oil, vitamin E, vitamin B5 <5% (w/w) in the treatment group and cleansing gel formulation containing 3% TTO only as a control group. The mean OSDI scores and the average Demodex population per eyelash decreased and fluorescein tear breakup time increased significantly in both treatment groups suggesting that basic washing gel containing only 3% TTO is also as effective as the other formula in reducing the related symptoms and complaints of patients (LOE II). No adverse effects of either formulation were reported (Ergun et al., 2019).
In an open-label trial by Gao et al., topical 5% TTO ointment was used to treat ocular pruritis associated with ocular demodicosis. Twice daily lid massage with 5% TTO ointment for 5 min was recommended to patients for 4 weeks. Demodex counts decreased from 5.5 to 0.7 per 8 epilated lashes and 16 out of 24 patients were totally free of pruritis and the remaining eight patients had different degrees of relief (LOE III-2). Lid massage with 5% TTO ointment for a period of 5 min twice per day was able to stimulate mites to emigrate from the lash follicle in the same manner as 50% TTO solution performed weekly but without the side-effects such as inflammation and irritation as mentioned in this study (Gao et al., 2012).
Another open-label controlled trial compared treatment options for Demodex blepharitis by Hirsch-Hoffmann et al., daily topical 5% TTO ointment or 0.02% TTO foam, topical or systemic metronidazole or systemic ivermectin with daily lid hygiene only as a control group for 8 weeks. The complete eradication rate and the improvement of symptoms were 6 and 40.5% in the 0.02% TTO foam group compared to 0 and 20% in topical 5% TTO ointment, respectively (LOE III-3). No adverse effects of topical TTO formulations were reported in this study (Hirsch-Hoffmann et al., 2015).
In a case series study, 5% TTO solution lid toileting followed by 5% TTO ointment at night for 12 weeks in patients with Demodex-related chronic primary conjunctivitis and dry eye disease resulted in 100 and 95% improvement of symptoms, respectively (LOE IV) (Nicholls et al., 2016). In an open-label retrospective clinical study, 50% TTO weekly lid scrub and 0.5 mL TTO shampoo lid scrub twice daily for 3 weeks had a 96.8% success rate of preventing recurrence of chalazion associated with demodicosis (LOE IV) (Yam et al., 2014). Another clinical study demonstrated that 50% TTO weekly lid scrub by clinicians and 0.5 mL TTO shampoo lid scrub twice daily for 1 month and then once daily thereafter is effective in eradicating ocular demodicosis (LOE IV) (Gao et al., 2005). In addition, an open-label trial and a case study about Demodex blepharitis showed that eyelid cleanser containing T4O eyelid rub twice daily alone resulted in significant improvement of ocular symptoms and complete eradication of mites, respectively (LOE IV) (Tighe et al., 2013; Evren Kemer et al., 2020).
Topical TTO, typically in diluted form, is usually well-tolerated. Local adverse effects are mostly skin and ocular allergic or irritant reactions to the oil, in which irritant reactions were usually concentration dependent (Hammer et al., 2006). Contact sensitization and allergic contact dermatitis reactions have been reported for TTO at 5–10%; however, latest prevalence rates in the USA suggested that only 1.4% of patients referred for patch testing had a positive reaction to TTO (Larson and Jacob, 2012). Clinical resistance of Demodex to TTO or its monoterpenes constituents has not been reported (Lam et al., 2018). The TTO and T4O dosage, direction and administration form showing effectiveness against the human demodicosis are summarized in Table 2.
Table 2.
TTO dosage and direction in treating human demodicosis
| NHMRC | Diseases | Age (years); gender | Sample size (n) | Dosage form | Administration (dose, route and time) | Effect | Reference |
|---|---|---|---|---|---|---|---|
| II | Blepharitis | Average age: 53.7 ± 10.3; male 34%, female 66% | 106 | Topical | 50% TTO lid scrub weekly followed by 10% TTO lid scrub daily for 1 month | Decrease in average Demodex count and OSDI score from 4.0 ± 2.5 to 3.2 ± 2.3 per 8 cilia and 34.5 ± 10.7 to 24.1 ± 11.9 respectively, and complete eradication of Demodex in 23.6% of patients post-treatment | (Koo et al., 2012) |
| II | Blepharitis | 49.6 ± 17.1; gender not specified | 28 | Topical | Dr Organic Tea Tree Face Wash™ (38% terpinen-4-ol) lid scrub nightly for 4 weeks | Decrease in mean Demodex count from 4.9 to 1.9 post-treatment | (Murphy et al., 2018) |
| II | Blepharitis | Mean age: 57.52 ± 14.22; male 46.7%, female 53.3% | 75 | Topical | 7.5% TTO eyelash shampoo twice daily for 4 weeks | Decrease in average Demodex count from 6.33 to 0 and 12.46 to 4.15 per eyelash in 36% and 64% patients respectively, and reduction in itching, burning, foreign body sensation, redness and cylindrical dandruff of eye post-treatment | (Karakurt and Zeytun, 2018) |
| II | Blepharitis | Mean age: 53.16 ± 9.59; male 37.5%, female 62.5% | 24 | Topical | Blefar-ex® Plus cleansing gel formulation containing 3% (w/w) TTO plus calendula oil, borage oil, vitamin E, vitamin B5 less than 5% (w/w) twice daily for 1 month | Demodex presence decreased from 54.2 to 20.6%, mean OSDI score decreased, TNF-α, IL-1β and IL-6 tear cytokines decreased | (Ergun et al., 2019) |
| II | Blepharitis | Mean age: 48.80 ± 13.22; male 52%, female 48% | 25 | Topical | Basic washing gel formulation containing 3% (w/w) TTO twice daily for 1 month | Demodex presence decreased from 42 to 27.8%, mean OSDI score decreased, TNF-α tear cytokines decreased | (Ergun et al., 2019) |
| III-2 | Ocular demodicosis | 37.2 ± 15.6; male 42%, female 58% | 24 | Topical | 5% TTO ointment lid massage twice per day for 4 weeks | Decrease in mean Demodex count from 5.5 ± 1.6 to 0.7 ± 0.8 per 8 epilated lashes with complete eradication of Demodex in 11 patients post-treatment | (Gao et al., 2012) |
| III-3 | Blepharitis | Not specified | 7 and 45 for ointment and foam regimen respectively | Topical | 5% TTO ointment to lid margins and lashes nightly for 2 months or 0.02% diluted TTO foam to clean eyelids, eyebrow and face skin daily for 2 months | Complete eradication of Demodex in 0% patients and 20% with improvement of symptoms for 5% TTO regimen post-treatment; complete eradication of Demodex in 6% patients and 40.5% with improvement of symptoms for 0.02% diluted TTO regimen after 2 months | (Hirsch-Hoffmann et al., 2015) |
| IV | Blepharitis | Mean age: 52.8 ± 15.8; male 53.6%, female 46.7% | 30 | Topical | 0.1% Terpinen-4-ol soaked wipes to eyelids twice daily for 2 weeks, followed by 7–10 day break followed by the same treatment again; patients were examined after the first and second cycle of treatment and after 1 year | Significant improvement in Schirmer test, ocular surface disease index, lid margin score, meibomian gland expressibility scores, and Oxford grade after the first cycle of treatment; the improvement in symptoms and tear function tests of patients after the second cycle was significantly better than in pre-treatment levels |
(Evren Kemer et al., 2020) |
| IV | Blepharitis | Average age: 48.3 ± 18.9; male 40%, female 60% | 10 | Topical | 50% TTO weekly lid scrub and 10% TTO shampoo lid scrub twice daily for 1 month | Decrease in mean Demodex count from 3.8 ± 2.2 to 0.2 ± 0.4 per eye, significant decrease in mean tear concentrations of cytokines for IL-1β and IL-17 from 1141.5 ± 440.3 to 561.7 ± 261.0 and 1,907.8 ± 861.0 to 1124.2 ± 545.1 pg mL−1 respectively, and complete eradication of Demodex in 10 of 13 eyes post-treatment | (Kim et al., 2011a) |
| IV | Blepharitis | Average age: 49.3 ± 17; male 66.7%, female 33.3% | 6 | Topical | 50% TTO lid scrub weekly and tea tree shampoo lid scrub daily for 6 weeks | Decrease in mean Demodex count from 6.8 ± 2.8 to 1 ± 0.9 per 8 lashes, all patients showed subjective improvement of ocular surface irritation and pain with complete disappearance of conjunctival redness and regression of corneal superficial vascularization at mean follow-up of 7.9 ± 7.7 months | (Kheirkhah et al., 2007) |
| IV | External ocular diseases | Average age: 62; male 25%, female 75% | 333 | Topical | Eyelid toileting followed by 5% tea tree ointment nightly for 3 months | 91% of patients with improvement of symptoms post-treatment | (Nicholls et al., 2016) |
| IV | Ocular demodicosis | 60.2 ± 11.6; male 54.5%, female 45.5% | 11 | Topical | 50% TTO office lid scrub weekly and 0.5 mL tea tree shampoo lid scrub twice daily for 4 weeks | Complete eradication of Demodex in 8 patients in <4 weeks with 9 patients experiencing 50–100% improvement in symptoms | (Gao et al., 2007) |
| IV | Ocular demodicosis | 59.86 ± 8.7; gender not specified | 9 | Topical | 50% TTO office lid scrub weekly and 0.5 mL tea tree shampoo lid scrub twice daily for 4 weeks | Complete eradication of Demodex in 5 and 2 patients in 3 and 4 weeks respectively | (Gao et al., 2005) |
| IV | Blepharoconjunctivitis | Age range: 2.5–11; male 41.7%, female 58.3% | 12 | Topical | 50% TTO eyelid scrubs 3 times weekly for 4–6 weeks or 5% TTO ointment eyelid massage twice daily for 4–6 weeks | Decrease in Demodex count with resolution of ocular irritation and inflammation in all patients after 1 week, all corneal signs resolved in 2 weeks | (Liang et al., 2010) |
| III-3 | Recurrent chalazion | 39.1 ± 10.2; male 46.7%, female 53.3% | 30 | Topical | 50% TTO lid scrub weekly and 0.5 mL tea tree shampoo lid scrub twice daily for 3 weeks | 96.8% success rate of preventing recurrent chalazion associated with demodicosis at mean follow-up of 10.0 ± 3.0 months | (Yam et al., 2014) |
| IV | Blepharitis | 60; male | 1 | Topical | 5% TTO and 50% tea tree lid scrub for 3 months | Improvement of blepharitis with reversal of ectropion, light microscopy of multiple eyelash confirmed eradication of Demodex post-treatment | (Galea et al., 2014) |
| IV | Blepharitis | 61; female | 1 | Topical | Cliradex™ (lid cleanser containing water and T4O) eyelid rub twice daily for 8 weeks | Marked resolution of symptoms and repeated examination showed clearer lashes and no Demodex post-treatment | (Tighe et al., 2013) |
Metronidazole
Metronidazole is a synthetic nitroimidazole derivative, which is active against parasitic and anaerobic bacterial infections by inhibiting nucleic acid synthesis thereby disrupting the DNA of microbial cells. Since Demodex infection is one of the important factors in rosacea (Holmes, 2013), metronidazole's mechanism of action in rosacea is related both to the agent's anti-inflammatory and anti-oxidant effects (Schaller et al., 2016). Metronidazole interferes with granulocytic neutrophil release of reactive oxygen species (ROS) production and inactivating existing ROS, which decreases the release of further pro-inflammatory cytokines (Narayanan et al., 2007). Topical metronidazole has demonstrated variable efficacy in various clinical studies (Kocak et al., 2002; Hsu et al., 2009). A randomized double-blind placebo-controlled study for Demodex-related papulopustular rosacea showed that topical metronidazole 0.75% twice a day for 8 weeks could significantly improve erythema, the number of papules and pustules, and lowered the Demodex count compared to placebo (Kocak et al., 2002) (LOE II). In one case series on dermatological demodicosis, it was demonstrated that topical metronidazole 2% twice daily for 45 days appeared to be effective in controlling Demodex infection in terms of the Demodex count but long-term benefits and risks are unknown (Forton et al., 1998) (LOE III-3). An open trial study on Demodex acne rosacea demonstrated that topical metronidazole 0.75% gel twice daily for 8 weeks resulted in marked clinical symptoms improvement and complete remission and eradication of Demodex (LOE III-3) (Ozturkcan et al., 2004). Moreover, in some report series studies of Demodex-related dermatological conditions, oral metronidazole may be effective eradicating mites and relieve the overall dermatological signs and symptoms but relapse happened post-treatment in some cases (LOE IV) (Hsu et al., 2009; Helou et al., 2016). Adverse effects include the gastrointestinal side-effects such as metallic taste in the mouth, nausea, vomiting, a disulfiram-like reaction with ingestion of alcohol (Swygard et al., 2004) and neurological side-effects such as seizure, encephalopathy and ataxia (Sunderkotter et al., 2006). Metronidazole is safe in pregnancy, breastfeeding and young infants (Hall et al., 1983; Passmore et al., 1988; Einarson et al., 2000), although it has carcinogenic potential (Sunderkotter et al., 2006). The metronidazole dosage, direction and administration form showing effectiveness against the human demodicosis are summarized in Table 3.
Table 3.
Metronidazole dosage and direction in treating human demodicosis
| NHMRC | Diseases | Age (years); gender | Sample size (n) | Dosage form | Administration (dose, route and time) | Effect | Reference |
|---|---|---|---|---|---|---|---|
| II | Papulopustular rosacea | Mean age: 51; male 23.8%, female 76.2% | 20 | Topical | 0.75% gel twice daily for 2 months | Decrease in mean Demodex count, erythema score, papules and pustules from 2.60 ± 0.74 to 2.00 ± 0.56, 2.85 ± 0.36 to 1.40 ± 0.68, 8.00 ± 6.70 to 2.90 ± 2.80 and 4.90 ± 4.78 to 2.40 ± 3.10 respectively post-treatment | (Kocak et al., 2002) |
| III-3 | Acne rosacea | Mean age: 46.96 ± 1.74; male 27.6%, female 72.4% | 29 | Topical | 0.75% gel twice daily for 2 months | 73.3, 26.7 and 17.2% patients showed marked clinical improvement, complete remission and complete eradication of Demodex respectively post-treatment | (Ozturkcan et al., 2004) |
| III-3 | Blepharitis | Not specified | 5 for topical or oral regimen | Topical or oral | 2% ointment nightly for 2 months or 500 mg twice daily for 10 days | Complete eradication of Demodex in 0% patients and 20% with improvement of symptoms for topical or oral regimen after 2 months | (Hirsch-Hoffmann et al., 2015) |
| III-3 | Demodicosis | Not specified | 6 | Topical | 2% twice daily for 45 days | Normalization of Demodex count in 2 patients and reduced Demodex count in 4 patients post-treatment | (Forton et al., 1998) |
| IV | Acne rosacea | 64; male | 1 | Oral | 500 mg twice daily for 4 weeks | Complete resolution at 3rd week follow-up | (Hsu et al., 2009) |
| IV | Demodex abscess | 53; male | 1 | Oral | 250 mg three times daily for 2 weeks | Complete recovery after 2 weeks and at 9 months follow-up respectively, repeated scrapings remained negative for Demodex | (Schaller et al., 2003) |
| IV | Demodex folliculitis | 38; female | 1 | Oral | 250 mg three times daily for 1 week | Complete resolution at 15th month follow-up | (Hsu et al., 2009) |
| IV | Demodex folliculitis | 27; female | 1 | Oral and topical | Oral 250 mg three times daily for 3 weeks and topical 0.75% gel twice daily for 3 weeks | Complete resolution at 82nd month follow-up with two relapses at 40th and 47th month | (Hsu et al., 2009) |
| IV | Demodex folliculitis | 40; male | 1 | Oral | 1 g daily for 3 weeks | Papulopustular eruption resolved without Demodex mites on previously affected areas post-treatment | (Aydogan et al., 2006) |
| IV | Demodex folliculitis | 27 and 50; male | 2 | Oral | 500 mg twice daily for 2 weeks or 2 months | Complete cessation of hair loss, erythema and pustules post-treatment | (Helou et al., 2016) |
| IV | Demodex folliculitis | 7; male | 1 | Topical | Cream twice daily for 9 months | Improvement within 1 month without resolution of rash after 9 months | (Herron et al., 2005) |
| IV | Demodicosis | 53; male | 1 | Oral and topical | Oral 250 mg three times daily for 3 weeks and topical 0.75% gel twice daily for 3 weeks | Complete resolution at 2nd week follow-up | (Hsu et al., 2009) |
| IV | Demodicosis | 18; female | 1 | Oral and topical | Oral 250 mg three times daily for 3 weeks and topical 0.75% gel twice daily for 3 weeks | Complete resolution at 2nd week follow-up | (Hsu et al., 2009) |
| IV | Demodicosis | 52; male | 1 | Oral | 250 mg three times daily for 1 week | Complete resolution without follow-up | (Hsu et al., 2009) |
| IV | Eosinophilic folliculitis | 42; female | 1 | Topical | 0.75% gel twice daily for 2 weeks | Complete resolution without follow-up | (Hsu et al., 2009) |
| IV | Neutrophilic sebaceous adenitis | 61; male | 1 | Topical | Gel for 3 days | Disappearance of plaque after 3 days | (Liaqat et al., 2015) |
| IV | Plaque forming demodicosis | 88; female | 1 | Topical | 0.75% gel twice daily for 3 months | Improvement after 1 month and complete resolution post-treatment | (Fichtel et al., 2005) |
| IV | Rosacea | 60; female | 1 | Topical | 0.75% gel twice daily for 1 week | Complete resolution without follow-up | (Hsu et al., 2009) |
| IV | Rosacea-like demodicosis | Age range: 10 months–5 years; male 50%, female 50% | 8 | Topical | 1% gel twice daily for 3–4 weeks | Complete eradication of Demodex in 50% patients post-treatment and complete recovery in all patients at 12–38th month follow-up | (Patrizi et al., 1997) |
Ivermectin
Ivermectin is a macrocyclic lactone derived from the soil bacterium Streptomyces avermectinius. Except for its well-known anti-onchocerca and anti-filarial effects in the world public health campaigns, ivermectin also demonstrates anti-parasitic activities against many other parasitic infections, such as human demodicosis. The anti-parasitic activity of ivermectin is possibly due to its selectively binding to glutamate or γ-aminobutyric acid (GABA) in the peripheral motor synapses of neurons, resulting in the permanent opening of chloride ion channels. The chloride ion influx might inhibit the neuronal and muscular activities in the parasite and subsequently cause paralysis and death of the parasite (Dourmishev et al., 2005; Kobylinski et al., 2012). The anti-inflammatory properties of ivermectin are likely due to the inhibition of phosphorylation of the MAPKs, JNK and p38 pathways as well as blocking the translocation of the transcription factor NF-κB that results in decreasing LPS-induced inflammation (Zhang et al., 2008; Zhang et al., 2009). The reduction of LPS-induced inflammation may decrease neutrophil phagocytosis and chemotaxis, inhibit pro-inflammatory cytokines such as IL-1β, IL-8 and TNF-α, and upregulate the anti-inflammatory cytokine IL-10 (Stein et al., 2014; Abokwidir and Fleischer, 2015). Compared with metronidazole, ivermectin is superior in reducing inflammatory lesions, with a faster onset of action for papulopustular rosacea (Taieb et al., 2016). The benefit of ivermectin in Demodex-related dermatological conditions is probably due to its anti-parasitic, anti-inflammatory and immunomodulatory properties. A single-blind, randomized controlled trial in patients with anterior blepharitis or skin lesions caused by Demodex evaluated the efficacy of ivermectin compared to ivermectin plus metronidazole. Two doses of ivermectin 200 μg kg−1 orally given 1 week apart or metronidazole 250 mg orally three times daily for 2 weeks in addition to the two doses of ivermectin. At week 4, there was a significant decrease in Demodex count, 71.6% showed complete remission on combined therapy, 26.7% showed marked clinical improvement and <2% of patients showed no clinical improvement, and compared favourably to those patients on ivermectin therapy alone (LOE III-1). No adverse events were reported (Salem et al., 2013). An open-label trial about treatment options in Demodex blepharitis, oral ivermectin 6 mg single dose and repeated after 2 weeks improved symptoms in 35% and eradicated Demodex in 6% at 8 weeks follow-up without any adverse effects reported (LOE III-3) (Hirsch-Hoffmann et al., 2015). Ivermectin can reduce the mite count (LOE IV) (Holzchuh et al., 2011; Ruini et al., 2017), and relieve the Demodex-related signs and symptoms in both ocular and dermatological demodicosis (LOE IV) (Filho et al., 2011; Friedman et al., 2017; Kaser et al., 2017). However, there has been one human Demodex ivermectin-resistant case reported recently (LOE IV) (Hervás Ontiveros et al., 2014). Adverse effects of topical ivermectin are mostly mild. They include dermatological side-effects such as skin irritation, allergic dermatitis, exacerbation of rosacea, erythema, pruritus and general flushing (Taieb et al., 2015). Systemic ivermectin treatment may result in transient and mild systemic adverse reactions including anorexia, asthenia, headache, arthralgia, myalgia, fever, eosinophilia, and maculopapular rashes (Hengge et al., 2006). Ivermectin should be avoided in pregnancy, breastfeeding and children weighing <15 kg (Hay et al., 2012). The ivermectin dosage, direction, administration form showing effectiveness against the human demodicosis are summarized in Table 4.
Table 4.
Ivermectin dosage and direction in treating human demodicosis
| NHMRC | Diseases | Age (years); gender | Sample size (n) | Dosage form | Administration (dose, route and time) | Effect | Reference |
|---|---|---|---|---|---|---|---|
| III-1 | Acne vulgaris, blepharitis, peri-oral dermatitis or rosacea | Mean age: 36.71 ± 12.4; male 48.4%, female 51.6% | 60 | Oral | 200 μg kg−1 2 doses 1 week apart | Decrease in mean Demodex density from 12.3 ± 3.2 to 2.3 ± 2.7, 12.8 ± 6.8 to 5.3 ± 5.4, 21.3 ± 7.5 to 1.7 ± 1.7 and 51.7 ± 20.8 to 31.4 ± 18.9 mites per cm2 for acne vulgaris, blepharitis, peri-oral dermatitis and rosacea respectively at 4th week follow-up | (Salem et al., 2013) |
| III-3 | Blepharitis | Not specified | 32 | Oral | 6 mg single dose and repeated after 2 weeks | Complete eradication of Demodex in 6% patients and 35% with improvement of symptoms after 2 months | (Hirsch-Hoffmann et al., 2015) |
| IV | Blepharitis | Mean age: 50.4 ± 21.0; male 25%, female 75% | 12 | Oral | 200 μg kg−1 2 doses 1 week apart | Decrease in average number of mites from 4.79 to 0.54 in superior and inferior lashes with improvement in tear break-up time, and Schirmer I test results 28 days post-treatment | (Holzchuh et al., 2011) |
| IV | Blepharitis | Mean age: 65.75; male 69.02%, female 31.58% | 19 | Oral | 6 mg twice for daily and repeated after 2 weeks | Symptoms improved in 84.3% patients with complete eradication of Demodex 3 months post-treatment | (Filho et al., 2011) |
| IV | Demodex folliculitis | 46; female | 1 | Oral | 12 mg single dose | Complete resolution of skin lesions within 24 h | (Cotliar and Frankfurt, 2013) |
| IV | Demodicosis | 25; female | 1 | Oral and topical | Oral 200 μg kg−1 single dose followed by topical ivermectin (10% dilution in propylene glycol) locally for 1 month 1 month later | Clinical improvement with decrease in papulopustules and only dead parasites on lesion scrapings after oral treatment, papulopustular lesions reappeared 1 month later which was resolved by topical treatment | (Clyti et al., 2006) |
| IV | Demodicosis | 38; female | 1 | Oral | 200 μg kg−1 single dose | Eradication of demodicosis | (Clyti et al., 2006) |
| IV | Demodicosis | Age not specified; female | 1 | Oral | 200 μg kg−1 single dose | Dead or only slightly mobile Demodex in scrapings 1 week post-treatment and eradication of demodicosis at day 15 | (Clyti et al., 2006) |
| IV | Demodicosis | 34; male | 1 | Topical | 1% nightly for 2 weeks | Significant improvement after 2 weeks | (Friedman et al., 2017) |
| IV | Papulopustular rosacea | 63; male | 1 | Topical | 1% cream for 4 weeks | Clinical improvement with reduction of Demodex density and inflammatory components within 4 weeks | (Kaser et al., 2017) |
| IV | Papulopustular rosacea | Mean age: 56; male 25%, female 75% | 50 | Topical | 1% nightly over 16 weeks | Complete eradication of Demodex and decreased inflammatory lesions post-treatment | (Trave et al., 2019) |
| IV | Papulopustular rosacea | Average age: 52.3; 40% male, 60% female | 20 | Topical | 1% cream daily over 12 weeks | Decrease in mean Demodex count from 99.9 to 3.8 per cm2 after 6 weeks and to 0.8 cm2 after 12 weeks of treatment with 80% of patients reaching therapeutic success | (Schaller et al., 2017) |
| IV | Papulopustular rosacea | Mean age: 58; male 50%, female 50% | 10 | Topical | Soolantra™ 10 mg g−1 Crème daily for 4 weeks | Reduction in erythematic papulopustules and subjective symptoms, > 50% reduction of number of mites in 50% of patients, and 10% of patients had complete recovery with disappearance of mites after 4 weeks | (Ruini et al., 2017) |
| IV | Papulopustular rosacea or peri-oral dermatitis | Mean age: 9.8 ± 2.2; male 40%, female 60% | 15 | Oral or topical | Oral 200–250 μg kg−1 single dose or topical 1% cream daily for 3 months | 93% of patients achieved complete or almost complete clearance of lesions at mean follow-up of 11.9 ± 7.1 months | (Noguera-Morel et al., 2017) |
| IV | Rosacea | 12; female | 1 | Oral | 12 mg single dose | Improvement of lesions and progressive resolution of ocular symptoms at 1 month follow-up | (Brown et al., 2014) |
Permethrin
Permethrin is a synthetic pyrethroid agent derived from Chrysanthemum cinerariifolium (Casida, 1980). It is insecticidal and scabicidal with selectively low mammalian toxicity properties (Casida, 1980). Permethrin may slow the rate of the voltage-gated sodium channel closure of arthropods, resulting in prolonged depolarization of nerve-cell membranes that could disrupt neurotransmission, causing nerve depolarization and hyper-excitation result in muscle paralysis and death of the parasite (Zlotkin, 1999). The neurotoxic effect of permethrin on vertebrates is relatively mild compared to invertebrates due to structural differences in voltage-gated sodium channels between vertebrates and invertebrates (Zlotkin, 1999). Moreover, it may also interfere with the GABA receptor chloride ionophore complexes and neurotransmitters (Imamura et al., 2000; Wang et al., 2016). The anti-demodicosis mechanism is unknown, but topical 5% permethrin twice daily for 8–12 weeks can decrease the Demodex mite count (LOE II) (Raoufinejad et al., 2016), and improve the erythema score (LOE II) (Kocak et al., 2002) and dermatological signs and symptoms (LOE IV) (Morras et al., 2003). One of the randomized, double-blind, placebo-controlled studies evaluated the effect of permethrin 5% cream or placebo twice daily in the management of Demodex papulopustular rosacea. From baseline to day 60, the extent of erythema, the number of papules and Demodex count showed a significant reduction in the permethrin group (LOE II). Side-effects were evaluated and no complications were reported during the study (Kocak et al., 2002). However, it is important to mention that while permethrin did cause some skin irritation and erythema, it did not result in discontinuation of therapy in some Demodex clinical studies (Forton et al., 1998; Jansen et al., 2001; Morras et al., 2003) (LOE III-3 to LOE IV). Permethrin is poorly absorbed through the skin and the small percentage absorbed is then metabolized rapidly and excreted in the urine in the form of inactive metabolites (Tomalik-Scharte et al., 2005). Adverse effects including dermatological effects such as pruritus, numbness, erythema, paresthesia; systematic effects such as headache, dizziness, muscle spasms, convulsions and dystonic action of the neck have been reported (Coleman et al., 2005; Raoufinejad et al., 2016). Topical use of permethrin is safe for pregnancy, breastfeeding and infants more than 2 months old (Hay et al., 2012). The strength, direction and administration form showing effectiveness against the human demodicosis are summarized in Table 5.
Table 5.
Permethrin dosage and direction in treating demodicosis
| NHMRC | Diseases | Age (years); gender | Sample size (n) | Dosage form | Administration (dose, route and time) | Effect | Reference |
|---|---|---|---|---|---|---|---|
| II | Papulopustular rosacea | Mean age: 51; male 23.8%, female 76.2% | 23 | Topical | 5% cream twice daily for 2 months | Decrease in mean Demodex count, erythema score, papules and pustules from 2.20 ± 1.04 to 0.65 ± 0.71, 2.60 ± 0.48 to 1.34 ± 0.7, 6.04 ± 7.60 to 1.73 ± 2.20 and 2.30 ± 3.73 to 0.56 ± 1.27 respectively post-treatment | (Kocak et al., 2002) |
| II | Papulopustular rosacea | Mean age: 42.2 ± 7.8; male 15%, female 85% | 20 | Topical | 5% twice daily for 12 weeks | Decrease in median Demodex density from 274.1 to 8.1 with reductions of papules, pustules and telangiectasia post-treatment | (Raoufinejad et al., 2016) |
| III-2 | Demodicosis | Mean age: 35.7 ± 11.40; male 12.5%, female 87.5% | 26 | Topical | 5% cream twice daily for 15 days | Complete eradication of median Demodex in patients who had erythema, facial itching and pityriasiform squame lesions respectively post-treatment | (Karincaoglu et al., 2004) |
| III-3 | Demodicosis | Not specified | 6 | Topical | 1% once every 2 days for 45 days | Average Demodex decreased by 20.3% with normalization of Demodex count in 0 patients and reduced Demodex count in 4 patients post-treatment | (Forton et al., 1998) |
| IV | Blepharitis | Mean age: 57.2 ± 16.8; male 38%, female 62% | 21 | Topical | 5% cream daily for 6 months | Decrease in mean Demodex count from 1.36 ± 1.233 to 0.48 ± 0.6 per eyelash with improvement in blepharitic symptoms and clinical findings at 4–6th month follow-up | (Hecht et al., 2019) |
| IV | Demodex folliculitis | 21 months; male | 1 | Topical | 5% cream for three consecutive nights weekly for 3 weeks | Incomplete resolution post-treatment | (Herron et al., 2005) |
| IV | Demodicosis | 2; female | 1 | Topical | 5% cream for 1 night | Eruption cleared after overnight treatment | (Sahn and Sheridan, 1992) |
| IV | Rosacea-like demodicosis | 4; male | 1 | Topical | 1.5% nightly for 1 week | Complete disappearance of lesions in 1 month and 1 year follow-up | (Morras et al., 2003) |
| IV | Rosacea-like demodicosis | 35; male | 1 | Topical | 5% cream twice daily for 4 weeks | Complete resolution of skin lesions and pruritus with few Demodex post-treatment | (Jansen et al., 2001) |
Crotamiton
Crotamiton is a pale yellow oil with a faint amine-like odour used as an anti-scabies, anti-bacterial and anti-pruritic for many dermatological conditions (Hausen and Kresken, 1988). It is a common scabies treatment recommended for newborn babies and infants (Hengge et al., 2006). The anti-pruritic effect is believed due to crotamiton's moderate inhibition of histamine, serotonin and PAR-2 agonist that is TRPV-1 independent and produces the counter-irritation and cooling effect (Sekine et al., 2012). Crotamiton can reduce the Demodex mite count (LOE IV) (Purcell et al., 1986) and improve the Demodex-related dermatological signs and symptoms (LOE IV) (Bikowski and Del Rosso, 2009; Hsu et al., 2009). An open-label, randomized study with patients suffering from a variety of Demodex skin conditions showed that 10% crotamiton was applied daily for 45 days, achieved normalization of Demodex count which was observed after the treatment period in one patient only and reduction of Demodex count occurred in other participants (LOE III-3) (Forton et al., 1998). Adverse effect included dermatological side-effects such as flushing, irritation, conjunctivitis and contact dermatitis (Hengge et al., 2006). It is safe for pregnancy, breastfeeding and young infants (Hay et al., 2012). The strength, direction, administration form showing effectiveness against the human demodicosis are summarized in Table 6.
Table 6.
Crotamiton therapy dosage and direction in treating human demodicosis
| NHMRC | Diseases | Age (years); gender | Dosage form | Administration (dose, route and time) | Effect | Reference |
|---|---|---|---|---|---|---|
| IV | Atopic dermatitis | 14 months; male | Topical | Cream daily for 3 nights | Lesions cleared post-treatment | (Won et al., 1993) |
| IV | Demodex dermatitis | Mean age: 50.4; male 44.4%, female 55.6% | Topical | 10% twice daily | 50% reduction in erythema, dryness, scaling, roughness, papules and/or pustules in 56 of 62 patients at average follow-up of 18.6 days | (Bikowski and Del Rosso, 2009) |
| IV | Demodex folliculitis | 49; male | Topical | Cream twice daily for 17 days | Decreased number of Demodex to 8 in affected area and pruritic eruption resolved in 72 h | (Purcell et al., 1986) |
| IV | Acne-rosacea like demodicosis | 1; female | Topical | 10% daily for 1 week | Complete resolution without follow-up | (Hsu et al., 2009) |
| IV | Demodicosis | 24; female | Topical | Cream daily | Disappearance of lesions 5 days post-treatment and dermatitis lasted for about 1 month | (Banuls et al., 1991) |
| III-3 | Demodicosis | Not specified | Topical | 10% daily for 45 days | Average Demodex decreased by 64.4% with normalization of Demodex count in 1 of 6 patients and reduced Demodex count in 6 of 6 patients post-treatment | (Forton et al., 1998) |
| IV | External genitalia demodicosis | 43; male | Topical | Cream for 3 nights | Clearing of multiple, match-head-sized, skin-coloured papulous lesions on penile shaft and scrotum | (Hwang et al., 1998) |
Lindane
Lindane, also known as γ hexachlorocyclohexane or benzene hexachloride, is an organochloride of the cyclohexane family. It acts on the GABA-gated chloride channels as a neurotoxin in arthropods, leading to paralysis and death of the parasite (Turberg, 2015). One of the open-label, randomized trials with patients suffering from a variety of dermatological Demodex conditions demonstrated that topical lindane 1% every second day for 15 days could reduce Demodex mite count but failed to achieve normalization of mites (LOE III-3). Longer treatment is not recommended due to its potential risks of intoxication (Forton et al., 1998). A case series study about dermatological demodicosis showed that lindane topical 1% daily for 2 weeks resulted in complete resolution of any clinical signs of pityriasis folliculorum demodicosis even at the 3 months follow-up (LOE IV) (Hsu et al., 2009). The adverse effects of lindane include dermatological side-effects such as irritation and allergic contact dermatitis; as well as neurological side-effects such as insomnia, irritability, vertigo, convulsions, restlessness and collapse (Hengge et al., 2006). Lindane needs to be avoided in pregnancy, breastfeeding and infant (Hay et al., 2012). The strength, direction, administration form showing effectiveness against the human demodicosis are summarized in Table 7.
Table 7.
Lindane therapy dosage and direction in treating human demodicosis
| NHMRC | Diseases | Age; gender | Dosage form | Administration (dose, route and time) | Effect | Reference |
|---|---|---|---|---|---|---|
| IV | Demodex folliculitis | 45; male | Topical | 1% lotion for 1 night | Complete resolution at 2nd week follow-up | (Ashack et al., 1989) |
| IV | Demodicosis | Not specified | Topical | 1% once every 2 days for 15 days | Normalization of Demodex in 0 patients and reduction of Demodex in 4 of 6 patients after 15 days | (Forton et al., 1998) |
| IV | Otitis externa and myringitis | 84; female | Topical | Daily for 10 days | Complete eradication of mites, remission of all symptoms and normal clinical presentation of ear at 1 year follow-up | (Klemm et al., 2009) |
| IV | Papulonodular demodicosis | 49; male | Topical | 1% lotion for 3 consecutive nights | Clearance of lesions overnight with normalization of Demodex post-treatment | (Dominey et al., 1989a) |
| IV | Rosacea | 28; female | Topical | 1% daily for 2 weeks | Complete resolution at 3rd month follow-up | (Hsu et al., 2009) |
Miscellaneous treatments
Doxycycline
Doxycycline is a board-spectrum bacteriostatic antibiotic that inhibits the bacterial ribosomal protein synthesis unit to prevent bacteria from reproducing through the inhibition of protein synthesis. Doxycycline inhibits the production and activity of matrix metalloproteinases (MMPs, especially MMP-9) directly and kallikrein (KLK) indirectly (Kim et al., 2005; Di Nardo et al., 2016). In vitro study shows corneal epithelial cells from human telomerase immortalized corneal epithelial cell line (hTCEpi) exposed to Demodex endogenous bacterial B. oleronius proteins can upregulate the expression of many pro-inflammatory mediators including IL-1β, IL-6, IL-8, TNF-α, cathelicidin, MMP-3 and MMP-9 (O'Reilly et al., 2012; McMahon et al., 2016). The chitin from the exoskeleton of Demodex may activate TLR2 receptors in keratinocyte, resulting in increased IL-8, TNF-α, cyclooxygenase-2 and the inflammasome, which subsequently increased KLK-5, LL-37, cathelicidin and MMP-9 (Casas et al., 2012; Woo et al., 2016). Increased MMP-9 levels have been observed in patients with ocular rosacea, and with some of these patients displaying recurrent corneal erosion, therapeutic treatment often includes the use of the doxycycline (Dursun et al., 2001). In addition, the inhibition of the nitric oxide (NO) synthase activity by doxycycline might explain the anti-inflammatory action in rosacea-associated vasodilation (Woo et al., 2016). Doxycycline has been demonstrated to inhibit neutrophil activity and several pro-inflammatory reactions including those associated with phospholipase A2, endogenous NO, TNF-α, IL-6, IL-8 and IL-10 (Bikowski, 2003; Baldwin, 2006; Jantzie and Todd, 2010). However, oral doxycycline is never used as a monotherapy in treating Demodex-related conditions. It is always combined with other therapeutic agents such as metronidazole or prednisolone to control the disease (LOE IV) (Hsu et al., 2009; Sattler et al., 2015; Douglas and Zaenglein, 2019). A case study of D. folliculorum-associated oculocutaneous rosacea showed that patients using oral doxycycline 100 mg daily and topical tacrolimus for 8 weeks achieved temporary improvement in the Demodex skin lesions but the ocular symptoms persisted. However, the patient had a flare 4 weeks after discontinuing doxycycline and restarting the doxycycline did not result in improvement (LOE IV) (Brown et al., 2014). Sub-anti-microbial dosing of doxycycline, e.g. 20 mg twice a day or 40 mg daily, was likely as effective or more effective than anti-microbial doses (⩾50 mg per day), in decreasing the release of pro-inflammatory cytokines and downregulating the production of ROS (Wise, 2007), but with much fewer adverse effects such as nausea, diarrhoea and lower the risk of developing bacteria resistance (van Zuuren et al., 2015; Asai et al., 2016). The adverse effects of doxycycline include oesophageal erosion, gastrointestinal discomfort and photosensitivity (Smith and Leyden, 2005). Doxycycline is not recommended in pregnancy, breastfeeding and infant (Aupee et al., 2009).
Benzyl benzoate
Benzyl benzoate is an organic ester that can be rapidly hydrolysed to benzoic acid and benzyl alcohol which are neurotoxic to the mites and active against their ova (Buffet and Dupin, 2003). One of the open-label, randomized studies with patients suffering from a variety of Demodex skin diseases showed that benzyl benzoate 10% applied to the affected area twice daily can reduce Demodex mite count by 98.2% and improve dermatological signs and symptoms with only moderate irritation (LOE III-3) (Forton et al., 1998). Adverse effects including transient skin irritation, burning sensation and post-treatment eczematous reaction happened in high strength preparations of ≥25% (Brooks and Grace, 2002). Topical use of benzyl benzoate is safe during second or third trimester pregnancy (Mytton et al., 2007), breastfeeding (Hengge et al., 2006) and in children that are more than 1 year old (Sunderkotter et al., 2007).
Pilocarpine
Pilocarpine is a well-known treatment for glaucoma in humans. It is a cholinergic parasympathomimetic agent acting primarily as a non-selective muscarinic agonist that may cause paralysis of mites’ respiration and mobility (Celorio et al., 1989; Fulk et al., 1996). A case series on ocular demodicosis demonstrated that 4% pilocarpine gel spread once in the evening on the base of eyelashes, and removed in the morning for 2 weeks, could reduce the number of mites and alleviate the related symptoms (LOE IV) (Fulk et al., 1996). No side-effects were observed secondary to pilocarpine when applied to the lid margins only (Fulk et al., 1996). Another study also confirms its efficacy for the miticidal effect (LOE IV) (Celorio et al., 1989). Pilocarpine is not recommended during pregnancy, and the use of pilocarpine in breastfeeding mothers and young children should be done cautiously (Coppens et al., 2010).
Other treatments
Several topical treatments have been found useful to relieve or control human Demodex conditions (LOE II to IV), such as sulphur (Robinson, 1965; Nakagawa et al., 1996; Forton et al., 1998; Sarro et al., 1998; Gao et al., 2005; Herron et al., 2005), 0.01–0.02% hypochlorous acid (Bachir and Bitton, 2015; Roan, 2016), 0.25% povidone-iodine gel (Pelletier et al., 2017), 99.9% ethyl-ether solution (Hervás Ontiveros et al., 2014), 2% mercury oxide ointment (Rodríguez et al., 2005; Anane et al., 2011), 100% alcohol (Gao et al., 2005), camphorated oil (Czepita et al., 2007; Liu et al., 2010), salicylic acid cream (Hoekzema et al., 1995; Karincaoglu et al., 2004), azithromycin (Kim et al., 2011b), erythromycin (Sanchez-Viera et al., 1992), sodium sulfacetamide (Herron et al., 2005) and tretinoin (Dominey et al., 1989b). Many of these treatments alone are often insufficiently effective in managing the condition (Salem et al., 2013). Further investigations are needed to understand their mechanisms of action and toxicities to determine the optimum dosage and length of treatment in demodicosis. The dosage, direction and administration form of miscellaneous treatments showing effectiveness against the human demodicosis are summarized in Table 8.
Table 8.
Miscellaneous treatment dosage and direction in treating human demodicosis
| NHMRC | Diseases | Age (years); gender | Sample size (n) | Drug | Dosage form | Administration (dose, route and time) | Effect | Reference |
|---|---|---|---|---|---|---|---|---|
| II | Rosacea | Mean age: 46.9; male 78.2%, female 21.8% | 23 | Precipitated sulphur | Topical | 3% precipitated sulphur twice daily for 28 days | Rosacea responded clinically | (Robinson, 1965) |
| III-1 | Blepharitis | 49.6 ± 16.9; gender not specified | 30 | OcuSoft™ Lids Scrub Plus (0.5% 1, 2-Octanediol) | Topical | OcuSoft™ Lids Scrub Plus wipes nightly for 4 weeks | Decrease in mean Demodex count from 3.8 to 1.9 post-treatment | (Murphy et al., 2018) |
| III-3 | Acne rosacea | Mean age: 49.5 ± 2.33; male 33%, female 66.7% | 27 | Benzoyl peroxide erythromycin (5% benzoyl peroxide and 3% erythromycin) | Topical | Benzoyl peroxide-erythromycin gel twice daily for 2 months | 91.7, 8.3 and 40.7% patients showed marked clinical improvement, complete remission and complete eradication of Demodex respectively post-treatment | (Ozturkcan et al., 2004) |
| III-3 | Demodicosis | Median age: 63.5; male 30%, female 70% | 20 | Blephadex™ (Glycerin, Aloe Barbadensis Leaf Juice, Cocamidopropyl Betaine, Cocos Nucifera oil, Lauryl Glucoside, Melaleuca alternifolia Leaf oil, Sodium Laureth Sulfate) | Topical | Blephadex™ eyelid wipes daily for 30 days | 50% reduction in Demodex burden post-treatment and overall comfort improved over time in treated eyes | (Wong et al., 2019) |
| III-3 | Demodicosis | Not specified | 5 | Benzyl benzoate | Topical | 10% twice daily for 45 days | Average Demodex decreased by 98.5% with normalization of Demodex count in 5 patients and reduced Demodex count in 5 patients post-treatment | (Forton et al., 1998) |
| III-3 | Demodicosis | Not specified | 5 | Sublimed sulphur | Topical | 10% sublimed sulphur once every 2 days for 45 days | Average Demodex decreased by 6.4% with normalization of Demodex count in 2 patients and reduced Demodex count in 3 patients post-treatment | (Forton et al., 1998) |
| III-3 | Demodicosis or rosacea | Mean age: 44.8 ± 12.7; male 12.5%, female 87.5% | 72 | Sulphur-sodium sulfacetamide or crotamiton or permethrin | Topical | 5% sulphur-sodium sulfacetamide or 10% crotamiton lotion or 5% permethrin cream twice daily for 8 weeks | Decrease in median Demodex count from 10 to 3, 17 to 6, and 14.5 to 6 per cm2 for sulphur-sodium sulfacetamide, crotamiton and permethrin treatment respectively post-treatment | (Sarac, 2019) |
| IV | Blepharitis | 95; female | 1 | 0.25% PVP-I in a dimethylsulfoxide vehicle | Topical | PVP-I gel to the lash surface from the skin side | Remarkable improvements in signs and symptoms 1 week post treatment; changes to the anterior eyelid was preserved, the corneal punctate erosions were no longer present, and the posterior eyelid meibum was less viscous at 1 month | (Pelletier et al., 2017) |
| IV | Blepharitis | 53; male | 1 | Ethyl ether (99.9% ethyl ether, 0.02% water, density 0.713 g mL−1) | Topical | Ether lid massage daily for 2 weeks | Improvement of symptoms and disappearance of palpebral crusty lesions post-treatment | (Hervás Ontiveros et al., 2014) |
| IV | Demodex folliculitis | 43; male | 1 | Sulphur | Topical | 6% sulphur lotion twice daily for 2 weeks | Complete eradication of Demodex and resolution of pustular lesion post-treatment | (Nakagawa et al., 1996) |
| IV | Demodicosis | 39; male | 1 | Aquaphor™ (3% sulphur in hydrophilic petrolatum) | Topical | Aquaphor™ for 2 weeks | Lesion cleared and no mites present post-treatment | (Sarro et al., 1998) |
| IV | Demodicosis | Not specified | Not specified | Camphor oil | Topical | 100, 75, 50 or 25–20% camphor oil | 100% cure at concentrations of 100, 75, and 50% with decreased Demodex density at concentrations of 25-20% post-treatment | (Morsy et al., 2002) |
| IV | Ocular demodicosis | Age range: 68–83; male 20%, female 80% | 10 | Pilocarpine | Topical | 4% pilocarpine gel nightly for 2 weeks | Decrease in average number of mites from 2.4 to 0.7 post-treatment | (Fulk et al., 1996) |
| IV | Rosacea | 4; female | 1 | Erythromycin | Oral | 250 mg erythromycin daily | Sustained improvement | (Sanchez-Viera et al., 1992) |
| IV | Rosacea | 52; female | 1 | Azythromycin | Oral | 500 mg daily for 2 weeks | Facial erythema and swelling reduced after 2 weeks followed by reduction in papular lesions, most lesions disappeared after 10 weeks | (Kim et al., 2011b) |
Combination therapy
Many combination regimens have been trialled for human demodicosis, especially TTO or T4O with other topical treatments. In a double-blind, randomized controlled clinical trial of Demodex-related rosacea by Ebneyamin et al., twice daily topical 2.5% permethrin with 100% TTO gel for 12 weeks is used to treat Demodex-related rosacea. Demodex density on the intervention side of the face was significantly reduced at week 12 (P value = 0.001) (LOE II). Clinical presentations, symptoms and global assessments showed papules, pustules, non-transient erythema, burning and stinging sensations had improvement in the treatment group after 12 weeks (P values < 0.05) (LOE II). Patients in the intervention group only experienced mild-to-moderate adverse effects that did not result in discontinuation of the treatment (Ebneyamin et al., 2020). In a triple-blinded randomized controlled clinical trial on dry eye after phacoemulsification cataract surgery by Mohammadpour et al., treatment combinations including daily artificial tears, topical steroid drops and eyesol shampoos with TTO daily for 4 weeks were used in the intervention group and the control group had the same treatment combinations without the TTO. Results demonstrated that a greater significant improvement in tear break-up time test, corrected distance visual acuity, osmolarity and ocular surface disease index score and a dramatic reduction in the number of Demodex, respectively, in the treatment group (LOE II) (Mohammadpour et al., 2019). A randomized prospective double-masked trial in Demodex blepharitis by Epstein et al. by using T4O lid scrubs with microblepharoexfoliation (MBE) twice daily for 8 weeks as a treatment showed in-office MBE with T4O lid scrubs has no significant improvement over sham scrubs. The results between the intervention and the control group were not statistically meaningful, possibly because of the low sample size of the study. Overall, patients in the treatment group with T4O tolerated the medicated eyelid scrubs well; with only a low incident of transient burning (LOE III-1) (Epstein et al., 2020). In a multicentre, open, randomized, two-parallel group comparative study by Messaoud et al., a cleaning wipe that contains both T4O 2.5% and hyaluronic acid 0.2% was used to treat Demodex anterior blepharitis daily or twice a day for 29 days. Overall ocular discomfort hyperaemia was significantly reduced from baseline (P < 0.0001) in both groups at day 8 (−3.6 ± 0.3 in daily and −4.0 ± 0.4 in twice daily treatment groups) and day 29 (−5.7 ± 0.4 daily and −6.8 ± 0.7 twice daily treatment groups), respectively (LOE III-2). Eyelid margin hyperaemia improved progressively at day 8 and day 29 as well for both groups (LOE III-2). Total disappearance of cylindrical dandruff was reported in 30.4% of patients in daily and 43.5% in twice daily treatment groups, respectively (LOE III-2). The T4O-based product was well tolerated and patients were willing to continue that for long-term use (Messaoud et al., 2019).
Other combination treatments such as metronidazole, ivermectin and permethrin also were trialled. A single-blind, randomized controlled trial in patients with ocular or dermatological demodicosis, oral metronidazole and ivermectin combined therapy showed a significant reduction in mite count in acne, peri-oral dermatitis, anterior blepharitis and rosacea group compared with the oral ivermectin treatment alone (LOE III-1) (Salem et al., 2013). This regimen was significantly better at reducing the mite count to the normal level in the rosacea and anterior blepharitis groups (Salem et al., 2013). In one of the single-blind, randomized clinical trials, the efficacy of metronidazole was compared with ornidazole when paired with the administration of anti-inflammatory agents betamethasone and ebastine of mites folliculitis. Ornidazole had a significantly higher effective rate compared to metronidazole in controlling the disease. Up to 12 weeks after finishing the treatment course, metronidazole-treated patients had significantly higher rates of mite relapse and new lesion occurrence. (LOE III-1) (Luo et al., 2016). The fulminant rosacea-like eruption with multiple D. folliculorum mites show excellent response to oral and topical metronidazole combination treatments, and dermatosis gradually cleared within 1 month, and the residual erythema on the patients’ cheeks was resolved 9 months later (LOE IV) (Hoekzema et al., 1995). The oral metronidazole and ivermectin combination therapy also showed subjective and objective improvement in blepharitis (LOE IV) (Nath et al., 2012). A trial of combined oral metronidazole and prednisolone for 3 weeks in conjunction with topical metronidazole and lindane emulsion showed a gradual reduction in pustules in tuberous-pustular demodicosis (LOE IV) (Grossmann et al., 1999). Facial mites disappeared with complete remission without recurrence in rosacea-like demodicosis by using topical and oral metronidazole for 2 months and yellow mercury ointment for 15 days (LOE IV) (Anane et al., 2011). Topical camphor oil and oral metronidazole were given for 15 days, and satisfactory results were achieved without observable side-effects (LOE IV) (El-Shazly et al., 2004). Oral ivermectin and topical permethrin were used to treat acute lymphoblastic leukaemia-associated demodicosis (LOE IV). No apparent adverse effects were experienced and eruption resolved 5 weeks later (Damian and Rogers, 2003). The oral ivermectin and topical permethrin combination regimen were also used for resolution of the rosacea-like folliculitis (LOE IV) (Allen et al., 2007). The combination treatment of dosage, direction and administration form showing effectiveness against the human demodicosis are summarized in Table 9.
Table 9.
Combination therapy dosage and direction in treating human demodicosis
| NHMRC | Diseases | Age (years); gender | Sample size (n) | Dosage form | Administration (dose, route and time) | Effect | Reference |
|---|---|---|---|---|---|---|---|
| II | Papulopustular rosacea | Age range: 18–60; male 25.4%, female 71.4% | 35 | Topical | 2.5% permethrin with tea tree oil gel formulation twice daily for 12 weeks | Reduction of mites, non-transient erythema, papule, pustules, dry appearance, burning and stinging post-treatment | (Ebneyamin et al., 2020) |
| II | Dry eye disease | Mean age: 66.37 ± 8.83; male 21%, female 79% | 33 | Topical | Artificial tears, topical steroid drops and eyesol shampoos with tea tree oil for 1 month | Significant decrease in Demodex count, tear break-up time, osmolarity and ocular surface disease index scores were significantly better than control at 1 month follow-up | (Mohammadpour et al., 2019) |
| III-1 | Acne vulgaris, blepharitis, peri-oral dermatitis or rosacea | Mean age: 36.71 ± 12.4; male 45%, female 55% | 60 | Oral | 250 mg metronidazole three times daily for 2 weeks and 200 μg kg−1 ivermectin 2 doses 1 week apart | Decrease in mean Demodex density from 12.9 ± 6.1 to 0.5 ± 1.2, 15 ± 5.7 to 0.2 ± 0.4, 21.9 ± 6.8 to 0.2 ± 0.4 and 51.5 ± 26.3 to 5.5 ± 19.5 mites per cm2 for acne vulgaris, blepharitis, peri-oral dermatitis and rosacea respectively at 4th week follow-up | (Salem et al., 2013) |
| III-1 | Blepharitis | Mean age: 34; male 33%, female 67% | 45 | Topical | Palpebral hygiene with neutral shampoo three times daily or palpebral hygiene with neutral shampoo and topical 0.75% metronidazole gel twice daily or palpebral hygiene with neutral shampoo and antibiotic cream comprised (3.5% neomycin and 10% polymixin with 0.5% dexamethasone) three times daily | Significant improvement of signs and symptoms with all regimen | (Arrua et al., 2015) |
| III-1 | Blepharitis | Mean age: 71 ± 5.8; male 46.1%, female 53.9% | 26 | Topical | Monthly in-office microblepharoexfoliation, followed by Cliradex™ (terpinen-4-ol) lid scrub twice daily for 2 months | Reduction in Demodex infestation from 4.7 to 3.6 per 4 lashes after first month and 2.6 per 4 lashes after second month | (Epstein et al., 2020) |
| III-1 | Blepharitis | Mean age: 49.86 ± 19.7; gender not specified | 28 | Topical | BlephEx™ (microblepharoexfoliation) with OcuSoft™ Lid Scrub Plus foam (0.5% 1, 2-octanediol) nightly for 4 weeks | Decrease in mean Demodex count and from 6.5 to 2.7 after 4 weeks | (Murphy et al., 2018) |
| III-1 | Demodex folliculitis | Age range: 20–45; male 21%, female 79% | 100 | Oral, injection and topical | Oral 500 mg ornidazole three times daily for 2 weeks, 1 mL compound betamethasone injection intramuscularly or oral 10 mg ebastine daily for 3 weeks, topical 1 g recombinant bovine basic fibroblast growth factor gel three times daily on lesion for 2 weeks beginning on day 7 post-ornidazole, oral anti-histamine for 4 weeks for patients who used ebastine | Overall effective rate of 94% and only 6 patients with Demodex mite relapse after 2 weeks | (Luo et al., 2016) |
| III-1 | Demodex folliculitis | Age range: 20–45; male 28%, female 72% | 100 | Oral, injection, and topical | Oral 200 mg metronidazole four times daily for 2 weeks, 1 mL compound betamethasone injection intramuscularly or 10 mg ebastine daily for 3 weeks, topical 1 g recombinant bovine basic fibroblast growth factor gel three times daily on lesion for 2 weeks beginning on day 7 post-metronidazole, oral anti-histamine for 4 weeks for patients who used ebastine | Overall effective rate of 78% and only 26 patients with Demodex mite relapse after 2 weeks | (Luo et al., 2016) |
| III-1 | Blepharitis | Mean age: 55.4 ± 19.1; gender not specified | 30 | Topical | Ivermectin 0.1% Metronidazole 1% gel on days 0, 15 and 30 | Complete eradication of Demodex in 96.6% of patients, significant reduction of inflammation signs found in all patients | (Avila et al., 2020) |
| III-2 | Demodicosis | Mean age: 35.7 ± 11.40; male 12.5%, female 87.5% | 6 | Topical | 5% permethrin cream twice daily and 2% salicylic acid for 1 month | Complete eradication of median Demodex in 2 of 3 patients who had acneiform lesions and complete eradication of median Demodex in all patients with granulomatous rosacea like lesion and pustular folliculitis post-treatment | (Karincaoglu et al., 2004) |
| III-2 | Anterior blepharitis | Mean age: 52 ± 16.2; male 62.5%, female 37.5% | 24 | Topical | BlephaDemodex® (preservative-free cleansing lotion containing 2.5%T4O and 0.2% hyaluronic acid) once daily in the evening 29 days | Overall ocular discomfort significantly reduced, ocular symptom associated with Demodex blepharitis was improved, eyelid margin hyperaemia significantly reduced at day 8 and day 29, total disappearance of cylindrical dandruff in 30.4% of patients; follow-up on day 8 and 29 | (Messaoud et al., 2019) |
| III-2 | Anterior blepharitis | Mean age: 56.5 ± 15.1; male 50%, female 50% | 24 | Topical | BlephaDemodex® (preservative-free cleansing lotion containing 2.5%T4O and 0.2% hyaluronic acid) twice daily in morning and evening for 29 days | Overall ocular discomfort was significantly reduced, ocular symptom associated with Demodex blepharitis was improved, eyelid margin hyperaemia significantly reduced at day 8 and day 29, total disappearance of cylindrical dandruff in 43.5% of patients; follow-up on day 8 and 29 | (Messaoud et al., 2019) |
| III-3 | Rosacea | Age range: 34–77; male 36%, female 64% | 25 | Oral and topical | Topical 2% metronidazole cream or gel twice daily combined with oral 50 mg doxycycline or minocycline daily | Decrease in mean Demodex from 115 to 96.2 and 86.2 to 58.5 for 8 × 8 and 5 × 5 mm2 mosaic respectively at mean follow-up of 53.5 days | (Sattler et al., 2015) |
| III-3 | Rosacea or demodicosis | Mean age: 48.1 ± 1.0; male 23%, female 77% | 195 | Topical | 10% crotamiton cream daily and 12% benzyl benzoate with 10% crotamiton cream nightly for 2 months | Decrease in mean Demodex count from 88 ± 8 to 28 ± 3 per cm2 with 20% patients without symptoms at mean follow-up of 2.7 ± 0.2 months | (Forton and De Maertelaer, 2020) |
| III-3 | Rosacea or demodicosis | Mean age: 47.1 ± 1.2; male 34%, female 66% | 171 | Topical | 12% benzyl benzoate and 10% in cetomacrogol cream twice daily for 2 months | Decrease in mean Demodex count from 103 ± 9to 23 ± 4 per cm2 with 40% patients without symptoms at mean follow-up of 2.7 ± 0.2 months | (Forton and De Maertelaer, 2020) |
| III-3 | Rosacea or demodicosis | Mean age: 45.1 ± 3.1; male 46%, female 54% | 28 | Topical | Moisturizing cream daily and 20–24% benzyl benzoate with 10% crotamiton in cetomacrogol cream nightly for 2 months | Decrease in mean Demodex count from 78 ± 19 to 22 ± 6 per cm2 with 54% patients without symptoms at mean follow-up of 2.7 ± 0.2 months | (Forton and De Maertelaer, 2020) |
| IV | Pityriasis folliculorum | Mean age: 38; female 100% | 3 | Topical | 0.01% tretinoin gel nightly for 4 weeks and 1% lindane lotion weekly for 4 weeks | Responded to treatment regimen | (Dominey et al., 1989b) |
| IV | Acute lymphoblastic leukaemia-associated Demodex | 6; male | 1 | Oral and topical | Oral ivermectin 200 μg kg−1; topical steroid for 2 days and 5% permethrin cream for 2 nights for 2 weeks | The eruption gradually cleared 5 weeks after additional treatments of oral ivermectin and topical permethrin at 6th and 7th week post-treatment | (Damian and Rogers, 2003) |
| IV | Blepharitis | 22, male | 1 | Oral | 12 mg ivermectin two doses 10 days apart followed by 400 mg metronidazole three times daily for 7 days | Subjective and objective improvement post-treatment | (Nath et al., 2012) |
| IV | Blepharitis | 59; female | 1 | Topical | Theralid™ and Cliradex™ (tea tree oil-based eyelid cleansers) for 6 weeks followed by hypochlorous acid cleanser for 2 weeks | Improved symptoms and reduction in the cylindrical dandruff post-treatment | (Bachir and Bitton, 2015) |
| IV | Blepharitis | 30; female | 1 | Topical | Pulse dosing with Cliradex™, followed by hypochlorous acid cleanser for 2 weeks | Improved symptoms and reduction in the cylindrical dandruff post-treatment | (Bachir and Bitton, 2015) |
| IV | Blepharoconjunctivitis | 30; female | 1 | Oral and topical | Oral 2% metronidazole and topical lid scrubs | 50% reduction in number of Demodex post-treatment | (Junk et al., 1998) |
| IV | Crusted demodicosis | 7; female | 1 | Oral and topical | Oral 200 μg kg−1 ivermectin once weekly for 10 weeks, topical 5% permethrin lotion for 3 nights followed by oral 30 mg kg−1 erythromycin and topical metronidazole cream | Lesions improved greatly within following 3 months | (Guerrero-Gonzalez et al., 2014) |
| IV | Demodicosis | 2; female | 1 | Topical | 5% Permethrin cream nightly for 3 nights followed by metronidazole 0.75% cream twice daily | Symptoms improved after 2 weeks without recurrence at 6 month follow-up | (Douglas and Zaenglein, 2019) |
| IV | Demodicosis | 13; male | 1 | Topical and oral | 5% Permethrin cream weekly overnight for 4 weeks with 100 mg doxycycline oral daily for 8 weeks | Improvement of mild erythema of the forehead and cheeks with a few pustules, and limited Demodex was found at 3 month follow-up | (Douglas and Zaenglein, 2019) |
| IV | Demodicosis | 19 months; male | 1 | Topical | 5% Permethrin cream once weekly for 2 weeks and 0.75% metronidazole cream daily for maintenance | Improvement of symptoms | (Douglas and Zaenglein, 2019) |
| IV | Demodicosis | 5; female | 1 | Topical | 0.75% Metronidazole cream twice daily, resulting in some improvement followed by 5% permethrin cream topically once a week for 2 weeks after 1 month | Improvement of symptoms | (Douglas and Zaenglein, 2019) |
| IV | Demodicosis | 11; female | 1 | Topical and oral | 5% permethrin cream weekly for a month and 20 mg doxycycline orally twice daily, as well as 0.75% metronidazole cream daily to the affected area | Improvement within 2 weeks; 2-month follow-up, examination showed a few small papules; no signs of demodicosis were found at 9-month follow-up | (Douglas and Zaenglein, 2019) |
| IV | Demodex folliculitis | 10; male | 1 | Topical | 10% sodium sulfacetamide and 5% sulphur lotion | Eruption resolved completely | (Herron et al., 2005) |
| IV | Demodex folliculitis | 3; female | 1 | Topical | 5% permethrin cream for 5 consecutive nights for 4 weeks, followed by metronidazole cream twice daily and 0.05% desonide cream daily | Improvement of facial eruption post-treatment | (Herron et al., 2005) |
| IV | Demodex folliculitis | 5; male | 1 | Topical | 5% permethrin cream for 3 consecutive nights for 7 weeks followed by metronidazole cream twice daily for 2 months | Symptoms resolved and rash improved after permethrin but did not clear after 7 weeks or metronidazole regimen | (Herron et al., 2005) |
| IV | Demodex folliculitis | 68; female | 1 | Topical | Daily lid scrub with polyhexamethylene biguanide, 1,2 hexanediol and 1,2-octanediol and erythromycin ointment twice daily | Complete resolution of symptoms after 4 weeks | (Yun et al., 2013) |
| IV | Demodex folliculitis | 24; female | 1 | Oral and topical | Oral 500 mg metronidazole three times daily for 2 weeks and topical 1% lindane for 2 weeks | Complete resolution at 3rd month follow-up | (Hsu et al., 2009) |
| IV | Demodex scalp folliculitis | 73; female | 1 | Oral and topical | Topical metronidazole gel along with fusidic acid cream and oral betamethasone-17-valerate for several weeks | Complete cessation of hair loss and signs of inflammation post-treatment | (Helou et al., 2016) |
| IV | Demodex scalp folliculitis | 16; male | 1 | Oral and topical | Oral 250 mg metronidazole three times daily and topical 5% minoxidil solution | Complete cessation of hair loss, erythema and pustules post-treatment | (Helou et al., 2016) |
| IV | Demodex scalp folliculitis | 75; male | 1 | Oral and topical | Topical permethrin plus oral metronidazole | Good response to treatment within few days, with clearance of lesions; regression of disease after 7 months | (Fernandez-Flores and Alija, 2009) |
| IV | Demodex scalp folliculitis | 57; male | 1 | Topical | 10% sulfacetamide and 5% sulphur cream twice daily in addition to a 2 weeks course of 2.5% selenium sulphide shampoo daily | Scalp cleared of erythematous plaque with hyper keratosis and pustules at 3rd month follow-up | (Sanfilippo and English, 2005) |
| IV | Demodicosis | 34; male | 1 | Oral and topical | Oral 250 mg doxycycline daily for 3 weeks and oral prednisolone 10 mg twice daily tapered in 3 weeks; topical lindane for 2 weeks | Complete resolution at 10th month follow-up with relapse in 1st and 2nd month | (Hsu et al., 2009) |
| IV | Demodicosis | 44; female | 1 | Oral and topical | Oral 250 mg metronidazole three times daily for 3 weeks, 10 mg prednisolone twice daily tapered in 3 weeks; topical 0.75% metronidazole gel twice daily for 3 weeks | Complete resolution at 6th week follow-up | (Hsu et al., 2009) |
| IV | Demodicosis | 3; female | 1 | Topical and oral | 0.75% metronidazole cream twice daily for 2 weeks followed by 1% lindane cream nightly for 2 weeks and oral 250 mg erythromycin daily for 1 week | Lesions gradually cleared after metronidazole followed by complete resolution of skin eruption after lindane regimen | (Castanet et al., 1997) |
| IV | Demodicosis | 56; male | 1 | Oral and topical | Oral ivermectin 200 μg kg−1 single dose followed by topical 5% permethrin cream weekly | Dramatic response obtained within 2 weeks and no recurrence after a year | (Aquilina et al., 2002) |
| IV | Demodicosis | 75; male | 1 | Oral and topical | Oral 500 mg metronidazole twice daily, scales were removed with topical 5% salicylic acid in white petrolatum twice daily for 2 days followed by 1% metronidazole cream twice daily | Excellent response to treatment with oral therapy was ceased on day 15, dermatosis gradually cleared within the next month with topical metronidazole, resolution of residual erythema on cheeks 9 months later | (Hoekzema et al., 1995) |
| IV | Demodicosis | 76; male | 1 | Oral and topical | Oral 200 μg kg−1 ivermectin single dose followed by topical 5% permethrin for 4 weeks | Visible improvement starting after 2 weeks, complete resolution after 5 weeks | (Eismann et al., 2010) |
| IV | Demodicosis | 56; male | 1 | Topical | Metronidazole and lindane twice daily for 3 months | Treatment was successful after 3 months | (Kim et al., 2014) |
| IV | Demodicosis | 32; male | 1 | Oral and topical | Oral 200 μg kg−1 ivermectin single dose followed by bland oil-in-water preparation after 1 month | Reduction of pruritus within 2 weeks, no Demodex mites in skin scrapings with reduction in size and intensity of inflammation within 4 weeks | (Forstinger et al., 1999) |
| IV | Demodicosis | 37; female | 1 | Oral and topical | Oral metronidazole for 2 months and topical yellow mercury ointment for 15 days | Disappearance of facial mites and complete remission post-treatment | (Anane et al., 2011) |
| IV | Demodicosis | 43; female | 1 | Oral and topical | 250 mg metronidazole three times daily and topical crotamiton ointment daily | Marked improvement of lesion with mild erythema after 6 weeks of treatment | (Park et al., 2011) |
| IV | Demodicosis | 38; female | 1 | Oral and topical | Oral 750 mg metronidazole daily and 100 mg daily prednisolone for 3 weeks followed by topical 0.15% lindane emulsion and 2% metronidazole cream | Treatment led to gradual regression of pustules after 3 weeks | (Grossmann et al., 1999) |
| IV | Granulomatous rosacea | 48; female | 1 | Oral and topical | Oral 500 mg metronidazole three times daily tapered in 3 weeks, 5 mg prednisolone three times daily tapered in 3 weeks; topical 0.75% metronidazole gel twice daily for 8 weeks | Complete resolution at 7th week follow-up | (Hsu et al., 2009) |
| IV | Papulonodular demodicosis | 51; male | 1 | Topical | 1% lindane lotion and 1% permethrin cream rinse daily | Expressed follicular contents revealed few Demodex and lesions cleared after 1 week of treatment | (Dominey et al., 1989a) |
| IV | Papulopustular rosacea | 68; male | 1 | Oral and topical | Oral ivermectin and topical 5% permethrin cream | Resolution of folliculitis | (Allen et al., 2007) |
| IV | Peri-oral dermatitis | 47; female | 1 | Oral | 250 mg metronidazole three times daily for 3 weeks, 0.03% tacrolimus twice daily for 5 weeks and then changed to 1% pimecrolimus for another 11 weeks | Complete resolution at 7th week follow-up | (Hsu et al., 2009) |
| IV | Phymatous rosacea | 57; male | 1 | Oral and injection | Oral 100 mg minocycline twice daily and intralesional triamcinolone injections every 3 months | Ear partially diminished in size and external auditory canal was wider after 8 months | (Carlson et al., 2008) |
| IV | Pityriasis folliculorum | 37; female | 1 | Topical | 0.01% tretinoin gel nightly for 2 weeks and 1% lindane lotion weekly for 2 weeks | Responded to treatment regimen | (Dominey et al., 1989b) |
| IV | Pityriasis folliculorum | 34; female | 1 | Topical | 0.05% tretinoin cream alternate nights for 10 days and 1% lindane lotion alternate nights for 10 days | Responded to treatment regimen | (Dominey et al., 1989b) |
| IV | Pityriasis folliculorum | 31; female | 1 | Oral and topical | Oral isotretinoin 0.5 mg kg−1 daily for 14 weeks and topical 1% permethrin cream rinse daily for 2 weeks | Responded to treatment regimen | (Dominey et al., 1989b) |
| IV | Rosacea | Age not specified; female | 15 | Oral and topical | Oral 500 mg metronidazole and topical 1/3 diluted camphor oil with glycerol daily for 15 days | Treatment successful | (El-Shazly et al., 2004) |
| IV | Rosacea-like demodicosis | 2; male | 1 | Oral and topical | Oral erythromycin 250 mg daily and topical metronidazole daily | Remarkable improvement after 2 months | (Barrio et al., 1996) |
Conclusion
There are a large number of reports of human demodicosis indicating sustained interest and needs for efficacious treatments. However, only limited double-blinded randomized controlled trials were done and many treatments have not been compared with each other. Five treatments have emerged but further work is required to vigorously evaluate their efficacy. TTO and T4O, metronidazole, ivermectin and permethrin have shown promising results in reducing mite counts and dermatological symptoms in Demodex infection. TTO or T4O topical treatment should be considered as the first-line therapy because of its satisfactory clinical results with minimal side-effects. Systemic treatments such as oral ivermectin or oral metronidazole could be added, which may also decrease recurrence in complicated cases. As a second-line treatment or in severe cases, topical permethrin is another alternative to be considered as an option based on consistent data showing moderate to satisfactory clinical improvement in Demodex-associated skin condition. However, side-effects such as skin irritation of permethrin should be carefully monitored. Treatment with benzyl benzoate or crotamiton may also be considered as they did demonstrate moderate anti-demodicosis properties. Although these treatments are effective, their repeated use may result in the development of resistance. These problems have highlighted the need for the development of new alternatives. Moreover, the goal of treatment should be to reduce the mite count below a certain threshold and restore the ocular and dermatological ecology to a balanced state. This should control the symptoms and achieve remissions. In refractory cases, such as immunocompromised and systemic disease patients, combination therapies such as TTO or T4O with other topical or systemic treatments may be needed to relieve the symptoms and prevent recurrence. Therefore, further research should focus on the efficacy, formulations and toxicity to improve the miticidal potency and pharmacological stability for human demodicosis.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
All contributing members declare there is no conflict of interest.
Ethical standards
Not applicable.
References
- Abokwidir M and Fleischer AB (2015) An emerging treatment: topical ivermectin for papulopustular rosacea. The Journal of Dermatological Treatment 26, 379–380. [DOI] [PubMed] [Google Scholar]
- Abu-Samra MT and Shuaib YA (2014) A study on the nature of association between Demodex mites and bacteria involved in skin and meibomian gland lesions of demodectic mange in cattle. Veterinary Medicine International 2014, 413719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akcinar UG, Unal E and Dogruman Al F (2018) Demodex spp. as a possible aetiopathogenic factor of acne and relation with acne severity and type. Postepy Dermatologii I Alergologii 35, 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akilov OE and Mumcuoglu KY (2003) Association between human demodicosis and HLA class I. Clinical and Experimental Dermatology 28, 70–73. [DOI] [PubMed] [Google Scholar]
- Allen KJ, Davis CL, Billings SD and Mousdicas N (2007) Recalcitrant papulopustular rosacea in an immunocompetent patient responding to combination therapy with oral ivermectin and topical permethrin. Cutis 80, 149–151. [PubMed] [Google Scholar]
- Anane S, Mokni M and Beltaief O (2011) [Rosacea-like demodicidosis and chronic blepharitis]. Annales de Dermatologie et de Venereologie 138, 30–34. [DOI] [PubMed] [Google Scholar]
- Aquilina C, Viraben R and Sire S (2002) Ivermectin-responsive Demodex infestation during human immunodeficiency virus infection. A case report and literature review. Dermatology (Basel, Switzerland) 205, 394–397. [DOI] [PubMed] [Google Scholar]
- Arrua M, Samudio M, Farina N, Cibils D, Laspina F, Sanabria R, Carpinelli L and Mino de Kaspar H (2015) Comparative study of the efficacy of different treatment options in patients with chronic blepharitis. Archivos de la Sociedad Espanola de Oftalmologia 90, 112–118. [DOI] [PubMed] [Google Scholar]
- Asai Y, Tan J, Baibergenova A, Barankin B, Cochrane CL, Humphrey S, Lynde CW, Marcoux D, Poulin Y, Rivers JK, Sapijaszko M, Sibbald RG, Toole J, Ulmer M and Zip C (2016) Canadian clinical practice guidelines for Rosacea. Journal of Cutaneous Medicine and Surgery 20, 432–445. [DOI] [PubMed] [Google Scholar]
- Ashack RJ, Frost ML and Norins AL (1989) Papular pruritic eruption of Demodex folliculitis in patients with acquired immunodeficiency syndrome. Journal of the American Academy of Dermatology 21, 306–307. [DOI] [PubMed] [Google Scholar]
- Aupee O, Almeras D, Le Garlantezec P and Bohand X (2009) [Doxycycline]. Med Trop (Mars) 69, 556–558. [PubMed] [Google Scholar]
- Avila MY, Martinez-Pulgarin DF and Rizo Madrid C (2020) Topical ivermectin-metronidazole gel therapy in the treatment of blepharitis caused by Demodex Spp.: a randomized clinical trial. Contact Lens & Anterior Eye 20, 1–6. doi: 10.1016/j.clae.2020.04.011 [DOI] [PubMed] [Google Scholar]
- Aydogan K, Alver O, Tore O and Karadogan SK (2006) Facial abscess-like conglomerates associated with Demodex mites. Journal of the European Academy of Dermatology and Venereology: JEADV 20, 1002–1004. [DOI] [PubMed] [Google Scholar]
- Aylesworth R and Vance JC (1982) Demodex folliculorum and Demodex brevis in cutaneous biopsies. Journal of the American Academy of Dermatology 7, 583–589. [DOI] [PubMed] [Google Scholar]
- Bachir V and Bitton E (2015) Treatment of Demodex with hypochlorous acid: case report. University of Montreal School of Optometry. https://www.aaopt.org/detail/knowledge-base-article/treatment-demodex-hypochlorous-acid-case-report (Accessed 20 August, 2020). [Google Scholar]
- Baima B and Sticherling M (2002) Demodicidosis revisited. Acta Dermato-Venereologica 82, 3–6. [DOI] [PubMed] [Google Scholar]
- Baldwin HE (2006) Oral therapy for rosacea. Journal of Drugs in Dermatology: JDD 5, 16–21. [PubMed] [Google Scholar]
- Banuls J, Ramon D, Aniz E, Jorda E and Torres V (1991) Papular pruritic eruption with human immunodeficiency virus infection. International Journal of Dermatology 30, 801–803. [DOI] [PubMed] [Google Scholar]
- Barrio J, Lecona M, Hernanz JM, Sanchez M, Gurbindo MD, Lazaro P and Barrio JL (1996) Rosacea-like demodicosis in an HIV-positive child. Dermatology (Basel, Switzerland) 192, 143–145. [DOI] [PubMed] [Google Scholar]
- Basta-Juzbasic A, Subic JS and Ljubojevic S (2002) Demodex folliculorum in development of dermatitis rosaceiformis steroidica and rosacea-related diseases. Clinics in Dermatology 20, 135–140. [DOI] [PubMed] [Google Scholar]
- Benk Silfeler D, Keskin Kurt R, Kaya OA, Yengil E, Hamamci B, Okyay AG and and Beyazit A (2015) Demodex folliculorum in polycystic ovary syndrome patients. European Review for Medical and Pharmacological Sciences 19, 1141–1145. [PubMed] [Google Scholar]
- Bevins CL and Liu FT (2007) Rosacea: skin innate immunity gone awry? Nature Medicine 13, 904–906. [DOI] [PubMed] [Google Scholar]
- Biernat MM, Rusiecka-Ziolkowska J, Piatkowska E, Helemejko I, Biernat P and Gosciniak G (2018) Occurrence of Demodex species in patients with blepharitis and in healthy individuals: a 10-year observational study. Japanese Journal of Ophthalmology 62, 628–633. [DOI] [PubMed] [Google Scholar]
- Bikowski JB (2003) Subantimicrobial dose doxycycline for acne and rosacea. Skinmed 2, 234–245. [DOI] [PubMed] [Google Scholar]
- Bikowski JB and Del Rosso JQ (2009) Demodex dermatitis: a retrospective analysis of clinical diagnosis and successful treatment with topical crotamiton. The Journal of Clinical and Aesthetic Dermatology 2, 20–25. [PMC free article] [PubMed] [Google Scholar]
- Brooks PA and Grace RF (2002) Ivermectin is better than benzyl benzoate for childhood scabies in developing countries. Journal of Paediatrics and Child Health 38, 401–404. [DOI] [PubMed] [Google Scholar]
- Brown M, Hernandez-Martin A, Clement A, Colmenero I and Torrelo A (2014) Severe Demodex folliculorum-associated oculocutaneous rosacea in a girl successfully treated with ivermectin. JAMA Dermatology 150, 61–63. [DOI] [PubMed] [Google Scholar]
- Buffet M and Dupin N (2003) Current treatments for scabies. Fundamental & Clinical Pharmacology 17, 217–225. [DOI] [PubMed] [Google Scholar]
- Carlson JA, Mazza J, Kircher K and Tran TA (2008) Otophyma: a case report and review of the literature of lymphedema (elephantiasis) of the ear. The American Journal of Dermatopathology 30, 67–72. [DOI] [PubMed] [Google Scholar]
- Carson CF, Mee BJ and Riley TV (2002) Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrobial Agents and Chemotherapy 46, 1914–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas C, Paul C, Lahfa M, Livideanu B, Lejeune O, Alvarez-Georges S, Saint-Martory C, Degouy A, Mengeaud V, Ginisty H, Durbise E, Schmitt AM and Redoules D (2012) Quantification of Demodex folliculorum by PCR in rosacea and its relationship to skin innate immune activation. Experimental Dermatology 21, 906–910. [DOI] [PubMed] [Google Scholar]
- Casida JE (1980) Pyrethrum flowers and pyrethroid insecticides. Environmental Health Perspectives 34, 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanet J, Monpoux F, Mariani R, Ortonne JP and Lacour JP (1997) Demodicidosis in an immunodeficient child. Pediatric Dermatology 14, 219–220. [DOI] [PubMed] [Google Scholar]
- Celorio J, Fariza-Guttman E and Morales V (1989) Pilocarpine as a coadjuvant treatment of blepharo-conjunctivitis caused by Demodex folliculorum. Investigative Ophthalmology And Visual Science Suppl 30, 40. [Google Scholar]
- Cheng AM, Sheha H and Tseng SC (2015) Recent advances on ocular Demodex infestation. Current Opinion in Ophthalmology 26, 295–300. [DOI] [PubMed] [Google Scholar]
- Chovatiya RJ and Colegio OR (2016) Demodicosis in renal transplant recipients. American Journal of Transplantation 16, 712–716. [DOI] [PubMed] [Google Scholar]
- Clyti E, Nacher M, Sainte-Marie D, Pradinaud R and Couppie P (2006) Ivermectin treatment of three cases of demodecidosis during human immunodeficiency virus infection. International Journal of Dermatology 45, 1066–1068. [DOI] [PubMed] [Google Scholar]
- Coleman CI, Gillespie EL and White CM (2005) Probable topical permethrin-induced neck dystonia. Pharmacotherapy 25, 448–450. [DOI] [PubMed] [Google Scholar]
- Comin VM, Lopes LQ, Quatrin PM, de Souza ME, Bonez PC, Pintos FG, Raffin RP, Vaucher Rde A, Martinez DS and Santos RC (2016) Influence of Melaleuca alternifolia oil nanoparticles on aspects of Pseudomonas aeruginosa biofilm. Microbial Pathogenesis 93, 120–125. [DOI] [PubMed] [Google Scholar]
- Coppens G, Stalmans I and Zeyen T (2010) Glaucoma medication during pregnancy and nursing. Bulletin de la Societe Belge D'ophtalmologie 314, 33–36. [PubMed] [Google Scholar]
- Cotliar J and Frankfurt O (2013) Demodex folliculitis mimicking acute graft-vs-host disease. JAMA Dermatology 149, 1407–1409. [DOI] [PubMed] [Google Scholar]
- Cox SD, Mann CM, Markham JL, Bell HC, Gustafson JE, Warmington JR and Wyllie SG (2000) The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). Journal of Applied Microbiology 88, 170–175. [DOI] [PubMed] [Google Scholar]
- Czepita D, Kuzna-Grygiel W, Czepita M and Grobelny A (2007) Demodex folliculorum and Demodex brevis as a cause of chronic marginal blepharitis. Annales Academiae Medicae Stetinensis 53, 63–67, discussion 67. [PubMed] [Google Scholar]
- Damian D and Rogers M (2003) Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. International Journal of Dermatology 42, 724–726. [DOI] [PubMed] [Google Scholar]
- Dhingra KK, Saroha V, Gupta P and Khurana N (2009) Demodex-associated dermatologic conditions – a coincidence or an etiological correlate. Review with a report of a rare case of sebaceous adenoma. Pathology – Research and Practice 205, 423–426. [DOI] [PubMed] [Google Scholar]
- Di Nardo A, Holmes AD, Muto Y, Huang EY, Preston N, Winkelman WJ and Gallo RL (2016) Improved clinical outcome and biomarkers in adults with papulopustular rosacea treated with doxycycline modified-release capsules in a randomized trial. Journal of the American Academy of Dermatology 74, 1086–1092. [DOI] [PubMed] [Google Scholar]
- Dokuyucu R, Kara OA, Yula E, Ustun I, Bayram F and Gokce C (2016) The presence of Demodex folliculorum in various obese groups according to BMI levels. Archives of Iranian Medicine 19, 210–214. [PubMed] [Google Scholar]
- Dominey A, Rosen T and Tschen J (1989a) Papulonodular demodicidosis associated with acquired immunodeficiency syndrome. Journal of the American Academy of Dermatology 20, 197–201. [DOI] [PubMed] [Google Scholar]
- Dominey A, Tschen J, Rosen T, Batres E and Stern JK (1989b) Pityriasis folliculorum revisited. Journal of the American Academy of Dermatology 21, 81–84. [DOI] [PubMed] [Google Scholar]
- Douglas A and Zaenglein AL (2019) A case series of demodicosis in children. Pediatric Dermatology 36, 651–654. [DOI] [PubMed] [Google Scholar]
- Dourmishev AL, Dourmishev LA and Schwartz RA (2005) Ivermectin: pharmacology and application in dermatology. International Journal of Dermatology 44, 981–988. [DOI] [PubMed] [Google Scholar]
- Dursun D, Kim MC, Solomon A and Pflugfelder SC (2001) Treatment of recalcitrant recurrent corneal erosions with inhibitors of matrix metalloproteinase-9, doxycycline and corticosteroids. American Journal of Ophthalmology 132, 8–13. [DOI] [PubMed] [Google Scholar]
- Ebbelaar CCF, Venema AW and Van Dijk MR (2018) Topical ivermectin in the treatment of papulopustular rosacea: a systematic review of evidence and clinical guideline recommendations. Dermatol Ther (Heidelb) 8, 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebneyamin E, Mansouri P, Rajabi M, Qomi M, Asgharian R and Azizian Z (2020) The efficacy and safety of permethrin 2.5% with tea tree oil gel on rosacea treatment: a double-blind, controlled clinical trial. Journal of Cosmetic Dermatology 19, 1426–1431. [DOI] [PubMed] [Google Scholar]
- Einarson A, Ho E and Koren G (2000) Can we use metronidazole during pregnancy and breastfeeding? Putting an end to the controversy. Canadian Family Physician Medecin de Famille Canadien 46, 1053–1054. [PMC free article] [PubMed] [Google Scholar]
- Eismann R, Bramsiepe I, Danz B, Wohlrab J, Marsch W and Fiedler E (2010) Abscessing nodular demodicosis – therapy with ivermectin and permethrin. Journal of the European Academy of Dermatology and Venereology: JEADV 24, 79–81. [DOI] [PubMed] [Google Scholar]
- El-Shazly AM, Hassan AA, Soliman M, Morsy GH and Morsy TA (2004) Treatment of human Demodex folliculorum by camphor oil and metronidazole. Journal of the Egyptian Society of Parasitology 34, 107–116. [PubMed] [Google Scholar]
- Enginyurt O, Karaman U, Cetin F and Ozer A (2015) The prevalence of Demodex species and its relationship with the metabolic syndrome in women of Malatya province, Turkey. Jundishapur Journal of Microbiology 8, e24322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein IJ, Rosenberg E, Stuber R, Choi MB, Donnenfeld ED and Perry HD (2020) Double-masked and unmasked prospective study of terpinen-4-ol lid scrubs with microblepharoexfoliation for the treatment of Demodex blepharitis. Cornea 39, 408–416. [DOI] [PubMed] [Google Scholar]
- Erbagci Z, Erbagci I and Erkilic S (2003) High incidence of demodicidosis in eyelid basal cell carcinomas. International Journal of Dermatology 42, 567–571. [DOI] [PubMed] [Google Scholar]
- Ergun SB, Saribas GS, Yarayici S, Elmazoglu Z, Cardak A, Ozogul C, Ilhan MN, Karasu C and Evren Kemer O (2019) Comparison of efficacy and safety of two tea tree oil-based formulations in patients with chronic blepharitis: a double-blinded randomized clinical trial. Ocular Immunology and Inflammation, published online ahead of print, 2019 Aug 20, 1–10. doi: 10.1080/09273948.2019.1644349. [DOI] [PubMed] [Google Scholar]
- Evren Kemer O, Karaca EE and Ozek D (2020) Efficacy of cyclic therapy with terpinen-4-ol in Demodex blepharitis: is treatment possible by considering Demodex's life cycle? European Journal of Ophthalmology ,published online ahead of print, 2020 Apr 24, doi: 10.1177/1120672120919085 [DOI] [PubMed] [Google Scholar]
- Fernandez-Flores A and Alija A (2009) Scalp folliculitis with Demodex: innocent observer or pathogen? The Brazilian Journal of Infectious Diseases 13, 81–82. [DOI] [PubMed] [Google Scholar]
- Fichtel JC, Wiggins AK and Lesher JL Jr (2005) Plaque-forming demodicidosis. Journal of the American Academy of Dermatology 52, 59–61. [DOI] [PubMed] [Google Scholar]
- Filho PA, Hazarbassanov RM, Grisolia AB, Pazos HB, Kaiserman I and Gomes JA (2011) The efficacy of oral ivermectin for the treatment of chronic blepharitis in patients tested positive for Demodex Spp. British Journal of Ophthalmology 95, 893–895. [DOI] [PubMed] [Google Scholar]
- Forstinger C, Kittler H and Binder M (1999) Treatment of rosacea-like demodicidosis with oral ivermectin and topical permethrin cream. Journal of the American Academy of Dermatology 41, 775–777. [DOI] [PubMed] [Google Scholar]
- Forton FMN and De Maertelaer V (2020) Treatment of rosacea and demodicosis with benzyl benzoate: effects of different doses on Demodex density and clinical symptoms. Journal of the European Academy of Dermatology and Venereology: JEADV 34, 365–369. [DOI] [PubMed] [Google Scholar]
- Forton F, Seys B, Marchal JL and Song AM (1998) Demodex folliculorum and topical treatment: acaricidal action evaluated by standardized skin surface biopsy. British Journal of Dermatology 138, 461–466. [DOI] [PubMed] [Google Scholar]
- Friedman P, Sabban EC and Cabo H (2017) Usefulness of dermoscopy in the diagnosis and monitoring treatment of demodicidosis. Dermatology Practical & Conceptual 7, 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulk GW, Murphy B and Robins MD (1996) Pilocarpine gel for the treatment of demodicosis – a case series. Optometry and Vision Science 73, 742–745. [DOI] [PubMed] [Google Scholar]
- Galea M, Sharma R, Srinivasan S and Roberts F (2014) Demodex blepharitis mimicking eyelid sebaceous gland carcinoma. Clinical & Experimental Ophthalmology 42, 208–210. [DOI] [PubMed] [Google Scholar]
- Gao YY, Di Pascuale MA, Li W, Baradaran-Rafii A, Elizondo A, Kuo CL, Raju VK and Tseng SC (2005) In vitro and in vivo killing of ocular Demodex by tea tree oil. British Journal of Ophthalmology 89, 1468–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y-Y M. D., Di Pascuale M. A. M. D., Elizondo A. M. D. and Tseng S. C. G. M. D. P (2007) Clinical treatment of ocular demodecosis by lid scrub with tea tree oil. [Article]. Cornea February 2007 26, 136–143. [DOI] [PubMed] [Google Scholar]
- Gao Y, Xu D, Huang L, Wang R and Tseng S (2012) Treatment of ocular itching associated with ocular demodicosis by 5% tea tree oil ointment. Cornea 31, 14–17. [DOI] [PubMed] [Google Scholar]
- Gazi U, Gureser AS, Oztekin A, Karasartova D, Kosar-Acar N, Derici MK, Artuz F, Mumcuoglu KY and Taylan-Ozkan A (2019) Skin-homing T-cell responses associated with Demodex infestation and rosacea. Parasite Immunology 41, e12658. [DOI] [PubMed] [Google Scholar]
- Georgala S, Katoulis AC, Kylafis GD, Koumantaki-Mathioudaki E, Georgala C and Aroni K (2001) Increased density of Demodex folliculorum and evidence of delayed hypersensitivity reaction in subjects with papulopustular rosacea. Journal of the European Academy of Dermatology and Venereology: JEADV 15, 441–444. [DOI] [PubMed] [Google Scholar]
- Gokce C, Aycan-Kaya O, Yula E, Ustun I, Yengil E, Sefil F, Rizaoglu H, Gultepe B and Bayram F (2013) The effect of blood glucose regulation on the presence of opportunistic Demodex folliculorum mites in patients with type 2 diabetes mellitus. Journal of International Medical Research 41, 1752–1758. [DOI] [PubMed] [Google Scholar]
- Grossmann B, Jung K and Linse R (1999) [Tubero-pustular demodicosis]. Der Hautarzt 50, 491–494. [DOI] [PubMed] [Google Scholar]
- Guerrero-Gonzalez GA, Herz-Ruelas ME, Gomez-Flores M and Ocampo-Candiani J (2014) Crusted demodicosis in an immunocompetent pediatric patient. Case Reports in Dermatological Medicine 2014, 458046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachfi W, Slama D, Ben Lasfar N, Mnif K, Bellazreg F, Fathallah A and Letaief A (2019) Demodicosis revealing an HIV infection. New Microbes and New Infections 31, 100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P, Kaye CM, McIntosh N and Steele J (1983) Intravenous metronidazole in the newborn. Archives of Disease in Childhood 58, 529–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer KA, Carson CF, Riley TV and Nielsen JB (2006) A review of the toxicity of Melaleuca alternifolia (tea tree) oil. Food and Chemical Toxicology 44, 616–625. [DOI] [PubMed] [Google Scholar]
- Hausen BM and Kresken J (1988) The sensitizing capacity of crotamiton. Contact Dermatitis 18, 298–299. [DOI] [PubMed] [Google Scholar]
- Hay RJ, Steer AC, Engelman D and Walton S (2012) Scabies in the developing world – its prevalence, complications, and management. Clinical Microbiology and Infection 18, 313–323. [DOI] [PubMed] [Google Scholar]
- Hecht I, Melzer-Golik A, Sadi Szyper N and Kaiserman I (2019) Permethrin cream for the treatment of Demodex blepharitis. Cornea 38, 1513–1518. [DOI] [PubMed] [Google Scholar]
- Helou W, Avitan-Hersh E and Bergman R (2016) Demodex folliculitis of the scalp: clinicopathological study of an uncommon entity. The American Journal of Dermatopathology 38, 658–663. [DOI] [PubMed] [Google Scholar]
- Hengge UR, Currie BJ, Jager G, Lupi O and Schwartz RA (2006) Scabies: a ubiquitous neglected skin disease. The Lancet. Infectious Diseases 6, 769–779. [DOI] [PubMed] [Google Scholar]
- Herron MD, O'Reilly MA and Vanderhooft SL (2005) Refractory Demodex folliculitis in five children with acute lymphoblastic leukemia. Pediatric Dermatology 22, 407–411. [DOI] [PubMed] [Google Scholar]
- Hervás Ontiveros A, Hurtado-Sarrió M, Udaondo P, García-Delpech S, Salom D and Díaz-Llopis M (2014). Ethyl ether: an old ally against oral ivermectin resistant Demodex blepharitis. Archivos de la Sociedad Española de Oftalmología (English Edition) 89, 85–86. [DOI] [PubMed] [Google Scholar]
- Hirsch-Hoffmann S, Kaufmann C, Banninger PB and Thiel MA (2015) Treatment options for Demodex blepharitis: patient choice and efficacy. Klinische Monatsblatter Fur Augenheilkunde 232, 384–387. [DOI] [PubMed] [Google Scholar]
- Hitraya-Low M, Ahmad RC and Cordoro KM (2016) Facial eruption in a 5-year-old child with acute lymphoblastic leukemia. Pediatric Dermatology 33, 671–672. [DOI] [PubMed] [Google Scholar]
- Hoda S and Cheng E (2019) Itching for attention: Demodex infestation of the nipple. International Journal of Surgical Pathology 27, 524–525. [DOI] [PubMed] [Google Scholar]
- Hoekzema R, Hulsebosch HJ and Bos JD (1995) Demodicidosis or rosacea: what did we treat? British Journal of Dermatology 133, 294–299. [DOI] [PubMed] [Google Scholar]
- Holmes AD (2013) Potential role of microorganisms in the pathogenesis of rosacea. Journal of the American Academy of Dermatology 69, 1025–1032. [DOI] [PubMed] [Google Scholar]
- Holzchuh FG, Hida RY, Moscovici BK, Villa Albers MB, Santo RM, Kara-Jose N and Holzchuh R (2011) Clinical treatment of ocular Demodex folliculorum by systemic ivermectin. American Journal of Ophthalmology, 151, 1030–1034 e1031. [DOI] [PubMed] [Google Scholar]
- Hsu CK, Hsu MM and Lee JY (2009) Demodicosis: a clinicopathological study. Journal of the American Academy of Dermatology 60, 453–462. [DOI] [PubMed] [Google Scholar]
- Huynh Q, Phan TD, Thieu VQQ, Tran ST and Do H-S (2012) Extraction and refining of essential oil from Australian tea tree. Melaleuca alterfornia, and the antimicrobial activity in cosmetic products.
- Hwang SM, Yoo MS, Ahn SK and Choi EH (1998) Demodecidosis manifested on the external genitalia. International Journal of Dermatology 37, 634–636. [DOI] [PubMed] [Google Scholar]
- Imamura L, Hasegawa H, Kurashina K, Hamanishi A, Tabuchi A and Tsuda M (2000) Repression of activity-dependent c-fos and brain-derived neurotrophic factor mRNA expression by pyrethroid insecticides accompanying a decrease in Ca(2+) influx into neurons. Journal of Pharmacology and Experimental Therapeutics 295, 1175–1182. [PubMed] [Google Scholar]
- Inci M, Kaya OA, Inci M, Yula E, Gokce H, Rifaioglu MM, Demirtas O and Yengil E (2012) [Investigating Demodex folliculorum in patients with urological cancer]. Turkiye Parazitolojii Dergisi 36, 208–210. [DOI] [PubMed] [Google Scholar]
- ISO (2017). Oil of Melaleuca, terpinen-4-ol type (tea tree oil). https://www.iso.org/standard/69082.html.
- Jacob S, VanDaele MA and Brown JN (2019) Treatment of Demodex-associated inflammatory skin conditions: a systematic review. Dermatologic Therapy 32, e13103. [DOI] [PubMed] [Google Scholar]
- Jalbert I and Rejab S (2015) Increased numbers of Demodex in contact lens wearers. Optometry and Vision Science 92, 671–678. [DOI] [PubMed] [Google Scholar]
- Jansen T, Kastner U, Kreuter A and Altmeyer P (2001) Rosacea-like demodicidosis associated with acquired immunodeficiency syndrome. British Journal of Dermatology 144, 139–142. [DOI] [PubMed] [Google Scholar]
- Jantzie LL and Todd KG (2010) Doxycycline inhibits proinflammatory cytokines but not acute cerebral cytogenesis after hypoxia-ischemia in neonatal rats. Journal of Psychiatry & Neuroscience 35, 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junk AK, Lukacs A and Kampik A (1998) [Topical administration of metronidazole gel as an effective therapy alternative in chronic Demodex blepharitis – a case report]. Klinische Monatsblatter Fur Augenheilkunde 213, 48–50. [DOI] [PubMed] [Google Scholar]
- Karakurt Y and Zeytun E (2018) Evaluation of the efficacy of tea tree oil on the density of Demodex mites (Acari: Demodicidae) and ocular symptoms in patients with demodectic blepharitis. Journal of Parasitology 104, 473–478. [DOI] [PubMed] [Google Scholar]
- Karincaoglu Y, Bayram N, Aycan O and Esrefoglu M (2004) The clinical importance of Demodex folliculorum presenting with nonspecific facial signs and symptoms. The Journal of Dermatology 31, 618–626. [DOI] [PubMed] [Google Scholar]
- Kaser S, Ruini C, Ezmerli M, von Braunmuhl T, Hartmann D, Ruzicka T and Reinholz M (2017) EGFRI-induced papulopustular rosacea-like rash successfully treated with topical ivermectin. Journal of the European Academy of Dermatology and Venereology: JEADV 31, e302–e304. [DOI] [PubMed] [Google Scholar]
- Kaya S, Selimoglu MA, Kaya OA and Ozgen U (2013) Prevalence of Demodex folliculorum and Demodex brevis in childhood malnutrition and malignancy. Pediatrics International 55, 85–89. [DOI] [PubMed] [Google Scholar]
- Kaya OA, Akkucuk S, Ilhan G, Guneri CO and Mumcuoglu K (2019) The importance of Demodex mites (Acari: Demodicidae) in patients with sickle cell Anemia. Journal of Medical Entomology 56, 599–602. [DOI] [PubMed] [Google Scholar]
- Keskin Kurt R, Aycan Kaya O, Karateke A, Silfeler DB, Soylu Karapinar O, Akkoca AN and Hakverdi and AU (2014) Increased density of Demodex folliculorum mites in pregnancies with gestational diabetes. Medical Principles and Practice: International Journal of the Kuwait University, Health Science Centre 23, 369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirkhah A, Casas V, Li W, Raju VK and Tseng SC (2007) Corneal manifestations of ocular Demodex infestation. American Journal of Ophthalmology 143, 743–749. [DOI] [PubMed] [Google Scholar]
- Kim HS, Luo L, Pflugfelder SC and Li DQ (2005) Doxycycline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Investigative Ophthalmology & Visual Science 46, 840–848. [DOI] [PubMed] [Google Scholar]
- Kim JH, Chun YS and Kim JC (2011a) Clinical and immunological responses in ocular demodecosis. Journal of Korean Medical Science 26, 1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Oh YS and Choi EH (2011b) Oral azithromycin for treatment of intractable rosacea. Journal of Korean Medical Science 26, 694–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JT, Lee SH, Chun YS and Kim JC (2011c) Tear cytokines and chemokines in patients with Demodex blepharitis. Cytokine 53, 94–99. [DOI] [PubMed] [Google Scholar]
- Kim BR, Park HS, Yoon HS and Cho S (2014) Demodicidosis on the arms of a patient with pemphigus foliaceus. Journal of the American Academy of Dermatology 71, e92–e93. [DOI] [PubMed] [Google Scholar]
- Klemm E, Haroske G and Wollina U (2009) Otitis externa and myringitis due to demodicidosis. Acta Dermatovenerologica Alpina, Pannonica, Et Adriatica 18, 73–76. [PubMed] [Google Scholar]
- Kobylinski KC, Foy BD and Richardson JH (2012) Ivermectin inhibits the sporogony of Plasmodium falciparum in Anopheles gambiae. Malaria Journal 11, 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocak M, Yagli S, Vahapoglu G and Eksioglu M (2002) Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. A randomized double-blind placebo-controlled study. Dermatology (Basel, Switzerland) 205, 265–270. [DOI] [PubMed] [Google Scholar]
- Kokacya MH, Yengil E, Kaya OA and Sahpolat M (2015) The frequency of Demodex Spp in depression patients. Erciyes Tıp Dergisi/Erciyes Medical Journal 36, 166–169. [Google Scholar]
- Kokaçya MH, Hamamcı B, Çöpoglu ÜS and Kaya ÖA (2016a) Demodex parazytes in schizophrenia. Journal of Clinical and Analytical Medicine 7, 6–9. [Google Scholar]
- Kokaçya MH, Kaya ÖA, Çöpoğlu ÜS and Elmacıoğlu S (2016b) Prevalence of Demodex Spp among alcohol-dependent patients. Cukurova Medical Journal 41, 259–263. [Google Scholar]
- Koo H, Kim TH, Kim KW, Wee SW, Chun YS and Kim JC (2012) Ocular surface discomfort and Demodex: effect of tea tree oil eyelid scrub in Demodex blepharitis. Journal of Korean Medical Science 27, 1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey N, Kavanagh K and Tseng SC (2009) Under the lash: Demodex mites in human diseases. Biochem (Lond) 31, 2–6. [PMC free article] [PubMed] [Google Scholar]
- Lam NSK, Long XX, Griffin RC, Chen MK and Doery JC (2018) Can the tea tree oil (Australian native plant: Melaleuca alternifolia Cheel) be an alternative treatment for human demodicosis on skin? Parasitology 145, 1510–1520. [DOI] [PubMed] [Google Scholar]
- Larson D and Jacob SE (2012) Tea tree oil. Dermatitis: Contact, Atopic, Occupational, Drug 23, 48–49. [DOI] [PubMed] [Google Scholar]
- Liang L, Safran S, Gao Y, Sheha H, Raju VK and Tseng SC (2010) Ocular demodicosis as a potential cause of pediatric blepharoconjunctivitis. Cornea 29, 1386–1391. [DOI] [PubMed] [Google Scholar]
- Liang L, Ding X and Tseng SC (2014) High prevalence of Demodex brevis infestation in chalazia. American Journal of Ophthalmology 157, 342–348 e341. [DOI] [PubMed] [Google Scholar]
- Liaqat M, Wilson LH, Wada D, Florell SR and Bowen AR (2015) Neutrophilic sebaceous adenitis with intralobular Demodex mites: a case report and review of the literature. The American Journal of Dermatopathology 37, 315–318. [DOI] [PubMed] [Google Scholar]
- Liu J, Sheha H and Tseng SC (2010) Pathogenic role of Demodex mites in blepharitis. Current Opinion in Allergy and Clinical Immunology 10, 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low WL, Kenward K, Britland ST, Amin MC and Martin C (2017) Essential oils and metal ions as alternative antimicrobial agents: a focus on tea tree oil and silver. International Wound Journal 14, 369–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Sun YJ, Zhang L and Luan XL (2016) Treatment of mites folliculitis with an ornidazole-based sequential therapy: a randomized trial. Medicine (Baltimore) 95, e4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraghi S, Rafiei A and Kaydani GA (2013) Human demodicosis: a report of 5 cases. Jundishapur Journal of Microbiology, published online ahead of print, 6. doi: 10.5812/jjm.7465. [DOI] [Google Scholar]
- McMahon F, Banville N, Bergin DA, Smedman C, Paulie S, Reeves E and Kavanagh K (2016) Activation of neutrophils via IP3 pathway following exposure to Demodex-associated bacterial proteins. Inflammation 39, 425–433. [DOI] [PubMed] [Google Scholar]
- Messaoud R, El Fekih L, Mahmoud A, Ben Amor H, Bannour R, Doan S and Khairallah M (2019) Improvement in ocular symptoms and signs in patients with Demodex anterior blepharitis using a novel terpinen-4-ol (2.5%) and hyaluronic acid (0.2%) cleansing wipe. Clinical Ophthalmology (Auckland, N.Z.) 13, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills C, Cleary BJ, Gilmer JF and Walsh JJ (2004) Inhibition of acetylcholinesterase by tea tree oil. Journal of Pharmacy and Pharmacology 56, 375–379. [DOI] [PubMed] [Google Scholar]
- Mohammadpour M, Maleki S and Khorrami-Nejad M (2019) The effect of tea tree oil on dry eye treatment after phacoemulsification cataract surgery: a randomized clinical trial. European Journal of Ophthalmology, published online ahead of print, doi: 10.1177/1120672119867642 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG and The PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine 6, e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran EM, Foley R and Powell FC (2017) Demodex and rosacea revisited. Clinics in Dermatology 35, 195–200. [DOI] [PubMed] [Google Scholar]
- Morras PG, Santos SP, Imedio IL, Echeverria ML and Hermosa JM (2003) Rosacea-like demodicidosis in an immunocompromised child. Pediatric Dermatology 20, 28–30. [DOI] [PubMed] [Google Scholar]
- Morsy TA, Morsy GH and Sanad EM (2002) Eucalyptus globulus (camphor oil) in the treatment of human demodicidosis. Journal of the Egyptian Society of Parasitology 32, 797–803. [PubMed] [Google Scholar]
- Murphy O, O'Dwyer V and Lloyd-McKernan A (2018) The efficacy of tea tree face wash, 1, 2–octanediol and microblepharoexfoliation in treating Demodex folliculorum blepharitis. Contact Lens & Anterior Eye 41, 77–82. [DOI] [PubMed] [Google Scholar]
- Mytton OT, McGready R, Lee SJ, Roberts CH, Ashley EA, Carrara VI, Thwai KL, Jay MP, Wiangambun T, Singhasivanon P and Nosten F (2007) Safety of benzyl benzoate lotion and permethrin in pregnancy: a retrospective matched cohort study. BJOG: An International Journal of Obstetrics and Gynaecology 114, 582–587. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sasaki M, Fujita K, Nishimoto M and Takaiwa T (1996) Demodex folliculitis on the trunk of a patient with mycosis fungoides. Clinical and Experimental Dermatology 21, 148–150. [PubMed] [Google Scholar]
- Narayanan S, Hunerbein A, Getie M, Jackel A and Neubert RH (2007) Scavenging properties of metronidazole on free oxygen radicals in a skin lipid model system. Journal of Pharmacy and Pharmacology 59, 1125–1130. [DOI] [PubMed] [Google Scholar]
- Nath AK, Timshina DK, Thappa DM and Sinclair R (2012) Demodex in an aerobic environment on the eyelashes. The Australasian Journal of Dermatology 53, 159–160. [DOI] [PubMed] [Google Scholar]
- National Health and Medical Research Council (2009) NHMRC additional levels of evidence and grades for recommendations for developers of guidelines. https://www.nhmrc.gov.au/sites/default/files/images/appendix-f-levels-of-evidence.pdf.
- Navel V, Mulliez A, Benoist d'Azy C, Baker JS, Malecaze J, Chiambaretta F and Dutheil F (2019) Efficacy of treatments for Demodex blepharitis: a systematic review and meta-analysis. The Ocular Surface 17, 655–669. [DOI] [PubMed] [Google Scholar]
- Nicholls SG, Oakley CL, Tan A and Vote BJ (2016) Demodex treatment in external ocular disease: the outcomes of a Tasmanian case series. International Ophthalmology 36, 691–696. [DOI] [PubMed] [Google Scholar]
- Nogueira MN, Aquino SG, Rossa Junior C and Spolidorio DM (2014) Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1beta, IL-6 and IL-10 on human macrophages. Inflammation Research 63, 769–778. [DOI] [PubMed] [Google Scholar]
- Noguera-Morel L, Gerlero P, Torrelo A and Hernandez-Martin A (2017) Ivermectin therapy for papulopustular rosacea and periorificial dermatitis in children: a series of 15 cases. Journal of the American Academy of Dermatology 76, 567–570. [DOI] [PubMed] [Google Scholar]
- Norn MS (1971) Demodex folliculorum. Incidence, regional distribution, pathogenicity. Danish Medical Bulletin 18, 14–17. [PubMed] [Google Scholar]
- O'Reilly N, Gallagher C, Reddy Katikireddy K, Clynes M, O'Sullivan F and Kavanagh K (2012) Demodex-associated Bacillus proteins induce an aberrant wound healing response in a corneal epithelial cell line: possible implications for corneal ulcer formation in ocular rosacea. Investigative Ophthalmology and Visual Science 53, 3250–3259. [DOI] [PubMed] [Google Scholar]
- Ozer A, Karaman U, Degerli S, Colak C, Karadan M and Karci E (2012) Investigation of Demodex Spp. prevalence among managers and workers of health hazard bearing and sanitary establishment. Journal of the Formosan Medical Association 111, 30–33. [DOI] [PubMed] [Google Scholar]
- Ozturkcan S, Ermertcan AT, Sahin MT and Afsar FS (2004) Efficiency of benzoyl peroxide-erythromycin gel in comparison with metronidazole gel in the treatment of acne rosacea. The Journal of Dermatology 31, 610–617. [DOI] [PubMed] [Google Scholar]
- Park SB, Jeong NJ, Lee Y and Im M (2011) Unilateral demodicidosis in a patient with seborrheic dermatitis. Korean Journal of Medical Mycology 16, 67–70. [Google Scholar]
- Passmore CM, McElnay JC, Rainey EA and D'Arcy PF (1988) Metronidazole excretion in human milk and its effect on the suckling neonate. British Journal of Clinical Pharmacology 26, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrizi A, Neri I, Chieregato C and Misciali M (1997) Demodicidosis in immunocompetent young children: report of eight cases. Dermatology (Basel, Switzerland) 195, 239–242. [DOI] [PubMed] [Google Scholar]
- Pelletier JS, Capriotti K, Stewart KS and Capriotti JA (2017) Demodex blepharitis treated with a novel dilute povidone-iodine and DMSO system: a case report. Ophthalmology and Therapy 6, 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta Guardia CA (2015) Demodex folliculorum: its association with oily skin surface rather than rosacea lesions. International Journal of Dermatology 54, e14–e17. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Hayes TJ and Dixon SL (1986) Pustular folliculitis associated with Demodex folliculorum. Journal of the American Academy of Dermatology 15, 1159–1162. [DOI] [PubMed] [Google Scholar]
- Rai M, Paralikar P, Jogee P, Agarkar G, Ingle AP, Derita M and Zacchino S (2017) Synergistic antimicrobial potential of essential oils in combination with nanoparticles: emerging trends and future perspectives. International Journal of Pharmaceutics 519, 67–78. [DOI] [PubMed] [Google Scholar]
- Raoufinejad K, Mansouri P, Rajabi M, Naraghi Z and Jebraeili R (2016) Efficacy and safety of permethrin 5% topical gel vs placebo for rosacea: a double-blind randomized controlled clinical trial. Journal of the European Academy of Dermatology and Venereology: JEADV 30, 2105–2117. [DOI] [PubMed] [Google Scholar]
- Rather PA and Hassan I (2014) Human Demodex mite: the versatile mite of dermatological importance. Indian Journal of Dermatology 59, 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roan V (2016) Eliminating the mite-Y menace. Review of Optometry, https://www.reviewofoptometry.com/article/spotlight-on-demodex-eliminating-the-mitey-menace (Accessed 20 August 2020). [Google Scholar]
- Robinson TW (1965) Demodex folliculorum and rosacea. A clinical and histological study. Archives of Dermatology 92, 542–544. [PubMed] [Google Scholar]
- Rodríguez AE, Ferrer C and Alió JL (2005) Chronic blepharitis and Demodex. Archivos de la Sociedad Española de Oftalmología 80, 635–642. [DOI] [PubMed] [Google Scholar]
- Rufli T and Mumcuoglu Y (1981) The hair follicle mites Demodex folliculorum and Demodex brevis: biology and medical importance. A review. Dermatologica 162, 1–11. [DOI] [PubMed] [Google Scholar]
- Ruini C, Sattler E, Hartmann D, Reinholz M, Ruzicka T and von Braunmuhl T (2017) Monitoring structural changes in Demodex mites under topical ivermectin in rosacea by means of reflectance confocal microscopy: a case series. Journal of the European Academy of Dermatology and Venereology: JEADV 31, e299–e301. [DOI] [PubMed] [Google Scholar]
- Sahn EE and Sheridan DM (1992) Demodicidosis in a child with leukemia. Journal of the American Academy of Dermatology 27, 799–801. [DOI] [PubMed] [Google Scholar]
- Salem DA, El-Shazly A, Nabih N, El-Bayoumy Y and Saleh S (2013) Evaluation of the efficacy of oral ivermectin in comparison with ivermectin-metronidazole combined therapy in the treatment of ocular and skin lesions of Demodex folliculorum. International Journal of Infectious Diseases: IJID 17, e343–e347. [DOI] [PubMed] [Google Scholar]
- Sanchez-Viera M, Hernanz JM, Sampelayo T, Gurbindo MD, Lecona M and Soto-Melo J (1992) Granulomatous rosacea in a child infected with the human immunodeficiency virus. Journal of the American Academy of Dermatology 27, 1010–1011. [DOI] [PubMed] [Google Scholar]
- Sanfilippo AM and English JC, 3rd (2005) Resistant scalp folliculitis secondary to Demodex infestation. Cutis 76, 321–324. [PubMed] [Google Scholar]
- Sarac G (2019) A comparison of the efficacy and tolerability of topical agents used in facial Demodex treatment. Journal of Cosmetic Dermatology 18, 1784–1787. [DOI] [PubMed] [Google Scholar]
- Sarro RA, Hong JJ and Elgart ML (1998) An unusual demodicidosis manifestation in a patient with AIDS. Journal of the American Academy of Dermatology 38, 120–121. [DOI] [PubMed] [Google Scholar]
- Sattler EC, Hoffmann VS, Ruzicka T, Braunmuhl TV and Berking C (2015) Reflectance confocal microscopy for monitoring the density of Demodex mites in patients with rosacea before and after treatment. British Journal of Dermatology 173, 69–75. [DOI] [PubMed] [Google Scholar]
- Schaller M, Sander CA and Plewig G (2003) Demodex abscesses: clinical and therapeutic challenges. Journal of the American Academy of Dermatology 49, S272–S274. [DOI] [PubMed] [Google Scholar]
- Schaller M, Schofer H, Homey B, Hofmann M, Gieler U, Lehmann P, Luger TA, Ruzicka T and Steinhoff M (2016) Rosacea management: update on general measures and topical treatment options. Journal der Deutschen Dermatologischen Gesellschaft 14(Suppl 6), 17–27. [DOI] [PubMed] [Google Scholar]
- Schaller M, Gonser L, Belge K, Braunsdorf C, Nordin R, Scheu A and Borelli C (2017) Dual anti-inflammatory and anti-parasitic action of topical ivermectin 1% in papulopustular rosacea. Journal of the European Academy of Dermatology and Venereology: JEADV 31, 1907–1911. [DOI] [PubMed] [Google Scholar]
- Sekine R, Satoh T, Takaoka A, Saeki K and Yokozeki H (2012) Anti pruritic effects of topical crotamiton, capsaicin, and a corticosteroid on pruritogen-induced scratching behavior. Experimental Dermatology 21, 201–204. [DOI] [PubMed] [Google Scholar]
- Smith K and Leyden JJ (2005) Safety of doxycycline and minocycline: a systematic review. Clinical Therapeutics 27, 1329–1342. [DOI] [PubMed] [Google Scholar]
- Sonmez OU, Yalcin ZG, Karakece E, Ciftci IH and Erdem T (2013) Associations between Demodex species infestation and various types of cancer. Acta Parasitologica 58, 551–555. [DOI] [PubMed] [Google Scholar]
- Souza ME, Lopes LQ, Bonez PC, Gundel A, Martinez DS, Sagrillo MR, Giongo JL, Vaucher RA, Raffin RP, Boligon AA and Santos RC (2017) Melaleuca alternifolia nanoparticles against Candida species biofilms. Microbial Pathogenesis 104, 125–132. [DOI] [PubMed] [Google Scholar]
- Stein L, Kircik L, Fowler J, Tan J, Draelos Z, Fleischer A, Appell M, Steinhoff M, Lynde C, Liu H and Jacovella J (2014) Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. Journal of Drugs in Dermatology: JDD 13, 316–323. [PubMed] [Google Scholar]
- Sunderkotter C, Herrmann M and Jappe U (2006) [Antimicrobial therapy in dermatology]. Journal der Deutschen Dermatologischen Gesellschaft 4, 10–27. [DOI] [PubMed] [Google Scholar]
- Sunderkotter C, Mayser P, Folster-Holst R, Maier WA, Kampen H and Hamm H (2007) Scabies. Journal der Deutschen Dermatologischen Gesellschaft 5, 424–430. [DOI] [PubMed] [Google Scholar]
- Swygard H, Sena AC, Hobbs MM and Cohen MS (2004) Trichomoniasis: clinical manifestations, diagnosis and management. Sexually Transmitted Infections 80, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb A, Ortonne JP, Ruzicka T, Roszkiewicz J, Berth-Jones J, Peirone MH, Jacovella J and Ivermectin Phase III Study Group (2015) Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. British Journal of Dermatology 172, 1103–1110. [DOI] [PubMed] [Google Scholar]
- Taieb A, Khemis A, Ruzicka T, Baranska-Rybak W, Berth-Jones J, Schauber J, Briantais P, Jacovella J, Passeron T and Ivermectin Phase III Study Group (2016) Maintenance of remission following successful treatment of papulopustular rosacea with ivermectin 1% cream vs metronidazole 0.75% cream: 36-week extension of the ATTRACT randomized study. Journal of the European Academy of Dermatology and Venereology: JEADV 30, 829–836. [DOI] [PubMed] [Google Scholar]
- Tarkowski W, Moneta-Wielgos J and Mlocicki D (2015) Demodex sp. as a potential cause of the abandonment of soft contact lenses by their existing users. Biomed Research International 2015, 259109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatu AL, Ionescu MA, Clatici VG and Cristea VC (2016) Bacillus cereus strain isolated from Demodex folliculorum in patients with topical steroid-induced rosaceiform facial dermatitis. Anais Brasileiros De Dermatologia 91, 676–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe S, Gao YY and Tseng SC (2013) Terpinen-4-ol is the most active ingredient of tea tree oil to kill Demodex mites. Translational Vision Science & Technology 2, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalik-Scharte D, Lazar A, Meins J, Bastian B, Ihrig M, Wachall B, Jetter A, Tantcheva-Poor I, Mahrle G and Fuhr U (2005) Dermal absorption of permethrin following topical administration. European Journal of Clinical Pharmacology 61, 399–404. [DOI] [PubMed] [Google Scholar]
- Trave I, Merlo G, Cozzani E and Parodi A (2019) Real-life experience on effectiveness and tolerability of topical ivermectin in papulopustular rosacea and antiparasitic effect on Demodex mites. Dermatologic Therapy 32, e13093. [DOI] [PubMed] [Google Scholar]
- Turberg A (2015) Ectoparasiticides: antagonists and modulators of chloride channels. In Mehlhorn H (ed), Encyclopedia of Parasitology. Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 1–10. [Google Scholar]
- Ugras M, Miman O, Karincaoglu Y and Atambay M (2009) The prevalence of Demodex folliculorum on the scrotum and male perineal skin. Turkiye Parazitolojii Dergisi 33, 28–31. [PubMed] [Google Scholar]
- Vanam HP, Mohanram KK, Siv Rami Reddy R., Poojari SS, Anuradha PR and Kandi V (2018) First report of concomitant tinea faciei and pityriasis folliculorum: a dermatomicrobiological rarity. Cureus 10, e3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zuuren EJ, Fedorowicz Z, Carter B, van der Linden MM and Charland L (2015) Interventions for rosacea. Cochrane Database of Systematic Reviews 2015. doi: doi: 10.1002/14651858.CD003262.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Arzola J, Reyes-Velasco L, Segura-Salvador A, Marquez-Navarro A, Diaz-Chiguer DL and Nogueda-Torres B (2012) Prevalence of Demodex mites in eyelashes among people of Oaxaca, Mexico. Acta Microbiologica et Immunologica Hungarica 59, 257–262. [DOI] [PubMed] [Google Scholar]
- Wang X, Martinez MA, Dai M, Chen D, Ares I, Romero A, Castellano V, Martinez M, Rodriguez JL, Martinez-Larranaga MR, Anadon A and Yuan Z (2016) Permethrin-induced oxidative stress and toxicity and metabolism. A review. Environmental Research 149, 86–104. [DOI] [PubMed] [Google Scholar]
- Wise RD (2007) Submicrobial doxycycline and rosacea. Comprehensive Therapy 33, 78–81. [DOI] [PubMed] [Google Scholar]
- Won JH, Ahn SK and Lee SH (1993) Unusual manifestation of demodicidosis in a child. International Journal of Dermatology 32, 822. [DOI] [PubMed] [Google Scholar]
- Wong K, Flanagan J, Jalbert I and Tan J (2019) The effect of blephadex eyelid wipes on Demodex mites, ocular microbiota, bacterial lipase and comfort: a pilot study. Contact Lens & Anterior Eye 42, 652–657. [DOI] [PubMed] [Google Scholar]
- Woo YR, Lim JH, Cho DH and Park HJ (2016) Rosacea: molecular mechanisms and management of a chronic cutaneous inflammatory condition. International Journal of Molecular Sciences 17, 1562. doi: 10.3390/ijms17091562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam JC, Tang BS, Chan TM and Cheng AC (2014) Ocular demodicidosis as a risk factor of adult recurrent chalazion. European Journal of Ophthalmology 24, 159–163. [DOI] [PubMed] [Google Scholar]
- Yamaoka T, Murota H, Tani M and Katayama I (2014) Severe rosacea with prominent Demodex folliculorum in a patient with HIV. The Journal of Dermatology 41, 195–196. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Yamaguchi R, Itoh T, Toh U, Nakagawa S and Kage M (2014) Detection of Demodex folliculorum from nipple discharge. Diagnostic Cytopathology 42, 236–237. [DOI] [PubMed] [Google Scholar]
- Yun SH, Levin F and Servat J (2013) Demodex folliculitis presenting as periocular vesiculopustular rash. Orbit (Amsterdam, Netherlands) 32, 370–371. [DOI] [PubMed] [Google Scholar]
- Zari J, Abdolmajid F, Masood M, Vahid M and Yalda N (2008) Evaluation of the relationship between androgenetic alopecia and Demodex infestation. Indian Journal of Dermatology 53, 64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Song Y, Ci X, An N, Ju Y, Li H, Wang X, Han C, Cui J and Deng X (2008) Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflammation Research 57, 524–529. [DOI] [PubMed] [Google Scholar]
- Zhang X, Song Y, Xiong H, Ci X, Li H, Yu L, Zhang L and Deng X (2009) Inhibitory effects of ivermectin on nitric oxide and prostaglandin E2 production in LPS-stimulated RAW 264.7 macrophages. International Immunopharmacology 9, 354–359. [DOI] [PubMed] [Google Scholar]
- Zhao YE, Guo N and Wu LP (2009) The effect of temperature on the viability of Demodex folliculorum and Demodex brevis. Parasitology Research 105, 1623–1628. [DOI] [PubMed] [Google Scholar]
- Zhong J, Tan Y, Li S, Peng L, Wang B, Deng Y and Yuan J (2019) The prevalence of Demodex folliculorum and Demodex brevis in cylindrical dandruff patients. Journal of Ophthalmology 2019, 8949683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotkin E (1999) The insect voltage-gated sodium channel as target of insecticides. Annual Review of Entomology 44, 429–455. [DOI] [PubMed] [Google Scholar]