Abstract
The metacestode of Echinococcus multilocularis is the etiological agent of alveolar echinococcosis. The metacestode stage used for research is maintained in rodents by serial passages. In order to determine whether cryopreservation of E. multilocularis metacestodes would be suitable for long-term maintenance and replace serial passages, isolates of different geographic origin were cryopreserved in 1984–1986. The aim of the current study was to test the viability of cryopreserved isolates following long-term cryopreservation (up to 35 years) and to determine the phylogenetic clades these isolates belonged to. Cryopreserved isolates were tested for viability in vitro and in vivo in gerbils. In vitro results of 5 isolates indicated protoscolex survival in 13 of 17 experiments (76%) and metacestode survival in 5 of 12 (42%) in vivo experiments. In vivo results showed ‘abortive lesions’ in 13 of the 36 animals, 15 were negative and 8 harboured proliferating metacestode tissue containing protoscoleces. Genetic analysis confirmed the isolates belonged to European, Asian and North-American clades. In conclusion, the results of the current study indicate that metacestodes of E. multilocularis are able to survive long-term cryopreservation. Therefore, cryopreservation is a suitable method for long-term storage of E. multilocularis metacestode isolates and reduces the number of experimental animals.
Key words: Alveolar echinococcosis, cryopreservation, Echinococcus multilocularis, fox tapeworm
Introduction
The larval (metacestode) stage of the fox tapeworm Echinococcus multilocularis, which is distributed across the Northern Hemisphere (Deplazes et al., 2017), is the etiological agent of the zoonotic disease alveolar echinococcosis (AE) (Eckert and Deplazes, 2004). The life cycle of E. multilocularis involves two hosts. The sexually reproducing adult worm inhabits the small intestine of carnivorous definitive hosts – primarily foxes and other canids, including domestic dogs. Wild rodents and other small mammals act as intermediate hosts harbouring the asexually proliferating metacestode, which resides in the liver and other organs in the form of small vesicular structures (Thompson, 1986, 2017; Romig et al., 2017). Intermediate and accidental hosts (e.g. humans) acquire the infection by ingestion of eggs which are produced by the adult worms and are shed in the feces to the environment by definitive hosts (Romig et al., 2017).
AE in humans is a rather rare or sporadic occurrence, but often lethal if it is not diagnosed and treated in an early stage of the infection (WHO/OIE, 2001; Eckert and Deplazes, 2004; Kern et al., 2017). The World Health Organization has prioritized AE as one of the 20 neglected zoonotic diseases. The global burden of the disease is substantial with an estimated 666 433 disability-adjusted life years (DALYs) and approximately 18 235 new AE cases per annum globally (Torgerson et al., 2010; WHO, 2020). As AE is one of the most dangerous zoonoses, research on the metacestode stage of E. multilocularis is of great significance and interest. For example, the metacestode is the focus of AE research for developing novel treatment options, including chemotherapy, but as well as studying the complex host–parasite interplay and immunomodulatory aspects of AE progression (Brehm and Koziol, 2017; Gottstein et al., 2017; Lundström-Stadelmann et al., 2019).
Traditionally, metacestodes of various E. multilocularis isolates/strains are maintained in rodents, typically in the highly susceptible Meriones spp. or laboratory mice (Romig and Bilger, 1999). Towards this purpose, the animals are infected through intraperitoneal (i.p.) injection of metacestode material. Due to the tumour-like proliferation and aggressive nature of the metacestode in rodents, the infection is terminated by euthanasia of the animals before they exhibit clinical signs. Therefore, the material has to be transferred to a new series of animals approximately every 2–3 months. This, however, means that relatively large numbers of experimental animals are required for mere strain maintenance purposes. Furthermore, during years of serial passage, the parasite biological behaviour might change/adapt – e.g. the metacestode is developing quickly and the animals have to be euthanised before protoscolex production within the metacestode is reached (Deplazes, personal communication).
In the framework of the R3-Program (Reduce, Refine, Replace) on alternatives to animal experimentation, a series of studies were already initiated in the mid-1980s at the Institute of Parasitology (IPZ) in Switzerland (University of Zurich), including cryopreservation trials of a number of different life cycle stages of various parasite species (Eckert, 1988). One of the aims was to develop a protocol for the cryopreservation of E. multilocularis metacestode material (Eckert and Ramp, 1985). Small tissue fragments (fragment size 0.1–3.5 mm3) or tissue blocks (TB) (0.2 g) of different isolates were cryopreserved according to a three-step protocol (pre-cooling for 30 min at 2–4°C, a three-step freezing protocol: 30 min at −28°C; 30 min at −80°C, plunge into liquid nitrogen), which was determined to be superior to a two-step protocol. It was shown that dimethylsulfoxide (DMSO, final concentration 5%) and glycerol (10%) are suitable cryoprotectants. After 1 week of cryopreservation between 46% and 100% of the samples contained viable metacestode material exhibiting proliferative capacity as judged by experimental infection of Meriones unguiculatus (Eckert and Ramp, 1985). Later, it was reported that the cryopreserved E. multilocularis isolates were still viable after 2 years (Eckert, 1988).
In principal, cryopreservation protocols are currently routinely implemented for various unicellular organisms (e.g. Miyake et al., 2004). Cryopreservation of multi-cellular organisms, however, still presents a challenge as different cell types typically have a variety of different requirements that would allow for their survival following storage in liquid nitrogen (James, 2004). Protocols for cryopreservation of multi-cellular aggregates have been developed for e.g. hepatocyte spheroids (Magalhães et al., 2008), and it was subsequently demonstrated that freezing hepatocytes in a tissue-like configuration (organoid) yields higher survivability rates than freezing them as a monolayer (Magalhães et al., 2012). Concerning multi-cellular parasites, cryopreservation protocols have been implemented with varying success rates of viability for a number of different larval stages of helminth species, such as Haemonchus contortus (Ramp et al., 1986), Trichinella spiralis (Andermatt-Mettler et al., 1987; Pozio et al., 1988) and Schistosoma mansoni (James, 1980; Cohen and Eveland, 1984). In contrast, studies documenting the long-term storage of cryopreserved parasite material are scarce. So far it has been shown that H. contortus (3rd stage larvae, exsheathed) is able to survive 10 years of cryopreservation and retain its infectivity for animals (Rew and Campbell, 1983, as cited in Eckert, 1988). Nevertheless, reports and effects of long-term cryostorage on viability for parasite material remain to be established.
The main aim of the current study was to determine to which degree the cryopreserved E. multilocularis metacestode isolates are still viable, following 35 years of storage in liquid nitrogen.
Material and methods
Biological Material
Echinococcus multilocularis metacestode material was analysed from five isolates that were previously cryopreserved between 1984 and 1986 at the IPZ, Zurich (Eckert and Ramp, 1985). Isolate A/1 was originally isolated in Alaska, St. Lawrence Island by Dr R.L. Rausch and transferred to Switzerland in a red-backed vole (Clethrionomys rutilus) in 1968. Isolate CDN/1 was of Canadian rodent origin, obtained from Dr G. Lubinsky in 1984. Both isolates were then maintained at the IPZ by serial passages in Mongolian gerbils/jirds (M. unguiculatus). Isolates CH/1, CH/11 and CH/22 originated from the livers of human patients in Switzerland and were propagated in gerbils since 1976, 1980 and 1984, respectively.
Method Of cryopreservation in 1984–1986 and thawing procedure
As described by Eckert and Ramp, (1985), the metacestode material was placed into liquid nitrogen as 1.0 mL aliquots containing either 0.1 mL of small tissue fragments (STF; fragment size 0.1–3.5 mm3), or 0.2 g TB suspended in Eagle's Minimal Essential Medium with Earle's salts (EMEM/A; Gibco Europe, CH-Basel), with the addition of 200 U/mL of penicillin and 200 μg/mL streptomycin. Either DMSO at 5% concentration or glycerol with a concentration of 10% was added as a cryoprotectant (Table 1).
Table 1.
Viability testing in vitro of E. multilocularis metacestode material after long-term (33–35 years) cryopreservation
| Isolate | Date of cryopreservation | Experiment no. | Tissue type | Cryoprotectant | Results | |
|---|---|---|---|---|---|---|
| Protoscoleces moving | Presence of vesicles | |||||
| A/1 | 19-09-84 | 1 | STF | 5% DMSO | no | no |
| 19-09-84 | 2 | STF | 10% glycerol | yes | yes | |
| 22-01-85 | 3 | TB | 5% DMSO | yes | no | |
| 06-03-86 | 4 | TB | 10% glycerol | no | no | |
| CDN/1 | 27-09-84 | 5 | STF | 5% DMSO | yes | no |
| 27-09-84 | 6 | STF | 10% glycerol | yes | yes | |
| 22-01-85 | 7 | TB | 5% DMSO | yes | no | |
| 24-01-86 | 8 | TB | 10% glycerol | yes | no | |
| CH/11 | 11-09-84 | 9 | STF | 5% DMSO | yes | yes |
| 13-09-84 | 10 | STF | 10% glycerol | yes | yes | |
| 20-03-85 | 11 | TB | 5% DMSO | yes | no | |
| 01-07-85 | 12 | TB | 10% glycerol | no | no | |
| CH/22 | 20-09-84 | 13 | STF | 5% DMSO | yes | yes |
| 22-01-85 | 14 | STF | 10% glycerol | yes | no | |
| 26-06-85 | 15 | TB | 10% glycerol | yes | yes | |
| CH/1 | 09-01-85 | 16 | TB | 5% DMSO | no | no |
| 06-03-86 | 17 | TB | 10% glycerol | yes | no | |
A, Alaska; CDN, Canada; CH, Switzerland; STF, small tissue fragments; TB, tissue block.
In the current study samples of the 5 above-mentioned metacestode isolates were studied which had been cryopreserved for up to 35 years (from 1984 to 1986 until 2019) according to different protocols (marked as experiments 1–17 in Table 1), varying in the geographical origin of the parasite isolates, the type of parasite material (STF or TB), and the cryoprotectant (DMSO or glycerol). Only samples were used that had been cryopreserved using a 3-step cooling procedure (Eckert and Ramp, 1985), which had been shown to be superior in terms of parasite survival. Briefly, the 3-step cooling procedure included: pre-cooling phase for 30 min at 2–4 °C; cooling for 30 min at −28 °C; followed by cooling for 30 min at −80 °C, and finally plunging into liquid nitrogen at −196°C (Eckert and Ramp, 1985). For our experiments, the cryotubes containing the samples were placed for 2–3 min in a 37°C water bath for rapid thawing. The further steps of material preparation are described below under sections 2.3 and 2.4.
Viability Testing in vitro
All 5 isolates were used for viability testing in vitro. Following thawing, the samples were transferred into 10 mL of pre-warmed (37°C) fresh Dulbecco's Modified Eagle's Medium (DMEM, high glucose, Sigma-Aldrich, Buchs, Switzerland) to dilute the cryoprotectant. After centrifugation at 200 g for 5–10 min the supernatant was removed without disturbing the pellet. This wash step was repeated twice. For the in vitro culturing of the parasite material, a co-culturing technique was used as essentially described by Spiliotis and Brehm (2009). Briefly, following the third wash step, 4 mL of conditioned sterile-filtered (0.2 μm syringe filter, Sarstedt, Germany) culture medium was added to the parasite material and placed into tissue culture flasks (25 cm2, BioSwisstech, Switzerland). The sterile-filtered conditioned culture medium was obtained from routinely co-cultivated unfrozen E. multilocularis in vitro cultures through collecting the supernatant. The quantity of metacestode material per flask for STF was 0.3–0.5 mL, and 0.6–1.0 g for TB (3–5 cryotubes per flask). The parasite cultures were incubated at 37°C and 5% CO2 in the presence of a monolayer of NIH/3T3 fibroblasts (Mus musculus, NIH/Swiss strain; ATCC CRL-1658, LGC Standards) and 10 mL of DMEM, supplemented with 20 μL/mL penicillin-streptomycin (stock 10 000 U/mL penicillin, 10 mg/mL streptomycin; Sigma-Aldrich, Buchs, Switzerland) and 10% heat-inactivated fetal calf serum (FCS; Bioconcept AG, Switzerland). Acidified culture medium was replaced with fresh medium three times a week for the duration of 8 weeks. Motility of protoscoleces, as well as presence and proliferation of metacestode vesicles, were recorded and used as preliminary indicators of parasite viability.
Viability Testing in vivo
In order to assess the viability of the parasite, 36 m. unguiculatus were infected with sample material through intraperitoneal (i.p.) injection. These studies were authorized by the Cantonal Veterinary Office of Zurich, Switzerland (permission no. ZH139/15 and ZH068/19). All procedures concerning in vivo experiments were conducted following the ethical standards of the guidelines of the Swiss Animals Protection Law. Extra care was taken to reduce the suffering of animals and to ensure the highest ethical and humane standards. Animals were kept at +21°C with a 12 h light/dark rhythm, whereas food was provided ad libitum. Three different isolates (A/1, CDN/1, CH/11) were selected for in vivo viability testing, with each of the isolates having been cryopreserved using four different protocols including STF or TB with the addition of either DMSO or glycerol (marked as experiments numbered 1–12 in Table 2). Animals were euthanised using CO2.
Table 2.
Vibility testing in vivo (Meriones unguiculatus) of E. multilocularis metacestode material following long-term (33–35 years) cryopreservation
| Isolate | Date of cryopreservation | Experiment no. | Tissue type | Cryoprotectant | Animals | ||
|---|---|---|---|---|---|---|---|
| Infected | Positive for metacestodes | With abortive lesions | |||||
| A/1 | 19-09-84 | 1 | STF | 5% DMSO | 3 | 0 | 0 |
| 19-09-84 | 2 | STF | 10% glycerol | 3 | 0 | 1 | |
| 22-01-85 | 3 | TB | 5% DMSO | 3 | 0 | 2 | |
| 06-03-86 | 4 | TB | 10% glycerol | 3 | 0 | 3 | |
| CDN/1 | 27-09-84 | 5 | STF | 5% DMSO | 3 | 2 | 1 |
| 27-09-84 | 6 | STF | 10% glycerol | 3 | 0 | 2 | |
| 22-01-85 | 7 | TB | 5% DMSO | 3 | 1 | 1 | |
| 24-01-86 | 8 | TB | 10% glycerol | 3 | 0 | 0 | |
| CH/11 | 11-09-84 | 9 | STF | 5% DMSO | 3 | 1 | 2 |
| 13-09-84 | 10 | STF | 10% glycerol | 3 | 2 | 0 | |
| 20-03-85 | 11 | TB | 5% DMSO | 3 | 2 | 0 | |
| 01-07-85 | 12 | TB | 10% glycerol | 3 | 0 | 1 | |
| Altogether: | 36 | 8 | 13 | ||||
A, Alaska; CDN, Canada; CH, Switzerland; STF, small tissue fragments; TB, tissue blocks.
The thawing procedure was as described above, whereas pre-warmed 1x phosphate-buffered saline (PBS) was used for the dilution of cryoprotectant. The parasite material (both STF and TB) was subsequently pushed through a sterile sieve, suspended in PBS, centrifuged at 200 g for 5–10 min, followed by removal of supernatant without disturbing the pellet. The samples for the i.p. injections were prepared from the packed sediment of 10–20 cryotubes, resulting in a dose of 200–300 μL suspension per animal. Taking into account the possible variability of metacestode infections in rodents, three gerbils were infected per tested cryopreserved sample.
Necropsy was performed 12 weeks post-inoculation (p.i.). Parasite material was considered as viable when the proliferation of metacestode tissue was observed. Subsequently, metacestode material obtained under sterile conditions from the infected animals was washed in 1x PBS, pushed through a sterile sieve, followed by culturing in vitro as described above to test for the proliferation of vesicles and the presence of protoscoleces.
DNA Extraction, polymerase chain reaction (PCR), sequencing and phylogenetic network
In order to determine the phylogeographic clades (Asian, North American, European) of the cryopreserved isolates (A/1, CDN/1, CH/11, CH/22), the mitochondrial genes of cytochrome b (cob, 1068 bp), NADH dehydrogenase subunit 2 (nad2, 882 bp) and cytochrome c oxidase subunit 1 (cox1, 1608 bp) were sequenced as described by Nakao et al. (2009). Genomic DNA was extracted from the metacestode tissue using the Qiagen DNeasy Blood and Tissue Kit (Hilden, Germany) according to the manufacturer's protocol. PCR primer pairs for each gene and amplification were as previously described in Nakao et al. (2009), while the Qiagen Multiplex PCR Kit (Hilden, Germany) was used for amplification reactions. Moreover, the MiniElute Kit (Qiagen, Hilden, Germany) was used for PCR product purification according to the manufacturer's protocols. Purified PCR products were sent for sequencing to Microsynth (Switzerland) with the same primers as for PCR. Consensus sequences were assembled using the program Geneious 2019.1.1 (Biomatters Ltd.), whereas for Clustal W multiple sequence alignment, as well as to manually check and correct the sequences for errors, the program BioEdit v.7.2.5 was employed (Thompson et al., 1994; Hall, 1999). Sequences of cob, nad2 and cox1 were concatenated and the phylogenetic network was constructed using the Network v4.612 software (Bandelt et al., 1999; http://www.fluxus-engineering.com, Fluxus Technology Ltd. 2004), with both indels and point mutations considered. In order to identify to which of the geographic clades the cryofrozen samples belonged to, sequences of the respective genes from the GenBank database were also included in the phylogenetic network analysis (AB461395–AB461420; AB477009–AB477012; Nakao et al., 2009).
Results
In Vitro testing
Protoscolex motility was observed for all of the five isolates cultured in vitro in one or more of the 4 experiments per isolate (Table 1), corresponding to 13 of all 17 experiments (76%). Comparison of types of the tissue showed that protoscolex movement was observed in 7 out of 8 experiments of STF (87%), and 6 out of 9 experiments of TB (67%). Assessment of the two different cryoprotectants indicated protoscolex movement in 6 out of 8 for DMSO (75%), and in 7 out 9 for glycerol (79%). Presence of metacestode vesicles was observed in 6 out of 17 experiments (35%), with 5 instances for STF and 1 for TB. The morphology of vesicles appeared to be similar to the unfrozen vesicles from the regular cycle of passage (Fig. S1).
In Vivo testing
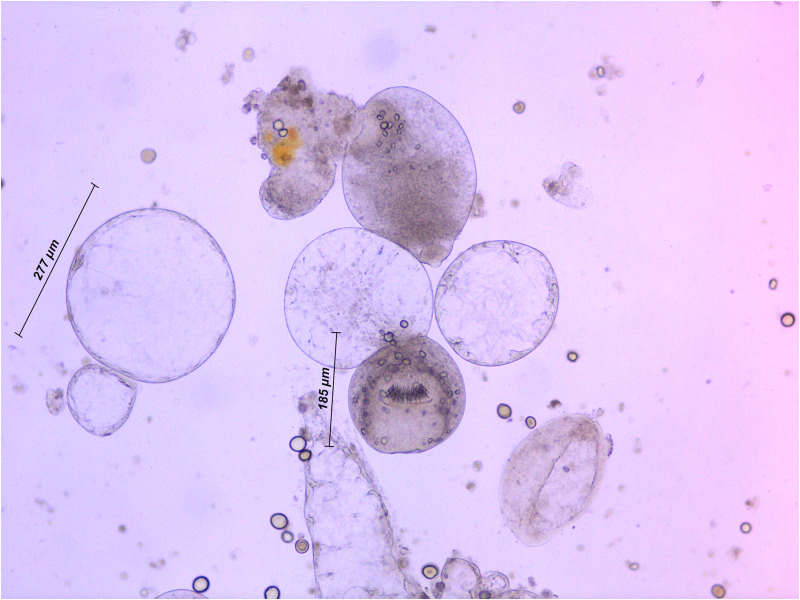
Overall 21 out of 36 necropsied gerbils presented signs of a metacestode infection (Table 2), including 8 (22%) animals with typical parasite proliferation (Fig. S2) and 13 with small abortive lesions (36%). The remaining 15 animals were negative. Regular parasite proliferation was not observed in 12 gerbils inoculated with the material of the Alaskan isolate (A/1), whereas 3 of 12 animals of the CDN/1 group (Canadian isolate) and 5 of 12 of animals of CH/11 group (Swiss isolate) harboured typical metacestode tissue. If the positive results are referred only by the two latter groups, the infection rate was 8 of 24 (33%). Material obtained from the 8 positive animals was cultured in vitro, and the presence of protoscoleces and proliferation of vesicles were observed in all 8 cases (Fig. 1).
Fig. 1.
Echinococcus multilocularis vesicles and protoscolex (invaginated) cultured in vitro. The material was obtained from a gerbil (Meriones unguiculatus) 12 weeks post-inoculation with the CDN/1 isolate cryopreserved for 35 years.
Sequencing And phylogenetic network
The complete mtDNA genes of cob and nad2 were successfully sequenced for isolates A/1, CDN/1, CH/11 and CH/22, whereas the full cox1 sequences were obtained for CDN/1, CH/11 and CH/22 and uploaded to GenBank . The cox1 sequence of A/1 was, however, 442 bp shorter when compared to the reference sequence of AB461418 . Notably, the sequence quality was high for the whole amplified cox1 gene region for A/1 . As such, the isolate A/1 was excluded from the phylogenetic network analysis of the concatenated 3 full mtDNA genes (3558 bp in total), and the geographic haplotype cluster it putatively belonged to was identified based on cob, nad2 and the available partial cox1 (concatenated sequence of 3116 bp). Accession numbers MT429271-MT429278, MT469888, MT461409-MT461411.
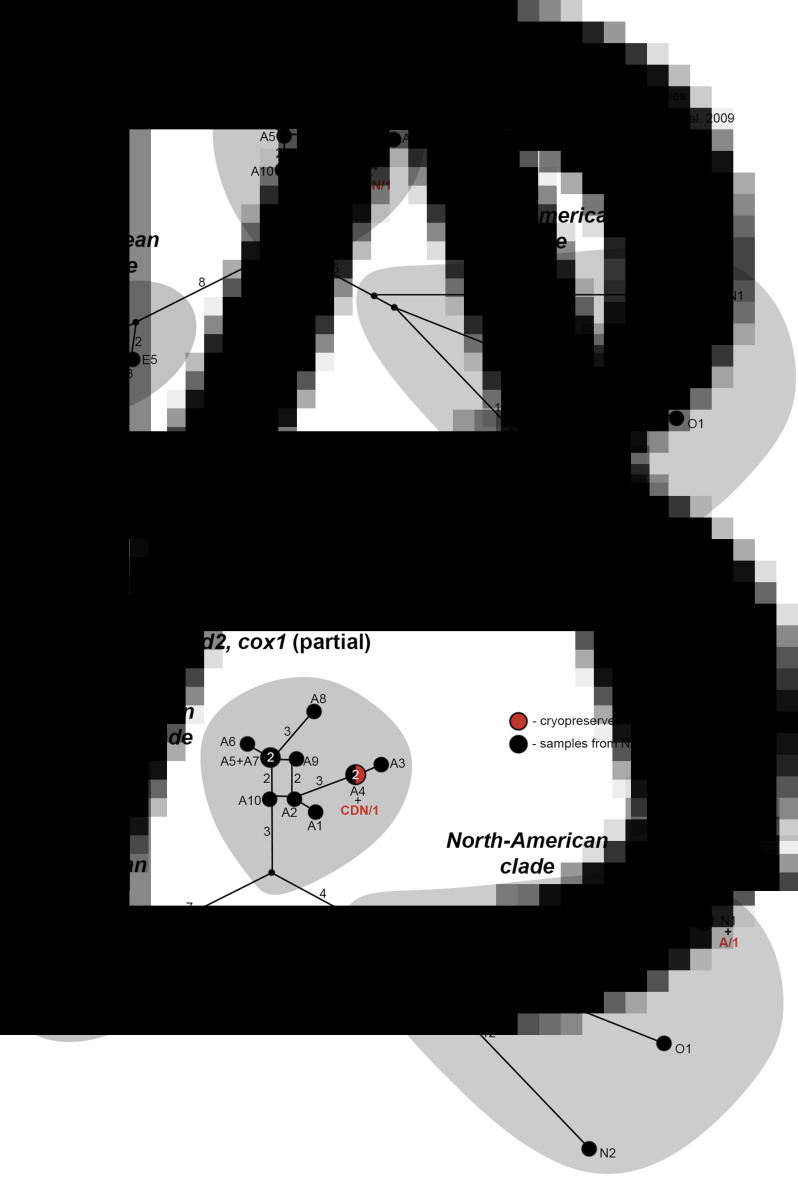
The phylogenetic network analysis based on a total of 3558 bp revealed that the isolate CDN/1, which was originally isolated in Canada, clustered into a clade together with Asian haplotypes (Fig. 2a). Whereas, as expected, the Swiss isolates CH/11 and CH/22 were shown to belong to the European clade. Based on 3116 bp (cob, nad2, partial cox1) of mtDNA, the Alaskan isolate A/1 from St. Lawrence island was shown to belong to the North American clade, and its available sequence was genetically more similar to the haplotype N1, comprising other samples originating from Alaska, St. Lawrence Island (Fig. 2b; Nakao et al., 2009).
Fig. 2.
(A) Median-joining phylogenetic network of concatenated (3,558 bp) mitochondrial genes cob, nad2 and cox1 for the cryopreserved Echinococcus multilocularis isolates analysed in the current study and relevant reference sequences obtained from GenBank (Nakao et al., 2009). Black circles represent sequences published by Nakao et al. (2009), while orange represents cryopreserved isolates. Numbers above the lines indicate the number of mutations, and the numbers inside the circles signify the number of samples comprising the haplotype. Abbreviations: CH, Switzerland; CDN, Canada; E, Europe; A, Asia; N, North-America; O, Mongolia. (B) Median-joining phylogenetic network (3116 bp), essentially the same as Fig. 1a, but with the shortened cox1 (1166 bp) sequence and the addition of the Alaskan isolate (A/1).
Discussion
The current study has shown that metacestode isolates of E. multilocularis are able to survive long-term cryopreservation in liquid nitrogen up to 33–35 years without losing their viability and their proliferative capacity as documented by in vitro and in vivo experiments. This applies to the metacestode isolates initially obtained from rodents or humans from Canada and Switzerland, respectively. The isolate from Alaska appeared to be viable in vitro but for unknown reasons, it did not proliferate in the in vivo experiments. In vitro viability tests of all 5 isolates had positive results in 13 of 17 experiments (76%; Table 1) and the 3 isolates used for in vivo infections had positive results in 5 of 12 (42%) experiments (Tables 1 and 2). In the current study, the proliferation of metacestode tissue was observed in only 8 of 36 animals, and was slightly higher in 2 of the isolates that showed regular metacestode proliferation (8 of 24 animals; 33%). In previous experiments by Eckert and Ramp (1985) parasite proliferation of 46% to 100% was observed in large groups of each 24 gerbils inoculated with material that had been cryopreserved for only 1 week. When comparing the results, methodological differences between the two studies must be taken into account, in particular the method of infection which in the 1980s was transplantation of complete TBs and injection of STF without pushing the material through a sieve. Despite these differences, our study shows that cryopreservation is suitable for long-term metacestode isolate maintenance and for reducing the number of animals traditionally used in serial passages. However, in order to compensate for a rather wide variability of the results sufficient quantities of metacestode material should be cryopreserved. When judging the results of cryopreservation several variables have to be considered, for example, composition of the original metacestode material (ratio parasite/host and viable/necrotic tissue, low or high number of vesicles, with or without protoscoleces), method of material preparation for viability tests, culture system, material quantities used, as well as the susceptibility of experimental animals.
The movement of protoscoleces in vitro immediately after thawing documents that this differentiated parasite life-cycle stage survived cryopreservation. Theoretically, it is likely possible to cryopreserve purified protoscoleces as well. However, due to the complex laborious process of excluding microvesicles and ensuring that only isolated protoscoleces remain (Gottstein et al., 1992), it is not advantageous to cryopreserve merely protoscoleces. It is also important to note that the motility of protoscoleces does not, however, indicate a proliferative capacity towards vesicle production with a surrounded laminated layer (LL), since purified protoscoleces of E. multilocularis have been shown not to be able to form metacestode material after i.p. injection in susceptible rodents (Gottstein et al., 1992). On the other hand, the production of vesicles with characteristic intact germinal (GL) and LL is a more suitable indication of viability in vitro. Additional confirmation of viability may be obtained by determination of metacestode proliferation in vivo.
In intermediate and accidental hosts metacestodes proliferate by the production of multiple vesicles attributable to undifferentiated cells of the germinal layer (GL) (reviewed by Thompson, 1986). An interesting question remains whether proliferation after cryopreservation occurs by surviving micro-vesicles or isolated totipotent stem cells from the GL. In a study by Spiliotis et al. (2008) it was demonstrated that E. multilocularis primary cells obtained from the GL and cultured in vitro, formed vesicles and subsequently synthesized the LL upon co-cultivation with rat hepatocytes, indicating that GL stem cells likely have complete regenerative capabilities to form vesicles. In another study, it was shown that E. multilocularis vesicles, which were punctured with a needle in vitro, failed to proliferate in vivo (Gottstein et al., 2002). The authors concluded that an intact LL is likely to be vital for parasite survival and proliferation in vivo, as the LL acts as a physical defence barrier against the immune system of the host, thus also providing protection for the proliferating GL cells. On the other hand, a study by Mehlhorn et al. (1983) postulated based on electronmicroscopy that root-like protrusions of the GL that are not protected by a LL may infiltrate the surrounding host tissue. However, so far there has been no further confirmation of this observation. Therefore, it seems likely that micro-vesicles with an intact LL that survived cryopreservation are responsible for metacestode proliferation in vitro and in vivo, but the role of single surviving stem-cells cannot be excluded.
Sequencing of the three mtDNA genes showed, as expected, that the cryopreserved isolates originating from Switzerland grouped together with other European isolates, whereas the Canadian isolate showed more genetic similarity to reference sequences from Asia (Fig. 2a). The presence of E. multilocularis so-called Asian-types in North America has been shown by previous studies as well, and it has been hypothesized that this might be explained by the formation of a land bridge between Asia and North-America during the last ice age, allowing for the dispersal of the definitive hosts (e.g. red foxes) and of E. multilocularis between the continents (Nakao et al., 2009). The nad2 and cob genes of the cryopreserved isolate from Alaska, St. Lawrence Island (A/1) showed 100% similarity to GenBank reference sequences from the same geographic origin (haplotype N1, Nakao et al., 2009). Interestingly, the cox1 sequence of this isolate was, however, 442 bp shorter than the reference sequences, whereas the sequence quality remained high in the corresponding and flanking regions. Multiple sequencing attempts and additionally cloning the gene region resulted in the same conclusion. One possible explanation for this could be that this was a pseudogene, and with the current primers and Sanger sequencing, we could not sequence the full length of the cox1 gene region in the mitogenome. Recently it was shown that the mtDNA genome of the closely related species E. granulosus sensu stricto (s.s.) contains a relatively long repeat region of fragments of mtDNA genes (Kinkar et al., 2019). This repeat region could not be previously determined with traditional Sanger sequencing technology. Towards this end, PacBio long-read sequencing helped overcome this problem, and as such in the future the PacBio technology would also be beneficial to implement for the Alaskan A/1 isolate to elucidate on the hypothesis whether this was a pseudogene or whether this is a significantly different isolate, with rearrangements within the mitogenome.
In conclusion, the results of the current study indicate that the metacestode stage of different E. multilocularis geographic isolates is able to survive long-term cryopreservation and retain their viability. Therefore, cryopreservation is a suitable method for long-term storage of metacestode isolates for further research reducing the number of experimental animals that would otherwise be needed to propagate the parasite in serial passages.
Acknowledgements
The authors gratefully acknowledge Claudia Bremen for her skilful assistance with looking after the animals and helping with the experiments.
Financial support
These studies were partially supported by grants of the Swiss National Research Foundation, the Federal Veterinary Office, the R3 Research Foundation (Eckert, 1988). Animal experiments were performed according to the ethical standards and licences approved by the Cantonal Veterinary Office of Zurich, Switzerland.
Ethical standards
All procedures concerning in vivo experiments were conducted following the ethical standards of the guidelines of the Swiss Animals Protection Law. Extra care was taken to reduce the suffering of animals and to ensure the highest ethical and humane standards. Ethical permission numbers for the experiments carried out within the current study were the following: ZH139/15 and ZH068/19.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S003118202000075X.
click here to view supplementary material
Conflict of interest
None
References
- Andermatt-Mettler I, Eckert J, Ramp Th and Gottstein B (1987) Cryopreservation of Dictyocaulus viviparus third-stage larvae and Trichinella spiralis muscle larvae. Parasitology Research 73, 358–365. [DOI] [PubMed] [Google Scholar]
- Bandelt HJ, Forster P and Rohl A (1999) Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution 16, 37–48. [DOI] [PubMed] [Google Scholar]
- Brehm K and Koziol U (2017) Echinococcus-host interactions at cellular and molecular levels. Advances in Parasitology 95, 147–212. [DOI] [PubMed] [Google Scholar]
- Cohen LM and Eveland LK (1984) Cryopreservation of Schistosoma mansoni sporocysts. Journal of Parasitology 70, 592. [PubMed] [Google Scholar]
- Deplazes P, Rinaldi L, Alvarez Rojas CA, Torgerson PR, Harandi MF, Romig T, Antolova D, Schurer JM, Lahmar S, Cringoli G, Magambo J, Thompson RC and Jenkins EJ (2017) Global distribution of alveolar and cystic echinococcosis. Advances in Parasitology 95, 315–493. [DOI] [PubMed] [Google Scholar]
- Eckert J (1988) Cryopreservation of parasites. Experientia 44, 873–877. [DOI] [PubMed] [Google Scholar]
- Eckert J and Deplazes P (2004) Biological, epidemiological and clinical aspects of echinococcosis: a zoonoses of increasing concern. Clinical Microbiology Reviews 17, 107–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert J and Ramp T (1985) Cryopreservation of Echinococcus multilocularis metacestodes and subsequent proliferation in rodents (Meriones). Zeitschrift für Parasitenkunde 71, 777–787. [DOI] [PubMed] [Google Scholar]
- Gottstein B, Deplazes P and Aubert M (1992) Echinococcus multilocularis: immunological study on the “Em2-positive” laminated layer during in vitro and in vivo post-oncospheral and larval development. Parasitology Research 78, 291–297. [DOI] [PubMed] [Google Scholar]
- Gottstein B, Dai WJ, Walker M, Stettler M, Müller N and Hemphill A (2002) An intact laminated layer is important for the establishment of secondary Echinococcus multilocularis infection. Parasitology Research 88, 822–828. [DOI] [PubMed] [Google Scholar]
- Gottstein B, Soboslay P, Ortona E, Wang J, Siracusano A and Vuitton DΑ (2017) Immunology of alveolar and cystic echinococcosis (AE and CE). Advances in Parasitology 96, 1–54. [DOI] [PubMed] [Google Scholar]
- Hall TA (1999) Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41, 95–98. [Google Scholar]
- James ER (1980) Cryopreservation of Schistosoma mansoni schistosomula using 40% v/v (10 m) methanol and rapid cooling. Cryo-Letters 1, 535–544. [Google Scholar]
- James ER (2004) Parasite cryopreservation by vitrification. Cryobiology 49, 201–210. [DOI] [PubMed] [Google Scholar]
- Kern P, Menezes da Silva A, Akhan O, Müllhaupt B, Vizcaychipi KA, Budke C and Vuitton DA (2017) The echinococcoses: diagnosis, clinical management and burden of disease. Advances in Parasitology 96, 259–369. [DOI] [PubMed] [Google Scholar]
- Kinkar L, Korhonen PK, Cai H, Gauci CG, Lightowlers MW, Saarma U, Jenkins DJ, Li J, Li J, Young ND and Gasser RB (2019) Long-read sequencing reveals a 4.4 kb tandem repeat region in the mitogenome of Echinococcus granulosus (Sensu Stricto) genotype G1. Parasites and Vectors 12, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström-Stadelmann B, Rufener R, Ritler D, Zurbriggen R and Hemphill A (2019) The importance of being parasiticidal… an update on drug development for the treatment of alveolar echinococcosis. Food and Waterborne Parasitology 15, e00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães R, Wang XW, Gouk SS, Lee KH, Ten CM, Yu H and Kuleshova LL (2008) Vitrification successfully preserves hepatocyte spheroids. Cell Transplantation 17, 813–828. [DOI] [PubMed] [Google Scholar]
- Magalhães R, Nugraha B, Pervaiz S, Yu H and Kuleshova LL (2012) Influence of cell culture configuration on the post-cryopreservation viability of primary rat hepatocytes. Biomaterials 33, 829–836. [DOI] [PubMed] [Google Scholar]
- Mehlhorn H, Eckert J and Thompson RCA (1983) Proliferation and metastases formation of larval Echinococcus multilocularis. II. Ultrastructural investigations. Zeitschrift für Parasitenkunde 69, 749–763. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Karanis P and Uga S (2004) Cryopreservation of protozoan parasites. Cryobiology 48, 1–7. [DOI] [PubMed] [Google Scholar]
- Nakao M, Xiao N, Okamoto M, Yanagida T, Sako Y and Ito A (2009) Geographic pattern of genetic variation in the fox tapeworm Echinococcus multilocularis. Parasitology International 58, 384–389. [DOI] [PubMed] [Google Scholar]
- Pozio E, Rossi P and Scrimitore E (1988) Studies on the cryopreservation of Trichinella species. Experimental Parasitology 67, 182–189. [DOI] [PubMed] [Google Scholar]
- Ramp T, Eckert J and Christen C (1986) Gefrierkonservierung dritter Larvenstadien von Trichostrongyliden der Wiederkäuer. Schweizer Archive für Tierheilkunde 128, 79–86. [PubMed] [Google Scholar]
- Rew RS and Campbell WC (1983) Infectivity of Haemonchus contortus after freezing for ten years over liquid nitrogen. Journal of Parasitology 69, 251–252. [PubMed] [Google Scholar]
- Romig T and Bilger B (1999) Animal models for echinococcosis. In Zak O and Sander MA (eds), Handbook of Animal Models of Infection. London, UK: Academic Press, pp. 877–884. doi: 10.1016/B978-012775390-4/50244-X. [DOI] [Google Scholar]
- Romig T, Deplazes P, Jenkins D, Giraudoux P, Massolo A, Craig PS, Wassermann M, Takahashi K and de la Rue M (2017) Ecology and life cycle patterns of Echinococcus species. Advances in Parasitology 95, 213–314. [DOI] [PubMed] [Google Scholar]
- Spiliotis M and Brehm K (2009) Axenic in vitro cultivation of Echinococcus multilocularis metacestode vesicles and the generation of primary cell cultures. In Rupp S and Sohn K (eds), Host-Pathogen Interactions. Methods in Molecular Biology. Totowa, USA: Humana Press, vol. 470, pp. 245–262. 10.1007/978-1-59745-204-5_17. [DOI] [PubMed] [Google Scholar]
- Spiliotis M, Lechner S, Tappe D, Scheller C, Krohne G and Brehm K (2008) Transient transfection of Echinococcus multilocularis primary cells and complete in Vitro regeneration of metacestode vesicles. International Journal for Parasitology 38, 1025–1039. [DOI] [PubMed] [Google Scholar]
- Thompson RCA (1986) Biology and systematics of Echinococcus. In Thompson RCA (ed.), The Biology of Echinococcus and Hydatid Disease. Hemel Heampstead, Herts, UK: George Allen & Unwin Publishers, pp. 5–43. [Google Scholar]
- Thompson RC (2017) Biology and systematics of Echinococcus. Advances in Parasitology 95, 65–109. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG and Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Research 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson PR, Keller K, Magnotta M and Ragland N (2010) The global burden of alveolar echinococcosis. PLoS Neglected Tropical Diseases 4, e722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation (2020) Echinococcosis. Fact Sheet. Available at https://www.who.int/news-room/fact-sheets/detail/echinococcosis (last accessed 12.03.2020).
- World Health Organisation/World Organisation for Animal Health (2001) WHO/OIE manual on Echinococcosis in humans and animals: a public health problem of global concern. In Eckert J, Gemmell MA, Meslin F-X and Pawloski ZS (eds), Paris, France: World Health Organization/World Organization for Animal Health, pp. 265. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S003118202000075X.
click here to view supplementary material