Abstract
To assess the role of white-tailed deer (Odocoileus virginianus, WTD) in the epidemiology of toxoplasmosis, we conducted a national survey of WTD across the USA for Toxoplasma gondii infection. To do this, we combined serology with parasite isolation to evaluate the prevalence and genetic diversity of T. gondii in this game species. From October 2012 to March 2019, serum and tissues were collected from 914 WTD across the USA. Serum samples were screened for antibodies to T. gondii, and then the tissues of seropositive WTD were bioassayed in mice. Antibodies were detected in 329 (36%) of 914 WTD tested by the modified agglutination test (positive reaction at 1:25 or higher). Viable T. gondii was isolated from the heart of 36 WTD from 11 states. Three of the 36 isolates were pathogenic but not highly virulent to outbred Swiss Webster mice and all 36 isolates could be propagated further in cell culture and were genotyped. For genotyping, DNA extracted from cell culture-derived tachyzoites was characterized by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) using the genetic markers SAG1, SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1 and Apico. Genotyping revealed seven ToxoDB PCR-RFLP genotypes, including 24 isolates for genotype #5 (haplogroup 12), four isolates for #2 (type III, haplogroup 3), three isolates for genotypes #1 (type II, haplogroup 2), two isolates for genotypes #3 (type II, haplogroup 2) and one isolate each for #39, #221 and #224. Genotype #5 was the most frequently isolated, accounting for 66.6% (24 of 36) of the isolates. Combining the 36 isolates from this study with previously reported 69 isolates from WTD, 15 genotypes have been identified. Among these, 50.4% (53/105) isolates belong to genotype #5. Our results indicate moderate genetic diversity of T. gondii in WTD. The results also indicate that undercooked venison should not be consumed by humans or fed to cats.
Key words: Isolation, Toxoplasma gondii, toxoplasmosis, USA, white-tailed deer (Odocoileus virginianus)
Introduction
The protozoan Toxoplasma gondii infects virtually all warm-blooded animals, including birds, humans, livestock and marine mammals (Dubey, 2010). Humans become infected postnatally by ingesting tissue cysts from undercooked meat of infected animals, or by consuming food or drink contaminated with T. gondii oocysts. In the USA, white-tailed deer (Odocoileus virginianus, WTD) are harvested by hunters and infected meat that is consumed by humans or other hosts can result in T. gondii infection. Cases of clinical toxoplasmosis including ocular manifestations have been documented in humans who consumed undercooked venison (Sacks et al., 1983; Ross et al., 2001; Schumacher et al., 2018; Gaulin et al., 2020).
Deer are popular game animals in several countries (Aubert et al., 2010). In the USA, the WTD population is estimated at approximately 30 million, with nearly 6 million deer harvested annually (Adams and Ross, 2013). Viscera are removed from most harvested deer in the field to preserve the meat, and the viscera is left on site or buried in a shallow grave. Tissues of these deer may be scavenged by mesocarnivores, which can then become infected.
Few states have strict rules with respect to disposal of the carcases of deer hit by vehicles. Bobcats (Lynx rufus) and cougars (Puma concolor) scavenge on deer, and if they eat infected tissues they can excrete T. gondii oocysts in the environment. Since deer–vehicle accidents are common in the USA (Adams and Ross, 2013), there are many opportunities for felids to become infected.
Since deer are herbivores, ingestion of food or water contaminated with oocysts is likely the main mode of post-natal transmission. Thus, WTD are considered an excellent indicator of contamination of the environment by T. gondii oocysts among other hosts, including feral chickens, wild swine and many other species wild ungulates. The objective of the present investigation was to estimate seroprevalence, isolate and characterize T. gondii from WTD across the USA.
Materials and methods
Animals and sampled areas
The United States Department of Agriculture's Wildlife Services (WS) removes deer from select areas where populations are not naturally regulated due to the lack of predators and hunting restrictions. Samples are opportunistically collected from these deer by WS’ National Wildlife Disease Program (NWDP). For this study, sera and hearts were collected from 914 WTD from October 2012 through March 2019 in 19 states (Table 1) following NWDP protocols (Cervid Health Procedures Manual, June 2015). Sex, age group (juvenile or adult), date of killing and location information were recorded for most WTD; not all data were available for all WTD (Table 1). Samples were submitted for T. gondii testing to the USDA's Animal Parasitic Diseases Laboratory (APDL) in Beltsville, Maryland.
Table 1.
Prevalence of T. gondii in WTD (O. virginianus) collected across the USA from 2012 to 2019
| Yeara | Samples received | Male | Female | Juvenile | Sub-adult | Adult | MAT positive [%; (95% CI)] | Samples bioassayed | T. gondii isolates |
|---|---|---|---|---|---|---|---|---|---|
| 2012 | 241 | 121 | 119 | 97 | 0 | 143 | 21.2 (16.5–26.8) | 40 | 12 |
| 2013 | 602 | 217 | 382 | 131 | 0 | 468 | 43.2 (39.3–47.2) | 110 | 21 |
| 2015 | 12 | 4 | 8 | 1 | 0 | 11 | 50.0 (25.4–74.6) | 6 | 0 |
| 2016 | 2 | 2 | 0 | 0 | 0 | 2 | 0 (0–65.8) | 0 | 0 |
| 2017 | 48 | 14 | 34 | 7 | 3 | 38 | 22.9 (13.3–36.5) | 11 | 3 |
| 2018 | 4 | 2 | 2 | 1 | 0 | 3 | 25.0 (4.6–69.9) | 0 | 0 |
| 2019 | 5 | 2 | 3 | 2 | 0 | 3 | 0 (0.0–43.5) | 0 | 0 |
| Total (% positive) | 914 | 362 (29.8) | 548 (40.0) | 239 (19.2) | 3 (0.0) | 668b (42.1) | 36.0 (33.0–39.2) | 167 | 36 |
No samples received in 2014.
Age and sex not recorded for four WTD.
Serology
Sera were tested for antibodies to T. gondii by the modified agglutination test (MAT) as described by Dubey and Desmonts (1987). Sera were screened at 1:25, 1:50, 1:100 and 1:200 dilutions or higher. Deer with positive reaction at 1:25 dilution were considered infected with T. gondii.
Isolation by bioassay in mice
The heart was selected as the organ for isolation of T. gondii because of convenience and because in food animals it is most commonly infected with T. gondii (Dubey, 2010). Myocardium samples (50 g) were homogenized in saline, digested in acidic pepsin, centrifuged and aliquots of homogenates were inoculated subcutaneously into 3–5 outbred albino Swiss Webster (SW) mice, and/or one or two interferon gamma gene knock out (KO) mice, which are especially susceptible to toxoplasmosis (Dubey, 2010). Inoculated mice that became ill were euthanized and tissue imprints of lungs and brains were examined for T. gondii tachyzoites or tissue cysts, respectively (Dubey, 2010). Survivors were bled at the earliest on 45 days post-inoculation (p.i.) and a 1:25 dilution of serum was tested for T. gondii antibodies by MAT. Mice were euthanized 46 days p.i. or later and brains of all mice were examined for tissue cysts as described previously (Dubey, 2010). The inoculated mice were considered infected with T. gondii when tachyzoites or tissue cysts were detected in their tissues.
Ethical considerations
All experimental procedures were approved by the Institutional Animal Care and Use Committee (Protocol no. 15-017), United States Department of Agriculture, Beltsville, Maryland. Outbred SW and KO mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) in compliance with the Institutional Animal Ethics Committee guidelines. The WTD were euthanized in the field, often in remote locations and tissues were shipped overnight by the collector with ice packs. Consequently, by the time tissues were received at the USDA laboratory, they often were contaminated with bacteria and not suited for cell culture to isolate T. gondii. The number of T. gondii in tissues of naturally infected large animal tissues is low (estimated one tissue cyst per 50–100 g) and the probability of isolation of T. gondii is very low unless large numbers of mice (10 or more) are used (Dubey et al., 1995; Dubey, 2010). To balance the possibility of isolating parasites and using the minimum number of mice, we decided to use 3–5 mice for the bioassay of each deer sample in the current study.
All mice used in the current study were treated humanely and examined twice daily for any signs of illness. A veterinarian was assigned exclusively to the toxoplasmosis project. Any sick mice were euthanized because our objective was isolation of T. gondii and not testing for mortality. We wanted to collect mouse tissues aseptically for cultivation in cell culture or subpassage to other mice.
In vitro cultivation
Lungs or brain from infected mice were seeded on to CV1 cell culture flasks and tachyzoites were harvested from the medium as previously described (Dubey, 2010).
Genotyping of DNA samples
In our experience, for successful genotyping of T. gondii strains from asymptomatic naturally infected animals, it is necessary to obtain good quality parasite DNA with minimal contamination of host tissue. Therefore, parasites isolated from mouse tissues were expanded in cell culture. Genotyping of DNA samples by multi-locus polymerase chain reaction (PCR)-restriction fragment polymorphism (RFLP) markers were carried out following the previously reported protocol (Su et al., 2010; Su and Dubey, 2020). Samples with missing data for one to three of the 10 PCR-RFLP markers, which otherwise matched with previously reported genotype were designated as ‘likely’ of that genotype.
Results
Antibodies to T. gondii were detected in 36% (329 of 914) of WTD (Table 1). Among 362 male and 548 female WTD tested (sex was unidentified for four), seroprevalence was higher in females (40.0%) than in males (29.8%). Seroprevalence were higher in adults than juveniles (Table 1).
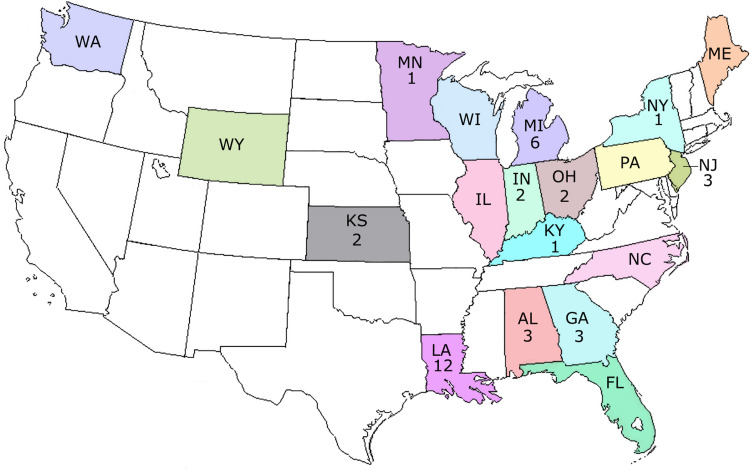
Of the 329 seropositive deer, hearts from 167 were bioassayed (Table 1; Supplementary Table 1). The selection was based on antibody titres and the number of samples received and the availability of mice. Viable T. gondii was isolated from 36 WTD collected in 11 states (Table 2, Fig. 1) (Supplementary Table 1). The rate of isolating viable parasites was positively associated with MAT titres, with the isolation rates of 10.7, 17.9, 33.3, 59 and 50% for MAT titres of 1:200, 1:400, 1:800, 1:1600 and ⩾1:3200, respectively. Tissue cysts were found in brains of all seropositive mice.
Table 2.
Collection information for T. gondii isolates from WTD (O. virginianus) collected across the USA from 2012 to 2019
| WTD | Bioassays in mice | ||||||
|---|---|---|---|---|---|---|---|
| Isolate number | Statea | County | Collection date | MAT | SW (no. of infected/no. of inoculated) | KO (no. infected/no. inoculated) | Genotype |
| (1) TgWTDAL1 | AL | Clay | 4/8/2013 | 800 | 3/3 | ND | #5 |
| (2) TgWTDAL2 | AL | Coosa | 4/2/2013 | 800 | 3/3 | ND | #5 |
| (3) TgWTDAL3 | AL | Tallapoosa | 5/23/2013 | 400 | 2/3 | 2/2 | #5 |
| (4) TgWTDGA1 | GA | Greene | 11/5/2012 | >3200 | 4/4 | 1/1 | #5 |
| (5) TgWTDGA2 | GA | Greene | 2/13/2013 | 200 | 1/3 | ND | #5 |
| (6) TgWTDGA3 | GA | Gwinnett | 11/27/2012 | 200 | 3/4 | 1/1 | #224 |
| (7) TgWTDIN1 | IN | Porter | 3/20/2013 | >3200 | 2/2 | ND | #2 |
| (8) TgWTDIN2 | IN | Porter | 3/13/2013 | 400 | 2/3 | ND | #2 |
| (9) TgWTDKS1 | KS | Jewell | 10/8/2017 | >3200 | 4/4 | 1/1 | #5 |
| (10) TgWTDKS2 | KS | Wabaunsee | 10/3/2017 | 1600 | 0/4 | 1/1 | #5 |
| (11) TgWTDKY1 | KY | Scott | 1/20/2013 | >3200 | 1/4 | 1/1 | #39 |
| (12) TgWTDLA1 | LA | Bossier | 12/12/2012 | 800 | 3/4 | 1/1 | #5 |
| (13) TgWTDLA2 | LA | East Feliciana | 11/10/2012 | 1600 | 1/4 | 0/1 | #5 |
| (14) TgWTDLA3 | LA | East Feliciana | 11/10/2012 | >3200 | 3/4 | 1/1 | #5 |
| (15) TgWTDLA4 | LA | East Feliciana | 11/10/2012 | 400 | 4/4 | 1/1 | #5 |
| (16) TgWTDLA5 | LA | East Feliciana | 11/10/2012 | 800 | 1/4 | 0/1 | #5 |
| (17) TgWTDLA6 | LA | East Feliciana | 4/24/2013 | 800 | 3/3 | 1/1 | #5 |
| (18) TgWTDLA7 | LA | East Feliciana | 4/24/2013 | 200 | 3/3 | 1/2 | #5 |
| (19) TgWTDLA8 | LA | Iberville | 11/24/2012 | 1600 | 4/4 | 1/1 | #5 |
| (20) TgWTDLA9 | LA | Union | 10/27/2012 | 1600 | 4/4 | 1/1 | #5 |
| (21) TgWTDLA10 | LA | Vernon | 10/27/2012 | 800 | 4/4 | 1/1 | #5 |
| (22) TgWTDLA11 | LA | Vernon | 10/27/2012 | 1600 | 4/4 | 1/1 | #5 |
| (23) TgWTDLA12 | LA | Vernon | 10/27/2012 | 400 | 2/4 | 0/1 | #5 |
| (24) TgWTDMI1 | MI | Alpena | 4/2/2013 | 200 | 2/3 | 1/1 | #3 |
| (25) TgWTDMI2 | MI | Alpena | 4/6/2013 | 1600 | 3/3 | ND | #5 |
| (26) TgWTDMI3 | MI | Alpena | 6/10/2013 | 1600 | 2/3 | 1/1 | #5 |
| (27) TgWTDMI4 | MI | Alpena | 7/8/2013 | 200 | 1/3 | 1/1 | #5 |
| (28) TgWTDMI5 | MI | Alpena | 7/9/2013 | 800 | 1/3 | 1/1 | #5 |
| (29) TgWTDMI6 | MI | Alpena | 7/23/2013 | 1600 | 3/3 | 1/1 | #5 likely |
| (30) TgWTDMN1 | MN | Hennepin | 2/15/2013 | 1600 | 3/3 | 1/1 | #1 |
| (31) TgWTDNJ1 | NJ | Essex | 1/22/2013 | 400 | 0/3 | 1/1 | #2 |
| (32) TgWTDNJ2 | NJ | Essex | 1/29/2013 | 1600 | 0/3 | 1/1 | #1 |
| (33) TgWTDNJ3 | NJ | Essex | 1/29/2013 | 200 | 0/3 | 1/1 | #221 |
| (34) TgWTDNY1 | NY | Dutchess | 1/13/2017 | >1600 | 1/4 | 0/1 | #2 |
| (35) TgWTDOH1 | OH | Cuyahoga | 1/15/2013 | 1600 | 0/4 | 1/1 | #1 |
| (36) TgWTDOH2 | OH | Cuyahoga | 1/15/2013 | 1600 | 1/4 | 1/1 | #3 |
AL, Alabama; GA, Georgia; IN, Indiana; KS, Kansas; KY, Kentucky; LA, Louisiana; MI, Michigan; MN, Minnesota; NJ, New Jersey; NY, New York; OH, Ohio.
SW, Swiss Webster albino mice; KO, interferon-γ knockout mice; ND, not done.
No isolates from FL, Florida; IL, Illinois; PA, Pennsylvania; ME, Maine; NC, North Carolina, WA, Washington; WI, Wisconsin and WY, Wyoming.
Fig. 1.
Map of USA showing the T. gondii isolates from WTD.
The SW mice that were inoculated with digest of hearts from three of the 36 infected WTD had clinical signs of T. gondii infection, and three mice died or were euthanized at 13, 41 and 46 days p.i. (Table 3). All three isolates were from Louisiana but from different counties and hunted on different dates (Table 3).
Table 3.
Isolates of pathogenic T. gondii identified in WTD collected across the USA from 2012 to 2019
| Isolate number | WTD ID | Collection date | State | County | Age class | Sex | MAT | Bioassayed in SW micea | Genotype | |
|---|---|---|---|---|---|---|---|---|---|---|
| No. of mice infected with T. gondii | No. of dead mice (day of death) | |||||||||
| 15 TgWTDLA4 | TOX00684 | 11/10/2012 | LA | East Feliciana | Adult | Female | 400 | 4 | 1 (46) | #5 |
| 20 TgWTDLA9 | TOX00667 | 10/27/2012 | LA | Union | Adult | Female | 1600 | 4 | 1 (13) | #5 |
| 21 TgWTDLA10 | TOX00660 | 10/27/2012 | LA | Vernon | Adult | Female | 800 | 4 | 1 (41) | #5 |
Thirty-six isolates were genotyped (Table 4); typing results for individual isolates are shown in Supplementary Table 2. The results revealed seven ToxoDB genotypes, including 24 isolates for genotype #5 (haplogroup 12), four isolates for genotype #2 (type III, haplogroup 3), three isolates for genotype #1 (type II, haplogroup 2), two isolates for genotypes #3 (type II, haplogroup 2), one isolate each for #39, #221 and #224. Genotype #5 was the most frequently isolated, accounting for 66.6% (24 of 36) of the isolates.
Table 4.
T. gondii isolates from WTD (O. virginianus) bioassayed samples and PCR-RFLP genotype per state collected from 2012 to 2019
| Statea | Samples bioassayed | No. of isolates | ToxoDB-PCR-RFLP genotype |
|---|---|---|---|
| AL | 7 | 3 | #5 |
| FL | 5 | 0 | – |
| GA | 9 | 3 | #5, #224 |
| IL | 10 | 0 | – |
| IN | 9 | 2 | #2 |
| KS | 4 | 2 | #5 |
| KY | 1 | 1 | #39 |
| LA | 24 | 12 | #5 |
| MI | 32 | 6 | #3, #5 |
| MN | 10 | 1 | #1 |
| NC | 1 | 0 | – |
| NJ | 34 | 3 | #1, #2, #221 |
| NY | 3 | 1 | #2 |
| OH | 5 | 2 | #1, #3 |
| PA | 12 | 0 | – |
| WA | 1 | 0 | – |
| Total | 167 | 36 | #1 (n = 3), #2 (n = 4), #3 (n = 2), #5 (n = 24), #39 (n = 1), #221 (n = 1), #224 (n = 1) |
No samples were bioassayed from ME, WI and WY.
Discussion
Nearly one-third of humans have been exposed to T. gondii, but not all develop clinical symptoms of disease. It is unknown how the severity of toxoplasmosis in immunocompetent hosts is linked to the parasite strain, host variability or to other factors. Attention has been focused on the genetic variability among T. gondii isolates from apparently healthy and sick hosts (Grigg and Sundar, 2009). Historically, T. gondii was considered clonal with low genetic diversity and grouped into three types, namely I, II and III (Howe and Sibley, 1995; Sibley and Ajioka, 2008). However, recent studies have revealed a greater genetic diversity of T. gondii, particularly isolates from Brazil (Shwab et al., 2018). A recent update indicated 279 genetic variants (Su and Dubey, 2020). Severe cases of toxoplasmosis have been reported in immunocompetent patients in association with atypical T. gondii genotypes (Ajzenberg et al., 2004; Demar et al., 2007; Elbez-Rubinstein et al., 2009; Lorenzi et al., 2016). However, little is known about the association of genotype and clinical disease in animals and humans in the USA (Dubey, 2010).
In the current study, 36% of WTD were seropositive, and these data are in accord with several regional surveys with seroprevalences of 28.7–74.4% (Table 5). These results are comparable because in all surveys listed in Table 5, the same MAT was used to detect T. gondii antibodies. WTD are the most common wild cervids in the USA and they are spreading from rural to urban areas. WTD have been imported to Europe (Jokelainen et al., 2010). Nothing has been published concerning the genotypes of T. gondii in WTD in Europe, but it would be most interesting to learn whether they may have played any role in introducing or propagating parasite strains otherwise considered endemic to North America.
Table 5.
Summary of prevalence of T. gondii in WTD from previous reports in the USAa
| State | No. tested | No. of seropositive (MAT, ⩾1:25) |
% pos. samples | No. of T. gondii isolates | ToxoDB PCR-RFLP genotypes | Reference |
|---|---|---|---|---|---|---|
| Alabama | 16 | 7 | 44.0 | ND | ND | Lindsay et al. (1991) |
| Alabama | 50 | 17 | 34.0 | 4 | ND | Lindsay et al. (1997) |
| Illinois | 443 | 247 | 55.9 | ND | ND | Hollis-Etter et al. (2019) |
| Iowa | 62 | 20 | 32.2 | ND | ND | Dubey et al. (2009) |
| Iowa | Foetuses from 61 deer | 43 | 70.5 | 6 | #1 (type II) – 4 isolates #4–2 isolates |
Dubey et al. (2008) |
| Minnesota | Foetuses from 27 deer | 15 | 55.5 | 9 | #4–5 isolates #2 (type III) – 2 isolates #54–1 isolate #74–1 isolate |
Dubey et al. (2008) |
| Minnesota | 1367 | 410 | 30.0 | ND | ND | Vanek et al. (1996) |
| Minnesota | 170 | 91 | 53.5 | ND | ND | Dubey et al. (2009) |
| Minnesota | 487 | 110 | 22.5 | 1 | #146 | Dubey et al. (2014a) |
| Mississippi | 73 | 21 | 46.0 | 19 | #2 – 1 isolate #5 – 18 isolates |
Dubey et al. (2004, 2014a) |
| New Jersey | 264 | 76 | 28.7 | 9 | #2 – 2 isolates #3 – 1 isolate #4 – 1 isolate #216 – 1 isolate #220 – 1 isolate #221 – 3 isolates |
Dubey et al. (2013a) |
| Ohio | 749 | 557 | 74.4 | 4 | #1 – 2 isolates #2 – 1 isolate #3 – 1 isolate |
Dubey et al. (2014a) |
| Ohio | 444 | 261 | 58.8 | ND | ND | Ballash et al. (2015) |
| Pennsylvania | 79 | 49 | 62.0 | 7 | #3 – 2 isolates #4 – 3 isolates #216 – 2 isolates |
Dubey et al. (2014b) |
| Pennsylvania | 593 | 357 | 60.0 | ND | ND | Humphreys et al. (1995) |
| Southeast | 241 | 99 | 41.0 | 13 | #5 – 10 isolates #154 – 1 isolate #167 – 1 isolate #216 – 1 isolate |
Gerhold et al. (2017) |
| Nationwide | 914 | 329 | 35.9 | 36 | 7 ToxoDB genotypes | Current study |
In addition to these reports, Yu et al. (2013) reported ToxoDB PCR-RFLP genotype #5 for the isolate TgWtdAL from the heart and tongue of a WTD from Alabama.
In the current study, there was relatively a low to moderate diversity of the isolates identified. Genotypes #1, #2, #3, #4, #39 and #221 have previously been identified in animals in the USA, with the first three being most common in animals on farms (Jiang et al., 2018). Genotype #224 was previously reported in dog from Grenada (Dubey et al., 2013b).
From previously published reports, 69 isolates from WTD in the USA have been genotyped (Table 5). Among these isolates, 13 ToxoDB PCR-RFLP genotypes were identified, including #1, #2, #3, #4, #5, #54, #74, #146, #154, #167, #216, #220 and #221. From these genotypes, #5 (haplotype 12) accounted for 42% (29 of 69), was the most frequently isolated. Genotype #4 (also known as haplotype 12) accounted for 16% (11 of 69). Genotypes #1 and #3 together known as type II and haplogroup 2, accounted for 15% (10 of 69). Combining the 36 isolates from our current study with previously reported 69 isolates from WTD, 15 genotypes are identified, including #1, #2, #3, #4, #5, #39, #54, #74, #146, #154, #167, #216, #220, #221 and #224. Among these, 50.4% (53/105) isolates were characterized as genotype #5. Genotypes #1 and #3 (together as type II, haplogroup 2) accounted for 14.3% (15/105) of the isolates. Genotype #4 (haplotype 12) accounted for 10.5% (11/105) and genotype #2 (type II, haplogroup 3) accounted for 9.5% (10/105). The other 10 genotypes were less frequently identified. A recent national survey of feral swine in the USA indicated a similar pattern, in which genotype #5 was dominant and accounted for 57% of 76 isolates genotyped (Dubey et al., 2020). Based on these two studies, dominance of genotype #5 suggests sylvatic transmission of T. gondii in wildlife in the USA.
Although bobcats are the most likely hosts involved in the sylvatic cycle of T. gondii in the USA (Dubey, 2010; VanWormer et al., 2014; Verma et al., 2017), other wild cats may participate in both feral and domestic cycles introducing atypical genotypes to domestic cats, thereby facilitating transmission of potentially more pathogenic genotypes to humans, domestic animals and wildlife (VanWormer et al., 2014). The partition of T. gondii genotypes such as #1, #2 and #3 among domestic animals and #5 in wildlife is mainly due to distinct sylvatic and domestic transmission cycles, though both cycles overlap to a certain degree (Jiang et al., 2018; Shwab et al., 2018). Recent evidence indicates that the strains of T. gondii prevalent in wildlife can also cause clinical disease in humans (Jokelainen et al., 2018; Pomares et al., 2018) and domesticated animals (Dubey and Prowell, 2013; Crouch et al., 2019), indicating a plausible route for introduction of virulent strains of T. gondii from the sylvatic cycle.
Our results revealed low to moderate genetic diversity of T. gondii in WTD in the USA, with genotype #5 (haplogroup 12) identified as the most dominant type in the USA. The contribution of WTD to the epidemiology of T. gondii deserves additional scrutiny. Considering the high rate of exposure of WTD to T. gondii in the USA, results affirm that the environment is highly contaminated with oocysts.
Acknowledgements
We would like to thank many WS employees who collected and submitted samples for this project.
Financial support
This research was supported in part by an appointment to the Agricultural Research Service (ARS) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the U.S. Department of Agriculture (USDA). ORISE is managed by ORAU under DOE contract number DE-SC 0014664. All opinions expressed in this paper are the authors' and do not necessarily reflect the policies and views of USDA, ARS, DOE or ORAU/ORISE.
Ethical standards
All experimental procedures were approved by the Institutional Animal Care and Use Committee (Protocol no. 15-017), United States Department of Agriculture, Beltsville, Maryland.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020000451.
click here to view supplementary material
Conflict of interest
None.
References
- Adams K and Ross M (2013) QDMA's WhitetailReport 2013. An annual report on the status of white-tailed deer, the foundation of the hunting industry in North America. 1–67.
- Ajzenberg D, Bañuls AL, Su C, Dumètre A, Demar M, Carme B and Dardé ML (2004) Genetic diversity, clonality and sexuality in Toxoplasma gondii. International Journal for Parasitology 34, 1185–1196. [DOI] [PubMed] [Google Scholar]
- Aubert D, Ajzenberg D, Richomme C, Gilot-Fromont E, Terrier ME, de Gevigney C, Game Y, Maillard D, Gibert P, Dardé ML and Villena I (2010) Molecular and biological characteristics of Toxoplasma gondii isolates from wildlife in France. Veterinary Parasitology 171, 346–349. [DOI] [PubMed] [Google Scholar]
- Ballash GA, Dubey JP, Kwok OCH, Shoben AB, Robison TL, Kraft TJ and Dennis PM (2015) Seroprevalence of Toxoplasma gondii in white-tailed deer (Odocoileus virginianus) and free-roaming cats (Felis catus) across a suburban to urban gradient in northeastern Ohio. EcoHealth 12, 359–367. [DOI] [PubMed] [Google Scholar]
- Crouch EEV, Mittel LD, Southard TL, Cerqueira-Cézar CK, Murata FHA, Kwok OCH, Su C and Dubey JP (2019) Littermate cats rescued from a shelter succumbed to acute, primary toxoplasmosis associated with TOXO DB genotype #4, generally circulating in wildlife. Parasitology International 72, 101942. [DOI] [PubMed] [Google Scholar]
- Demar M, Ajzenberg D, Maubon D, Djossou F, Panchoe D, Punwasi W, Valery N, Peneau C, Daigre JL, Aznar C, Cottrelle B, Terzan L, Dardé ML and Carme B (2007) Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clinical Infectious Diseases 45, e88–e95. [DOI] [PubMed] [Google Scholar]
- Dubey JP (2010) Toxoplasmosis of Animals and Humans, 2nd Edn. Boca Raton, FL: CRC Press. [Google Scholar]
- Dubey JP and Desmonts G (1987) Serological responses of equids fed Toxoplasma gondii oocysts. Equine Veterinary Journal 19, 337–339. [DOI] [PubMed] [Google Scholar]
- Dubey JP and Prowell M (2013) Ante-mortem diagnosis, diarrhea, oocyst shedding, treatment, isolation, and genetic typing of Toxoplasma gondii associated with clinical toxoplasmosis in a naturally infected cat. Journal of Parasitology 99, 158–160. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Thulliez P and Powell EC (1995) Toxoplasma gondii in Iowa sows: comparison of antibody titers to isolation of T. gondii by bioassays in mice and cats. Journal of Parasitology 81, 48–53. [PubMed] [Google Scholar]
- Dubey JP, Graham DH, de Young RW, Dahl E, Eberhard ML, Nace EK, Won K, Bishop H, Punkosdy G, Sreekumar C, Vianna MCB, Shen SK, Kwok OCH, Sumners JA, Demarais S, Humphreys JG and Lehmann T (2004) Molecular and biologic characteristics of Toxoplasma gondii isolates from wildlife in the United States. Journal of Parasitology 90, 67–71. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Velmurugan GV, Ulrich V, Gill J, Carstensen M, Sundar N, Kwok OCH, Thulliez P, Majumdar D and Su C (2008) Transplacental toxoplasmosis in naturally-infected white-tailed deer: isolation and genetic characterisation of Toxoplasma gondii from foetuses of different gestational ages. International Journal for Parasitology 38, 1057–1063. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Jenkins MC, Kwok OCH, Zink RL, Michalski ML, Ulrich V, Gill J, Carstensen M and Thulliez P (2009) Seroprevalence of Neospora caninum and Toxoplasma gondii antibodies in white-tailed deer (Odocoileus virginianus) from Iowa and Minnesota using four serologic tests. Veterinary Parasitology 161, 330–334. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Randall AR, Choudhary S, Ferreira LR, Verma SK, Oliveira S, Kwok OCH and Su C (2013a) Occurrence, isolation, and genetic characterization of Toxoplasma gondii from white-tailed deer (Odocoileus virginianus) in New Jersey. Journal of Parasitology 99, 763–769. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Tiwari K, Chikweto A, DeAllie C, Sharma R, Thomas D, Choudhary S, Ferreira LR, Oliveira S, Verma SK, Kwok OCH and Su C (2013b) Isolation and RFLP genotyping of Toxoplasma gondii from the domestic dogs (Canis familiaris) from Grenada, West Indies revealed high genetic variability. Veterinary Parasitology 197, 623–626. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Dennis PM, Verma SK, Choudhary S, Ferreira LR, Oliveira S, Kwok OCH, Butler E, Carstensen M and Su C (2014a) Epidemiology of toxoplasmosis in white tailed deer (Odocoileus virginianus): occurrence, congenital transmission, correlates of infection, isolation, and genetic characterization of Toxoplasma gondii. Veterinary Parasitology 202, 270–275. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Van Why K, Verma SK, Choudhary S, Kwok OCH, Khan A, Behinke MS, Sibley LD, Ferreira LR, Oliveira S, Weaver M, Stewart R and Su C (2014b) Genotyping Toxoplasma gondii from wildlife in Pennsylvania and identification of natural recombinants virulent to mice. Veterinary Parasitology 200, 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP, Cerqueira-Cézar CK, Murata FHA, Verma SK, Kwok OCH, Pedersen K, Rosenthal BM and Su C (2020) Genotyping of viable Toxoplasma gondii from the first national survey of feral swine revealed evidence for sylvatic transmission cycle, and presence of highly virulent parasite genotypes. Parasitology 147, 295–302. doi: 10.1017/S0031182019001586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbez-Rubinstein A, Ajzenberg D, Dardé ML, Cohen R, Dumètre A, Yera H, Gondon E, Janaud JC and Thulliez P (2009) Congenital toxoplasmosis and reinfection during pregnancy: case report, strain characterization, experimental model of reinfection, and review. Journal of Infectious Diseases 199, 280–285. [DOI] [PubMed] [Google Scholar]
- Gaulin C, Ramsay D, Thivierge K, Tataryn J, Courville A, Martin C, Cunningham P, Désilets J, Morin D and Dion R (2020) Acute toxoplasmosis among Canadian deer hunters associated with consumption of undercooked deer meat hunted in the United States. Emerging Infectious Diseases 26, 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhold RW, Saraf P, Chapman A, Zou X, Hickling G, Stiver WH, Houston A, Souza M and Su C (2017) Toxoplasma gondii seroprevalence and genotype diversity in select wildlife species from the southeastern United States. Parasites & Vectors 10, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg ME and Sundar N (2009) Sexual recombination punctuated by outbreaks and clonal expansions predicts Toxoplasma gondii population genetics. International Journal for Parasitology 39, 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis-Etter KM, Anchor CL, Chelsvig JE, Dubey JP and Warner RE (2019) Suburban white-tailed deer seropositive for Toxoplasma gondii from Chicago, Illinois. Parasitology Research 118, 2271–2276. [DOI] [PubMed] [Google Scholar]
- Howe DK and Sibley LD (1995) Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. Journal of Infectious Diseases 172, 1561–1566. [DOI] [PubMed] [Google Scholar]
- Humphreys JG, Stewart RL and Dubey JP (1995) Prevalence of Toxoplasma gondii antibodies in sera of hunter-killed white-tailed deer in Pennsylvania. American Journal of Veterinary Research 56, 172–173. [PubMed] [Google Scholar]
- Jiang T, Shwab EK, Martin RM, Gerhold RW, Rosenthal BM, Dubey JP and Su C (2018) A partition of Toxoplasma gondii genotypes across spatial gradients and among host species, and decreased parasite diversity towards areas of human settlement in North America. International Journal for Parasitology 48, 611–619. [DOI] [PubMed] [Google Scholar]
- Jokelainen P, Näreaho A, Knaapi S, Oksanen A, Rikula U and Sukura A (2010) Toxoplasma gondii in wild cervids and sheep in Finland: north-south gradient in seroprevalence. Veterinary Parasitology 171, 331–336. [DOI] [PubMed] [Google Scholar]
- Jokelainen P, Murat JB and Nielsen HV (2018) Direct genetic characterization of Toxoplasma gondii from clinical samples from Denmark: not only genotypes II and III. European Journal of Clinical Microbiology and Infectious Diseases 37, 579–586. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Blagburn BL, Dubey JP and Mason WH (1991) Prevalence and isolation of Toxoplasma gondii from white-tailed deer in Alabama. Journal of Parasitology 77, 62–64. [PubMed] [Google Scholar]
- Lindsay DS, Sundermann CA, Dubey JP and Blagburn BL (1997) Update on Toxoplasma gondii infections in wildlife and exotic animals from Alabama. Journal of the Alabama Academy of Science 68, 246–254. [Google Scholar]
- Lorenzi H, Khan A, Behnke MS, Namasivayam S, Swapna LS, Hadjithomas M, Karamycheva S, Pinney D, Brunk BP, Ajioka JW, Ajzenberg D, Boothroyd JC, Boyle JP, Dardé ML, Diaz-Miranda MA, Dubey JP, Fritz HM, Gennari SM, Gregory BD, Kim K, Saeij JPJ, Su C, White MW, Zhu XQ, Howe DK, Rosenthal BM, Grigg ME, Parkinson J, Liu L, Kissinger JC, Roos DS and Sibley LD (2016) Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nature Communications 7, 10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomares C, Devillard S, Holmes TH, Olariu TR, Press CJ, Ramirez R, Talucod J, Estran R, Su C, Dubey JP, Ajzenberg D and Montoya JG (2018) Genetic characterization of Toxoplasma gondii DNA samples isolated from humans living in North America: an unexpected high prevalence of atypical genotypes. Journal of Infectious Diseases 218, 1783–1791. [DOI] [PubMed] [Google Scholar]
- Ross RD, Stec LA, Werner JC, Blumenkranz MS, Glazer L and Williams GA (2001) Presumed acquired ocular toxoplasmosis in deer hunters. Retina 21, 226–229. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Delgado DG, Lobel HO and Parker RL (1983) Toxoplasmosis infection associated with eating undercooked venison. American Journal of Epidemiology 118, 832–838. [DOI] [PubMed] [Google Scholar]
- Schumacher A, Kazmierczak J, Moldenhauer E, Hanhly T, Montoya J, Press C, Letzer L, Elbadawi S, Smiley S and Davis J (2018) Toxoplasmosis associated with venison consumption during a retreat – Wisconsin, September–October 2017. Presented at 67th Annual Epidemic Intelligence Service (EIS) Conference, 16–19 April 2018, Atlanta, Georgia, USA.
- Shwab EK, Saraf P, Zhu XQ, Zhou DH, McFerrin BM, Ajzenberg D, Schares G, Hammond-Aryee K, van Helden P, Higgins SA, Gerhold RW, Rosenthal BM, Zhao X, Dubey JP and Su C (2018) Human impact on the diversity and virulence of the ubiquitous zoonotic parasite Toxoplasma gondii. Proceedings of the National Academy of Sciences of the United States of America 115, E6956–E6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley LD and Ajioka JW (2008) Population structure of Toxoplasma gondii: clonal expansion driven by infrequent recombination and selective sweeps. Annual Review of Microbiology 62, 329–351. [DOI] [PubMed] [Google Scholar]
- Su C and Dubey JP (2020) Isolation and genotyping of Toxoplasma gondii strains. Methods in Molecular Biology 2071, 49–80. [DOI] [PubMed] [Google Scholar]
- Su C, Shwab EK, Zhou P, Zhu XQ and Dubey JP (2010) Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology 137, 1–11. [DOI] [PubMed] [Google Scholar]
- Vanek JA, Dubey JP, Thulliez P, Riggs MR and Stromberg BE (1996) Prevalence of Toxoplasma gondii antibodies in hunter-killed white-tailed deer (Odocoileus virginianus) in four regions of Minnesota. Journal of Parasitology 82, 41–44. [PubMed] [Google Scholar]
- VanWormer E, Miller MA, Conrad PA, Grigg ME, Rejmanek D, Carpenter TE and Mazet JAK (2014) Using molecular epidemiology to track Toxoplasma gondii from terrestrial carnivores to marine hosts: implications for public health and conservation. PLoS Neglected Tropical Diseases 8, e2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SK, Sweeny AR, Lovallo MJ, Calero-Bernal R, Kwok OC, Jiang T, Su C, Grigg ME and Dubey JP (2017) Seroprevalence, isolation, and co-infection of multiple Toxoplasma gondii strains in individual bobcats (Lynx rufus) from Mississippi, USA. International Journal for Parasitology 47, 297–303. [DOI] [PubMed] [Google Scholar]
- Yu L, Shen J, Su C and Sundermann CA (2013) Genetic characterization of Toxoplasma gondii in wildlife from Alabama, USA. Parasitology Research 112, 1333–1336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020000451.
click here to view supplementary material