Abstract
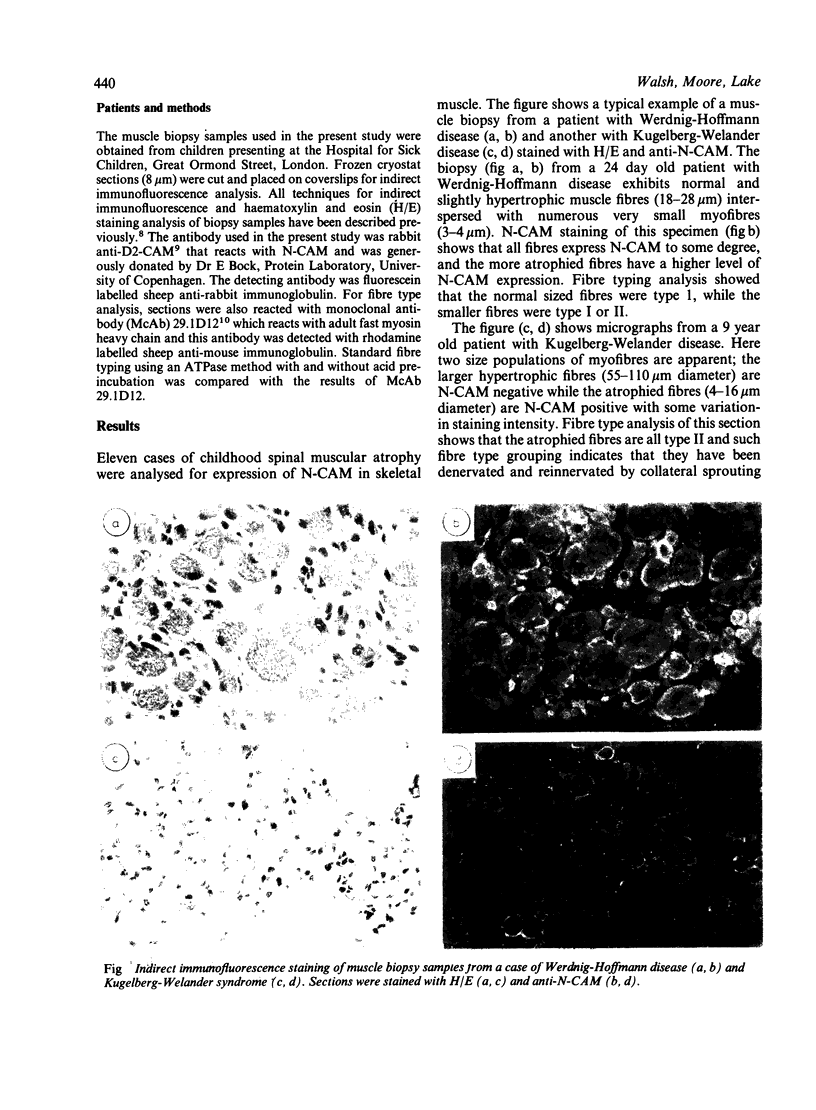
Immunocytochemical analysis utilising antibody to neural cell adhesion molecule (N-CAM) was carried out on skeletal muscle biopsies from patients with childhood spinal muscular atrophy. Children with both Werdnig-Hoffmann and Kugelberg-Welander disease showed positive N-CAM reactivity. There were however differences in the N-CAM expression profiles in these two sets of patients. All myofibres were positive for N-CAM in the Werdnig-Hoffmann patients. This included both the normal sized fibres and the atrophic fibres. In contrast only the atrophic fibres were positive in the Kugelberg-Welander patients. No reactivity was found associated with the large hypertrophic fibres. It is likely that in the Werdnig-Hoffmann patients the positive N-CAM reactivity reflects unstable innervation of myofibres that had been previously innervated. A similar mechanism may operate in the Kugelberg-Welander patients, but the innervation of the hypertrophic fibres is more stable as they are able to repress N-CAM expression. These results contrast with a lack of N-CAM expression found previously on muscle biopsies from adults with denervation disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Covault J., Sanes J. R. Neural cell adhesion molecule (N-CAM) accumulates in denervated and paralyzed skeletal muscles. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4544–4548. doi: 10.1073/pnas.82.13.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Modulation of cell adhesion during induction, histogenesis, and perinatal development of the nervous system. Annu Rev Neurosci. 1984;7:339–377. doi: 10.1146/annurev.ne.07.030184.002011. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Fidziańska A. Morphological differences between the atrophied small muscle fibres in amyotrophic lateral sclerosis and Werdnig-Hoffmann disease. Acta Neuropathol. 1976 Apr 26;34(4):321–327. doi: 10.1007/BF00696561. [DOI] [PubMed] [Google Scholar]
- Hurko O., Walsh F. S. Human fetal muscle-specific antigen is restricted to regenerating myofibers in diseased adult muscle. Neurology. 1983 Jun;33(6):737–743. doi: 10.1212/wnl.33.6.737. [DOI] [PubMed] [Google Scholar]
- Ibsen S., Berezin V., Nørgaard-Pedersen B., Bock E. Enzyme-linked immunosorbent assay of the D2-glycoprotein. J Neurochem. 1983 Aug;41(2):356–362. doi: 10.1111/j.1471-4159.1983.tb04750.x. [DOI] [PubMed] [Google Scholar]
- Moore S. E., Hurko O., Walsh F. S. Immunocytochemical analysis of fibre type differentiation in developing skeletal muscle. J Neuroimmunol. 1984 Dec;7(2-3):137–149. doi: 10.1016/s0165-5728(84)80014-4. [DOI] [PubMed] [Google Scholar]
- Moore S. E., Walsh F. S. Nerve dependent regulation of neural cell adhesion molecule expression in skeletal muscle. Neuroscience. 1986 Jun;18(2):499–505. doi: 10.1016/0306-4522(86)90170-3. [DOI] [PubMed] [Google Scholar]
- Moore S. E., Walsh F. S. Specific regulation of N-CAM/D2-CAM cell adhesion molecule during skeletal muscle development. EMBO J. 1985 Mar;4(3):623–630. doi: 10.1002/j.1460-2075.1985.tb03675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. A., Robbins N. Cell proliferation in denervated muscle: identity and origin of dividing cells. Neuroscience. 1982 Jul;7(7):1823–1833. doi: 10.1016/0306-4522(82)90040-9. [DOI] [PubMed] [Google Scholar]
- Rieger F., Grumet M., Edelman G. M. N-CAM at the vertebrate neuromuscular junction. J Cell Biol. 1985 Jul;101(1):285–293. doi: 10.1083/jcb.101.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel S. P., Bender A. N., Engel W. K. Extrajunctional acetylcholine receptors. Alterations in human and experimental neuromuscular diseases. Arch Neurol. 1976 Nov;33(11):751–758. doi: 10.1001/archneur.1976.00500110019004. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Grumet M., Edelman G. M. Neural cell adhesion molecule mediates initial interactions between spinal cord neurons and muscle cells in culture. J Cell Biol. 1983 Jul;97(1):145–152. doi: 10.1083/jcb.97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y. Muscle fibre type differentiation and satellite cell population in Werdnig-Hoffmann disease. J Neurol Sci. 1985 Apr;68(1):75–87. doi: 10.1016/0022-510x(85)90051-6. [DOI] [PubMed] [Google Scholar]
- Schmid-Antomarchi H., Renaud J. F., Romey G., Hugues M., Schmid A., Lazdunski M. The all-or-none role of innervation in expression of apamin receptor and of apamin-sensitive Ca2+-activated K+ channel in mammalian skeletal muscle. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2188–2191. doi: 10.1073/pnas.82.7.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh F. S., Moore S. E. Expression of cell adhesion molecule, N-CAM, in diseases of adult human skeletal muscle. Neurosci Lett. 1985 Aug 16;59(1):73–78. doi: 10.1016/0304-3940(85)90217-4. [DOI] [PubMed] [Google Scholar]



