Summary
Background
The prevalence and impact of alcohol withdrawal syndrome (AWS) in patients with alcohol-associated hepatitis (AH) are unknown. In this study, we aimed to investigate the prevalence, predictors, management, and clinical impact of AWS in patients hospitalized with AH.
Methods
A multinational, retrospective cohort study enrolling patients hospitalized with AH at 5 medical centres in Spain and in the USA was performed between January 1st, 2016 to January 31st, 2021. Data were retrospectively retrieved from electronic health records. Diagnosis of AWS was based on clinical criteria and use of sedatives to control AWS symptoms. The primary outcome was mortality. Multivariable models controlling for demographic variables and disease severity were performed to determine predictors of AWS (adjusted odds ratio [OR]) and the impact of AWS condition and management on clinical outcomes (adjusted hazard ratio [HR]).
Findings
In total, 432 patients were included. The median MELD score at admission was 21.9 (18.3–27.3). The overall prevalence of AWS was 32%. Lower platelet levels (OR = 1.61, 95% CI 1.05–2.48) and previous history of AWS (OR = 2.09, 95% CI 1.31–3.33) were associated with a higher rate of incident AWS, whereas the use of prophylaxis decreased the risk (OR = 0.58, 95% CI 0.36–0.93). The use of intravenous benzodiazepines (HR = 2.18, 95% CI 1.02–4.64) and phenobarbital (HR = 2.99, 95% CI 1.07–8.37) for AWS treatment were independently associated with a higher mortality. The development of AWS increased the rate of infections (OR = 2.24, 95% CI 1.44–3.49), the need for mechanical ventilation (OR = 2.49, 95% CI 1.38–4.49), and ICU admission (OR = 1.96, 95% CI 1.19–3.23). Finally, AWS was associated with higher 28-day (HR = 2.31, 95% CI 1.40–3.82), 90-day (HR = 1.78, 95% CI 1.18–2.69), and 180-day mortality (HR = 1.54, 95% CI 1.06–2.24).
Interpretation
AWS commonly occurs in patients hospitalized with AH and complicates the hospitalization course. Routine prophylaxis is associated with a lower prevalence of AWS. Prospective studies should determine diagnostic criteria and prophylaxis regimens for AWS management in patients with AH.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Keywords: Alcohol withdrawal syndrome, Alcohol-associated hepatitis, Benzodiazepines, Alcohol use disorder
Research in context.
Evidence before this study
Alcohol withdrawal syndrome (AWS) is common in hospitalized patients with alcohol use disorder and is associated with increased morbidity and mortality. Recent excessive alcohol intake is key diagnostic criterion for alcohol-associated hepatitis (AH). Thus, hospitalized patients with AH are at high risk of developing AWS. We searched PubMed for studies published by 1st January 2021 for articles in English using the search term “alcohol withdrawal hepatitis” and “alcohol-associated hepatitis”. There are no original articles evaluating the prevalence, clinical characteristics, and management of AWS in patients with liver disease. This fact influences that most liver societies do not recommend homogeneous protocols to manage AWS. This is the first study evaluating the burden, outcomes, and treatment strategies of AWS in patients hospitalised with AH.
Added value of this study
This is the first study evaluating AWS in patients with AH. Our findings highlight the high burden of AWS in patients with AH. We also demonstrated that the development of AWS is negatively associated with increased morbidity and mortality, regardless of AH severity. Centres that provided universal AWS prophylaxis to all patients with AH had a lower prevalence of AWS along with overall better outcomes. Patients with AH who developed AWS received a higher dose of benzodiazepines (BZD) compared to previously reported in patients without AH. This is counterintuitive as sedatives mainly have hepatic metabolism and can cause hepatic encephalopathy, suggesting that current assessment scales based on symptom-triggered approach need further validation in patients with AH. The use of intravenous BZD and phenobarbital were associated with a higher mortality rate compared to oral BZD.
Implications of all the available evidence
Our novel findings underscore the high rate and clinical impact of AWS in patients with AH and can serve as proof of concept for frequent monitoring of AWS-related symptoms. Our data also should warn clinicians on overutilization of sedatives in patients with AH as it is associated with worse outcomes. As current AWS assessment scores are not validated in patients with AH, we recommend favouring clinical judgment for dosing sedatives on the basis of scaling assessment. Our findings highlight the urgent need to conduct clinical trials to 1) assess the efficacy of AWS prophylaxis and 2) compare different AWS treatment modalities in patients with AH.
Introduction
Excessive alcohol use is associated with a high risk of morbidity and mortality, accounting for more than 3 million deaths per year worldwide.1,2 Over the last decade, the prevalence of alcohol use disorder (AUD) has increased at an alarming rate with a greater rise in women, youth, and racial minorities.3 About 50% of patients with excessive alcohol intake develop some degree of alcohol withdrawal syndrome (AWS) after abrupt cessation or reduction in alcohol intake.4 The presentation of AWS ranges from mild symptoms such as irritability, tachycardia, and tremulousness to severe forms with seizure and delirium tremens (characterized by alteration in mental status and severe autonomic hyperactivity).5 When patients with AUD are admitted into the hospital, regardless of the reason for admission, they are at risk for developing AWS. Based on a recent systematic review, among patients hospitalized for any medical condition with a history of excessive alcohol use, 2–7% develop severe AWS.6
Prolonged, heavy alcohol drinking is the most common aetiology of advanced liver disease globally.7 In parallel with the epidemic of AUD, the incidence and mortality of alcohol-associated liver disease (ALD) are on the rise representing about half of all liver-related mortality.2 While the majority of patients with ALD exhibit chronic hepatic changes such as steatosis or cirrhosis, a subgroup of patients present with alcohol-associated hepatitis (AH), a form of acute-on-chronic liver failure (ACLF) manifesting as rapid onset of jaundice and systemic inflammation in the setting of prolonged heavy alcohol use.8 Importantly, about 75% of patients with AH has undiagnosed cirrhosis at the time of initial presentation.9 Prolonged and heavy alcohol consumption coupled with the need for hospitalization puts the patients AH at a high risk for the development of AWS.
The incidence and clinical impact of AWS in patients with liver disease are unknown.10 It is plausible that early identification and appropriate management of AWS is associated with favourable outcomes. Moreover, the safety of current therapies has not been validated in patients with profound liver failure such as AH.11,12 Although practice guidelines for the management of AH include some comments on management of AWS, there is little evidence to support the use of AWS prophylaxis. Subsequently, significant variation among different centres.13,14 While some European centres have adopted prophylactic pharmacotherapy in patients at high risk for the development of AWS, universal prophylaxis is highly uncommon in US centres. Furthermore, most tertiary care centres adopt control of AWS symptoms guided by assessment scales; however, other centres use clinical judgment for dosing of sedatives to control AWS symptoms.15
In this multi-national study, we aimed to describe the prevalence, clinical characteristics, management strategies, and outcomes of AWS in patients hospitalized with AH. We also explore the potential beneficial effects of prophylactic therapy in patients admitted with AH by comparing centres with different management strategies.
Methods
Study design and population
We performed a multinational, observational study identifying patients hospitalized with AH consecutively admitted between January 1st, 2016 and January 31st, 2021. Patients from 5 tertiary medical centres were included in this study: 4 centres from Spain (Clinic University Hospital of Valencia, University Hospital of Canarias, Vall d’Hebron University Hospital of Barcelona, and Santa Creu i Sant Pau Hospital of Barcelona) and one centre from the US (University of Pittsburgh Medical Center). All patients were aged 18 years or older at the time of hospitalization for AH. The diagnosis of AH was based on criteria by the National Institute on Alcohol Abuse and Alcoholism (NIAAA).13,14 Patients with histologic confirmation of AH (definite AH) and patients with clinical characteristics of AH without any confounding findings (probable AH) were included. Briefly, the clinical diagnostic criteria of AH include: 1) history of alcohol use of >60 g/day in men and >40 g/day in women, 2) an aspartate aminotransferase (AST) elevated >1.5 times the upper limit of normal but <400 U/l with AST/ALT ratio >1.5, 3) Serum γ-glutamyl transpeptidase (GGT) levels >80 mg/dL, 4) altered coagulation tests [prolonged prothrombin time and/or international ratio (INR) values], and 5) serum bilirubin levels >3 mg/dL. Severe AH was defined as MELD score >20. Exclusion criteria include 1) presence of other identifiable causes of liver disease such as viral or autoimmune hepatitis, 2) alternative diagnosis on liver biopsy, 3) hepatocellular carcinoma and/or other malignancies, and 4) other extrahepatic severe illness with low life expectancy. The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and had the a priori approval of the institutional review boards from all participating hospitals (2021/139, CHUC_2021_33, PR (AG)404/2021, and STUDY 19090128). Informed consent from patients was deemed unnecessary by the ethics committees for this retrospective study. The study was conducted and reported in compliance with the STROBE guidelines for cohort studies.
Data collection
Data were retrospectively retrieved from electronic health records. Patients with a diagnosis of AH admitted to the participating centres were consecutively enrolled. Demographic data, clinical features, laboratory tests, management strategies, and outcomes were recorded for every patient. Longitudinal laboratory variables were collected and used to calculate model for end-stage liver disease (MELD), albumin-bilirubin-INR-creatinine (ABIC), and ACLF scores at different time points of admission. The development of new organ failure during hospitalization was captured. Acute kidney injury (AKI) was defined according to the International Ascites Club. Respiratory failure was defined as SpO2/FiO2 ≤214. Cardiovascular failure was defined as mean arterial pressure less than 65 mmHg or need for vasopressors. Altered mental status was defined based on the presence of hepatic encephalopathy grade II or higher.15 Then, we calculated acute-on-chronic liver failure (ACLF) score and categorized patients into 4 classes: no ACLF, ACLF grade I, ACLF grade II, and ACLF grade III.16 Empirical use of antibiotic in patients with severe AH was not standard of care in any of participating centres. Use of antibiotics was based on clinician's judgement.
Data related to AWS were captured by clinicians with experience taking care of patients hospitalized with AH. The diagnosis of AWS was made based on the clinical judgment of the primary clinician following the patient and the need for sedative therapy to control AWS symptoms. Medications used for control of AWS were categorized into benzodiazepines and phenobarbital. The route of therapy [oral versus intravenous (IV)] was recorded as well. Patients who received both oral and IV therapy were categorized in the IV group. In Spanish centres, phenobarbital is not utilized for AWS treatment. The amount of benzodiazepine was detailed in diazepam-equivalent format (https://www.benzo.org.uk/bzequiv.htm). The duration of AWS therapy was determined by the time a patient required medication to control AWS-related symptoms. AWS severity was categorized into severe and non-severe based on the presence of hallucination, delirium tremens, and/or seizures. The severity of AWS symptoms was quantified by clinical institute withdrawal assessment-alcohol revised (CIWA-Ar) scoring system. We also obtained clinical complications associated with AWS, such as intubation and ICU admission. Participating centers shared similar ICU admission protocols and none of them considered having alcohol use disorder or AH as a contraindication for ICU admission. Of note, the clinical protocols for the management of AWS differed between US and Spanish centres. In Spain, AWS prophylaxis is universally administered in all patients with a history of recent excessive alcohol use, including all patients admitted with AH. As a standard of care protocol, AWS prophylaxis is given at the time of admission to all patients with AH. Prophylaxis is largely based on clomethiazole.17 Clomethiazole is a thiamine derivative with a GABA agonist effect commonly used for the treatment of AWS symptoms. It has been shown clomethiazole efficacy to control AWS symptoms is not inferior to chlordiazepoxide.18 Use of clomethiazole as a prophylaxis agent in patients at risk for AWS is common in several European countries such as Germany or Spain.19 In the US, prophylaxis is rarely used and patients do not receive any pharmacologic therapy until AWS symptoms start. To conduct a comparison, cases were defined as patients diagnosed with AH by an attending physician and requiring sedative therapy to control symptoms of AWS.8 As controls, we included all patients with AH who did not develop AWS during the index episode. The primary outcome of the study was 28-day, 90-day, and 180-day mortality.
Statistical analysis
Qualitative and quantitative variables were reported as absolute frequencies/percentages and median with interquartile range (IQR), respectively. Comparisons between groups were conducted using Mann–Whitney U test for continuous variables and chi-square or Fisher test for categorical variables, as appropriated. For time-to-event analysis, the day of admission for AH was defined as the baseline point (time 0). Censoring time was defined as the last available patient encounter, time of death, or study closure at 180-days, whichever occurred first. Time-to-event analysis was performed using Kaplan–Meier method. Survival curves were compared using the log-rank test to identify parameters modifying 28-day, 90-day, and 180-day mortality. Multivariable Cox proportional hazard regression analyses was performed to determine the independent contribution of AWS on mortality, adjusted for age, MELD score, ALCF class, in-hospital infection, hepatic encephalopathy (HE), and corticosteroid use (all considered confounding factors of AH mortality). A second model of multivariable Cox regression was performed to determine in patients who developed AWS the independent contribution of AWS management on mortality, adjusted for age, MELD, HE and severity of AWS (all considered confounding factors influencing AWS management and mortality). The use of Cox proportional hazards models needs two assumptions that were checked as follows: (I) Sc. Schoenfeld plot tested that survival curves for different strata have hazard functions that are proportional over the time t, and (II) Martingale and Schoenfeld residuals plots tested that the relationship between the log hazard and each covariate was linear. The results of multivariable Cox analysis are presented as estimated hazard ratios (HR) with corresponding 95% confidence interval (95% CI) and p-values. Collinearity was assessed among the variables included in the multivariable analysis by using variance inflation factors (VIF). VIFs value of less than 5 show absence of any significant collinearity. In addition, a multivariable binary logistic regression model adjusted for age and MELD score was performed to evaluate factors associated with AWS (predictors and clinical outcomes). We tested assumptions for logistic regression models that included independence of errors, linearity in the logit for continuous variables, absence of multicollinearity, and lack of strongly influential outliers. The results of multivariable logistic regression are presented as estimated odds ratio (OR) with corresponding 95% CI and p-values. All tests were two-sided and a p-value less than 0.05 was considered statistically significant. All analyses were performed with the IBM SPSS Statistics for Windows, Version 25.0, Armonk, NY. The software Statistica 14.0.1.25 (tibco Software Inc.) was used to check the Cox proportional hazard model assumptions.
Role of the funding source
There was no funding source for the study. All authors had full access to the data in this study and accept responsibility for the decision to submit the manuscript for publication.
Results
Patient characteristic
The clinical characteristics, relevant laboratory markers, complications, and outcomes of patients with AH are depicted in Table 1. In total, 432 patients with probable (n = 393) or definite diagnosis (n = 39) of AH were included. Patients were admitted consecutively to participating centres between January 2016 and January 2021. Of them, 55% (n = 239) were recruited in the US and 45% (n = 193) in Spain (Supplementary Table S1). The median MELD score was 21.9 (18.3–27.3). Prevalence of severe AH and treatment with corticosteroids was 64% (n = 277) and 44% (n = 192), respectively. The median MELD score and Maddrey's discriminant function (mDF) were significantly higher in patients treated with corticosteroids: MELD of 20 (16–26) vs. 23 (20–28), p < 0.0010; and mDF of 45 (23–80) vs. 65 (44–83), p < 0.0010. Overall, 24.5% (n = 106) of patients met criteria for ACLF (6% grade I, 10.6% grade II and 7.9% grade III), with a mean CLIF-C ACLF score of 51 ± 9. Antibiotic therapy was used in 58% (n = 249) of the patients, being their main indication of use an active infection (n = 159), prophylaxis in the setting of GI bleeding (n = 25), endotracheal intubation (n = 9), and presence of ACLF with signs of systemic inflammation response (n = 12). The overall mortality rate at 28 days, 90 days, and 180 days were 17.3%, 27.3%, and 33.6%, respectively. Only 20 patients (4.7%) underwent early liver transplantation.
Table 1.
Clinical characteristics of patients with alcohol-associated hepatitis.
| Characteristic | Total (n = 432) |
|---|---|
| Anthropometric and history | |
| Age, years, median (IQR) | 48 (40–56) |
| Sex, female, n (%) | 157 (36.3%) |
| Sex, male, n (%) | 275 (63.7%) |
| Race, white, n (%) | 391 (90.5%) |
| BMI, kg/m2, median (IQR) | 27.9 (24.2–32.5) |
| History of AH, n (%) | 134 (31.1%) |
| History of AWS, n (%) | 116 (26.8%) |
| History of hepatic encephalopathy, n (%) | 91 (21.1%) |
| Corticosteroid use, n (%) | 192 (44.4%) |
| Data on Admission | |
| Creatinine (mg/dL), median (IQR) | 0.77 (0.6–1.2) |
| Na (mmol/L), median (IQR) | 133 (128–137) |
| Bilirubin (mg/dL), median (IQR) | 9.9 (6.3–19.1) |
| INR, median (IQR) | 1.7 (1.4–2.1) |
| Albumin (g/dL), median (IQR) | 2.6 (2.2–3) |
| Platelets (×109/L), median (IQR) | 114 (65–178) |
| MELD score, median (IQR) | 21.9 (18.3–27.3) |
| MELD score >20, n (%) | 277 (64%) |
| ABIC score, median (IQR) | 7.5 (6.6–8.8) |
| Complications | |
| AWS, n (%) | 140 (32.4%) |
| Gastrointestinal bleeding, n (%) | 72 (16.6%) |
| Ascites, n (%) | 267 (61.8%) |
| Infections, n (%) | 173 (40.1%) |
| AKI, n (%) | 140 (32.4%) |
| Encephalopathy, n (%) | 165 (38.2%) |
| Shock, n (%) | 34 (7.8%) |
| Need for intubation, n (%) | 58 (13.4%) |
| Clinical outcomes | |
| ICU admission, n (%) | 118 (27.3%) |
| Length of stay (days), median (IQR) | 11.0 (6.0–22.0) |
| ICU stay (days), median (IQR) | 5.0 (2.0–10.0) |
| Mortality on day 28, n (%) | 75 (17.3%) |
| Mortality on day 90, n (%) | 118 (27.3%) |
| Mortality on day 180, n (%) | 145 (33.6%) |
Abbreviations: ABIC, Age-bilirubin-INR-creatinine score; AH, Alcohol-associated hepatitis; AKI, Acute kidney injury; AWS, Alcohol withdrawal syndrome; BMI, Body mass index; ICU, Intensive care unit; INR, International ratio; IQR, Interquartile range; MELD, Model for end stage liver disease.
Prevalence, severity, and risk factors
The clinical features of AWS during hospitalization are described in Table 2. During hospitalization, 32.4% (n = 140) of patients developed AWS. In most cases (95%), symptoms of AWS started within the initial three days of hospitalization and lasted for 5 days. Next, we explored the clinical and laboratory parameters associated with the development of AWS (Supplementary Table S2). There was no significant difference in the degree of liver dysfunction based on MELD score between patients with and without AWS. Among patients who underwent liver biopsy, histologic severity of AH determined by alcoholic hepatitis histologic score (AHHS) was not associated with the development of AWS (Supplementary Table S3). In the univariate analysis, patients who developed AWS were younger (46 vs. 49 years, p = 0.0060) and more commonly had a previous history of AWS (37.1% vs. 22.2%, p = 0.0010). Among laboratory parameters at admission, platelet levels were inversely associated with the rate of AWS (124.1/dL vs. 139.7/dL, p = 0.045). To identify independent predictors of AWS, a multivariable logistic regression analysis was performed. After controlling for age and MELD score, previous history of AWS (OR 2.09, 95% CI 1.31–3.33) and lower platelet levels (OR 1.61, 95% CI 1.05–2.48) were independently associated with a higher rate of AWS (Supplementary Table S2). Importantly, we found that the use of prophylaxis at the time of admission decreased the risk of AWS by 42% (OR 0.58, 95% CI 0.36–0.93, p = 0.024).
Table 2.
Clinical features and management of alcohol withdrawal syndrome.
| Total (N = 140) | USA (N = 89) | Spain (N = 51) | p-value | |
|---|---|---|---|---|
| Timing | ||||
| AWS onset (days), median (IQR) | 1 (0–2.5) | 1 (0–3) | 1 (0–2) | |
| Severity | ||||
| WAS score on admission | 4 (0–16) | 5 (0–18) | 2 (0–6) | 0.0020 |
| Maximum WAS score, median (IQR) | 8 (0–21) | 10 (0–24) | 4 (0–11) | 0.33 |
| Hallucinations, n (%) | 23 (16.4%) | 15 (16.9%) | 8 (15.7%) | 0.86 |
| Delirium tremens, n (%) | 10 (7.1%) | 5 (5.6%) | 5 (9.8%) | 0.36 |
| Seizure, n (%) | 5 (3.5%) | 3 (3.4%) | 2 (3.9%) | 0.87 |
| AWS duration (days), median (IQR) | 4 (2–6) | 3 (2–6) | 5 (3–9) | 0.011 |
| Management | ||||
| Use of prophylaxis, n (%) | 51 (36.4%) | 0 (0%) | 51 (100%) | <0.0010 |
| BZD use, n (%) | 128 (91.4%) | 87 (97.8%) | 41 (80.4%) | <0.0010 |
| BZD use route, n (%) | 0.036 | |||
| PO only | 51 (39.8%) | 28 (32.2%) | 23 (56.1%) | |
| IV only | 55 (43.0%) | 42 (48.3%) | 13 (31.7%) | |
| PO and IV | 22 (17.2%) | 17 (19.5%) | 5 (12.2%) | |
| BZD dose (mg), median (IQR) | 45 (20–148) | 45 (22–180) | 35 (15–105) | 0.12 |
| Phenobarbital use, n (%) | 12 (8.6%) | 12 (13.5%) | 0 (0%) | 0.0060 |
Abbreviations: AWS, Alcohol withdrawal syndrome; BZD, Benzodiazepine; IQR, Interquartile range; IV, Intravenous; mg, milligrams; PO, Per oral; WAS, Withdrawal assessment scale.
Management
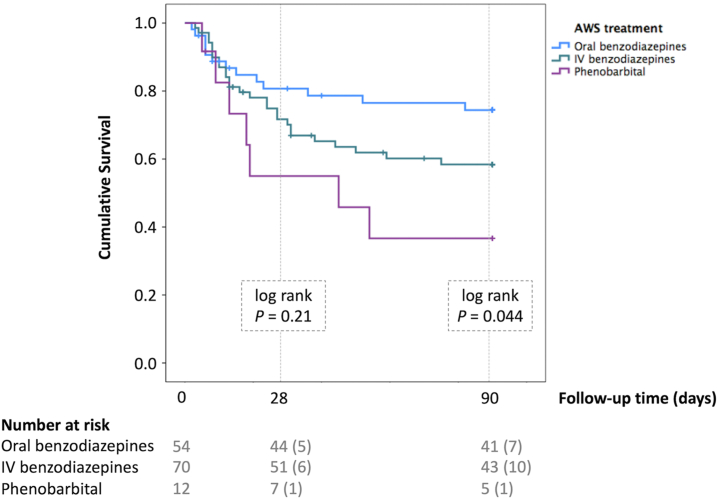
We next studied the impact of different modalities of AWS treatment. Table 2 details the management strategies to control AWS symptoms. We compared the management strategies between US and Spanish cohorts. While most patients admitted with AH in Spain received prophylaxis with clomethiazole at the time of admission, no patient received prophylactic therapy in the US. Remarkably, patients from the US had higher severity of AWS symptoms scaled by CIWA-Ar score (10 vs. 4, p = 0.0020). In the US, the dosage and route of sedatives to control AWS symptoms were dictated by CIWA-Ar scale assessment, whereas in Spanish centres clinical judgment by clinicians was main driver of sedative use. BZDs were more frequently administered via IV route in the US, compared to Spanish centres (48.3% vs. 31.7%, p = 0.032). Phenobarbital was used in a small proportion of patients (8.6%) as adjunctive therapy to BZD. Next, we performed a multivariable Cox regression to assess the effect of different management strategies on patient outcomes. After controlling for age, HE, and severity of AH and AWS, we found that the use of BZD via IV route (HR 2.18, 95% CI 1.02–4.64) and phenobarbital (HR 2.99, 95% CI 1.07–8.37) were associated with a higher rate of 90-day mortality compared to the oral-only route (Supplementary Table S4). Fig. 1 illustrates Kaplan Meir curves depicting the impact of different treatment modalities on the mortality of patients with AH who developed AWS.
Fig. 1.
Kaplan Meier curves comparing the survival between different treatment strategies to control Alcohol Withdrawal Syndrome in Alcohol-associated Hepatitis patients. The blue line corresponds to oral benzodiazepines, the green line to intravenous benzodiazepine, and the purple line to phenobarbital. Raw p-values are presented. Abbreviations: IV, Intravenous; AWS, Alcohol withdrawal syndrome.
Although AWS and HE are two distinct conditions, they can have common symptoms and co-exist. In an attempt to differentiate both conditions, ammonia levels were tested in a subgroup of patient (n = 163). Ammonia levels correlated with HE grades (r = 0.55, p = 0.011) but not with CIWA-Ar scale assessment (r = 0.20, p = 0.070). Among patients with severe AH and AWS, ammonia levels were higher in patients with HE [96 (75–126) ug/dL vs. 79 (53–111) ug/dL; p = 0.11].
Clinical outcomes
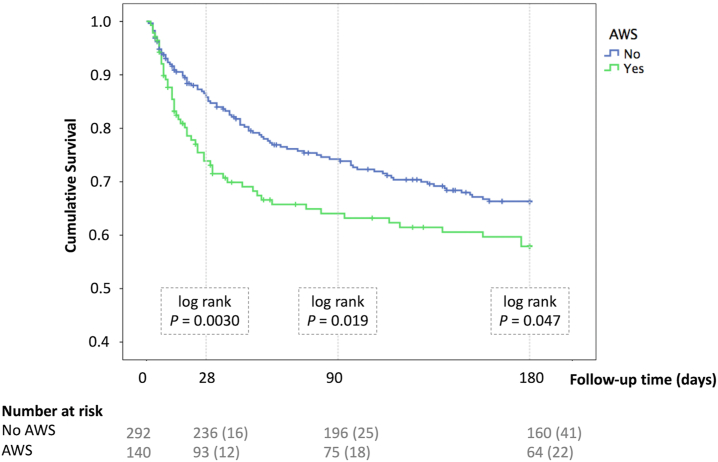
We finally investigated the clinical impact of AWS. Table 3 summarizes the association between AWS and clinical outcomes. After controlling for MELD score and age, the development of AWS was associated with increased risk of in-hospital infection (OR 2.24, 95% CI 1.44–3.49), altered mental status (OR 2.41, 95% CI 1.52–3.83), ICU admission (OR 1.96, 95% CI 1.19–3.23), as well as the need for mechanical ventilation (OR 2.49, 95% CI 1.38–4.49). As illustrated in Fig. 2, we used Kaplan–Meier curves to compare the short-term and long-term mortality between patients with and without AWS during AH hospitalization. A multivariable Cox regression analysis to determine the independent effect of AWS on mortality was adjusted for age, MELD, ACLF class, infection, HE, and corticosteroid use. There was no significant collinearity among variables (Supplementary Table S5). We found that the development of AWS independently increased the risk of short-term and long-term mortality. For 28-day mortality, the adjusted-HR was 2.31, 95% CI 1.40–3.82 (p = 0.0010); for 90-day mortality, the adjusted-HR was 1.78, 95% CI 1.18–2.69 (p = 0.0060); and for 180-day mortality, the adjusted-HR was 1.54, 95% CI 1.06–2.24 (p = 0.023). Supplementary Table S6 shows the complete multivariable analysis for the determination of factors associated with short and long-term mortality. These results indicate that the development of AWS negatively impact the clinical course of AH.
Table 3.
Impact of alcohol withdrawal syndrome on outcomes of patients with alcohol-associated hepatitis.
| Univariate Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Clinical outcomes | AWS | No AWS | p-value | ORb | 95% CI | p-value |
| AKI, n (%) | 45 (32.1%) | 95 (32.9%) | 0.88 | 1.29 | 0.75–2.25 | 0.36 |
| Cardiovascular failure, n (%) | 16 (14.0%) | 18 (7.6%) | 0.054 | 2.18 | 0.96–4.95 | 0.062 |
| HE during hospitalization, n (%) | 67 (48.2%) | 98 (33.9%) | 0.0040 | 2.41 | 1.52–3.83 | <0.0010 |
| Infections, n (%) | 73 (53.3%) | 100 (34.6%) | <0.0010 | 2.24 | 1.44–3.49 | <0.0010 |
| Need for intubation, n (%) | 30 (21.4%) | 28 (9.6%) | 0.0010 | 2.49 | 1.38–4.49 | 0.0030 |
| ACLF, n (%) | 32 (22.9%) | 74 (25.3%) | 0.57 | 1.29 | 0.68–2.45 | 0.44 |
| CLIF-C ACLFa (mean ± SD) | 50 ± 10 | 51 ± 8 | 0.76 | 1.01 | 0.95–1.08 | 0.68 |
| ICU admission, n (%) | 50 (36.2) | 68 (23.6) | 0.0060 | 1.96 | 1.19–3.23 | 0.0090 |
| ICU stay ≥7 days, n (%) | 16 (32.0) | 26 (38.8) | 0.45 | 0.66 | 0.29–1.53 | 0.33 |
| Hospitalization stay ≥7 days, n (%) | 107 (76.4) | 205 (71.2) | 0.25 | 1.75 | 1.05–2.91 | 0.031 |
| Mortality | AWS | No AWS | p-value | HRc | 95% CI | p-value |
| Mortality on day 28, n (%) | 35 (27.1) | 40 (14.6) | 0.0030 | 2.31 | 1.40–3.82 | 0.0010 |
| Mortality on day 90; n (%) | 47 (38.5) | 71 (26.8) | 0.019 | 1.78 | 1.18–2.69 | 0.0060 |
| Mortality on day 180, n (%) | 54 (45.4) | 91 (36.5) | 0.047 | 1.54 | 1.06–2.24 | 0.023 |
Abbreviations: ACLF, Acute-on-chronic liver failure; AKI, Acute kidney injury; AWS, Alcohol withdrawal syndrome; CI, Confidence interval; HE, Hepatic encephalopathy; HR, Hazard ratio; ICU, Intensive care unit; OR, Odds ratio.
CLIF-C score was only calculated in patients who developed ACLF.
For clinical outcome variables, the effect of AWS was adjusted by age and MELD score.
For mortalities, the effect of AWS was adjusted by age, MELD, ACLF class, hepatic encephalopathy, infection, and corticosteroid use.
Fig. 2.
Kaplan–Meier curve comparing survival between patients with (green line) and without (blue line) Alcohol Withdrawal Syndrome during hospitalization for Alcohol-associated Hepatitis. Raw p-values are presented. Abbreviations: AWS, Alcohol withdrawal syndrome.
Discussion
Despite the high prevalence of AWS in AH, this is the first study investigating the clinical characteristics, management, and outcomes of AWS in patients hospitalized with AH. Our results revealed 6 key findings. First, the development of AWS is common in patients hospitalized with AH, affecting up to one-third of patients. Second, we found that patients with AH developing AWS received high doses of BZD and frequently through the IV route. Third, we showed that IV BZD and phenobarbital use were associated with worse clinical outcomes. Fourth, AWS complicated hospitalization course by increasing the risk of in-hospital infections, need for mechanical ventilation, and ICU admission. Fifth, we identified higher short-term and long-term mortality in patients with AH who developed AWS. Finally, we observed a significant disparity in the management strategies between centres in the US and Spain. Strikingly, AWS prophylaxis at admission in patients with AH was associated with a lower risk of AWS development and related complications.
In our study, up to one-third of patients with AH developed clinically significant AWS. In a USA cohort of patients at the Veterans Health Administration, patients with cirrhosis had higher prevalence of AWS.12 A small study in patients with WDS undergoing a liver biopsy, found histopathologic findings consistent with AH in 45% of patients.20 The high prevalence of AWS in patients with AH in our study is due to the fact that most of them have a history of recent excessive alcohol intake prior to hospitalization. In agreement with previous reports, we showed that younger age and prior history of AWS increase the risk of AWS in patients with AH.21 Unlike earlier reports in patients without AH, we did not find any difference in the prevalence of AWS in AH between female and male sex.12 The high prevalence of AWS in patients with AH highlights the importance of close monitoring in all patients admitted with AH and strongly suggests that early prophylaxis should be instituted in order to prevent the development and complications of AWS.
While there are no randomized controlled trials in the management of AWS in patients with AH, we assessed various management strategies to provide insight into the safety of current treatment modalities and pave the way for future studies. We found that patients with AH predominantly received a high dose of BZD and, in half of the cases, through an IV route. When comparing our results with previous studies in the management of AWS in patients without AH, a higher dosage of BZD was utilized in our cohort of patients with AH.22,23 We also found the use of BZD via IV route was independently associated with higher morbidity and mortality. BZD dosing and route of administration are driven by a symptoms-triggered approach using withdrawal assessment scales.23, 24, 25, 26 Higher BZD requirement in patients with AH developing AWS may be related to overestimation of AWS severity by current scaling scores. The lack of validity of common AWS scoring systems in face of other acute illness were previously shown during postoperative care, leading to inappropriately higher dose of sedative-hypnotics.27 We hypothesize that systemic inflammation related to AH may have led to the overestimation of AWS severity, which subsequently led to higher BZD dose and higher frequency of IV routes.28, 29, 30, 31 Validating current withdrawal severity scores in patients with AH represents an urgent need to avoid over-utilization of BZD in patients with AWS. Our results also warn clinicians to use the minimum dose of BZD possible to control the symptoms and avoid IV route and barbiturates, if possible, in patients with AH.
Poor outcomes in patients with AH who received high dose of BZD or phenobarbital can be related to triggering or aggravating HE, which in turn, increase the risk of aspiration and in-hospital infection.5,32, 33, 34, 35, 36 AWS and HE have some common and distinctive symptoms and can co-exist, making the differential diagnosis quite challenging. Currently, there is no specific diagnostic test to reliably differentiate these two conditions and clinician judgement (i.e., timing of symptoms onset, and presenting symptoms) is used for this purpose, raising concern over misdiagnosis. Although ammonia level do not guide clinical management in HE, we showed that ammonia was slightly higher in severe AH patients with AWS and co-existing HE.37 Furthermore, ammonia levels correlated with HE grades but not CIWA-Ar scale. Importantly, development of HE and AWS are not mutually exclusive and they can co-exist in about of half of patients with AH.38 It is conceivable that patients with a poor outcome after IV BZD administration could have, at least, some degree of concomitant HE. Prospective studies should identify novel specific diagnostic tools to differentiate between HE and AWS. Additional causes that mimic AWS such as Wernicke's encephalopathy should also be further investigated.
Our findings indicate that the development of AWS is associated with a more than two-fold risk of short-term mortality in patients admitted with AH. The negative impact of AWS was independent of the severity of liver dysfunction, highlighting the appropriate management of AWS as major part of AH care to improve overall outcomes. While overall mortality of AWS in patients admitted with alcohol intoxication is relatively low, the clinical outcomes in patients admitted with any other indication are less favourable.39, 40, 41 While there is no study on patients with AH, previous reports showed that patients with advanced liver disease are at higher risk of developing severe AWS and its related complications.42 Altered mental status resulting in a higher need for intubation and risk of aspiration pneumonia is a potential mechanism explaining poor outcomes in patients with AH who develop AWS. AWS can trigger alteration in mental status directly or secondary to the use of sedatives required to control psychomotor agitation related to AWS.
An important finding of our study is the potential beneficial role of AWS prophylaxis.43,44 Given the high burden and impact of AWS, an attempt to achieve an effective preventive strategy was pursued by few studies.45,46 However, there are no head-to-head trial comparing the outcomes of prophylaxis with no prophylaxis in patients at risk for developing AWS.47 Based on expert opinion, AWS prophylaxis is suggested in patients at risk for the development of severe AWS and not actively experiencing AWS.48 Given the low level of evidence, significant variation exists among centres. A small clinical trial compared clonidine vs. diazepam as prophylaxis agents in patients at risk of AWS undergoing surgery; clonidine resulted in a lower rate of post-operative AWS.49 Clomethiazole, a fast-acting barbiturate-like drug, has been widely used for the treatment and prophylaxis of AWS in Europe.17,50 Clomethiazole was found similarly effective to BZD for treatment and prophylaxis of AWS.43,51 To the best of our knowledge, no study has evaluated the effect of prophylaxis against AWS in patients with AH. After controlling for age and AH severity, we found that centres adopting prophylaxis strategy had a significantly lower rate of infection, need for mechanical ventilation, and mortality. Results of this study strongly suggest beneficial effects of the prophylactic regimen in all patients admitted with AH. Further randomized controlled trials is urgently warranted to confirm this observation.
Our study encompassed several strengths. First, to the best of our knowledge, this is the first study investigating the clinical characteristics, management, and outcomes of AWS in patients with AH. Given the high prevalence of both AH and AWS, the clinical message of this study is highly relevant to patient care. Second, despite the multi-centre design, the study leadership was central to ensuring universal methods for patient selection, variable definitions, and data collection. Third, all clinical data were collected by clinician-investigators with extensive experience in the management of patients with AH and AWS. This should enhance the accuracy of data by limiting the risk of inappropriate coding and documentation. Forth, detailed clinical data on specific time-points were available and our study contained very minimal missing data (n = 13 due to non-reported critical laboratory values). And fifth, long-term follow up was available in all patients, which allowed us to assess long-term outcomes. There are, however, several limitations. First, Spanish and US cohorts differs in several clinical aspects, severity, and management strategies of AWS. Differences represent the heterogenous manner in which this complication is being managed, influenced by the lack of scientific evidence and recommendations. Although fully adjusted multivariable analysis were performed, considering together both cohorts can raise concern on the applicability of the results as it may be influenced by local healthcare systems and standard of care protocols. Nevertheless, this study allowed to compare two cohorts with different clinical management representing a unique opportunity to assess the potential usefulness of AWS prophylaxis and the risk of different therapies. Our preliminary results can raise awareness of clinically relevant issues that should now be confirmed in clinical trials. Second, retrospective design of study incurs selection bias on prevalence and outcomes of AWS and confounding variables. We included patients admitted consecutively in all participating centres to minimize this limitation. All participating centres were tertiary care hospitals with high level of complexity, so a referral bias was likely to occur. Thus, our results may not reflect the burden of AWS in community centres. We attempted to control potential confounding by conducting analysis adjusted by multiple variables and presenting the independent measure of AWS impact on mortality and other clinical outcomes, fully weighed with respect to critical confounders. Finally, the diagnosis of AWS and its related complications relied on subjective judgment of attending physician. To reduce this limitation, we confirmed their diagnosis with the dose of as-needed sedative use and vital signs to decrease the subjective assessments in diagnosis of AWS.
In conclusion, this multi-center study demonstrates that AWS is common in patients admitted with AH. AWS complicates the clinical course of patients with AH by increasing the risk of hepatic encephalopathy, infection, and the need for mechanical ventilation. AWS independently increased the short-term and long-term mortality of AH. The higher dose of sedative agents via IV route to control AWS symptoms is associated with worse outcomes. Comparing the prophylaxis strategies between centres, our findings suggest that adoption of universal prophylaxis with clomethiazole may be beneficial to prevent AWS and its related complications.
Contributors
DMA: Writing-Original Draft, Methodology, accessed and verified the underlying data, responsible for the decision to submit the manuscript.
AG: Writing-Original Draft, Methodology, accessed and verified the underlying data, responsible for the decision to submit the manuscript.
CGM: Resources, Project administration, accessed and verified the underlying data.
AJ: Formal analysis.
AAZ: Resources.
DM-A: Formal analysis.
AJS: Writing - Review & Editing.
KB: Resources.
AB: Resources.
AH: Resources.
CP: Resources.
EAT: Resources.
JS: Resources.
WKC: Resources.
HWC: Resources.
AV: Resources.
MVC: Writing - Review & Editing.
HG: Resources.
PL: Resources.
VR: Writing - Review & Editing.
ADR: Writing - Review & Editing.
RB: Conceptualization, Supervision, Writing - Review & Editing, responsible for the decision to submit the manuscript.
All authors concur with the decision to submit the manuscript.
Data sharing statement
De-identified individual participant data collected during the study will be made available to request following publication, access to this data will be granted to researchers who provide a methodologically sound proposal. Proposals should be directed to the corresponding author.
Declaration of interests
The authors declare no conflict of interest regarding this manuscript.
Acknowledgements
DMA is recipient of a Joan Rodés award Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation (JR22/00002). This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102046.
Appendix A. Supplementary data
References
- 1.Mokdad A.H., Marks J.S., Stroup D.F., Gerberding J.L. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 2.Axley P.D., Richardson C.T., Singal A.K. Epidemiology of alcohol consumption and societal burden of alcoholism and alcoholic liver disease. Clin Liver Dis. 2019;23(1):39–50. doi: 10.1016/j.cld.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Grant B.F., Chou S.P., Saha T.D., et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry. 2017;74(9):911–923. doi: 10.1001/jamapsychiatry.2017.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuckit M.A. Alcohol-use disorders. Lancet. 2009;373(9662):492–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- 5.Kosten T.R., O'Connor P.G. Management of drug and alcohol withdrawal. N Engl J Med. 2003;348(18):1786–1795. doi: 10.1056/NEJMra020617. [DOI] [PubMed] [Google Scholar]
- 6.Wood E., Albarqouni L., Tkachuk S., et al. Will this hospitalized patient develop severe alcohol withdrawal syndrome?: the rational clinical examination systematic review. JAMA. 2018;320(8):825–833. doi: 10.1001/jama.2018.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seitz H.K.,, Bataller R., Cortez-Pinto H., et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4(1):16. doi: 10.1038/s41572-018-0014-7. [DOI] [PubMed] [Google Scholar]
- 8.Rachakonda V., Bataller R., Duarte-Rojo A. Recent advances in alcoholic hepatitis. Frontline Gastroenterol. 2020;9 doi: 10.12688/f1000research.20394.1. F1000Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gougol A., Clemente-Sanchez A., Argemi J., Bataller R. Alcoholic hepatitis . Clin Liver Dis. 2021;18(2):90–95. doi: 10.1002/cld.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steel T.L., Afshar M., Edwards S., et al. Research needs for inpatient management of severe alcohol withdrawal syndrome: an official American thoracic society research statement. Am J Respir Crit Care Med. 2021;204(7):e61–e87. doi: 10.1164/rccm.202108-1845ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saitz R., O'Malley S.S. Pharmacotherapies for alcohol abuse. Withdrawal and treatment. Med Clin North Am. 1997;81(4):881–907. doi: 10.1016/s0025-7125(05)70554-x. [DOI] [PubMed] [Google Scholar]
- 12.Steel T.L., Malte C.A., Bradley K.A., Hawkins E.J. Use of electronic health record data to estimate the probability of alcohol withdrawal syndrome in a national cohort of hospitalized Veterans. J Addict Med. 2021;15(5):376–382. doi: 10.1097/ADM.0000000000000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singal A.K., Bataller R., Ahn J., Kamath P.S., Shah V.H. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol. 2018;113(2):175–194. doi: 10.1038/ajg.2017.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crabb D.W., Im G.Y., Szabo G., Mellinger J.L., Lucey M.R. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American association for the study of liver diseases. Hepatology. 2020;71(1):306–333. doi: 10.1002/hep.30866. [DOI] [PubMed] [Google Scholar]
- 15.Bajaj J.S., Cordoba J., Mullen K.D., et al. Review article: the design of clinical trials in hepatic encephalopathy--an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33(7):739–747. doi: 10.1111/j.1365-2036.2011.04590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustot T., Jalan R. Acute-on-chronic liver failure in patients with alcohol-related liver disease. J Hepatol. 2019;70(2):319–327. doi: 10.1016/j.jhep.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Vaishnav A., Lutsep H.L. GABA agonist: clomethiazole. Curr Med Res Opin. 2002;18(Suppl 2):s5–s8. doi: 10.1185/030079902125000651. [DOI] [PubMed] [Google Scholar]
- 18.Elsing C., Stremmel W., Grenda U., Herrmann T. Gamma-hydroxybutyric acid versus clomethiazole for the treatment of alcohol withdrawal syndrome in a medical intensive care unit: an open, single-center randomized study. Am J Drug Alcohol Abuse. 2009;35(3):189–192. doi: 10.1080/00952990902933852. [DOI] [PubMed] [Google Scholar]
- 19.Bahji A., Bach P., Danilewitz M., et al. Comparative efficacy and safety of pharmacotherapies for alcohol withdrawal: a systematic review and network meta-analysis. Addiction. 2022;117(10):2591–2601. doi: 10.1111/add.15853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrio E., Tomé S., Rodríguez I., et al. Liver disease in heavy drinkers with and without alcohol withdrawal syndrome. Alcohol Clin Exp Res. 2004;28(1):131–136. doi: 10.1097/01.ALC.0000106301.39746.EB. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed N., Kuo Y. Risk of alcohol withdrawal syndrome in hospitalized trauma patients: a national data analysis. Injury. 2022;53(1):44–48. doi: 10.1016/j.injury.2021.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Saitz R., Mayo-Smith M.F., Roberts M.S., Redmond H.A., Bernard D.R., Calkins D. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA. 1994;272(7):519–523. [PubMed] [Google Scholar]
- 23.Daeppen J.B., Gache P., Landry U., et al. Symptom-triggered vs. fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117–1121. doi: 10.1001/archinte.162.10.1117. [DOI] [PubMed] [Google Scholar]
- 24.Ismail M.F., Doherty K., Bradshaw P., O’Sullivan I., Cassidy E.M. Symptom-triggered therapy for assessment and management of alcohol withdrawal syndrome in the emergency department short-stay clinical decision unit. Emerg Med J. 2019;36(1):18–21. doi: 10.1136/emermed-2017-206997. [DOI] [PubMed] [Google Scholar]
- 25.Cassidy E.M., O’Sullivan I., Bradshaw P., Islam T., Onovo C. Symptom-triggered benzodiazepine therapy for alcohol withdrawal syndrome in the emergency department: a comparison with the standard fixed dose benzodiazepine regimen. Emerg Med J. 2012;29(10):802–804. doi: 10.1136/emermed-2011-200509. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan J.T., Sykora K., Schneiderman J., Naranjo C.A., Sellers E.M. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84(11):1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 27.Underwood K., Stupart D., Morgan F.H., et al. Can the alcohol withdrawal scale be applied to post-operative patients? ANZ J Surg. 2022;92(6):1377–1381. doi: 10.1111/ans.17334. [DOI] [PubMed] [Google Scholar]
- 28.Long B., Koyfman A. Clinical mimics: an emergency medicine-focused review of sepsis mimics. J Emerg Med. 2017;52(1):34–42. doi: 10.1016/j.jemermed.2016.07.102. [DOI] [PubMed] [Google Scholar]
- 29.Eloma A.S., Tucciarone J.M., Hayes E.M., Bronson B.D. Evaluation of the appropriate use of a CIWA-Ar alcohol withdrawal protocol in the general hospital setting. Am J Drug Alcohol Abuse. 2018;44(4):418–425. doi: 10.1080/00952990.2017.1362418. [DOI] [PubMed] [Google Scholar]
- 30.Hecksel K.A., Bostwick J.M., Jaeger T.M., Cha S.S. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83(3):274–279. doi: 10.4065/83.3.274. [DOI] [PubMed] [Google Scholar]
- 31.Michelena J., Altamirano J., Abraldes J.G., et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology. 2015;62(3):762–772. doi: 10.1002/hep.27779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prakash R., Mullen K.D. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2010;7(9):515–525. doi: 10.1038/nrgastro.2010.116. [DOI] [PubMed] [Google Scholar]
- 33.Grønbæk L., Watson H., Vilstrup H., Jepsen P. Benzodiazepines and risk for hepatic encephalopathy in patients with cirrhosis and ascites. United European Gastroenterol J. 2018;6(3):407–412. doi: 10.1177/2050640617727179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amato L., Minozzi S., Vecchi S., Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063. doi: 10.1002/14651858.CD005063.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ntais C., Pakos E., Kyzas P., Ioannidis J.P. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2005;(3):CD005063. doi: 10.1002/14651858.CD005063.pub2. [DOI] [PubMed] [Google Scholar]
- 36.Rudler M., Weiss N., Bouzbib C., Thabut D. Diagnosis and management of hepatic encephalopathy. Clin Liver Dis. 2021;25(2):393–417. doi: 10.1016/j.cld.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Haj M., Rockey D.C. Ammonia levels do not guide clinical management of patients with hepatic encephalopathy caused by cirrhosis. Am J Gastroenterol. 2020;115(5):723–728. doi: 10.14309/ajg.0000000000000343. [DOI] [PubMed] [Google Scholar]
- 38.Bajaj J.S., Nagy L.E. Natural history of alcohol-associated liver disease: understanding the changing landscape of pathophysiology and patient care. Gastroenterology. 2022;163(4):840–851. doi: 10.1053/j.gastro.2022.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith J.T., Sage M., Szeto H., et al. Outcomes after implementation of a benzodiazepine-sparing alcohol withdrawal order set in an integrated health care system. JAMA Netw Open. 2022;5(2) doi: 10.1001/jamanetworkopen.2022.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson J.A., Suelzer C.J., Eckert G.J., Zhou X.H., Dittus R.S. Risk factors for delirium tremens development. J Gen Intern Med. 1996;11(7):410–414. doi: 10.1007/BF02600188. [DOI] [PubMed] [Google Scholar]
- 41.Deng Z., Jin J., Li M., Wang S., Ma Y., Zheng Q. Alcohol withdrawal is associated with worse outcomes in patients undergoing primary total knee or total hip arthroplasty. J Arthroplasty. 2021;36(12):3870–3877.e5. doi: 10.1016/j.arth.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Vigouroux A., Garret C., Lascarrou J.B., et al. Alcohol withdrawal syndrome in ICU patients: clinical features, management, and outcome predictors. PLoS One. 2021;16(12) doi: 10.1371/journal.pone.0261443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sychla H., Gründer G., Lammertz S.E. Comparison of clomethiazole and diazepam in the treatment of alcohol withdrawal syndrome in clinical practice. Eur Addict Res. 2017;23(4):211–218. doi: 10.1159/000480380. [DOI] [PubMed] [Google Scholar]
- 44.Bonnet U., Lensing M., Specka M., Scherbaum N. Comparison of two oral symptom-triggered pharmacological inpatient treatments of acute alcohol withdrawal: clomethiazole vs. clonazepam. Alcohol Alcohol. 2011;46(1):68–73. doi: 10.1093/alcalc/agq081. [DOI] [PubMed] [Google Scholar]
- 45.Maldonado J.R., Sher Y., Das S., et al. Prospective validation study of the prediction of alcohol withdrawal severity scale (PAWSS) in medically ill inpatients: a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol Alcohol. 2015;50(5):509–518. doi: 10.1093/alcalc/agv043. [DOI] [PubMed] [Google Scholar]
- 46.Maldonado J.R., Sher Y., Ashouri J.F., et al. The “Prediction of Alcohol Withdrawal Severity Scale” (PAWSS): systematic literature review and pilot study of a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol. 2014;48(4):375–390. doi: 10.1016/j.alcohol.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Spies C.D., Dubisz N., Funk W., et al. Prophylaxis of alcohol withdrawal syndrome in alcohol-dependent patients admitted to the intensive care unit after tumour resection. Br J Anaesth. 1995;75(6):734–739. doi: 10.1093/bja/75.6.734. [DOI] [PubMed] [Google Scholar]
- 48.Maldonado J.R. Novel algorithms for the prophylaxis and management of alcohol withdrawal syndromes-beyond benzodiazepines. Crit Care Clin. 2017;33(3):559–599. doi: 10.1016/j.ccc.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 49.Dobrydnjov I., Axelsson K., Berggren L., Samarütel J., Holmström B. Intrathecal and oral clonidine as prophylaxis for postoperative alcohol withdrawal syndrome: a randomized double-blinded study. Anesth Analg. 2004;98(3):738–744. doi: 10.1213/01.ane.0000099719.97261.da. [DOI] [PubMed] [Google Scholar]
- 50.Herrán A., Vázquez-Barquero J.L. Treating alcohol dependence. Chlormethiazole is widely used in Europe. BMJ. 1997;315(7120):1466. [PMC free article] [PubMed] [Google Scholar]
- 51.Franz M., Dlabal H., Kunz S., Ulferts J., Gruppe H., Gallhofer B. Treatment of alcohol withdrawal: tiapride and carbamazepine versus clomethiazole. A pilot study. Eur Arch Psychiatry Clin Neurosci. 2001;251(4):185–192. doi: 10.1007/s004060170039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.