Abstract
Background
Influenza vaccination is recommended for adults regardless of human immunodeficiency virus (HIV) status. There may be facilitators or barriers to vaccinating people with HIV (PWH) that differ from people without HIV (PWoH). We sought to describe the uptake of influenza vaccination by HIV status and identify factors associated with vaccination.
Methods
We abstracted data from the electronic health records of PWH and PWoH in Kaiser Permanente Northern California during 6 influenza seasons (2013–2018). We determined vaccination uptake and used Poisson regression models to evaluate factors associated with vaccination in PWH and PWoH.
Results
9272 PWH and 194 393 PWoH matched by age, sex, and race/ethnicity were included (mean age: 48 vs 49 years; men: 91% vs 90%; White race: 53% for both groups). PWH were more likely to receive the influenza vaccine (65–69% across years for PWH and 37–41% for PWoH) with an adjusted risk ratio for all years of 1.48 (95% CI: 1.46–1.50). For PWH, lower vaccination uptake was associated with several factors that suggested more complex health needs, such as lower CD4 cell counts, higher HIV viral loads, prior depression diagnoses, having Medicare insurance, and having a higher number of comorbidities. Associations with vaccination uptake were attenuated in PWH, compared with PWoH, for smoking, alcohol, and demographic factors.
Conclusions
PWH had an almost 50% higher uptake of influenza vaccination than PWoH, possibly reflecting greater engagement with the healthcare system. We also found that PWH with more complex health needs had reduced vaccination uptake. Findings may inform outreach strategies to increase influenza vaccination in PWH.
Keywords: HIV, influenza vaccine, epidemiology, primary care, disparities
In a large cohort study of Kaiser Permanente Northern California members, people with HIV had a 50% higher influenza vaccine uptake than people without HIV. Factors associated with lower rates of vaccination were also identified.
Vaccination is an essential component of disease prevention for people with human immunodeficiency virus (HIV; PWH) and people without HIV (PWoH). While vaccine efficacy and safety have been demonstrated in PWH, including for the influenza vaccine, differences in vaccination rates by HIV status have been described in limited detail [1–3]. One reason it is often challenging to compare vaccination rates by HIV status is because the Centers for Disease Control and Prevention's (CDC’s) vaccination recommendations sometimes vary by vaccine and HIV status. However, the influenza vaccine is recommended for all adults regardless of HIV status, providing the opportunity to learn about vaccination differences between PWH and PWoH. Additionally, influenza is a preventable disease with substantial morbidity and mortality in both PWH and PWoH [4–6]. Understanding factors associated with influenza vaccination uptake may lead to improved strategies for increasing uptake of all vaccines.
For PWoH and PWH, the CDC recommends that all people aged 6 months and older without contraindications receive the annual influenza vaccine [7]. The Healthy People 2020 and subsequent Healthy People 2030 policy goals aim for 70% of eligible people to receive the influenza vaccine; however, only 50% of the US adult population was vaccinated during the 2020–2021 influenza season [8, 9]. The CDC HIV Medical Monitoring Project reports that 75–85% of PWH received the influenza vaccine during the 2013–2018 influenza seasons [10–15]. A few studies, however, have reported lower influenza vaccination rates for PWH in the United States, with ranges from 24% to 51% between 1990 and 2017 [16–18]. These variable ranges suggest that the true proportion of PWH in the United States who receive the influenza vaccine is unknown.
Numerous factors are associated with influenza vaccine uptake for PWoH and PWH. For PWoH, the CDC reports differences in vaccination rates by sex, race/ethnicity, and location [19]. Prior studies for PWoH, some of which were conducted in non-US populations, have also found associations between influenza vaccination and specific health comorbidities, including lower vaccination rates for people who smoked and people reporting unhealthy alcohol use [19–31]. Fewer studies evaluating these associated factors have been conducted for PWH, but some associations were found [16–18, 32, 33].
To better understand influenza vaccination uptake and factors associated with vaccination use by PWH, we evaluated electronic health record (EHR) data from Kaiser Permanente Northern California (KPNC). We compared the rates of influenza vaccination between adult PWH and race-, age-, and sex-matched PWoH in KPNC between the 2013 and 2018 influenza seasons. Additionally, we used EHR data to identify disparities in vaccination rates by demographics, key comorbidities, HIV-specific markers, smoking, alcohol use, and number of outpatient visits. We hypothesized that influenza vaccination rates are higher in PWH compared with PWoH and that there are disparities by participant characteristics despite an integrated healthcare system model with stably insured people.
METHODS
Population and Study Design
The source population for this study was adult PWH and PWoH (≥18 years of age) in KPNC, a large, private, nonprofit integrated health system providing care to more than 4 million members. Participants were active KPNC members between 1 July 2013 (rollout of routine unhealthy alcohol-use screening in KPNC) and 31 December 2021. Persons with HIV were identified from the KPNC HIV registry (described previously), which includes all known cases of HIV infection among KPNC members [34]. Persons without HIV were frequency matched 20:1 to PWH by age (5-year categories), sex, race/ethnicity, and observation start year (2013–2021). The source population included 15 604 PWH and 312 080 PWoH.
The final study population was limited to PWH and PWoH from the source population with active membership during at least 6 of the 7 months of at least 1 of the 2013 to 2018 influenza seasons, defined as 1st September to 31st March of the following year. The start of follow-up for each influenza season was defined as the latest of the following: start of influenza season (1 September YYYY), KPNC member enrollment start date, or 18th birthday. Participants contribute to more than 1 influenza season if they met the criteria for multiple influenza seasons. Participants were followed until evidence of vaccination in that season, or until they died, were lost to follow-up, or reached the end of the influenza season (31 March YYYY + 1).
Setting
Kaiser Permanente Northern California uses population management strategies to provide influenza vaccines to members. Multiple locations offer influenza vaccination in a single campus: primary care clinics, injection clinic, and seasonal influenza vaccine clinics. Population management tools, like a health maintenance alert system and mass electronic messaging, notify healthcare personnel and members when members are due for vaccination.
Measures
The main outcome of interest was influenza vaccine administration during the 2013 to 2018 influenza seasons. Participant influenza vaccination status was ascertained from the Kaiser Immunization Tracking System, which includes outside vaccinations reported by patients. The primary exposure of interest was HIV status. Additional key variables extracted from the EHR were age at the start of follow-up, sex, race/ethnicity (White, Black, Hispanic, other, unknown), alcohol use, smoking status (current or noncurrent), depression (Patient Health Questionnaire-9 [PHQ-9] ≥10; International Classification of Diseases, Ninth Revision [ICD-9]: 296.2x, 296.3x, 296.82, 298.0, 300.4, 301.12, 311; or International Classification of Diseases, Tenth Revision [ICD-10]: F32.x, F33.x, F34.1), calendar year (based on the start of influenza season), neighborhood deprivation index (NDI) score (categorized into quartiles), insurance type (commercial, Medicare, Medicaid, other), number of outpatient care visits in the 1 year prior to the start of influenza season, body mass index (BMI), and the Charlson comorbidity score in the 1 year prior to the start of influenza season. Kaiser Permanente Northern California medical assistants screen annually for unhealthy alcohol use [35]. Unhealthy alcohol use, based on clinical guidelines, was defined as either reporting any days of drinking 4+ drinks for women and men over age 65 or 5+ drinks for men aged 65 years and younger, or an average of 8+/15+ drinks in 1 week in the last 90 days [36]. Alcohol use was categorized as none, moderate (any use other than unhealthy), frequent unhealthy use (5+ days in the last 90 days with 4+/5+ drinks), and infrequent unhealthy use (<5 days in the last 90 days with 4+/5+ drinks or an average of 8+/15+ drinks in 1 week in the last 90 days). HIV-specific factors extracted from the EHR were HIV transmission risk status, AIDS status at diagnosis, CD4 cell count in the 1 year prior to the start of influenza season, and HIV viral load in the 1 year prior to the start of influenza season. Outpatient encounters include all in-person visits to a KPNC clinic, laboratory, or pharmacy.
Statistical Analysis
We first compared demographics (race/ethnicity, age, and sex) and other patient factors (alcohol use, smoking status, depression, calendar year, NDI, insurance, outpatient visits, and BMI) between PWH and PWoH using Pearson's chi-square test for categorical variables and t tests for continuous variables. Crude and adjusted risk ratios (RRs) were obtained from Poisson regression models with robust standard errors to account for repeated measures (participants could contribute to multiple influenza seasons) using Proc Genmod in SAS (version 9.4; SAS Institute, Cary, NC, USA). Adjusted models included terms for HIV status, race/ethnicity, age, sex, alcohol use, smoking status, calendar year, NDI, depression, insurance type, outpatient visits, and BMI. In separate models for each covariate, we included interaction terms for HIV × race/ethnicity, HIV × age group, HIV × sex, HIV × smoking status, HIV × alcohol use, HIV × depression, HIV × year, HIV × insurance, HIV × outpatient visits, and HIV × BMI. Finally. we ran a separate model for only PWH with additional adjustments for the HIV-specific measures (risk status, AIDS status, CD4 cell count, and viral load). Missing laboratory values were included as their own category.
Analyses were performed with SAS (version 9.4; SAS Institute). All statistical tests were 2-sided, and significance was defined as P < .05. The study was approved by KPNC and the University of California, San Francisco, Institutional Review Boards and included waivers of written informed consent.
RESULTS
The study sample included 9272 PWH and 194 393 PWoH who contributed a mean of 4 influenza seasons per subject and had similar matching characteristics: mean age at baseline (48 vs 49 years), men (91% vs 90%), and White race (53%) (Table 1). Persons with HIV, compared with PWoH, had a more frequent history of depression (38% vs 16%) and 20 or more outpatient encounters in the prior year (22% vs 8%). Persons with HIV, compared with PWoH, had a lower prevalence of overweight/obesity (60% vs 77%). Persons with HIV predominantly reported men who have sex with men (MSM) as their HIV risk factor (70%), most with CD4+ T-cell counts of 500 cells/µL or more (65%), and most with HIV RNA less than 200 copies/mL (86%).
Table 1.
Baseline Characteristics of Persons Living With and Without HIV
| PWH (n = 9272) |
PWoH (n = 194 393) |
P | |
|---|---|---|---|
| Age (years) | <.001 | ||
| 18–29 | 835 (9.0%) | 16 724 (8.6%) | |
| 30–49 | 3768 (40.6%) | 79 416 (40.9%) | |
| 50–64 | 3835 (41.4%) | 74 898 (38.5%) | |
| 65+ | 834 (9.0%) | 23 355 (12.0%) | |
| Men | 8418 (90.8%) | 175 085 (90.1%) | .023 |
| Race/ethnicity | <.001 | ||
| Black | 1434 (15.5%) | 31 942 (16.4%) | |
| Hispanic | 1720 (18.6%) | 36 308 (18.7%) | |
| White | 4870 (52.5%) | 102 771 (52.9%) | |
| Other | 932 (10.1%) | 20 160 (10.4%) | |
| Unknown | 316 (3.4%) | 3212 (1.7%) | |
| Alcohol usea | <.001 | ||
| None | 5349 (57.7%) | 99 853 (51.4%) | |
| Moderate | 2971 (32.0%) | 70 808 (36.4%) | |
| Unhealthy—infrequent | 604 (6.5%) | 15 324 (7.9%) | |
| Unhealthy—frequent | 264 (2.8%) | 7458 (3.8%) | |
| Smoking status | <.001 | ||
| Current | 835 (9.0%) | 13 612 (7.0%) | |
| Not current | 8437 (91.0%) | 180 781 (93.0%) | |
| Depression (PHQ-9 or ICD)b | 3492 (37.7%) | 31 969 (16.4%) | <.001 |
| Influenza season start yearc | <.001 | ||
| 2013 | 1478 (15.9%) | 26 155 (13.5%) | |
| 2014 | 3534 (38.1%) | 76 157 (39.2%) | |
| 2015 | 1433 (15.5%) | 31 835 (16.4%) | |
| 2016 | 977 (10.5%) | 18 158 (9.3%) | |
| 2017 | 663 (7.2%) | 12 407 (6.4%) | |
| 2018 | 1187 (12.8%) | 29 681 (15.3%) | |
| NDI quartile | <.001 | ||
| Quartile 1 | 2682 (28.9%) | 47 601 (24.5%) | |
| Quartile 2 | 1894 (20.4%) | 48 750 (25.1%) | |
| Quartile 3 | 2113 (22.8%) | 48 301 (24.8%) | |
| Quartile 4 | 2583 (27.9%) | 49 741 (25.6%) | |
| Insurance | <.001 | ||
| Commercial | 6986 (75.3%) | 160 163 (82.4%) | |
| Medicare | 1821 (19.6%) | 28 116 (14.5%) | |
| Medicaid | 353 (3.8%) | 4733 (2.4%) | |
| Other | 112 (1.2%) | 1381 (0.7%) | |
| Outpatient encountersd | <.001 | ||
| 0–9 | 3956 (42.7%) | 145 364 (74.8%) | |
| 10–19 | 3294 (35.5%) | 33 242 (17.1%) | |
| 20+ | 2022 (21.8%) | 15 787 (8.1%) | |
| BMI (kg/m2) | <.001 | ||
| Underweight/normal: <25.0 | 3653 (39.4%) | 41 873 (21.5%) | |
| Overweight/obesity: ≥25.0 | 5556 (59.9%) | 149 996 (77.2%) | |
| Charlson comorbidity scoree | <.001 | ||
| 0 | 6495 (70.0%) | 141 293 (72.7%) | |
| 1 | 1376 (14.8%) | 30 471 (15.7%) | |
| 2+ | 1401 (15.1%) | 22 629 (11.6%) | |
| HIV measures, n (%) | |||
| Risk status | |||
| MSM | 6531 (70.4%) | … | |
| Heterosexual | 1301 (14.0%) | … | |
| IDU | 653 (7.0%) | … | |
| Other | 81 (0.9%) | … | |
| Unknown | 706 (7.6%) | … | |
| CDC clinical AIDSf | |||
| No AIDS | 5332 (57.5%) | … | |
| AIDS | 3940 (42.5%) | … | |
| CD4 cells/mm3g | |||
| <200 | 370 (4.0%) | … | |
| 200–349 | 785 (8.5%) | … | |
| 350–499 | 1402 (15.1%) | … | |
| ≥500 | 6062 (65.4%) | … | |
| No test in period | 653 (7.0%) | … | |
| Viral load copies/mLg | |||
| <200 | 7979 (86.1%) | … | |
| ≥200 | 729 (7.9%) | … | |
| No test in period | 564 (6.1%) | … |
Abbreviations: BMI, body mass index; CDC, Centers for Disease Control and Prevention; HIV, human immunodeficiency virus; ICD, International Classification of Diseases; IDU, injection drug use; MSM, men who have sex with men; NDI, neighborhood deprivation index; PHQ-9, Patient Health Questionnaire-9; PWH, people with HIV; PWoH, people without HIV.
Moderate = any alcohol use other than unhealthy use. Unhealthy—infrequent = <5 days in the last 90 days with 4+/5+ drinks for women (and men over age 65)/men age 65 and younger or an average of 8+/15+ drinks in 1 week in the last 90 days. Unhealthy—frequent = 5+ days in the last 90 days with 4+/5+ drinks.
PHQ-9 >10; ICD-9: 296.2x, 296.3x, 296.82, 298.0, 300.4, 301.12, 311; or ICD-10: F32.x, F33.x, F34.1.
Defined as influenza season start year (ie, 1 September YYYY).
Number of outpatient care visits in the 1 year prior to the start of influenza season, including all in-person visits to a Kaiser Permanente Northern California clinic, laboratory, or pharmacy.
In the 1 year prior to the start of influenza season.
At the time of HIV diagnosis.
Measured in the 1 year prior to the start of influenza season; if multiple labs were obtained, the lab on the date closest to the start of influenza season was recorded.
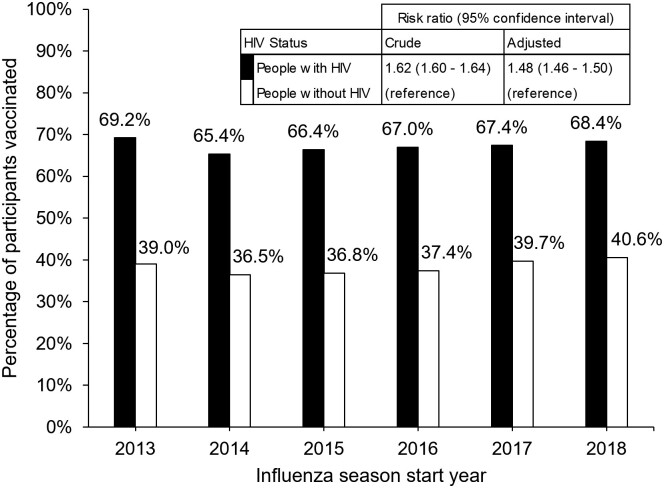
As shown in Figure 1, there were differences in the uptake of influenza vaccination by HIV status. Persons with HIV were more likely to receive the influenza vaccine during each of the 2013–2018 influenza seasons (2013: 69% vs 39%; 2014: 65% vs 37%; 2015: 66% vs 37%; 2016: 67% vs 37%; 2017: 67% vs 40%; 2018: 68% vs 41%) with an adjusted RR of 1.48 (1.46–1.50; P < .001).
Figure 1.
Uptake of influenza vaccination by HIV status and influenza season start year. Bar chart displays the percentage of people with HIV (black bars) and people without HIV (white bars) who received the influenza vaccine by influenza season start year (2013–2018). Crude and adjusted risk ratios comparing influenza vaccination uptake by HIV status for all years combined were obtained from Poisson regression models with robust standard errors. Adjusted models included terms for HIV status, race/ethnicity, age, sex, alcohol use, smoking status, calendar year, neighborhood deprivation index, depression, insurance type, outpatient visits, and body mass index. Abbreviation: HIV, human immunodeficiency virus.
Table 2 shows differences in influenza vaccination uptake stratified by HIV status. Black (adjusted RR: .75; 95% confidence interval [CI]: .74–.77) and Hispanic (.91; .90–.93) PWoH were less likely to receive the influenza vaccine compared with White PWoH. The effect was attenuated among Black (.89; .85–.93) PWH, but vaccination was more likely for Hispanic PWH compared with White PWH (1.14; 1.11–1.18). Older participants among both PWoH (30–49 years: 1.63; 1.58–1.68; 50–64 years: 2.20; 2.13–2.27; ≥65 years: 2.73; 2.64–2.83) and PWH (30–49 years: 1.38; 1.28–1.49; 50–64 years: 1.55; 1.44–1.67; ≥65 years: 1.42; 1.32–1.53) were increasingly more likely to get vaccinated compared with PWoH aged 18–29 years old, with attenuated results for PWH. Female PWoH were more likely to receive the influenza vaccine compared with male PWoH (1.11; 1.09–1.13), with attenuated results for PWH.
Table 2.
Adjusted Risk Ratios for Factors Associated With Influenza Vaccination Uptake, Stratified by HIV Status
| PWH | PWoH | ||||
|---|---|---|---|---|---|
| Risk Factor | Adjusteda Risk Ratio (95% CI) | P | Adjusteda Risk Ratio (95% CI) | P | Type 3 Effect, P |
| Race | <.001 | ||||
| Black | .89 (.85–.93) | <.001 | .75 (.74–.77) | <.001 | |
| Hispanic | 1.14 (1.11–1.18) | <.001 | .91 (.90–.93) | <.001 | |
| Other | 1.18 (1.13–1.23) | <.001 | 1.20 (1.18–1.22) | <.001 | |
| Unknown | 1.12 (1.04–1.20) | .001 | .87 (.84–.91) | <.001 | |
| White | … | (ref) | … | (ref) | |
| Age group (years) | <.001 | ||||
| 18–29 | … | (ref) | … | (ref) | |
| 30–49 | 1.38 (1.28–1.49) | <001 | 1.63 (1.58–1.68) | <.001 | |
| 50–64 | 1.55 (1.44–1.67) | <.001 | 2.20 (2.13–2.27) | <.001 | |
| 65+ | 1.42 (1.32–1.53) | <.001 | 2.73 (2.64–2.83) | <.001 | |
| Sex | <.001 | ||||
| Female | 1.00 (.96–1.05) | .89 | 1.11 (1.09–1.12) | <.001 | |
| Male | … | (ref) | … | (ref) | |
| Smoking status | <.001 | ||||
| Current | .92 (.87–.98) | .006 | .68 (.67–.70) | <.001 | |
| Not current | … | (ref) | … | (ref) | |
| Alcohol use | <.001 | ||||
| None | .97 (.95–.99) | .009 | .97 (.96–.98) | <.001 | |
| Moderate | … | (ref) | … | (ref) | |
| Unhealthy infrequent | 1.00 (.96–1.05) | .99 | .93 (.92–.95) | <.001 | |
| Unhealthy frequent | 1.03 (.95–1.11) | .54 | .81 (.79–.83) | <.001 | |
| Depression | <.001 | ||||
| Yes | .95 (.93–.98) | <.001 | 1.04 (1.03–1.06) | <.001 | |
| No | … | (ref) | … | (ref) | |
| Calendar year | <.001 | ||||
| 2013 | … | (ref) | … | (ref) | |
| 2014 | .95 (.92–.98) | .003 | .93 (.92–.94) | <.001 | |
| 2015 | .96 (.93–1.00) | .032 | .91 (.90–.92) | <.001 | |
| 2016 | .96 (.93–.99) | .014 | .89 (.88–.90) | <.001 | |
| 2017 | .94 (.91–.97) | <.001 | .91 (.90–.92) | <.001 | |
| 2018 | .96 (.93–1.00) | .027 | .93 (.92–.94) | <.001 | |
| NDI quartile | <.001 | ||||
| Quartile 1 | … | (ref) | … | (ref) | |
| Quartile 2 | .96 (.93–.99) | .010 | .94 (.93–.95) | <.001 | |
| Quartile 3 | .99 (.96–1.02) | .66 | .91 (.90–.92) | <.001 | |
| Quartile 4 | .99 (.96–1.02) | .37 | .86 (.85–.87) | <.001 | |
| Insurance | <.001 | ||||
| Medicare | .90 (.88–.93) | <.001 | 1.30 (1.28–1.32) | <.001 | |
| Medicaid | .98 (.92–1.04) | .48 | 1.04 (1.01–1.08) | .014 | |
| Commercial | … | (ref) | … | (ref) | |
| Outpatient encounters | <.001 | ||||
| 0–9 | … | (ref) | … | (ref) | |
| 10–19 | 1.07 (1.04–1.09) | <.001 | 1.27 (1.26–1.28) | <.001 | |
| 20+ | 1.06 (1.04–1.09) | <.001 | 1.37 (1.36–1.39) | <.001 | |
| BMI (kg/m2) | <.001 | ||||
| ≥25.0 | 1.06 (1.04–1.08) | <.001 | .99 (.98–1.00) | .15 | |
| <25.0 | … | (ref) | … | (ref) | |
| Charlson comorbidity score | <.001 | ||||
| 0 | … | (ref) | … | (ref) | |
| 1 | .96 (.93–.98) | .001 | 1.16 (1.14–1.17) | <.001 | |
| 2+ | .86 (.84–.88) | <.001 | 1.17 (1.16–1.18) | <.001 | |
Abbreviations: BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; NDI, neighborhood deprivation index; PWH, people with HIV; PWoH, people without HIV; ref, reference.
Adjusted for HIV status and all covariates in the table. In separate models for each covariate in the table, we included interaction terms for HIV status and the measure of interest.
Persons without HIV with 10–19 outpatient encounters (adjusted RR: 1.27; 95% CI: 1.26–1.28) and 20 or more outpatient encounters (1.37; 1.36–1.39) in the year prior to the start of influenza season were more likely to receive the influenza vaccine compared with PWoH with 0–9 outpatient encounters, but this effect was attenuated among PWH (10–19 outpatient encounters: 1.07; 1.04–1.09; ≥20 outpatient encounters: 1.06; 1.04–1.09). Similarly, PWoH with a higher Charlson comorbidity score were more likely to receive the influenza vaccine compared with PWoH with a Charlson comorbidity score of 0 (score 1: 1.16; 1.14–1.17; score ≥2: 1.17; 1.16–1.18), but this effect was in the opposite direction for PWH (score 1: .96; .93–.98; score ≥2: .86; .84–.88).
Persons without HIV who reported no alcohol use (adjusted RR: .97; 95% CI: .96–.98), less frequent unhealthy alcohol use (.93; .92–.95), and more frequent unhealthy alcohol use (.81; .79–.83) were all less likely to receive the influenza vaccine compared with PWoH who reported moderate alcohol use. Persons with HIV who reported no alcohol use (.97; .95–.99) were also less likely to receive the influenza vaccine compared with PWH who reported moderate alcohol use. Unlike PWoH, PWH who reported less frequent or more frequent unhealthy alcohol use were equally likely to receive the influenza vaccine compared with those reporting moderate alcohol use. People who smoked were less likely to get vaccinated compared with people who did not smoke among both PWoH (.68; .67–.70) and PWH (.92; .87–.98), with attenuated results for PWH.
For PWH, some HIV-specific factors of interest were also associated with influenza vaccination (Table 3). Persons with HIV who reported HIV transmission risk status as heterosexual (adjusted RR: .95; 95% CI: .91–.99) were less likely to receive the influenza vaccine compared with MSM. Compared with PWH with a CD4 count of 500 cells/mm3 or greater, those with a CD4 count of 200–349 cells/mm3 (.96; .92–.99) and less than 200 cells/mm3 (.89; .84–.95) were less likely to receive the influenza vaccine. Similarly, PWH with a HIV viral load of 200 copies/mL or greater were less likely to receive the influenza vaccine compared with those with a HIV viral load of less than 200 copies (.81; .77–.86). AIDS status at diagnosis was not associated with vaccination uptake.
Table 3.
Adjusted Risk Ratios for HIV Risk Factors Associated With Influenza Vaccination Uptake Among Persons With HIV
| Adjusteda Risk Ratio (95% CI) | P | |
|---|---|---|
| Transmission risk status | ||
| Heterosexual | .95 (.91–.99) | .023 |
| IDU | .98 (.94–1.02) | .37 |
| Other | .86 (.74–1.01) | .058 |
| Unknown | .98 (.94–1.02) | .32 |
| MSM | … | (ref) |
| AIDS status at diagnosis | ||
| CDC AIDS | 1.02 (.99–1.04) | .18 |
| Pre-AIDS | … | (ref) |
| CD4 cell count | ||
| <200 cells/mm3 | .89 (.84–.95) | <.001 |
| 200–349 cells/mm3 | .96 (.92–.99) | .010 |
| 350–499 cells/mm3 | .98 (.96–1.01) | .16 |
| ≥500 cells/mm3 | … | (ref) |
| No test in period | 1.02 (.99–1.06) | 0.22 |
| HIV viral load | ||
| <200 | … | (ref) |
| ≥200 | .81 (.77–.86) | <.001 |
| No test in period | .77 (.73–.82) | <.001 |
Abbreviations: CDC, Centers for Disease Control and Prevention; CI, confidence interval; HIV, human immunodeficiency virus; IDU, injection drug use; MSM, men who have sex with men; ref, reference.
Adjusted for covariates in table as well as race/ethnicity, age, sex, smoking status, alcohol use, calendar year, neighborhood deprivation index, depression, insurance status, outpatient encounters, body mass index, and modified Charlson comorbidity score.
DISCUSSION
In a large cohort study of adult PWH and demographically matched PWoH with access to care, we found that PWH had an almost 50% higher uptake of influenza vaccination than PWoH. Few studies have directly compared PWH with PWoH on vaccination rates, especially the influenza vaccine. Although 1 study suggested that PWH had less intention of getting a coronavirus disease 2019 (COVID-19) vaccination than PWoH, the association was not maintained after adjustment for confounders [1]. In this cohort, PWH had a high vaccination rate approaching the CDC's estimates (75–85%) and a higher rate than reported in other studies (24–51%) [10–18]. Persons without HIV, however, had a lower rate than the national average (50%) [8]. The rate of vaccination for PWoH was similar to the CDC's estimate for those without high-risk conditions (37–40%). Thus, the PWoH in this study may better reflect a younger and healthy cohort that is less likely to be vaccinated compared with older, unhealthy adults [8]. While the vaccination uptake for PWH was high, neither cohort meets the Healthy People 2030 goal of 70% [8].
Kaiser Permanente Northern California implements a number of strategies designed to maximize annual influenza vaccination rates. Population management tools alert healthcare personnel and members when vaccinations are due. Additionally, KPNC offers vaccination at multiple locations, including walk-up clinics, to improve access. Furthermore, advice to get the vaccine is given at most outpatient encounters by a variety of healthcare personnel, such as physicians, medical assistants, case managers, and pharmacists. An important finding was that PWH, compared with PWoH, had a substantially higher number of outpatient encounters in the 1 year prior to the start of the influenza season. The higher number of encounters was expected since PWH engage with the clinic, pharmacy, and laboratory multiple times per year for routine care. Thus, PWH may have more opportunity to receive the influenza vaccine, and in fact, a higher number of outpatient encounters was associated with vaccination uptake. An important unmeasured factor that may further contribute to higher vaccination rates for PWH is the dedicated case managers at many HIV clinics who often facilitate engagement in care and following through with healthcare recommendations, including vaccination.
Many factors were identified that could be used for targeted outreach among PWH to further increase influenza vaccination rates, some of which are new findings. Importantly, PWH who were more medically complicated were less likely to be vaccinated, including those with higher Charlson comorbidity scores, CD4 count less than 350 cells/mm3, HIV viral load 200 copies/mL or higher, prior depression diagnoses, and Medicare insurance. In contrast, PWoH who had a higher Charlson comorbidity score, depression, and Medicare insurance were more likely to be vaccinated. The reason for this difference is unknown, but the prioritization of actively managed medical issues, such as HIV treatment and counseling, opportunistic infection prevention, and management of social determinants of health, during outpatient appointments may lead to less time to discuss routine vaccination for PWH. These findings align with those in other studies: higher vaccination rates were identified in those with lower HIV viral loads [17, 18, 32] and those with higher CD4 cell counts [18, 32], further suggesting that PWH who are less adherent to medications, or with reduced engagement in care, may be less likely to be vaccinated.
We identified other factors in PWH associated with reduced vaccination uptake, including those who were Black, reported current smoking, reported no alcohol use, and lived in neighborhoods with a lower NDI. One study similarly found that PWH with a lower educational level had a lower uptake of influenza vaccination [33]. In addition, for PWH, those who were Hispanic, being older than 18–29 years, being overweight/obese, or having a higher number of outpatient encounters were associated with a higher uptake of influenza vaccination. Associations of older age and a higher number of outpatient visits with a greater uptake of influenza vaccination were similarly found in other studies [17, 18, 32]. All of these findings were attenuated in PWH compared with PWoH, which suggests more uniform implementation of influenza vaccination recommendations in PWH than in PWoH in KPNC.
Interestingly, abstaining from alcohol use was associated with lower vaccine uptake; however, the effect was small and, in the context of a large sample size revealing many small differences, unlikely to be clinically relevant. Of greater interest, PWoH with unhealthy alcohol use were less likely to be vaccinated than those with moderate alcohol use, but this association was not seen in PWH. This may again be related to increased engagement with the medical system for routine HIV treatment, which may link them into further encounters for alcohol-use disorder treatment and result in more uniform implementation of vaccination recommendations. The association of smoking and reduced vaccination uptake was similarly attenuated in PWH. Influenza vaccination is important for people who smoke and use alcohol because using these substances is associated with increased influenza morbidity and mortality [37, 38]. Besides 1 study from France reporting no association of influenza vaccination with smoking in PWH, other studies have not assessed the relationship of influenza vaccination to smoking and alcohol use by PWH [32].
The study has some limitations. First, some factors, such as smoking and alcohol use were not collected systematically for research purposes. However, KPNC routinely assesses smoking and alcohol use at clinic visits; for example, 85% and 93% of PWoH and PWH, respectively, from the same source population had been screened for alcohol use. Second, the study may not be generalizable to all vaccines based on an individual's acceptance of influenza vaccination. Third, findings from this cohort of stably insured people may not be generalizable to underinsured or uninsured populations. However, almost 25% of the cohort had Medicare or Medicaid insurance and were relatively evenly distributed across the 4 NDI quartiles, suggesting applicability to a large portion of the US population. Additionally, prior research suggests similarity between KPNC members and the general population in Northern California [39].
Conclusions
In a large cohort study of stably insured KPNC adults, we found that PWH had an almost 50% higher uptake of receiving the influenza vaccine than PWoH. We believe that the higher uptake was driven by greater engagement in healthcare for PWH compared with PWoH. We also found that PWH with more complex health needs, including those with lower CD4 cell counts, detectable HIV viral loads, a greater number of comorbidities, Medicare insurance, and prior depression diagnoses, were associated with lower uptake of influenza vaccination. Combined, these findings generate opportunities to systematically target populations to increase influenza vaccination rates to reach the Healthy People 2030 target. Reassuringly, other demographic associations with vaccination uptake were attenuated in PWH compared with PWoH, demonstrating more uniform implementation of vaccine recommendations for PWH.
Contributor Information
Brandon M Imp, Department of Adult and Family Medicine, Kaiser Permanente Oakland Medical Center, Oakland, California, USA.
Tory Levine, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA.
Derek D Satre, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA; Department of Psychiatry and Behavioral Sciences, University of California San Francisco, San Francisco, California, USA.
Jacek Skarbinski, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA; Department of Infectious Diseases, Kaiser Permanente Oakland Medical Center, Oakland, California, USA.
Mitchell N Luu, Department of Adult and Family Medicine, Kaiser Permanente Oakland Medical Center, Oakland, California, USA.
Stacy A Sterling, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA; Department of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California, USA.
Michael J Silverberg, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA; Department of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California, USA; Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, California, USA.
Notes
Acknowledgments. The authors thank the people that contributed to this research.
Financial support. This work was supported by the National Institute on Alcohol Abuse and Alcoholism (grant numbers U01AA026230, K24AA025703) and the Kaiser Permanente Northern California Graduate Medical Education Program's HIV Medicine Fellowship (paid to B.I.).
References
- 1. Kaida A, Brotto LA, Murray MCM, et al. Intention to receive a COVID-19 vaccine by HIV status among a population-based sample of women and gender diverse individuals in British Columbia, Canada. AIDS Behav 2022; 26:2242–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang W, Sun H, Atiquzzaman M, Sou J, Anis AH, Cooper C. Influenza vaccination for HIV-positive people: systematic review and network meta-analysis. Vaccine 2018; 36:4077–86. [DOI] [PubMed] [Google Scholar]
- 3. Hechter RC, Qian L, Tartof SY, et al. Vaccine safety in HIV-infected adults within the vaccine safety datalink project. Vaccine 2019; 37:3296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention . Burden of influenza. 2022. Available at: https://www.cdc.gov/flu/about/burden/index.html. Accessed 20 July 2022.
- 5. Centers for Disease Control and Prevention . Upcoming 2020–2021 influenza season. 2021. Available at: https://www.cdc.gov/flu/season/faq-flu-season-2020-2021.htm. Accessed 20 July 2022.
- 6. Cohen C, Simonsen L, Sample J, et al. Influenza-related mortality among adults aged 25–54 years with AIDS in South Africa and the United States of America. Clin Infect Dis 2012; 55:996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 influenza season. MMWR Recomm Rep 2021; 70:1–28. Available at:https://www.cdc.gov/mmwr/volumes/70/rr/rr7005a1.htm. Accessed 20 July 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Healthy People 2030 . Increase the proportion of people who get the flu vaccine every year—IID-09. Available at: https://health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-proportion-people-who-get-flu-vaccine-every-year-iid-09. Accessed 20 July 2022.
- 9. Immunization and Infectious Diseases | Healthy People 2020. Available at: https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed 20 July 2022.
- 10. Bradley H, Frazier E, Huang P, et al. Behavioral and clinical characteristics of persons receiving medical care for HIV infection. Medical monitoring project, United States 2013 cycle (June 2013–May 2014). Available at: https://stacks.cdc.gov/view/cdc/94014. Accessed 6 December 2022. [PubMed]
- 11. Bradley H, Frazier E, Huang P, et al. Behavioral and clinical characteristics of persons receiving medical care for HIV infection. Medical monitoring project, United States 2014 cycle (June 2014–May 2015). Available at: https://stacks.cdc.gov/view/cdc/94013. Accessed 6 December 2022. [PubMed]
- 12. Beer L, Tie Y, Wu K, et al. Behavioral and clinical characteristics of persons with diagnosed HIV infection—medical monitoring project, United States 2015 cycle (June 2015–May 2016). Available at: https://stacks.cdc.gov/view/cdc/94012. Accessed 29 January 2023.
- 13. Beer L, Tie Y, Wu K, et al. Behavioral and clinical characteristics of persons with diagnosed HIV infection—medical monitoring project, United States 2016 cycle (June 2016–May 2017). Available at: https://stacks.cdc.gov/view/cdc/94011. Accessed 6 December 2022.
- 14. Beer L, Tie Y, Wu K, et al. Behavioral and clinical characteristics of persons with diagnosed HIV infection—medical monitoring project, United States, 2017 cycle (June 2017–May 2018). Available at: https://stacks.cdc.gov/view/cdc/94010. Accessed 29 January 2023.
- 15. Beer L, Tie Y, Wu K, et al. Behavioral and clinical characteristics of persons with diagnosed HIV infection—medical monitoring project, United States 2018 cycle (June 2018–May 2019)— Surveillance Special Report 25. Available at: https://stacks.cdc.gov/view/cdc/94009. Accessed 29 January 2023.
- 16. Durham MD, Buchacz K, Armon C, et al. Seasonal influenza vaccination rates in the HIV outpatient study—United States, 1999–2013. Clin Infect Dis 2015; 60:976–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Durham MD, Buchacz K, Armon C, Patel P, Wood K, Brooks JT. Rates and correlates of influenza vaccination among HIV-infected adults in the HIV Outpatient Study (HOPS), USA, 1999–2008. Prev Med 2011; 53:89–94. [DOI] [PubMed] [Google Scholar]
- 18. Gallagher KM, Juhasz M, Harris NS, Teshale EH. Adult and adolescent spectrum of HIV disease group. Predictors of influenza vaccination in HIV-infected patients in the United States, 1990–2002. J Infect Dis 2007; 196:339–46. [DOI] [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention . Flu vaccination coverage, United States, 2020–21 influenza season | FluVaxView | seasonal influenza (flu). 2021. Available at: https://www.cdc.gov/flu/fluvaxview/coverage-2021estimates.htm. Accessed 20 July 2022.
- 20. Roller-Wirnsberger R, Lindner S, Kolosovski L, et al. The role of health determinants in the influenza vaccination uptake among older adults (65+): a scope review. Aging Clin Exp Res 2021; 33:2123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takayama M, Wetmore CM, Mokdad AH. Characteristics associated with the uptake of influenza vaccination among adults in the United States. Prev Med 2012; 54:358–62. [DOI] [PubMed] [Google Scholar]
- 22. Tian C, Wang H, Wang W, Luo X. Characteristics associated with influenza vaccination uptake among adults. J Public Health 2019; 41:e267–73. [DOI] [PubMed] [Google Scholar]
- 23. Bhugra P, Grandhi GR, Mszar R, et al. Determinants of influenza vaccine uptake in patients with cardiovascular disease and strategies for improvement. JAHA 2021; 10:e019671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grandhi GR, Mszar R, Vahidy F, et al. Sociodemographic disparities in influenza vaccination among adults with atherosclerotic cardiovascular disease in the United States. JAMA Cardiol 2021; 6:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O’Halloran AC, Lu P-J, Williams WW, Bridges CB, Singleton JA. Influenza vaccination coverage among people with high-risk conditions in the U.S. Am J Prev Med 2016; 50:e15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singleton JA, Wortley P, Lu P-J. Influenza vaccination of persons with cardiovascular disease in the United States. Tex Heart Inst J 2004; 31:22–7. [PMC free article] [PubMed] [Google Scholar]
- 27. Harrison SM, Wei MY, Lamerato LE, Petrie JG, Toth Martin E. Multimorbidity is associated with uptake of influenza vaccination. Vaccine 2018; 36:3635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marrie RA, Walld R, Bolton JM, et al. Uptake of influenza vaccination among persons with inflammatory bowel disease, multiple sclerosis or rheumatoid arthritis: a population-based matched cohort study. CMAJ Open 2021; 9:E510–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eder M, Omic H, Gorges J, et al. Influenza vaccination uptake and factors influencing vaccination decision among patients with chronic kidney or liver disease. PLoS One 2021; 16:e0249785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park JK, Lee S, Lee JE, et al. The association between smoking status and influenza vaccination coverage rate in Korean adults: analysis of the 2010–2012 Korea National Health and Nutrition Examination Survey. Korean J Fam Med 2018; 39:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amy Liu I-L, Tanenbaum HC, Qian L, Sy LS, Chen W, Jacobsen SJ. Trends in influenza vaccine uptake and severe influenza-related outcomes at Kaiser Permanente Southern California, 2007–2017. Perm J 2021; 25:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Valour F, Cotte L, Voirin N, et al. Vaccination coverage against hepatitis A and B viruses, Streptococcus pneumoniae, seasonal flu, and A(H1N1)2009 pandemic influenza in HIV-infected patients. Vaccine 2014; 32:4558–64. [DOI] [PubMed] [Google Scholar]
- 33. Tsachouridou O, Georgiou A, Naoum S, et al. Factors associated with poor adherence to vaccination against hepatitis viruses, Streptococcus pneumoniae and seasonal influenza in HIV-infected adults. Hum Vaccin Immunother 2019; 15:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS 2009; 23:2337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mertens JR, Chi FW, Weisner CM, et al. Physician versus non-physician delivery of alcohol screening, brief intervention and referral to treatment in adult primary care: the ADVISe cluster randomized controlled implementation trial. Addict Sci Clin Pract 2015; 10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: a clinician's guide, updated 2005 edition. Revised 2016. U.S. Department of Health and Human Services [NIH Publication No. 07–3769]. Available at: https://pubs.niaaa.nih.gov/publications/clinicianGuide/guide/intro/data/resources/Clinicians%20Guide.pdf. Accessed 28 February 2023.
- 37. Reacher M, Warne B, Reeve L, et al. Influenza-associated mortality in hospital care: a retrospective cohort study of risk factors and impact of oseltamivir in an English teaching hospital, 2016 to 2017. Euro Surveill 2019; 24:1900087. Available at:https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2019.24.44.1900087. Accessed 20 July 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Han L, Ran J, Mak Y-W, et al. Smoking and influenza-associated morbidity and mortality: a systematic review and meta-analysis. Epidemiology 2019; 30:405–17. [DOI] [PubMed] [Google Scholar]
- 39. Gordon N. Similarity of the adult Kaiser Permanente membership in Northern California to the insured and general population in Northern California: statistics from the 2011–12 California Health Interview Survey. Oakland, CA: Kaiser Permanente Division of Research, 2015. Available at:https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/compare_kp_ncal_chis2017-18.pdf. Accessed 28 February 2023.