Abstract
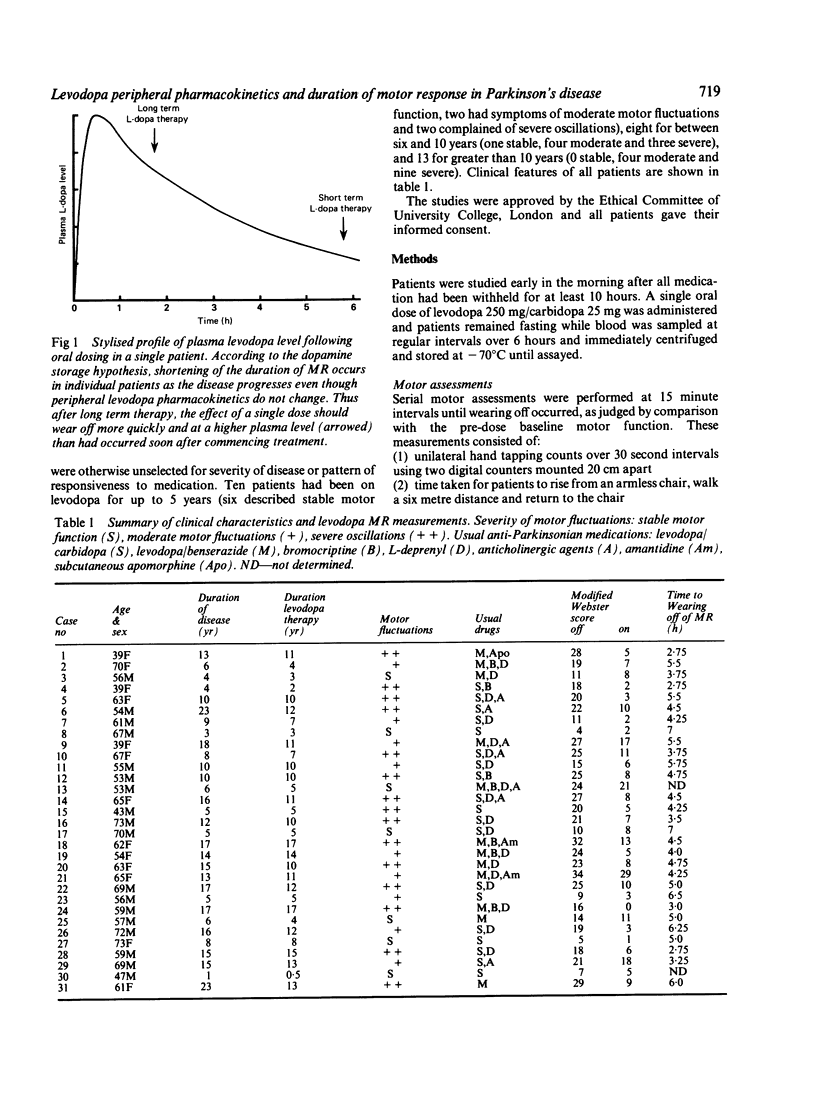
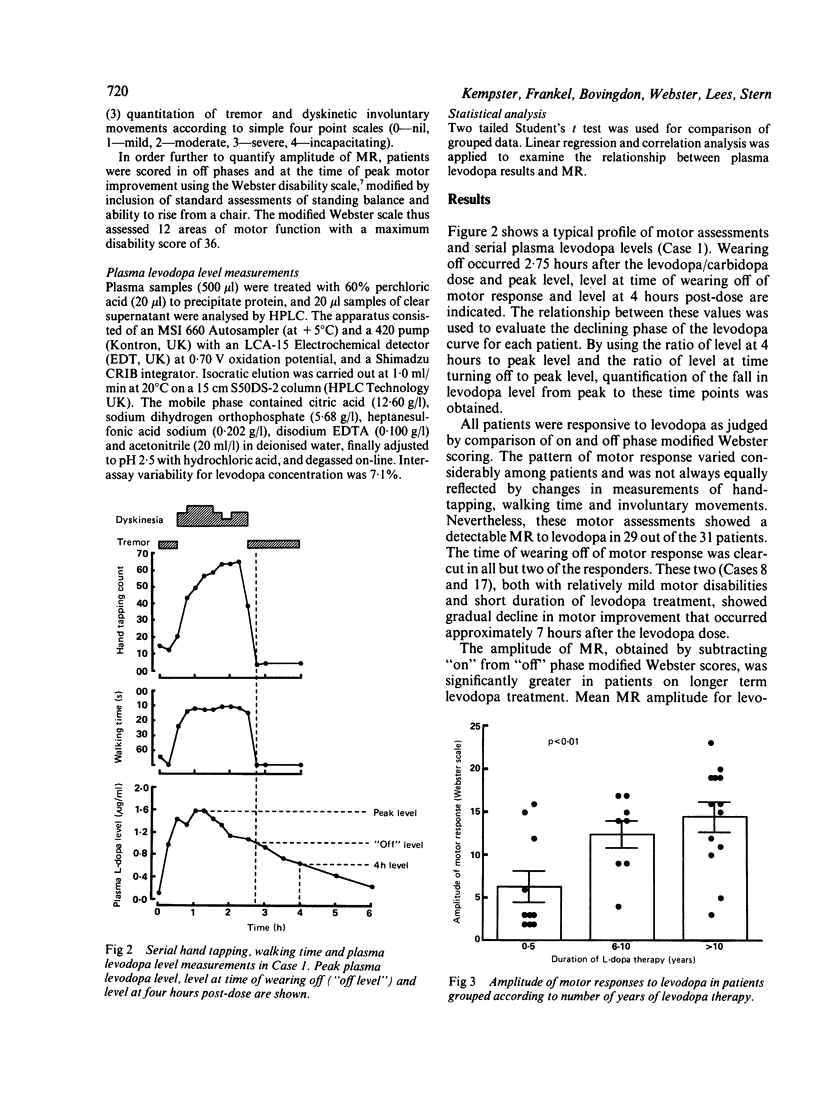
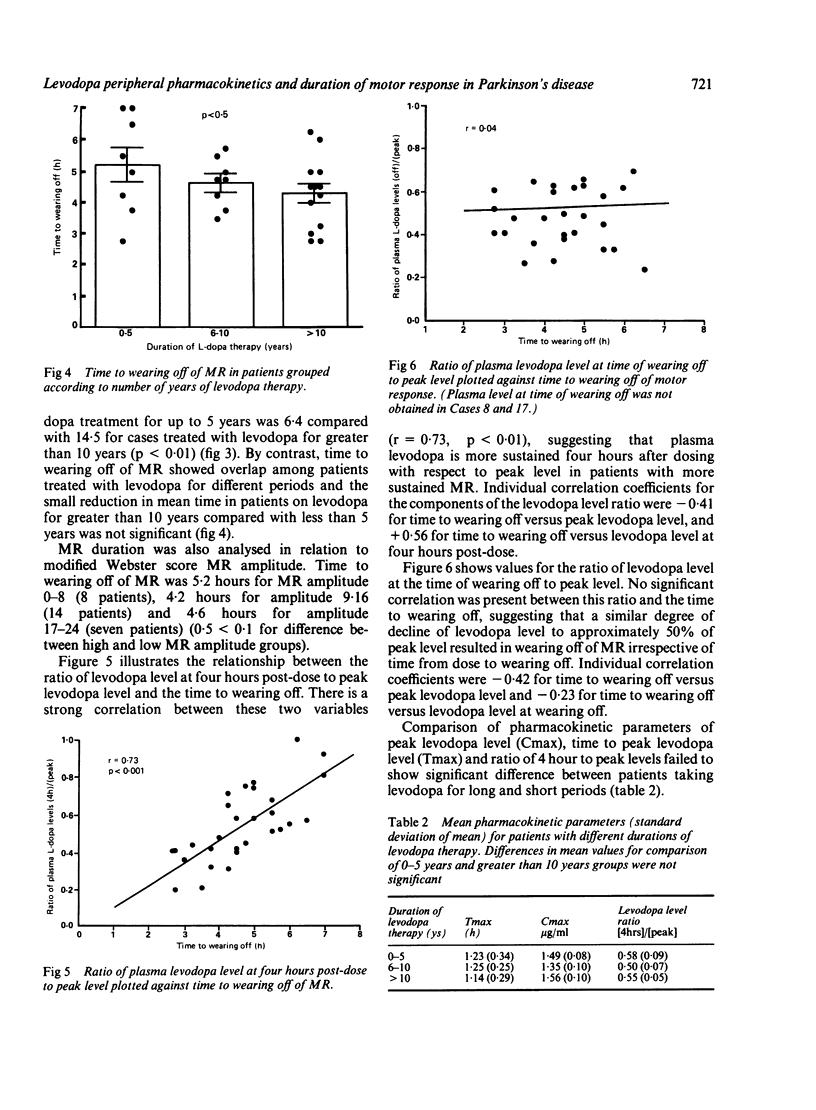
To assess the relative influence of central pharmacodynamic and peripheral pharmacokinetic factors on the duration of motor response to levodopa, the relationship between motor function and plasma levodopa levels was studied in 31 Parkinsonian patients. Duration of benefit from single levodopa doses while fasting depended on the degree to which the plasma levodopa level had declined over four hours; wearing off occurred when the plasma levodopa level had fallen to approximately 50% of peak concentration, irrespective of the duration of the motor response. Whilst the amplitude of motor response to levodopa is likely to be modified by alternations in dopamine receptor stimulation and sensitivity as the disease progresses, it is proposed that the duration of response is primarily determined by levodopa peripheral pharmacokinetics rather than by central pharmacodynamic factors associated with dopamine storage capacity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fahn S. "On-off" phenomenon with levodopa therapy in Parkinsonism. Clinical and pharmacologic correlations and the effect of intramuscular pyridoxine. Neurology. 1974 May;24(5):431–441. doi: 10.1212/wnl.24.5.431. [DOI] [PubMed] [Google Scholar]
- Gancher S. T., Nutt J. G., Woodward W. R. Peripheral pharmacokinetics of levodopa in untreated, stable, and fluctuating parkinsonian patients. Neurology. 1987 Jun;37(6):940–944. doi: 10.1212/wnl.37.6.940. [DOI] [PubMed] [Google Scholar]
- Gancher S. T., Nutt J. G., Woodward W. Response to brief levodopa infusions in parkinsonian patients with and without motor fluctuations. Neurology. 1988 May;38(5):712–716. doi: 10.1212/wnl.38.5.712. [DOI] [PubMed] [Google Scholar]
- Kempster P. A., Gibb W. R., Stern G. M., Lees A. J. Asymmetry of substantia nigra neuronal loss in Parkinson's disease and its relevance to the mechanism of levodopa related motor fluctuations. J Neurol Neurosurg Psychiatry. 1989 Jan;52(1):72–76. doi: 10.1136/jnnp.52.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders K. L., Palmer A. J., Quinn N., Clark J. C., Firnau G., Garnett E. S., Nahmias C., Jones T., Marsden C. D. Brain dopamine metabolism in patients with Parkinson's disease measured with positron emission tomography. J Neurol Neurosurg Psychiatry. 1986 Aug;49(8):853–860. doi: 10.1136/jnnp.49.8.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Parkes J. D. Success and problems of long-term levodopa therapy in Parkinson's disease. Lancet. 1977 Feb 12;1(8007):345–349. doi: 10.1016/s0140-6736(77)91146-1. [DOI] [PubMed] [Google Scholar]
- Melamed E., Hefti F., Pettibone D. J., Liebman J., Wurtman R. J. Aromatic L-amino acid decarboxylase in rat corpus striatum: implications for action of L-dopa in parkinsonism. Neurology. 1981 Jun;31(6):651–655. doi: 10.1212/wnl.31.6.651. [DOI] [PubMed] [Google Scholar]
- Shoulson I., Glaubiger G. A., Chase T. N. On-off response. Clinical and biochemical correlations during oral and intravenous levodopa administration in parkinsonian patients. Neurology. 1975 Dec;25(12):1144–1148. doi: 10.1212/wnl.25.12.1144. [DOI] [PubMed] [Google Scholar]
- Webster D. D. Critical analysis of the disability in Parkinson's disease. Mod Treat. 1968 Mar;5(2):257–282. [PubMed] [Google Scholar]


