Summary
Background
HexaBody®-CD38 (GEN3014) is a hexamerization-enhanced human IgG1 that binds CD38 with high affinity. The E430G mutation in its Fc domain facilitates the natural process of antibody hexamer formation upon binding to the cell surface, resulting in increased binding of C1q and potentiated complement-dependent cytotoxicity (CDC).
Methods
Co-crystallization studies were performed to identify the binding interface of HexaBody-CD38 and CD38. HexaBody-CD38-induced CDC, antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), trogocytosis, and apoptosis were assessed using flow cytometry assays using tumour cell lines, and MM patient samples (CDC). CD38 enzymatic activity was measured using fluorescence spectroscopy. Anti-tumour activity of HexaBody-CD38 was assessed in patient-derived xenograft mouse models in vivo.
Findings
HexaBody-CD38 binds a unique epitope on CD38 and induced potent CDC in multiple myeloma (MM), acute myeloid leukaemia (AML), and B-cell non-Hodgkin lymphoma (B-NHL) cells. Anti-tumour activity was confirmed in patient-derived xenograft models in vivo. Sensitivity to HexaBody-CD38 correlated with CD38 expression level and was inversely correlated with expression of complement regulatory proteins. Compared to daratumumab, HexaBody-CD38 showed enhanced CDC in cell lines with lower levels of CD38 expression, without increasing lysis of healthy leukocytes. More effective CDC was also confirmed in primary MM cells. Furthermore, HexaBody-CD38 efficiently induced ADCC, ADCP, trogocytosis, and apoptosis after Fc-crosslinking. Moreover, HexaBody-CD38 strongly inhibited CD38 cyclase activity, which is hypothesized to relieve immune suppression in the tumour microenvironment.
Interpretation
Based on these preclinical studies, a clinical trial was initiated to assess the clinical safety of HexaBody-CD38 in patients with MM.
Funding
Genmab.
Keywords: Antibody, CD38, Complement-dependent cytotoxicity, Hematologic neoplasms, Daratumumab, Multiple myeloma
Research in context.
Evidence before this study
We searched PubMed to identify hexamerization-enhanced CD38-targeting monoclonal antibodies in development before December 1, 2022, by using the search terms “CD38 antibody”, “complement-dependent cytotoxicity”, “hexamerization”, “hexabody”, and combinations thereof. This search retrieved previous studies from our group, in which we showed that the introduction of specific mutations in the Fc region of CD38 antibodies enhanced formation of hexamers upon binding to CD38 on the cell surface and resulted in faster and more robust CDC than their wild-type counterparts.1,2 Furthermore, Schutze et al. have confirmed the enhanced CDC activity of CD38 antibody variants with a hexamerization-enhancing mutation.3
Two unmodified IgG1 antibodies targeting CD38 have been approved for the treatment of patients with multiple myeloma (MM): daratumumab and isatuximab. The anti-tumour activity of these approved CD38 antibodies is mediated by effector mechanisms such as complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and apoptosis. In addition, CD38 mAbs showed immunomodulatory effects through interactions with CD38-expressing immune cells, which is thought to ultimately lead to the deep and durable responses observed in MM patients. However, not all patients respond to anti-CD38 therapy and many patients eventually develop progressive disease. Progression during daratumumab treatment has been associated with low CD38 expression levels and reduced sensitivity to complement-mediated tumour cell lysis, pointing to CDC as an important effector mechanism in vivo. Also, low clinical response rates were observed with anti-CD38 monotherapy in other haematological indications that express low levels of CD38, such as NHL.
Added value of this study
In preclinical studies we show that HexaBody-CD38, a next generation CD38-specific IgG1 with enhanced CDC activity due to a hexamerization-enhancing mutation (E430G), induced potent CDC in MM, B-NHL and acute myeloid leukaemia (AML) cell lines in vitro and showed strong anti-tumour activity in B-NHL and AML PDX models in vivo. Especially in cell lines with lower levels of CD38 expression HexaBody-CD38 showed increased CDC potency compared to daratumumab, without increasing lysis of healthy leukocytes. More effective CDC was also confirmed in primary MM cells. Furthermore, preincubation of cell lines with daratumumab or isatuximab did not inhibit HexaBody-CD38 from reaching its full capacity to induce CDC. In addition to its strong CDC potency, HexaBody-CD38 could efficiently activate FcγR-expressing effector cells, such as natural killer cell and macrophages, to kill tumour cells. Moreover, HexaBody-CD38 strongly inhibited CD38 cyclase activity, which is hypothesized to relieve immune suppression in the tumour microenvironment.
Implications of all the available evidence
This work provides preclinical evidence that increasing antibody hexamerization can further increase CDC potency of CD38 targeting antibodies. The enhanced CDC potency of HexaBody-CD38 is hypothesized to increase clinical activity compared to already approved CD38 targeting antibodies in tumours with lower levels of CD38 expression, such as B-NHL. The safety and preliminary efficacy of HexaBody-CD38 are currently being evaluated in a first-in-human trial in MM patients (NCT04824794).
In a broader context, this work illustrates that introducing a hexamerization enhancing mutation can unlock potent CDC activity in monoclonal antibodies that demonstrate minimal CDC as wild-type, with minimal engineering. Enhanced CDC increases overall cytotoxic capacity and broadens the effector mechanisms contributing to anti-tumour activity. Whereas the current manuscript focuses on CD38-expressing haematological malignancies, enhanced hexamerization to enhance CDC may also be considered for therapeutic antibodies targeting other haematological malignancies4 or solid cancers. Clinical validation of the safety and efficacy of HexaBody-CD38 could provide rationale to apply enhanced IgG hexamerization also in other monoclonal antibodies designed to deplete specific cell populations, in cancer or other disease areas.
Introduction
The multifunctional cell surface molecule CD38 is a validated target for anti-cancer therapy, given its high expression in MM.5 Other haematological malignancies, including AML and DLBCL, also express CD38 albeit more heterogeneously and at lower levels.6, 7, 8, 9 Currently, two CD38-targeting therapeutic antibodies have been approved for the treatment of MM: daratumumab (DARZALEX®; Janssen Biotech) and isatuximab (SARCLISA®, Sanofi-Aventis).10 Fc-mediated effector functions, including CDC, ADCC, and ADCP, as well as apoptosis induction and CD38 enzyme inhibition may contribute to the anti-tumour activity of CD38 antibodies. Whereas CD38 antibodies have demonstrated strong efficacy against MM with a favourable safety profile, not all MM patients respond to treatment, and many eventually develop progressive disease. Furthermore, low clinical response rates were observed with CD38 antibodies in haematological malignancies including non-Hodgkin lymphoma (NHL) subtypes and ALL, which express lower levels of CD38 compared to MM.11,12 In MM patients, resistance to daratumumab has been associated with low CD38 expression and increased expression of CRP.13 Increasing CD38 expression with all-trans retinoic acid (ATRA) has been evaluated to improve the efficacy of daratumumab in patients with daratumumab-refractory MM, but with minimal success.14 Alternatively, improving the CDC activity of antibodies may overcome these limitations.1
Here we present HexaBody®-CD38 (GEN3014), a next-generation CD38-specific IgG1 molecule with enhanced CDC activity. HexaBody-CD38 contains the hexamerization-enhancing mutation E430G, which facilitates the natural process of antibody hexamer formation upon binding to antigens on the cell surface, through increased intermolecular Fc–Fc interactions.1 Membrane-bound IgG hexamers efficiently bind the hexavalent complement component C1q, the first step in the classical pathway of complement activation, thereby potentiating or unlocking highly efficient CDC.1
We evaluated effector mechanisms, including CDC, as well as the preclinical anti-tumour activity of HexaBody-CD38 and benchmarked against its parental antibody without the E430G mutation and the clinically validated CD38 antibodies daratumumab and isatuximab.
Methods
Ethics
The study site ethics committee from UMC Amsterdam approved the protocol for ex vivo studies using bone marrow mononuclear cells (BMMCs) obtained from bone marrow aspirates from MM patients (METC 2017.008). Studies were performed according to the principles of the Declaration of Helsinki, the International Conference on Harmonization, and the Guidelines for Good Clinical Practice. All patients gave written informed consent. Animal experiments complied with the ARRIVE guidelines and were carried out in accordance with the EU Directive 2010/63/EU for animal experiments. The protocols were approved by the local Ethical committees (AVD244002016-385-01 and EPO “Reg 0010/19”).
Patient and healthy donor cells
Ex vivo CDC activity of HexaBody-CD38 was assessed in 15 BMMC samples obtained from 14 MM patients with newly diagnosed (ND) MM (n = 4), daratumumab-naïve relapsed/refractory (RR) MM (n = 3) or daratumumab-refractory RRMM (n = 8). From one patient, two samples were obtained at different time points. The clinical characteristics of these patients are shown in Table 1. Experiments with MM patient samples were performed at the UMC Amsterdam using their established protocol15 and analysed blinded to the disease status of the patients. Buffy coats and heparinized blood from de-identified healthy blood donors were obtained from Sanquin (Amsterdam). Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats using Lymphocyte Separation Medium (Corning, 25-072-CI) and LeucoSep™-tubes (Greiner, 227290).
Table 1.
Baseline characteristics of MM patients from which primary samples were obtained.
| MM patients (n = 15)a | |
|---|---|
| Age (median in years, IQR) | 70 (63–75.8) |
| Male sex (n, %) | 12 (80%) |
| M-protein (n, %) | |
| IgG kappa | 6 (40%) |
| IgG lambda | 1 (7%) |
| IgA kappa | 1 (7%) |
| IgA lambda | 1 (7%) |
| Light chain only | 4 (27%) |
| Unknown | 2 (13%) |
| Disease stage | |
| Newly diagnosed MM | 4 (27%) |
| Relapsed/refractory MM | 11 (73%) |
| Prior lines of treatment (median, IQR) | 5 (3.5–6) |
| Refractory tob (n, %) | |
| Lenalidomide | 8 (53%) |
| Bortezomib | 6 (40%) |
| Daratumumab | 8 (53%) |
From one patient with relapsed/refractory disease, two samples were obtained at different timepoints before and after daratumumab treatment.
Refractory disease is defined as progressive disease during therapy, no response (less than partial response), or progressive disease within 60 days of stopping treatment, according to the International Uniform Response Criteria for Multiple Myeloma.
Antibodies and cells
Antibodies produced at Genmab were released for in vitro or in vivo use according to internal specifications, which included assessment of the concentration (A280; ≥0.25 mg/mL), the presence of particles by visual analysis, purity by capillary electrophoresis SDS (Non-Reducing: ≥90%; Reducing: ≥95%), the presence of monomers by HP-SEC (≥95.0% monomer), mass check by mass spectrometry, and endotoxin concentration (≤0.5 EU/mg). The CD38-binding domain of HexaBody-CD38 was derived from antibody clone 028, described previously,16 and cloned into an expression vector coding an IgG1 Fc-backbone containing the hexamerization-enhancing E430G Fc mutation.1 HexaBody-CD38 is a Genmab proprietary antibody. In some experiments, the parental clone without the E430G Fc mutation was included (IgG1-CD38-028), containing a functionally irrelevant K409R or delK mutation as indicated. Commercially available mAb used are listed in Table S1. The HIV-1 gp120-specific mAb b1217 was included as non-binding antibody, and is referred to as IgG1-ctrl (wild-type IgG1) or Hx-ctrl (IgG1 containing the E430G mutation). Cell lines were cultured at 37 °C and 5% CO2. Details of the cell lines and their culture medium used are listed in Table S2. No STR profiling of cell lines has been performed after receiving them directly from ATCC or DSMZ. Mycoplasma contamination was excluded by monthly testing of cell lines using the MycoAlert Mycoplasma Detection Kit (Lonza, LT07-318).
Crystallization, data collection, structure determination, and refinement
CD38:HexaBody-CD38 complex formation, crystallization, crystallographic data collection, processing, and structure determination are described in the Supplementary Methods. The crystal structure of HexaBody-CD38 Fab in complex with CD38 can be accessed using PDB identification number 8BYU.
Binding affinity determination and crossblock
Target binding affinity and potential crossblock of human CD38-specific antibodies was determined by Biolayer Interferometry (BLI). Details are described in the Supplementary Methods.
Binding of HexaBody-CD38 to CD38-positive cell lines
Binding of HexaBody-CD38 to the B-NHL cell lines Daudi, NALM-16 and Wien-133 cells was evaluated using flow cytometry. The R-Phycoerythrin (R-PE)-conjugated goat anti-human IgG F (ab’)2 (Jackson, cat# 109-116-098, RRID AB_2337678) was used as detection antibody. Experimental curves were fitted using four-variable nonlinear regression models with sigmoidal dose-responses using GraphPad Prism software version 9.0.0. Some experiments were performed in the presence of 10 μg/mL daratumumab or isatuximab. After pre-incubation for 15 min with daratumumab or isatuximab, fluorescein isothiocyanate (FITC-) labelled HexaBody-CD38 was added and fluorescence intensity was evaluated after 15 min, 1 h, 4 h, or 24 h incubation. (Pre-) incubation with IgG1-ctrl or without antibody were included as negative controls. Fluorescence intensity was determined by flow cytometry as a measure of antibody-binding.
In vitro CDC assay
Cells were incubated with HexaBody-CD38 for 15 min after which normal human serum (Sanquin, Amsterdam, the Netherlands) was added to a final concentration of 20%. CDC was allowed for 45 min unless otherwise specified. Finally, cells were incubated with the viability markers propidium iodide (Sigma-Aldrich, cat# P4864) or TO-PRO-3 (Invitrogen, cat# T3605) and the % dead cells was determined by flow cytometry. Some experiments were performed in the presence of 10 μg/mL daratumumab or isatuximab. NHS, as a source of complement, was added to start CDC after HexaBody-CD38 was allowed to bind for 15 min to cells with or without pre-incubation with daratumumab, isatuximab, or IgG1-ctrl at a saturating concentration (10 μg/mL). CDC was evaluated by flow cytometry after 45 min, 4 h, or 24 h. The % lysis was calculated as 100% minus the % TO-PRO-3-negative (viable) cells.
Cytotoxicity experiments of healthy leukocytes and erythrocytes are described in the Supplementary Methods.
Semi-quantitative cell surface expression analysis
Antibody-binding sites (ABS) were quantified by flow cytometry using QIFIKIT (DAKO, cat# K0078) according to the manufacturer's instructions. Briefly, cells were incubated with mouse antibodies specific for the relevant human antigens (Table S1). After incubation, the cells were stained alongside Set-Up and Calibration Beads with FITC-conjugated goat-anti-mouse F (ab’)2 (DAKO, cat# F0479). For each sample, the ABS, as an estimate for the number of target molecules expressed on the plasma membrane, was calculated using the geometric mean fluorescence intensity (gMFI) of the antibody-stained cells and the equation of the calibration curve, calculated using GraphPad Prism Software.
Correlation analysis between CDC and expression of CD38 and CRP
The relationship between CDC activity of HexaBody-CD38 and daratumumab (EC50 and Emax) with density of CD38, CD46, CD55 and CD59 was investigated by multiple linear regression analysis. For each cell line the average of the estimated EC50 and Emax between replicates was used after logit transformation. Details can be found in the Supplementary Methods.
ADCC assay
Calcein AM-labelled (Life Technologies, Carlsbad, CA) Daudi cells were incubated with HexaBody-CD38 for 15 min. Thereafter, healthy donor PBMCs were added at an effector to target (E:T) cell ratio of 20:1 and co-cultures were pelleted and incubated for 4 h at 37 °C. Cell viability was monitored by flow cytometry using the viability marker FVS700 (BD Biosciences, cat# 564997, RRID AB_2869637), while expression of cell lineage markers was evaluated using a mixture of fluorochrome-conjugated antibodies (see Table S1). Loss of Daudi cells relative to the untreated control was used as a measure for ADCC.
ADCP assay
Calcein AM-labelled Daudi cells were opsonized with HexaBody-CD38 for 15 min, after which macrophages were added at an E:T ratio of 2:1. Macrophages were derived from healthy donor PBMC-derived CD14+ monocytes that were cultured in GMP DC medium (CellGenix, cat# 20801-0500) supplemented with 50 ng/mL macrophage colony-stimulating factor (M-CSF, GIBCO, cat# PHC9501) for 6 or 7 days. Co-cultures were incubated for 24 h at 37 °C. Flow cytometry was used to monitor cell viability (using TO-PRO-3) and macrophages that had phagocytosed Daudi cells (CD11b+/Calcein+ cells). The percentage loss of Daudi cells was determined from the % non-phagocytosed Daudi cells (CD11b−/calcein AM+/CD19+ cells) relative to the average number of CD11b−/calcein AM+/CD19+ cells in the no antibody samples. The % macrophages with phagocytic activity was determined by evaluating the % of CD11b+/calcein AM + CD19low cells.
Trogocytosis assay
Wien-133 target cells were labelled with the cytosolic dye cell trace violet (CTV, Invitrogen, cat# 34557) and the plasma membrane with PKH-26 (Sigma-Aldrich, cat# PKH26GL-1 KT). The cells were opsonized with HexaBody-CD38 and co-cultured with healthy donor PBMC-derived CD14+ monocytes for 90 min at 37 °C. The % PKH-26+ and CTV+ cells was evaluated by flow cytometry in CD11b+ monocytes as a measure for transfer of membrane or cytosol, respectively. Trogocytosis was evaluated by flow cytometry as the transfer of cell membrane dye from target cells to CD14+ monocytes in absence of transfer of cytosol.
Apoptosis assay
Daudi cells were opsonized with 0.63 μg/mL HexaBody-CD38 in presence or absence of a polyclonal goat anti-human-IgG Fcγ-fragment-specific antibody (Jackson, cat# 109-006-098, RRID AB_2337551). After incubation for 72 h at 37 °C, early and late apoptotic cells were determined by flow cytometry as the % of PI−/Annexin V+ (early apoptotic) and PI+/Annexin V+ (late apoptotic) cells in the total cell population, respectively.
Enzymatic assays
Daudi cells in 20 mM Tris-HCl (pH 7.4) were seeded in a white opaque 96-well microplate (PerkinElmer), and incubated with HexaBody-CD38 for 15–20 min. Then, the NAD+ analog NGD+ was added and the conversion to fluorescent cGDPR was measured on an EnVision Plate Reader (PerkinElmer) as a measure of CD38 cyclase activity.18 To evaluate CD38 hydrolase activity, the NAD+ analog ε-NAD+ was added and the conversion to fluorescent ε-ADPR was measured by fluorescence spectroscopy.19 CD38 cyclase and hydrolase activity was calculated by determining the slope (arbitrary fluorescence units/min) of the linear part of the cGDPR or ε-ADPR production curve using linear regression analysis (GraphPad Prism software).19 CD38 cyclase and hydrolase activity was calculated by determining the slope (arbitrary fluorescence units/min) of the linear part of the cGDPR or ε-ADPR production curve using linear regression analysis (GraphPad Prism software).
In vivo experiments
Dose–response studies were performed in PDX models Ly12638, AML11810, and AML13990 (EPO, Berlin, Germany) using female CB17-SCID mice aged 5–8 weeks (Janvier Labs). Tumour fragments (2–3 mm3) were implanted subcutaneously under anaesthesia and analgesia. Mice were randomized into different treatment arms at a tumour volume of 100–200 mm3 by stratification. In case of very heterogeneous growth rates of the PDX models, animals were included in the study using rolling enrolment. Depending on the study, 7 to 10 mice were allocated to each treatment group. Group size was determined to achieve a power of 80% to detect statistically significant changes between treatment groups, based on prior experience with the growth with this model and the estimated effect of the treatment. Mice were intravenously (IV) injected with 0.2 mL HexaBody-CD38 at doses of 0.1–10 mg/kg once weekly (QW) for three weeks (depending on the model 3–5 doses were compared to control mice). Mice were dosed once weekly based on the regular human IgG-like plasma half-life of HexaBody-CD38. Control mice were IV injected with Hx-ctrl, as indicated. Mice were observed at least twice weekly for clinical signs of illness and their body weight was monitored. Mice with a tumour volume >2000 mm3, or in case of serious clinical signs were removed from the experiment. The persons involved in the study were blinded to the nature of the treatments. Animals were housed under SPF conditions.
PK of HexaBody-CD38 was evaluated in tumour-free female SCID mice aged 11–12 weeks (Envigo), with 3–4 mice in each dose group. Exposure of HexaBody-CD38 was evaluated in tumour-bearing mice using the AML PDX model AML11810 as described above. Details of these studies can be found in the Supplementary Methods.
CDC in primary multiple myeloma cells ex vivo
Cryopreserved primary BMMC samples were thawed, washed and plated in a 96-well round bottom plate. Cells were opsonized with HexaBody-CD38 or daratumumab at a final antibody concentration range of 0.01–10 μg/mL for 15 min at room temperature (RT). NHS (Sanquin, cat# B0625/B0626) was added to each well at a final concentration of 20% and incubated for 45 min at 37 °C. After staining with fluorescent antibodies directed at cell lineage markers and CD38, the viability marker 7-AAD was added, and cells were evaluated by flow cytometry (LSR Fortessa, BD). Viable plasma cells were identified as 7-AAD−/CD3−/CD14−/CD38+/CD138+ cells, using established protocols and reagents available at the UMC Amsterdam as described previously15 with small adaptations: CD269 and CD274 were removed, and CD14-APC (BD 345787 RRID AB_2868813) was added. Background lysis was determined as the mean % lysis in presence of an isotype control antibody plus 2 × the SD. Non-malignant cells including NK cells (CD56dim/CD3−/CD138−), T cells, (CD3+), B cells (CD19+) and monocytes (CD14+) were also evaluated.
Data analysis and statistics
Dose–response curves were generated using four-parameter non-linear regression analysis (Graphpad Prism 8.0 software; equation log (agonist) vs response (variable slope)). Half maximal effective concentration (EC50) values were determined from these curves. EC50 values between different experimental conditions were compared using a one-way ANOVA or mixed effect analysis, in case of missing values, followed by a Tukeys test or a paired T-test if only two conditions were compared (Graphpad Prism). When comparing the percentage lysis or enzyme activity between multiple experimental conditions statistical significance was evaluated using a Friedman test with uncorrected Dunn's post hoc test or a Wilcoxon matched-pairs signed rank test when only two conditions were compared (Graphpad Prism).
For our CDC cell line panel concentration-response, maximum response (Emax) and EC50 values were evaluated with multi-level nonlinear mixed effects modelling using Rstudio v1.0.153 and the library nlme (Linear and Nonlinear Mixed Effects Models) v3.1-137. The relationship between CDC activity of HexaBody-CD38 and daratumumab (EC50 and Emax) with density of CD38, CD46, CD55 and CD59 was investigated by multiple linear regression analysis. For each cell line the average of the estimated EC50 and Emax between replicates was used after logit transformation. More details regarding this analysis can be found in the Supplementary Methods.
In in vivo PDX models differences in tumour volumes between treatment groups were compared using Mann Whitney test. Mantel–Cox analysis of Kaplan–Meier curves applying a tumour-size cut off of 500 (AML models) or 1000 mm3 (DLBCL model) was performed to determine differences in tumour growth between the groups.
Role of funders
Genmab was involved in study design, analysis, interpretation, and submission decision.
Results
HexaBody-CD38 binds to CD38 with high affinity
HexaBody-CD38 showed high affinity binding to human CD38 with a dissociation constant (KD) value in the nanomolar range (1.1 ± 0.17 nM), as determined by BLI (Fig. S1a). Co-crystallization studies of the Fab domain of HexaBody-CD38 with the extracellular domain of CD38 identified a unique binding epitope that is non-overlapping with the catalytic site (Fig. S2). Most of the buried solvent accessible area is contributed by the heavy chain and the binding interface is stabilized by extensive van der Waals contacts and polar contacts including residues Arg78 and Asp202 on CD38 and residues Lys74 and Arg103, and Trp32 and Arg38 on the HexaBody-CD38 heavy and light chains, respectively. Flow cytometry analysis confirmed dose-dependent binding of HexaBody-CD38 to cell lines expressing variable CD38 levels on the plasma membrane (Daudi (∼200,000 ABS/cell), Wien-133 (∼100,000 ABS/cell) and NALM-16 cells (∼50,000 ABS/cell)), with an overall average EC50 in the subnanomolar range (Table 2).
Table 2.
Functional characteristics of HexaBody-CD38 in comparison with daratumumab.
| Functional Characteristics | HexaBody-CD38 | daratumumab | Significance |
|---|---|---|---|
| Binding | |||
| Binding to soluble CD38 (Affinity, KD; average ± SD) | 1.1 ± 0.17 nM | 3.9 ± 0.48 nM | P = 0.0058a CI 1.9–3.8 |
| Binding to membrane-expressed CD38 (Apparent affinity, EC50; average ± SD) | 0.087 ± 0.042 μg/mL | 0.148 ± 0.038 μg/mL | P = 0.10161a CI −0.0298 to 0.152 |
| CDC | |||
| EC50 (geometric mean; geometric standard deviation [GSD]) | 0.044 μg/mL; GSD 1.52 | 0.31 μg/mL; GSD 1.40 | P < 0.0001b |
| Max lysis (average ± SD) | 72 ± 29% | 44 ± 38% | P < 0.0001b |
| ADCC Loss of Daudi cells | |||
| EC50 (average ± SD) | 0.290 ± 0.231 ng/mL | 2.192 ± 2.376 ng/mL | P = 0.1204a CI −0.0002837 to 0.001797 |
| Max loss (average ± SD) | 42 ± 9% | 40 ± 11% | P = 0.1602c |
| ADCP Macrophages with phagocytic activity | |||
| EC50 (average ± SD) | 2.719 ± 2.080 ng/mL | 7.517 ± 6.828 ng/mL | P = 0.0305d CI −0.009108 to −0.0004880 |
| Max activity (average ± SD) | 25 ± 12% | 25 ± 11% | ND |
| ADCP Loss of Daudi cells | |||
| EC50 (average ± SD) | 5.779 ± 7.554 ng/mL | 16.248 ± 24.21 ng/mL | P = 0.2593d CI −0.03325 to 0.008984 |
| Max loss (average ± SD) | 68 ± 22% | 67 ± 25% | ND |
| Trogocytosis | |||
| EC50 (average ± SD) | 8.79 ± 2.49 ng/mL | 17.99 ± 1.82 ng/mL | P = 0.0218e CI 0.002451–0.01596 |
| Apoptosis | |||
| Mean max % of early apoptotic cells after crosslinking | 39% | 39% | ND |
| Mean max % of late apoptotic cells after crosslinking | 12% | 11% | ND |
| CD38 cyclase activity | |||
| EC50 (average ± SD) | 0.419 ± 0.097 μg/mL | 0.179 ± 0.038 μg/mL | P = 0.0085e CI 0.07884–0.4028 |
| Max inhibition (%) | 59 ± 2% | 37 ± 4% | P = 0.4386f |
| CD38 hydrolase activity | |||
| EC50 (average ± SD) | 0.335 ± 0.115 μg/mL | 0.028 ± 0.008 μg/mL | P = 0.0114e CI 0.08747–0.5268 |
| Max stimulation (%) | 20 ± 4% | 16 ± 3% | P = 0.3017f |
ADCC, Antibody-dependent cellular cytotoxicity; ADCP, Antibody-dependent cell-mediated phagocytosis; BLI, BioLayer Interferometry; CDC, Complement-dependent cytotoxicity; EC50, Half maximal effective concentration; GSD, Geometric standard deviation; max, maximum; SD, Standard deviation. ND, Not determined, statistical testing was not performed if results were clearly not different.
Paired T test.
F-test.
Wilcoxon matched-pairs signed rank test.
Mixed effects analysis with Tukeys test, due to missing values.
One-way ANOVA, Tukey multiple comparison test.
Friedman test with uncorrected Dunn's posthoc test.
HexaBody-CD38 induces highly potent CDC
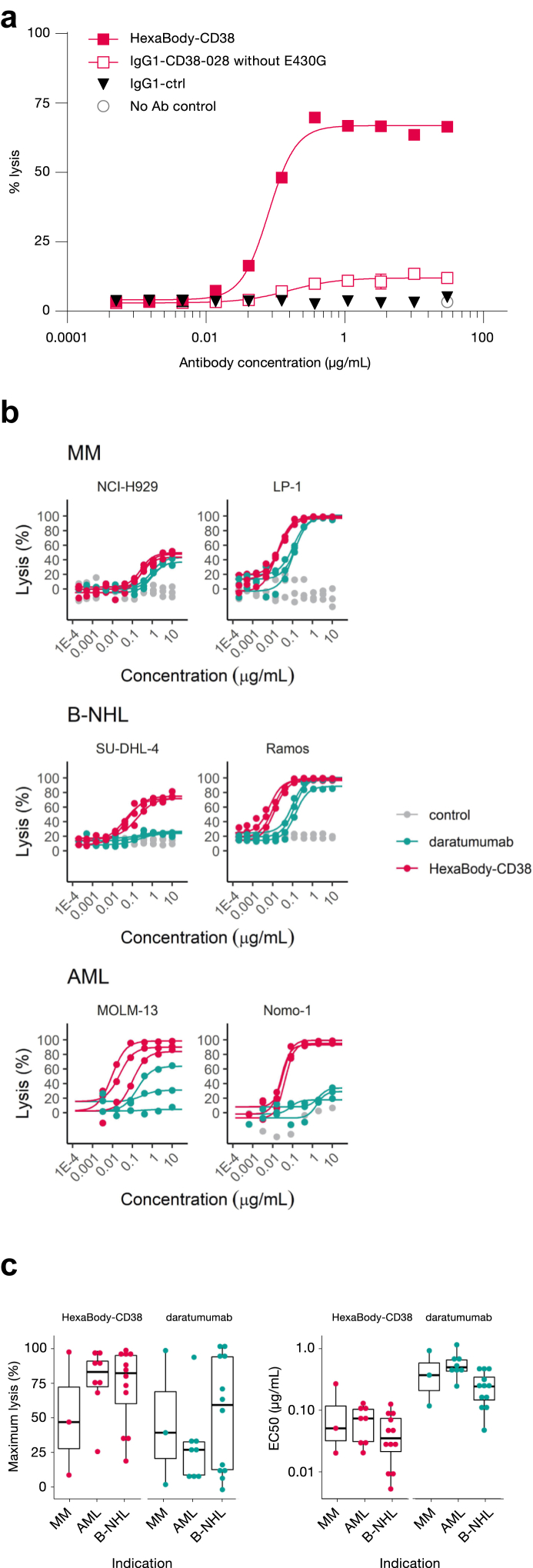
Introduction of the E430G mutation was previously shown to enhance CDC activity for various monoclonal antibodies.1 We selected HexaBody-CD38 from a panel of hexamerization-enhanced CD38 antibodies based on excellent CDC potency, as shown in the CD38+ Daudi cell line (Fig. 1a). Of note, the wild-type parental antibody IgG1-CD38-028 showed minimal CDC activity, confirming that CDC activity of HexaBody-CD38 could be attributed to the E430G mutation (Fig. 1a).
Fig. 1.
HexaBody-CD38 induces potent CDC in vitro. (a) CDC activity of HexaBody-CD38 and IgG1-CD38-028 (parental antibody without the E430G mutation) in Daudi cells. Non-binding antibody IgG1-ctrl was used as a negative control. The results of one representative experiment (out of three) with averages of duplicate measurements is shown. (b) HexaBody-CD38-induced CDC was determined in a panel of 27 cell lines. Six representative cell lines were selected and grouped per indication (AML, B-NHL and MM) and the graphs show lysis (CDC) against concentration (μg/mL) on semi-log scale. For each cell line individual observations of independent experiments are presented as symbols, and curves show the concentration-responses per replicate per compound simulated by a four-parameter logistic model. The control antibody used in these experiments is Hx-ctrl in the MM and B-NHL cell lines, and IgG1-ctrl in AML cell lines. Cell lines were tested in at least three different experiments. (c) Boxplots of average maximum lysis (left panel) and EC50 values (right panel) are shown of the 23 cell lines with dose–response curves to HexaBody-CD38 and/or daratumumab-induced CDC, as shown in b. Boxplots represent median plus interquartile range of each cell line, grouped per indication.
The CDC activity of HexaBody-CD38 was further evaluated in a panel of 27 human tumour cell lines derived from haematological malignancies, including MM, AML, and B-NHL. HexaBody-CD38 induced dose-dependent CDC in 23 out of the 27 tumour cell lines tested (Fig. 1b, Fig. S3), with an average maximum tumour cell lysis (Emax) of 72% and a geometric mean EC50 of 0.044 μg/mL (Fig. 1c, Table 2).
The CDC activity of HexaBody-CD38 was benchmarked against that of daratumumab, which was previously shown to induce complement-dependent tumour cell kill.16 On average, maximal tumour cell kill by HexaBody-CD38 was higher than for daratumumab (72% versus 44%, P < 0.0001) in the 23 HexaBody-CD38-responsive cell lines and the EC50 for CDC activity of daratumumab was 7-fold higher (0.31 μg/mL versus 0.044 μg/mL, P < 0.0001; Fig. 1c, Table 2). The outstanding CDC potency of HexaBody-CD38 was most evident in 19 cell lines with expression levels between 75,000 and 190,000 CD38 ABS/cell: HexaBody-CD38 induced ≥25% CDC in 16, whereas daratumumab only in 9 cell lines (Fig. 2a and b).
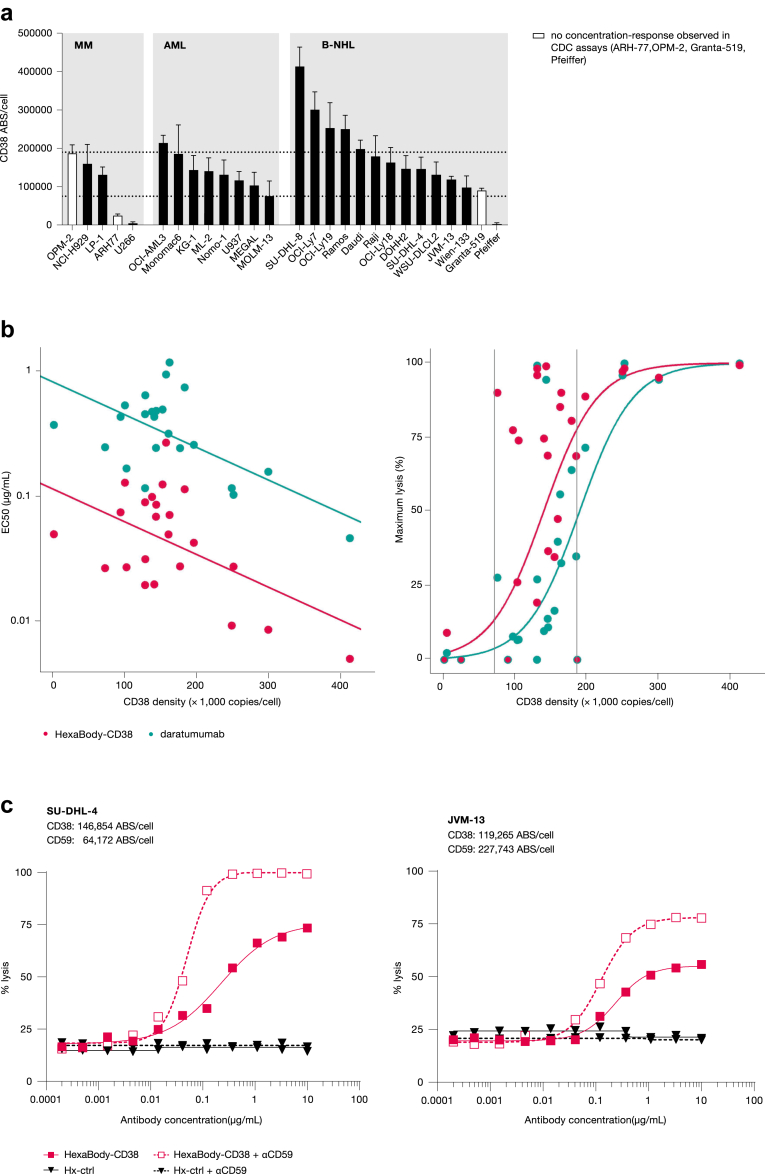
Fig. 2.
Impact of CD38 expression levels on CDC induction by HexaBody-CD38. (a) CD38 molecule expression (ABS) on the cell surface of 27 human tumour cell lines. Cell lines were grouped per indication and the white bars represent cell lines that did not show a concentration-response by HexaBody-CD38 in CDC assays (see Fig. 1). (b) Correlation plots of EC50-value (left panel) or maximum lysis (right panel) and CD38 density are shown. The position of the dots represents the average CDC value of a compound (HexaBody-CD38 or daratumumab) and CD38 expression levels observed for each cell line. Data points completely overlapping for HexaBody-CD38 and daratumumab are shown in red with green border. Curves represent the fitted linear relationships. For maximum lysis, the data were first linearized by logit transformation (ln [Emax/(100—Emax]), and then back transformed. (c) Antibody-induced CDC of SU-DHL-4 and JVM-13 cells was determined by flow cytometry in the presence of a CD59 blocking antibody. Representative examples out of two experiments are shown.
Thus, HexaBody-CD38 showed considerably higher CDC capacity in vitro compared with daratumumab, including in cell lines that were not sensitive to daratumumab-induced CDC. This could be attributed to the E430G hexamerization-enhancing mutation in its IgG Fc domain.
HexaBody-CD38-mediated CDC is impacted by CD38 and CRP expression levels
CD38 surface expression in the cell line panel ranged from ∼2100 to 415,000 ABS/cell, with most cell lines expressing between 100,000 and 300,000 CD38 molecules per cell (Fig. 2a). CDC potency of HexaBody-CD38 significantly correlated with CD38 expression level, i.e., lower EC50 values were observed at higher levels of target expression (variance F test, F value = 7.0928; P value = 0.01077). In addition, a significant non-linear (sigmoidal) correlation (variance F test, F value = 37.796; P value < 0.0001) was observed between maximum lysis induced by HexaBody-CD38 and CD38 expression level (Fig. 2b).
In line with this, HexaBody-CD38 was unable to induce CDC (defined as <25% maximum kill) of ARH77, Pfeiffer, and U266 cells, which display relatively low CD38 expression (<75,000 CD38 ABS/cell; Fig. 1, Fig. 2a). However, also cell lines expressing higher levels of CD38, such as OPM-2 (∼190,000 ABC/cell) and Granta-519 (∼90,000 ABC/cell), were not responsive to HexaBody-CD38-induced CDC (Fig. 1, Fig. 2a). In contrast, HexaBody-CD38 effectively induced CDC of MOLM-13 cells, which express relatively low levels of CD38 (∼77,000 ABC/cell). This suggests that in addition to CD38 expression, other factors affect the CDC efficiency of HexaBody-CD38. CRP were reported to limit the CDC efficacy of daratumumab,13 and high expression of these proteins by OPM-2 and Granta-519 cells (Fig. S4) may explain the observed unresponsiveness to HexaBody-CD38-induced CDC. In line with this, HexaBody-CD38-mediated CDC in the cell line panel negatively correlated with CD55 expression level (variance F test, F value = 9.1076; P value = 0.0043), and the maximum tumour cell lysis negatively correlated with expression levels of CD46 and CD59 combined (variance F test, F value = 22.248, 2 degrees of freedom; P value < 0.0001) (Fig. S5). Blockade of CD59 in two cell lines with variable CD59 expression levels resulted in enhanced CDC induction by HexaBody-CD38 (Fig. 2c), confirming the direct impact of this CRP on HexaBody-CD38-mediated CDC.
Overall, these data show a positive correlation between HexaBody-CD38-induced CDC and the level of CD38 expression and demonstrated that HexaBody-CD38 can unlock complement-mediated tumour cell lysis in in vitro models with no or modest sensitivity to daratumumab. Expression of CRP negatively impacted the efficiency of CDC induction by HexaBody-CD38 in vitro.
HexaBody-CD38 does not induce lysis of leukocytes or erythrocytes from healthy donors ex vivo
To exclude that the enhanced CDC potency of HexaBody-CD38 would lead to increased cytotoxicity in leukocyte subsets expressing low levels of CD38, lysis of healthy donor leukocytes and erythrocytes was assessed ex vivo. HexaBody-CD38 (20 μg/mL) did not induce lysis of monocytes, B cells, T cells, granulocytes or erythrocytes, and only minimal lysis of NK cells (median 13% lysis, range 9.5–55.1%; Fig. S6a, b). NK-cell lysis induced by HexaBody-CD38 was in the same range as by daratumumab (median 8%, range 4.5–21.2%).
HexaBody-CD38 induces Fc gamma receptor-mediated effector mechanisms
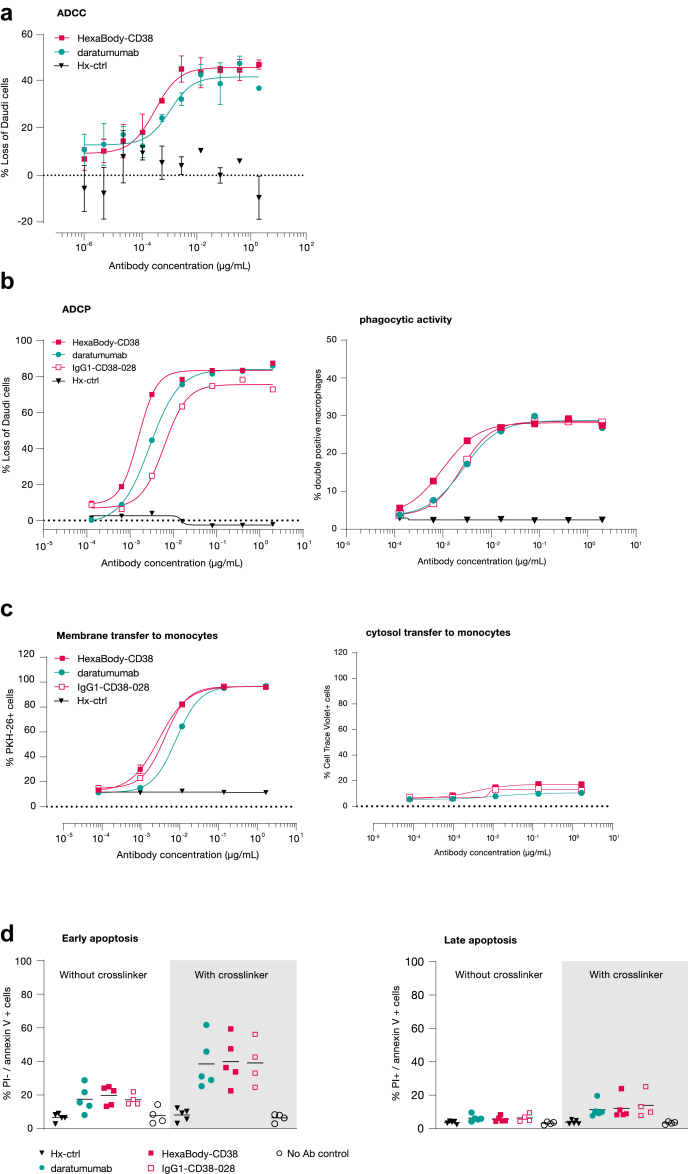
HexaBody-CD38 induced dose-dependent ADCC (Fig. 3a), ADCP (Fig. 3b), trogocytosis (Fig. 3c), and Fc-crosslinking-dependent apoptosis (Fig. 3d) of CD38-expressing tumour cells in vitro. In general, the potency of HexaBody-CD38, the parental antibody of HexaBody-CD38, and daratumumab for these effector mechanisms was in the same range, although it should be noted that HexaBody-CD38 showed a small but statistically significant increase in potency (lower EC50) compared to daratumumab for ADCP and trogocytosis (Fig. 3, Table 2, Fig. S7). This indicates that the E430G mutation did not impact FcγR-mediated effector functions of HexaBody-CD38.
Fig. 3.
HexaBody-CD38 exerts FcγR-mediated effector functions. (a) ADCC was evaluated in calcein AM-labelled Daudi cells opsonized with indicated antibodies that were co-cultured for 4 h with human PBMC. Loss of Daudi cells relative to the ‘no antibody’ control was used as a measure for ADCC. Dose–response curves from one representative responding PBMC donor are shown (out of 10/22 responding PBMC donors), with error bars indicating variation of technical duplicates in the experiment. (b) ADCP was evaluated in calcein AM-labelled Daudi cells opsonized with indicated antibodies that were co-cultured for 24 h with CD11b+ human macrophages. The left panel shown the percentage loss of Daudi cells. The right panel shows the % macrophages with phagocytic activity. In each panel dose–response curves from one representative donor (n = 10) are shown. (c) Trogocytosis was evaluated in CTV- and PKH-26-labeled Wien-133 cells opsonized with indicated antibodies that were co-cultured with monocytes for 90 min. The left panel shows the % PKH-26+ monocytes as a measure for transfer of fluorescently labelled membrane. The right panel shows the percentage of CTV+ monocytes as a measure for transfer of fluorescently labelled cytosol. In each panel dose–response curves from one representative donor (n = 7) are shown. (d) Apoptosis was evaluated in Daudi cells that were incubated with 0.63 μg/mL antibody in the absence or presence of Fc fragment-specific goat anti-human IgG crosslinker. Cells were characterized as early (left panel) and late (right panel) apoptotic cells by flow cytometry. Results from 5 independent experiments are shown.
HexaBody-CD38 modulates CD38 enzyme activity
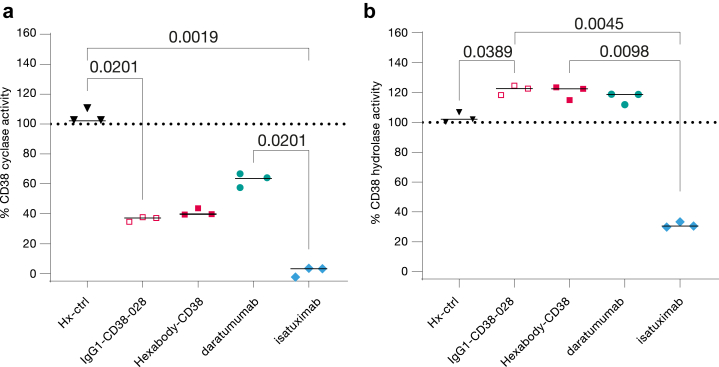
Being an ecto-enzyme, CD38 has both cyclase and hydrolase activity, catalysing the conversion of NAD+ or NADP to the Ca2+ mobilizing metabolites cADPR, ADPR, or NAADP.20 Binding of mAbs to CD38 has previously been shown to affect its cyclase and hydrolase activities,21,22 which is hypothesized to reverse the immunosuppressive tumour microenvironment by inhibiting production of adenosine.20 In enzyme inhibition assays in vitro, HexaBody-CD38 induced significant inhibition of the CD38 cyclase activity (up to 59%, Fig. 4a), and a small increase of hydrolase activity (up to 20%; Fig. 4b). HexaBody-CD38 modulated the CD38 enzyme activities to the same extent as the parental antibody without E430G mutation, indicating that enzyme modulation is a characteristic of the Fab domain specificity. In comparison to daratumumab, HexaBody-CD38 more effectively inhibited CD38 cyclase activity (59% versus 37%), while the effect on hydrolase activity was comparable (20% and 16% increase, respectively). In line with earlier reports,21 isatuximab induced effective inhibition of both the CD38 cyclase (98%) and hydrolase (69%) activities (Fig. 4).
Fig. 4.
HexaBody-CD38 modulates CD38 enzyme activity. The (a) cyclase and (b) hydrolase activity of CD38 expressed on Daudi cells in vitro after incubation with IgG1-CD38-028, HexaBody-CD38, daratumumab, or isatuximab at a concentration of 10 μg/mL. Enzyme activity is expressed as % of the no antibody control (dotted line). Results and the median from three independent experiments are shown. Statistical significance of differences was evaluated using a Friedman test with uncorrected Dunn's post hoc test.
HexaBody-CD38 is monomeric in solution and shows typical IgG1-like pharmacokinetics in mice
Monoclonal antibodies carrying the E430G mutation were shown to be monomeric in solution and are not expected to show accelerated clearance in vivo.1 Before we evaluated the therapeutic potential of HexaBody-CD38 in mouse models, we confirmed that incubation of HexaBody-CD38 in NHS did not result in the production of C4d in vitro (Fig. S8a), indicating that HexaBody-CD38 does not induce fluid-phase, antigen-independent complement activation. The pharmacokinetics (PK) of HexaBody-CD38 in mice were comparable to wild-type IgG1 (Fig. S8b and c; Table S5 and S6). These results are in line with our previous observations showing that HexaBody molecules only form hexameric antibody structures upon target binding on a cell surface.1
Anti-tumour activity of HexaBody-CD38 in patient-derived xenograft models of AML and B-NHL in mice
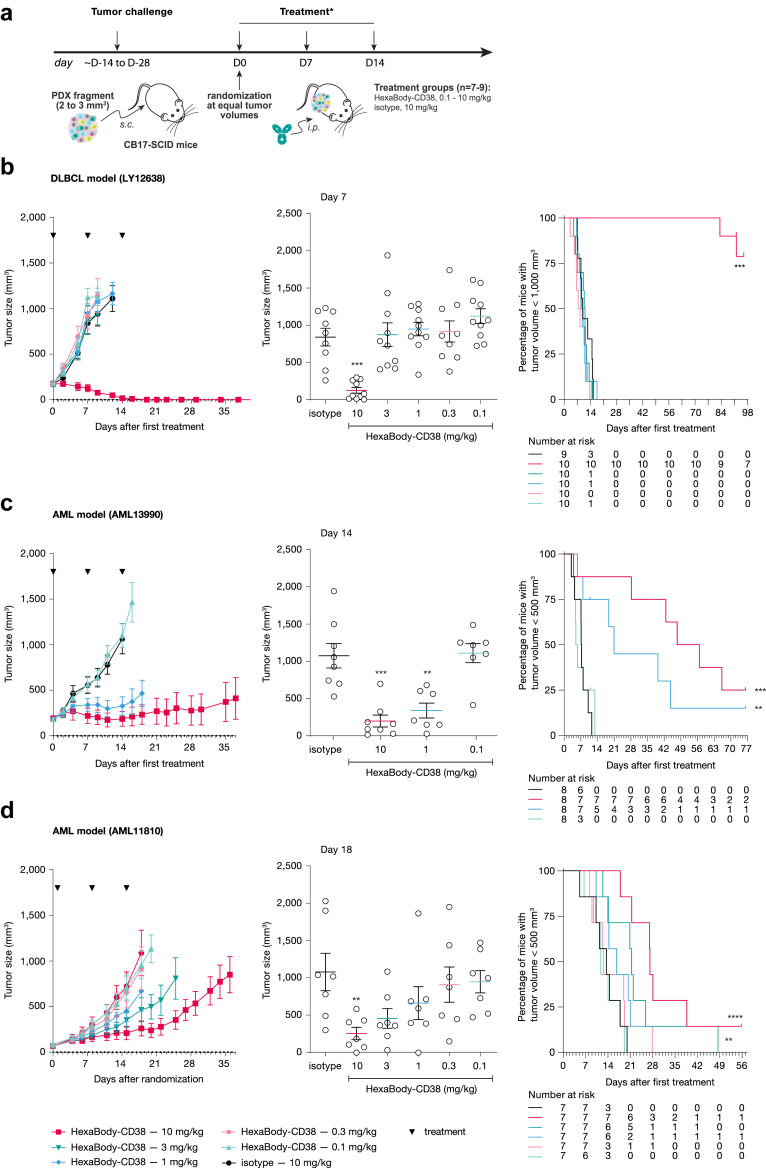
Anti-tumour activity of HexaBody-CD38 has been tested in three PDX models with doses of 0.1–10 mg/kg (Fig. 5a). Treatment with 10 mg/kg HexaBody-CD38 induced complete tumour regression in the DLBCL patient-derived xenograft (PDX) model Ly12638, whereas lower doses did not induce anti-tumour effect (Fig. 5b, Table S7). In AML models, HexaBody-CD38 induced inhibition of tumour growth at doses of 1 mg/kg (AML13990) or 3 mg/kg (AML11810) and higher (Fig. 5c and d, Table S7). Anti-tumour activity was associated with prolonged progression-free survival in all models (Fig. 5, Table S7). Of note, the contribution of mouse lytic complement to the anti-tumour activity in these models is limited23 and therapeutic activity is therefore thought to be driven mainly through FcγR-mediated effector functions. In both the AML11810 and AML13990 models, the majority of evaluated tumours from the control animals showed high tumour cellularity, a low to moderate blood vessel content and CD38 expression in tumours (Fig. S9). Treatment with CD38 decreased tumour size, but remaining tumours showed comparable histology and CD38 expression as control animals (Fig. S9).
Fig. 5.
Evaluation of Dose-dependent anti-tumour activity of HexaBody-CD38 on established B-NHL and AML PDX tumours in vivo. (a) Experimental set up of the evaluation of the anti-tumour activity of HexaBody-CD38 in PDX models in vivo. ∗: treatment started 1 day after randomization in the AML11810 model. Dose-dependent anti-tumour activity of HexaBody-CD38 (3 weekly IV injections) was evaluated in (b) B-NHL PDX model Ly12638 and in AML PDX models (c) AML13990 and (d) AML11810. Treatment with isotype control antibody Hx-ctrl (10 mg/kg) was included as negative control. Left panels show mean tumour growth ± SEM for each treatment group in time. Middle panels show the tumour size for each individual mouse plotted per treatment on the last day that all groups were complete: Day 7 for model Ly12638 (b), Day 14 for model AML13990 (c), and Day 18 for model AML11810 (d). C). ∗∗P < 0.01, ∗∗∗P < 0.001; Mann Whitney test versus Hx-ctrl. Right panels show the % of mice with tumour sizes smaller than 1000 or 500 mm3, as indicated, in Kaplan–Meier plots. ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0005; Mantel Cox Analysis versus Hx-ctrl.
Hexabody-CD38 induces CDC in the presence of daratumumab or isatuximab
Patients enrolled in clinical trials evaluating HexaBody-CD38 may have received prior CD38 mAb treatment and could have residual daratumumab or isatuximab present in the circulation. Therefore, we studied the binding and CDC activity of HexaBody-CD38 in the presence of saturating concentrations of daratumumab and isatuximab.
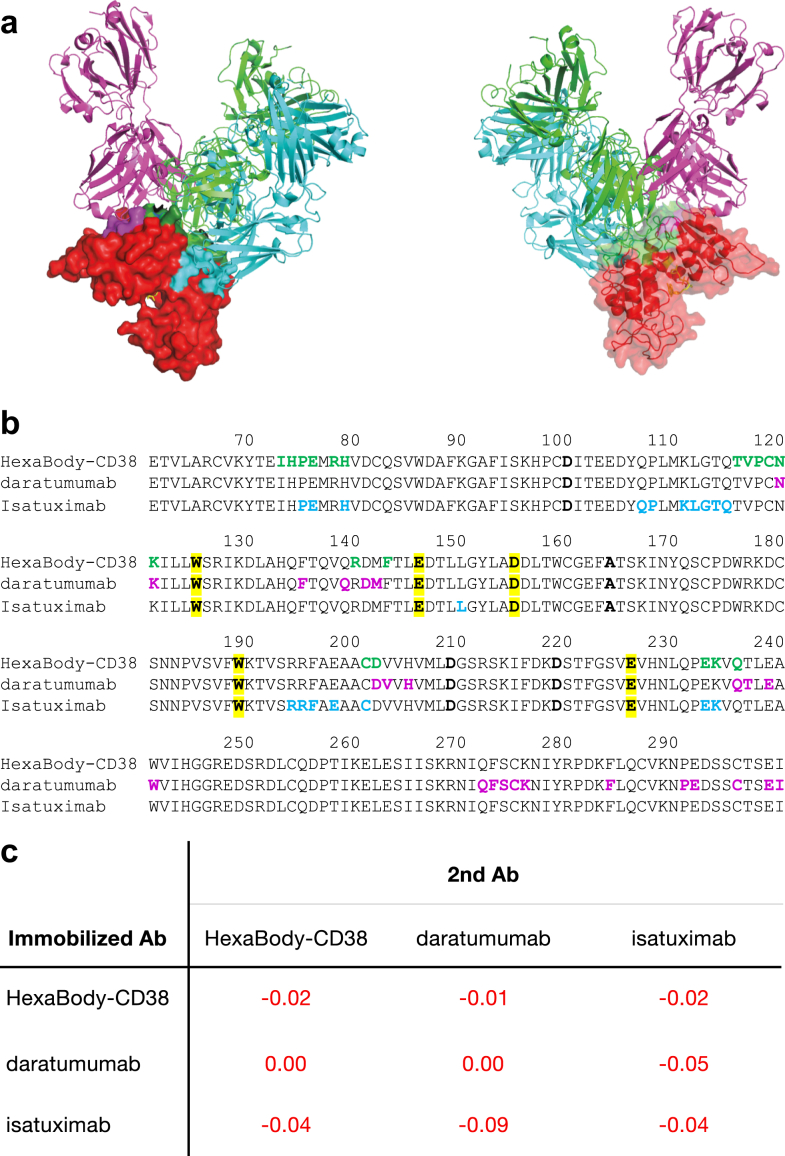
HexaBody-CD38 binds to an epitope on CD38 that is distinct but partially overlapping with the epitopes of daratumumab and isatuximab. Overlaying the crystal structure of HexaBody-CD38 with those reported for daratumumab and isatuximab suggests that simultaneous binding would be blocked due to steric hindrance (Fig. 6a and b). Indeed, antibody crossblock analysis using BLI demonstrated that HexaBody-CD38, daratumumab and isatuximab compete for binding to CD38 (Fig. 6c).
Fig. 6.
Binding competition between HexaBody-CD38, daratumumab, and isatuximab to CD38. HexaBody-CD38 recognizes a unique epitope on CD38 that is distinct from daratumumab and isatuximab. (a) Overlayed co-crystal structures of the Fab-arms of HexaBody-CD38 (green cartoon), daratumumab (7DHA24; magenta cartoon), and isatuximab (4CMH21; cyan cartoon) with soluble CD38 (red surface, transparent in left panel), aligned on the CD38 structures shown from the front and back (left and right panel). CD38 residues that are within a 3.9 Å contact radius with the Fabs are indicated on the surface of CD38 in green, magenta and cyan for HexaBody-CD38, daratumumab and isatuximab, respectively, whereas residues that interact with more than one Fab are shown in dark green. The catalytic site is indicated by a substrate analogue in yellow, superimposed from a published complex of CD38 with PDB code 3P5S.25 (b) Primary amino acid sequence of CD38, numbered according to Uniprot accession P28907-1, with contact residues coloured the same as in (a). Catalytic residues20 are highlighted in yellow. Four point mutants that were introduced to remove glycosylation sites are indicated in bold and are not contact residues in the complexes. (c) CD38 crossblock analysis for HexaBody-CD38, daratumumab, and isatuximab was performed by BLI assay with recombinant human CD38. The numbers in the matrix indicate the response of the second antibody (nm). All responses were <0.1 nm and were considered to be blocking pairs.
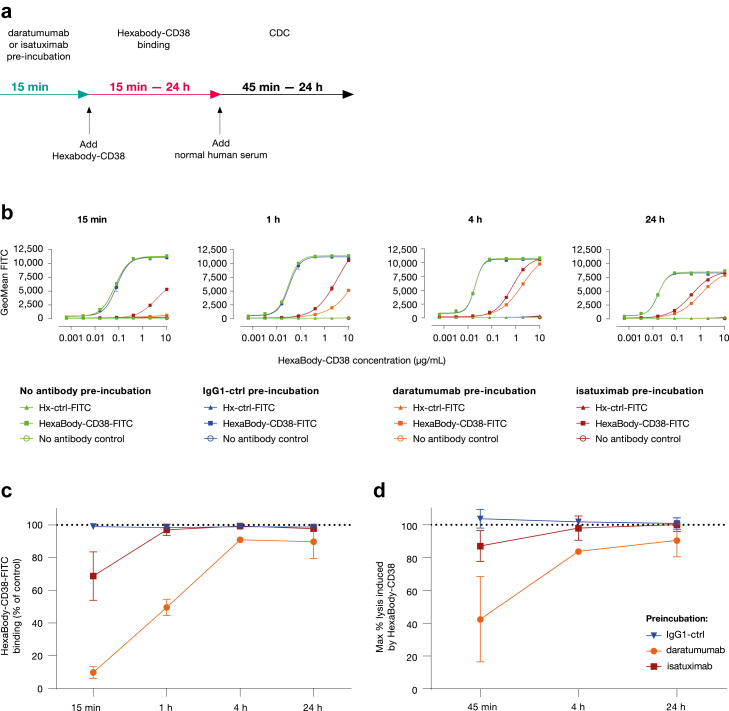
In line with this, binding of HexaBody-CD38 to Wien-133 cells, after 15 min of incubation, was at least partially blocked when cells were opsonized with daratumumab or isatuximab (Fig. 7a–c). When prolonging incubation to 1 h, HexaBody-CD38 binding increased (Fig. 7b and c), and maximal binding, compared with the control without competing antibody, was achieved after 4–24 h incubation (Fig. 7b and c). This indicates that HexaBody-CD38 can partially displace CD38-bound daratumumab and isatuximab over time. The longer time required for displacement of daratumumab compared to isatuximab matches with the relatively higher CD38 binding affinity of daratumumab (3.9 nM) versus isatuximab (6.5 nM; Fig. S1). In the presence of daratumumab or isatuximab, EC50 values for HexaBody-CD38 binding increased, indicating that higher concentrations of HexaBody-CD38 are needed for optimal binding.
Fig. 7.
Binding and CDC induction of HexaBody-CD38 in the presence daratumumab or isatuximab. (a) Experimental set-up to determine the binding and CDC activity of HexaBody-CD38 on Wien-133 cells in the presence of daratumumab or isatuximab. (b) Dose-dependent binding of FITC-conjugated HexaBody-CD38 is shown over time in the presence of daratumumab or isatuximab at a saturating concentration (10 μg/mL). After 15 min incubation with a concentration range of HexaBody-CD38-FITC, the cells were either directly evaluated by flow cytometry (15 min) or incubated for an extended time to evaluate binding after 1 h, 4 h, or 24 h incubation. (Pre-) incubation with IgG1-ctrl or without antibody were included as negative controls. Fluorescence intensity is shown as a measure of antibody-binding. Representative experiments from at least three independent experiments are shown. (c) HexaBody-CD38 (10 μg/mL) binding in the presence of IgG1-ctrl, daratumumab, or isatuximab over time as percentage of HexaBody-CD38 binding without antibody pre-incubation. (d) The capacity of HexaBody-CD38 (10 μg/mL) to induce CDC of Wien-133 cells in the presence of equal amounts of daratumumab or isatuximab over time. CDC was evaluated by flow cytometry after 45 min, 4 h, or 24 h. (Pre-) incubation with IgG1-ctrl, or without antibody were included as negative controls. The % lysis was calculated as 100% minus the % TO-PRO-3-negative (viable) cells. Mean ± SD of antibody binding (c) and lysis (d) are shown from at least three independent experiments (except for the 4 h timepoint in the presence of daratumumab, which was only included once).
Next, HexaBody-CD38-induced CDC was evaluated in the presence of daratumumab and isatuximab in Wien-133 cells, which are sensitive for CDC induced by HexaBody-CD38, but not by daratumumab or isatuximab (Fig. S10). When CDC was allowed for 45 min, HexaBody-CD38-mediated CDC was partially blocked in the presence of daratumumab or isatuximab (Fig. 7d). When allowing CDC for 4 or 24 h, HexaBody-CD38 induced >80% of maximum lysis in the presence of daratumumab or isatuximab as compared to HexaBody-CD38-induced CDC in absence of another CD38-targeting antibody (Fig. 7d). Of note, when HexaBody-CD38 binding was extended to 24 h after which CDC was allowed for only 45 min, HexaBody-CD38 also induced >90% of maximum CDC in the presence of daratumumab or isatuximab (data not shown), indicating that the displacement of CD38-bound antibody by HexaBody-CD38 caused the delayed CDC induction.
Thus, HexaBody-CD38 is capable of inducing CDC in the presence of daratumumab or isatuximab, suggesting that the capacity of HexaBody-CD38 to induce CDC may not be impacted in patients that were recently treated with daratumumab or isatuximab.
HexaBody-CD38 induces CDC of primary MM cells ex vivo
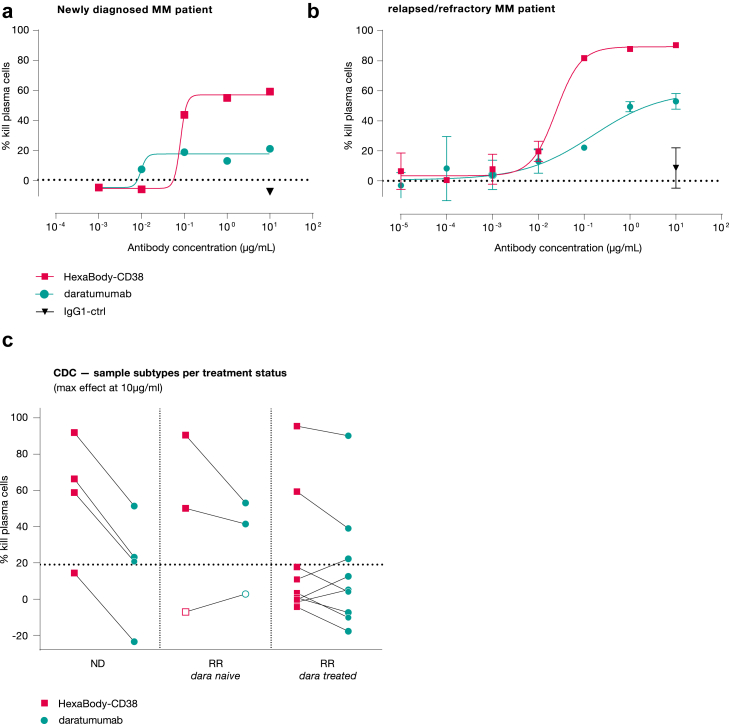
The sensitivity of primary MM cells to HexaBody-CD38-induced CDC was evaluated in BMMC samples obtained from NDMM and RRMM patients ex vivo (Table 1). HexaBody-CD38 induced dose-dependent CDC of malignant plasma cells in 3 out of 4 NDMM patient samples, 2 out of 3 daratumumab-naïve RRMM patient samples, and 2 out of 9 daratumumab-treated RRMM patient samples evaluated (Fig. 8). The average maximal tumour cell kill in the responding patient samples (defined as maximal tumour cell lysis >19%) was 73%, with a concentration-response relationship comparable to what was observed in cell lines (Fig. 8a and b). When compared with daratumumab, HexaBody-CD38 induced more effective CDC of primary MM cells, with an average maximal tumour cell lysis of 73% compared to 46% for daratumumab in responding samples.
Fig. 8.
HexaBody-CD38 induced potent CDC of primary MM cells ex vivo. CDC induction by HexaBody-CD38 and daratumumab in primary MM cells was determined ex vivo. Dose-dependent CDC by HexaBody-CD38 and daratumumab in a newly diagnosed MM patient sample (a) and a daratumumab-naïve RR patient sample (b). (c) CDC activity of 10 μg/mL HexaBody-CD38 and daratumumab in ND (n = 4), daratumumab-naïve RR (n = 3) and daratumumab-treated RR (n = 9) MM patient samples. The dotted line represents the background lysis (19%). Lines between HexaBody-CD38 and daratumumab symbols link values observed in the same samples. Open symbols represent CDC activity observed after incubation with 1 μg/mL instead of 10 μg/mL antibody.
HexaBody-CD38 induced tumour cell lysis in two daratumumab-treated RRMM patient samples that had been sampled 39 and 112 weeks after the last daratumumab dosing. Tumour cells present in these samples showed higher CD38 expression levels compared to samples that were obtained within 8 weeks after discontinuation of daratumumab (data not shown). This is in line with previous observations showing that CD38 levels in MM patients initially declined after daratumumab, and returned to pre-treatment levels approximately 6 months after discontinuation of treatment.13 However, one RRMM patient sample obtained 59 weeks after the last daratumumab dose showed sustained low CD38 expression and was not responsive to HexaBody-CD38-mediated CDC. In line with a lack of cytotoxicity previously noted in healthy donor leukocytes, HexaBody-CD38 induced only minimal or no CDC of non-malignant NK cells, monocytes, B cells, and T cells that were present in these BMMC samples (Fig. S6c).
Discussion
HexaBody-CD38 is a next-generation CD38 antibody that binds with high affinity to human CD38 and carries the hexamerization-enhancing mutation E430G. HexaBody-CD38 is designed to induce strong anti-tumour activity in patients with CD38-expressing hematopoietic malignancies through highly potent CDC, in addition to FcγR-mediated effector functions. Furthermore, HexaBody-CD38 inhibits CD38 cyclase activity, which may relieve immune suppression in the tumour microenvironment.
The parental antibody clone of Hexabody-CD38 without E430G mutation in its Fc domain did not induce significant CDC, showing that the hexamerization-enhancing mutation is essential for the high CDC potency of HexaBody-CD38.
The CDC activity of HexaBody-CD38 was confirmed in a panel of tumour cell lines of MM, AML, and B-NHL origin. The sensitivity of these cell lines to HexaBody-CD38-mediated CDC positively correlated with CD38 expression levels. Previously it was shown that MM patients with a partial response or better to daratumumab showed significantly higher pre-treatment levels of CD38 on MM cells compared with nonresponders.13 Our preclinical data suggest that a similar association may be expected for HexaBody-CD38. However, HexaBody-CD38 was able to induce CDC in tumour cell lines that were not sensitive to daratumumab, and the potency of HexaBody-CD38 across cell lines was significantly higher than that of daratumumab. Increased potency of HexaBody-CD38 was most evident in cell lines with low to intermediate levels of CD38 expression. Since the level of CD38 expression was associated with response in MM patients treated with daratumumab,13 this may indicate enhanced therapeutic potential of HexaBody-CD38 compared to daratumumab in MM patients with lower levels of CD38 expression. Indeed, HexaBody-CD38 induced more effective CDC of primary MM cells when compared to daratumumab. It should be noted that the sample size was relatively small and more data across naïve, RR (with and without prior daratumumab treatment) would be required for better understanding. In addition to MM, HexaBody-CD38 may provide therapeutic opportunity in indications with lower levels of CD38 expression, in which daratumumab did not have clinical activity, such as B-NHL.11 Of note, HexaBody-CD38 showed preclinical anti-tumour activity in murine PDX models of AML and DLBCL in vivo, supporting clinical investigation of HexaBody-CD38 in these CD38 positive hematologic malignancies. It should be noted that these PDX models were selected based on expression of CD38.
Importantly, the increased capacity of HexaBody-CD38 to induce cytotoxicity in tumour cells with lower CD38 expression levels did not come at the cost of increased specific lysis of healthy leukocytes and erythrocytes ex vivo. This is most likely explained by low CD38 expression levels in these cell populations, while expression levels of CRP are relatively high (data not shown). Although HexaBody-CD38 and daratumumab induced only minimal killing of NK cells ex vivo, daratumumab-induced a rapid and strong reduction of NK cell count in patients,26 which could be attributed to ADCC activity and may also be expected after treatment with HexaBody-CD38. However, NK cells are not completely depleted by daratumumab in patients, and NK-cell reduction was not related to efficacy or the safety profile of daratumumab.26
Expression of CRP, such as CD46, CD55, and CD59, may limit the clinical efficacy of CD38 antibodies, as shown for daratumumab.13 In a panel of cell lines we showed that higher CRP expression levels correlated with lower sensitivity to HexaBody-CD38 in vitro. Whether CRP expression impacts Hexabody-CD38 efficacy in the clinic will be evaluated in an ongoing clinical trial.
In addition to CDC, HexaBody-CD38 induced FcγR-mediated effector functions, including ADCC, ADCP, trogocytosis, and induction of apoptosis after Fc-crosslinking in CD38-expressing cells in vitro. The potency of HexaBody-CD38 for these effector mechanisms was in the same range as daratumumab. This indicates that CDC is the main differentiating effector mechanism of HexaBody-CD38 compared with daratumumab. Previous studies showed that the CD38 mAbs daratumumab, isatuximab, felzartamab, and mezagitamab demonstrated comparable ADCC, ADCP, trogocytosis, and crosslinker-mediated apoptosis in vitro, while daratumumab demonstrated higher CDC activity and more efficient depletion of MM cells in the presence of human serum.27 This suggests that the potent CDC activity of HexaBody-CD38 not only outperforms that of daratumumab, but also that of isatuximab, felzartamab, and mezagitamab.
HexaBody-CD38 modulated the CD38 cyclase and hydrolase activities, which was independent of the E430G mutation in the Fc domain and appeared to be a characteristic of the Fab domain specificity even though the epitope does not overlap with the catalytic site (Fig. S2). CD38 enzymatic activity is hypothesized to contribute to production of immunosuppressive adenosine, in particular under acidic pH conditions in the MM bone marrow niche.28 It remains uncertain whether antibody-mediated inhibition of CD38 enzymatic activity, by itself, significantly contributes to the anti-tumour and immune stimulating activity of CD38 antibodies. CD38 mAbs may also reduce CD38 enzyme activity in the tumour microenvironment indirectly, through selective killing of CD38high cells or trogocytosis.29 By targeting cells with lower CD38 expression levels, HexaBody-CD38 may reduce CD38 in the tumour microenvironment to a larger extent than daratumumab, possibly leading to stronger reduction of CD38-dependent metabolism and a less immunosuppressive tumour microenvironment.
In summary, the mechanism of action of HexaBody-CD38 consists of multiple effector mechanisms including CDC, FcγR-mediated effector functions and inhibition of CD38 enzymatic activity. While effector functions overlap between daratumumab and isatuximab, HexaBody-CD38 shows a particular increase in CDC potency. Although the association between daratumumab resistance and expression of CRP in MM patients13 suggests a clinically relevant contribution of CDC to antitumour activity of daratumumab, it is not known which effector mechanisms are the key drivers of clinical benefit of CD38 mAb treatment in MM patients. As a consequence, it is not known if enhanced CDC activity will drive increased anti-tumour activity of HexaBody-CD38 compared to other CD38 mAbs in the clinical setting.
Treatment with daratumumab reduces CD38 expression on MM cells initially, after which CD38 expression levels gradually return to baseline levels approximately 6 months after the last daratumumab dose.13 This suggests that, over time, tumour cells in patients who had prior exposure to CD38 mAbs may express CD38 levels sufficient for targeting with HexaBody-CD38. Indeed, HexaBody-CD38 was able to effectively kill MM cells from daratumumab-relapsed patients, in particular longer (>26 weeks) after the last daratumumab dose, presumably after daratumumab wash-out. To mimic a situation where MM patients are dosed with HexaBody-CD38 within a timeframe that would allow for the presence of residual daratumumab or isatuximab present in their circulation, we confirmed the CDC activity of HexaBody-CD38 in the presence of these CD38 mAbs in cell lines. Even though Hexabody-CD38, daratumumab and isatuximab all compete for binding to CD38, HexaBody-CD38 was able to displace CD38-bound daratumumab and isatuximab over time, plausibly because of its higher binding affinity and induce CDC of daratumumab- and isatuximab-resistant cells. However, MM cells from patients shortly (0–8 weeks) after the last daratumumab dose were not responsive to HexaBody-CD38 in this assay, possibly because of low CD38 expression levels. Furthermore, the short duration of the CDC assay (45 min) may have limited the CDC capacity of HexaBody-CD38 in samples from patients who were recently exposed to daratumumab.
The safety and preliminary efficacy of HexaBody-CD38 are currently being evaluated in a first-in-human trial in MM patients (NCT04824794), in which exploratory biomarker analyses are included to study the clinical mechanism of action and pharmacodynamics.
Contributors
Ida H. Hiemstra: conceptualisation, data curation, formal analysis, methodology, project administration, supervision, validation, visualisation, writing—original draft, writing—review & editing.
Kim C.M. Santegoets: conceptualisation, data curation, formal analysis, methodology, project administration, supervision, validation, visualisation, writing—original draft, writing—review & editing.
Maarten L. Janmaat: conceptualisation, visualisation, writing—original draft, writing—review & editing.
Bart E.C.G. De Goeij: conceptualisation, data curation, formal analysis, methodology, project administration, supervision, writing—review & editing.
Wessel ten Hagen: data curation, investigation, methodology, validation, visualisation, writing—review & editing.
Sanne van Dooremalen: data curation, investigation, methodology, validation, writing—review & editing.
Peter Boross: data curation, formal analysis, methodology, project administration, supervision, validation, writing—review & editing.
Jeroen van den Brakel: data curation, formal analysis, investigation, methodology, visualisation, writing—review & editing.
Sieto Bosgra: data curation, formal analysis, software, validation, visualisation, writing—review & editing.
Grietje Andringa: data curation, investigation, methodology, validation, writing—review & editing.
Berris Van Kessel: data curation, investigation, methodology, validation, writing—review & editing.
Dennis Verzijl: data curation, formal analysis, methodology, project administration, supervision, validation, visualisation, writing—review & editing.
Rick Hibbert: data curation, formal analysis, methodology, project administration, supervision, validation, visualisation, writing—review & editing.
Kristine A. Frerichs: methodology, supervision, writing—review & editing.
Tuna Mutis: methodology, supervision, writing—review & editing.
Niels W. C. J. Van De Donk: methodology, supervision, writing—review & editing.
Tahamtan Ahmadi: supervision, writing—review & editing.
David Satijn: conceptualisation, supervision, writing—review & editing.
A. Kate Sasser: supervision, writing—review & editing.
Esther C. W. Breij: conceptualisation, project administration, supervision, validation, writing—review & editing. All authors read and approved the final version of the manuscript.
Data sharing statement
The crystal structure of HexaBody-CD38 Fab in complex with CD38 has been deposited with the Protein Data Bank with accession number 8BYU. All other data are available in the main text or the supplementary materials. Raw data obtained in the current study are available from the corresponding author under a confidentiality agreement.
Declaration of interests
IH, KS, MJ, BdG, WtH, SvD, PB, JvdB, SB, GA, BvK, DV, RH, TA, DS, AS, and EB are (former) employees of Genmab and own Genmab warrants and/or stock. BdG, GA, TA, and DS are co-inventors on patents related to the data described in the manuscript. NvdD received research funding from Janssen Pharmaceuticals, Amgen, Celgene, Novartis, Cellectis, and BMS, and served as a consultant or advisor to Janssen, Amgen, Celgene, BMS, Takeda, Roche, Novartis, Bayer, Adaptive, and Servier. TM has received research support from Janssen Pharmaceuticals, Genmab, Takeda, Onkimmune, and Gilead. KF declares no competing interests.
Acknowledgments
The authors would like to thank Rajaa Snijdewint—Nkairi, Laurens Kil, Gilbert van den Tillaart, Marie Lecomte, Veronique Roolker-Knaup, Amber van Kooten Niekerk, and Leonardo Astolfi Rosado for scientific input and/or technical support.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104663.
Appendix A. Supplementary data
References
- 1.de Jong R.N., Beurskens F.J., Verploegen S., et al. A novel platform for the potentiation of therapeutic antibodies based on antigen-dependent formation of IgG hexamers at the cell surface. PLoS Biol. 2016;14(1) doi: 10.1371/journal.pbio.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diebolder C.A., Beurskens F.J., de Jong R.N., et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343(6176):1260–1263. doi: 10.1126/science.1248943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schutze K., Petry K., Hambach J., et al. CD38-Specific biparatopic heavy chain antibodies display potent complement-dependent cytotoxicity against multiple myeloma cells. Front Immunol. 2018;9:2553. doi: 10.3389/fimmu.2018.02553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oostindie S.C., van der Horst H.J., Kil L.P., et al. DuoHexaBody-CD37((R)), a novel biparatopic CD37 antibody with enhanced Fc-mediated hexamerization as a potential therapy for B-cell malignancies. Blood Cancer J. 2020;10(3):30. doi: 10.1038/s41408-020-0292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin P., Owens R., Tricot G., Wilson C.S. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004;121(4):482–488. doi: 10.1309/74R4-TB90-BUWH-27JX. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim S., Keating M., Do K.A., et al. CD38 expression as an important prognostic factor in B-cell chronic lymphocytic leukemia. Blood. 2001;98(1):181–186. doi: 10.1182/blood.v98.1.181. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y., McKenna R.W., Asplund S.L., Kroft S.H. Comparison of immunophenotypes of small B-cell neoplasms in primary lymph node and concurrent blood or marrow samples. Am J Clin Pathol. 2002;118(5):758–764. doi: 10.1309/11J6-0U42-VF4E-WA02. [DOI] [PubMed] [Google Scholar]
- 8.Keyhani A., Huh Y.O., Jendiroba D., et al. Increased CD38 expression is associated with favorable prognosis in adult acute leukemia. Leuk Res. 2000;24(2):153–159. doi: 10.1016/s0145-2126(99)00147-2. [DOI] [PubMed] [Google Scholar]
- 9.Rodig S.J., Vergilio J.A., Shahsafaei A., Dorfman D.M. Characteristic expression patterns of TCL1, CD38, and CD44 identify aggressive lymphomas harboring a MYC translocation. Am J Surg Pathol. 2008;32(1):113–122. doi: 10.1097/PAS.0b013e3180959e09. [DOI] [PubMed] [Google Scholar]
- 10.van de Donk N., Richardson P.G., Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131(1):13–29. doi: 10.1182/blood-2017-06-740944. [DOI] [PubMed] [Google Scholar]
- 11.Salles G., Gopal A.K., Minnema M.C., et al. Phase 2 study of daratumumab in relapsed/refractory mantle-cell lymphoma, diffuse large B-cell lymphoma, and follicular lymphoma. Clin Lymphoma Myeloma Leuk. 2019;19(5):275–284. doi: 10.1016/j.clml.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Martin T.G., Corzo K., Chiron M., et al. Therapeutic opportunities with pharmacological inhibition of CD38 with isatuximab. Cells. 2019;8(12):1522. doi: 10.3390/cells8121522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nijhof I.S., Casneuf T., van Velzen J., et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128(7):959–970. doi: 10.1182/blood-2016-03-703439. [DOI] [PubMed] [Google Scholar]
- 14.Frerichs K.A., Minnema M.C., Levin M.D., et al. Efficacy and safety of daratumumab combined with all-trans retinoic acid in relapsed/refractory multiple myeloma. Blood Adv. 2021;5(23):5128–5139. doi: 10.1182/bloodadvances.2021005220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frerichs K.A., Broekmans M.E.C., Marin Soto J.A., et al. Preclinical activity of JNJ-7957, a novel BCMAxCD3 bispecific antibody for the treatment of multiple myeloma, is potentiated by daratumumab. Clin Cancer Res. 2020;26(9):2203–2215. doi: 10.1158/1078-0432.CCR-19-2299. [DOI] [PubMed] [Google Scholar]
- 16.de Weers M., Tai Y.T., van der Veer M.S., et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–1848. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 17.Burton D.R., Pyati J., Koduri R., et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266(5187):1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 18.Graeff R.M., Walseth T.F., Fryxell K., Branton W.D., Lee H.C. Enzymatic synthesis and characterizations of cyclic GDP-ribose. A procedure for distinguishing enzymes with ADP-ribosyl cyclase activity. J Biol Chem. 1994;269(48):30260–30267. [PubMed] [Google Scholar]
- 19.Muller H.M., Muller C.D., Schuber F. NAD+ glycohydrolase, an ecto-enzyme of calf spleen cells. Biochem J. 1983;212(2):459–464. doi: 10.1042/bj2120459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee H.C. Structure and enzymatic functions of human CD38. Mol Med. 2006;12(11-12):317–323. doi: 10.2119/2006-00086.Lee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deckert J., Wetzel M.C., Bartle L.M., et al. SAR650984, A novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin Cancer Res. 2014;20(17):4574–4583. doi: 10.1158/1078-0432.CCR-14-0695. [DOI] [PubMed] [Google Scholar]
- 22.van de Donk N.W., Janmaat M.L., Mutis T., et al. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016;270(1):95–112. doi: 10.1111/imr.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ong G.L., Mattes M.J. Mouse strains with typical mammalian levels of complement activity. J Immunol Methods. 1989;125(1-2):147–158. doi: 10.1016/0022-1759(89)90088-4. [DOI] [PubMed] [Google Scholar]
- 24.Lee H.T., Kim Y., Park U.B., Jeong T.J., Lee S.H., Heo Y.S. Crystal structure of CD38 in complex with daratumumab, a first-in-class anti-CD38 antibody drug for treating multiple myeloma. Biochem Biophys Res Commun. 2021;536:26–31. doi: 10.1016/j.bbrc.2020.12.048. [DOI] [PubMed] [Google Scholar]
- 25.Egea P.F., Muller-Steffner H., Kuhn I., et al. Insights into the mechanism of bovine CD38/NAD+glycohydrolase from the X-ray structures of its Michaelis complex and covalently-trapped intermediates. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0034918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casneuf T., Xu X.S., Adams H.C., 3rd, et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv. 2017;1(23):2105–2114. doi: 10.1182/bloodadvances.2017006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinder M., Bahlis N.J., Malavasi F., et al. Comparison of CD38 antibodies in vitro and ex vivo mechanisms of action in multiple myeloma. Haematologica. 2021;106(7):2004–2008. doi: 10.3324/haematol.2020.268656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horenstein A.L., Quarona V., Toscani D., et al. Adenosine generated in the bone marrow niche through a CD38-mediated pathway correlates with progression of human myeloma. Mol Med. 2016;22:694–704. doi: 10.2119/molmed.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krejcik J., Frerichs K.A., Nijhof I.S., et al. Monocytes and granulocytes reduce CD38 expression levels on myeloma cells in patients treated with daratumumab. Clin Cancer Res. 2017;23(24):7498–7511. doi: 10.1158/1078-0432.CCR-17-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.