ABSTRACT
Background: Pre-and post-traumatic hypothalamic–pituitary–adrenal (HPA) axis markers have been studied to predict posttraumatic stress disorder (PTSD) risk, but its acute reactivity cannot be measured in real-life settings. Experimental paradigms can depict the cortisol response to stimuli that simulate traumatic events.
Objective: To review experimental studies on the cortisol response to traumatic stimuli and the correlation between cortisol and PTSD symptoms.
Method: Experimental, (un-)published studies in German or English from any year were eligible if they confronted non-traumatized humans with traumatic stimuli, assessed cortisol before, during or after stimulus presentation and subsequent PTSD symptoms. The literature was searched via PubMed, PubPsych, PsychINFO, PsycArticle, Web of Science, EMBASE, ProQuest and ClinicalTrials.gov up to 16th February 2021. Risk of bias was assessed with the Cortisol Assessment List. Multilevel-meta-analyses were conducted under the random effects model. The standardized mean change (dSMC) indicated the cortisol response. Coefficient r indicated the correlations between cortisol and PTSD symptoms.
Results: 14 studies, investigating 1004 individuals, were included. A cortisol response was successfully induced between 21 and 40 min post-presentation onset (kobservations = 25, dSMC = 0.15 [.03; .26]). Cortisol was not associated with overall or cluster-level PTSD symptoms. On a symptom-level, higher pre-presentation onset cortisol was correlated with lower state tension (k = 8, r = −.18 [−.35; −.01]), higher state happiness (k = 8, r = −.34 [−.59; −.03], variable inverted) and lower state anger (k = 9, r = −.14 [−.26; −.01]). Higher post-presentation onset cortisol was correlated with higher state happiness (k = 16, r = −.20 [−.33; −.06]) and lower state sadness (k = 17, r = −.16 [−.25; −.05]), whereas cortisol response was positively correlated with state anxiety (k = 9, r = .16 [0.04; 0.27]).
Conclusions: Experimental paradigms effectively induce a cortisol response. Higher basal cortisol, higher cortisol, as measured after traumatic stimulus presentation, and a lower cortisol response were associated with more adaptive emotional reactions. These markers did not predict longer-term PTSD symptoms.
KEYWORDS: Prognostic biomarker, trauma film, glucocorticoid, endocrine, intrusion
HIGHLIGHTS
Experimental trauma paradigms successfully induced a cortisol response.
Cortisol was predictive for single state, emotion-related symptoms, but not overall PTSD symptoms.
Trauma paradigms shed light into the immediate post-trauma period that is hard to capture in real life, but the gap between experimental and naturalistic settings is difficult to overcome.
Abstract
Antecedentes: Se han estudiado los marcadores del eje hipotálamo-pituitario-suprarrenal (HPA) pretraumático y postraumático para predecir el riesgo de trastorno de estrés postraumático (TEPT), pero su reactividad aguda no puede ser medida en escenarios de la vida real. Los paradigmas experimentales pueden representar la respuesta del cortisol a los estímulos que simulan eventos traumáticos.
Objetivo: Revisar los estudios experimentales sobre la respuesta del cortisol a los estímulos traumáticos y la correlación entre el cortisol y los síntomas del TEPT.
Método: Los estudios experimentales (no) publicados en alemán o inglés de cualquier año fueron elegibles si confrontaron a humanos no traumatizados con estímulos traumáticos, evaluaron el cortisol antes, durante o después de la presentación del estímulo y los síntomas de TEPT subsecuentes. Se realizaron búsquedas en la literatura a través de PubMed, PubPsych, PsychINFO, PsycArticle, Web of Science, EMBASE, ProQuest y ClinicalTrials.gov hasta el 16 de febrero de 2021. El riesgo de sesgo se evaluó con la Lista de evaluación de cortisol. Se realizaron metaanálisis multinivel bajo el modelo de efectos aleatorios. El cambio medio estandarizado (dSMC) indicó la respuesta del cortisol. El coeficiente r indicó las correlaciones entre el cortisol y los síntomas de TEPT.
Resultados: Se incluyeron 14 estudios que investigaron a 1004 personas. Se indujo con éxito una respuesta de cortisol entre 21 y 40 minutos después del inicio de la presentación (Kobservaciones = 25, dSMC = 0,15 [0,03; 0,26]). El cortisol no se asoció con síntomas generales o a nivel de grupos sintomáticos de TEPT. A nivel de síntomas, un nivel más alto de cortisol antes de la presentación se correlacionó con un estado de tensión más bajo (k = 8, r = −0,18 [−0,35; −0,01]), estado de felicidad más alto (k = 8, r = − .34 [−.59; −.03], variable invertida) y menor estado de ira (k = 9, r = −.14 [−.26; −.01]). Un nivel más alto de cortisol posterior a la presentación se correlacionó con un estado de felicidad más alto (k = 16, r = −0,20 [−0,33; −0,06]) y un estado de tristeza más bajo (k = 17, r = −0,16 [−0,25]; −0,05]), mientras que la respuesta de cortisol se correlacionó positivamente con el estado de ansiedad (k = 9, r = 0,16 [0,04; 0,27]).
Conclusiones: Los paradigmas experimentales inducen efectivamente una respuesta de cortisol. El cortisol basal más alto, el cortisol más alto, medido después de la presentación del estímulo traumático, y una respuesta de cortisol más baja se asociaron con reacciones emocionales más adaptativas. Estos marcadores no predijeron los síntomas del TEPT a largo plazo.
PALABRAS CLAVE: Biomarcador pronóstico, película de trauma, glucocorticoide, endocrino, intrusión
Abstract
背景:已经研究了创伤前和创伤后下丘脑-垂体-肾上腺 (HPA) 轴标志物来预测创伤后应激障碍 (PTSD) 风险,但无法在现实生活中测量其急性反应性。实验范式可以描述皮质醇对模拟创伤事件刺激的反应。
目的:综述关于皮质醇对创伤性刺激的反应以及皮质醇与 PTSD 症状之间相关性的实验研究。
方法:任何一年以德语或英语发表的实验性、(未)发表、让未受创伤的人受到创伤性刺激并在刺激呈现之前、期间或之后评估皮质醇以及随后的 PTSD 症状的研究都是合格的。 通过 PubMed、PubPsych、PsychINFO、PsycArticle、Web of Science、EMBASE、ProQuest 和 ClinicalTrials.gov 搜索了截至 2021 年 2 月 16 日的文献。使用皮质醇评估列表评估了偏倚风险。 在随机效应模型下进行了多水平元分析。标准化平均变化 (dSMC) 表示皮质醇反应。系数 r 表示皮质醇和 PTSD 症状之间的相关性。
结果:纳入了 14 项研究,考查了 1,004 人。在出现后 21–40 分钟期间成功诱导皮质醇反应(kobservations = 25,dSMC = 0.15 [0.03; 0.26])。皮质醇与整体或症状簇水平的 PTSD 症状无关。在症状水平上,较高的呈现前初始皮质醇与较低的状态紧张 (k = 8, r = −.18 [−.35; −.01])、较高的状态幸福感 (k = 8, r = − .34 [−.59; −.03],变量反向计分)和较低的状态愤怒(k = 9,r = −.14 [−.26; −.01])相关。较高的呈现后初始皮质醇与较高的幸福状态 (k = 16, r = −.20 [−.33; −.06]) 和较低的悲伤状态 (k = 17, r = −.16 [−.25]; −.05]) 相关,而皮质醇反应与状态焦虑呈正相关 (k = 9, r = .16 [.04; .27])。
结论:实验范式有效地诱导皮质醇反应。较高的基线皮质醇、较高的皮质醇(在呈现创伤性刺激后测量)和较低的皮质醇反应与更适应性的情绪反应相关。这些标志物不能预测长期的 PTSD 症状。
关键词: 预后生物标志物, 创伤影片, 糖皮质激素, 内分泌, 闯入
1. Theoretical background
After exposure to a traumatic event, that is, actual or threatened death, serious injury or sexual violence (American Psychiatric Association, 2013), most individuals suffer from acute, but transient distress. This can be considered a normal response to an extraordinary stressor (Bonanno & Loss, 2004). While many individuals are resilient to traumatic stress (Galatzer-Levy et al. 2018), some develop mental disorders. The most prominent disorders are acute stress disorder, with prevalence rates between 14.1% and 36.0% within the first month post-trauma (Geoffrion et al., 2020), and posttraumatic stress disorder (PTSD), with an estimated lifetime prevalence rate of 5.6% among trauma-exposed individuals (Koenen et al., 2017). In order to provide targeted preventive interventions for individuals who are at increased risk for PTSD, it is necessary to understand how they differ from resilient individuals.
To address this question, many studies analysed pre-, peri-, and post-traumatic factors and their association with later PTSD development. Studies on brain-derived prognostic markers focused particularly on post-traumatic factors collected in emergency departments or other facilities for acute post-trauma health care (Schultebraucks et al., 2020). These studies highlighted that a smaller hippocampal volume (Gilbertson et al., 2002), decreased functional cortico-limbic connectivity (Harnett et al., 2021), as well as hyperreactivity of limbic brain regions might contribute to later PTSD development (Stevens et al., 2017). The importance of enhanced limbic activity was confirmed by studies focusing on pre-traumatic biological risk factors (Admon et al., 2009; McLaughlin et al., 2014). Information on such pre-traumatic factors can best be collected by following up on cohorts with increased risk for trauma exposure, such as first responders or military personnel (Berger et al., 2012; Hoge et al., 2004).
Besides structural and functional neuroimaging approaches, PTSD risk can be informed by other prognostic biomarkers, including (epi-)genetic markers, neuromodulators, autonomic markers or hormones (as reviewed in Pitman et al. (2012)). Among these, hypothalamic–pituitary–adrenal (HPA) axis markers, as derived from tissues such as blood or saliva which capture the immediate cortisol response within minutes after a stressor, as well as from as hair, fingernails, or urine, which reflect cumulative cortisol output from several hours (urine) to months (hair, fingernails), have most commonly been studied (Olff & van Zuiden, 2017), albeit often with inconsistent results (Engel et al., 2022). In order to address these inconsistencies and correctly interpret previous evidence, it seems necessary to distinguish between long- and short-term markers of HPA axis regulation, as well as to disentangle the respective impact of trauma exposure and PTSD symptoms. An integrative, theoretical model suggests that trauma exposure causes a short-term increase, but a subsequent decrease of cortisol output. That way, following a dose–response-relationship, cumulative trauma is suggested to lead to a chronic HPA axis downregulation, in line with a biological ‘building block’ effect, which increases PTSD risk (Steudte-Schmiedgen et al., 2016). Consequently, lower pre-traumatic cortisol, a higher cortisol response and lower post-traumatic cortisol are hypothesized as prognostic biomarkers for an increased PTSD risk. However, although the ‘biological building block model’ suggests increased cortisol output in the immediate aftermath of a trauma in individuals at risk, the model has so far mainly been tested with data on hair cortisol, which is not a suitable marker to depict HPA axis reactivity.
A meta-analysis of seven studies conducted in acute post-trauma healthcare settings failed to show that cortisol in blood, saliva or urine measured within 72 h post-trauma are predictive for PTSD symptoms from one up to six months later (Morris et al., 2016). While assessing humans in such settings within hours post-trauma is the closest timepoint to capture immediate responses to the event, this comes with two relevant limitations. First, such settings restrict the assessment of pre-trauma cortisol levels and thus, researchers are unable to assess individual baseline levels. Second, aftereffects of a strong immediate HPA axis reactivity might still be reflected in cortisol output some hours after the traumatic stressor, but immediate HPA axis reactivity cannot exactly be captured. In other words, fine-grained investigations of their cortisol responses during trauma are simply not feasible in real-life settings. Fortunately, experimental paradigms offer at least an approximate solution to this problem. Exposing individuals to stimuli with traumatic content (e.g. violent films or pictures), these paradigms allow for psychological and biological measurements before, after, and - extending the limitations of real-life investigations – even during stimulus presentation. Thereby, they enable a clear distinction between pre-, peri- and posttraumatic risk factors for PTSD and are suitable to precisely depict what happens when humans are exposed to traumatic stimuli, on a psychological and biological level. Previous evaluations of experimental trauma paradigms showed that they are effective in inducing PTSD symptoms (Holmes & Bourne, 2008; James et al., 2016). Thus, these paradigms seem suitable to investigate HPA axis reactivity to traumatic stress as a prognostic biomarker for PTSD symptoms.
This study aimed to systematically review all empirical studies that related cortisol concentrations before, during or after exposure to traumatic stimuli (i.e. related to actual or threatened death, serios injury or sexual violence) to subsequent PTSD symptoms. Our main research questions were defined along the PFO framework for prognostic systematic reviews (Munn et al., 2018): Our population of interest were humans without prior trauma exposure who did not suffer from PTSD symptoms. We investigated cortisol concentrations measured before and after the presentation of traumatic stimuli, as well as the cortisol response to the traumatic stimuli – as reflected in summary parameters reflecting multiple measurements – as prognostic factor to predict subsequent PTSD symptoms as outcome.
We investigated the following research questions:
Are there differences between cortisol concentrations measured before and after onset of the traumatic stimuli presentation?
Are cortisol concentrations before the presentation of traumatic stimuli correlated with subsequent PTSD symptoms?
Are cortisol concentrations after the presentation of traumatic stimuli correlated with subsequent PTSD symptoms?
Is the cortisol response to traumatic stimuli correlated with subsequent PTSD symptoms?
2. Methods
This systematic review was conducted in accordance with the PRISMA 2020 guidelines (Page et al., 2021). Adherence to the guidelines is documented in supplementary material 1. The systematic review was preregistered: https://osf.io/9yb7s. Deviations from the preregistration are documented in supplementary material 2. All data and analysis scripts are available at: https://osf.io/tbgch/.
2.1. Eligibility criteria
The inclusion and exclusion criteria are listed in Table 1.
Table 1.
Inclusion and exclusion criteria.
| Domain | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Participants |
|
|
| Interventionb/ Experiment |
|
|
| Comparison |
|
|
| Outcomes |
|
|
| Study designs |
|
|
| Report characteristics |
|
|
aStudies were only be eligible if the cortisol samples were collected at the day of the experiment.
If cortisol concentrations or outcome values were influenced by an intervention that was administered or by the potential effects of different experimental conditions, the study was not excluded, but the potential impact factor was extracted and data were extracted separately for different interventions or experimental conditions.
2.2. Literature search
We conducted a systematic literature search in accordance with the recommendations by Lipsey and Wilson (2001). We searched the following electronic databases: PubMed, PubPsych, PsychINFO, PsycArticle, Web of Science and EMBASE up to December, 21st 2020. We additionally searched ProQuest for dissertations (15 February 2021) and ClinicalTrials.gov for unpublished studies (16 February 2021). We also searched the grey literature by examining conference abstracts and by contacting experts in the field. Additionally, we applied snowball search methods by examining reference lists of review articles and primary studies that were already considered eligible for inclusion. Our electronic search strategy is presented in supplementary material 3. The literature search was conducted by two trained researchers (SK and SE).
To select eligible studies, in a first step, SK screened titles and abstracts of all records identified by the literature search and decided if they met the inclusion criteria. SE checked the quality of this first screening step by independently screening titles and abstracts of a randomly selected subpool of 30% of all records. We manually calculated the degree of agreement, which was very high (99%). In case of discrepancies, the respective record was transferred to the next step: the full-text screening. This step was conducted in duplo (SK and SE). The reports of those studies that meet all inclusion criteria were included in the qualitative part of the systematic review and, if sufficient data were available, they were also included in the meta-analyses.
2.3. Data extraction
Two trained researchers (SK and SE) independently extracted the data of interest, using a standardized form. In case of discrepancies, a third trained researcher (SL) made an independent decision.
We extracted the following data for the qualitative analyses: bibliographic details of the report; participants description; number and age of participants; percentage of women; description of the traumatic stimuli; duration of the stimulus presentation; time of day of the experiment; type of cortisol sample; unit of cortisol concentrations; description of parameters used to reflect the cortisol response (formula, M, SD or SEM).
For the meta-analyses on the cortisol response, we extracted: M and SD or SEM of cortisol, as well as the timing of collection in relation to the onset of the traumatic stimuli presentation (pre, peri or post-presentation cortisol). For post-presentation cortisol, we also extracted the number of minutes since onset of the presentation. If several pre-presentation cortisol values were reported, we chose those assessed shortest before presentation onset.
For the meta-analyses on the correlation between cortisol and PTSD symptoms, we additionally extracted the respective r values.
If relevant data was only depicted in a figure, we extracted them using a plot digitizer (Rohatgi, 2015). The average deviations between the values extracted with the plot digitizer by SK and SE were low (2.04%). If data was neither reported in the text, nor depicted in a figure, we contacted the corresponding authors.
The risk of bias related to HPA axis reactivity assessment was rated independently by two trained researchers (SL and SE), using the Cortisol Assessment List (CoAL; Laufer et al., 2022). The list evaluates the quality of cortisol assessments based on: sampling protocol, consideration of state covariates, consideration of trait covariates and exclusion criteria. If ratings were discrepant, the decision was re-evaluated and discussed until agreement was achieved.
2.4. Meta-analyses
2.4.1. Cortisol response to experimentally-induced traumatic stress
To examine potential differences between cortisol levels before and after traumatic stimuli presentation (research question 1), we conducted a multilevel-meta-analysis, which enabled us to consider several observations from the same sample. There was considerable variance in the number of minutes between traumatic stimuli presentation onset and post-presentation cortisol assessment, which suggested the necessity to conduct a time-sensitive analysis of the cortisol response. We thus conducted four separate meta-analyses for the timeframes: 0–20, 21–40, 41–60 and >60 min since presentation onset. Studies on standardized stress tests, such as the Trier Social Stress Test (TSST), showed that cortisol peaks between 10 and 30 min after stress cessation (Foley & Kirschbaum, 2010). The active component of the TSST takes about 20 min (Labuschagne et al., 2019). Therefore, the cortisol response could especially be expected in the second (21–40 min), and possibly still in the third time window (41–60 min). We used the standardized mean change (dSMC) to indicate differences between pre- and post-presentation onset cortisol, applying a raw score standardization with heteroscedastic population variances (Bonett, 2008).
2.4.2. Correlation between cortisol and subsequent PTSD symptoms
To examine the correlations between pre-presentation cortisol (research question 2), post-presentation cortisol (research question 3) or the cortisol response (research question 4) and PTSD symptoms, we again conducted multilevel-meta-analyses. Correlations were indicated by the coefficient r. PTSD symptoms were first grouped according to the DSM-5 symptom cluster level (e.g. intrusion symptoms) and then on a single-symptom level (e.g. dissociation).
2.4.3. Heterogeneity of effects and risk of bias assessment
In order to determine heterogeneity of effects, Q and I2 were used (Borenstein et al., 2011). Given a non-significant Q (p ≥ .05), effects were interpreted as significant if p < .05. Given a significant Q statistic (p < .05) and I2 values of 25, 50, and 75, effects were interpreted as lowly, moderately and highly heterogeneous, respectively. In these cases, moderator analyses were applied. As moderators, we considered participants’ sex, CoAL ratings and impact of the experimental condition (dampening, not influencing or increasing the cortisol response). If a homogeneous and significant effect was based on n ≥ 6 primary studies, we tested risk of publication bias using Egger's regression test (Egger et al., 1997) and the trim-and-fill procedure (Duval & Tweedie, 2000). All meta-analyses were conducted under the random effects model, using the software R (RStudio Team, 2022) and the meta-analysis packages metafor (Viechtbauer, 2010), dmetar (Harrer et al., 2019) metaviz (Kossmeier et al., 2020).
3. Results
3.1. Qualitative analyses
3.1.1. Description of included studies
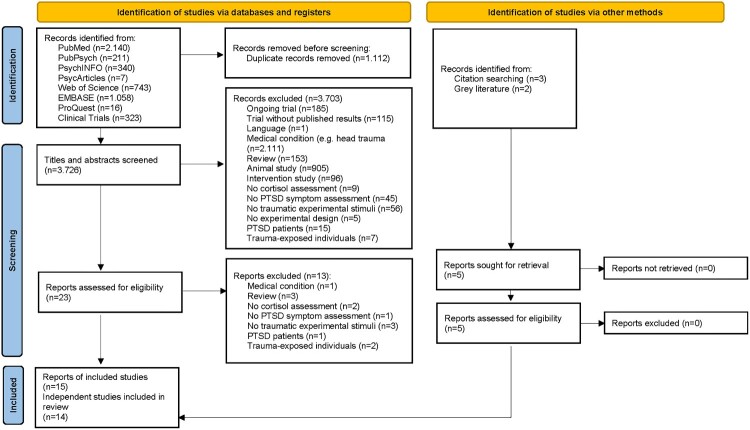
The results of the study selection process are shown in Figure 1. 15 reports of 14 independent studies, summarizing data of 1004 individuals, were included. Supplementary material 4 includes a full list of the reports. Details of the participants and experimental setups are shown in Table 2. Participants were mostly recruited in university contexts and therefore relatively young, healthy and highly educated. Women were overrepresented (732 individuals, 72.91%). Most studies used a trauma film (n = 12) as stimulus. Among trauma films, a scene from the film Irréversible, directed by Gaspar Noé which depicts sexual and physical violence, was most frequently used (n = 9). Stimulus presentation times ranged between 2 and 15 min. All studies evaluated the effects of psychological and/or biological variables on subsequent PTSD symptoms. In n = 9 studies, these variables were experimentally manipulated: Hilberdink et al. (2021) investigated the impact of exposure to a socially-evaluated cold pressor test (CPT), as compared to a warm water control condition, before the trauma film. Cheung et al. (2015) applied the socially-evaluated CPT, as well, but two days after the trauma film. In one group, the socially-evaluated CPT was combined with a reactivation induction of the traumatic memory, in another group, a warm water control condition was combined with the reactivation and a third group was exposed to the socially-evaluated CPT without reactivation. Schultebraucks et al. (2019) investigated the impact of the biological responses to participation in the TSST, versus an active control condition (placebo TSST) on processing of the traumatic stimuli. In the study conducted by Lass-Hennemann et al. (2018), three groups watched a trauma film but engaged in different activities afterwards. One group actively engaged with therapy dogs, a second group watched a film depicting dogs and a third group spent the time alone, instructed to relax. Marks (2018) evaluated the effects of different memory reconsolidation strategies (negative, neutral or scrambled cues, as presented before, or neutral cues, presented after an extinction session that took place 48 h after the participants watched the trauma film). Rombold et al. (2016a) compared the effects of clonidine (0.15 mg), yohimbine (10 mg) and placebo and in another study (Rombold et al., 2016b) they evaluated the effects of hydrocortisone (20 mg) versus placebo, all administered before the trauma film. Scheele et al. (2019) compared the effects of repeated intranasal oxytocin (6 daily doses of 24 IU) versus placebo administration, as applied after the trauma film. Lastly, Kamboj et al. (2020) compared the effects of single doses of hydrocortisone (30 mg), propranolol (80 mg) and placebo administration, also administered directly after the trauma film. If possible, subsamples assigned to different experimental conditions or interventions within one study were entered separately in the meta-analyses. Some of these experimental conditions or interventions have an impact on the cortisol response. We categorized them into (a) dampening the cortisol response (clonidine, oxytocin), (b) not influencing the cortisol response (any experimental conditions or interventions applied after presentation of the traumatic stimuli; control and placebo conditions) or (c) increasing the cortisol response (socially-evaluated CPT, yohimbine, hydrocortisone, TSST). We used this categorization for moderator analyses.
Figure 1.
Literature search and flow of study selection.
Table 2.
Participants and experimental setup.
| Study | Participants | n | % Women | Age M (SD) | Experimental stimuli | Duration of presentation (in min) | Begin of experimental session |
|---|---|---|---|---|---|---|---|
| Cheung et al. (2015) | Undergraduate psychology students | 21 (socially-evaluated CPT + reactivation)b | 52.38 (socially-evaluated CPT + reactivation)b | 19.43 (1.75) (socially-evaluated CPT + reactivation)b | Trauma film (real life car accidents with serious injury and death) | 10.00 | 1:00 pm – 6:00 pm |
| 21 (warm water control + reactivation)b | 52.38 (warm water control + reactivation)b | 20.33 (2.89) (warm water control + reactivation)b | |||||
| 21 (socially-evaluated CPT only)b | 57.14 (socially-evaluated CPT only)b | 19.90 (2.14) (socially-evaluated CPT only)b | |||||
| Chou et al. (2014) | Healthy students and individuals from the general public | 58 | 44.83 | 24.16 (4.22) | Trauma film (real life car accidents with serious injury and death) | 13.67 | 1:30 pm – 6:00 pm |
| Hilberdink et al. (2022) | Healthy, university educated participants | 29 (socially-evaluated CPT)b | 0.00 | 22.52 (4.83) (socially-evaluated CPT)b | Trauma film (Irréversible, sexual and physical violence) | 15.00 | Afternoon |
| 34 (warm water control condition)b | 22.90 (3.89) (warm water control condition)b | ||||||
| Holz et al. (2017) | Healthy students | 60 | 50.00 | 23.17 (2.76) | Trauma film (Irréversible) | 11.00 | 1:00 pm – 6:00 pm |
| Kamboj et al. (2020) | Healthy individuals from a university locale | 88 | 100.00 | 23.76 (3.65) (placebo)b 23.20 (3.46) (propranolol)b 23.65 (3.12) (hydrocortisone) |
Trauma film (Irréversible) | 15.00 | 2:00 pm – 5:00 pm |
| Lass-Hennemann et al. (2018) | Healthy university students | 20 (dog interaction)b | 100.00 | 22.95 (2.87) (dog interaction)b | Trauma film (Irréversible) | 11.00 | 2:00 pm – 6:00 pm |
| 20 (dog film)b | 22.25 (3.32) (dog film)b | ||||||
| 20 (relaxation)b | 22.65 (2.39) (relaxation)b | ||||||
| Liberzon et al. (1999) | Healthy non-veteran controls | 14 | 0.00 | 45.4 (6.1) | Combat sounds | 3.00 | 9:00 am |
| Marks (2018) | Students | 173 | 56.60 | 19.27 (1.66) | Trauma film (The Last King of Scotland, mutilation and death) | 10.00 | N/Av |
| Nicholson et al. (2014) | Non-trauma exposed controls | 20 | 60.00 | 21.80 (5.72) | 20 negative emotional pictures from the International Affective Picture Systema | 2.00 | 12:00 pm – 6:00 pm |
| Rombold et al. (2016a) | Healthy university students | 38 (clonidine)b | 100.00 | 23.30 (3.60) (clonidine)b | Trauma film (Irréversible) | 14.67 | N/Av |
| 38 (placebo)b | 23.10 (3.20) (placebo)b | ||||||
| 38 (yohimbine)b | 23.40 (4.50) (yohimbine)b | ||||||
| Rombold et al. (2016b) | Healthy university students | 30 (hydrocortisone)b | 100.00 | 23.00 (3.32) (hydrocortisone)b | Trauma film (Irréversible) | 14.67 | 1:30 pm |
| 30 (placebo)b | 22.70 (3.41) (placebo)b | ||||||
| Scheele et al. (2019) | Healthy individuals from the general public | 16 (oxytocin, strong trauma disclosure)b | 100.00 | 23.31 (4.20) | Trauma film (Irréversible) | 15.00 | N/Av |
| 16 (oxytocin, weak trauma disclosure) b | |||||||
| 15 (placebo, strong trauma disclosure)b | |||||||
| 15 (placebo, weak trauma disclosure)b | |||||||
| Schultebraucks et al. (2019) | Healthy university students | 60 (TSST)b | 100.00 | 22.88 (2.78) (TSST)b | Trauma film (Irreversible) | 14.67 | 2:00 pm |
| 62 (placebo TSST)b | 23.89 (3.42) (placebo TSST)b | ||||||
| Trautmann et al. (2018) | Healthy individuals from a university environment | 47 | 55.30 | 24.50 (4.20) | Trauma film (Irreversible) | 15.00 | 1:00 pm – 8:00 pm |
Lang et al. (2008) bcortisol concentrations and outcome values were potentially influenced by allocation of participants to different experimental conditions. Therefore, the values are reported separately for each condition N/Av = not available; N/Ap = not applicable; CPT = cold pressor test; TSST = Trier social stress test.
3.1.2. Description of cortisol assessments
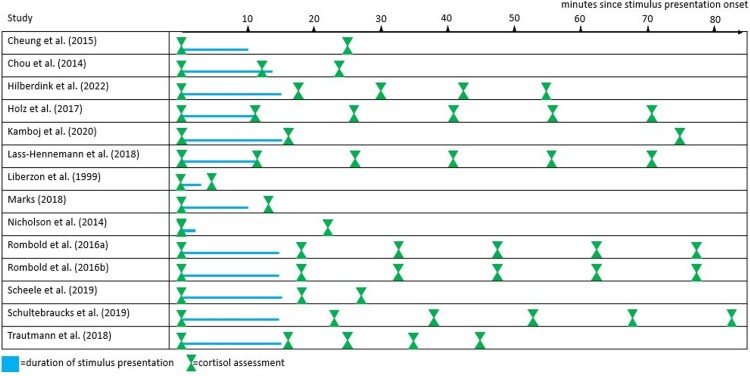
Figure 2 gives an overview of the timing of cortisol assessments with respect to the experimental setup. Supplementary material 5 provides more detailed information about the cortisol assessments. Cortisol was mostly measured in saliva. The CoAL ratings for each study and domain are depicted in supplementary material 6. The CoAL global scores indicated sufficient cortisol data quality (M = 67.79, SD = 17.75). All studies had a baseline cortisol assessment. Only n = 1 study investigated cortisol during stimulus presentation. As the maximal duration of stimulus presentation was 15 min, it can be assumed that the cortisol response is reflected in some of the post-presentation concentrations. The number of cortisol assessments ranged between 2 and 6.
Figure 2.
Overview of the timing of the traumatic stimulus presentation and cortisol assessments.
3.1.3. Description of PTSD symptom assessments
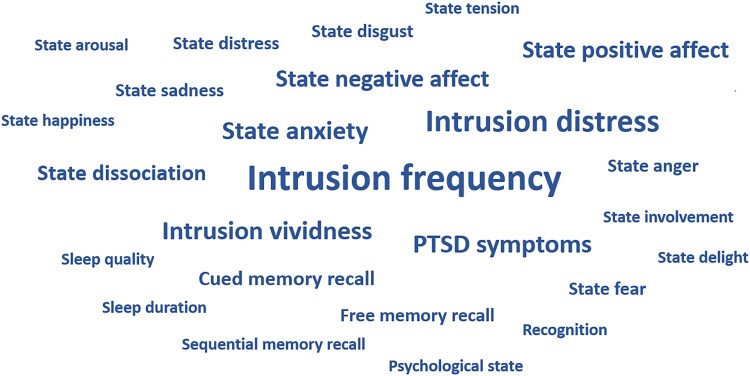
The most frequently measured PTSD symptoms are shown in Figure 3. Details about the PTSD symptom assessments are shown in supplementary material 7. Intrusion frequency, distress and vividness, representing the DSM-5 symptom cluster B, were the most frequently investigated outcomes (investigated in n = 12, n = 9 and n = 6 studies, respectively). Cluster C (avoidance) was not directly investigated, only as part of overall PTSD symptom questionnaires (n = 6 studies). Cluster D (negative alterations in cognitions and mood) was mostly measured via the assessment of emotional states before and after presentation of the traumatic stimuli (e.g. state anxiety, n = 6) or via tests that assessed the ability to remember the traumatic stimuli (e.g. cued memory recall, n = 3, this, just as some other parameters, was inverted for the meta-analyses, as more recalled items represent a better memory performance and thus lower expression of the symptom ‘inability to remember an important aspect of the traumatic event’). Cluster E (marked alterations in arousal and reactivity) was also measured comparatively seldom, by assessments of state anger (n = 2), state arousal or sleep (n = 1, respectively). Time points of outcome assessments ranged from immediately after the traumatic stimulus presentation – mostly for the assessment of emotional states – up to 4 weeks after the experimental session – for the assessment of overall PTSD symptoms related to the stimuli.
Figure 3.
PTSD symptoms, as assessed as outcomes in the primary studies. The size of the words indicates the frequency of their assessments.
3.2. Meta-analyses
Altogether, thanks to the efforts of the primary studies’ corresponding authors, data from 12 out of 14 independent studies could be used for meta-analyses.
3.2.1. Quantifying the cortisol response to experimentally-induced traumatic stress
Concerning research question 1, the difference between pre-presentation cortisol and cortisol measured within the first time window (0–20 min post-presentation onset) was not significant (k = 17 observations, dSMC = 0.03, SEM = 0.06, p = .60). Significantly higher cortisol values were observed within the second time window (21–40 min post-presentation onset), indicating a significant cortisol response to the traumatic stimuli (k = 25, dSMC = 0.15, SEM = 0.06 p = .01). Regarding the third and fourth time windows (41–60 and >60 min post-presentation onset), the differences were non-significant, again (k = 20, dSMC = 0.12, SEM = 0.06 p = .05 and k = 19, dSMC = 0.02, SEM = 0.10 p = .81).
The significant effect size that indicated differences between cortisol measured before and within 21–40 min after onset of the presentation was moderately heterogeneous (Q = 59.57, p < .01, I2 = 64.63). We thus applied moderator analyses to explore heterogeneity. Specifically, we tested the impact of participants’ sex, CoAL ratings and impact of the experimental condition on the cortisol response. None of the moderators significantly reduced heterogeneity (sex: Q = 57.60, p < .01; I2 = 69.89; CoAL: Q = 47.83, p < .01, I2 = 49.98; experimental condition: Q = 56.88, p < .01, I2 = 69.54). Only the CoAL score was identified as a significant moderator (dSMC = 0.01, SEM = 0.00 p = .01), indicating a higher effect size, i.e. greater differences between pre- and post-presentation onset cortisol, in studies with higher cortisol assessment quality.
In addition to testing the impact of the experimental condition as a moderator (which had limited value, as in k = 21 observations, no impact was assumed whereas an increasing or decreasing impact was only assumed in k = 4 and k = 1 observations, respectively), we repeated our analyses only within those observations which were not influenced by any experimental manipulation. This subgroup analysis showed the same pattern of results: No significant effects for the first, third and fourth time windows (k = 13, dSMC = −0.04, SEM = 0.06, p = .51; k = 14, dSMC = 0.11, SEM = 0.08, p = .16 and k = 11, dSMC = 0.07, SEM = 0.12, p = .56, respectively), but a significant difference in cortisol from pre- to 21–40 min post-presentation onset (k = 19, dSMC = 0.13, SEM = 0.06, p = .03) – an effect size with low heterogeneity (Q = 40.61, p < .01, I2 = 53.27).
Finally, we calculated an exploratory quadratic model to predict differences between pre-presentation cortisol and current cortisol levels by using minutes after presentation onset as a predictor. The quadratic term of this model was significant (k = 81, z = −4.46, p < .01). We estimated the peak of the quadratic distribution, which showed that cortisol levels differed the most from baseline levels 37 min after presentation onset.
3.2.2. Correlation between cortisol and subsequent PTSD symptoms
3.2.2.1. Correlation between pre-presentation cortisol and subsequent PTSD symptoms
Concerning research question 2, results showed that pre-presentation cortisol concentrations were not significantly correlated with cluster B (k = 60, r = −.04 [−.10; .02], p = .21), cluster D (k = 73, r = −.08 [−.19; .02], p = .12), cluster E (k = 10, r = −.10 [−.17; .04], p = .17) or overall PTSD symptoms (k = 9, r = −.02 [−.11; .07], p = .64). The correlation with cluster C symptoms could not be tested, lacking sufficient data. On a single symptom level, higher pre-presentation cortisol was significantly correlated with lower state tension (k = 8, r = −.18 [−.36; −.01], p = .04), with a homogeneous effect estimate (Q = 8.37, p = .30). Another homogeneous, significant association was found for state happiness (k = 8, r = −.36 [−.68; −.03], p = .03; Q = 11.08, p = .14): higher pre-presentation cortisol was correlated with higher happiness after the experiment (the variable happiness was inverted, as higher happiness represents a lower expression of the symptom ‘inability to experience positive emotions’). A significant association was found for state anger (k = 9, r = −.14 [−.26; −.01], p = .03), indicating that higher pre-presentation cortisol was correlated with lower anger after stimulus presentation. This effect was homogeneous (Q = 3.21, p = .92). For these three effects, publication bias could not be tested, as the number of independent studies (n) was below our predefined criteria of n ≥ 6. We detected no further significant correlations between pre-presentation cortisol and single PTSD symptoms.
3.2.2.2. Correlation between post-presentation cortisol and subsequent PTSD symptoms
With regard to research question 3, post-presentation cortisol concentrations were also not significantly correlated with cluster B (k = 241, r = .01 [−.05; .06], p = .81), cluster D (k = 147, r = −.03 [−.10; .05], p = .50), cluster E (k = 18, r = −.03 [−.12; .07], p = .57) or overall PTSD symptoms (k = 52, r = .05 [−.01; .11], p = .10). Again, lacking sufficient data, the correlation with cluster C symptoms could not be tested. On a single symptom level, higher post-presentation cortisol was significantly correlated with higher state happiness (k = 16, r = −.20 [−.34; −.06], p = .01). The effect estimate was homogeneous (Q = 5.07, p = .99). Further, higher post-presentation cortisol was significantly correlated with lower state sadness (k = 17, r = −.16 [−.26; −.05], p < .01), with a homogeneous effect estimate (Q = 4.51, p > .99). Again, with n < 6, publication bias was not tested for these effects, and no further significant correlations between post-presentation cortisol and single PTSD symptoms were detected.
3.2.2.3. Correlation between the cortisol response and subsequent PTSD symptoms
Concerning research question 4, the cortisol response to the traumatic stimuli was also neither significantly correlated with overall PTSD symptoms (k = 15, r = .05 [−.02; .12], p = .17), nor with PTSD symptom clusters (cluster B: k = 73, r = .04 [−.03; .11], p = .25; cluster C: not tested due to lack of data; cluster D: k = 53, r = .07 [−.01; .14], p = .07; cluster E: k = 11, r = .03 [−.01; .16], p = .59). On a symptom level, a significant association was only found for state anxiety (k = 9, r = .16 [.04; .28], p = .01), indicating that a higher cortisol response was correlated with higher anxiety after stimulus presentation. This effect was homogeneous (Q = 4.61, p = .80). With n < 6, publication bias was not tested.
4. Discussion
This systematic review and meta-analysis summarized 14 independent studies which evaluated the effects of traumatic stimuli, mostly trauma films such as Irréversible, on the cortisol response and subsequent PTSD symptoms. Our results show that presentation of traumatic stimuli to mostly female, young, healthy and highly educated individuals successfully induced a cortisol response. Cortisol concentrations peaked within 21–40 min post-presentation onset, albeit the effects varied considerably between studies. This heterogeneity was partly related to the quality of cortisol assessments: A greater effect size was detected in studies with higher quality of cortisol assessment, i.e. better control of confounding variables. Concerning the association between cortisol and subsequent PTSD symptoms, no significant effects were found on the overall symptom or symptom cluster levels. Yet, more refined single-symptom analyses showed that cortisol affected some psychological state variables after stimulus presentation: Higher cortisol concentrations pre- and post-presentation onset were associated with lower state PTSD symptoms (pre: lower state tension; higher state happiness; lower state anger; post: higher state happiness; lower state sadness). In turn, a higher cortisol response to the traumatic stimuli was associated with higher state anxiety.
4.1. Interpretation of results
The qualitative analyses showed that most included studies investigated HPA axis reactivity to vivid traumatic stimuli, especially trauma films. They built upon previous evidence showing that trauma film paradigms have psychological effects, evoking transient PTSD symptoms (Holmes & Bourne, 2008). Our meta-analysis additionally showed that these paradigms have biological effects, stimulating the HPA axis and increasing cortisol concentrations between 21 and 40 min after traumatic stressor onset with peaking concentrations around 37 min afterwards. This answers the question about the comparability between naturalistic (e.g. emergency departments) and experimental settings: Realistically, the time window between 21 and 40 min after traumatic stress is extremely difficult to capture in actual trauma survivors. At the same time, cortisol concentrations measured within hours afterwards, as they have been evaluated in the meta-analysis by Morris et al. (2016) are indicators of HPA axis recovery, rather than reactivity. This confirms that the gap between real-life and experimental trauma research is extremely difficult to overcome.
Our results further showed that, in order to depict a cortisol response, it is crucial to control for variables that influence cortisol measurements, such as time of day, food, caffeine and medication intake, just to name a few examples. For a full list of variables that should be considered in HPA axis reactivity studies, interested researchers might be referred to Laufer et al. (2022). The necessity to apply strict inclusion criteria is reflected in the included studies’ sample selections. Our qualitative analyses showed that participants were mostly recruited at universities. Thus, the investigated samples were presumably younger, healthier and higher educated than both, the average population and average trauma survivor populations. Therefore, the samples should not be considered as representative. The generalizability of our findings to more diverse populations still needs evaluation. This is especially challenging regarding the participants’ health status. Responses to traumatic stimuli might be different in participants with previous PTSD or other psychiatric disorders, many of which have been shown to modulate HPA axis reactivity (e.g. PTSD (Morris et al., 2012; Schumacher et al., 2019)) or depression (Brand et al., 2015; Strawbridge et al., 2017). It can, however, be ethically challenging to expose individuals with preexisting disorders to burdensome traumatic stimuli such as the trauma film, as discussed by James et al. (2016). Furthermore, including exclusively healthy participants increases internal consistency through reducing unsystematic influencing factors on the experiment, such as higher arousal through preexisting PTSD.
Concerning the correlation between cortisol and PTSD symptoms, a predictive value was only found on a single symptom level. Here, emotion-related symptoms were affected by cortisol, whereas cognition-related symptoms remained unaffected. This is surprising, as there is strong evidence from basic research towards a modulating effect of cortisol on memory (de Quervain et al., 2017). Further, intrusions, the hallmark symptom of PTSD that most clearly distinguishes this disorder from other adverse consequences of trauma, such as depression or anxiety disorders, were the most frequently investigated outcome and therefore, the meta-analysis on the effect of cortisol on intrusions had the largest power. Nevertheless, no significant correlation was detected. However, instead of concluding that emotion-related symptoms are generally more strongly modulated by the HPA axis, the mode of assessment should be considered as an alternative explanation: While cognition-related symptoms and intrusions were most frequently investigated within days after the experiment, emotions were mostly assessed as state variables, minutes up to hours afterwards. Thus, our meta-analyses provide evidence towards a modulating influence of cortisol on temporary emotional states, but not on PTSD symptoms, as measured more long-term. There is a neurobiological rationale behind the different assessment timepoints of emotional versus cognitive symptoms. The primary studies’ focus on immediate emotional states could be explained by the amygdaloid efferents to the paraventricular nucleus (PVN) of the hypothalamus (Feldman et al., 1995). More specifically, the visceral emotional response to the traumatic stimuli most likely triggered an immediate stimulation of the PVN resulting in the described correspondence between the cortisol response and emotion-related symptoms in the short-term assessments. An effect on cognitive symptoms would require the involvement of higher-order prefrontal mechanisms, which have been shown to be affected in PTSD (Hayes et al., 2012) and are most likely to manifest in the days to weeks following the acute post-trauma period.
Cortisol's influence on the emotional symptoms might be explained by the anticipation of a stressful event, which affects HPA axis reactivity (Gaab et al., 2005; Pulopulos et al., 2020). Possibly, the observed predictive value of participants’ cortisol concentrations and responses on emotional PTSD symptoms is a function of their respective anticipatory capacity. If participants anticipated not to have sufficient resources to adequately process the traumatic stress, this could result in homeostatic overload (Romero et al., 2009), which would be in line with the observed decreased pre-presentation onset cortisol concentrations followed by the subsequent hyper-response in those individuals later showing higher emotional PTSD-related symptoms (Boucher & Plusquellec, 2019).
It should be noted that the emotional symptoms identified in the current analysis (i.e. lack of happiness, tension and anxiety), are not unique to PTSD, but comprise the basic symptomatology of other psychological disorders like depression and anxiety disorders. This again points to a rather general mechanism of processing external and internal stressors to maintain homeostasis. Additionally, although we decided to consider such state measurements as outcome, per definition, PTSD is not characterized by negative emotions in general, but by persistent negative alterations in mood. These changes are supposed to occur over a period of at least one month. However, such long-term effects were not empirically supported by our study.
At first glance, the direction of the effects that were detected on a single symptom level seems to be in line with the ‘biological building block model’ (Steudte-Schmiedgen et al., 2016): Pre-presentation cortisol was associated with lower PTSD symptoms, but higher reactivity was associated with higher PTSD symptoms. Also seemingly supporting the model, post-presentation cortisol was associated with lower PTSD symptoms. However, according to the model, lower cortisol concentrations post-trauma represent a consequence of maladaptive adjustment, possible after the overwhelming experience of cumulative trauma, and thereby increase subsequent PTSD risk. However, we only considered cortisol concentrations that were measured within minutes up to hours after the experiment. We did not investigate long-term adaptation of the HPA axis. As noted before, due to the short duration of the traumatic stimulus presentation and the delay of the cortisol peak after stressor onset (Gaab et al., 2002), most of the post-presentation cortisol concentrations that we considered still indicate HPA axis reactivity. Interpreting them this way, our findings do not completely match with the suggested ‘biological building block model’. However, as discussed before, this model is based on cumulative cortisol measures. It provides a well-founded theoretical assumption on HPA axis reactivity and recovery, but, lacking access to the immediate post-trauma period, these assumptions can hardly be empirically tested in acutely traumatized individuals. Again, the gap between experimental and naturalistic settings cannot fully be overcome, which limits the possibilities to directly compare our findings to such from real-life settings.
4.2. Limitations and future directions
Concerning our finding that intrusions were the most frequently investigated outcome, it needs to be critically discussed that cluster B symptoms were the only ones specifically addressed by our search strategy. When defining search terms, researchers need to balance two requirements: identifying as many relevant reports as possible while obtaining as few irrelevant reports as possible. In order to meet the first requirement, we decided to include the intrusion-related terms (‘intrusi*’, ‘nightmare*’, ‘flashback*’ as these are the hallmark symptoms of PTSD. In order to meet the second requirement, we decided not to include terms related to other symptoms, such as ‘mood’, ‘anxiety’ or ‘avoidance’, as they occur in a broad range of mental disorders and are therefore unspecific for PTSD. Due to our additional search methods – particularly the snowball search and the information exchange with experts – it is unlikely, however, it cannot be ruled out that due to our search terms, no studies were found that examined avoidance as an outcome. Further, we did not consider any psychophysiological measurements as outcomes. Instead, we exclusively focused on self-report symptom measures. However, this might explain why relatively few cluster symptoms E symptoms (only self-reported arousal, sleep and anger) were considered, limiting the power of the related meta-analysis.
Another limitation of our study needs to be addressed, namely, the definition of experimental stimuli as traumatic, which we left to the primary study authors. Most included studies used trauma films, but one used combat sounds (Liberzon et al., 1999) and another one used pictures (Nicholson et al., 2014), explicitly stating that they have the potential to induce intrusive memories, thereby explicitly defining them as potentially traumatic. However, it is possible that other studies exist, which also showed pictures related to death, injury or sexual violence and evaluated their impact to PTSD-unspecific symptoms, such as positive or negative affect, but did not explicitly define them as traumatic and therefore did not meet our inclusion criteria. In order not to change our pre-defined inclusion criteria, we decided to not restrict our study to trauma film paradigms.
Future research might extend the pool of experimental trauma paradigms by such that require even more involvement by the participants, such as simulation of military events or accidents in military or surgical stress trainings (Goldberg et al., 2018). It is also possible that expressive writing about traumatic events (Tamagawa et al., 2013) might increase participants’ involvement and thereby produce strong biological responses. Lastly, aside from HPA axis reactivity, other acute neurobiological markers most likely respond to traumatic stimuli and might play an additional role in the development of PTSD symptoms (Pitman et al., 2012). Such neurobiological markers, for example, heart rate (variability) or skin conductance, hold high potential for knowledge through further experimental studies. To gain access to the stress response immediately post-trauma aftermath, technology-based assessments via wearables might be used. Such technologies do not allow for cortisol measurements, but indicators of the autonomic nervous system, such as heart rate (variability) or electrodermal activity, can be assessed and thereby complement our understanding of the role of the biological stress response systems in trauma processing.
4.3. Conclusion
Experimental paradigms have been proven successful to induce traumatic stress and related symptoms, particularly intrusions (Holmes & Bourne, 2008; James et al., 2016). This study showed that they also successfully induce a cortisol response. However, just as previous research on pre – and post-traumatic HPA axis regulation in real-life settings could not detect a clear prospective effect of cortisol on subsequent PTSD symptoms (Engel et al., 2022; Morris et al., 2016), our meta-analysis did not detect such an effect on experimentally induced symptoms. Comparing our findings on cortisol's impact on temporary emotional states with previous research in real-life settings shows that it remains a challenge to bridge the gap between experimental and naturalistic research. Particularly, knowledge about the immediate post-trauma period is still in the dark. To date, it can only be approached by experimental approaches, but in the future, technological advances might enable us to track the body's stress system responses to actual trauma in real time.
Supplementary Material
Acknowledgements
All authors would like to thank Sophie Kill (SK) for her support in the literature research, study selection and data extraction. We are also grateful to all primary study authors who provided us with the data for the meta-analyses. Their effort clearly increased the informative value of this study.
Funding Statement
This study did not receive funding. It was preregistered at: https://osf.io/9yb7s
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contribution
SE designed the study. SK and SE performed the literature search, study selection and data extraction. SL supported the extraction process in case of disagreements. SE contacted the authors of the primary studies for which relevant data were missing. SL and SE performed the risk of bias rating. LS and SE planned and performed the meta-analyses. SE drafted the manuscript. SL, HK, LS, SSch and CK revised the manuscript for important intellectual content.
Data availability statement
All data is available here: https://osf.io/tbgch/
References
- Admon, R., Lubin, G., Stern, O., Rosenberg, K., Sela, L., Ben-Ami, H., & Hendler, T. (2009). Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences, 106(33), 14120–14125. 10.1073/pnas.0903183106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association. https://psychiatryonline.org/doi/book/10.1176appi.books.9780890425596 [Google Scholar]
- Berger, W., Coutinho, E. S. F., Figueira, I., Marques-Portella, C., Luz, M. P., Neylan, T. C., Marmar, C. R., & Mendlowicz, M. V. (2012). Rescuers at risk: A systematic review and meta-regression analysis of the worldwide current prevalence and correlates of PTSD in rescue workers. Social Psychiatry and Psychiatric Epidemiology, 47(6), 1001–1011. 10.1007/s00127-011-0408-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno, G., & Loss, T. (2004). And human resilience: Have we underestimated the human capacity to thrive after extremely aversive events? American Psychologist, 59(1), 20–28. 10.1037/0003-066X.59.1.20 [DOI] [PubMed] [Google Scholar]
- Bonett, D. G. (2008). Confidence intervals for standardized linear contrasts of means. Psychological Methods, 13(2), 99–109. 10.1037/1082-989X.13.2.99 [DOI] [PubMed] [Google Scholar]
- Borenstein, M., Hedges, L. V., Higgins, J. P. T., & Rothstein, H. R. (2011). Introduction to Meta-Analysis. Wiley. [Google Scholar]
- Boucher, P., & Plusquellec, P. (2019). Acute stress assessment from excess cortisol secretion: Fundamentals and perspectives. Front Endocrinol, 10, 749. 10.3389/fendo.2019.00749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand, S. J., Möller, M., & Harvey, B. H. (2015). A review of biomarkers in mood and psychotic disorders: A dissection of clinical vs. preclinical correlates. Current Neuropharmacology, 13(3), 324–368. 10.2174/1570159X13666150307004545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, J., Garber, B., & Bryant, R. A. (2015). The role of stress during memory reactivation on intrusive memories. Neurobiology of Learning and Memory, 123, 28–34. 10.1016/j.nlm.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Chou, C.-Y., La Marca, R., Steptoe, A., & Brewin, C. R. (2014). Biological responses to trauma and the development of intrusive memories: An analog study with the trauma film paradigm. Biological Psychology, 103, 135–143. 10.1016/j.biopsycho.2014.08.002 [DOI] [PubMed] [Google Scholar]
- de Quervain, D., Schwabe, L., & Roozendaal, B. (2017). Stress, glucocorticoids and memory: Implications for treating fear-related disorders. Nature Reviews Neuroscience, 18(1), 7–19. 10.1038/nrn.2016.155 [DOI] [PubMed] [Google Scholar]
- Duval, S., & Tweedie, R. (2000). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56(2), 455–463. 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- Egger, M., Davey Smith, G., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, S., Klusmann, H., Laufer, S., Kapp, C., Schumacher, S., & Knaevelsrud, C. (2022). Biological markers in clinical psychological research - A systematic framework applied to HPA axis regulation in PTSD. Compr Psychoneuroendocrinol, 11, 100148. 10.1016/j.cpnec.2022.100148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, S., Conforti, N., & Weidenfeld, J. (1995). Limbic pathways and hypothalamic neurotransmitters mediating adrenocortical responses to neural stimuli. Neuroscience & Biobehavioral Reviews, 19(2), 235–240. 10.1016/0149-7634(94)00062-6 [DOI] [PubMed] [Google Scholar]
- Foley, P., & Kirschbaum, C. (2010). Human hypothalamus–pituitary–adrenal axis responses to acute psychosocial stress in laboratory settings. Neuroscience & Biobehavioral Reviews, 35(1), 91–96. 10.1016/j.neubiorev.2010.01.010 [DOI] [PubMed] [Google Scholar]
- Gaab, J., Hüster, D., Peisen, R., Engert, V., Heitz, V., Schad, T., Schürmeyer, T. H., & Ehlert, U. (2002). Hypothalamic-pituitary-adrenal axis reactivity in chronic fatigue syndrome and health under psychological, physiological, and pharmacological stimulation. Psychosomatic Medicine, 64(6), 951–962. 10.1097/01.psy.0000038937.67401.61 [DOI] [PubMed] [Google Scholar]
- Gaab, J., Rohleder, N., Nater, U. M., & Ehlert, U. (2005). Psychological determinants of the cortisol stress response: The role of anticipatory cognitive appraisal. Psychoneuroendocrinology, 30(6), 599–610. 10.1016/j.psyneuen.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Galatzer-Levy, I. R., Huang, S. H., & Bonanno, G. A. (2018). Trajectories of resilience and dysfunction following potential trauma: A review and statistical evaluation. Clinical Psychology Review, 63, 41–55. 10.1016/j.cpr.2018.05.008 [DOI] [PubMed] [Google Scholar]
- Geoffrion, S., Goncalves, J., Robichaud, I., Sader, J., Giguère, C-É, Fortin, M., Lamothe, J., Bernard, P., & Guay, S. (2020). Systematic review and meta-analysis on acute stress disorder: Rates following different types of traumatic events. Trauma, Violence, & Abuse, 23(1), 213–223. 10.1177/1524838020933844 [DOI] [PubMed] [Google Scholar]
- Gilbertson, M. W., Shenton, M. E., Ciszewski, A., Kasai, K., Lasko, N. B., Orr, S. P., & Pitman, R. K. (2002). Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience, 5(11), 1242–1247. 10.1038/nn958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, M. B., Mazzei, M., Maher, Z., Fish, J. H., Milner, R., Yu, D., & Goldberg, A. J. (2018). Optimizing performance through stress training - An educational strategy for surgical residents. The American Journal of Surgery, 216(3), 618–623. 10.1016/j.amjsurg.2017.11.040 [DOI] [PubMed] [Google Scholar]
- Harnett, N. G., van Rooij, S. J. H., Ely, T. D., Lebois, L. A. M., Murty, V. P., Jovanovic, T., Hill, S. B., Dumornay, N. M., Merker, J. B., Bruce, S. E., House, S. L., Beaudoin, F. L., An, X., Zeng, D., Neylan, T. C., Clifford, G. D., Linnstaedt, S. D., Germine, L. T., Bollen, K. A., … Stevens, J. S. (2021). Prognostic neuroimaging biomarkers of trauma-related psychopathology: Resting-state fMRI shortly after trauma predicts future PTSD and depression symptoms in the AURORA study. Neuropsychopharmacology, 46(7), 1263–1271. 10.1038/s41386-020-00946-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrer, M., Cuijpers, P., Furukawa, T., & D, D. (2019). Ebert, dmetar: Companion R package for the guide’Doing meta-analysis in R’. R package version 0.0. 9000.
- Hayes, J. P., Hayes, S. M., & Mikedis, A. M. (2012). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood & Anxiety Disorders, 2(9). 10.1186/2045-5380-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilberdink, C. E., van Zuiden, M., Schrantee, A., Korosi, A., Kaiser, A., Zhutovsky, P., Ginty, A. T., Ensink, J. B. M., Lindauer, R. J. L., Vrijkotte, T. G. M., & de Rooij, S. R. (2021). Dysregulated functional brain connectivity in response to acute social-evaluative stress in adolescents with PTSD symptoms. European Journal of Psychotraumatology, 12(1), 1880727. 10.1080/20008198.2021.1880727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge, C. W., Castro, C. A., Messer, S. C., McGurk, D., Cotting, D. I., & Koffman, R. L. (2004). Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine, 351(1), 13–22. 10.1056/NEJMoa040603 [DOI] [PubMed] [Google Scholar]
- Holmes, E. A., & Bourne, C. (2008). Inducing and modulating intrusive emotional memories: A review of the trauma film paradigm. Acta Psychologica, 127(3), 553–566. 10.1016/j.actpsy.2007.11.002 [DOI] [PubMed] [Google Scholar]
- Holz, E., Lass-Hennemann, J., & Michael, T. (2017). Analogue PTSD symptoms are best predicted by state rumination. Journal of Experimental Psychopathology, 8(2), 192–213. 10.5127/jep.050915 [DOI] [Google Scholar]
- James, E. L., Lau-Zhu, A., Clark, I. A., Visser, R. M., Hagenaars, M. A., & Holmes, E. A. (2016). The trauma film paradigm as an experimental psychopathology model of psychological trauma: Intrusive memories and beyond. Clinical Psychology Review, 47, 106–142. 10.1016/j.cpr.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Kamboj, S. K., Gong, A. T., Sim, Z., Rashid, A. A., Baba, A., Iskandar, G., Das, R. K., & Curran, H. V. (2020). Reduction in the occurrence of distressing involuntary memories following propranolol or hydrocortisone in healthy women. Psychological Medicine, 50(7), 1148–1155. 10.1017/S0033291719001028 [DOI] [PubMed] [Google Scholar]
- Koenen, K. C., Ratanatharathorn, A., Ng, L., McLaughlin, K. A., Bromet, E. J., Stein, D. J., Karam, E. G., Meron Ruscio, A., Benjet, C., Scott, K., Atwoli, L., Petukhova, M., Lim, C. C. W., Aguilar-Gaxiola, S., Al-Hamzawi, A., Alonso, J., Bunting, B., Ciutan, M., de Girolamo, G., … Kessler, R. C. (2017). Posttraumatic stress disorder in the World Mental Health Surveys. Psychological Medicine, 47(13), 2260–2274. 10.1017/S0033291717000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossmeier, M., Tran, U. S., & Voracek, M. (2020). Visualizing meta-analytic data with R package metaviz. R Package Version, 3(1). [Google Scholar]
- Labuschagne, I., Grace, C., Rendell, P., Terrett, G., & Heinrichs, M. (2019). An introductory guide to conducting the trier social stress test. Neuroscience & Biobehavioral Reviews, 107, 686–695. 10.1016/j.neubiorev.2019.09.032 [DOI] [PubMed] [Google Scholar]
- Lang, P., Bradley, M., & Cuthbert, B. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Lass-Hennemann, J., Schäfer, S. K., Römer, S., Holz, E., Streb, M., & Michael, T. (2018). Therapy dogs as a crisis intervention after traumatic events? – An experimental study. Frontiers in Psychology, 9, 1627. 10.3389/fpsyg.2018.01627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer, S., Engel, S., Lupien, S., Knaevelsrud, C., & Schumacher, S. (2022). The cortisol assessment list (CoAL) A tool to systematically document and evaluate cortisol assessment in blood, urine and saliva. Compr Psychoneuroendocrinol, (100108). 10.1016/j.cpnec.2021.100108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon, I., Abelson, J. L., Flagel, S. B., Raz, J., & Young, E. (1999). Neuroendocrine and psychophysiologic responses in PTSD: A symptom provocation study. Neuropsychopharmacology, 21(1), 40–50. 10.1016/S0893-133X(98)00128-6 [DOI] [PubMed] [Google Scholar]
- Lipsey, M. W., & Wilson, D. B. (2001). Practical Meta-Analysis. SAGE Publications. [Google Scholar]
- Marks, E. H. (2018). Reducing intrusive memories of real-world stimuli via memory reconsolidation.
- McLaughlin, K. A., Busso, D. S., Duys, A., Green, J. G., Alves, S., Way, M., & Sheridan, M. A. (2014). Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depression and Anxiety, 31(10), 834–842. 10.1002/da.22284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, M. C., Compas, B. E., & Garber, J. (2012). Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clinical Psychology Review, 32(4), 301–315. 10.1016/j.cpr.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, M. C., Hellman, N., Abelson, J. L., Rao, U., & Cortisol, H. R. (2016). Cortisol, heart rate, and blood pressure as early markers of PTSD risk: A systematic review and meta-analysis. Clinical Psychology Review, 49, 79–91. 10.1016/j.cpr.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn, Z., Stern, C., Aromataris, E., Lockwood, C., & Jordan, Z. (2018). What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Medical Research Methodology, 18(5). 10.1186/s12874-017-0468-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, E. L., Bryant, R. A., & Felmingham, K. L. (2014). Interaction of noradrenaline and cortisol predicts negative intrusive memories in posttraumatic stress disorder. Neurobiology of Learning and Memory, 112, 204–211. 10.1016/j.nlm.2013.11.018 [DOI] [PubMed] [Google Scholar]
- Olff, M., & van Zuiden, M. (2017). Neuroendocrine and neuroimmune markers in PTSD: Pre-, peri- and post-trauma glucocorticoid and inflammatory dysregulation. Current Opinion in Psychology, 14, 132–137. 10.1016/j.copsyc.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., … McKenzie, J. E. (2021). PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ, 372(160), 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman, R. K., Rasmusson, A. M., Koenen, K. C., Shin, L. M., Orr, S. P., Gilbertson, M. W., Milad, M. R., & Liberzon, I. (2012). Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience, 13(11), 769–787. 10.1038/nrn3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulopulos, M. M., Baeken, C., & De Raedt, R. (2020). Cortisol response to stress: The role of expectancy and anticipatory stress regulation. Hormones and Behavior, 117, 104587. 10.1016/j.yhbeh.2019.104587 [DOI] [PubMed] [Google Scholar]
- Rombold, F., Wingenfeld, K., Renneberg, B., Hellmann-Regen, J., Otte, C., & Roepke, S. (2016a). Influence of the noradrenergic system on the formation of intrusive memories in women: An experimental approach with a trauma film paradigm. Psychological Medicine, 46(12), 2523–2534. 10.1017/S0033291716001379 [DOI] [PubMed] [Google Scholar]
- Rombold, F., Wingenfeld, K., Renneberg, B., Schwarzkopf, F., Hellmann-Regen, J., Otte, C., & Roepke, S. (2016b). Impact of exogenous cortisol on the formation of intrusive memories in healthy women. Journal of Psychiatric Research, 83, 71–78. 10.1016/j.jpsychires.2016.08.005 [DOI] [PubMed] [Google Scholar]
- Rohatgi, A. 2015. WebPlotDigitizer. https://automeris.io/WebPlotDigitizer/. [Google Scholar]
- Romero, L. M., Dickens, M. J., & Cyr, N. E. (2009). The reactive scope model — A new model integrating homeostasis, allostasis, and stress. Hormones and Behavior, 55(3), 375–389. 10.1016/j.yhbeh.2008.12.009 [DOI] [PubMed] [Google Scholar]
- RStudio Team . (2022). RStudio: Integrated development for R. Boston, MA: RStudio Inc.
- Scheele, D., Lieberz, J., Goertzen-Patin, A., Engels, C., Schneider, L., Stoffel-Wagner, B., Becker, B., & Hurlemann, R. (2019). Trauma disclosure moderates the effects of oxytocin on intrusions and neural responses to fear. Psychotherapy and Psychosomatics, 88(1), 61–63. 10.1159/000496056 [DOI] [PubMed] [Google Scholar]
- Schultebraucks, K., Rombold-Bruehl, F., Wingenfeld, K., Hellmann-Regen, J., Otte, C., & Roepke, S. (2019). Heightened biological stress response during exposure to a trauma film predicts an increase in intrusive memories. Journal of Abnormal Psychology, 128(7), 645–657. 10.1037/abn0000440 [DOI] [PubMed] [Google Scholar]
- Schultebraucks, K., Shalev, A. Y., Michopoulos, V., Grudzen, C. R., Shin, S.-M., Stevens, J. S., Maples-Keller, J. L., Jovanovic, T., Bonanno, G. A., Rothbaum, B. O., Marmar, C. R., Nemeroff, C. B., Ressler, K. J., & Galatzer-Levy, I. R. (2020). A validated predictive algorithm of post-traumatic stress course following emergency department admission after a traumatic stressor. Nature Medicine, 26(7), 1084–1088. 10.1038/s41591-020-0951-z [DOI] [PubMed] [Google Scholar]
- Schumacher, S., Niemeyer, H., Engel, S., Cwik, J. C., Laufer, S., Klusmann, H., & Knaevelsrud, C. (2019). HPA axis regulation in posttraumatic stress disorder: A meta-analysis focusing on potential moderators. Neuroscience & Biobehavioral Reviews, 100, 35–57. 10.1016/j.neubiorev.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Steudte-Schmiedgen, S., Kirschbaum, C., Alexander, N., & Stalder, T. (2016). An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: Insight from recent hair cortisol findings. Neuroscience & Biobehavioral Reviews, 69, 124–135. 10.1016/j.neubiorev.2016.07.015 [DOI] [PubMed] [Google Scholar]
- Stevens, J. S., Kim, Y. J., Galatzer-Levy, I. R., Reddy, R., Ely, T. D., Nemeroff, C. B., Hudak, L. A., Jovanovic, T., Rothbaum, B. O., & Ressler, K. J. (2017). Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biological Psychiatry, 81(12), 1023–1029. 10.1016/j.biopsych.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge, R., Young, A. H., & Cleare, A. J. (2017). Biomarkers for depression: Recent insights, current challenges and future prospects. Neuropsychiatric Disease and Treatment, 13, 1245–1262. 10.2147/NDT.S114542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagawa, R., Moss-Morris, R., Martin, A., Robinson, E., & Booth, R. J. (2013). Dispositional emotion coping styles and physiological responses to expressive writing. British Journal of Health Psychology, 18(3), 574–592. 10.1111/bjhp.12004 [DOI] [PubMed] [Google Scholar]
- Trautmann, S., Reineboth, M., Trikojat, K., Richter, J., Hagenaars, M. A., Kanske, P., & Schäfer, J. (2018). Susceptibility to others' emotions moderates immediate self-reported and biological stress responses to witnessing trauma. Behaviour Research and Therapy, 110, 55–63. 10.1016/j.brat.2018.09.001 [DOI] [PubMed] [Google Scholar]
- Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is available here: https://osf.io/tbgch/