Abstract
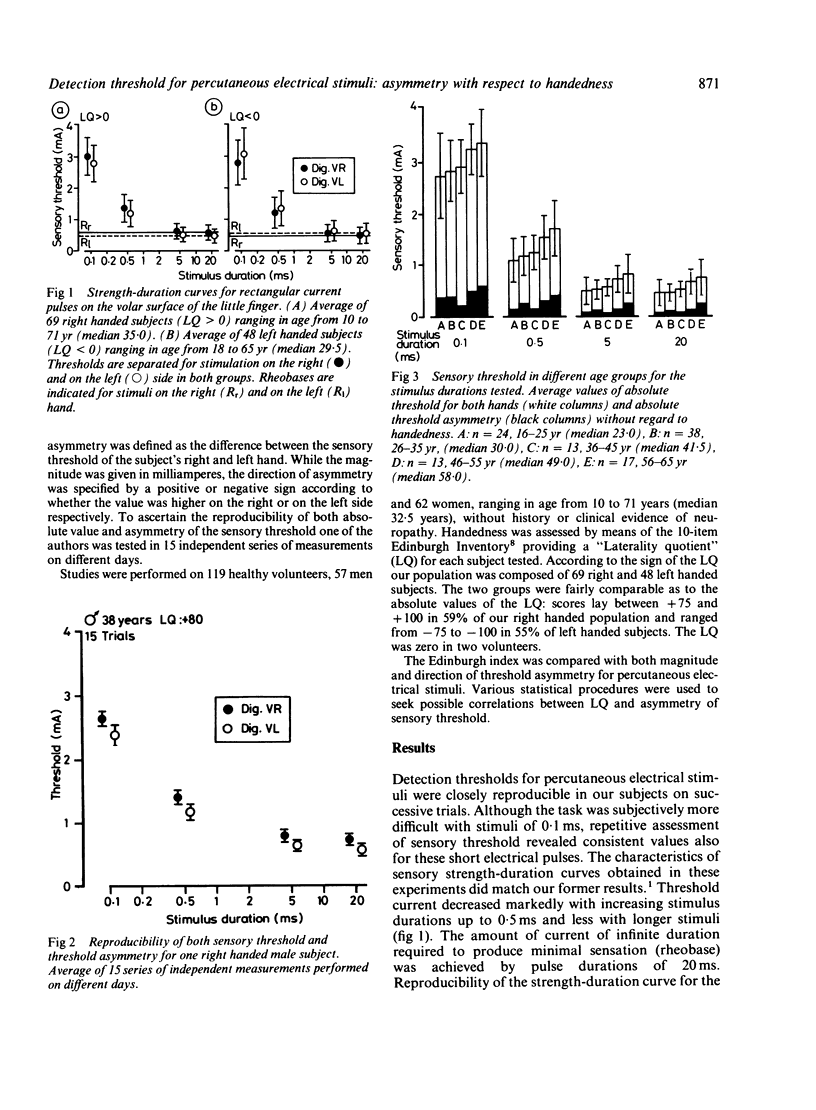
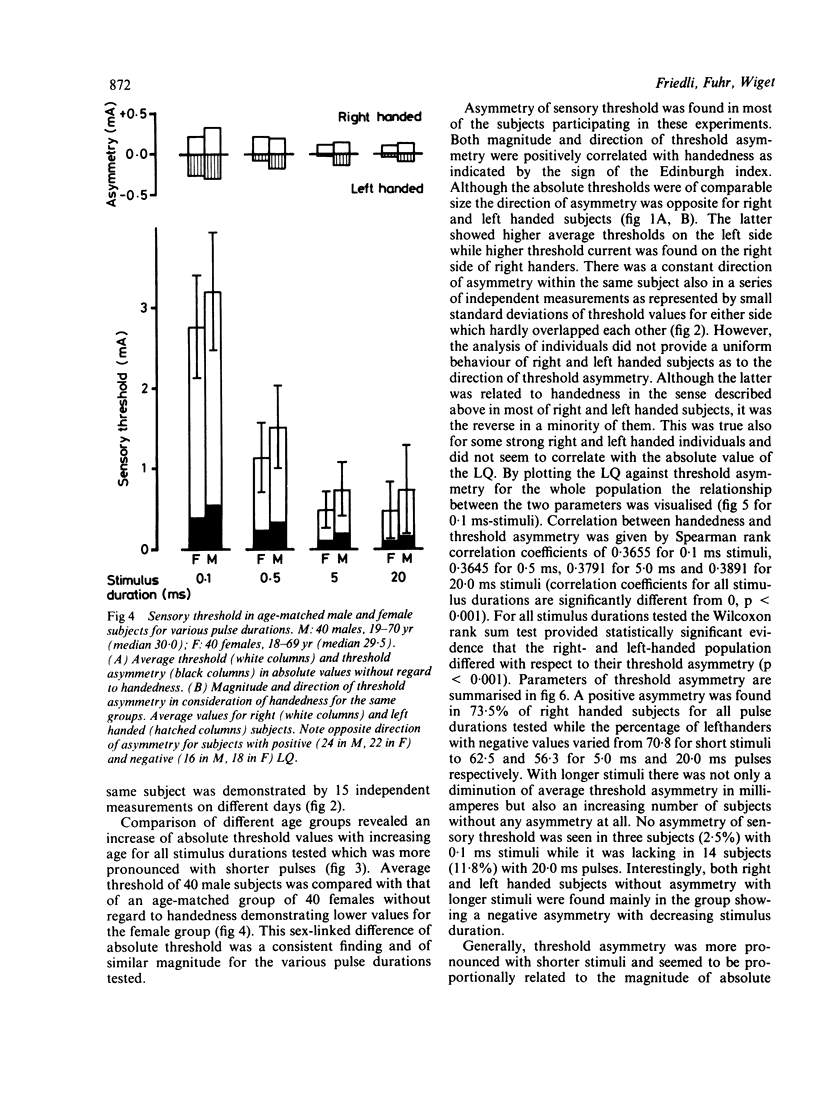
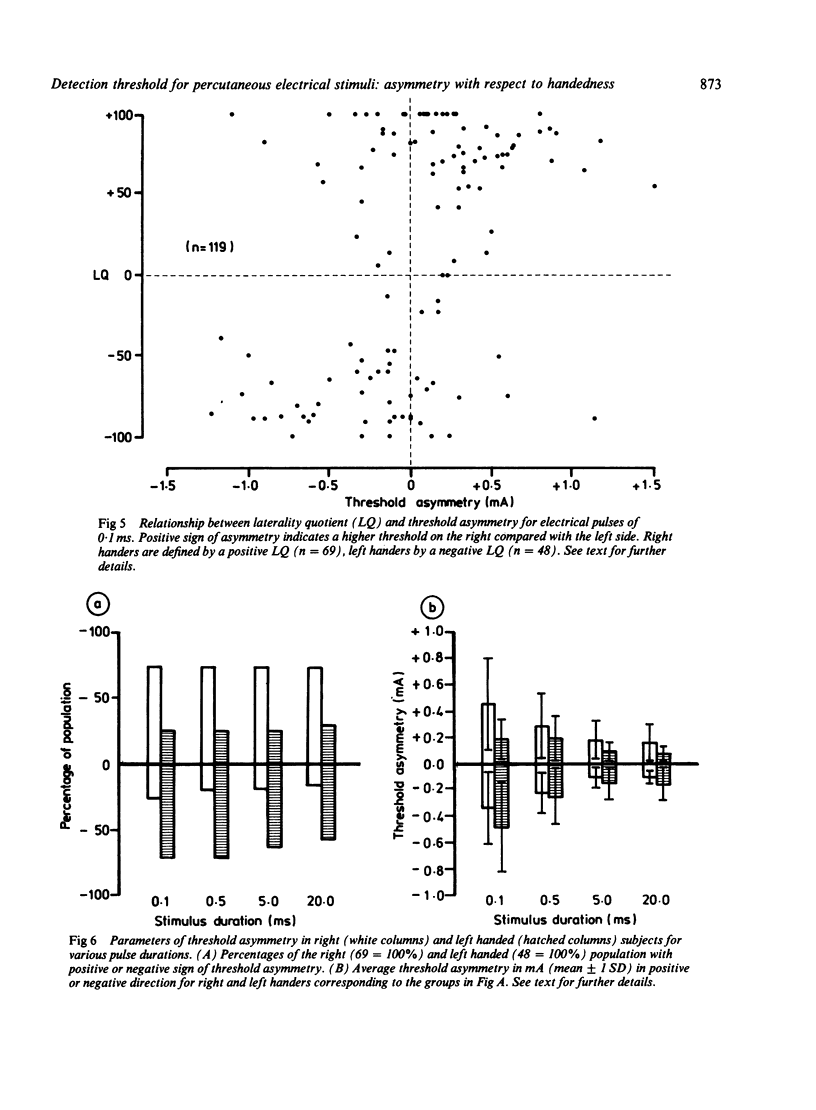
Sensory strength-duration curves were obtained using percutaneous true square-wave pulses ranging from 0.1 to 20.0 ms produced by an isolated constant current stimulator. In 119 healthy volunteers sensory thresholds were measured bilaterally by stimulating the distal phalange of the little finger. In order to examine the relationship of sensory threshold and handedness the latter was assessed by means of the Edinburgh Inventory. An asymmetry of sensory threshold was found for all the subjects and this was more pronounced with shorter stimuli. Of right-handers tested 73.5% had a lower threshold on the left side while 70.8% of left-handers had a lower threshold on the right side. Although threshold asymmetry is associated with handedness this is not necessarily due to cerebral lateralization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colbourn C. J. Can laterality be measured? Neuropsychologia. 1978;16(3):283–289. doi: 10.1016/0028-3932(78)90021-0. [DOI] [PubMed] [Google Scholar]
- Cruz Martínez A., Barrio M., Pérez Conde M. C., Ferrer M. T. Electrophysiological aspects of sensory conduction velocity in healthy adults. 2. Ratio between the amplitude of sensory evoked potentials at the wrist on stimulating different fingers in both hands. J Neurol Neurosurg Psychiatry. 1978 Dec;41(12):1097–1101. doi: 10.1136/jnnp.41.12.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt J. E., Cheron G. Somatosensory evoked potentials to finger stimulation in healthy octogenarians and in young adults: wave forms, scalp topography and transit times of parietal and frontal components. Electroencephalogr Clin Neurophysiol. 1980 Dec;50(5-6):404–425. doi: 10.1016/0013-4694(80)90007-3. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., O'Brien P. C., Bushek W., Oviatt K. F., Schilling K., Stevens J. C. Clinical vs quantitative evaluation of cutaneous sensation. Arch Neurol. 1976 Sep;33(9):651–655. doi: 10.1001/archneur.1976.00500090057011. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Zimmerman I. R., O'Brien P. C., Ness A., Caskey P. E., Karnes J., Bushek W. Introduction of automated systems to evaluate touch-pressure, vibration, and thermal cutaneous sensation in man. Ann Neurol. 1978 Dec;4(6):502–510. doi: 10.1002/ana.410040605. [DOI] [PubMed] [Google Scholar]
- ETTLINGER G., WARRINGTON E., ZANGWILL O. L. A further study of visual-spatial agnosia. Brain. 1957 Sep;80(3):335–361. doi: 10.1093/brain/80.3.335. [DOI] [PubMed] [Google Scholar]
- Friedli W. G., Meyer M. Strength-duration curve: a measure for assessing sensory deficit in peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1984 Feb;47(2):184–189. doi: 10.1136/jnnp.47.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhstorfer H., Lindblom U., Schmidt W. C. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry. 1976 Nov;39(11):1071–1075. doi: 10.1136/jnnp.39.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N., Galaburda A. M. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol. 1985 May;42(5):428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Goldberg J. M., Lindblom U. Standardised method of determining vibratory perception thresholds for diagnosis and screening in neurological investigation. J Neurol Neurosurg Psychiatry. 1979 Sep;42(9):793–803. doi: 10.1136/jnnp.42.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWKES G. R. Cutaneous discrimination of electrical intensity. Am J Psychol. 1961 Mar;74:45–53. [PubMed] [Google Scholar]
- Heckmann J. R. Excitability curve: a new technique for assessing human peripheral nerve excitability in vivo. Neurology. 1972 Mar;22(3):224–230. doi: 10.1212/wnl.22.3.224. [DOI] [PubMed] [Google Scholar]
- Higgens J. D., Tursky B., Schwartz G. E. Shock-elicited pain and its reduction by concurrent tactile stimulation. Science. 1971 May 21;172(3985):866–867. doi: 10.1126/science.172.3985.866. [DOI] [PubMed] [Google Scholar]
- Lascelles R. G., Thomas P. K. Changes due to age in internodal length in the sural nerve in man. J Neurol Neurosurg Psychiatry. 1966 Feb;29(1):40–44. doi: 10.1136/jnnp.29.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B., Perl E. R. A comparison of four tests for assessing the pain sensitivity of different subjects and test areas. Pain. 1977 Aug;3(4):353–365. doi: 10.1016/0304-3959(77)90065-3. [DOI] [PubMed] [Google Scholar]
- Martinez A. C., Perez Conde M. C., del Campo F., Barrio M., Gutierrez A. M., Lopez E. Sensory and mixed conduction velocity in infancy and childhood. I. Normal parameters in median, ulnar and sural nerves. Electromyogr Clin Neurophysiol. 1978 Nov-Dec;18(6):487–504. [PubMed] [Google Scholar]
- Martínez A. C., Conde M. C., Campo F. D., Mingo P., Ferrer M. T. Ratio between the amplitude of sensory evoked potentials at the wrist in both hands of left-handed subjects. J Neurol Neurosurg Psychiatry. 1980 Feb;43(2):182–184. doi: 10.1136/jnnp.43.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod J. G. Digital nerve conduction in the carpal tunnel syndrome after mechanical stimulation of the finger. J Neurol Neurosurg Psychiatry. 1966 Feb;29(1):12–22. doi: 10.1136/jnnp.29.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B., Taylor L. Right-hemisphere superiority in tactile pattern-recognition after cerebral commissurotomy: evidence for nonverbal memory. Neuropsychologia. 1972 Apr;10(1):1–15. doi: 10.1016/0028-3932(72)90038-3. [DOI] [PubMed] [Google Scholar]
- Neary D., Ochoa J., Gilliatt R. W. Sub-clinical entrapment neuropathy in man. J Neurol Sci. 1975 Mar;24(3):283–298. doi: 10.1016/0022-510x(75)90248-8. [DOI] [PubMed] [Google Scholar]
- O'Sullivan D. J., Swallow M. The fibre size and content of the radial and sural nerves. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):464–470. doi: 10.1136/jnnp.31.5.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi A., O'Brien P. C., Okazaki H., Dyck P. J. Morphometry of myelinated fibers of fasciculus gracilis of man. J Neurol Sci. 1976 Feb;27(2):163–172. doi: 10.1016/0022-510x(76)90058-7. [DOI] [PubMed] [Google Scholar]
- Powers R. E., Swick H. M., McQuillen M. P. Human nerve excitability. J Neurol Neurosurg Psychiatry. 1978 Jul;41(7):642–648. doi: 10.1136/jnnp.41.7.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollman G. B. Behavioral assessment of peripheral nerve function. Neurology. 1975 Apr;25(4):339–342. doi: 10.1212/wnl.25.4.339. [DOI] [PubMed] [Google Scholar]
- SEMMES J., WEINSTEIN S., GHENT L., TEUBER H. L. CORRELATES OF IMPAIRED ORIENTATION IN PERSONAL AND EXTRAPERSONAL SPACE. Brain. 1963 Dec;86:747–772. doi: 10.1093/brain/86.4.747. [DOI] [PubMed] [Google Scholar]
- Veale J. L., Mark R. F., Rees S. Differential sensitivity of motor and sensory fibres in human ulnar nerve. J Neurol Neurosurg Psychiatry. 1973 Feb;36(1):75–86. doi: 10.1136/jnnp.36.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. A., McQuillen M. P. Hypoexcitability of ulnar nerve in patients with normal motor nerve conduction velocities. Neurology. 1973 Jan;23(1):78–83. doi: 10.1212/wnl.23.1.78. [DOI] [PubMed] [Google Scholar]


