Abstract
Objective
The use of digital health is a novel way to improve access to comprehensive pulmonary rehabilitation for people with chronic obstructive pulmonary disease (COPD). This study aims to determine if a home-based pulmonary rehabilitation program supported by mobile health (mHealth) technology is equivalent to center-based pulmonary rehabilitation in terms of improvements in exercise capacity and health status in people with COPD.
Methods
This study is a prospective, multicenter, equivalence randomized controlled trial (RCT) with intention-to-treat analysis. A hundred participants with COPD will be recruited from 5 pulmonary rehabilitation programs. Following randomization, participants will be assigned in a concealed manner to receive either home-based pulmonary rehabilitation supported by mHealth or center-based pulmonary rehabilitation. Both programs will be 8 weeks and will include progressive exercise training, disease management education, self-management support, and supervision by a physical therapist. Co-primary outcome measures will be the 6-Minute Walk Test and the COPD Assessment Test. Secondary outcome measures will include the St George’s Respiratory Questionnaire, the EuroQol 5 Dimension 5 Level, the modified Medical Research Council dyspnea scale, the 1-minute sit-to-stand test, the 5 times sit-to-stand test, the Hospital Anxiety and Depression Scale, daily physical activity levels, health care utilization, and costs. Outcomes will be measured at baseline and at the end of the intervention. Participant experience will be assessed through semi-structured interviews at the end of the intervention. Utilization of health care and costs will be measured again after 12 months.
Impact
This study will be the first rigorous RCT to examine the effects of a home-based pulmonary rehabilitation program supported by mHealth technology that includes comprehensive clinical outcome evaluation, assessment of daily physical activity, a health economic analysis, and qualitative analysis. If findings demonstrate that there is equivalence in clinical outcomes, that the mHealth program costs the least amount (and is thus cost-effective), and that the mHealth program is acceptable to participants, such programs should be widely implemented to improve access to pulmonary rehabilitation.
Keywords: Chronic Obstructive Pulmonary Disease, Exercise, mHealth, Physical Activity, Pulmonary Rehabilitation, Quality of Life
Introduction
Pulmonary rehabilitation (PR) is a core, non-pharmacological intervention in the management of chronic obstructive pulmonary disease (COPD) with strong evidence of effectiveness.1–3 PR traditionally consists of structured programs of exercise training and education, supervised in a center,2 but access to programs worldwide is limited.4 Even before the coronavirus disease 2019 (COVID-19) pandemic, research had focused on investigating alternative PR delivery models such as unsupervised home-based programs and real time supervised tele-rehabilitation.5–7 Previous research has shown that PR participants demonstrated a substantial engagement with technology and were willing to use technology for rehabilitation.8 There has been increasing interest in the delivery of health care through digital platforms,9 with interest increasing exponentially during the COVID-19 pandemic.6 The provision of a comprehensive home-based PR program with multiple components tailored to the individual,10 including self-management education, exercise training sessions, symptom and exacerbation monitoring, and feedback through digital systems, is now possible.9 Two randomized controlled trials (RCTs) have investigated the use of web-based (online) PR programs, demonstrating similar outcomes to center-based programs in improvements in exercise capacity and health–related quality of life (HRQoL).11,12 These studies did not include any cost-effectiveness or qualitative analysis and were conducted prior to the pandemic with lower rates of completion reported in the web-based groups compared with the center-based groups.11,12 Mobile health (mHealth) technology is a further advancement on web-based programs whereby patients can access their PR program on their smartphones. Although there have been studies confirming the feasibility and acceptability of short-term home-based mHealth PR programs in COPD,13,14,15,16 to date only a single study, with limited outcomes, has examined the use of mHealth platforms to deliver an initial home-based PR program, demonstrating similar results in terms of disease impact and HRQoL compared with center-based PR.17 To date, no home-based PR program using smartphone technology has been rigorously evaluated with comprehensive outcomes. Therefore, the primary aim of this study is to determine, in people with COPD, whether a home-based mHealth PR program (mobile pulmonary rehabilitation [m-PR]) is equivalent to center-based PR in terms of improvements in functional exercise capacity and health status. The secondary aims are to: (a) determine if the m-PR program results in equivalent outcomes to center-based PR in terms of HRQoL, breathlessness, lower limb endurance and strength, psychological status, and daily physical activity levels; (b) assess the costs, outcomes, and cost-effectiveness of the m-PR program to determine whether it offers value for money; (c) evaluate participant acceptability and experience with both programs; and (d) evaluate the rate of adherence to exercise prescription, completion rates, and occurrence of adverse events in both programs.
Methods
Design
This study is a prospective, single blind (assessor), multi-center, equivalence, RCT (Fig. 1).
Figure 1.
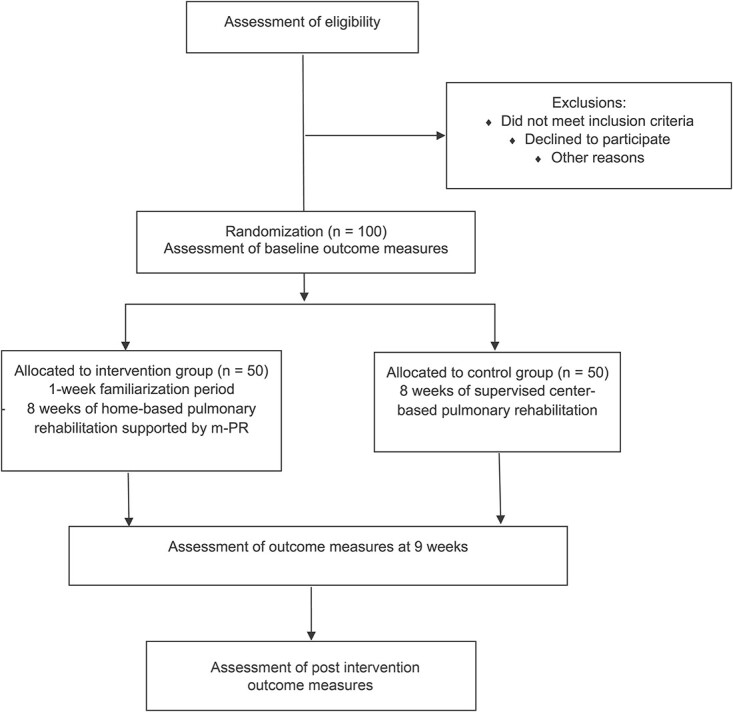
Study participation flow. m-PR = mobile pulmonary rehabilitation.
Participants
People will be eligible for inclusion if they are aged over 18 years and have a medical diagnosis of COPD (forced expiratory volume in 1 second (FEV1) /forced vital capacity ratio of <0.7; FEV1 < 80% predicted normal) and have access to, and regular use of, the internet via a smartphone. Participants will be excluded if they have had an acute exacerbation of COPD in the previous 2 weeks or have musculoskeletal, cardiovascular, or neurological conditions that prevent exercise assessments or exercise training such as severe mobility issues due to arthritis or a stroke, if they are dependent on a walking frame, or have unstable cardiac disease. Participants will also be excluded if they are unable to safely exercise due to oxygen desaturation (SpO2 < 85%), have participated in supervised exercise training within the last 12 months, have limited English language skills which might hinder their understanding of the m-PR platform, or have limitations in the use of smartphone technology (eg, not interested in using mobile technology, non-correctable vision, cognitive or dexterity impairment). Participants will receive written and verbal information about the study and provide informed consent. This study has been approved by the Ethics Committee of the Northern Sydney Local Health District. The trial is registered with the Australian and New Zealand Clinical Trial Registry: ACTRN12619001253190.
Recruitment
Participants will be recruited following their initial PR assessment at 5 sites in the Northern Sydney Local Health District, Sydney, Australia (Royal North Shore Hospital, Hornsby Ku-ring-gai Hospital, Ryde Hospital, Mona Vale Community Health Centre, Brookvale Community Health Centre).
Randomization
Following informed consent, participants will be randomized using a computer-generated random sequence in a 1:1 ratio and allocated in a concealed manner to the intervention group or control group. Randomization will be stratified according to exercise capacity (6-Minute Walk Distance [6MWD] < or ≥400 m)18 and lung function (FEV1 < or ≥40% predicted).19
Intervention Group
Following allocation to the intervention, the study coordinator will undertake a visit to each participant’s home. During the visit, the m-PR app (Fig. 2) will be uploaded onto the participants’ own smartphone, and a 30-minute education session on use of the m-PR app will be provided, together with a hard copy instruction manual. The m-PR platform contains both a patient app and a clinician web-based portal. The m-PR app contains a survey for daily symptom monitoring, weekly collection of outcome measures including the COPD Assessment Test (CAT) and modified Medical Research Council dyspnea scale (mMRC), daily goal setting for certain behaviors such as step count, and a rehabilitation program of individualized exercises and education support through videos (Tab. 1). A virtual “Lung Foundation Australia” Action Plan with medication list is integrated within the m-PR app. Health coaching will be provided to participants through regular notifications and through weekly phone or video-conference contact with the physical therapist. The m-PR clinician portal allows the physical therapist to track each participant’s progress and individualize the participant’s exercise and education program, health coaching notifications, and Action Plan information. Participants will be able to track their own progress within the m-PR app to review their data entry and targets (eg, exercise prescription, daily steps). Following the home visit, participants will be asked to practice accessing and becoming familiarized with the m-PR app for a week and have the ability to contact the physical therapist for assistance. After 1 week, the participant will commence the 8-week m-PR program (Tab. 1) and have weekly contact with the physical therapist.
Figure 2.
Examples of m-PR (mobile pulmonary rehabilitation) app screens.
Table 1.
Components of the m-PR Programa
| Program Component | Functionality |
|---|---|
| Daily symptom monitoring | Participants will be instructed to record their daily symptoms (breathlessness, cough, sputum color, sputum volume, wheeze, fever, exacerbation) on the m-PR app and will be able to track their symptoms over time (score and graph). Symptoms will discussed during the weekly contact between participants and physical therapist and if symptoms deteriorate initiation of COPD Action Plan will be encouraged and/or review with the participants medical officer or respiratory physician facilitated. |
| Exercise program | Participants will receive an individually prescribed (by the physical therapist following the exercise assessment at the pulmonary rehabilitation center) exercise program (8 weeks × 3 sessions per week) supported by exercise videos. The exercise sessions will include aerobic exercise, using a modality accessible to the participant, which is usually ground-based walking (or use of stationary cycle or treadmill if available). Initial aerobic exercise prescription will be for 20-min duration (excluding rests) based on the participant’s baseline 6MWT (best of 2 tests) with intensity commencing at 80% of the average speed achieved during the 6MWT for ground-based walking (Wootton et al, 201422) or treadmill training and 60% of the peak work rate estimated from the 6MWT for cycle training (Hill et al, 200823). Aerobic training will be progressed, by increasing the duration by 5 min after every 3 sessions, to a maximum of 40 min. Intensity will be increased once 40 min of aerobic exercise is achieved and will be based on using the modified dyspnea and rate of perceived exertion (RPE) scales with the aim of a “moderate” to “somewhat severe/strong” level of exertion or breathlessness (whichever is greater) being achieved during training. Resistance training using functional activities and equipment that are readily accessible in the home environment will also be prescribed. Lower limb functional exercises, such as squats and sit-to-stand, will begin at 2 sets of 10 repetitions. Exercises will be progressed once 3 sessions have been completed with an additional 1 set of 10 repetitions to a maximum of 3 sets of 10 repetitions. Hand weights will be added in 1 kg (eg, 500 g in each hand initially) increments once 3 sets of 10 repetitions have been achieved. Upper limb functional exercises will begin at 1 set of 10 repetitions with items in each hand weighing 500 g. Exercises will be progressed once 3 sessions have been completed with an additional 1 set of 10 repetitions until 3 sets of 10 repetitions have been completed. Weights will be increased in 500 g increments once 3 sets of 10 repetitions have been achieved for 3 consecutive sessions. Intensity will be based on using the modified dyspnea and RPE scales with the aim of “moderate” to “somewhat severe/strong” level of dyspnea or exertion (whichever is the highest) being achieved during training. Participants will be instructed to indicate when each exercise is completed by recording date, time, duration, sets, and repetitions of each exercise on the m-PR app, which will then be transferred through to the clinician portal and reviewed by the physical therapist prior to the weekly contact. |
| Educational videos | Participants will receive disease management education sessions consisting of videos (see Tab. 2), scheduled and automatically delivered to the m-PR app each week. The sessions will be personalized for each participant (eg, the smoking cessation video will only be included if the participant is a current smoker) and will be available to watch again at any time during the program. |
| Electronic COPD action plan | If the participant has a current COPD Action Plan, the details, including medications, will be uploaded by the physical therapist to the participants m-PR app and this will automatically populate the electronic Lung Foundation Australia COPD action plan on the m-PR app. If the participant does not have a COPD Action Plan, a paper copy will be given to the participant as part of the initial education session and participants will be asked to take the COPD Action Plan to their local medical officer or respiratory physician to complete and then the details will be uploaded to the m-PR app. |
| Physical activity plan | An individual plan for physical activity (daily steps) will be prescribed for participants and uploaded to the m-PR app each week. An activity tracker (Fitbit Inspire, Fitbit Inc, San Francisco, CA, USA) will be provided to participants to automatically track daily step count. The participant will be instructed to input their daily step count data into the m-PR app on a daily basis. Initial weekly step target will start at the earliest in week 2 of the m-PR program and will be set to increase by 5% average daily step count from the previous week. Step goals will be progressed if the previous week’s goal was achieved and will increase by 5% of the new average daily step count. If the previous week’s goal was not achieved or the participant is experiencing an exacerbation then the daily step goal will be kept the same or reduced. |
| Inhaler technique videos | Instructional videos for inhaler technique from Lung Foundation Australia’s website will be uploaded to each participant’s m-PR app based on their prescribed medications in the second week of the program and will be available to be watched again. |
| CAT questionnaire | Participants will be asked to complete the CAT once a week via the m-PR app. The CAT scores will be used to track the participant’s health status during the m-PR program. |
| mMRC | Participants will be asked to complete the mMRC once a week via the m-PR app. The mMRC scores will be used to track the participant’s level of breathlessness during activity during the m-PR program. |
| Notifications | A minimum of 3 standard notifications designed to educate, motivate, and support participants will be sent each week. Reminders to complete the exercise sessions, watch the education videos, and complete the physical activity program will also be sent. The physical therapist will have a choice of 49 other pre-written notifications to send to the participant’s as clinically indicated, covering topics such as COPD management, how to overcome barriers to exercise, nutrition, smoking cessation, mental health, and wellbeing. |
| Menu | The m-PR app includes a menu function that provides participants with a frequently asked questions section, which includes information on how to use the m-PR app, a safety guide that provides information regarding exercising safely at home and what to do in the event of abnormal symptoms during exercise, breathlessness, and effort scales to help guide the participant with intensity of exercise, pictures, and instructions of how the stretch, and the ability to review all data that has been entered into the m-PR app to allow the participant to track their progress. Links to websites are also provided within the m-PR app which include heart attack warning signs, local air quality and pollen count, the Australian guide to healthy eating and the Lung Foundation Australia website. |
| Clinician web portal | This portal is where the physical therapists upload the required components of the m-PR program to be shown on the participant’s app. Regular monitoring will be undertaken by the study coordinator to ensure that regular usage of the m-PR app is occurring for all intervention group participants. This is a secure system, with all information kept fully confidential (deidentified and coded). Data will only be accessible by the physical therapist and research team. Any data or interaction that the participant has with the m-PR app (eg, exercise data, use of the educational videos, use of COPD Action Plan) will be able to be analyzed to determine the participant level of engagement with the m-PR program. |
6MWT = 6-Minute Walk Test; app = application; CAT = COPD Assessment Test; COPD = chronic obstructive pulmonary disease; mMRC = modified Medical Research Council dyspnea scale; m-PR = mobile pulmonary rehabilitation.
Table 2.
Educational Content and Place in m-PR Programa
| Video/Audio Title | Place in m-PR | |
|---|---|---|
| 1 | Introduction to pulmonary rehabilitation and m-PR | Week 1 |
| 2 | Relaxation—progressive muscle relaxation | Weeks 1,5, 8 |
| 3 | Breathlessness management techniques | Week 2 |
| 4 | How do your lungs work? | Week 2 |
| 5 | Mindfulness of breath | Weeks 2 and 6 |
| 6 | Understanding your lung disease and breathlessness | Week 3 |
| 7 | Airway clearance techniques | Week 3 |
| 8 | Smoking cessation | Week 3b |
| 9 | Knowing your medications and action plans | Week 4 |
| 10 | Patient experience video | Week 4 |
| 11 | Managing your lung disease | Week 4 |
| 12 | Mindfulness—body scan | Weeks 4, 7 |
| 13 | Managing fatigue | Week 5 |
| 14 | Nutrition—general healthy eating | Week 6 |
| 15 | Nutrition—healthy eating and weight gain | Week 6b |
| 16 | Nutrition—healthy eating and weight loss | Week 6b |
| 17 | Anxiety and depression and coping strategies | Week 7 |
| 18 | Supportive care and end of life issues | Week 8 |
| 19 | Inhaler demonstration video (personalized to each participant) | Week 2 |
m-PR = Mobile pulmonary rehabilitation
Video only uploaded to participant m-PR app if indicated.
Control Group
Participants in the control group will attend the center-based PR program twice a week for 8 weeks according to current best practice guidelines.20 Each group-based session will consist of supervised, individually prescribed exercise training and self-management education. At least 30 minutes of aerobic exercise training will be performed, under the supervision of a physical therapist, plus resistance exercises using functional activities such as stair climbing and sit-to-stand, as well as free weight exercises for the upper limbs. The exercise prescription and progression will replicate the intervention group exercise protocols (Tab. 1). Participants will also be encouraged to exercise at home once a week and to record adherence in a paper diary. Self-management education during supervised sessions will include disease management, covering standard topics including management of exacerbations and use of COPD action plans, understanding medications, and ongoing participation in exercise.21
Measures
At baseline, all participants will either complete pulmonary function tests (PFTs) or have recent PFT results (ie, within the last 6 months if clinically stable) recorded. Anthropometric measurements (height, weight) will be recorded along with a medical history including current medications and co-morbidities. All primary and secondary outcome measures will be collected at baseline and post intervention by an assessor, blinded to group allocation.
Primary Outcome Measures
This study has 2 co-primary outcome measures: functional exercise capacity and health status. Functional exercise capacity will be measured by the 6-Minute Walk Test (6MWT) with 2 tests performed according to protocol24 and the best result recorded. Pulse rate and oxygen saturation will be monitored during the 6MWT using a pulse oximeter (RAD-5v Masimo Corporation, Irvine, CA, USA). Dyspnoea and rate of perceived exertion will be recorded before and after each 6MWT using a modified 0–10 Borg scale.25 Health status will be assessed using the paper-based CAT,26 which is an 8-item questionnaire that measures the impact of COPD on patients’ wellbeing and daily life.
Secondary Outcome Measures
HRQoL will be measured by the St George’s Respiratory Questionnaire (SGRQ). The SGRQ has 53 items examining the impact of respiratory disease across 3 domains (ie, symptoms, activity limitations, impact of disease). The total score is calculated from all 3 domains with a lower score indicating better HRQoL.27 Level of dyspnea will be assessed using the mMRC28 where the participant can rate the extent to which their breathlessness affects mobility on a 1–5 stage scale. Lower limb endurance will be assessed using the 1-minute sit-to-stand test29 during which the participant is instructed to stand up and sit down as many times as possible during 1 minute with the number of repetitions completed recorded as the outcome. Functional lower limb strength will be assessed by the 5 times sit-to-stand test30 in which the participant is instructed to stand up and sit down as quickly as possible 5 times and the time taken is recorded. For both the 1-minute sit-to-stand test and the 5 times sit-to-stand tests, a 46 to 48 cm high chair (dependent on site) will be used. Two tests will be performed at each time-point to account for a learning effect, with 10 minutes of rest between tests, and the best result will be recorded. The same height of chair will be used for the sit-to-stand tests at baseline and post intervention assessment. Psychological status will be assessed using the Hospital Anxiety and Depression Scale (HADS). The HADS is a self-administered questionnaire consisting of 14 items (7 each for anxiety and depression). Scores for both anxiety and depression range from 0 to 21, with scores above 11 indicating that psychological assessment may be required.31 Daily physical activity levels will be measured using the activPAL4 activity monitor (PAL Technologies Ltd, Glasgow, Scotland, UK), which is a small, lightweight sensor attached to the anterior mid-thigh using a waterproof adhesive dressing. The device will be worn for 7 days, 24 hours a day. Data collected will include time spent in moderate/vigorous physical activity, step counts and time spent standing and walking.
Health Economic Analysis of the M-PR Program
All relevant resources used for the m-PR and center-based programs will be recorded and valued using micro-costing.32 The resources compiled will include health care resources associated with: (1) m-PR, such as the time taken by the physical therapist to interact with the portal and develop and implement exercise programs, education, and health coaching, as well as the time taken by the participants to interact with the app and complete other program components; (2) the center-based program, such as the time used at each center, and the time taken by the physical therapist to plan exercises and education and supervise participants, out-of-pocket costs to participants for attending the center and time taken to complete and record exercises at home; and (3) both programs, such as the cost of medicines prescribed by health professionals for COPD, emergency department (ED) care, hospital admissions, other clinic visits, general practitioner and specialist visits, nursing and allied health visits, and the cost of equipment (eg, hand weights). Resource information will be collected from several sources, such as participant questionnaires and administrative data from sites. With an equivalent co-primary outcome assumed for this study, it will be important to assess whether m-PR is the least costly program for providing PR, so that the desired health outcomes can be obtained or not. For the cost-utility analysis, participants will complete the non-specific health condition and utility-founded EuroQol 5 dimensions of health, 5 response options questionnaire (EQ-5D-5L)33 at baseline, post intervention, and 12 months post intervention. Quality adjusted life years (QALYs) will be the main outcome for the cost-utility analysis. Differences in the means of other outcomes between the 2 groups will also be used in the denominators of incremental cost-effectiveness ratios (ICERs) to summarize, for instance, the economic cost per clinically meaningful improvement in the 6MWT and CAT, adverse events (eg, fall or other injury related to participating in exercise that requires medical care) avoided, and unplanned hospital admission avoided. Environmental costs of both programs will be calculated from the estimated carbon dioxide equivalents of travel, exercising, and use of the m-PR platform.
Participant Experience
Participant experience will be measured by 2 questionnaires on completion of the study: (i) a custom-designed satisfaction survey, and (ii) the Physical Activity Enjoyment Scale,34 which will explore participants’ experiences and satisfaction with m-PR or the center-based PR program. In addition to the questionnaires, at completion of the 8-week intervention period, a subset of participants in each group will be invited to complete individual, semi-structured interviews to provide a critical realist evaluation of their experiences of each program. The interviewer will be unknown to the participants. Interviews will be audio recorded and transcribed verbatim and will be analyzed thematically using NVivo 1.5.1 (940) (Qualitative Solutions & Research Pty Ltd, Australia).
Participants in the intervention group only will be able to rate their experience of using the m-PR platform using 2 questionnaires on completion of the study, (i) The System Usability Scale, which is a 10-item scale giving a global view of subjective assessments of usability of digital systems35 and (ii) The Mobile Application Rating Scale: User version,36 which examines the engagement, functionality, esthetics, and information quality within the m-PR app.
Adherence, Completion Rates, and Adverse Events
A comparison of rates of adherence to the exercise session prescription, program completion, and occurrence of minor and major adverse events will be recorded for participants in both groups. In the intervention group, usage of the m-PR app, including weekly completion of assigned tasks, will be analyzed through the clinician web-portal that receives data directly from the participant’s m-PR app.
Data Analysis
Statistical analysis will be performed for both the intention-to-treat (ITT) population and per-protocol population, with a P value of <.05 considered significant. ITT analysis will include all participants in the groups they were randomized to regardless of their level of adherence to either program. Per-protocol analysis will include participants in both groups who adhered to at least 70% of the prescribed exercise training sessions. All main analyses will be performed using SPSS (Version 22 for Windows, IBM Corp, Armonk, NY, USA). Linear mixed models will be used to determine whether there are significant differences in clinical and health outcomes over time between the intervention group and the control group. Data will be presented as the mean and 95% CI for the differences between the 2 groups with equivalence limits determined for each outcome (eg, for the primary outcome of 6MWD the equivalence limit will be set as −25 m37 and for the CAT score the equivalence limit will be set as −2 points38 which are both based on the minimum clinically important differences respectively). Standard frameworks for conducting health economic analyses, including collating information and presenting results,39,40 will be used in this study. ICERs will be calculated as the economic cost per unit of the outcomes obtained at the end of the study using the mean value of resources used expressed in dollars and outcomes expressed in either ordinary units (eg, QALYs gained) or dollars in the intervention group compared with the control group. The bootstrapping procedure will be applied to the trial data to create 10,000 simulated samples. These samples will be used to calculate the means and standard deviations for the costs and outcomes and the 95% CIs for the ICERs. The mean costs and outcomes generated will be drawn onto the cost-effectiveness plane, with suitable willingness-to-pay lines imposed so that acceptability of m-PR can be assessed from the perspective of the health care provider. A range of sensitivity analyses will be undertaken, including assessing the change in the ICERs when the type of costs incorporated changes. The last part of the economic analysis will be to identify the predictors for the occurrence of crucial events (such as hospital admissions) and outcomes (such as exercise capacity and HRQoL) over time using longitudinal/time-to-event data methods. For the qualitative data, deidentified interview transcripts will be open coded and thematically analyzed by 2 researchers. Subsequently, researchers will interpret the data in relation to the 7 components of the theoretical framework of acceptability (affective attitude, burden, ethicality, intervention coherence, opportunity costs, perceived effectiveness, and self-efficacy).41
Sample Size
The size of the study was calculated with consideration of preliminary evidence for the equivalence of m-PR as compared with gold standard center-based PR.42 The co-primary focus was to obtain an estimate for the lower bound of the 95% CI for the 6MWT and the CAT. For the 6WMT, assuming no difference between intervention group and control group and a SD of 56 m for 6MWD,43 88 participants will be required to estimate the lower 95% CI bound for the mean difference to be no >25 m.24 For the CAT, assuming no difference between intervention group and control group and a SD of 7 for total CAT score,38 86 participants will be required to estimate the lower 95% CI bound for the mean difference to be no >3.8 points. To account for a 15% drop-out rate, a total of 100 participants will be required (50 in each group).
Role of the Funding Source
The funders played no role in the drafting or approval of this manuscript.
Discussion
This will be the first rigorous multi-center RCT to determine, in people with COPD, if a comprehensive home-based PR program delivered via mHealth technology is equivalent to center-based PR in terms of improvements in exercise capacity and health status. In addition, comparisons of the effect of both programs on HRQoL, breathlessness, lower limb endurance and strength, psychological status, and daily physical activity levels will be examined. A health economic analysis investigating the cost-effectiveness of m-PR as well as assessment of acceptability and participant experience of both programs will also be conducted. If the findings of the current study show equivalence of outcomes between both programs and if m-PR is found to be cost-effective and acceptable to people with COPD, this will contribute strong evidence for the implementation of m-Health programs as a strategy to improve access to PR.
Acknowledgments
The authors acknowledge the contributions of the NSLHD Chronic Disease Community Rehabilitation Service at Royal North Shore Hospital, Hornsby Ku-ring-gai Hospital, Ryde Hospital, Mona Vale Hospital, and Brookvale Community Health Centre. The authors also acknowledge the CSIRO Software engineers Derek Ireland and Simon Gibson for the creation of the m-PR app.
Contributor Information
Sally L Wootton, Chronic Disease Community Rehabilitation Service, Northern Sydney Local Health District, North Ryde, NSW, Australia; Faculty of Medicine and Health, Sydney School of Health Sciences, University of Sydney, Sydney, NSW, Australia.
Marita T Dale, Faculty of Medicine and Health, Sydney School of Health Sciences, University of Sydney, Sydney, NSW, Australia.
Jennifer A Alison, Faculty of Medicine and Health, Sydney School of Health Sciences, University of Sydney, Sydney, NSW, Australia; Allied Health, Sydney Local Health District, NSW, Australia.
Sarah Brown, Chronic Disease Community Rehabilitation Service, Northern Sydney Local Health District, North Ryde, NSW, Australia; Faculty of Medicine and Health, Sydney School of Health Sciences, University of Sydney, Sydney, NSW, Australia; Department of Physiotherapy, Royal North Shore Hospital, St Leonards, NSW, Australia.
Hannah Rutherford, Faculty of Medicine and Health, Sydney School of Health Sciences, University of Sydney, Sydney, NSW, Australia; Directorate of Strategy, Innovation and Improvement, South Eastern Sydney Local Health District, Sydney, NSW, Australia.
Andrew S L Chan, Chronic Disease Community Rehabilitation Service, Northern Sydney Local Health District, North Ryde, NSW, Australia; Department of Respiratory and Sleep Medicine, Royal North Shore Hospital, St Leonards, NSW, Australia; Faculty of Medicine and Health, Northern Clinical School, The University of Sydney, Sydney, NSW, Australia.
Marlien Varnfield, Australian eHealth Research Centre, CSIRO, Brisbane, Queensland, Australia.
Ian A Yang, The Prince Charles Hospital and The University of Queensland, Brisbane, Queensland, Australia.
Michelle Cunich, Boden Initiative, Charles Perkins Centre, Central Clinical School, Faculty of Medicine and Health, The University of Sydney, Camperdown, NSW, Australia; Implementation and Policy, Cardiovascular Initiative, The University of Sydney, Camperdown, NSW, Australia; Sydney Institute for Women, Children and their Families, Sydney Local Health District , Camperdown, NSW, Australia; The ANZAC Research Institute, Concord Repatriation General Hospital, Concord, Australia; Sydney Health Economics Collaborative, Sydney Local Health District, Sydney, NSW, Australia.
Sarah Dennis, Faculty of Medicine and Health, Sydney School of Health Sciences, University of Sydney, Sydney, NSW, Australia; South West Sydney Allied Health Research Collaboration, South West Sydney Local Health District, Liverpool, NSW, Australia; Ingham Institute for Applied Medical Research, Liverpool, NSW, Australia.
Zoe J McKeough, Faculty of Medicine and Health, Sydney School of Health Sciences, University of Sydney, Sydney, NSW, Australia.
Author Contributions
The study was conceived by S.L. Wootton, Z.J. McKeough, J.A. Alison, M.T. Dale, A.S.L. Chan, I.A. Yang, M. Varnfield, S. Dennis and M. Cunich. All authors contributed to the study design. S.L. Wootton wrote the first draft of the manuscript. All authors contributed to the writing of the manuscript and critically reviewed it. All authors approved the final version of the manuscript.
Funding
This project was funded from grants from the Translational Research Grant Scheme, NSW Health, and from a seeding grant from Chronic and Complex Care, Primary and Community Health, Northern Sydney Local Health District (NSLHD). The m-PR app development was funded by a NSLHD innovation grant, a Metro North Hospital and Health Service seeding grant, and the Australian Government Commonwealth Scientific and Industrial Research Organisation (CSIRO). PhD scholarship funding for S. Brown was provided by the Better Breathing Foundation and a Waratah Foundation grant. The content is solely the responsibility of the authors and does not necessarily represent the official view of NSW Health, NSLHD, The University of Sydney, CSIRO, Better Breathing Foundation, or Lung Foundation Australia.
Ethics Approval
This study was approved by the Northern Sydney local Health District Human Research Ethics Committee (2019/ETH00368).
Clinical Trial Registration
The trial is registered with the Australian and New Zealand Clinical Trial Registry (ACTRN12619001253190).
Disclosures
Z.J. McKeough is the managing director of the Better Breathing Foundation, which has contributed PhD scholarship funding to this project. All other authors declare that they have no competing interests.
Data Availability
Data will be stored according to, and as required by, the ethics committee and will be available from the authors on request.
References
- 1. McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;CD003793. 10.1002/14651858.CD003793.pub3. PMID: 25705944; PMCID: PMC10008021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alison JA, McKeough ZJ, Johnston K, et al. Australian and New Zealand pulmonary rehabilitation guidelines. Respirology. 2017;22:800–819. 10.1111/resp.13025. [DOI] [PubMed] [Google Scholar]
- 3. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society / European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–e64. 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 4. Houchen-Wolloff L, Spitzer KA, Candy S. Access to services around the world. In: Holland AE, Dal Corso S, Spruit MA, eds., Pulmonary Rehabilitation (ERS Monograph). Sheffield: European Respiratory Society; 2021: 258–272. [Google Scholar]
- 5.Hanssen H, Nolan CM. Emerging models. In: Holland AE, Dal Corso S, Spruit MA, eds. Pulmonary Rehabilitation (ERS Monograph). Sheffield, European Respiratory Society; 2021:294–310. [Google Scholar]
- 6. Michaelchuk W, Oliveira A, Marzolini S, et al. Design and delivery of home-based telehealth pulmonary rehabilitation programs in COPD: a systematic review and meta-analysis. Int J Med Inform. 2022;162:104754. [DOI] [PubMed] [Google Scholar]
- 7. Holland AE, Mahal A, Hill CJ, et al. Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial. Thorax. 2017;72:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seidman Z, McNamara R, Wootton S, et al. People attending pulmonary rehabilitation demonstrate a substantial engagement with technology and willingness to use telerehabilitation: a survey. J Physiother. 2017;63:175–181. [DOI] [PubMed] [Google Scholar]
- 9. Watson A, Wilkinson TMA. Digital healthcare in COPD management: a narrative review on the advantages, pitfalls, and need for further research. Ther Adv Respir Dis. 2022;16:17534666221075493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spruit MA, Wouters EFM. Organizational aspects of pulmonary rehabilitation in chronic respiratory diseases. Respirology. 2019;24:838–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bourne S, DeVos R, North M, et al. Online versus face-to-face pulmonary rehabilitation for patients with chronic obstructive pulmonary disease: randomised controlled trial. BMJ Open. 2017;7:e014580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chaplin E, Hewitt S, Apps L, et al. Interactive web-based pulmonary rehabilitation programme: a randomised controlled feasibility trial. BMJ Open. 2017;7:e013682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon H, Lee S, Jung EJ, Kim S, Lee JK, Kim DK, et al. An mHealth Management Platform for Patients with Chronic Obstructive Pulmonary Disease (efil breath): Randomized Controlled Trial. JMIR Mhealth Uhealth. 2018;6:e10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng N, Sheng L, Jiang W, Hao Y, Wei S, Wang B, et al. A home-based pulmonary rehabilitation mHealth system to enhance the exercise capacity of patients with COPD: development and evaluation. BMC Med Inform Decis Mak. 2021;21:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whittaker R, Dobson R, Candy S, et al. Mobile pulmonary rehabilitation: feasibility of delivery by a mobile phone-based program. Front Comput Sci. 2021;3:3. 10.3389/fcomp.2021.546960. [DOI] [Google Scholar]
- 16. Rassouli F, Boutellier D, Duss J, et al. Digitalizing multidisciplinary pulmonary rehabilitation in COPD with a smartphone application: an international observational pilot study. Int J Chron Obstruct Pulmon Dis. 2018;13:3831–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang Y, Liu F, Guo J, et al. Evaluating an intervention program using WeChat for patients with chronic obstructive pulmonary disease: randomized controlled trial. J Med Internet Res. 2020;22:e17089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jenkins S, Cecins N, Camarri B, et al. . Regression equations to predict 6-minute walk distance in middle-aged and elderly adults. Physiother Theory Pract. 2009;25:516–522. [DOI] [PubMed] [Google Scholar]
- 19. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
- 20. Alison JA, McKeough ZJ, Johnston K, et al. Australian and New Zealand pulmonary rehabilitation guidelines. Respirology. 2017;22:800–819. 10.1111/resp.13025. [DOI] [PubMed] [Google Scholar]
- 21.Lung Foundation Australia. Pulmonary Rehabilitation Toolkit. Accessed March 29, 2022. https://pulmonaryrehab.com.au/.
- 22.Wootton SL, Ng C, McKeough ZJ, et al. Ground based walking training improves quality of life and exercise capacity in COPD. Eur. Respir. J. 2014;44:885–894. [DOI] [PubMed] [Google Scholar]
- 23.Hill, K, Jenkins SC, Cecins N, et al. Estimating maximum work rate during incremental cycle ergometry testing from six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2008;89:1782–1787. [DOI] [PubMed] [Google Scholar]
- 24. Holland MA, Spruit T, Troosters MA, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–1446. [DOI] [PubMed] [Google Scholar]
- 25. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 26. Jones PW, Harding G, Berry P, Wiklund I, Chen W-H, Kline LN. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34:648–654. [DOI] [PubMed] [Google Scholar]
- 27. Jones PW, Quirk FH, Baveystock CM. The St George's respiratory questionnaire. Respir Med. 1991;85:25–31. [DOI] [PubMed] [Google Scholar]
- 28. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. [DOI] [PubMed] [Google Scholar]
- 29. Ozalevli S, Ozden A, Itil O, Akkoclu A. Comparison of the Sit-to-Stand test with 6 Min Walk Test in patients with chronic obstructive pulmonary disease. Respir Med. 2007;101:286–293. [DOI] [PubMed] [Google Scholar]
- 30. Jones SE, Kon SS, Canavan JL, et al. The five-repetition sit-to-stand test as a functional outcome measure in COPD. Thorax. 2013;68:1015–1020. [DOI] [PubMed] [Google Scholar]
- 31. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 32. Tekin RN, Şahin B. Comparison of top down and bottom up cost approaches in colon and rectal cancer treatment. Health. 2021;13:90–109. [Google Scholar]
- 33. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kendzierski D, DeCarlo KJ. Physical activity enjoyment scale: two validation studies. J Sport Exerc Psychol. 1991;13:50–64. [Google Scholar]
- 35. Brooke J. SUS: A “Quick and Dirty” Usability Scale. London: Taylor and Francis; 1996. [Google Scholar]
- 36. Stoyanov SR, Hides L, Kavanagh DJ, et al. Development and validation of the user version of the mobile application rating scale (uMARS). JMIR Mhealth Uhealth. 2016;4:e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1447–1478. [DOI] [PubMed] [Google Scholar]
- 38. Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respir Med. 2014;2:195–203. [DOI] [PubMed] [Google Scholar]
- 39. NHMRC (National Health and Medical Research Council) (2001). How to Compare the Costs and Benefits: Evaluation of the Economic Evidence. Canberra, NHMRC. Accessed date June 1, 2022. https://www.nhmrc.gov.au/sites/default/files/images/how-to-compare-costs-and-benefits-evaluation-of-the-economic-evidence.pdf.
- 40. Drummond M. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- 41. Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017;17:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holland AE, Cox NS, Houchen-Wolloff L, et al. Defining modern pulmonary rehabilitation: an official american thoracic society workshop report. Ann Am Thorac Soc. 2021;18:e12–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Karapolat H, Atasever A, Atamaz F, et al. Do the benefits gained using a short-term pulmonary rehabilitation program remain in COPD patients after participation? Lung. 2007;185:221–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be stored according to, and as required by, the ethics committee and will be available from the authors on request.



