Abstract
Dietary supplementation with nutraceuticals can promote optimal immune system activation, modulating different pathways that enhance immune defenses. Therefore, the immunity-boosting effects of nutraceuticals encompass not only immunomodulatory but also antioxidant, antitumor, antiviral, antibacterial, and antifungal properties, with therapeutic effects against diverse pathological conditions. However, the complexity of the pathways that regulate the immune system, numerous mechanisms of action, and heterogeneity of the immunodeficiencies, and subjects treated make their application in the clinical field difficult. Some nutraceuticals appear to safely improve immune system function, particularly by preventing viral and bacterial infections in specific groups, such as children, the elderly, and athletes, as well as in frail patients, such as those affected by autoimmune diseases, chronic diseases, or cancer. Several nutraceuticals, such as vitamins, mineral salts, polyunsaturated omega-3 fatty acids, many types of phytocompounds, and probiotic strains, have the most consolidated evidence in humans. In most cases, further large and long-term randomized clinical trials are needed to confirm the available preliminary positive data.
Keywords: dietary supplements, immunomodulation, infections, nutraceuticals
INTRODUCTION
The immune system comprises structures, mediators, and biological processes that protect the body from infection. Innate immunity includes pre-existing immune responses to infection that have evolved to recognize pathogens and protect the human body. Conversely, acquired immunity develops later, after exposure to foreign agents, and is specific to each agent. Epithelial barriers, phagocytic cells, dendritic cells, natural killer (NK) cells, and various plasma proteins are the main components of innate immunity. The primary responses are inflammation, by which phagocytes are recruited and activated to eliminate microbes, and defenses mediated by NK and dendritic cells (Paludan et al., 2021).
Specific responses rely on lymphocytes and their products, including antibodies. Two types of acquired immunity exist: humoral immunity, which principally protects against extracellular pathogens and their toxins, and cell-mediated immunity, which defends against intracellular pathogens. Humoral immunity is mediated by B lymphocytes (or B-cells) originating in the bone marrow and their secreted products, antibodies, or immunoglobulins (Igs). Meanwhile, cellular immunity is mediated by T lymphocytes (or T cells) originating in the thymus (Marshall et al., 2018).
Immune system function has been associated with not only health but also longevity (Santoro et al., 2021). Immunosenescence, which refers to the decline in immune system function over time, increases the risk of infections, chronic inflammatory and autoimmune diseases, and cancer in humans (Xu et al., 2020). Therefore, elderly individuals have been suggested to have compromised innate and acquired immune function. Consequently, correcting and preventing these dysfunctions in the elderly is one of the primary goals of modern medicine (Borgoni et al., 2021).
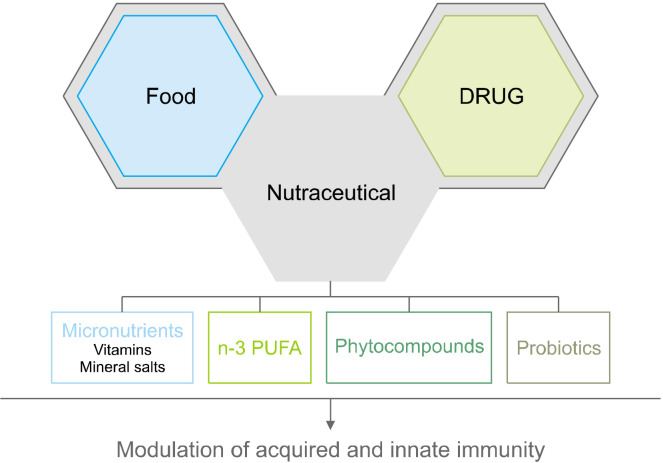
The term nutraceutical was coined by Stephen DeFelice as a syncretic neologism of the words “nutrient” and “pharmaceutical”, representing a modern approach to food science. Indeed, a nutraceutical is a “food or part of a food that provides medical or health benefits, including the prevention and/or treatment of a disease” (Santini et al., 2018). As such, nutraceuticals, which comprise several bioactive derivatives from edible sources, possess therapeutic properties that can modulate the immune system (Fig. 1) and have demonstrated immunity-boosting effects encompassing not only active-modulatory but also antioxidant, antitumor, antiviral, antibacterial, and antifungal properties. Hence, nutraceuticals provide preventive, health-promoting, and therapeutic effects against diverse pathological conditions, such as diabetes, hypertension, arthritis, obesity, and allergy (Ooi and Pak, 2021).
Fig. 1.
Various nutraceutical compounds can modulate innate and adaptive immune responses. n-3 PUFA, polyunsaturated omega-3 fatty acids.
The present review aims to highlight evidence regarding the immunomodulating properties of nutraceutical compounds and their potential therapeutic activities, summarizing several types of nutraceuticals and their diverse effects on the immune system.
VITAMINS
Vitamin A
Vitamin A (or retinoic acid) plays a peculiar role in intestinal innate immunity and homeostatic maintenance of the gut barrier (Biesalski, 2016). This vitamin has three main forms: retinoic acid, retinol, and retinal. Retinoic acid is the main active metabolite and is partly synthesized by intestinal dendritic cells (Huang et al., 2018).
Evidence suggests that vitamin A modulates the immune response in dendritic cells, is involved in the development and differentiation of B and T cells, and affects macrophage activity by regulating cytokine release, principally tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-12. In particular, one study showed that in vitro vitamin A supplementation modulated B lymphocyte activation, differentiation, and cytokine production (Blomhoff et al., 1992).
Vitamin A deficiency can affect T cell immunocompetence at different levels, including lymphopoiesis, distribution, expression of surface molecules, and cytokine production. T cell-macrophage co-cultures treated with retinoic acid showed a reduction in interferon (IFN)-γ production and an increase in IL-4 secretion by T cells, influencing T-helper cell (Th)1/Th2 differentiation pathways (Roy and Awasthi, 2019). Studies have proposed that vitamin A plays an immunomodulatory role during autoimmune and inflammatory diseases given its potential to modulate regulatory T cells (Tregs), a subpopulation of T cells involved in the maintenance of the immune tolerance and regulation of the autoimmune response (Lu et al., 2010; Lu et al., 2014).
Several studies have shown that inflammatory conditions increase retinoic acid synthesis and signal transmission. Indeed, vitamin A deficient rats demonstrated impaired antibody production after introducing bacterial antigens (Pasatiempo et al., 1990). Therefore, vitamin A deficiency may increase proinflammatory responses and impair normal antibody activity (Cantorna et al., 1995). Optimal vitamin A levels are required against enteric pathogens and colonic inflammation given that its deficiency is associated with more persistent infections (McDaniel et al., 2015).
Qi et al. (2016) have recently suggested a correlation between micronutrient deficiency in children (particularly in vitamin A) and infectious diseases of the respiratory and digestive systems. Estimates have shown that there are around 250 million vitamin A-deficient preschool children in developing countries, 10% of whom may die due to increased susceptibility to infection. Since around 1980, vitamin A supplementation has been one of the most successful interventions for childhood infections (Sommer et al., 1986; Brown and Noelle, 2015). Meanwhile, several types of infections may result in decreased systemic vitamin A levels due to reduced intestinal absorption and infection-induced anorexia (Sivakumar and Reddy, 1972). A meta-analysis of 21 clinical trials evaluated the preventive effects of vitamin A supplementation. Accordingly, six of the included studies showed that vitamin A administration, following World Health Organization guidelines (WHO, 1997), during infancy may reduce diarrhea-specific mortality by 30% and all-cause mortality by 25% in children aged between 6 months and 5 years in developing countries (Imdad et al., 2011).
Overall, evidence suggests that healthy populations have significantly higher serum vitamin A levels than do tuberculosis patients. Indeed, a longitudinal cohort study demonstrated that vitamin A deficiency is dose-dependently correlated with the occurrence of tuberculosis (Aibana et al., 2017) and that its supplementation decreased the incidence of tuberculosis in patients with human immunodeficiency virus (HIV) (Campa et al., 2017).
Vitamin C
Vitamin C is an essential micronutrient with anti-inflammatory and antioxidant activities. It promotes collagen synthesis, proliferation, and migration of fibroblasts necessary for stabilizing epithelial barriers and widely improves adaptive immunity by promoting T and B cell differentiation and proliferation (Huijskens et al., 2014; Kouakanou et al., 2020) and increasing antibody production (Qi et al., 2020). The role of vitamin C is not limited to the modulation of adaptative immunity. In fact, supplementation with vitamin C rich fruits for 4 weeks promotes microbial killing by increasing neutrophil migration in response to chemotaxis and suppresses neutrophil extracellular traps (NETs), a network of extracellular fibers produced by activated neutrophils (Bozonet et al., 2015). Vitamin C abolishes the lipopolysaccharide (LPS)-induced production of proinflammatory cytokines TNF-α and IL-6 (Molina et al., 2014; Shati et al., 2022) and may suppress LPS-induced gene expression in human macrophages via nuclear factor kappa-light-chain-enhancer of activated B-cell (NF-κB) (Parahuleva et al., 2013). Moreover, murine-activated B-cells showed a reduction in apoptosis induction levels after a pretreatment with vitamin C (Woo et al., 2010). Huijskens et al. (2015) showed that the in vitro administration of vitamin C may enhance the antitumoral capacity of NK cells.
A meta-analysis of randomized clinical trials (RCTs) showed that vitamin C does not reduce the incidence of colds, although it reduces their duration and severity with good tolerability. It has also been shown to aid subjects exposed to short periods of intense physical exercise (Hemilä and Chalker, 2013). Furthermore, vitamin C has been used to reduce immunodepression induced by intense exercise given its ability to mitigate lymphocyte reduction resulting from exercise and prevent upper respiratory tract infections (Moreira et al., 2007). In addition, vitamin C and E supplementation (500 mg+400 mg for 21 days) promotes increased lymphocyte counts following exercise (Petersen et al., 2001) but did not attenuate the increase in inflammatory cytokines when taken at a dosage of 1,500 mg/d (Nieman et al., 2002).
Vitamin D
Vitamin D is a steroid hormone that modulates immune functions at the cellular level, lowering inflammatory cytokine expression and increasing macrophage activity. Therefore, vitamin D inhibits B-cell proliferation, differentiation, and Ig secretion and blocks T cell proliferation, facilitating the induction of Tregs (Aranow, 2011). Evidence suggests that vitamin D may prevent the suppressive effects of the adaptive immune system on Th1 cell formation by reducing IFN-γ, IL-2, and IL-12 production (Carvalho et al., 2017).
The modulatory functions eventually promote a reduction in inflammatory cytokines-particularly IL-17 and IL-21-and increase in anti-inflammatory cytokines (such as IL-10). Vitamin D also acts on monocytes and dendritic cells, preventing monocytes from synthesizing inflammatory cytokines, such as IL-1, IL-6, IL-8, IL-12, and TNF-α. Moreover, vitamin D inhibits the differentiation and maturation of dendritic cells, preserving an immature phenotype (Sassi et al., 2018). Vitamin D also promotes the expression of antimicrobial peptides (AMPs), principally cathelicidin and defensin, that are present in NK cells, monocytes, neutrophils, and epithelial cells lining the respiratory tract, protecting the lungs from severe infections such as tuberculosis (Mawson, 2013).
Epidemiological studies show that vitamin D deficiency may increase the risk of autoimmune and respiratory diseases, including asthma (Wang et al., 2022). Values of 1,25-dihydroxy vitamin D3 [1,25(OH)2D3], the biologically active form of vitamin D, are considered sufficient, deficient, and insufficient if they are ≥30 ng/mL, between 21 and 29 ng/mL, and ≤20 ng/mL, respectively. Based on these values, estimates have shown that 1 billion people worldwide suffer from vitamin D insufficiency or deficiency (Dawson-Hughes et al., 2005).
A study on over 19,000 individuals also highlighted that vitamin D levels were associated with an increase in immune defenses such that subjects with vitamin D deficiency were more likely than others to have recently had an upper respiratory tract infection (Ginde et al., 2009). The administration of vitamin D (1,200 IU/d vs. placebo) over 4 months (from December to March) significantly reduced the incidence of influenza A in school-aged children by 42% (Urashima et al., 2010). Vitamin D deficiency has also been associated with various autoimmune diseases, including systemic lupus erythematosus (Athanassiou et al., 2022). More recently, vitamin D deficiency was proposed as a possible factor in the susceptibility, severity, and mortality of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (Jain and Parsanathan, 2020; Razdan et al., 2020).
Vitamin E
Vitamin E is essential for the normal functioning of immune cells, exerting multiple effects on innate immunity. For instance, it reduces the release of reactive oxygen species (ROS) by monocytes and their adhesion to the endothelium (Lee and Han, 2018). It also acts on T cells by suppressing the activity of the transcription factor NF-κB, thereby blocking Fas ligand expression and preventing T cell activation-induced cell death (Li-Weber et al., 2002; Lewis et al., 2019).
Vitamin E supplementation has been shown to enhance cell-mediated and humoral immune responses in various animal species. Notably, dietary interventions involving vitamin E increased lymphocyte proliferation, Ig and IL levels, and NK cell activity (Tanaka et al., 1979; Bendich et al., 1986; Meydani et al., 1986; Moriguchi et al., 1990; Wakikawa et al., 1999; Beharka et al., 2000; Ren et al., 2010). Moreover, several animal studies have revealed that vitamin E supplementation is involved in enhanced resistance against several pathogens (Tvedten et al., 1973; Hayek et al., 1997; Han et al., 2000).
Preclinical results in humans confirmed that administering 200 mg/d of vitamin E for 3 months may increase lymphocyte proliferation, IL-2 production, neutrophil function, and NK cell activity (De la Fuente et al., 2008). Moreover, a clinical trial that evaluated the effects of administering 200 IU of vitamin E (α-tocopherol) for 1 year in an elderly population residing in a nursing home showed that vitamin E supplementation protects against upper respiratory tract infections. In particular, supplementation reduced the incidence of colds and the number of subjects who contracted a cold. Thus, clinical studies have confirmed the efficacy of vitamin E supplementation (200 IU daily for 1 year), especially in preventing respiratory tract infections in the elderly (Meydani et al., 2004).
Vitamin B complex
B vitamins are also involved in immune system regulation. For instance, vitamin B1 influences mitochondrial membrane potential, apoptotic proteins, and protein kinases [p38 mitogen-activated protein kinases (p38-MAPK)]; suppresses NF-κB-induced oxidative stress; and exhibits anti-inflammatory properties. Vitamin B1 deficiency can cause T cell infiltration and chemokine (C-C motif) ligand 2 activation, as well as the overexpression of proinflammatory molecules, such as IL-1, TNF, IL-6, and arachidonic acid derivatives (Spinas et al., 2015).
Vitamin B2 (or riboflavin) exhibits antioxidant and anti-inflammatory effects. In fact, vitamin B2 supplementation in mice increased the phagocytic activity of macrophages infected with Staphylococcus aureus, decreasing the levels of IFN-γ, IL-6, and IL-1β (Dey and Bishayi, 2016).
Vitamin B6 modulates both humoral and cellular immunity. Specifically, vitamin B6 deficiency may alter lymphocyte differentiation and maturation, reduce delayed-type hypersensitivity responses, and indirectly alter antibody production. Dietary vitamin B6 supplementation (50∼100 mg/d) for 14 days improved the immune response in critically ill patients (Cheng et al., 2006). In addition, administering 100 mg/d of vitamin B6 for 12 weeks in patients with rheumatoid arthritis can suppress the release of proinflammatory IL-6 and TNF-α (Huang et al., 2010).
Vitamin B12, also known as cobalamin, is a water-soluble vitamin involved in DNA synthesis and the metabolism of fatty and amino acids. This vitamin is thought to modulate the immune system by keeping lymphocyte Treg counts within the normal range and reducing proinflammatory cytokines (Boran et al., 2021). Recently, studies have confirmed its involvement in enhancing CD8+ and NK cell activity. Vitamin B12 deficiency has been linked to reduced NK cell activity and a decrease in the number of circulating lymphocytes. Supplementation with this vitamin can alter these immunological responses by increasing CD8 T cell and NK cell activity. Moreover, Kavitha et al. (2022) found that vitamin B12 is a helpful modulator of the immunological and inflammatory conditions of patients with HIV.
MINERAL SALTS
Zinc is a micronutrient strongly implicated in immune function and antioxidant response. Its deficiency reduces the activity of acquired and innate immunity, thereby increasing the risk of frequent infections, cancer, and chronic diseases. It also helps maintain the integrity of mucosal membranes, the immune system’s first line of defense (Fraker and King, 2004). Zinc deficiency suppresses NK cell lytic activity (Haase and Rink, 2009), the development of proinflammatory Th17 and Th9 cells (Kitabayashi et al., 2010; Maywald et al., 2018), the oxidative burst of polymorphonuclear leukocytes (PMNs) (Moroni et al., 2005), and mast cell activation (Kabu et al., 2006). Therefore, zinc may modulate the CD34+ progenitor cell differentiation into NK cells in vitro (Muzzioli et al., 2007), with its supplementation promoting increased levels of IFN-γ-producing NK cells (Metz et al., 2007).
Selenium is one of the important trace elements found in whole grains and dairy products. It is involved in maintaining immune homeostasis (Kryukov et al., 2003), stimulating Th, cytotoxic T cell, and NK cell functions (Mehdi et al., 2013). Specifically, selenium reduces the levels of oxidized metallothioneins through glutathione peroxidase (a protein containing a selenocysteine residue that reduces oxidative damage) (Maret, 2003), which results in the activation of certain zinc-dependent antioxidant enzymes that consequently prevent oxidative stress. Selenium supplementation (400 μg/d) for 6 months improves NK cell cytotoxicity in elderly subjects (Wood et al., 2000). Finally, selenium deficiency in aged populations appears to be associated with increased IL-6 levels and a higher risk for mortality over a 5-year observation period (Walston et al., 2006). Selenium strongly reduces the expression of the target genes of NF-κB, a positive regulator of HIV activation from a latent state, interfering with controlled HIV replication (Makropoulos et al., 1996). Several clinical trials have assessed the reduction of blood selenium levels in HIV-infected patients at different stages of HIV and the positive effects of its supplementation on disease progression. Indeed, lower selenium levels were associated with opportunistic infections and increased HIV-related mortality (Baum et al., 1997). Notably, combined supplementation comprising selenium (80 μg daily) and vitamin E (25 mg daily) for 2 months significantly improved symptoms of HIV (Cirelli et al., 1991).
Copper is a trace element that contributes to immune system function, particularly of PMNs and monocytes (Maggini et al., 2007). Copper deficiency may alter cellular antioxidant systems by decreasing superoxide dismutase production, thereby increasing oxidative DNA damage and ultimately downregulating the immune response (Pan and Loo, 2000). Dietary copper intake generally satisfies the daily requirements as people age. Therefore, copper supplementation in the elderly is not always necessary. However, to correct immune system alterations, copper supplementation (up to 2 mg/d) in adults is possible (Festa and Thiele, 2011).
Several investigators have highlighted a potential link between magnesium levels in the blood and the body’s immune response given that magnesium acts as a co-factor in Ig synthesis, C3 convertase activation (complement activation), immune cell adhesion, antibody-dependent cytolysis, macrophage response to lymphokines, and Th-B-cell adhesion (Galland, 1988). Magnesium deficiency may be accompanied by the activation of macrophages and neutrophils and an increase in phagocytosis (Malpuech-Brugère et al., 2000).
POLYUNSATURATED OMEGA-3 FATTY ACIDS (n-3 PUFA)
n-3 PUFA, including docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), are essential molecules in humans that cannot be synthesized de novo (Davinelli et al., 2022). Fish (excluding farmed fish) is one of the main sources of these molecules, and EPA and DHA are found, for example, in algae (Sokoła-Wysoczańska et al., 2018).
n-3 PUFA can modulate inflammatory cell function through various mechanisms. Recently, EPA and DHA supplementation was proposed as a strong and effective preventive measure and a supportive strategy against SARS-CoV-2 infection (Szabó et al., 2020). However, n-3 PUFA also represent precursors of many lipid mediators, which can act as signaling molecules. Indeed, some EPA- and DHA-derived mediators exert anti-inflammatory properties; in particular, resolvins, protectins, and maresins have been the subject of numerous investigations (Cipollina, 2015). These oxidized forms of EPA and DHA are better activators of peroxisome proliferator-activated receptor γ than are parent lipids, leading to NF-κB inhibition-one of the mechanisms by which n-3 PUFA and their derivatives promote anti-inflammatory activities (Im, 2012).
In detail, RvE1 promotes the resolution of inflammation by inhibiting transendothelial migration and PMN infiltration and enhancing macrophage clearance PMN phagocytosis and apoptosis (Campbell et al., 2007; Schwab et al., 2007). Similar activities have been demonstrated by RvD1 and RvD2 detected mainly in human adipose tissue, although their concentrations were reduced in overweight/obese subjects (Clària et al., 2012). The addition of RvD1 and RvD2 to inflamed adipocytes can reduce IL-6 and IL-1β release and the monocyte migration phenomenon (Spite et al., 2009).
PHYTOCOMPOUNDS
Polyphenols
Polyphenols, which are non-energetic secondary metabolites produced by plants in response to stress, have been described as pharmacologically active compounds with immunomodulatory activity (Shakoor et al., 2021). The effects of polyphenols on innate immunity have been studied in various mouse models and clinical studies (Han et al., 2007; Magrone and Jirillo, 2014). Hydroxytyrosol, a polyphenol found in extra virgin olive oil, exhibits anti-inflammatory activity and represses proinflammatory and proatherosclerotic genes, thereby reducing the inflammatory capacity of peripheral blood mononuclear cells (PBMCs) (Granados-Principal et al., 2010). Carnosol and curcumin attenuate the increase in glycolysis and preserve the respiratory capacity of dendritic cells in response to LPS stimulation. They exert this immunosuppressive property through the AMP-activated protein kinase-dependent expression of heme oxygenase 1 (Campbell et al., 2019).
Pallauf et al. (2013) described that the molecular mechanisms by which dietary polyphenols (mainly flavonoids and resveratrol) enhance autophagy in aged cells involve the modulation of the activity of sirtuins (molecules that induce autophagocytosis) and the production and activation of kinases. The increased autophagy of PMNs, monocytes, and macrophages achieved by dietary polyphenol supplementation may reduce the risk of infectious and neoplastic diseases (Mukherjee et al., 2020; Patra et al., 2021). For example, the anticarcinogenic effects of curcumin, the polyphenol extracted from Curcuma longa, have been attributed to increased macrophage and NK cell activation (Zhang et al., 2007). Indeed, curcumin can enhance NO production by NK cells, exhibiting antitumor effects (Bhaumik et al., 2000). Other polyphenols have also shown immunomodulatory effects on the number and activity of NK cells. For instance, studies in animal models have shown that green tea catechin metabolites increase NK cell cytotoxicity (Kim et al., 2016) and that quercetin enhances NK cell lytic activity (Exon et al., 1998). Meanwhile, a clinical trial revealed that supplementation with juices rich in polyphenols (330 mL daily for 2 weeks) among healthy participants with a diet low in polyphenols increased lymphocyte proliferation, IL-2 secretion, and NK cell lytic activity (Bub et al., 2003).
Curcumin acts also on immune innate responses, such as NETs, an important extracellular killing mechanism. Indeed, in neutrophils treated with polybrominated diphenyl ethers, curcumin counteracted the toxic effects by reducing ROS and inhibited NET formation by interfering with NRF2 (Ye et al., 2021). Several studies have also reported that curcumin is involved in T lymphocyte proliferation and activation, claiming that it reduces T cell proliferation induced by compounds such as concanavalin A, phytohemagglutinin, and phorbol-12-myristate-13-acetate (Ranjan et al., 2004) and decreases IL-2 production through NF-κB modulation (Ranjan et al., 1998).
Resveratrol may decrease the number of Th17 cells in a rodent model of inflammatory arthritis (Xuzhu et al., 2012). These cells play a clear role in the progression and pathogenesis of several chronic inflammatory diseases, such as rheumatoid arthritis, multiple sclerosis, psoriasis, atopic dermatitis, and asthma (Zheng et al., 2007; Louten et al., 2009; Cavani et al., 2012). One study showed that grape seed proanthocyanidin extract also showed antiarthritic properties and that it upregulated the number of Tregs and maintained the balance between Th17/Treg, thereby attenuating inflammation (Ahmad et al., 2013).
In murine models of asthma, sesamin can reduce allergic inflammation induced by asthma and airway hyperresponsiveness (Lin et al., 2014). In another animal model of asthma, resveratrol promoted a significant reduction in asthmatic parameters such as the production of Th2 cytokines, airway hyperresponsiveness, and eosinophilia (Lee et al., 2009). In addition, the administration of a polyphenol-rich ethanolic extract from Boehmeria nivea may reduce allergic responses in a mouse model by suppressing mast cell-mediated inflammation, decreasing TNF-α, IL-1β, and IL-6 (Lim et al., 2020).
Several studies have shown the potential role of polyphenols in autoimmune diseases. Polyphenols may help regulate pancreatic β cells, type 1 diabetes, and complications associated with type 1 diabetes (Apaya et al., 2020). Quercetin treatment in mice with type 1 diabetes modulated Th1/Th2 balance, suggesting its glucose-lowering potential (Ravikumar and Kavitha, 2020). In addition, butein was involved in the prevention of cytokine-induced β cell damage and glucose-stimulated insulin secretion, thereby preventing the progression of type 1 diabetes (Jeong et al., 2011). Similarly, polyphenols isolated from Broussonetia kazinoki showed therapeutic potential by preventing cytokine-induced β cell damage and reducing/delaying the extent of pancreatic β cell damage in type 1 diabetes (Bae et al., 2015).
Polyphenols may also improve the quality of life in patients with rheumatoid arthritis. Khojah et al. (2018) reported that the dietary supplementation with a 1-g capsule of resveratrol for 3 months decreased joint swelling and tenderness by regulating proinflammatory cytokines. Moreover, epigallocatechin gallate (EGCG) decreased clinical symptoms in an animal model of rheumatoid arthritis (Morinobu et al., 2008). Similarly, grape polyphenols may mitigate inflammation, oxidative stress, and rheumatoid arthritis-associated symptoms (Park et al., 2011; Gonçalves et al., 2017; Stamer et al., 2017). Administering polyphenol extracts from extra virgin olive oil decreased the proinflammatory cytokines in arthritic mice, resulting in decreased diseases progression (Rosillo et al., 2014).
Polyphenols also play a role in the prevention and treatment of inflammatory bowel disease. They may reduce proinflammatory cytokines, regulate the activity of Tregs, and promote the growth of beneficial microbiota in the intestine. For example, dietary polyphenols from mango (principally gallotannins and gallic acid) improved the symptoms of inflammatory bowel disease in 10 subjects who received 200∼400 g/d of mango pulp for 8 weeks (Kim et al., 2020). Moreover, green tea polyphenols may exert beneficial effects against inflammatory bowel disease by reducing the expression of various proinflammatory cytokines and regulating the composition of the luminal microbiota (Rahman et al., 2018).
Phytocompounds from fungi
Various clinical studies have investigated the immunostimulant and tumor growth-inhibiting abilities of fungi. Such effects have been attributed mainly to not only β-glucans, particularly β-1,3-D-glucans, and β-1,6-D-glucans, but also polysaccharides, proteins, and other low molecular weight compounds contained therein. They inhibit tumor growth by stimulating the immune system (Vlassopoulou et al., 2021). In particular, the active substances derived from fungi intensify the innate and acquired immune response by activating immune effector cells-lymphocytes, macrophages, and NK cells-and the consequent production of cytokines, ILs, TNF-α, and INF-γ. Moreover, some specific extracts can modulate the differentiation of CD4+ T cells into Th1 and Th2, which are involved in chronic autoimmune and allergic diseases, respectively (van Steenwijk et al., 2021).
The antitumor and immunostimulant activities of Ganoderma lucidum have been associated with its polysaccharide content. Its immunostimulatory action in patients with lung cancer suggests its potential additive role in cancer treatment, noting that suppression of lymphocyte activation by phytohemagglutinin can be partially or completely antagonized by polysaccharides extracted from this fungus (Sun et al., 2014).
Another study evaluated the effects of administering G. lucidum for 6 weeks (2.5 or 5 g daily) in soccer players undergoing training including physical activity and exposure to hypoxia for 28 days. This type of exercise may act on the T lymphocyte structure. Study participants were divided into three groups and consumed either 5 or 2.5 g/d of G. lucidum extract or a placebo. At the end of the treatment, a significant reduction in the CD4+/CD8+ ratio was detected relative to the placebo group (Zhang et al., 2008). Thus, the administration of G. lucidum was able to regulate immune system activity, even in athletes.
Other studies have involved the mushroom Lentinula edodes (Shiitake), whose major active component is β-glucan. A clinical study on 52 healthy subjects was conducted to explore the effects of administering 5 or 10 g/d of mushrooms for 4 weeks. At the end of the treatment, the ex vivo proliferation of γδ T cells (60% more) and NK cells (2-fold more) had increased. In addition, both cell types showed an increased ability to express activation receptors. Furthermore, secretory IgA (sIgA) increased, whereas C-reactive protein decreased. Finally, the pattern of cytokines secreted after mushroom consumption changed significantly owing to increased levels of IL-4, IL-10, TNF-α, and IL-1α and a reduction in the macrophage inflammatory protein-1α/chemokine C-C ligand 3 ratio after shiitake intake. Thus, regular shiitake consumption may improve immune function, as evidenced by enhanced γδ T and NK cell proliferation and increased sIgA synthesis (Dai et al., 2015).
One placebo-controlled clinical trial showed that Agaricus blazei Murill Kyowa extract administration for 6 weeks to patients undergoing chemotherapy for cervical, ovarian, or endometrial cancer promoted a significant increase in NK cell activity among treated subjects (Ahn et al., 2004).
Another nutraceutical with immunostimulant potential is β-glucan, which is extracted from Pleurotus ostreatus and has been studied in sports contexts. Notably, evidence shows that its supplementation for 2 or 3 months (50 or 200 mg daily) can mitigate reductions of immune system responses induced by short-term, high-intensity exercise (Bobovčák et al., 2010; Bergendiova et al., 2011). Finally, the immunostimulatory effects of P. ostreatus have been evaluated in children. A clinical study observed 175 children aged 3 to 7 years who had more than five respiratory infections during the 12 months before school started. Participants were given a supplement with either β-glucan and vitamin C or vitamin C alone for 12 months. Notably, the study showed that 36% of children in the active treatment group (10 mg/kg daily for 6 months) did not suffer from respiratory infections, whereas only 21% of those in the placebo group did not suffer from the same. The frequency of influenza, flu-like illnesses, and the number of lower respiratory tract infections also decreased in the active treatment group (Jesenak et al., 2013).
Phytocompounds from Echinacea
Echinacea purpurea (L.), Echinacea angustifolia DC, and Echinacea pallida are frequently used as medicinal plants to prevent and treat colds, flu, and upper respiratory tract infections. The immunostimulant activity of Echinacea occurs via three main mechanisms: the activation of phagocytosis, the stimulation of fibroblasts, and the enhancement of respiratory activity resulting in increased leukocyte mobility (Burlou-Nagy et al., 2022). Several in vivo investigations have shown that Echinacea administration boosts innate immunity and the ability to fight pathogenic infections by activating neutrophils, macrophages, and NK cells (Khalaf et al., 2019). Caffeic acid derivatives, alkamides, ketoalkenes, polysaccharides, and glycoproteins are the molecules that mediate the immunostimulatory and anti-inflammatory activities of Echinacea (Aarland et al., 2017; Thomsen et al., 2018).
Echinacea halves the risk of recurrent respiratory infections in individuals with increased sensitivity and stress or those in a state of immunodeficiency. Similar results have been obtained in subjects with recurrent virologic infections. Clinical evidence also indicates that Echinacea might reduce the risk of recurrent respiratory infections and related complications (David and Cunningham, 2019). Another meta-analysis including 14 RCTs investigating the effects of this plant on the incidence and duration of the common cold found that Echinacea supplementation reduced the cases of the common cold by 58% and the duration of the cold by 1.4 days (Shah et al., 2007). In general, the duration of the studies varied from 4 to 12 weeks, highlighting that the enhancement of immune activity needs chronic administration and that the final efficacy strongly depends on the subject’s compliance.
Echinacea also possesses antiviral properties, especially those that work against influenza, herpes, and syphilis viruses. Potential mechanisms of action were related to the stimulation of IFN production (polysaccharide moiety binding to T lymphocyte receptors) and the inhibition of the viral hyaluronidase enzyme (reduced cell permeability of the virus) (Manayi et al., 2015). In addition, oral Echinacea extract intake enhances NK cell activity in C57BL/6N mice exposed to a forced swimming exercise by upregulating major histocompatibility complex II and Th1 CD4+ T cell responses (Park et al., 2021).
Phytocompounds from papaya
The papaya fruit (Carica papaya) is rich in biologically active components including chymopapain and papain. Papaya seed extract is promoted as a nutritional supplement that can strengthen the immune response (Kong et al., 2021). Indeed, numerous studies have shown that papayas exert significant anti-inflammatory and immunomodulatory activities via different mechanisms. However, the level of maturation, type of cultivation, and method used to extract different components may promote differing levels and types of bioactive phytochemicals (Pandey et al., 2015).
A meta-analysis that evaluated the efficacy of C. papaya extract in patients with Dengue fever showed an increased platelet count after the fourth day of treatment. However, after 48 h, no significant differences were detected between the active treatment and control groups. In addition, treated patients spent significantly fewer days in the hospital than did untreated patients. Thus, the reported data show that C. papaya leaf extract may increase platelet counts in patients with Dengue fever (Charan et al., 2016).
Phytocompounds from garlic
Owing to its antimicrobial and immunostimulant properties, garlic (Allium sativum L.) has been used to prevent and treat numerous diseases. Allicin is the main bioactive compound of garlic, which is produced from alliin by the enzyme alliinase. Immunomodulating proteins have also been isolated from garlic, particularly lectins or agglutinins (Melguizo-Rodríguez et al., 2022). The immunomodulatory effects of garlic have been attributed due to increased macrophage and NK cell activity and T and B lymphocyte production. Clinical studies have shown that garlic supplementation can reduce the number, duration, and severity of upper respiratory tract infections (Percival, 2016; Ried, 2016).
An oncological study investigated patients with colorectal, hepatic, or pancreatic cancer who were treated with aged garlic extract or a placebo for 6 months. At the end of the study, subjects treated with the active product (500 mg daily for 24 weeks) experienced an increase in the number and activity level of NK cells without suffering any side effects (Ishikawa et al., 2006).
Systemic inflammation during obesity is often accompanied by a reduced number of γδ T cells. This subset of cells represents the ability of innate lymphocytes to respond to dietary bioactive components (Poles et al., 2021). A recent clinical trial investigated the immunomodulatory effects of garlic in a population of obese adults. Compared to the placebo group, the aged garlic extract group demonstrated a significantly higher percentage of γδ T cells and lower IL-6 and TNF-α levels, which are one of the causes of systemic inflammation during obesity (Xu et al., 2018).
Phytocompounds from ginseng
The three most used species of ginseng are Asian or Korean ginseng (Panax ginseng), American ginseng (Panax quinquefolius), and Siberian ginseng or, more properly, Eleutherococcus (Eleutherococcus senticosus). Other types of ginseng include Japanese ginseng (Panax japonicus), Vietnamese ginseng (Panax vietnamensis), Indian ginseng (Whitania somnifera), Brazilian ginseng (Suma, Pfaffia paniculata), Peruvian ginseng (Maca, Lepidium meyenii), and wild ginseng (Aralia nudicaulis). The main active constituents (generally between 2∼9%) of ginseng are triterpene saponins known as ginsenosides. More than 100 different ginsenosides have been identified in P. ginseng. Furthermore, various minor constituents (such as essential oils and phytosterols) have been extracted and isolated from this plant’s root, stem, and leaves (Liu et al., 2021).
Few clinical studies have focused on the potential immunomodulatory properties of ginseng. For example, Liu et al. (1995) reported that the ginsenoside Rg1 stimulates lymphocyte proliferation in 10 young and 19 elderly people. In addition, Rg1 significantly improves lymphocyte membrane fluidity (possibly due to its antioxidant activity), thereby intensifying immune function. Moreover, in an RCT that included 20 healthy adults, supplementation with P. ginseng (300 mg/d) significantly increased phagocytic activity and chemotaxis of PBMCs (Scaglione et al., 1990). Finally, among 227 volunteers treated for 3 months with either a flu vaccine+placebo or 100 mg of ginseng extract (+flu vaccine), the prevalence of colds and the flu was significantly higher in the placebo group than in the active treatment group (42 cases vs. 15 cases) (Scaglione et al., 1996).
Phytocompounds from astragalus
Astragalus root (Astragalus membranaceus) occupies an important place in traditional Chinese medicine given its pleiotropic actions on the cardiovascular, nervous, and immune systems. Astragalus polysaccharide is one of the most significant bioactive components derived from the dry root. Several studies have shown its effects on several immune cells. In particular, it appears to promote the activities of macrophages, NK cells, dendritic cells, T cells, and microglia and induce the expression of various cytokines and chemokines (Li et al., 2022).
Hou et al. (1981) demonstrated that supplementation with 8 g/d of astragalus in 14 healthy volunteers for 2 months significantly increased the IFN-producing capacity of blood cells relative to placebo. This capacity was maintained 2 months after the treatment was discontinued.
Phytocompounds from Schisandra
Schisandra (Schisandra chinensis) has been used traditionally for years (mostly on an empirical basis) as a remedy for general fatigue and neurasthenia. As a nutraceutical, it fits with the profile and definition of an adaptogen. This property appears to be the leading explanation for its effects on the immune system, which suggests that it exerts a normalizing action that somehow restores an altered balance (in this case, immune defenses) (Nowak et al., 2019).
Dibenzocyclooctadiene lignans isolated from Schisandra positively impacted the redox status of monocytes, the maturation of dendritic cells, and the activation of T cells (Kortesoja et al., 2019). Cyanidin 3-rutinoside, the major anthocyanin pigment of Schisandra, ameliorated phorbol-12-myristate-13-acetate/A23187-induced allergic inflammation in vitro, suppressing inflammatory cytokines. Therefore, this anthocyanin inhibited the secretion of inflammatory cytokines, such as IL-6 and TNF-α, while also suppressing NF-κB phosphorylation (Jeon et al., 2019).
A clinical study evaluated the effects of supplementation comprising a combination of aqueous extracts of Schisandra, Rhodiola, Eleutherococcus, and Leuzea carthamoides roots on immunity in ovarian cancer patients. A total of 28 patients under drug treatment (cisplatin+cyclophosphamide) were treated with 270 mg/d of nutraceutical or placebo for 4 weeks at the end of their chemotherapy cycle. After 4 weeks, the treatment group showed significantly greater mean IgG and IgM levels than did the placebo group. This result indicates that such a combination of predominantly adaptogenic plant extracts can intensify immunity suppressed by pharmacological anticancer therapy in patients with ovarian cancer (Kormosh et al., 2006).
Lebedev et al. (1970) reported that the administration of Schizandra significantly decreased the morbidity rate among school children during an influenza epidemic in 1969. Among the studied school children, the morbidity rate was 19.5% in the control group and 12.5% in the Schisandra-treated group (net decrease=35.9%) after 1 month. The net decrease was even more significant (65%) after 2 months. In addition, the Schisandra-treated group demonstrated a shorter duration of influenza infections and less severe clinical manifestations than did the control group.
Phytocompounds from Rhodiola and Withania
Rhodiola roots and rhizomes are used in traditional Chinese medicine. Their pharmacologically active compounds include organic acids (gallic, caffeic, and chlorogenic acids), flavonoids, catechins, proanthocyanidins, tannins, and phenolic glycosides. In vitro studies have shown that Rhodiola kirilowii extract stimulates granulocyte activity and increases lymphocyte responses to mitogens (Wójcik et al., 2009). Long-term supplementation with R. kirilowii extracts in mouse mothers during pregnancy and lactation increased the percentage of granulocytes and decreased those of lymphocytes (Lewicki et al., 2017). In another study, Rhodiola rosea extract (0.5 or 1 g daily for 45 days) suppressed proinflammatory cytokines but did not enhance T cell-mediated immunity (Xu et al., 2013).
Withania somnifera (Ashwagandha), also called Indian ginseng or winter cherry, is used in Indian medicine like ginseng. It consists primarily of alkaloids (witanin, scopoletin, somniferin, isopelletierin, and anaferin), steroidal lactones (witanolides and witaferins), terpenoids with a tetracyclic skeleton such as cortisol (sitoindosides I∼IV) and essential oil (puranol), and various acylsteryl glycosides (Paul et al., 2021).
A clinical study evaluated the effects of W. somnifera on the immune system, particularly on four types of immune cells. Participants took W. somnifera root extract twice a day for 96 h and were subjected to peripheral blood sampling at 0, 24, and 96 h to determine differences in the cellular expression of CD4, CD8, CD19, CD56, and CD69 receptors via flow cytometry. After 96 h, the expression of all measured receptor types increased relative to their pretreatment levels, indicating a change in immune cell activation. In detail, a significant increase in CD4 expression was observed on CD3+ T cells, and CD56+ NK cells were activated (Mikolai et al., 2009).
PROBIOTICS
Some probiotic strains could play an important immunomodulatory role in several disorders, including allergic asthma, some atopic dermatitis, and rheumatoid arthritis. However, the mechanisms by which they operate have yet to be fully understood. The most plausible explanation for the antimicrobial and immunomodulatory effects of probiotics is related to the mechanisms of microbial fermentation by specific strains (mainly Bacteroides) that generate fatty acids and organic acids endowed with a series of pleiotropic activities (e.g., anti-inflammatory, hypotensive, chemo-preventive, antimicrobial, and immunostimulant activity) (Cristofori et al., 2021).
Another peculiarity of these microorganisms is that they act at the level of the intestinal mucosa by strengthening “tight junctions” and, therefore, the intestinal barrier. Thus, they exponentially reduce the translocation of pathogenic microorganisms into the bloodstream, thereby weakening the immune response. The strains that adhere the most securely to the intestinal mucosa are Lactobacilli and Bifidobacteria. It is apparent that the gut is a vital organ for immune homeostasis; therefore, more than 70% of immune cells are localized at the intestinal level, where probiotics interact with epithelial cells, dendritic cells, and macrophages through various pathways. In particular, they interact with Toll-like receptors, which are responsible for the body’s defense signal cascade (innate immunity), mediating gene expression (Sirisinha, 2016).
Probiotic supplementation could be a viable strategy for reducing the inflammatory state and preserving the immune system, especially in the elderly. For instance, evidence shows that supplementation with Bifidobacterium animalis increased the fecal concentration of polyamines. These molecules play an anti-inflammatory role in the intestine, thereby preserving the integrity of the intestinal mucosal barrier. These experimental data are supported by reports that supplementation with Bifidobacterium through yogurt in hospitalized elderly patients increases luminal polyamine content and reduces intestinal symptoms (Matsumoto and Benno, 2007).
Some interesting RCTs regarding the effects of probiotics on innate immunity have been available. For instance, in one study, supplementation with Bifidobacterium lactis in elderly subjects significantly increased T and NK cells and improved phagocytic functions (exerted by PMN and monocytes) and tumoricidal activity of NK cells themselves (Gill et al., 2001). In addition, probiotic treatment has been shown to improve vaccine efficacy, as evidenced by increased specific antibody titers, improved NK and PMN functionality, and decreased incidences of the flu and fevers (Namba et al., 2010). Probiotics also appear to have a control function in disorders caused by hyperstimulation leading to an excessive immune response (e.g., in atopic dermatitis, certain types of colitis, and autoimmune diseases such as rheumatoid arthritis) (Sharma and Im, 2018).
CONCLUSION
Some nutraceuticals appear to safely improve immune system function by preventing viral and bacterial infections in specific populations, such as children, the elderly, and athletes. They have also shown to benefit some frail patients, such as those affected by systemic lupus erythematosus, asthma, rheumatoid arthritis, diabetes mellitus, inflammatory bowel disease, acquired immune deficiency syndrome, and cancer. Table 1 and 2 summarize available preclinical and clinical studies described and discussed in the current review. In most cases, further large and long-term RCTs are needed to confirm the positive findings presented in this review and determine the recommended intake that would provide optimized or boosted immune functions.
Table 1.
Preclinical studies regarding the effects of nutraceuticals on the immune system
| Reference | Model | Nutraceutical | Intervention details (duration and oral dose) | Key findings |
|---|---|---|---|---|
| Pasatiempo et al., 1990 | Male Lewis rats | Vitamin A | Vitamin A deficient diet | ·Impaired antibody production against bacterial antigens |
| Cantorna et al., 1995 | B10.BR mice | Vitamin A | Deficient diet, 5 d | ·Increased proinflammatory response (IL-12 and IFN-γ) |
| McDaniel et al., 2015 | C57BL/6 mice | Vitamin A | Vitamin A deficient diet | ·Severe gut infection and increased mortality after exposition to Citrobacter rodentium |
| Qi et al., 2020 | C57BL/6 and ascorbic acid deficient Gulo—/— mice | Vitamin C | − | ·Block of plasma cell differentiation and reduced humoral immune response in deficient mice |
| Ren et al., 2010 | Male young and old C57BL/6 mice | Vitamin E | 6 wk, 500 mg/kg | ·Improved T cell proliferation (old) and IL-1β levels (young) |
| Bendich et al., 1986 | Young rats | Vitamin E | 8~10 wk, 50, 200 mg/kg | ·Improved T and B cell proliferation and responses to mitogens |
| Meydani et al., 1986 | Old mice | Vitamin E | 6 wk, 500 mg/kg | ·Increased lymphocyte proliferation and IL-2 levels |
| Wakikawa et al., 1999 | Young and old C57BL/6 mice | Vitamin E | 9 wk, 500 IU (500 mg) | ·Increased lymphocyte proliferation and IFN-γ levels (young) |
| Moriguchi et al., 1990 | Male F344 rats | Vitamin E | 7 d, 50, 100, 250, 500, 2,500 mg/kg | ·Increased lymphocyte proliferation and NK activity |
| Tanaka et al., 1979 | SL mice | Vitamin E | 6~12 wk, 200 mg/kg | ·Increased antibody response |
| Beherka et al., 2000 | C57BL/6NCrlBR mice | Vitamin E | 6 mon, 500 mg/kg | ·Reduced IL-6 levels and NOS production by macrophages |
| Han et al., 2000 | C57BL/6 mice | Vitamin E | 8 wk, 500 mg/kg | ·Reduced influenza titer ·Increased IL-2 and IFN-γ production |
| Hayek et al., 1997 | C57BL/6NIA mice | Vitamin E | 6 wk, 500 mg/kg | ·Reduced influenza A/PC/1/73 (H3N2) titer |
| Tvedten et al., 1973 | Sprague-Dawley rats | Vitamin E + Vitamin A | 6 wk, 180 mg/kg diet + 6,000 IU vitamin A | ·Increased Mycoplasma pulmonis resistance to infection |
| Malpuech-Brugère et al., 2000 | Male Winstar rats | Magnesium | 4~8 d, Mg deficient diet | ·Increased IL-6 levels ·Increased macrophages number and activity |
| Bhaumik et al., 2000 | Winstar rats | Curcumin | 20 d, 20 mg/d (intraperitoneal injection) | ·Increased NO production by NK cells |
| Exon et al., 1998 | Female Sprague-Dawley rats | Quercetin | 7 wk, 100 mg/kg | ·Increased NK cells activity |
| Ahmad et al., 2013 | BALB/c mice | Proanthocyanidin | Grape seed proanthocyanidin extract, 14 d, 25, 50, or 100 mg/kg | ·Upregulation of Treg cells ·Reduction of inflammatory mediators’ levels |
| Lin et al., 2014 | Male BALB/c mice | Sesamin | 6 d, 1 mg/kg, 10 mg/kg, and 20 mg/kg (intraperitoneal injection) | ·Decreased expression levels of IL-4, IL-5, IL-13, and serum IgE ·Reduced total inflammatory cells and eosinophils |
| Morinobu et al., 2008 | Male DBA/1 mice | EGCG | 15 d, 20 μg/g/d (intraperitoneal injection) | ·Ameliorated arthritis during disease course |
| Gonçalves et al., 2017 | Male Holtzman rats | Grape polyphenols | Merlot grape pomace extract, 23 d, 250 mg/kg/d | ·Normal oxidative stress indicators (GSH, GSGG, GSH/GSGG) |
| Stamer et al., 2017 | Female TNF transgenic mice | Grape polyphenols | Grape powder, 4 wk, 50~100 g/kg/d | ·Decreased inflammation induced formation of synovitis/enthesitis ·Downregulated TNF-mediated enhancement in transcript levels of cytokines (TNF and IL-1β), RANKL, MMP1&3, and CCL3/MIP1α |
| Lee et al., 2009 | Female BALB/c mice | Resveratrol | 32 d, 30 mg/kg (nebulization on days 28, 29, and 30) | ·Decrease of IL-4 and IL-5 in plasma and bronchoalveolar lavage fluid ·Suppression of airway hyperresponsiveness, eosinophilia, and mucus hypersecretion |
| Ravikumar and Kavitha, 2020 | Adult male BALB/c mice | Resveratrol | 12 d, 10~30 mg/kg | ·Decreased number of eosinophils and IL-4 levels ·Reduced the allergic airway inflammation by inhibiting inflammatory cell infiltration and mucous cell metaplasia ·Significant glucose reduction |
| Bae et al., 2015 | Male C57BL/6 mice | Kazinol C and isokazinol D | 3 d, 10 mg/kg (intraperitoneal injection) | ·Prevention of cytokine-induced β-cell damage |
| Khalaf et al., 2019 | Mature albino rats | Phytocompounds from Echinacea purpurea | Herbal formulation containing E. purpurea extract, 2 wk, 250 mg/kg | ·Increased activation of activating neutrophils, macrophages, and NK cells |
| Park et al., 2021 | C57BL/6N mice | Phytocompounds from E. purpurea | 2 wk, 50, 100, and 200 mg/kg | ·Increased NK cell activity, MHC II, CD4+ T cells, Th1 cytokines, and B-cell proliferation |
| Lewicki et al., 2017 | Pregnant BALB/c mice | Phytocompounds from Rhodiola kirilowii | R. kirilowii aqueous or 50% hydroalcoholic extracts, 28 d, 20 mg/kg/d | ·Increased percentage of granulocytes ·Decreased percentage of lymphocytes ·Stimulation of granulocyte phagocytosis |
IL, interleukin; IFN-γ, interferon-γ; NET, neutrophil extracellular trap; NK, natural killer; NOS, nitric oxide synthase; NO, nitric oxide; Treg, regulatory T cell; IgE, immunoglobulin E; GSH, reduced glutathione; GSGG, oxidized glutathione; TNF, tumor necrosis factor; RANKL, receptor activator of nuclear factor-κB ligand; MMP, matrix metalloproteinase; CCL3/MIP-1α, chemokine C-C ligand 3/macrophage inflammatory protein-1α; MHC II, major histocompatibility complex class II; CD, cluster of differentiation; Th1, T-helper 1 cells.
Table 2.
Clinical studies regarding the effects of nutraceuticals on the immune system
| Reference | Study population | Nutraceutical | Intervention details/status (duration and dose) | Key findings |
|---|---|---|---|---|
| Qi et al., 2016 | 684 healthy children (5 mon~12 yr) | Vitamin A | Vitamin A deficiency | ·Higher risk of acute respiratory tract infection and diarrhea |
| Sommer et al., 1986 | 5,939 preschool children | Vitamin A | Over 1 yr, 200,000 IU | ·Reduced mortality |
| Imdad et al., 2011 1) | Neonates, infants (1~6 mon) and children (6~59 mon) | Vitamin A | − | ·Reduced all-cause and diarrhea-specific mortality in children (6~59 mon) |
| Aibana et al., 2017 | 889 household contacts of pulmonary tuberculosis | Vitamin A | Vitamin A deficiency | ·Higher risk of occurrence of tuberculosis in a dose-dependent manner |
| Bozonet et al., 2015 | 14 young men (18~30 yr) with suboptimal plasma vitamin C status (<50 μmol/L) | Vitamin C | Two SunGold vitamin C rich kiwi fruit/d, 4 wk | ·Increased neutrophil chemotaxis |
| Hemilä et al., 2013 1) | 11,306 participants | Vitamin C | − | ·Reduction of duration and severity of colds |
| Moreira et al., 2007 1) | 1,603 athletes | Vitamin C | − | ·Reduction of the immunodepression induced by intense exercise and prevention upper respiratory tract infections |
| Petersen et al., 2001 | 20 male recreational runners | Vitamin C+Vitamin E | 21 d, 500 mg+400 mg vitamin E | ·Increased lymphocyte counts following exercise |
| Nieman et al., 2002 | 28 runners | Vitamin C | 8 d, 1,500 mg/d | ·No changes in inflammatory cytokines |
| Wang et al., 2022 1) | 5,711 children with asthma | Vitamin D | − | ·Increased risk of asthma in vitamin D deficient children |
| Ginde et al., 2012 | 18,883 participants (>12 yr) | Vitamin D | − | ·Serum 25(OH)D levels inversely associated with recent upper respiratory tract infections |
| Urashima et al., 2010 | 334 school children | Vitamin D | 4 mon, 1,200 IU/d | ·Reduction of the incidence of Influenza A |
| De La Fuente et al., 2008 | 33 elderly participants | Vitamin E | 3 mon, 200 mg/d | ·Increased lymphocyte proliferation, IL-2 production, neutrophil function, and NK cell activity |
| Meydani et al., 2004 | 617 elderly participants (>65 yr) | Vitamin E | 1 yr, 200 IU/d | ·Protective effect on upper respiratory tract infections, particularly the common cold |
| Cheng et al., 2006 | 51 intensive care patients | Vitamin B6 | 14 d, 50~100 mg/d | ·Increased total lymphocyte count and T-helper and T-suppressor cell levels |
| Huang et al., 2010 | 35 patients with rheumatoid arthritis | Vitamin B6 | 12 wk, 100 mg/d | ·Decreased plasma IL-6 and TNF-α levels significantly |
| Boran et al., 2021 | 611 healthy infants | Vitamin B12 | Vitamin B12 deficiency | ·Reduced percentage of Treg cells ·Increased proinflammatory cytokines levels |
| Tamura et al., 1999 | 19 patients | Vitamin B12 | Vitamin B12 deficiency | ·Decreased number of lymphocytes and CD8+ cells ·Higher CD4+/CD8+ ratio ·Suppressed NK cell activity |
| Kavitha et al., 2022 | 55 HIV positive patients | Vitamin B12 | Vitamin B12 deficiency | ·Reduced NK cell activity ·Increased CD8+ cells |
| Wood et al., 2000 | 45 elderly participants (57~84 yr) | Selenium | 6 mon, 400 μg/d | ·Increased number of T cells ·Improvement in NK cell cytotoxicity |
| Walston et al., 2006 | 619 elderly women | Selenium | Selenium deficiency | ·Increased IL-6 levels ·Increased risk of mortality |
| Baum et al., 1997 | 125 HIV positive patients | Selenium | Selenium deficiency | ·Increased risk of mortality |
| Cirelli et al., 1991 | 12 HIV positive patients | Selenium+Vitamin E | 2 mon, 80 μg/d+25 mg/d vitamin E | ·Symptomatic improvement |
| Bub et al., 2003 | 27 healthy men | Polyphenols (cyanidin glycosides and EGCG) | 2 polyphenol-rich juices, 2 wk, 330 mL/d | ·Increased lymphocyte proliferative responsiveness, IL-2 levels and NK lytic activity |
| Khojah et al., 2018 | 100 patients with rheumatoid arthritis | Resveratrol | 3 mon, 1 g/d | ·Reduced clinical markers and the disease activity score assessment ·Decreased levels of serum levels biochemical markers (i.e., IL-6 and TNF-α) |
| Kim et al., 2020 | 10 patients with inflammatory bowel disease | Polyphenols (gallotannins and gallic acid) | Mango (Mangifera indica L.) pulp, 8 wk, 200~400 g/d | ·Increased the abundance of Lactobacillus spp., Lactobacillus plantarum, Lactobacillus reuteri, and Lactobacillus lactis in the intestinal microbiome ·Improved symptoms of inflammatory bowel disease ·Decreased plasma levels of IL-8 |
| Zhang et al., 2008 | 40 male football players | Phytocompounds from Ganoderma lucidum | Extract isolated from the fruiting body of G. lucidum, 6 wk, 2.5~5.0 g/d | ·Reduction of the CD4+/CD8+ ratio |
| Dai et al., 2015 | 52 healthy participants (21~41 yr) | Phytocompounds from Lentinula edodes (Shiitake) | 4 wk, 5~10 g/d | ·Increased proliferation of γδ T cells and NK cells ·Increased levels of sIgA, IL-4, IL-10, TNF-α, and IL-1α ·Reduced ratio MIP-1α/CCL3 |
| Ahn et al., 2004 | 100 gynecological cancer patients (26~79 yr) | Phytocompounds from Agaricus blazei Murill Kyowa | 6 wk, three packs/d Every 3 wk | ·Increased NK cell activity |
| Bobovčák et al., 2010 | 20 elite athletes | β-Glucan (from Pleurotus ostreatus) | 2 mon, 50 mg/d | ·No reduction of NK cell activity after intensive exercise |
| Bergendiova et al., 2011 | 50 athletes | β-Glucan (from P. ostreatus) | 3 mon, 200 mg/d | ·Reduced incidence of upper respiratory tract infection symptoms ·Increased number of circulating NK cells |
| Jesenak et al., 2013 | 175 children (3~7 yr) | β-Glucan (from P. ostreatus) | 6 mon, 10 mg/kg/d | ·Decreased frequency of respiratory infections, influenza and flu-like illnesses |
| David and Cunningham, 2019 1) | Healthy participants | Phytocompounds from Echinacea purpurea | − | ·Reduced risk of recurrent respiratory infections and related complications |
| Shah et al., 2007 1) | Healthy or with cold participants | Phytocompounds from E. purpurea | − | ·Decreased incidence and duration of the common cold |
| Charan et al., 2016 1) | 377 patients with Dengue fever | Phytocompounds from papaya (Carica papaya) | − | ·Decrease in hospitalization days and increased platelet count |
| Ried, 2016 1) | 970 participants | Phytocompounds from garlic (Allium sativum L.) | − | ·Increased macrophage activity, NK cells, and production of T-and B-cells |
| Ishikawa et al., 2006 | Advanced colon, liver, or pancreatic cancer patients (>65 yr) | Phytocompounds from garlic (A. sativum L.) | Aged garlic extract, 24 wk, 500 mg/d | ·Increased NK cells number and activity |
| Xu et al., 2018 | 51 adults with obesity (25~65 yr) | Phytocompounds from garlic (A. sativum L.) | Aged garlic extract, 6 wk, 3, 6 g/d | ·Prevention of the increase of serum TNF-α and IL-6 concentrations ·Reduced blood LDL concentration |
| Scaglione et al., 1990 | 20 healthy adults | Phytocompounds from Panax ginseng | 8 wk, 200 mg/d | ·Increased phagocytic activity and chemotaxis of PBMCs |
| Scaglione et al., 1996 | 227 participants | Phytocompounds from P. ginseng | Standardized ginseng extract, 12 wk, 100 mg/d | ·Reduced prevalence of colds and the flu |
| Hou et al., 1981 | 14 healthy participants | Phytocompounds from Astragalus membranaceus | 2 mon, 8 g/d | ·Increased interferon-producing capacity of blood cells |
| Kormosh et al., 2006 | 28 ovarian cancer patients in chemotherapy | Phytocompounds from Schisandra chinensis, Leuzea carthamoides, Rhodiola rosea, Eleutherococcus senticosus | Dried ethanol/aqueous extracts, 4 wk, 270 mg/d | ·Boosted suppressed immunity ·Increased numbers of the T cell subclasses and mean amounts of IgG and IgM |
| Lebedev et al, 1970 | 346 school children | Phytocompounds from S. chinensis | S. chinensis seed tincture | ·Reduced rate, duration and clinical manifestations of influenza |
| Xu et al., 2013 | 15 male participants (HDBR experiment) | Phytocompounds from R. rosea | 45 d, 0.5~1.0 g/d | ·Decreased IFN-γ level ·Slowed upregulation of IL-1 family cytokines |
| Mikolai et al., 2009 | 5 participants | Phytocompounds from Withania somnifera | Root extract, 96 h, 12 mL/d | ·Activation of NK cells ·Increased CD4+ T cells |
| Gill et al., 2001 | 27 healthy elderly participants (63~84 yr) | Probiotic (Bifidobacterium lactis HN019) | 3 wk, 5×1010 CFU/d or 5×109 CFU/d | ·Increased helper and activated T cells and NK cells ·Elevated phagocytic capacity of mononuclear and PMN phagocytes and the tumoricidal activity of NK cells |
| Namba et al., 2010 | 27 elderly participants | Probiotic (Bifidobacterium longum BB536) | 5 wk, 1×1011 CFU/d | ·Lower number of participants who contracted influenza ·Increased NK cell activity and neutrophils bactericidal activity |
1)Meta-analysis.
25(OH)D, 25(OH) vitamin D; IL, interleukin; NK, natural killer; TNF-α, tumor necrosis factor-α; HIV, human immunodeficiency virus; CD, cluster of differentiation; ECGC, epigallocatechin gallate; sIgA, secretory immunoglobulin A; MIP-1α/CCL3, macrophage inflammatory protein-1α/chemokine C-C ligand 3; LDL, low-density lipoprotein; PBMC, peripheral blood mononuclear cell; IgG, immunoglobulin G; IgM, immunoglobulin M; HDBR, head-down bed rest; IFN-γ, interferon-γ; CFU, colony-forming unit; PMN, polymorphonuclear leukocyte.
Footnotes
FUNDING
None.
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Concept and design: all authors. Analysis and interpretation: all authors. Data collection: all authors. Writing the article: all authors. Critical revision of the article: all authors. Final approval of the article: all authors. Statistical analysis: all authors. Overall responsibility: all authors.
REFERENCES
- Aarland RC, Bañuelos-Hernández AE, Fragoso-Serrano M, Sierra-Palacios ED, Díaz de León-Sánchez F, Pérez-Flores LJ, et al. Studies on phytochemical, antioxidant, anti-inflammatory, hypoglycaemic and antiproliferative activities of Echinacea purpurea and Echinacea angustifolia extracts. Pharm Biol. 2017;55:649–656. doi: 10.1080/13880209.2016.1265989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad SF, Zoheir KM, Abdel-Hamied HE, Ashour AE, Bakheet SA, Attia SM, et al. Grape seed proanthocyanidin extract has potent anti-arthritic effects on collagen-induced arthritis by modifying the T cell balance. Int Immunopharmacol. 2013;17:79–87. doi: 10.1016/j.intimp.2013.05.026. [DOI] [PubMed] [Google Scholar]
- Ahn WS, Kim DJ, Chae GT, Lee JM, Bae SM, Sin JI, et al. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemotherapy. Int J Gynecol Cancer. 2004;14:589–594. doi: 10.1111/j.1048-891X.2004.14403.x. [DOI] [PubMed] [Google Scholar]
- Aibana O, Franke MF, Huang CC, Galea JT, Calderon R, Zhang Z, et al. Impact of vitamin A and carotenoids on the risk of tuberculosis progression. Clin Infect Dis. 2017;65:900–909. doi: 10.1093/cid/cix476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apaya MK, Kuo TF, Yang MT, Yang G, Hsiao CL, Chang SB, et al. Phytochemicals as modulators of β-cells and immunity for the therapy of type 1 diabetes: Recent discoveries in pharmacological mechanisms and clinical potential. Pharmacol Res. 2020;156:104754. doi: 10.1016/j.phrs.2020.104754. https://doi.org/10.1016/j.phrs.2020.104754. [DOI] [PubMed] [Google Scholar]
- Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881–886. doi: 10.2310/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanassiou L, Kostoglou-Athanassiou I, Tsakiridis P, Devetzi E, Mavroudi M, Fytas P, et al. Vitamin D levels in Greek patients with systemic lupus erythematosus. Lupus. 2022;31:125–132. doi: 10.1177/09612033211066462. [DOI] [PubMed] [Google Scholar]
- Bae UJ, Jang HY, Lim JM, Hua L, Ryu JH, Park BH. Polyphenols isolated from Broussonetia kazinoki prevent cytokine-induced β-cell damage and the development of type 1 diabetes. Exp Mol Med. 2015;47:e160. doi: 10.1038/emm.2015.16. https://doi.org/10.1038/emm.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MK, Shor-Posner G, Lai S, Zhang G, Lai H, Fletcher MA, et al. High risk of HIV-related mortality is associated with selenium deficiency. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:370–374. doi: 10.1097/00042560-199708150-00007. [DOI] [PubMed] [Google Scholar]
- Beharka AA, Han SN, Adolfsson O, Wu D, Smith D, Lipman R, et al. Long-term dietary antioxidant supplementation reduces production of selected inflammatory mediators by murine macrophages. Nutrition Res. 2000;20:281–296. doi: 10.1016/S0271-5317(99)00160-8. [DOI] [Google Scholar]
- Bendich A, Gabriel E, Machlin LJ. Dietary vitamin E requirement for optimum immune responses in the rat. J Nutr. 1986;116:675–681. doi: 10.1093/jn/116.4.675. [DOI] [PubMed] [Google Scholar]
- Bergendiova K, Tibenska E, Majtan J. Pleuran (β-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur J Appl Physiol. 2011;111:2033–2040. doi: 10.1007/s00421-011-1837-z. [DOI] [PubMed] [Google Scholar]
- Bhaumik S, Jyothi MD, Khar A. Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett. 2000;483:78–82. doi: 10.1016/S0014-5793(00)02089-5. [DOI] [PubMed] [Google Scholar]
- Biesalski HK. Nutrition meets the microbiome: micronutrients and the microbiota. Ann NY Acad Sci. 2016;1372:53–64. doi: 10.1111/nyas.13145. [DOI] [PubMed] [Google Scholar]
- Blomhoff HK, Smeland EB, Erikstein B, Rasmussen AM, Skrede B, Skjønsberg C, et al. Vitamin A is a key regulator for cell growth, cytokine production, and differentiation in normal B cells. J Biol Chem. 1992;267:23988–23992. doi: 10.1016/S0021-9258(18)35934-9. [DOI] [PubMed] [Google Scholar]
- Bobovčák M, Kuniaková R, Gabriž J, Majtán J. Effect of pleuran (β-glucan from Pleurotus ostreatus) supplementation on cellular immune response after intensive exercise in elite athletes. Appl Physiol Nutr Metab. 2010;35:755–762. doi: 10.1139/H10-070. [DOI] [PubMed] [Google Scholar]
- Boran P, Yildirim S, Karakoc-Aydiner E, Ogulur I, Ozen A, Haklar G, et al. Vitamin B12 deficiency among asymptomatic healthy infants: its impact on the immune system. Minerva Pediatr. 2021;73:59–66. doi: 10.23736/S2724-5276.16.04274-X. [DOI] [PubMed] [Google Scholar]
- Borgoni S, Kudryashova KS, Burka K, de Magalhães JP. Targeting immune dysfunction in aging. Ageing Res Rev. 2021;70:101410. doi: 10.1016/j.arr.2021.101410. https://doi.org/10.1016/j.arr.2021.101410. [DOI] [PubMed] [Google Scholar]
- Bozonet SM, Carr AC, Pullar JM, Vissers MC. Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold kiwifruit. Nutrients. 2015;7:2574–2588. doi: 10.3390/nu7042574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CC, Noelle RJ. Seeing through the dark: New insights into the immune regulatory functions of vitamin A. Eur J Immunol. 2015;45:1287–1295. doi: 10.1002/eji.201344398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bub A, Watzl B, Blockhaus M, Briviba K, Liegibel U, Müller H, et al. Fruit juice consumption modulates antioxidative status, immune status and DNA damage. J Nutr Biochem. 2003;14:90–98. doi: 10.1016/S0955-2863(02)00255-3. [DOI] [PubMed] [Google Scholar]
- Burlou-Nagy C, Bănică F, Jurca T, Vicaș LG, Marian E, Muresan ME, et al. Echinacea purpurea (L.) Moench: biological and pharmacological properties. A review. Plants. 2022;11:1244. doi: 10.3390/plants11091244. https://doi.org/10.3390/plants11091244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campa A, Baum MK, Bussmann H, Martinez SS, Farahani M, van Widenfelt E, et al. The effect of micronutrient supplementation on active TB incidence early in HIV infection in Botswana. Nutr Diet Suppl. 2017;2017:37–45. doi: 10.2147/NDS.S123545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, et al. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- Campbell NK, Fitzgerald HK, Fletcher JM, Dunne A. Plant-derived polyphenols modulate human dendritic cell metabolism and immune function via AMPK-dependent induction of heme oxygenase-1. Front Immunol. 2019;10:345. doi: 10.3389/fimmu.2019.00345. https://doi.org/10.3389/fimmu.2019.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, Nashold FE, Hayes CE. Vitamin A deficiency results in a priming environment conducive for Th1 cell development. Eur J Immunol. 1995;25:1673–1679. doi: 10.1002/eji.1830250629. [DOI] [PubMed] [Google Scholar]
- Carvalho JTG, Schneider M, Cuppari L, Grabulosa CC, Aoike DT, Redublo BMQ, et al. Cholecalciferol decreases inflammation and improves vitamin D regulatory enzymes in lymphocytes in the uremic environment: A randomized controlled pilot trial. PLoS One. 2017;12:e0179540. doi: 10.1371/journal.pone.0179540. https://doi.org/10.1371/journal.pone.0179540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavani A, Pennino D, Eyerich K. Th17 and Th22 in skin allergy. Chem Immunol Allergy. 2012;96:39–44. doi: 10.1159/000331870. [DOI] [PubMed] [Google Scholar]
- Charan J, Saxena D, Goyal JP, Yasobant S. Efficacy and safety of Carica papaya leaf extract in the dengue: A systematic review and meta-analysis. Int J Appl Basic Med Res. 2016;6:249–254. doi: 10.4103/2229-516X.192596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CH, Chang SJ, Lee BJ, Lin KL, Huang YC. Vitamin B6 supplementation increases immune responses in critically ill patients. Eur J Clin Nutr. 2006;60:1207–1213. doi: 10.1038/sj.ejcn.1602439. [DOI] [PubMed] [Google Scholar]
- Cipollina C. Endogenous generation and signaling actions of omega-3 fatty acid electrophilic derivatives. Biomed Res Int. 2015;2015:501792. doi: 10.1155/2015/501792. https://doi.org/10.1155/2015/501792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli A, Ciardi M, de Simone C, Sorice F, Giordano R, Ciaralli L, et al. Serum selenium concentration and disease progress in patients with HIV infection. Clin Biochem. 1991;24:211–214. doi: 10.1016/0009-9120(91)90601-A. [DOI] [PubMed] [Google Scholar]
- Clària J, Dalli J, Yacoubian S, Gao F, Serhan CN. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J Immunol. 2012;189:2597–2605. doi: 10.4049/jimmunol.1201272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol. 2021;12:578386. doi: 10.3389/fimmu.2021.578386. https://doi.org/10.3389/fimmu.2021.578386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Stanilka JM, Rowe CA, Esteves EA, Nieves C, Jr, Spaiser SJ, et al. Consuming Lentinula edodes (Shiitake) mushrooms daily improves human immunity: a randomized dietary intervention in healthy young adults. J Am Coll Nutr. 2015;34:478–487. doi: 10.1080/07315724.2014.950391. [DOI] [PubMed] [Google Scholar]
- David S, Cunningham R. Echinacea for the prevention and treatment of upper respiratory tract infections: A systematic review and meta-analysis. Complement Ther Med. 2019;44:18–26. doi: 10.1016/j.ctim.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Davinelli S, Medoro A, Intrieri M, Saso L, Scapagnini G, Kang JX. Targeting NRF2-KEAP1 axis by omega-3 fatty acids and their derivatives: Emerging opportunities against aging and diseases. Free Radic Biol Med. 2022;193:736–750. doi: 10.1016/j.freeradbiomed.2022.11.017. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, Hernanz A, Guayerbas N, Victor VM, Arnalich F. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic Res. 2008;42:272–280. doi: 10.1080/10715760801898838. [DOI] [PubMed] [Google Scholar]
- Dey S, Bishayi B. Riboflavin along with antibiotics balances reactive oxygen species and inflammatory cytokines and controls Staphylococcus aureus infection by boosting murine macrophage function and regulates inflammation. J Inflamm. 2016;13:36. doi: 10.1186/s12950-016-0145-0. https://doi.org/10.1186/s12950-016-0145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exon JH, Magnuson BA, South EH, Hendrix K. Dietary quercetin, immune functions and colonic carcinogenesis in rats. Immunopharmacol Immunotoxicol. 1998;20:173–190. doi: 10.3109/08923979809034816. [DOI] [PubMed] [Google Scholar]
- Festa RA, Thiele DJ. Copper: an essential metal in biology. Curr Biol. 2011;21:R877–R883. doi: 10.1016/j.cub.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- Galland L. Magnesium and immune function: an overview. Magnesium. 1988;7:290–299. [PubMed] [Google Scholar]
- Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. 2001;74:833–839. doi: 10.1093/ajcn/74.6.833. [DOI] [PubMed] [Google Scholar]
- Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves GA, Soares AA, Correa RCG, Barros L, Haminiuk CWI, Peralta RM, et al. Merlot grape pomace hydroalcoholic extract improves the oxidative and inflammatory states of rats with adjuvant-induced arthritis. J Funct Foods. 2017;33:408–418. doi: 10.1016/j.jff.2017.04.009. [DOI] [Google Scholar]
- Granados-Principal S, Quiles JL, Ramirez-Tortosa CL, Sanchez-Rovira P, Ramirez-Tortosa MC. Hydroxytyrosol: from laboratory investigations to future clinical trials. Nutr Rev. 2010;68:191–206. doi: 10.1111/j.1753-4887.2010.00278.x. [DOI] [PubMed] [Google Scholar]
- Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr. 2009;29:133–152. doi: 10.1146/annurev-nutr-080508-141119. [DOI] [PubMed] [Google Scholar]
- Han SN, Wu D, Ha WK, Beharka A, Smith DE, Bender BS, et al. Vitamin E supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunology. 2000;100:487–493. doi: 10.1046/j.1365-2567.2000.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Shen T, Lou H. Dietary polyphenols and their biological significance. Int J Mol Sci. 2007;8:950–988. doi: 10.3390/i8090950. [DOI] [Google Scholar]
- Hayek MG, Taylor SF, Bender BS, Han SN, Meydani M, Smith DE, et al. Vitamin E supplementation decreases lung virus titers in mice infected with influenza. J Infect Dis. 1997;176:273–276. doi: 10.1086/517265. [DOI] [PubMed] [Google Scholar]
- Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;2013:CD 000980. doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou YD, Ma GL, Wu SH, Li YY, Li HT. Effect of Radix Astragali seu Hedysari on the interferon system. Chin Med J. 1981;94:35–40. [PubMed] [Google Scholar]
- Huang SC, Wei JC, Wu DJ, Huang YC. Vitamin B6 supplementation improves pro-inflammatory responses in patients with rheumatoid arthritis. Eur J Clin Nutr. 2010;64:1007–1013. doi: 10.1038/ejcn.2010.107. [DOI] [PubMed] [Google Scholar]
- Huang Z, Liu Y, Qi G, Brand D, Zheng SG. Role of vitamin A in the immune system. J Clin Med. 2018;7:258. doi: 10.3390/jcm7090258. https://doi.org/10.3390/jcm7090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijskens MJ, Walczak M, Koller N, Briedé JJ, Senden-Gijsbers BL, Schnijderberg MC, et al. Technical advance: ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. J Leukoc Biol. 2014;96:1165–1175. doi: 10.1189/jlb.1TA0214-121RR. [DOI] [PubMed] [Google Scholar]
- Huijskens MJ, Walczak M, Sarkar S, Atrafi F, Senden-Gijsbers BL, Tilanus MG, et al. Ascorbic acid promotes proliferation of natural killer cell populations in culture systems applicable for natural killer cell therapy. Cytotherapy. 2015;17:613–620. doi: 10.1016/j.jcyt.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Im DS. Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Prog Lipid Res. 2012;51:232–237. doi: 10.1016/j.plipres.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Imdad A, Yakoob MY, Sudfeld C, Haider BA, Black RE, Bhutta ZA. Impact of vitamin A supplementation on infant and childhood mortality. BMC Public Health. 2011;11:S20. doi: 10.1186/1471-2458-11-S3-S20. https://doi.org/10.1186/1471-2458-11-s3-s20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Saeki T, Otani T, Suzuki T, Shimozuma K, Nishino H, et al. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J Nutr. 2006;136:816S–820S. doi: 10.1093/jn/136.3.816S. [DOI] [PubMed] [Google Scholar]
- Jain SK, Parsanathan R. Can vitamin D and L-cysteine co-supplementation reduce 25(OH)-vitamin D deficiency and the mortality associated with COVID-19 in African Americans? J Am Coll Nutr. 2020;39:694–699. doi: 10.1080/07315724.2020.1789518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YD, Aye A, Song YJ, Kim YH, Soh JR, Jin JS. Cyanidin 3-rutinoside, an anthocyanin pigment of Schisandra chinensis Baill, inhibits allergic inflammation. J Med Food. 2019;22:703–712. doi: 10.1089/jmf.2018.4346. [DOI] [PubMed] [Google Scholar]
- Jeong GS, Lee DS, Song MY, Park BH, Kang DG, Lee HS, et al. Butein from Rhus verniciflua protects pancreatic β cells against cytokine-induced toxicity mediated by inhibition of nitric oxide formation. Biol Pharm Bull. 2011;34:97–102. doi: 10.1248/bpb.34.97. [DOI] [PubMed] [Google Scholar]
- Jesenak M, Majtan J, Rennerova Z, Kyselovic J, Banovcin P, Hrubisko M. Immunomodulatory effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Int Immunopharmacol. 2013;15:395–399. doi: 10.1016/j.intimp.2012.11.020. [DOI] [PubMed] [Google Scholar]
- Kabu K, Yamasaki S, Kamimura D, Ito Y, Hasegawa A, Sato E, et al. Zinc is required for Fc epsilon RI-mediated mast cell activation. J Immunol. 2006;177:1296–1305. doi: 10.4049/jimmunol.177.2.1296. [DOI] [PubMed] [Google Scholar]
- Kavitha K, Saharia GK, Singh AK, Mangaraj M. Association of serum vitamin B12 with immuno-hematological parameters in treatment-naive HIV positive cases. J Family Med Prim Care. 2022;11:3784–3789. doi: 10.4103/jfmpc.jfmpc_2490_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf AA, Hussein S, Tohamy AF, Marouf S, Yassa HD, Zaki AR, et al. Protective effect of Echinacea purpurea (Immulant) against cisplatin-induced immunotoxicity in rats. Daru. 2019;27:233–241. doi: 10.1007/s40199-019-00265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khojah HM, Ahmed S, Abdel-Rahman MS, Elhakeim EH. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: a clinical study. Clin Rheumatol. 2018;37:2035–2042. doi: 10.1007/s10067-018-4080-8. [DOI] [PubMed] [Google Scholar]
- Kim H, Venancio VP, Fang C, Dupont AW, Talcott ST, Mertens-Talcott SU. Mango (Mangifera indica L.) polyphenols reduce IL-8, GRO, and GM-SCF plasma levels and increase Lactobacillus species in a pilot study in patients with inflammatory bowel disease. Nutr Res. 2020;75:85–94. doi: 10.1016/j.nutres.2020.01.002. [DOI] [PubMed] [Google Scholar]
- Kim YH, Won YS, Yang X, Kumazoe M, Yamashita S, Hara A, et al. Green tea catechin metabolites exert immunoregulatory effects on CD4+ T cell and natural killer cell activities. J Agric Food Chem. 2016;64:3591–3597. doi: 10.1021/acs.jafc.6b01115. [DOI] [PubMed] [Google Scholar]
- Kitabayashi C, Fukada T, Kanamoto M, Ohashi W, Hojyo S, Atsumi T, et al. Zinc suppresses Th17 development via inhibition of STAT3 activation. Int Immunol. 2010;22:375–386. doi: 10.1093/intimm/dxq017. [DOI] [PubMed] [Google Scholar]
- Kong YR, Jong YX, Balakrishnan M, Bok ZK, Weng JKK, Tay KC, et al. Beneficial role of Carica papaya extracts and phytochemicals on oxidative stress and related diseases: a mini review. Biology. 2021;10:287. doi: 10.3390/biology10040287. https://doi.org/10.3390/biology10040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormosh N, Laktionov K, Antoshechkina M. Effect of a combination of extract from several plants on cell-mediated and humoral immunity of patients with advanced ovarian cancer. Phytother Res. 2006;20:424–425. doi: 10.1002/ptr.1889. [DOI] [PubMed] [Google Scholar]
- Kortesoja M, Karhu E, Olafsdottir ES, Freysdottir J, Hanski L. Impact of dibenzocyclooctadiene lignans from Schisandra chinensis on the redox status and activation of human innate immune system cells. Free Radic Biol Med. 2019;131:309–317. doi: 10.1016/j.freeradbiomed.2018.12.019. [DOI] [PubMed] [Google Scholar]
- Kouakanou L, Xu Y, Peters C, He J, Wu Y, Yin Z, et al. Vitamin C promotes the proliferation and effector functions of human γδ T cells. Cell Mol Immunol. 2020;17:462–473. doi: 10.1038/s41423-019-0247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, et al. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- Lebedev AA. The effect of Schisandra seed tincture on morbidity rate among workers of Chirick shoe factory during the 1969 influenza epidemic. In: Brekhman II, Fruentov NK, editors. Medicinal Products of the Far East. Far East Branch of the USSR Academy of Science. Khabarovsk Medical Institute; 1970. pp. 115–119. [Google Scholar]
- Lee GY, Han SN. The role of vitamin E in immunity. Nutrients. 2018;10:1614. doi: 10.3390/nu10111614. https://doi.org/10.3390/nu10111614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Kim S, Kwon OK, Oh SR, Lee HK, Ahn K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int Immunopharmacol. 2009;9:418–424. doi: 10.1016/j.intimp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Lewicki S, Skopińska-Różewska E, Brewczyńska A, Zdanowski R. Administration of Rhodiola kirilowii extracts during mouse pregnancy and lactation stimulates innate but not adaptive immunity of the offspring. J Immunol Res. 2017;2017:8081642. doi: 10.1155/2017/8081642. https://doi.org/10.1155/2017/8081642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ED, Meydani SN, Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. 2019;71:487–494. doi: 10.1002/iub.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CX, Liu Y, Zhang YZ, Li JC, Lai J. Astragalus polysaccharide: a review of its immunomodulatory effect. Arch Pharm Res. 2022;45:367–389. doi: 10.1007/s12272-022-01393-3. [DOI] [PubMed] [Google Scholar]
- Li-Weber M, Weigand MA, Giaisi M, Süss D, Treiber MK, Baumann S, et al. Vitamin E inhibits CD95 ligand expression and protects T cells from activation-induced cell death. J Clin Invest. 2002;110:681–690. doi: 10.1172/JCI0215073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JY, Lee JH, Lee BR, Kim MA, Lee YM, Kim DK, et al. Extract of Boehmeria nivea suppresses mast cell-mediated allergic inflammation by inhibiting mitogen-activated protein kinase and nuclear factor-κB. Molecules. 2020;25:4178. doi: 10.3390/molecules25184178. https://doi.org/10.3390/molecules25184178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Shen ML, Zhou N, Lee CC, Kao ST, Wu DC. Protective effects of the polyphenol sesamin on allergen-induced TH2 responses and airway inflammation in mice. PLoS One. 2014;9:e96091. doi: 10.1371/journal.pone.0096091. https://doi.org/10.1371/journal.pone.0096091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang S, Liu H, Yang L, Nan G. Stimulatory effect of saponin from Panax ginseng on immune function of lymphocytes in the elderly. Mech Ageing Dev. 1995;83:43–53. doi: 10.1016/0047-6374(95)01618-A. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang H, Dai X, Zhu R, Chen B, Xia B, et al. A comprehensive review on the phytochemistry, pharmacokinetics, and antidiabetic effect of Ginseng. Phytomedicine. 2021;92:153717. doi: 10.1016/j.phymed.2021.153717. https://doi.org/10.1016/j.phymed.2021.153717. [DOI] [PubMed] [Google Scholar]
- Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. J Allergy Clin Immunol. 2009;123:1004–1011. doi: 10.1016/j.jaci.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Lu L, Lan Q, Li Z, Zhou X, Gu J, Li Q, et al. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci USA. 2014;11:E3432–E3440. doi: 10.1073/pnas.1408780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Zhou X, Wang J, Zheng SG, Horwitz DA. Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-β and retinoic acid. PLoS One. 2010;5:e15150. doi: 10.1371/journal.pone.0015150. https://doi.org/10.1371/journal.pone.0015150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggini S, Wintergerst ES, Beveridge S, Hornig DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007;98:S29–S35. doi: 10.1017/S0007114507832971. [DOI] [PubMed] [Google Scholar]
- Magrone T, Jirillo E. Disorders of innate immunity in human ageing and effects of nutraceutical administration. Endocr Metab Immune Disord Drug Targets. 2014;14:272–282. doi: 10.2174/1871530314666141010105540. [DOI] [PubMed] [Google Scholar]
- Makropoulos V, Brüning T, Schulze-Osthoff K. Selenium-mediated inhibition of transcription factor NF-κB and HIV-1 LTR promoter activity. Arch Toxicol. 1996;70:277–283. doi: 10.1007/s002040050274. [DOI] [PubMed] [Google Scholar]
- Malpuech-Brugère C, Nowacki W, Daveau M, Gueux E, Linard C, Rock E, et al. Inflammatory response following acute magnesium deficiency in the rat. Biochim Biophys Acta. 2000;1501:91–98. doi: 10.1016/S0925-4439(00)00018-1. [DOI] [PubMed] [Google Scholar]
- Manayi A, Vazirian M, Saeidnia S. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacogn Rev. 2015;9:63–72. doi: 10.4103/0973-7847.156353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W. Cellular zinc and redox states converge in the metallothionein/thionein pair. J Nutr. 2003;133:1460S–1462S. doi: 10.1093/jn/133.5.1460S. [DOI] [PubMed] [Google Scholar]
- Marshall JS, Warrington R, Watson W, Kim HL. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. 2018;14:49. doi: 10.1186/s13223-018-0278-1. https://doi.org/10.1186/s13223-018-0278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Benno Y. The relationship between microbiota and polyamine concentration in the human intestine: a pilot study. Microbiol Immunol. 2007;51:25–35. doi: 10.1111/j.1348-0421.2007.tb03887.x. [DOI] [PubMed] [Google Scholar]
- Mawson AR. Role of fat-soluble vitamins A and D in the pathogenesis of influenza: a new perspective. ISRN Infect Dis. 2013;2013:246737. doi: 10.5402/2013/246737. https://doi.org/10.5402/2013/246737. [DOI] [Google Scholar]
- Maywald M, Wang F, Rink L. Zinc supplementation plays a crucial role in T helper 9 differentiation in allogeneic immune reactions and non-activated T cells. J Trace Elem Med Biol. 2018;50:482–488. doi: 10.1016/j.jtemb.2018.02.004. [DOI] [PubMed] [Google Scholar]
- McDaniel KL, Restori KH, Dodds JW, Kennett MJ, Ross AC, Cantorna MT. Vitamin A-deficient hosts become nonsymptomatic reservoirs of Escherichia coli-like enteric infections. Infect Immun. 2015;83:2984–2991. doi: 10.1128/IAI.00201-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi Y, Hornick JL, Istasse L, Dufrasne I. Selenium in the environment, metabolism and involvement in body functions. Molecules. 2013;18:3292–3311. doi: 10.3390/molecules18033292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melguizo-Rodríguez L, García-Recio E, Ruiz C, De Luna-Bertos E, Illescas-Montes R, Costela-Ruiz VJ. Biological properties and therapeutic applications of garlic and its components. Food Funct. 2022;13:2415–2426. doi: 10.1039/D1FO03180E. [DOI] [PubMed] [Google Scholar]
- Metz CH, Schröder AK, Overbeck S, Kahmann L, Plümäkers B, Rink L. T-helper type 1 cytokine release is enhanced by in vitro zinc supplementation due to increased natural killer cells. Nutrition. 2007;23:157–163. doi: 10.1016/j.nut.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Meydani SN, Meydani M, Verdon CP, Shapiro AA, Blumberg JB, Hayes KC. Vitamin E supplementation suppresses prostaglandin E21 synthesis and enhances the immune response of aged mice. Mech Ageing Dev. 1986;34:191–201. doi: 10.1016/0047-6374(86)90034-5. [DOI] [PubMed] [Google Scholar]
- Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MF, et al. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA. 2004;292:828–836. doi: 10.1001/jama.292.7.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolai J, Erlandsen A, Murison A, Brown KA, Gregory WL, Raman-Caplan P, et al. In vivo effects of Ashwagandha (Withania somnifera) extract on the activation of lymphocytes. J Altern Complement Med. 2009;15:423–430. doi: 10.1089/acm.2008.0215. [DOI] [PubMed] [Google Scholar]
- Molina N, Morandi AC, Bolin AP, Otton R. Comparative effect of fucoxanthin and vitamin C on oxidative and functional parameters of human lymphocytes. Int Immunopharmacol. 2014;22:41–50. doi: 10.1016/j.intimp.2014.06.026. [DOI] [PubMed] [Google Scholar]
- Moreira A, Kekkonen RA, Delgado L, Fonseca J, Korpela R, Haahtela T. Nutritional modulation of exercise-induced immunodepression in athletes: a systematic review and meta-analysis. Eur J Clin Nutr. 2007;61:443–460. doi: 10.1038/sj.ejcn.1602549. [DOI] [PubMed] [Google Scholar]
- Moriguchi S, Kobayashi N, Kishino Y. High dietary intakes of vitamin E and cellular immune functions in rats. J Nutr. 1990;120:1096–1102. doi: 10.1093/jn/120.9.1096. [DOI] [PubMed] [Google Scholar]
- Morinobu A, Biao W, Tanaka S, Horiuchi M, Jun L, Tsuji G, et al. (-)-Epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum. 2008;58:2012–2018. doi: 10.1002/art.23594. [DOI] [PubMed] [Google Scholar]
- Moroni F, Di Paolo ML, Rigo A, Cipriano C, Giacconi R, Recchioni R, et al. Interrelationship among neutrophil efficiency, inflammation, antioxidant activity and zinc pool in very old age. Biogerontology. 2005;6:271–281. doi: 10.1007/s10522-005-2625-0. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Baidoo JNE, Fried A, Banerjee P. Using curcumin to turn the innate immune system against cancer. Biochem Pharmacol. 2020;176:113824. doi: 10.1016/j.bcp.2020.113824. https://doi.org/10.1016/j.bcp.2020.113824. [DOI] [PubMed] [Google Scholar]
- Muzzioli M, Stecconi R, Donnini A, Re F, Provinciali M. Zinc improves the development of human CD34+ cell progenitors towards Natural Killer cells and induces the expression of GATA-3 transcription factor. Int J Biochem Cell Biol. 2007;39:955–965. doi: 10.1016/j.biocel.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Namba K, Hatano M, Yaeshima T, Takase M, Suzuki K. Effects of Bifidobacterium longum BB536 administration on influenza infection, influenza vaccine antibody titer, and cell-mediated immunity in the elderly. Biosci Biotechnol Biochem. 2010;74:939–945. doi: 10.1271/bbb.90749. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Henson DA, McAnulty SR, McAnulty L, Swick NS, Utter AC, et al. Influence of vitamin C supplementation on oxidative and immune changes after an ultramarathon. J Appl Physiol. 2002;92:1970–1977. doi: 10.1152/japplphysiol.00961.2001. [DOI] [PubMed] [Google Scholar]
- Nowak A, Zakłos-Szyda M, Błasiak J, Nowak A, Zhang Z, Zhang B. Potential of Schisandra chinensis (Turcz.) Baill. in human health and nutrition: a review of current knowledge and therapeutic perspectives. Nutrients. 2019;11:333. doi: 10.3390/nu11020333. https://doi.org/10.3390/nu11020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi SL, Pak SC. Nutraceuticals in immune function. Molecules. 2021;26:5310. doi: 10.3390/molecules26175310. https://doi.org/10.3390/molecules26175310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallauf K, Giller K, Huebbe P, Rimbach G. Nutrition and healthy ageing: calorie restriction or polyphenol-rich "MediterrAsian" diet? Oxid Med Cell Longev. 2013;2013:707421. doi: 10.1155/2013/707421. https://doi.org/10.1155/2013/707421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paludan SR, Pradeu T, Masters SL, Mogensen TH. Constitutive immune mechanisms: mediators of host defence and immune regulation. Nat Rev Immunol. 2021;21:137–150. doi: 10.1038/s41577-020-0391-5. https://doi.org/10.1038/s41577-020-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Loo G. Effect of copper deficiency on oxidative DNA damage in Jurkat T-lymphocytes. Free Radic Biol Med. 2000;28:824–830. doi: 10.1016/S0891-5849(00)00165-9. [DOI] [PubMed] [Google Scholar]
- Pandey S, Shaw PN, Hewavitharana AK. Review of procedures used for the extraction of anti-cancer compounds from tropical plants. Anticancer Agents Med Chem. 2015;15:314–326. doi: 10.2174/1871520614666141114202104. [DOI] [PubMed] [Google Scholar]
- Parahuleva MS, Maj R, Hölschermann H, Parviz B, Abdallah Y, Erdogan A, et al. Regulation of monocyte/macrophage function by factor VII activating protease (FSAP) Atherosclerosis. 2013;230:365–372. doi: 10.1016/j.atherosclerosis.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Park MK, Park JS, Cho ML, Oh HJ, Heo YJ, Woo YJ, et al. Grape seed proanthocyanidin extract (GSPE) differentially regulates Foxp3+ regulatory and IL-17+ pathogenic T cell in autoimmune arthritis. Immunol Lett. 2011;135:50–58. doi: 10.1016/j.imlet.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Park SJ, Lee M, Kim D, Oh DH, Prasad KS, Eun S, et al. Echinacea purpurea extract enhances natural killer cell activity in vivo by upregulating MHC II and Th1-type CD4+ T cell responses. J Med Food. 2021;24:1039–1049. doi: 10.1089/jmf.2021.K.0064. [DOI] [PubMed] [Google Scholar]
- Pasatiempo AM, Kinoshita M, Taylor CE, Ross AC. Antibody production in vitamin A-depleted rats is impaired after immunization with bacterial polysaccharide or protein antigens. FASEB J. 1990;4:2518–2527. doi: 10.1096/fasebj.4.8.2110538. [DOI] [PubMed] [Google Scholar]
- Patra S, Pradhan B, Nayak R, Behera C, Das S, Patra SK, et al. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine. 2021;90:153554. doi: 10.1016/j.phymed.2021.153554. https://doi.org/10.1016/j.phymed.2021.153554. [DOI] [PubMed] [Google Scholar]
- Paul S, Chakraborty S, Anand U, Dey S, Nandy S, Ghorai M, et al. Withania somnifera (L.) Dunal (ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed Pharmacother. 2021;143:112175. doi: 10.1016/j.biopha.2021.112175. https://doi.org/10.1016/j.biopha.2021.112175. [DOI] [PubMed] [Google Scholar]
- Percival SS. Aged garlic extract modifies human immunity. J Nutr. 2016;146:433S–436S. doi: 10.3945/jn.115.210427. [DOI] [PubMed] [Google Scholar]
- Petersen EW, Ostrowski K, Ibfelt T, Richelle M, Offord E, Halkjaer-Kristensen J, et al. Effect of vitamin supplementation on cytokine response and on muscle damage after strenuous exercise. Am J Physiol Cell Physiol. 2001;280:C1570–C1575. doi: 10.1152/ajpcell.2001.280.6.C1570. [DOI] [PubMed] [Google Scholar]
- Poles J, Karhu E, McGill M, McDaniel HR, Lewis JE. The effects of twenty-four nutrients and phytonutrients on immune system function and inflammation: A narrative review. J Clin Transl Res. 2021;7:333–376. [PMC free article] [PubMed] [Google Scholar]
- Qi T, Sun M, Zhang C, Chen P, Xiao C, Chang X. Ascorbic acid promotes plasma cell differentiation through enhancing TET2/3-mediated DNA demethylation. Cell Rep. 2020;33:108452. doi: 10.1016/j.celrep.2020.108452. https://doi.org/10.1016/j.celrep.2020.108452. [DOI] [PubMed] [Google Scholar]
- Qi YJ, Niu QL, Zhu XL, Zhao XZ, Yang WW, Wang XJ. Relationship between deficiencies in vitamin A and E and occurrence of infectious diseases among children. Eur Rev Med Pharmacol Sci. 2016;20:5009–5012. [PubMed] [Google Scholar]
- Rahman SU, Li Y, Huang Y, Zhu L, Feng S, Wu J, et al. Treatment of inflammatory bowel disease via green tea polyphenols: possible application and protective approaches. Inflammopharmacology. 2018;26:319–330. doi: 10.1007/s10787-018-0462-4. [DOI] [PubMed] [Google Scholar]
- Ranjan D, Johnston TD, Wu G, Elliott L, Bondada S, Nagabhushan M. Curcumin blocks cyclosporine A-resistant CD28 costimulatory pathway of human T-cell proliferation. J Surg Res. 1998;77:174–178. doi: 10.1006/jsre.1998.5374. [DOI] [PubMed] [Google Scholar]
- Ranjan D, Chen C, Johnston TD, Jeon H, Nagabhushan M. Curcumin inhibits mitogen stimulated lymphocyte proliferation, NFkB activation, and IL-2 signaling. J Surg Res. 2004;121:171–177. doi: 10.1016/j.jss.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Ravikumar N, Kavitha CN. Immunomodulatory effect of quercetin on dysregulated Th1/Th2 cytokine balance in mice with both type 1 diabetes and allergic asthma. J Appl Pharm Sci. 2020;10:080–087. doi: 10.7324/JAPS.2020.103010. [DOI] [Google Scholar]
- Razdan K, Singh K, Singh D. Vitamin D levels and COVID-19 susceptibility: is there any correlation? Med Drug Discov. 2020;7:100051. doi: 10.1016/j.medidd.2020.100051. https://doi.org/10.1016/j.medidd.2020.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Pae M, Dao MC, Smith D, Meydani SN, Wu D. Dietary supplementation with tocotrienols enhances immune function in C57BL/6 mice. J Nutr. 2010;140:1335–1341. doi: 10.3945/jn.110.121434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried K. Garlic lowers blood pressure in hypertensive individuals, regulates serum cholesterol, and stimulates immunity: an updated meta-analysis and review. J Nutr. 2016;146:389S–396S. doi: 10.3945/jn.114.202192. [DOI] [PubMed] [Google Scholar]
- Rosillo MÁ, Alcaraz MJ, Sánchez-Hidalgo M, Fernández-Bolaños JG, Alarcón-de-la-Lastra C, Ferrándiz ML. Anti-inflammatory and joint protective effects of extra-virgin olive-oil polyphenol extract in experimental arthritis. J Nutr Biochem. 2014;25:1275–1281. doi: 10.1016/j.jnutbio.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Roy S, Awasthi A. Vitamin A and the immune system. In: Mahmoudi M, Rezaei N, editors. Nutrition and Immunity. Springer; 2019. pp. 53–73. [Google Scholar]
- Santini A, Cammarata SM, Capone G, Ianaro A, Tenore GC, Pani L, et al. Nutraceuticals: opening the debate for a regulatory framework. Br J Clin Pharmacol. 2018;84:659–672. doi: 10.1111/bcp.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro A, Bientinesi E, Monti D. Immunosenescence and inflammaging in the aging process: age-related diseases or longevity? Ageing Res Rev. 2021;71:101422. doi: 10.1016/j.arr.2021.101422. https://doi.org/10.1016/j.arr.2021.101422. [DOI] [PubMed] [Google Scholar]
- Sassi F, Tamone C, D'Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10:1656. doi: 10.3390/nu10111656. https://doi.org/10.3390/nu10111656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaglione F, Ferrara F, Dugnani S, Falchi M, Santoro G, Fraschini F. Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. Drugs Exp Clin Res. 1990;16:537–542. [PubMed] [Google Scholar]
- Scaglione F, Cattaneo G, Alessandria M, Cogo R. Efficacy and safety of the standardised ginseng extract G115 for potentiating vaccination against the influenza syndrome and protection against the common cold. Drugs Exp Clin Res. 1996;22:65–72. [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SA, Sander S, White CM, Rinaldi M, Coleman CI. Evaluation of echinacea for the prevention and treatment of the common cold: a meta-analysis. Lancet Infect Dis. 2007;7:473–480. doi: 10.1016/S1473-3099(07)70160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoor H, Feehan J, Apostolopoulos V, Platat C, Al Dhaheri AS, Ali HI, et al. Immunomodulatory effects of dietary polyphenols. Nutrients. 2021;13:728. doi: 10.3390/nu13030728. https://doi.org/10.3390/nu13030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Im SH. Probiotics as a potential immunomodulating pharmabiotics in allergic diseases: current status and future prospects. Allergy Asthma Immunol Res. 2018;10:575–590. doi: 10.4168/aair.2018.10.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shati AA, Zaki MSA, Alqahtani YA, Al-Qahtani SM, Haidara MA, Dawood AF, et al. Antioxidant activity of vitamin C against LPS-induced septic cardiomyopathy by down-regulation of oxidative stress and inflammation. Curr Issues Mol Biol. 2022;44:2387–2400. doi: 10.3390/cimb44050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirisinha S. The potential impact of gut microbiota on your health: Current status and future challenges. Asian Pac J Allergy Immunol. 2016;34:249–264. doi: 10.12932/AP0803. [DOI] [PubMed] [Google Scholar]
- Sivakumar B, Reddy V. Absorption of labelled vitamin A in children during infection. Br J Nutr. 1972;27:299–304. doi: 10.1079/BJN19720094. [DOI] [PubMed] [Google Scholar]
- Sokoła-Wysoczańska E, Wysoczański T, Wagner J, Czyż K, Bodkowski R, Lochyński S, et al. Polyunsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders-a review. Nutrients. 2018;10:1561. doi: 10.3390/nu10101561. https://doi.org/10.3390/nu10101561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A, Tarwotjo I, Djunaedi E, West KP, Jr, Loeden AA, Tilden R, et al. Impact of vitamin A supplementation on childhood mortality. A randomised controlled community trial. Lancet. 1986;1:1169–1173. doi: 10.1016/S0140-6736(86)91157-8. [DOI] [PubMed] [Google Scholar]
- Spinas E, Saggini A, Kritas SK, Cerulli G, Caraffa A, Antinolfi P, et al. Crosstalk between vitamin B and immunity. J Biol Regul Homeost Agents. 2015;29:283–288. [PubMed] [Google Scholar]
- Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer DK, Nizami SA, Lee FY, Soung DY. Whole grape alleviates inflammatory arthritis through inhibition of tumor necrosis factor. J Funct Foods. 2017;35:458–465. doi: 10.1016/j.jff.2017.06.018. [DOI] [Google Scholar]
- Sun LX, Li WD, Lin ZB, Duan XS, Li XF, Yang N, et al. Protection against lung cancer patient plasma-induced lymphocyte suppression by Ganoderma lucidum polysaccharides. Cell Physiol Biochem. 2014;33:289–299. doi: 10.1159/000356669. [DOI] [PubMed] [Google Scholar]
- Szabó Z, Marosvölgyi T, Szabó É, Bai P, Figler M, Verzár Z. The potential beneficial effect of EPA and DHA supplementation managing cytokine storm in coronavirus disease. Front Physiol. 2020;11:752. doi: 10.3389/fphys.2020.00752. https://doi.org/10.3389/fphys.2020.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura J, Kubota K, Murakami H, Sawamura M, Matsushima T, Tamura T, et al. Immunomodulation by vitamin B12: augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin Exp Immunol. 1999;116:28–32. doi: 10.1046/j.1365-2249.1999.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Fujiwara H, Torisu M. Vitamin E and immune response. I. Enhancement of helper T cell activity by dietary supplementation of vitamin E in mice. Immunology. 1979;38:727–734. [PMC free article] [PubMed] [Google Scholar]
- Thomsen MO, Christensen LP, Grevsen K. Harvest strategies for optimization of the content of bioactive alkamides and caffeic acid derivatives in aerial parts and in roots of Echinacea purpurea. J Agric Food Chem. 2018;66:11630–11639. doi: 10.1021/acs.jafc.8b03420. [DOI] [PubMed] [Google Scholar]
- Tvedten HW, Whitehair CK, Langham RF. Influence of vitamins A and E on gnotobiotic and conventionally maintained rats exposed to Mycoplasma pulmonis. J Am Vet Med Assoc. 1973;163:605–612. [PubMed] [Google Scholar]
- Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- van Steenwijk HP, Bast A, de Boer A. Immunomodulating effects of fungal beta-glucans: from traditional use to medicine. Nutrients. 2021;13:1333. doi: 10.3390/nu13041333. https://doi.org/10.3390/nu13041333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassopoulou M, Yannakoulia M, Pletsa V, Zervakis GI, Kyriacou A. Effects of fungal beta-glucans on health-a systematic review of randomized controlled trials. Food Funct. 2021;12:3366–3380. doi: 10.1039/D1FO00122A. [DOI] [PubMed] [Google Scholar]
- Wakikawa A, Utsuyama M, Wakabayashi A, Kitagawa M, Hirokawa K. Vitamin E enhances the immune functions of young but not old mice under restraint stress. Exp Gerontol. 1999;34:853–862. doi: 10.1016/S0531-5565(99)00055-8. [DOI] [PubMed] [Google Scholar]
- Walston J, Xue Q, Semba RD, Ferrucci L, Cappola AR, Ricks M, et al. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol. 2006;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ying Q, Zhu W, Chen J. Vitamin D and asthma occurrence in children: A systematic review and meta-analysis. J Pediatr Nurs. 2022;62:e60–e68. doi: 10.1016/j.pedn.2021.07.005. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO), author Vitamin A supplements a guide to their use in the treatment and prevention of vitamin A deficiency and xerophthalmia. 2th ed. WHO; 1997. [Google Scholar]
- Wójcik R, Siwicki AK, Skopińska-Rózewska E, Wasiutyński A, Sommer E, Furmanowa M. The effect of Chinese medicinal herb Rhodiola kirilowii extracts on cellular immunity in mice and rats. Pol J Vet Sci. 2009;12:399–405. [PubMed] [Google Scholar]
- Woo A, Kim JH, Jeong YJ, Maeng HG, Lee YT, Kang JS, et al. Vitamin C acts indirectly to modulate isotype switching in mouse B cells. Anat Cell Biol. 2010;43:25–35. doi: 10.5115/acb.2010.43.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SM, Beckham C, Yosioka A, Darban H, Watson RR. β-Carotene and selenium supplementation enhances immune response in aged humans. Integr Med. 2000;2:85–92. doi: 10.1016/S1096-2190(00)00009-3. [DOI] [PubMed] [Google Scholar]
- Xu C, Mathews AE, Rodrigues C, Eudy BJ, Rowe CA, O'Donoughue A, et al. Aged garlic extract supplementation modifies inflammation and immunity of adults with obesity: A randomized, double-blind, placebo-controlled clinical trial. Clin Nutr ESPEN. 2018;24:148–155. doi: 10.1016/j.clnesp.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Xu W, Wong G, Hwang YY, Larbi A. The untwining of immunosenescence and aging. Semin Immunopathol. 2020;42:559–572. doi: 10.1007/s00281-020-00824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Tan C, Li P, Zhang S, Pang X, Liu H, et al. Changes of cytokines during a spaceflight analog-a 45-day head-down bed rest. PLoS One. 2013;8:e77401. doi: 10.1371/journal.pone.0077401. https://doi.org/10.1371/journal.pone.0077401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuzhu G, Komai-Koma M, Leung BP, Howe HS, McSharry C, McInnes IB, et al. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann Rheum Dis. 2012;71:129–135. doi: 10.1136/ard.2011.149831. [DOI] [PubMed] [Google Scholar]
- Ye S, Li S, Ma Y, Hu D, Xiao F. Curcumin hinders PBDE-47-induced neutrophil extracellular traps release via Nrf2-associated ROS inhibition. Ecotoxicol Environ Saf. 2021;225:112779. doi: 10.1016/j.ecoenv.2021.112779. https://doi.org/10.1016/j.ecoenv.2021.112779. [DOI] [PubMed] [Google Scholar]
- Zhang HG, Kim H, Liu C, Yu S, Wang J, Grizzle WE, et al. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim Biophys Acta. 2007;1773:1116–1123. doi: 10.1016/j.bbamcr.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lin Z, Hu Y, Wang F. Effect of Ganoderma lucidum capsules on T lymphocyte subsets in football players on "living high-training low". Br J Sports Med. 2008;42:819–822. doi: 10.1136/bjsm.2007.038620. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]