Abstract
Objective:
Insulin resistance (IR) and compensatory hyperinsulinemia result in hyperandrogenism (HA) and oligo-ovulation in women with Polycystic Ovary Syndrome (PCOS), who can present with either apparent eumenorrhea or overt menstrual dysfunction (i.e. oligomenorrhea/amenorrhea). However, the determinants of the degree of menstrual dysfunction is unclear. The objective of this study was to examine the relation of abnormalities in menstrual cyclicity to HA and dynamic state IR in oligo-ovulatory PCOS women.
Design:
Prospective cross-sectional study.
Setting:
Tertiary-care academic center.
Patients:
Fifty-seven participants with PCOS (by NIH 1990 criteria) and 57 body mass index (BMI)-matched healthy controls.
Interventions:
The short insulin tolerance test (ITT).
Main Outcome Measure(s):
Menstrual cyclicity, SHBG, measures of HA (i.e. modified Ferriman-Gallwey [mF-G] score, total and free testosterone, DHEAS), and kITT derived from the short ITT.
Main results:
Adjusting for age, BMI and ethnicity, mean androgen measures were higher and SHBG trended lower, kITT was lower and the prevalence of IR higher, in PCOS than controls, independent of menstrual cyclicity. Optimal cut-off point for IR was set at kITT value of 3.57%/min or lower. Overall, 79% of PCOS had IR. To control further for the effect of ethnicity, a subgroup of 46 non-Hispanic white PCOS participants were studied; those who exhibited amenorrhea (n=15) or oligomenorrhea (n=19) had or tended towards having a lower kITT and a higher prevalence of IR than women with PCOS and oligoovulatory eumenorrhea (n=12). kITT trended lower and prevalence of IR higher in PCOS women with amenorrhea than those with oligomenorrhea. SHBG and HA measures were similar across the three menstrual groups.
Conclusions:
Oligo-ovulatory PCOS women with overt oligo/amenorrhea have greater degrees of IR, but not HA, compared to oligo-ovulatory eumenorrheic PCOS women, suggesting that IR and hyperinsulinemia, but not HA, play a role in determining the degree of menstrual dysfunction, which can be used as a clinical marker for the degree of IR in oligo-ovulatory PCOS.
Keywords: Menstrual dysfunction, dynamic state, insulin resistance, PCOS
INTRODUCTION
The Polycystic Ovary Syndrome (PCOS) is the most common endocrine-medical disorder in reproductive-aged women with a prevalence of 10–15%, and is characterized by hyperandrogenism (HA), ovulatory dysfunction (OD), and polycystic ovarian morphology (PCOM) (1). The disorder is associated with insulin resistance (IR), and compensatory hyperinsulinemia (2–4), which increase their risks of type 2 diabetes (T2DM) and cardiovascular disease (CVD). (1). IR also underlies, alone or in combination with HA, many of the reproductive features of the disorder, although surprisingly little has been done to define the phenotype of IR. Considering the high prevalence of PCOS and the associated metabolic dysfunction, the economic burden of the disorder is significant. For example, considering only reproductive age complications the economic burden of PCOS in the U.S. alone approximates 6 billion in current dollars, not considering obstetrical and pregnancy-related morbidities or complications in the post-menopausal period (5).
Given the high prevalence of IR, its associated morbidities in PCOS, the high economic burden, and the need to focus resources to those at the greatest risks of these morbidities, it would be useful to be able to readily identify PCOS women with the greatest risk of IR. Unfortunately, current direct measures of IR are expensive and cumbersome. Consequently, many studies of IR in PCOS have relied on fasting insulin and glucose levels, homeostasis model assessment of IR (HOMA-IR), quantitative insulin sensitivity check index (QUICKI), and oral glucose stimulated insulin sensitivity (e.g. Matsuda index) (68), understanding that these surrogate markers are of limited sensitivity and specificity (9).
One possible ready marker for IR in PCOS may be the phenotypic presentation of PCOS, which we currently can classify into A through D (i.e., phenotype A: HA+OD+PCOM; phenotype B: HA+OD; phenotype C: HA+PCOM; and phenotype D: PCOM+OD). IR and its associated morbidities are linked to PCOS phenotypes: the severity of IR and HA and cardiometabolic risks decrease with each successive PCOS phenotype, with classical phenotype (phenotype A or B) being the most affected, and those with non-hyperandrogenic PCOS (phenotype D) being least affected (10–14). Another marker may be the degree of menstrual dysfunction, as oligo-ovulatory or anovulatory PCOS women can present with either apparent eumenorrhea or overt menstrual dysfunction. Indeed, previous studies (6–8), including our own (15), have demonstrated a relationship between the degree of menstrual dysfunction and surrogate markers of IR. However, the relationship of the degree of menstrual dysfunction in PCOS to IR assessed directly and dynamically is not known.
The ‘gold standard’ test for the determination of IR is the euglycemic hyperinsulinemic clamp (16). However, this method is expensive and complex, prompting the use of other direct measures of in vivo insulin sensitivity, including the modified frequently sampled intravenous glucose tolerance test (mFSIVGTT) (17) and the short insulin tolerance test (ITT) (18–19), which have been shown to correlate with each other and with the euglycemic hyperinsulinemic clamp in the general population (17–20). However, the ITT is simpler, less expensive, and more convenient than either euglycemic hyperinsulinemic clamp or mFSIVGTT when performing large scale or epidemiologic studies and requires only six to eight blood samples for determination of the plasma glucose disappearance rate constant (kITT) (18–19). Despite these advantages, there are limited data on the use of ITT in PCOS (21–24).
We undertook the present study to test the hypothesis that the severity of menstrual dysfunction was associated with HA and dynamic state whole-body IR in women with PCOS. In line with this hypothesis, PCOS women with more severe degrees of OD and HA were expected to have higher degrees of IR.
SUBJECTS AND METHODS
Study population and protocol
One hundred and fourteen (57 healthy control and 57 PCOS) reproductive-aged participants were prospectively and consecutively recruited for the study. The two groups were matched for body mass index (BMI). The diagnosis of PCOS was based on the presence of oligo-ovulation, and clinical or biochemical hyperandrogenism, including a modified Ferriman–Gallwey (mF-G) hirsutism score (i.e., mF-G score ≥ 6) and hyperandrogenemia (i.e., total testosterone [T], free T and dehydroepiandrosterone sulfate [DHEAS] above normal) (25–26). Because we were interested in studying metabolic dysfunction, we selected for study those PCOS participants who met the 1990 National Institutes of Health consensus criteria, corresponding to Phenotypes A and B of the Rotterdam criteria (11), excluding other known endocrinopathies as previously described (25). A control group of healthy premenopausal women with long-term eumenorrhea and no evidence of hyperandrogenism or endocrine disorders were also prospectively recruited.
Study exclusion criteria included pregnancy state, other endocrine disorders, inability to assess menstruation or ovulation status (e.g. prior hysterectomy, bilateral oophorectomy, vaginal agenesis, postmenopausal or premenarcheal state) or use of any hormonal medication (including oral contraceptives, insulin-sensitizing agents, anti-diabetic medications, antiandrogens, or glucocorticoids) for at least 3 months preceding the evaluation. All subjects had normal thyroid-stimulating hormone, 17-hydroxyprogesterone, and prolactin levels, and underwent a standard 75-g oral glucose tolerance test to exclude type 2 diabetes mellitus (T2DM). Screening for Cushing’s syndrome and androgen-secreting neoplasms was performed if clinically indicated.
Research subjects were recruited through advertisements or, for PCOS, the clinical and research practice of reproductive endocrinology clinic (to R.A.) at the University of Alabama at Birmingham (UAB). To ensure comparable groups matched as closely as possible, we began to prospectively recruit PCOS subjects first, and matching controls were subsequently and prospectively recruited. An attempt was made to closely match PCOS participants and control participants within narrow ranges (i.e. ±3 kg/M2 in body mass index [BMI], ±5 years in age, and similar race) as previously described (27). Both the PCOS patients and controls were recruited over a similar period of time.
Protocol
All subjects completed a uniform questionnaire providing information regarding age, race, and menstrual history. Participants with PCOS were grouped according to the interval between episodes of vaginal bleeding (15). Women with 26- to 34-day bleeding intervals were considered eumenorrheic and their ovulatory function assessed by measuring a menstrual cycle day 22–24 progesterone (P4) level. Those women with a P4 level less than 4 ng/mL were considered oligo-ovulatory (i.e., classified as d 26–34, oligo-ovulatory eumenorrhea), whereas the remainder were considered to be ovulatory (i.e., classified as d 26–34, ovulatory eumenorrhea) (15; 28). PCOS women with 35 days to 3-month bleeding intervals were classified as oligomenorrheic and those with cycles >3 months as amenorrheic (25). All the PCOS subjects in this study were oligo-ovulatory, irrespective of their menstrual cyclicity.
Fasting baseline blood samples were obtained in all subjects in the follicular or pre-ovulatory phase of the cycle, unless amenorrheic when the sample was obtained at random. In addition to history, all subjects underwent a physical examination with blood sampling for hormone measurements, as previously described (25; 29), and were normoglycemic. In addition to height, weight, and modified Ferriman-Gallwey (mF-G) score, waist circumference (WC) was measured at the narrowest portion of the torso approximately midway between the lower costal margin and the iliac crest, and the hip circumference was measured over the widest portion of the gluteal and greater trochanteric region. The BMI and waist to hip ratio (WHR) were then calculated.
Fasting blood samples for circulating total T, free T, DHEAS, sex hormone binding globulin (SHBG), insulin, and glucose concentrations were obtained on days 3 through 8 (i.e. the follicular phase) of a spontaneous menstrual cycle or a Prometrium® (Solvay Pharmaceuticals, Marietta, GA)-induced withdrawal bleed. The study protocol was approved by the Institutional Review Board of UAB. All subjects provided written informed consent prior to participating in the study.
ITT
All subjects underwent an ITT between 8:00 and 9:00 am on days 3–8 of a spontaneous or induced withdrawal bleed. In brief, after an overnight fast, an intravenous (iv) catheter was placed in each forearm, one for blood sampling and a second for insulin injection. After 15 min. of rest, iv blood samples were drawn at −20, −15, and 0 min. ThereaUer, a bolus of 0.1 U/kg regular insulin (Humulin-R, Lilly, Indianapolis, IN) was injected iv at time 0 min. Venous samples were then drawn at +3, +6, +9, +12, 15 mins. Subjects were monitored continuously and closely for signs of significant hypoglycemia during the entire test and for 30 min. thereafter. Immediately after the test, or sooner if signs of significant hypoglycemia developed, a bolus 50 cc dose of 50% dextrose solution was administered iv. Plasma samples were collected into prechilled tubes containing EDTA (for insulin) or sodium fluoride potassium oxalate (for glucose). The plasma glucose and insulin levels at −20, −15 and 0 min. were averaged to yield respective fasting values.
Whole-body glucose/insulin kinetics were determined using the kITT (%/min) (i.e. the first order rate constant for plasma glucose disposal), as previously described (18; 30). To determine kITT values, plasma glucose levels were converted into their natural logarithm (Ln), and the slope during the first 3 – 15 minutes after insulin injection was then calculated using linear regression (Ln [glucose]) x time) and multiplied by 100 to obtain the constant rate of glucose decay per minute (%/minute) during the ITT (i.e., kITT) (18; 30). Overall, the smaller the kITT value the greater the degree of IR.
Hormonal and Biochemical analysis
For the ITT, plasma glucose levels were determined immediately after sampling using a Beckman glucose analyzer II (Beckman Inc., Brea, CA) and plasma insulin levels were determined by radioimmunoassay (RIA) (Medical Research Laboratories, Inc., Highland Heights, KY) as previously described (25). Serum samples were analyzed for total T, SHBG, DHEAS, and P4, per protocol. Total T was measured by an in-house radioimmunoassay [RIA] method after serum extraction, as previously described (31). SHBG binding activity was measured by diffusion equilibrium dialysis, using Sephadex G25 (Sigma-Aldrich Corp., St. Louis, MO) and [3H]T as the ligand, and the free T was calculated, as previously described (31 – 32). DHEAS and P4, were measured by direct RIA, using commercially available kits (DHEAS and P4 from Diagnostic Products Corp., Los Angeles, CA), as previously described (33). Samples were batched at regular intervals for analysis to minimize the impact of interassay variability and provide study subjects with timely information. The intra- and interassay variations for total T, SHBG, DHEAS, and P4 have been previously reported (25).
Statistical analysis
The Shapiro-Wilks W-test was used to determine whether continuous variables were normally distributed. All continuous variables, except for the mF-G score, reasonably followed a parametric distribution. For the analysis of menstrual dysfunction, participants with PCOS were divided into three groups (oligo-ovulatory eumenorrheic with cycles d. 26–34; oligomenorrheic with cycles d. 35 to 3 months, and amenorrheic with cycles >3 months. Intergroup differences were evaluated using the unpaired t test for normally distributed continuous variables (i.e. PCOS vs. controls) or ANOVA and Tukey for post hoc ANOVA test for multiple comparisons of normally distributed continuous variables (i.e. eumenorrheic vs. oligomenorrheic vs. amenorrheic groups), and the Wilcoxon rank-sum test for mF-G score. Values for comparing proportions were computed with the χ2 test with Yates correction or Fisher’s exact test as appropriate. Bivariate correlations between variables were analyzed using the Pearson correlation coefficient for all variables. Linear regression was used to adjust for differences in age, BMI and race. A receiver-operating-characteristic (ROC) curve was used, and the area under the curve (AUC) estimated, to assess the discrimination ability and to determine the optimal (in terms of sensitivity and specificity) threshold level of kITT that defined IR, as previously described (9). Based on our previous studies (15), a power analysis with a pooled standard deviation of 2.16, a β = 0.20, and an α = 0.05, based on t testing, indicated that a sample size of 20 participants per group was sufficient to detect a mean difference of 2 (%/min) (i.e. 20% change) in kITT value between PCOS women with amenorrhea and healthy control participants. Data were expressed as mean ± SE (standard error) or geometrical mean (range) if log transformed unless otherwise stated. A two-sided p <0.05 was considered statistically significant. All statistical analyses were conducted using the Stats Direct statistics software package, version 3.2.10 2020 (Cheshire, UK).
RESULTS
Baseline features of the study groups in all PCOS versus all controls
In all, we studied 114 non-diabetic consecutive subjects, 57 participants with PCOS (i.e. Phenotypes A and B of the Rotterdam criteria (11) and 57 healthy control participants (Table 1, Fig. 1, and Supplemental Table 1). The basic demographic, anthropometric, and endocrine characteristics of the PCOS and control participants are depicted in Table 1. Despite a proactive matching strategy, controls were slightly but significantly older than PCOS participants (31.3±0.9 yrs. vs. 25.2±1.0 yrs.; P=<0.001), and differences existed in racial composition between the two groups. Most of the PCOS participants (80.7%) were non-Hispanic White (non-HW), whereas fewer PCOS participants were of African American (AA) ancestry (19.3%) compared to controls (56;1% and 43.9%, respectively), a significant difference (P=0.009). By design, there was no difference in mean BMI between PCOS participants and control participants but the former group was numerically greater in weight, with the BMI in PCOS being 35.2±1.0 Kg/m2 compared to 32.5 Kg/m2 in controls (P=0.089). Therefore, the outcome variables were adjusted for age, BMI, and race.
Table 1.
Basal, endocrine, and metabolic characteristics of all PCOS and all BMI-matched control participants.
| Variables | PCOS (n = 57) | Control (n = 57) | P value Unadjusted for age, BMI, and race | P value Adjusted for age, BMI, and race |
|---|---|---|---|---|
| Age (y) | 25.2 ± 1.0 | 31.3 ± 0.9 | <.001a | NA |
| BMI (kg/m2) | 35.2 ± 1.0 | 32.5 ± 1.2 | .089 | NA |
| WHR | 0.86 ± 0.01 | 0.80 ± 0.01 | <.001a | .007a |
| Ethnicity (n, %) | ||||
| AA | 11 (19.3) | 25 (43.9) | .009a | NA |
| Non-Hispanic White | 46 (80.7) | 32 (56.1) | .009a | NA |
| Hirsutism (mF-G) score | 11.23 ± 0.66 | 0.02 ± 02 | <.001a | <.001a |
| Hormonal analysis | ||||
| Total T (ng/dL) | 86.2 ± 5.4 | 37.8 ±6.6 | <.001a | .007a |
| Free T (ng/dL) | 0.96 ± 0.07 | 0.32 ±0.05 | <.001a | .001a |
| DHEAS (ng/mL) | 2,746.9 ± 194.8 | 6,668 ± 126.2 | <.001a | .003a |
| SHBG (nmol/L) | 174 ± 11 | 220 ±27 | .079 | .333 |
| Metabolic analysis | ||||
| Fasting glucose (mg/dL) | 83.9 ±2.1 | 84.0 ± 1.5 | .972 | .404 |
| INSo (μlU/mL) | 34.7 (5.4–190) | 9.6 (4.3–28.5) | <.001a | .001a |
| kITT (%/min) | 2.54 ±0.21 | 5.44 ±0.31 | <.001a | .001a |
| Prevalence or IR (n, %) | 45 (79) | 10(17.5) | <.001a | NA |
Note:Variables are expressed as mean ± standard error of the mean or as number or percentage (and range). For this analysis P<.05 is considered significant. AA = African American; BMI = body mass index; DHEAS = dehydroepiandrosterone sulfate; INS0 = fasting insulin levels; IR = insulin resistance; kITT = rate constant for plasma glucose disappearance during insulin tolerance test; mF-G = modified Ferriman-Gallwey; NA = not applicable; PCOS = polycystic ovary syndrome; SHBG = sex hormone-binding globulin; T = testosterone; WHR = waist-to-hip ratio.
P values considered statistically significant.
Ezeh. Menstrual cyclicity and dynamic state IR. Fertil Steril 2020.
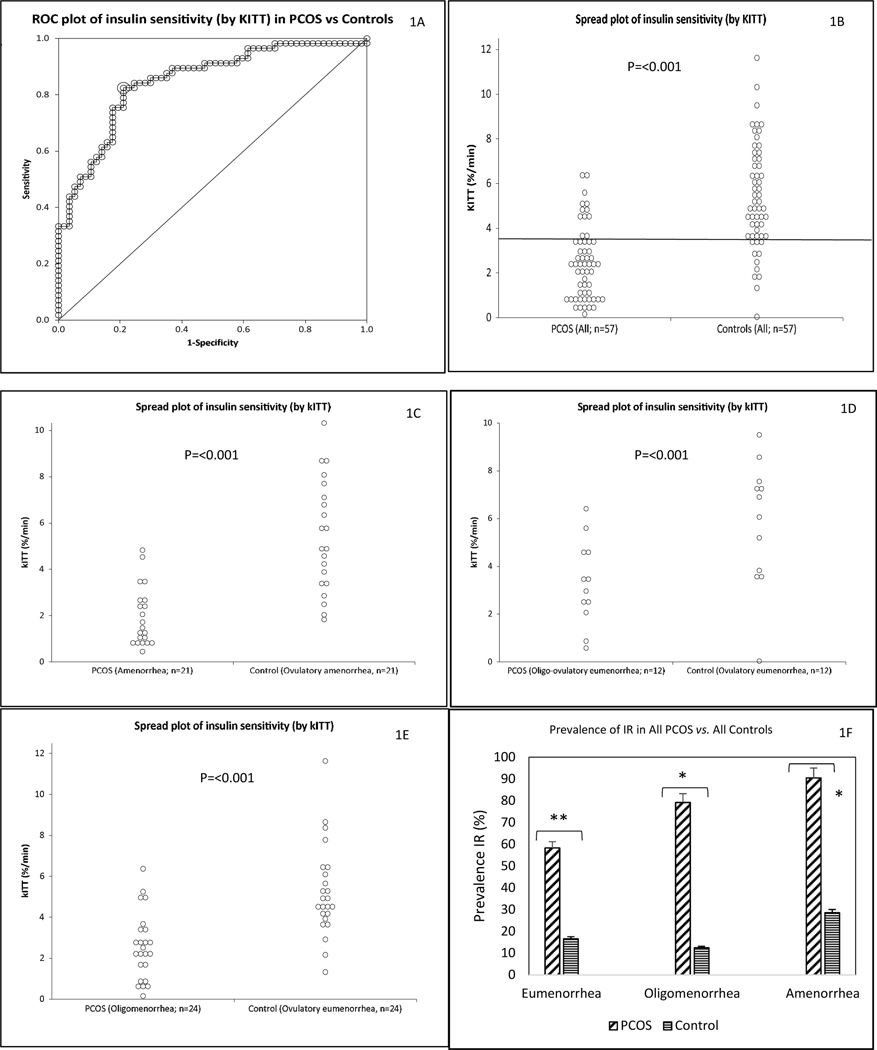
Fig. 1. Differences in measures of in vivo whole-body IR between PCOS and controls.
Subjects in the all cohort (57 PCOS and 57 BMI-matched controls adjusted for age, BMI and race) are depicted. IR was estimated by kITT (%/min) after a short insulin tolerance test. ROC plot of kITT values in PCOS (-ve) and controls (+ve), with the optimal cut-off value for kITT of 3.57 (%/min) (horizontal line) providing 79.7% specificity and 81.5% sensitivity in discriminating between PCOS and control participants (Fig. 1A, Fig. 1B). A scatter plot of differences in mean kITT values between PCOS and control participants for all the cohort (57 PCOS and 57 BMI-matched controls adjusted for age, BMI and race) (Fig. 1B), and for all the cohort subdivided according to menstrual categories (Fig. 1C, 1D and 1E for oligo-ovulatory eumenorrheic, oligomenorrheic and amenorrheic PCOS groups vs. respective ovulatory eumenorrheic groups, respectively) are also depicted. The prevalence of IR between each menstrual subtype of PCOS vs. respective ovulatory eumenorrheic groups is also shown (Fig. 1F).
AUC is area-under-the-curve; kITT is the rate constant for glucose disappearance during short ITT, and ROC is receiver operating characteristics (Fig. 1A).
**P=0.049 and *P<0.001 (Fig. 1F).
As expected, women with PCOS had significantly higher mF-G scores and baseline mean serum total T, free T, DHEAS levels than control, both before and after adjustment for age, BMI and race. Serum SHBG levels trended towards being lower by 26.4% in PCOS compared to control participants, although the difference did not reach significance. All 57 controls presented with normal ovulatory menstrual patterns (cycle length 26–34+ days). Of the 57 oligo-ovulatory PCOS women studied, 12 (26.1%) manifested clinically apparent eumenorrhea (cycle length 26–34 days), 24 (31.3%) oligomenorrhea (cycle length >35 days to 3 months), and 21 (32.6%) amenorrhea (cycles > 3 month) (Table 2, and Supplemental Tables 1 and 3).
Table 2.
Characteristics of the overall and subgroup of non-Hispanic White PCOS participants based on menstrual cyclicity.
| All PCOS | NHW PCOS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P value between groupsa | P value between groupsa | |||||||||||
| Variables | Eumeno (n = 12) | Oligo (n = 24) | Ameno (n = 21) | Eumeno vs. Oligo | Oligo vs. Ameno | Eumeno vs. Ameno | Eumeno (n = 12) | Oligo (n = 19) | Ameno (n = 15) | Eumeno vs. Oligo | Oligo vs. Ameno | Eumeno vs. Ameno |
| Age (y) | 24.1 ± 2.3 | 25.7 ± 1.7 | 25.3 ± 1.6 | .841 | .989 | .902 | 24.1 ±2.3 | 26.6 ± 1.9 | 25.1 ± 1.7 | .82 | .893 | .456 |
| BMI (kg/m2) | 33.5 ±2.6 | 35.0 ± 1.7 | 36.3 ± 1.4 | .855 | .855 | .603 | 33.5 ±2.6 | 36.1 ± 1.9 | 37.4 ± 1.8 | .647 | .846 | .932 |
| WHR | 0.83 ± 0.03 | 0.88 ± 0.02 | 0.85 ± 0.02 | .179 | .420 | .789 | 0.83 ± 0.03 | 0.89 ± 0.02 | 0.85 ± 0.03 | .095 | .340 | .786 |
| Ethnicity (n, %) | ||||||||||||
| AA | 0(0) | 5 (20.8) | 6 (28.6) | NA | .799 | NA | NA | NA | NA | NA | NA | NA |
| NHW | 12(100) | 19(79.2) | 15 (74.4) | NA | .799 | NA | NA | NA | NA | NA | NA | NA |
| Hirsutism (mF-G) score | 11.9 ± 1.0 | 11.0 ± 1.0 | 11.1 ± 1.2 | .860 | .998 | .891 | 11.9 ± 1.0 | 11.3 ± 1.3 | 11.3 ± 1.6 | .940 | .999 | .956 |
| Hormonal | ||||||||||||
| Total T (ng/dL) | 76.7 ±4.1 | 80.5 ±3.9 | 98.2 ± 13.6 | .960 | .315 | .310 | 76.7 ±4.1 | 78.5 ±4.6 | 103.3 ± 17.9 | .992 | .217 | .244 |
| Free T (ng/dL) | 0.83 ± 0.08 | 0.93 ± 0.06 | 1.07 ±0.18 | .862 | .688 | .464 | 0.83 ± 0.08 | 0.95 ± 0.07 | 1.14 ±0.24 | .851 | .609 | .366 |
| DHEAS (ng/mL) | 3,626.7 ± 467.3 | 2,525.8 ± 293.2 | 2,496.9 ± 280.7 | .082 | .998 | .081 | 3,626.7 ± 467.3 | 2,662.2 ± 317.3 | 2,678.3 ± 379.5 | .190 | .999 | .232 |
| SHBG (nmol/L) | 16.75 ± 1.49 | 16.31 ±0.96 | 19.15 ±2.75 | .988 | .511 | .713 | 167.5 ± 14.9 | 156.1 ± 10.5 | 180.0 ±35.3 | .936 | .598 | .850 |
| Metabolic | ||||||||||||
| Fasting glucose (mg/L) | 78.6 ± 1.7 | 81.3 ± 1.7 | 89.9 ± 5.0 | .864 | .151 | .107 | 78.6 ± 1.7 | 82.0 ±2.1 | 87.5 ± 3.8 | .665 | .294 | .087 |
| INSo (μlU/mL) | 58.9 ±23.1 | 46.9 ± 10.4 | 47.3 ± 12.5 | .838 | .999 | .863 | 58.9 ±23.1 | 41.9 ±9.9 | 51.1 ± 19.0 | .997 | .986 | .974 |
| kITT (%/min) | 3.33 ±0.51 | 2.63 ± 0.33 | 1.98 ±0.28 | .411 | .341 | .049b | 3.33 ±0.51 | 2.72 ± 0.41 | 1.81 ±0.29 | .597 | .082 | .029 |
| Prevalence of IR (n, %) | 7 (58.3) | 19(79.2) | 19(90.5) | .223 | .337 | .048b | 7 (58.3) | 14(73.7) | 14(93.3) | .204 | .085 | .046 |
Note:Variables are expressed as mean ± standard error of the mean unless otherwise indicated. For thisanalysis P<.05 is considered significant. AA = African American; ameno = amenorrhea; BMI = body mass index; DHEAS = dehydroepiandrosterone sulfate; eumeno = oligo-ovulatory eumenorrhea; INSO = fasting insulin levels; IR = insulin resistance; kITT = rate constant for plasma glucose disappearance during short insulin tolerance test; mF-G = modified Ferriman-Gallwey; NA = not applicable; NHW = Non-Hispanic White; oligo = oligomenorrhea; PCOS = polycystic ovary syndrome; SHBG = sex hormone-binding globulin; T = testosterone; WHR = waist-to-hip ratio.
Unadjusted for age and BMI.
Ezeh. Menstrual cyclicity and dynamic state IR. Fertil Steril 2020.
Metabolic function and relation to menstrual dysfunction in all PCOS versus all controls
The metabolic characteristics of the participants are depicted in Table 1 and Fig. 1. Fasting plasma glucose levels were similar between the groups, consistent with a nondiabetic population, and fasting insulin levels (INS0,) were higher in PCOS women than controls, both before and further adjustment for age, BMI, and race (Table 1).
Based on the ROC curve, the optimal cut-off value for kITT (i.e. the kITT cut-off value with highest sensitivity and specificity for diagnosing IR) was 3.57 %/min, which yielded a specificity of 79.7% and a sensitivity of 82.5% in discriminating between PCOS and controls (AUC= 0.85 [95% CI = 0.78 to 0.92]) (Figs. 1A and 1B). Consequently, a kITT value at or below 3.57 %/min was considered IR in our study, a value that is close to the median kITT value for PCOS and controls combined, i.e. 3.65 %/min (Figs. 1A and 1B). Mean kITT values were lower (by 36.9%) in PCOS than controls, both before and after adjustment for age, BMI, and race (Table 1 and Fig. 1B). The prevalence of IR assessed by the kITT was higher (by 61.5%) in PCOS than among controls (P < 0.001) (Table 1 and Supplemental Figs. 1A).
Compared with controls, mean kITT values were lower in all PCOS menstrual dysfunction subgroups (i.e. oligo-ovulatory eumenorrhea, oligomenorrhea, and amenorrhea), both before and after adjustment for age and BMI (Figs. 1C, 1D and 1E; Supplemental Table 1). Similarly, the prevalence of IR was higher in PCOS than controls, regardless of menstrual cyclicity (Figs. 1F; Supplemental Table 1).
We studied the influence of obesity on kITT values and prevalence of IR in PCOS and control participants (Supplemental Table 2). The prevalence of IR was 57.1% among the lean, 62.5% overweight, and 85.7% obese PCOS participants as compared with 15.4%, 14.3% and 30% of control participants, respectively. Similarly, mean kITT values were lower in obese and non-obese PCOS vs. controls (2.17±0.22 vs. 4.80±0.42 %/min; P= <0.001 and 3.56±0.44 vs. 6.1±0.43 %/min; P< 0.001, respectively). Overall, IR was greater in PCOS women, independent of BMI, although the increased IR in PCOS was exacerbated by obesity.
Metabolic function and relation to menstrual dysfunction in all PCOS
Basic, endocrine, and metabolic parameters for the entire PCOS cohort are depicted in Table 2, Figs. 2A and Supplemental Table 3). All the participants with apparent eumenorrhea (n=12) were of nonHispanic White (NHW) race. Among the participants with oligomenorrhea (n=24), 20.8% were of AA and 79.2% were of NHW ethnic background, as compared with those with amenorrhea (n=21) where 28.6% were AA and 74.4% NHW.
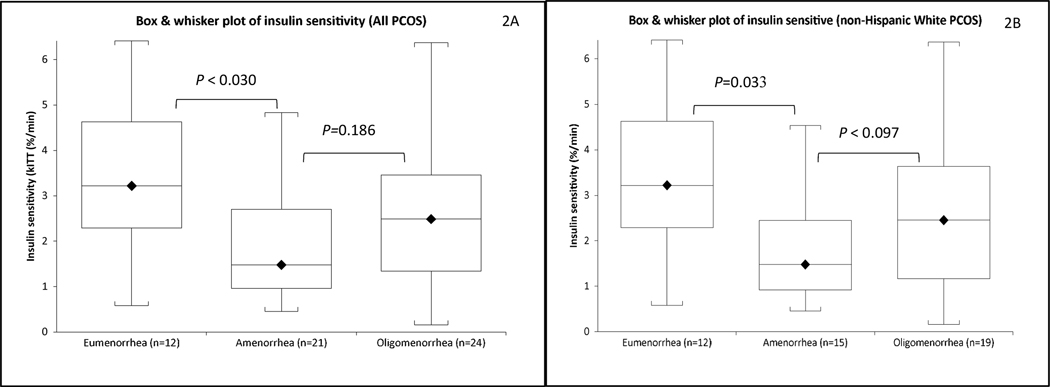
Fig. 2. Insulin sensitivity among women with PCOS, categorized by menstrual cyclicity (oligo-ovulatory eumenorrhea, oligomenorrhea and amenorrhea), are depicted.
The degree of insulin resistance (IR) estimated by mean kITT (adjusted for age and BMI) in all PCOS subjects (Fig. 2A), and a subgroup of NHW PCOS subjects (Fig. 2B) are shown.
(a) Median values are represented by dark diamond, and median by the horizontal lines in the boxes.
(b) The heights of the boxes denote the 25th to 75th ranges
(c) The upper and lower frames represent the maximal and minimal values, respectively.
The differences were adjusted for age and BMI
A comparison of the androgen measures between the three menstrual groups revealed no difference in mean mF-G score and circulating androgens levels, before and after adjusting for age and BMI (Supplemental Table 3), except that the difference in DHEAS levels became significantly higher in PCOS participants with amenorrhea than those with apparent eumenorrhea, after adjustment for age and BMI. Circulating SHBG levels were similar across the three menstrual groups (Table 2).
With respect to metabolic risk factors, the mean fasting glucose levels and INS0 were similar across the three menstrual groups (Table 2), while mean kITT value was lower (both before and after adjusting for age and BMI (Table 2, Figs. 2A and Supplemental Table 3) and prevalence of IR higher in oligo-ovulatory PCOS participants with amenorrhea than eumenorrheic participants (Table 2 and Supplemental Figs. 2A). Mean kITT value and prevalence of IR did not differ between PCOS participants with amenorrhea and those with oligomenorrhea (Table 2, Figs. 2A, and Supplemental Table 3 and Figs. 2A ).
The correlation of age, BMI, WHR, SHBG, and measures of clinical and biochemical HA with kITT across the three menstrual groups are depicted in Supplemental Table 4. In bivalent analysis, kITT value was associated with BMI (i.e., eumenorrhea [r=−0.63; P=0.028], oligomenorrhea [r=−0.59; P=0.003], and amenorrhea [r=−0.47; P=0.030), but not with age or mF-G, in all three PCOS menstrual subgroups. kITT value was positively associated with SHBG (r= 0.57; P=0.009), but only in participants with amenorrhea. The association of kITT negatively with free T (r=−0.76; P=0.004) and total T (r=−0.76; P=0.004), and positively and weakly with DHEAS (r=0.56; P=0.056) levels was observed in PCOS participants with apparent eumenorrhea only.
Metabolic function and relation to menstrual dysfunction in NHW PCOS
As noted, PCOS participants were more likely to be of non-Hispanic White (NHW) ancestry than controls (Table 1), and all PCOS participants who exhibited oligo-ovulatory eumenorrhea were NHW. To control for any possible confounding effects of race/ethnicity, the relation of menstrual dysfunction with insulin sensitivity and HA was re-assessed in the subgroup of 46 non-Hispanic white (NHW) oligoovulatory PCOS women, 12 (26.1%) of whom had apparent eumenorrhea, 19 (41.3%) oligomenorrhea, and 15 (32.6%) amenorrhea (Table 2, Figs. 2B, and Supplemental Table 5 and Figs. 2B ). There was no significant difference between participants with oligo-ovulatory PCOS and apparent eumenorrhea, oligomenorrhea, or amenorrhea in terms of mean age, BMI, WHR, fasting glucose levels, INS0, mF-G score, and circulating levels of SHBG, total and free T, before and after adjustment for age and BMI (Table 2 and Supplemental Table 5). In contrast to what was obtained when the entire 57 PCOS cohort was considered, circulating DHEAS levels were similar across the three menstrual groups.
Compared to PCOS participants with oligo-ovulatory eumenorrhea, mean kITT value was lower (by 45.7%) and prevalence of IR higher (by 35.0%) among those with amenorrhea (Table 2, Figs. 2B and Supplemental Table 5 and Figs. 2B), consistent with the results obtained when the entire 57 PCOS cohort (unadjusted for race) instead of the 46 NHW PCOS participants was considered. There was a trend towards a lower mean kITT value (by 18.3%) and higher prevalence of IR (by 15.4%) among those with oligomenorrhea (Table 2, Figs. 2B and Supplemental Table 5 and Figs. 2B). Similarly, NHW PCOS participants with amenorrhea tended towards having lower kITT (by 33.5%) and higher prevalence of IR than those with oligomenorrhea (by 19.6%).
The association of age, BMI, WHR, SHBG, and measures of clinical and biochemical HA with kITT value across the three menstrual groups are depicted in Supplemental Table 4. As expected, kITT value was strongly and negatively correlated with total T (r=−0.76; P=0.004) and free T (r=−0.76; P=0.004), and positively and weakly with DHEAS (r=−0.56; P=0.056), but only in PCOS participants with apparent eumenorrhea (Supplemental Table 4). The kITT value was associated with BMI (ie. eumenorrhea [r=−0.63; P=0.028]; oligomenorrhea [r=−0.67; P=0.002]; and amenorrhea [r=−0.47; P=0.038), but not with age or mF-G in all three PCOS menstrual subgroups. In contrast to the results obtained when all the 57 PCOS participants (unadjusted for race) instead of the 46 NHW PCOS participants were considered, circulating SHBG levels were similar across the three menstrual groups. Overall, severity of menstrual cyclicity did not track with measures androgenicity in PCOS.
DISCUSSION
The primary hypothesis of this cross-sectional prospective study was to investigate whether the severity of menstrual dysfunction is associated with dynamic state IR and HA in PCOS. Consistent with our hypothesis, we found that the severity of menstrual dysfunction tracked with the prevalence and degree of dynamic state insulin resistance (IR), after adjustment for factors known to predispose to IR, including age, BMI and race, confirming our prior observation using markers of basal state whole-body IR (i.e. HOMA-IR) and bridging a knowledge gap by studying insulin sensitivity in physiologic state. In terms of adverse metabolic risks, oligo-ovulatory PCOS women exhibiting amenorrhea had the worst insulin sensitivity (i.e. lowest mean kITT values) and highest prevalence of IR, followed by those presenting with oligomenorrhea; PCOS women with oligo-ovulatory eumenorrhea had the best insulin sensitivity (i.e. highest kITT values) and lowest prevalence of IR. Nonetheless, when compared with their respective healthy normoandrogenic, ovulatory and eumenorrheic control participants, all women with PCOS exhibited lower mean kITT values and higher prevalence of IR, regardless of whether they presented with amenorrhea, oligomenorrhea or clinically apparent eumenorrhea.
Contrary to our hypothesis, the severity of menstrual abnormalities did not track with the degree of HA -- the mean measures of HA (i.e. mF-G, and circulating levels of DHEAS, total and free T, and SHBG levels) were similar across the three menstrual groups. Although bivariate analysis revealed that insulin sensitivity was associated negatively with circulating total and free T levels, and positively with DHEAS levels, this association was observed in PCOS women with oligo-ovulatory eumenorrhea only. Cupisti et al. also did not observe an association between HA measures and the degree of menstrual dysfunction in PCOS (7), although our results contrast with those of Strowitzki et al., who reported higher free androgen index (FAI) and total T in amenorrheic than eumenorrheic PCOS (8). Collectively, our study indicates that menstrual pattern tracks with dynamic state IR rather than HA.
Consistent with previous studies, we confirmed that IR is very common in PCOS, independent of age, race, and adiposity (3, 21–24, 27, 34–35). The high prevalence of IR observed in 79% of the PCOS population in our study using a threshold kITT value of 3.57 %/min determined by the ROC curve is similar to the value of 85% reported in a study of women with PCOS defined by Rotterdam criteria assessed using the euglycemic hyperinsulinemic clamp (35), but higher than the 66% for clamp based studies (3), 53% for mFSIVGTT (35), and 65% - 69% for the few studies that used the ITT to assess IR (21, 24) in PCOS meeting the NIH-based criteria (i.e. Rotterdam A and B). Overall, the variance in the prevalence of IR perhaps reflect differences in the prevalence of obesity and racial composition of the participants studied, PCOS diagnosis, and disparities in methodologies or cut-off points of the variables used to define IR in these studies.
Our current study expands on the findings from previous reports (15) and more clearly addresses the question of whether the severity of menstrual dysfunction can predict IR in PCOS. Abnormalities in menstrual cycles are readily measurable and are frequently used as accurate surrogate markers of oligo-ovulation in reproductive-aged women in clinical settings. As many as 85% of PCOS women demonstrate overt menstrual abnormalities, although some oligo-ovulatory PCOS women can present with apparent eumenorrhea (1). These findings have significant potential public health implications, as they focus our attention on the value of the menstrual cycle, which is easy and inexpensive to obtain, to predict IR, which is more cumbersome to obtain, in PCOS.
Only a few studies have assessed the relationship between menstrual dysfunction and IR in PCOS. In our previous study of abnormal menstrual cycle in PCOS (by Rotterdam criteria), comparing PCOS women with menstrual dysfunction (polymenorrhea, ovulatory and non-ovulatory eumenorrhea, 35- to 45-day cycle intervals, oligomenorrhea and amenorrhea), using HOMA-IR as the measure of IR and healthy normoandrogenic women as the reference cohort, we found that only PCOS women with oligomenorrhea and amenorrhea, who comprise about 80% of study group, had higher mean HOMA-IR levels than controls, with those exhibiting amenorrhea having the highest HOMA-IR values (15). Panidis et al reported similar results using HOMA-IR and QUICK) (6). Others confirmed this association only in hyperandrogenic women with amenorrhea, but not oligomenorrhea, when compared with participants with eumenorrhea and PCOM using HOMA-IR (8) or insulin levels, QUICKI, and the glucose-insulin ratio (7) as measures of IR. These studies were fundamentally limited by the use of surrogate markers to estimate IR. In our current study, we focused on studying the relation of menstrual dysfunction to the magnitude and prevalence of dynamic state whole-body IR measured directly.
We could only find one study that have assessed the relationship between menstrual pattern and IR in dynamic state, but this was in the context of evaluating insulin sensitivity and menstrual pattern in women with PCOM (22). In that study, the investigators found that insulin sensitivity was reduced in 53 participants with HA presenting with oligomenorrhea/amenorrhea (and PCOM), compared to 19 subjects with HA exhibiting eumenorrhea (and PCOM) or 31 healthy controls (22). Both studies used the kITT, as we did, to determine insulin sensitivity and found that women with oligomenorrhea/amenorrhea had lower insulin sensitivity than control participants. Additionally, fasting insulin, glucose, total T, and SHBG levels did not differ significantly between hyperandrogenic participants with oligo/amenorrhea vs. those with eumenorrhea. Unlike our current study, the focus of that study was on women with HA+PCOM, the prevalence of IR was not assessed, those with amenorrhea and oligomenorrhea were combined as one group, and the participants with eumenorrhea were not hormonally screened for evidence of oligo-ovulation.
From mechanistic perspectives, our hypothesis that menstrual cycle pattern could track with IR and HA was based on the evidence that compensatory hyperinsulinemia from IR plays an important role in the pathophysiology of HA and ovulatory dysfunction in PCOS (14). Further, it was based on evidence gathered from the study of nonhuman primate models of PCOS (36), whereby a reduction in HA increases insulin sensitivity and ovulatory function in young adult female nonhuman primate (37). The findings of our current study that abnormalities in menstrual dysfunction tracked with IR and, by inference, hyperinsulinemia, but not HA, is supported by reports demonstrating that therapies that improve insulin sensitivity, whether via diet and exercise (38–39), insulin sensitizers (metformin, D-chiroinositol and peroxisome proliferator-activated receptor gamma [PPARγ] agonists) (40–41), bariatric surgery (42–43) or glucagon-like peptide-1 (GLP-1) analogs (44), significantly reduce circulating insulin levels and improve ovulation rates and menstrual irregularity. Our findings are further supported by the evidence that flutamide, an androgen receptor antagonist, decreased androgen levels but failed to improve insulin sensitivity in non-obese women with PCOS (45). Finally, it should be noted that wholebody IR is also associated with IR in the endometrium (46; 47), although whether whole-body or endometrial IR adversely affect menstrual cyclicity remains to be established.
The strengths of our study include use of well-phenotyped, matched PCOS and control participants, and measuring whole-body insulin-mediated glucose uptake directly using a robust method (i.e. ITT). The reported concerns about the use of the ITT due to frequent episodes of hypoglycemia and surges of counter regulatory hormones (e.g. glucagon, catecholamine, growth hormone and cortisol), which have the potential to reduce glucose disposal from plasma into insulin target tissues are addressed by our study’s use of the short (15 mins.) version of ITT used in this study, since hypoglycemia generally appears after 15 mins and the surge of counter regulatory hormones at 20–30 mins post insulin injection (18–19). Our study was limited by its cross-sectional nature, which made it difficult to determine the cause-effect relationship between menstrual dysfunction and IR or HA. Additionally, as we studied PCOS as defined by the 1990 NIH consensus criteria (i.e. Phenotypes A and B of the Rotterdam criteria (11) because of our focus on the metabolic aspects of the disorder, the results of this study cannot be readily generalized to other phenotypes of PCOS (i.e. phenotypes C and D). Furthermore, the sample size of subjects in different menstrual groups will need to be increased in future studies.
Overall, our results indicate that women with classic PCOS demonstrated higher prevalence and greater degree of dynamic state IR than matched controls overall, regardless of menstrual pattern or adiposity. Furthermore, PCOS women with overt oligo/amenorrhea had greater degrees of IR, but not HA, compared to oligo-ovulatory PCOS women with apparent eumenorrhea. These data suggest that: a) IR and hyperinsulinemia, but not HA, play a role in determining the degree of menstrual dysfunction in oligo-ovulatory women with PCOS; and b) the degree of menstrual dysfunction in oligo-ovulatory PCOS women could be used as a marker for the degree of IR in these women. Further studies will be required to determine the role of IR on endometrial integrity in PCOS and whether abnormalities in menstrual cyclicity can help define PCOS women at greater risk for metabolic complications, such as T2DM.
Supplementary Material
Supplemental Fig. 1. Differences in prevalence of IR between PCOS and controls. The difference in the prevalence of IR in PCOS participants (79.0%) vs. control participants (17.5%) for all the cohort (57 PCOS vs. 57 BMI-matched controls) (Supplemental Fig. 1A) is depicted. The difference in the prevalence of IR between all PCOS vs. control subdivided according to menstrual categories are also shown (Supplemental Fig. 1B).
Supplemental Fig. 2. Differences in prevalence of IR among women with PCOS, categorized by menstrual cyclicity (oligo-ovulatory eumenorrhea, oligomenorrhea and amenorrhea). The prevalence of insulin resistance among all PCOS subjects (Supplemental Fig. 2A) and the subgroup of non-Hispanic White participants with PCOS (Supplemental Fig. 2B) are shown.
Grants:
This work was supported by grants R01-DK073632 and R01-HD29364 from the NIH and an endowment of the Helping Hand of Los Angeles, Inc. (to R.A.).
Abbreviations:
- AA
African American
- AUC
Area under the curve
- BMI
body mass index
- DHEAS
dehydroepiandrosterone sulfate
- HA
hyperandrogenism
- HOMA-β%
homeostasis model of β-cell function
- HOMA-β%
homeostasis model of β-cell function
- kITT
the rate constant for plasma glucose disappearance
- mF-G
modified Ferriman-Gallwey hirsutism score
- NHW
non-Hispanic white
- OD
ovulatory dysfunction
- PCOS
polycystic ovary syndrome
- ITT
short insulin tolerance test
- T
testosterone
- T2DM
type 2 diabetes mellitus
- WHR
waist to hip ratio
Footnotes
Abbreviations of authors’ names and disclosure summary: Uche Ezeh (UE), Chima Ezeh (CE), Margareta 35 D. Pisarska (MDP), and Ricardo Azziz (RA) have nothing to declare.
Precis: The severity of menstrual dysfunction tracks with dynamic state insulin resistance, but not with hyperandrogenism, and could potentially be used to predict metabolic dysfunction in PCOS.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E; Escobar-Morreale HF; Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009; 91: 456–88. [DOI] [PubMed] [Google Scholar]
- 2.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012; 33: 981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989; 38: 1165–74. [DOI] [PubMed] [Google Scholar]
- 4.Ezeh U, Ida Chen YD, Azziz R. Racial and ethnic differences in the metabolic response of polycystic ovary syndrome Clin Endocrinol (Oxf). 2020; 93: 163–72. [DOI] [PubMed] [Google Scholar]
- 5.Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005; 90: 4650–58. [DOI] [PubMed] [Google Scholar]
- 6.Panidis D, Tziomalos K, Misichronis G, Papadakis E, Betsas G, Katsikis I, et al. Insulin resistance and endocrine characteristics of the different phenotypes of polycystic ovary syndrome: a prospective study. Hum Reprod. 2012; 27: 541–49. [DOI] [PubMed] [Google Scholar]
- 7.Cupisti S, Kajaia N, Dittrich R, Duezenli H, Beckmann WM, Mueller A. Body mass index and ovarian function are associated with endocrine and metabolic abnormalities in women with hyperandrogenic syndrome. Eur J Endocrinol. 2008; 158: 711–19. [DOI] [PubMed] [Google Scholar]
- 8.Strowitzki T, Capp E, von Eye Corleta H. The degree of cycle irregularity correlates with the grade of endocrine and metabolic disorders in PCOS patients. Eur J Obstet Gynecol Reprod Biol. 2010; 149: 178–81. [DOI] [PubMed] [Google Scholar]
- 9.Tosi F, Bonora E, Moghetti P. Insulin resistance in a large cohort of women with polycystic ovary syndrome: a comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum Reprod. 2017; 32: 2515–21. [DOI] [PubMed] [Google Scholar]
- 10.Jovanovic VP, Carmina E, Lobo RA. Not all women diagnosed with PCOS share the same cardiovascular risk profiles. Fertil Steril. 2010; 94: 826–32. [DOI] [PubMed] [Google Scholar]
- 11.Johnson T, Kaplan L, Ouyang P, Rizza R. National Institutes of Health evidence-based methodology workshop on polycystic ovary syndrome (PCOS). NIH EbMW Report. 2013. Available from https://prevention.nih.gov/programs-events/pathways-to-prevention/previous-workshops/pcos/workshop-resources. Bethesda, MD: National Institutes of Health; 1–14. [Google Scholar]
- 12.Moghetti P, Tosi F, Bonin C, Di Sarra D, Fiers T, Kaufman JM, et al. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2013; 98, E628–37. [DOI] [PubMed] [Google Scholar]
- 13.Escobar-Morreale HF. Reproductive endocrinology: Menstrual dysfunction--a proxy for insulin resistance in PCOS? Nat Rev Endocrinol. 2014; 10: 10–11. [DOI] [PubMed] [Google Scholar]
- 14.Rosenfield RL, Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr Rev. 2016; 37: 467–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brower M, Brennan K, Pall M, Azziz R. The severity of menstrual dysfunction as a predictor of insulin resistance in PCOS. J Clin Endocrinol Metab. 2013; 98: E1967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979; 237: E214–23. [DOI] [PubMed] [Google Scholar]
- 17.Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes. 1989; 38: 1512–27. [DOI] [PubMed] [Google Scholar]
- 18.Bonora E, Moghetti P, Zancanaro C, Cigolini M, Querena M, Cacciatori V, et al. Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J Clin Endocrinol Metab. 1989; 68: 374–78. [DOI] [PubMed] [Google Scholar]
- 19.Akinmokun A, Selby PL, Ramaiya K, Alberti KG. The short insulin tolerance test for determination of insulin sensitivity: a comparison with the euglycaemic clamp. Diabet Med. 1992; 9: 432–37. [DOI] [PubMed] [Google Scholar]
- 20.Okita K, Iwahashi H, Kozawa J, Okauchi Y, Funahashi T, Imagawa A, et al. Usefulness of the insulin tolerance test in patients with type 2 diabetes receiving insulin therapy. J Diabetes Investig. 2014; 5: 305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmina E, Koyama T, Chang L, Stanczyk FZ, Lobo RA. Does ethnicity influence the prevalence of adrenal hyperandrogenism and insulin resistance in polycystic ovary syndrome? Am J Obstet Gynecol. 1992;167: 1807–12. [DOI] [PubMed] [Google Scholar]
- 22.Robinson S, Kiddy D, Gelding SV, Willis D, Niththyananthan R, Bush A, et al. The relationship of insulin insensitivity to menstrual pattern in women with hyperandrogenism and polycystic ovaries. Clin Endocrinol (Oxf). 1993; 39: 351–55. [DOI] [PubMed] [Google Scholar]
- 23.Robinson S, Chan SP, Spacey S, Anyaoku V, Johnston DG, Franks S. Postprandial thermogenesis is reduced in polycystic ovary syndrome and is associated with increased insulin resistance. Clin Endocrinol (Oxf). 1992; 36: 537–43. [DOI] [PubMed] [Google Scholar]
- 24.Carmina E, Lobo RA. Use of fasting blood to assess the prevalence of insulin resistance in women with polycystic ovary syndrome. Fertil Steril. 2004; 82: 661–65. [DOI] [PubMed] [Google Scholar]
- 25.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Bots LR, Azziz R. Prevalence of polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998; 83: 3078–82. [DOI] [PubMed] [Google Scholar]
- 26.Chang WY, Knochenhauer ES, Bartolucci AA, Azziz R. Phenotypic spectrum of polycystic ovary syndrome: clinical and biochemical characterization of the three major clinical subgroups. Fertil Steril. 2005; 83: 1717–23. [DOI] [PubMed] [Google Scholar]
- 27.Ezeh U, Pall M, Mathur R, Dey D, Berman D, Chen IY et al. Effects of endogenous androgens and abdominal fat distribution on the interrelationship between insulin and non-insulin-mediated glucose uptake in females. J Clin Endocrinol Metab. 2013; 98: 1541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wathen NC, Perry L, Lilford RJ, Chard T. Interpretation of single progesterone measurement in diagnosis of anovulation and defective luteal phase: observations on analysis of the normal range. Br Med J (Clin Res Ed). 1984; 288 (6410): 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004; 89: 2745–9. [DOI] [PubMed] [Google Scholar]
- 30.Rezende LF, Santos GJ, Santos-Silva JC, Carneiro EM, Boschero AC. Ciliary neurotrophic factor (CNTF) protects non-obese Swiss mice against type 2 diabetes by increasing beta cell mass and reducing insulin clearance. Diabetologia. 2012; 55: 1495–1504. [DOI] [PubMed] [Google Scholar]
- 31.Boots LR, Potter S, Potter D, Azziz R. Measurement of total serum testosterone levels using commercially available kits: high degree of between-kit variability. Fertil Steril. 1998; 69: 286–92. [DOI] [PubMed] [Google Scholar]
- 32.Azziz R, Bradley EL Jr, Potter HD, Parker CR Jr, Boots LR. Chronic hyperinsulinemia and the adrenal androgen response to acute corticotropin-(1–24) stimulation in hyperandrogenic women. Am J Obstet Gynecol. 1995; 172: 1251–6. [DOI] [PubMed] [Google Scholar]
- 33.Azziz R, Hincapie LA, Knochenhauer ES, Dewailly D, Fox L, Boots LR. Screening for 21hydroxylase-deficient nonclassic adrenal hyperplasia among hyperandrogenic women: a prospective study. Fertil Steril. 1999; 72: 915–25. [DOI] [PubMed] [Google Scholar]
- 34.Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod. 2013; 28: 777–84. [DOI] [PubMed] [Google Scholar]
- 35.Legro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab 1998; 83: 2694–8. [DOI] [PubMed] [Google Scholar]
- 36.Abbott DH, Dumesic DA, Levine JE. Hyperandrogenic origins of polycystic ovary syndrome - implications for pathophysiology and therapy. Expert Rev Endocrinol Metab. 2019; 14: 131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varlamov O, Bishop CV, Handu M, Takahashi D, Srinivasan S, White A, et al. Combined androgen excess and Western-style diet accelerates adipose tissue dysfunction in young adult, female nonhuman primates. Hum Reprod. 2017; 32: 1892–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakubowicz DJ, Nestler JE. 17 alpha-Hydroxyprogesterone responses to leuprolide and serum androgens in obese women with and without polycystic ovary syndrome offer dietary weight loss. J Clin Endocrinol Metab. 1997; 82: 556–60. [DOI] [PubMed] [Google Scholar]
- 39.Lim SS, Hutchison SK, Van Ryswyk E, Norman RJ, Teede HJ, Moran LJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2019; 3(3): CD007506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002; 87: 1555–9. [DOI] [PubMed] [Google Scholar]
- 41.Morley LC, Tang T, Yasmin E, Norman RJ, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2017; 11(11): CD003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albers PH, Bojsen-Møller KN, Dirksen C, Serup AK, Kristensen DE, Frystyk J, et al. Enhanced insulin signaling in human skeletal muscle and adipose tissue following gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2015; 309: R510–24. [DOI] [PubMed] [Google Scholar]
- 43.Butterworth J, Deguara J, Borg CM. Bariatric Surgery, Polycystic Ovary Syndrome, and Infertility. J Obes. 2016; 2016:1871594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han Y, Li Y, He B. GLP-1 receptor agonists versus metformin in PCOS: a systematic review and meta-analysis. Reproductive BioMedicine Online. 2019; 39: 332–42. [DOI] [PubMed] [Google Scholar]
- 45.Sahin I, Serter R, Karakurt F, Demirbas B, Culha C, Taskapan C, et al. Metformin versus flutamide in the treatment of metabolic consequences of non-obese young women with polycystic ovary syndrome: a randomized prospective study. Gynecol Endocrinol. 2004; 19: 115–24. [DOI] [PubMed] [Google Scholar]
- 46.Qi J, Wang W, Zhu Q, He Y, Lu Y, Wang Y, et al. Local Cortisol Elevation Contributes to Endometrial Insulin Resistance in Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2018; 103: 2457–67. [DOI] [PubMed] [Google Scholar]
- 47.Ujvari D, Hulchiy M, Calaby A, Nybacka A, Byström B, Hirschberg AL. Lifestyle intervention upregulates gene and protein levels of molecules involved in insulin signaling in the endometrium of overweight/obese women with polycystic ovary syndrome. Hum Reprod. 2014; 29: 1526–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Differences in prevalence of IR between PCOS and controls. The difference in the prevalence of IR in PCOS participants (79.0%) vs. control participants (17.5%) for all the cohort (57 PCOS vs. 57 BMI-matched controls) (Supplemental Fig. 1A) is depicted. The difference in the prevalence of IR between all PCOS vs. control subdivided according to menstrual categories are also shown (Supplemental Fig. 1B).
Supplemental Fig. 2. Differences in prevalence of IR among women with PCOS, categorized by menstrual cyclicity (oligo-ovulatory eumenorrhea, oligomenorrhea and amenorrhea). The prevalence of insulin resistance among all PCOS subjects (Supplemental Fig. 2A) and the subgroup of non-Hispanic White participants with PCOS (Supplemental Fig. 2B) are shown.