Abstract
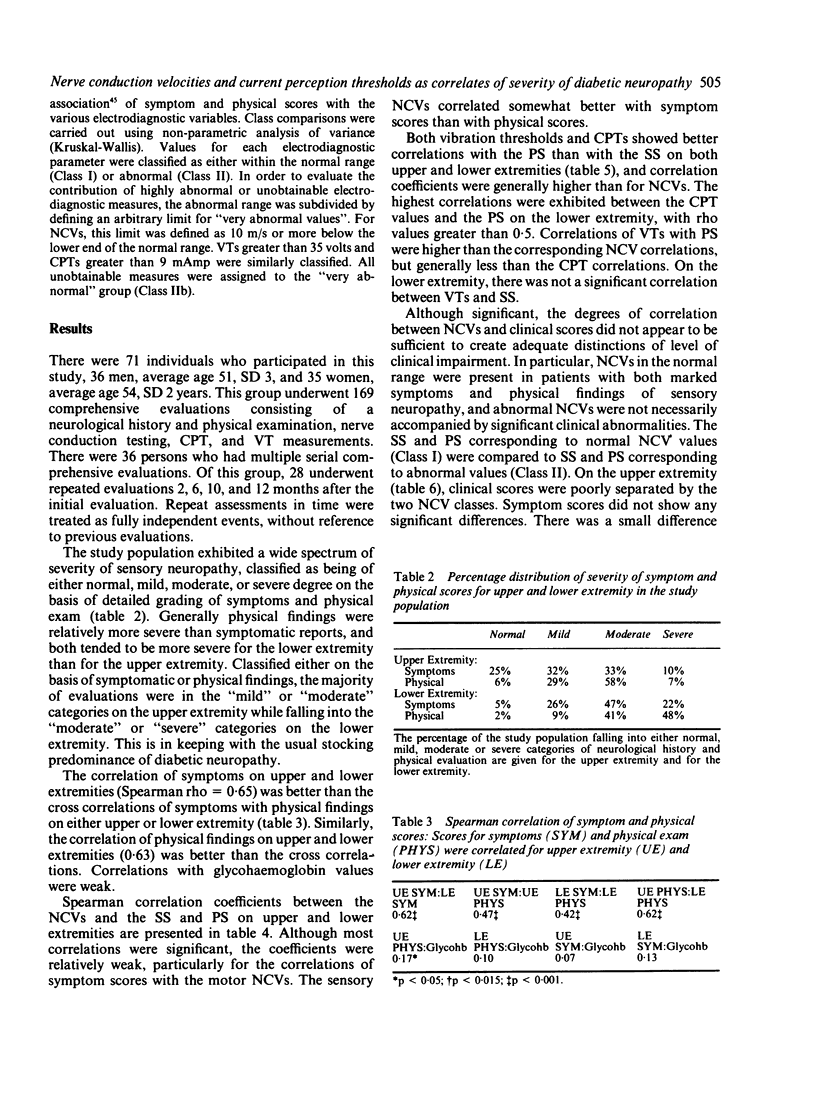
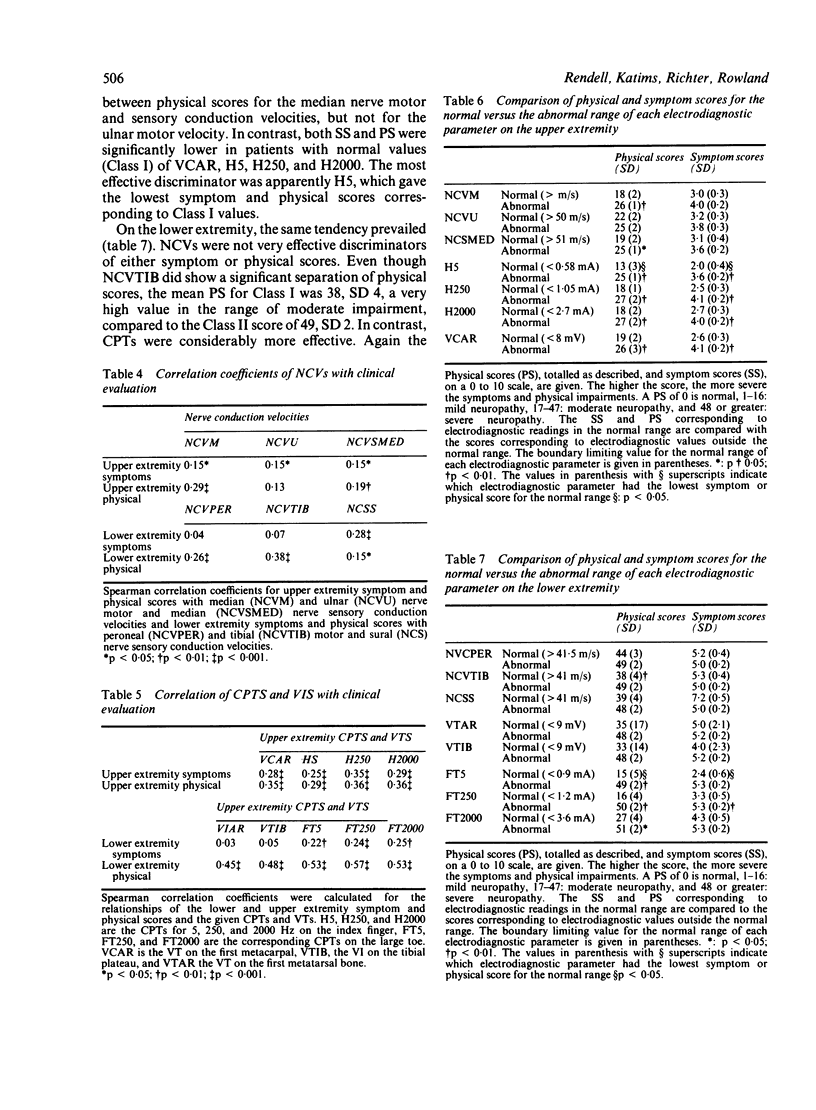
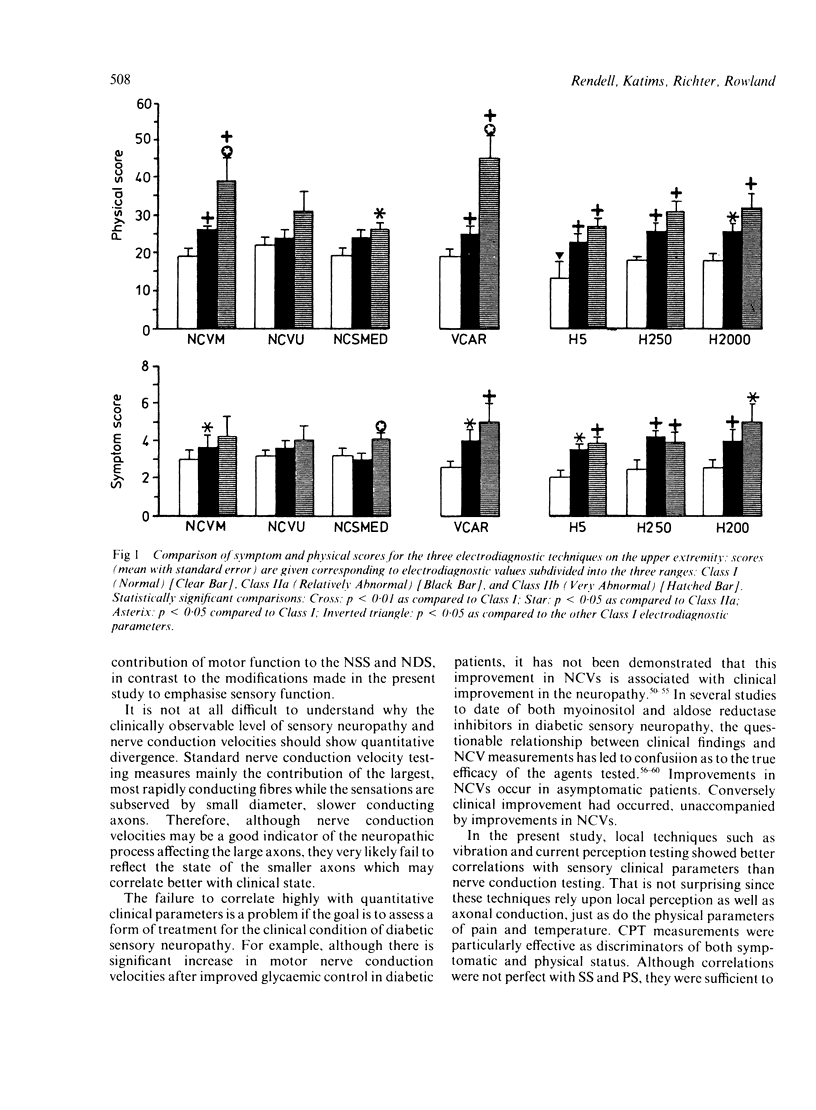
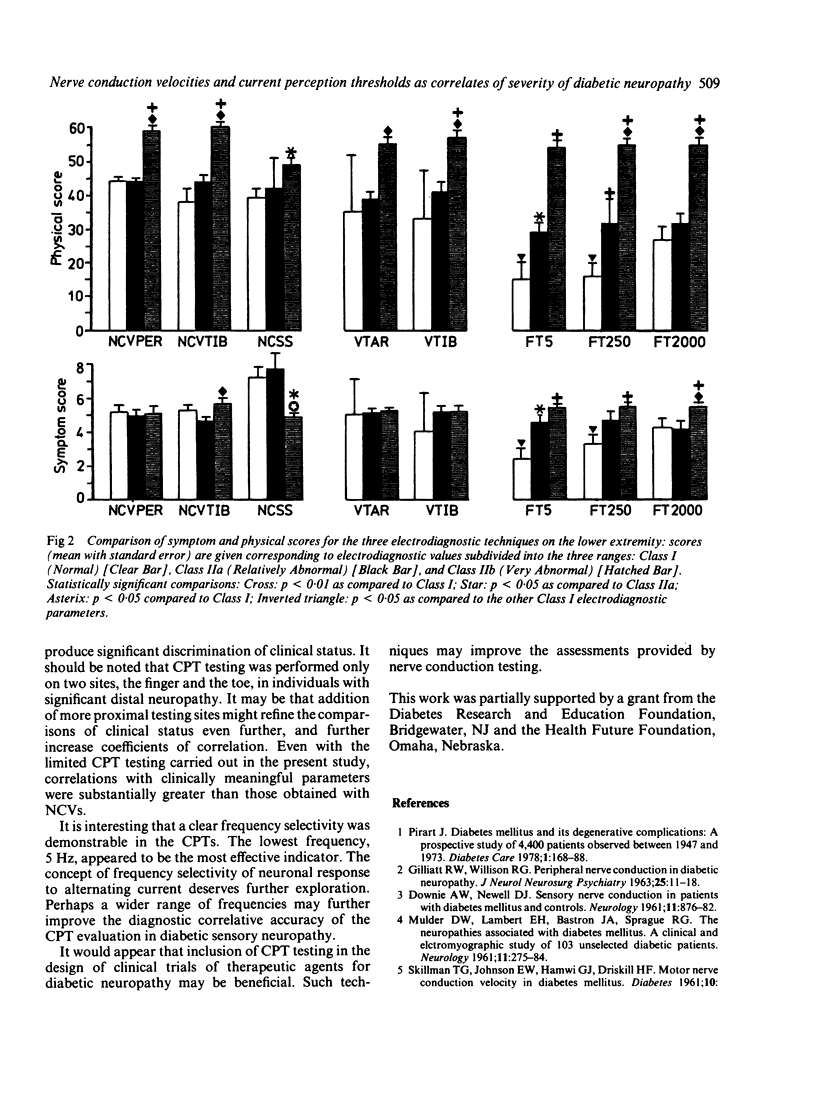
Nerve conduction velocities (NCVs) are the standard measurements used to confirm the presence or absence of diabetic neuropathy. NCVs were contrasted with the newer technique of measurement of alternating current perception thresholds (CPTs) in assessing the quantitative level of correlation with severity of diabetic sensory neuropathy. A very detailed, scored neurological history (symptoms) and physical examination, emphasising sensory assessment, was conducted on 71 individuals with diabetic neuropathy of varying degrees of severity. Sensory and motor NCVs and CPTs at 5, 250, and 2000 Hz of the upper and lower extremities were determined for these individuals. In addition, vibration thresholds (VTs) were measured as a third modality. Twenty eight individuals underwent repeated evaluations at 2, 6, 10 and 12 months after the initial procedures. Using the results of 169 complete evaluations, correlations were determined between physical scores (PS) and symptoms scores (SS) and NCVs. NCV correlations with the SS were weaker than with the PS. The strongest of the correlations were found between the PS and motor NCVs of the median nerve (rho = 0.29) and the tibial nerve (rho = 0.38). Normal NCVs were present in the face of very significant historical and physical abnormality. Correlations of the SS and PS with both VTs and CPTs were higher than with the NCVs. CPTs proved the more effective as predictors of both symptomatic and physical impairment. NCVs appear to lack the resolving power necessary to evaluate subtle differences in clinical state of diabetic sensory neuropathy. The supplementary use of current perception testing may improve the quantitative assessment of this condition.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER S. W., Jr Effects of 8-methoxypsoralen and ultraviolet light in human skin. Science. 1958 Apr 18;127(3303):878–878. doi: 10.1126/science.127.3303.878. [DOI] [PubMed] [Google Scholar]
- Behse F., Buchthal F., Carlsen F. Nerve biopsy and conduction studies in diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1977 Nov;40(11):1072–1082. doi: 10.1136/jnnp.40.11.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Sears T. A., Sherratt R. M. The spatial distribution of excitability and membrane current in normal and demyelinated mammalian nerve fibres. J Physiol. 1983 Aug;341:41–58. doi: 10.1113/jphysiol.1983.sp014791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. J., Martin J. R., Asbury A. K. Painful diabetic neuropathy. A morphometric study. Arch Neurol. 1976 Mar;33(3):164–171. doi: 10.1001/archneur.1976.00500030020004. [DOI] [PubMed] [Google Scholar]
- Burton C., Maurer D. D. Pain suppression by transcutaneous electronic stimulation. IEEE Trans Biomed Eng. 1974 Mar;21(2):81–88. doi: 10.1109/TBME.1974.324292. [DOI] [PubMed] [Google Scholar]
- Bütikofer R., Lawrence P. D. Electrocutaneous nerve stimulation--I: model and experiment. IEEE Trans Biomed Eng. 1978 Nov;25(6):526–531. [PubMed] [Google Scholar]
- Bütikofer R., Lawrence P. D. Electrocutaneous nerve stimulation-II: stimulus waveform selection. IEEE Trans Biomed Eng. 1979 Feb;26(2):69–75. doi: 10.1109/TBME.1978.326286. [DOI] [PubMed] [Google Scholar]
- Conomy J. P., Barnes K. L., Conomy J. M. Cutaneous sensory function in diabetes mellitus. J Neurol Neurosurg Psychiatry. 1979 Jul;42(7):656–661. doi: 10.1136/jnnp.42.7.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conomy J. P., Barnes K. L. Quantitative assessment of cutaneous sensory function in subjects with neurologic disease. J Neurol Sci. 1976 Dec;30(2-3):221–235. doi: 10.1016/0022-510x(76)90129-5. [DOI] [PubMed] [Google Scholar]
- DOWNIE A. W., NEWELL D. J. Sensory nerve conduction in patients with diabetes mellitus and controls. Neurology. 1961 Oct;11:876–882. doi: 10.1212/wnl.11.10.876. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Bushek W., Spring E. M., Karnes J. L., Litchy W. J., O'Brien P. C., Service F. J. Vibratory and cooling detection thresholds compared with other tests in diagnosing and staging diabetic neuropathy. Diabetes Care. 1987 Jul-Aug;10(4):432–440. doi: 10.2337/diacare.10.4.432. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Karnes J. L., Daube J., O'Brien P., Service F. J. Clinical and neuropathological criteria for the diagnosis and staging of diabetic polyneuropathy. Brain. 1985 Dec;108(Pt 4):861–880. doi: 10.1093/brain/108.4.861. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Sherman W. R., Hallcher L. M., Service F. J., O'Brien P. C., Grina L. A., Palumbo P. J., Swanson C. J. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol. 1980 Dec;8(6):590–596. doi: 10.1002/ana.410080608. [DOI] [PubMed] [Google Scholar]
- Fraser D. M., Campbell I. W., Ewing D. J., Murray A., Neilson J. M., Clarke B. F. Peripheral and autonomic nerve function in newly diagnosed diabetes mellitus. Diabetes. 1977 Jun;26(6):546–550. doi: 10.2337/diab.26.6.546. [DOI] [PubMed] [Google Scholar]
- GILLIATT R. W., WILLISON R. G. Peripheral nerve conduction in diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1962 Feb;25:11–18. doi: 10.1136/jnnp.25.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay K. H., Spack N., Loo S., Hirsch H. J., Ackil A. A. Aldose reductase inhibition: studies with alrestatin. Metabolism. 1979 Apr;28(4 Suppl 1):471–476. doi: 10.1016/0026-0495(79)90059-3. [DOI] [PubMed] [Google Scholar]
- Goldberg J. M., Lindblom U. Standardised method of determining vibratory perception thresholds for diagnosis and screening in neurological investigation. J Neurol Neurosurg Psychiatry. 1979 Sep;42(9):793–803. doi: 10.1136/jnnp.42.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf R. J., Halter J. B., Pfeifer M. A., Halar E., Brozovich F., Porte D., Jr Glycemic control and nerve conduction abnormalities in non-insulin-dependent diabetic subjects. Ann Intern Med. 1981 Mar;94(3):307–311. doi: 10.7326/0003-4819-94-3-307. [DOI] [PubMed] [Google Scholar]
- Gregersen G. Diabetic neuropathy: influence of age, sex, metabolic control, and duration of diabetes on motor conduction velocity. Neurology. 1967 Oct;17(10):972–980. doi: 10.1212/wnl.17.10.972. [DOI] [PubMed] [Google Scholar]
- Gregersen G. Variations in motor conduction velocity produced by acute changes of the metabolic state in diabetic patients. Diabetologia. 1968 Nov;4(5):273–277. doi: 10.1007/BF01309900. [DOI] [PubMed] [Google Scholar]
- Gregersen G. Vibratory perception threshold and motor conduction velocity in diabetics and non-diabetics. Acta Med Scand. 1968 Jan-Feb;183(1-2):61–65. doi: 10.1111/j.0954-6820.1968.tb10440.x. [DOI] [PubMed] [Google Scholar]
- Halar E. M., Graf R. J., Halter J. B., Brozovich F. V., Soine T. L. Diabetic neuropathy: a clinical, laboratory and electrodiagnostic study. Arch Phys Med Rehabil. 1982 Jul;63(7):298–303. [PubMed] [Google Scholar]
- Handelsman D. J., Turtle J. R. Clinical trial of an aldose reductase inhibitor in diabetic neuropathy. Diabetes. 1981 Jun;30(6):459–464. doi: 10.2337/diab.30.6.459. [DOI] [PubMed] [Google Scholar]
- JOSS G. AN UNUSUAL PHENOMENON IN THE ELECTRICAL PROPERTIES OF MAMMALIAN SKIN. Nature. 1964 Jan 25;201:418–419. doi: 10.1038/201418a0. [DOI] [PubMed] [Google Scholar]
- Judzewitsch R. G., Jaspan J. B., Polonsky K. S., Weinberg C. R., Halter J. B., Halar E., Pfeifer M. A., Vukadinovic C., Bernstein L., Schneider M. Aldose reductase inhibition improves nerve conduction velocity in diabetic patients. N Engl J Med. 1983 Jan 20;308(3):119–125. doi: 10.1056/NEJM198301203080302. [DOI] [PubMed] [Google Scholar]
- Katims J. J., Long D. M., Ng L. K. Transcutaneous nerve stimulation. Frequency and waveform specificity in humans. Appl Neurophysiol. 1986;49(1-2):86–91. [PubMed] [Google Scholar]
- Katims J. J., Naviasky E. H., Ng L. K., Rendell M., Bleecker M. L. New screening device for assessment of peripheral neuropathy. J Occup Med. 1986 Dec;28(12):1219–1221. [PubMed] [Google Scholar]
- Katims J. J., Naviasky E. H., Rendell M. S., Ng L. K., Bleecker M. L. Constant current sine wave transcutaneous nerve stimulation for the evaluation of peripheral neuropathy. Arch Phys Med Rehabil. 1987 Apr;68(4):210–213. [PubMed] [Google Scholar]
- LAWRENCE D. G., LOCKE S. Motor nerve conduction velocity in diabetes. Arch Neurol. 1961 Nov;5:483–489. doi: 10.1001/archneur.1961.00450170021003. [DOI] [PubMed] [Google Scholar]
- Lamontagne A., Buchthal F. Electrophysiological studies in diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1970 Aug;33(4):442–452. doi: 10.1136/jnnp.33.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer M., Long D. M. Transcutaneous neural stimulation for relief of pain. IEEE Trans Biomed Eng. 1976 Jul;23(4):341–345. doi: 10.1109/tbme.1976.324595. [DOI] [PubMed] [Google Scholar]
- MULDER D. W., LAMBERT E. H., BASTRON J. A., SPRAGUE R. G. The neuropathies associated with diabetes mellitus. A clinical and electromyographic study of 103 unselected diabetic patients. Neurology. 1961 Apr;11(4):275–284. doi: 10.1212/wnl.11.4.275. [DOI] [PubMed] [Google Scholar]
- McNeal D. R. Analysis of a model for excitation of myelinated nerve. IEEE Trans Biomed Eng. 1976 Jul;23(4):329–337. doi: 10.1109/tbme.1976.324593. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E. A. Electrical stimulation of sensory nerves with skin electrodes for research, diagnosis, communication and behavioral conditioning: a survey. Med Biol Eng. 1968 Nov;6(6):637–651. doi: 10.1007/BF02474726. [DOI] [PubMed] [Google Scholar]
- Pietri A., Ehle A. L., Raskin P. Changes in nerve conduction velocity after six weeks of glucoregulation with portable insulin infusion pumps. Diabetes. 1980 Aug;29(8):668–671. doi: 10.2337/diab.29.8.668. [DOI] [PubMed] [Google Scholar]
- Reilly J. P., Freeman V. T., Larkin W. D. Sensory effects of transient electrical stimulation--evaluation with a neuroelectric model. IEEE Trans Biomed Eng. 1985 Dec;32(12):1001–1011. doi: 10.1109/TBME.1985.325509. [DOI] [PubMed] [Google Scholar]
- SIGEL H. Cutaneous sensory threshold stimulation with high frequency square-wave current. I. The relationship of electrode dimensions to the sensory threshold. J Invest Dermatol. 1952 Jun;18(6):441–445. doi: 10.1038/jid.1952.53. [DOI] [PubMed] [Google Scholar]
- SIGEL H. Cutaneous sensory threshold stimulation with high frequency square-wave current. II. The relationship of body site and of skin diseases to the sensory threshold. J Invest Dermatol. 1952 Jun;18(6):447–451. doi: 10.1038/jid.1952.54. [DOI] [PubMed] [Google Scholar]
- STEINESS I. Vibratory perception in diabetics; a biothesiometric study. Acta Med Scand. 1957 Sep 20;158(5):327–335. doi: 10.1111/j.0954-6820.1957.tb15497.x. [DOI] [PubMed] [Google Scholar]
- STEINESS I. Vibratory perception in normal subjects; a biothesiometric study. Acta Med Scand. 1957 Sep 20;158(5):315–325. doi: 10.1111/j.0954-6820.1957.tb15496.x. [DOI] [PubMed] [Google Scholar]
- Seligman L. J. Physiological stimulators: from electric fish to programmable implants. IEEE Trans Biomed Eng. 1982 Apr;29(4):270–284. doi: 10.1109/TBME.1982.324944. [DOI] [PubMed] [Google Scholar]
- Sjölund B. H., Eriksson M. B. The influence of naloxone on analgesia produced by peripheral conditioning stimulation. Brain Res. 1979 Sep 14;173(2):295–301. doi: 10.1016/0006-8993(79)90629-2. [DOI] [PubMed] [Google Scholar]
- Ward J. D., Barnes C. G., Fisher D. J., Jessop J. D., Baker R. W. Improvement in nerve conduction following treatment in newly diagnosed diabetics. Lancet. 1971 Feb 27;1(7696):428–430. doi: 10.1016/s0140-6736(71)92415-9. [DOI] [PubMed] [Google Scholar]


