Abstract
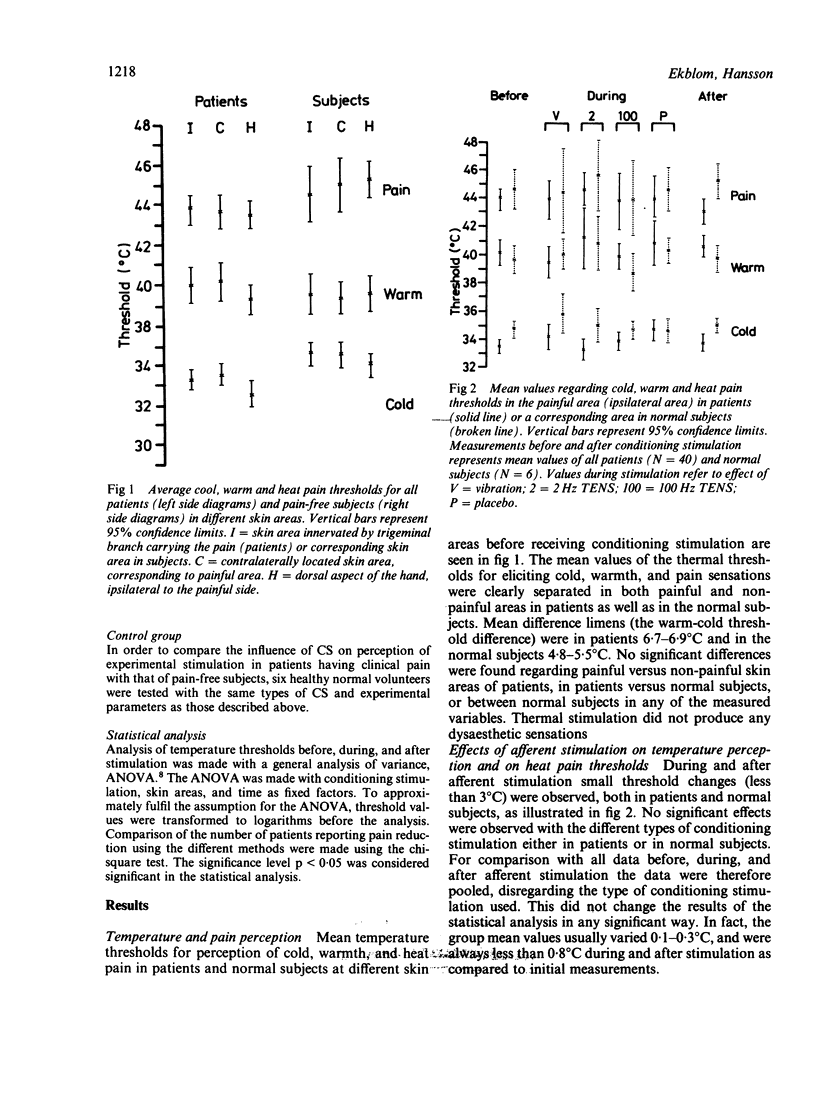
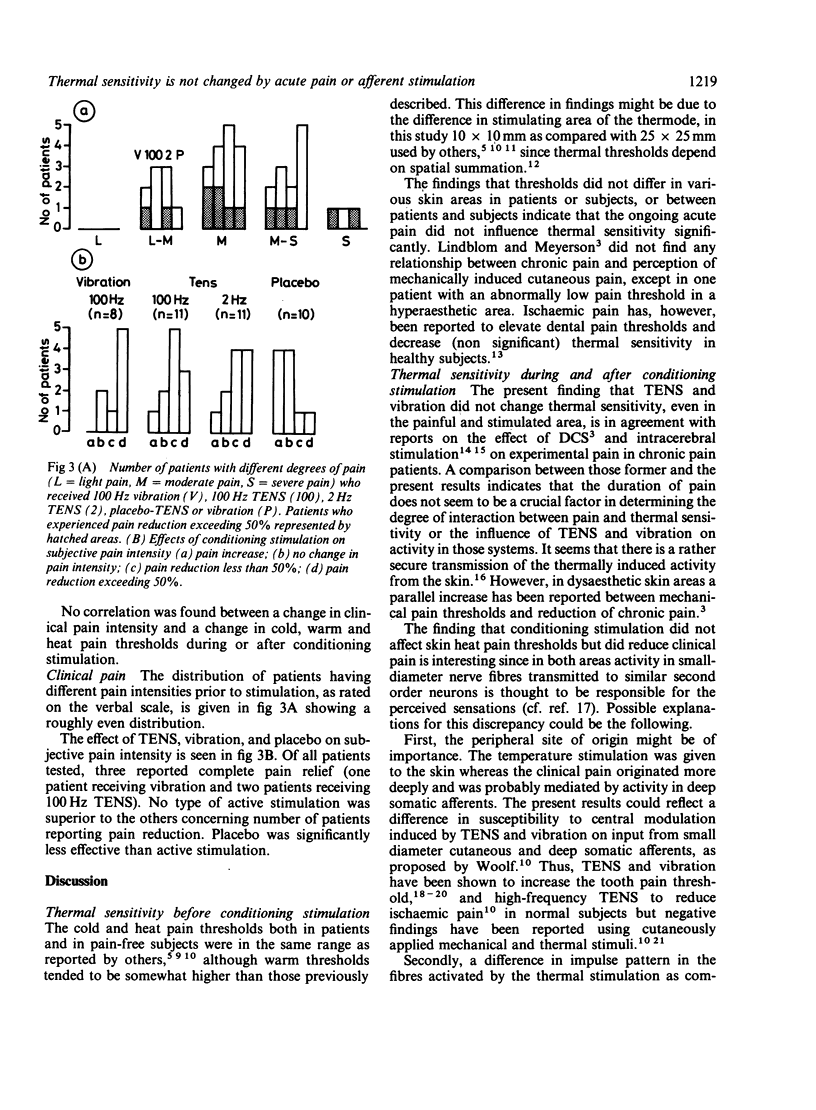
The effect of conditioning stimulation on thermal sensitivity and clinical pain was studied in 40 patients and six healthy subjects. Thresholds regarding cold, warm and heat pain perception did not differ significantly between the painful and non-painful skin areas in patients or between patients and healthy subjects before stimulation. The patients received either 100 Hz TENS, 2 Hz TENS, 100 Hz vibration, or placebo. No significant changes in thermal sensitivity were observed during and after conditioning stimulation in any of the test groups, although 24/40 (60%) of the patients reported reduction of their clinical pain intensity. The results indicate that (a) thermal sensitivity is not influenced by the presence of clinical pain, (b) the effects of stimulation on thermal sensitivity (thresholds) and clinical pain are not closely related, (c) central inhibitory effects of TENS and vibration are crucial for their pain relieving capacity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S. A., Holmgren E. On acupuncture analgesia and the mechanism of pain. Am J Chin Med (Gard City N Y) 1975 Oct;3(4):311–334. doi: 10.1142/s0192415x75000396. [DOI] [PubMed] [Google Scholar]
- Callaghan M., Sternbach R. A., Nyquist J. K., Timmermans G. Changes in somatic sensitivity during transcutaneous electrical analgesia. Pain. 1978 Aug;5(2):115–127. doi: 10.1016/0304-3959(78)90033-7. [DOI] [PubMed] [Google Scholar]
- Campbell J. N., Taub A. Local analgesia from percutaneous electrical stimulation. A peripheral mechanism. Arch Neurol. 1973 May;28(5):347–350. doi: 10.1001/archneur.1973.00490230083012. [DOI] [PubMed] [Google Scholar]
- Fagius J., Wahren L. K. Variability of sensory threshold determination in clinical use. J Neurol Sci. 1981 Jul;51(1):11–27. doi: 10.1016/0022-510x(81)90056-3. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H., Lindblom U., Schmidt W. C. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry. 1976 Nov;39(11):1071–1075. doi: 10.1136/jnnp.39.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson P., Ekblom A. Afferent stimulation induced pain relief in acute oro-facial pain and its failure to induce sufficient pain reduction in dental and oral surgery. Pain. 1984 Nov;20(3):273–278. doi: 10.1016/0304-3959(84)90016-2. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y., Adams J. E., Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977 Jul 8;197(4299):183–186. doi: 10.1126/science.301658. [DOI] [PubMed] [Google Scholar]
- Ignelzi R. J., Nyquist J. K. Direct effect of electrical stimulation on peripheral nerve evoked activity: implications in pain relief. J Neurosurg. 1976 Aug;45(2):159–165. doi: 10.3171/jns.1976.45.2.0159. [DOI] [PubMed] [Google Scholar]
- Janko M., Trontelj J. V. Transcutaneous electrical nerve stimulation: a microneurographic and perceptual study. Pain. 1980 Oct;9(2):219–230. doi: 10.1016/0304-3959(80)90009-3. [DOI] [PubMed] [Google Scholar]
- Nathan P. W., Rudge P. Testing the gate-control theory of pain in man. J Neurol Neurosurg Psychiatry. 1974 Dec;37(12):1366–1372. doi: 10.1136/jnnp.37.12.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara A. Experimental pain and transcutaneous electrical nerve stimulation at high frequency. Appl Neurophysiol. 1980;43(6):290–297. doi: 10.1159/000102268. [DOI] [PubMed] [Google Scholar]
- Pertovaara A., Kemppainen P., Johansson G., Karonen S. L. Dental analgesia produced by non-painful low-frequency stimulation is not influenced by stress or reversed by naloxone. Pain. 1982 Aug;13(4):379–384. doi: 10.1016/0304-3959(82)90006-9. [DOI] [PubMed] [Google Scholar]
- Pertovaara A., Kemppainen P., Johansson G., Karonen S. L. Ischemic pain nonsegmentally produces a predominant reduction of pain and thermal sensitivity in man: a selective role for endogenous opioids. Brain Res. 1982 Nov 11;251(1):83–92. doi: 10.1016/0006-8993(82)91276-8. [DOI] [PubMed] [Google Scholar]
- Pertovaara A., Kojo I. Influence of the rate of temperature change on thermal thresholds in man. Exp Neurol. 1985 Mar;87(3):439–445. doi: 10.1016/0014-4886(85)90174-8. [DOI] [PubMed] [Google Scholar]
- Price D. D., Hu J. W., Dubner R., Gracely R. H. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977 Feb;3(1):57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- Woolf C. J. Transcutaneous electrical nerve stimulation and the reaction to experimental pain in human subjects. Pain. 1979 Oct;7(2):115–127. doi: 10.1016/0304-3959(79)90003-4. [DOI] [PubMed] [Google Scholar]


