Abstract
This study of SARS-CoV-2 mRNA vaccination in 14 persons with HIV (PWH) demonstrated uniformly high anti-SARS-CoV-2 receptor binding domain (RBD) antibody titres after two doses, despite varied titres after a single dose. The majority of vaccine reactions were mild and no adverse events occurred.
Early studies indicate that PWH may appear to be at an increased risk of severe COVID-19 infection, potentially due to increased rates of multimorbidity [1–3]. Although the original SARS-CoV-2 mRNA vaccine trials found near-universal robust immune responses in the general population [4,5], certain immunocompromised populations appear to mount much lower antibody titres [6–10]. Antibody response to SARS-CoV-2 vaccination in PWH has not been reported, and furthermore, given lower antibody response in PWH to common viral vaccine targets such as hepatitis B [11], it is important to evaluate SARS-CoV-2 vaccine immunogenicity in PWH. After a single dose of a SARS-CoV-2 mRNA vaccine, we demonstrated that PWH showed detectable, yet variable antibody responses, including low titres among persons with CD4+ T cell counts less than 200 cells/μl [12]. We thus aimed to study boosted antibody response and safety of the two-dose SARS-CoV-2 mRNA vaccine in PWH.
PWH at least 18 years old were recruited to this prospective observational cohort from 7 December 2020 to 25 April 2021 via social media outreach to national HIV/AIDS organizations. As previously described [12], self-reported demographics, SARS-CoV-2 infection history, most recent HIV viral load (detectable/undetectable), most recent CD4 count (<200, 200–350, 350–499 or ≥500 cells/μl), presence/absence of current antiretroviral therapy (ART) and duration of ART treatment (<6 months or ≥6 months) were collected using the Research Electronic Data Capture (REDCap) tool, a secure, web-based software platform designed to support data capture for research studies [13].
Prior to dose 2 (titre 1, T1) and 1 month after D2 (titre 2, T2), participants underwent SARS-CoV-2 antibody testing on the semi-quantitative Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay, which measures total antibody (IgM, IgG) to the RBD, a critical target of neutralizing antibodies within the spike protein encoded by the mRNA vaccines [14]. Results ranged from < 0.4 to > 250 U/ml (upper reported assay limit) with a positive response defined by the manufacturer as ≥ 0.8 U/ml. One week after each dose, participants completed a reactogenicity questionnaire indicating local (pain, swelling or erythema) and systemic symptoms experienced (fatigue, headache, myalgia, chills, fever, diarrhoea or vomiting) on an ordinal scale (similar to reporting in original vaccine trials [15,16]): none, mild (does not interfere with activity), moderate (some interference with activity) or severe (prevents daily activity). Major adverse events were also assessed (i.e. incident anaphylaxis, neurologic diagnoses or infections including SARS-CoV-2). This study was approved by the Johns Hopkins Institutional Review Board (IRB00248540) and participants provided informed consented electronically.
We studied 14 PWH who reported receiving two doses (dose 1 [D1] and dose 2 [D2]) of a SARS-CoV-2 mRNA vaccine (Supplemental Table, http://links.lww.com/QAD/C238). The median (IQR) age was 62 (56, 70), 13 (93%) were male, 12 (86%) were white and none had a prevaccination history of COVID-19. At vaccination, all were on ART for at least 6 months and 13 (93%) had an undetectable HIV viral load. Two (14%) had CD4+ cell counts less than 200 cells/μl, whereas one (7%), three (21%) and eight (57%) had CD4+ cell counts of 200–349, 350–499, at least 500 cells/μl, respectively.
Five (36%) received the Pfizer/BioNTech BNT162b2 vaccine and nine (64%) received the Moderna mRNA-1273 vaccine. T1 samples were collected on 10 (71%) participants, whereas all 14 (100%) had T2 samples. Median (IQR) time between D1 and T1 was 21 (16, 27) days, and 29 (28, 32) days between D2 and T2. Median (IQR) T1 was 76 (5, 149) U/ml and all participants had a T2 > 250 U/ml apart from 239 U/ml in one participant with a CD4+ cell count less than 200 cells/μl (Fig. 1).
Fig. 1. Anti-SARS-CoV-2 receptor binding domain antibody titres of people with HIV on antiretroviral therapy who underwent two-dose SARS-CoV-2 mRNA vaccination.
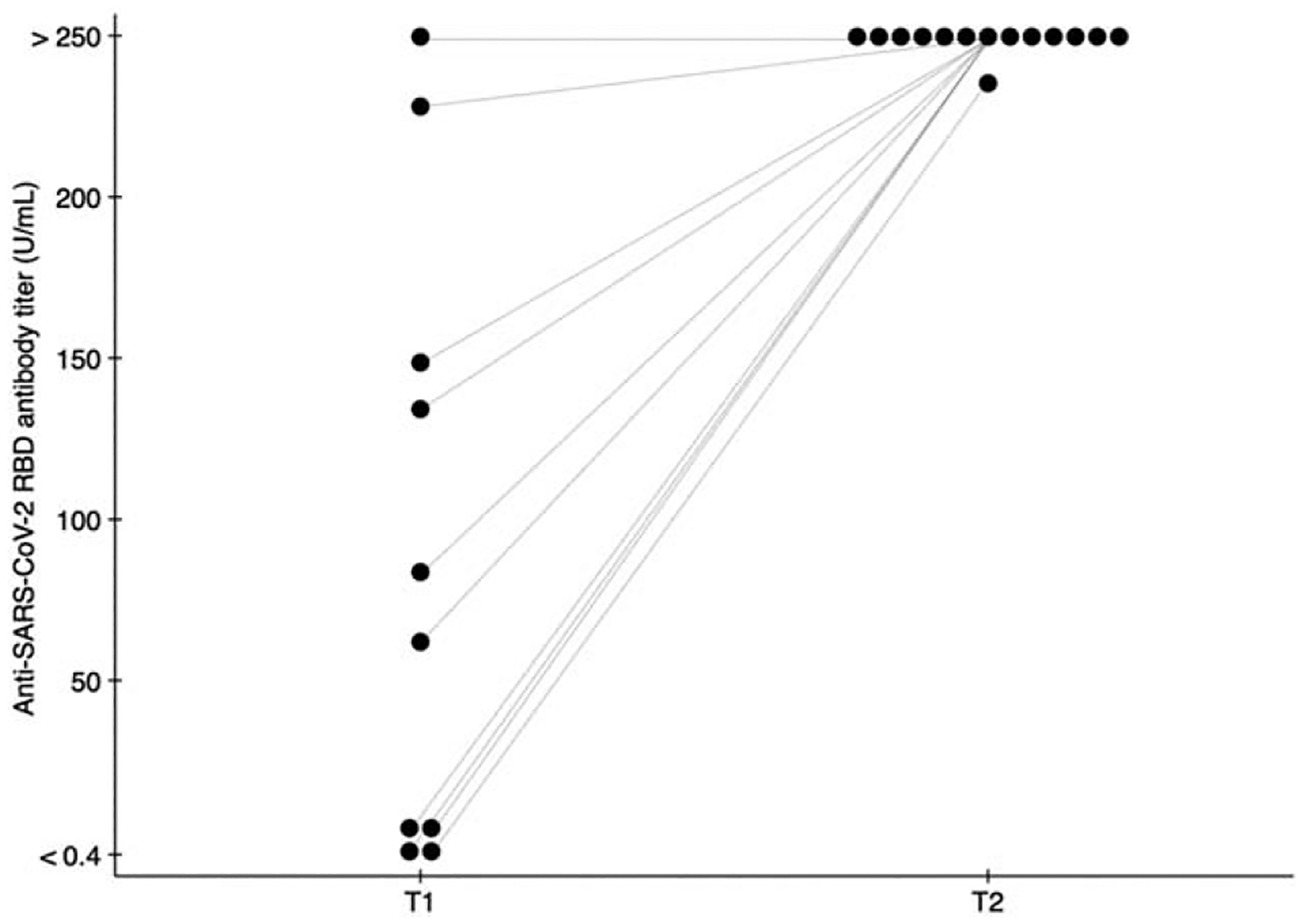
T1 (titre 1, n = 10) and T2 (titre 2, n = 14) denote anti SARS-CoV-2 RBD titres measured before and 1 month after the second dose of SARS-CoV-2 mRNA vaccination; lines indicate the change in an individual participant’s titre. Assay results could range from <0.4 to >250 U/ml.
The majority of local and systemic reactions were mild (Supplemental Figure, http://links.lww.com/QAD/C237). Local mild or moderate symptoms were reported by 12 (86%) after D1 and 13 (93%) after D2, most commonly pain (12, 86% after D1 and 13, 93% after D2). Systemic symptoms were reported by 10 (71%) after D1 and nine (64%) after D2, most commonly fatigue (six, 43% after D1 and eight, 57% after D2). One (7%) participant reported severe headache after D2. All local and systemic reactions were more common after D2 apart from local injection site erythema (Supplemental Figure, http://links.lww.com/QAD/C237). No participant experienced anaphylaxis or developed a new infection or a neurologic condition.
In this study of antibody response to two-dose SARS-CoV-2 mRNA vaccination in PWH with excellent virologic control on ART, all participants developed high titres of anti-RBD antibodies. Reactions were generally mild, increased after D2 and comparable to those seen in both the original trials [15,16] and in other immunocompromised populations [17,18]. Although titres after a single dose varied across a range of CD4+ cell counts, all participants had titres near or above the upper reported assay limit after two doses. Although no specific titre has been precisely correlated with protection from COVID-19 after vaccination, plasma antibody titres from 15 to 133 U/ml using the Roche Elecsys anti-RBD assay have been correlated with neutralizing serum activity in vitro [19,20]. In addition, the post-D2 titres observed in this study are comparable to those seen in immunocompetent, HIV-uninfected populations (i.e. median titres >250 U/ml after D2) [21,22]. These results contrast with antibody response after SARS-CoV-2 mRNA vaccination in other immunocompromised patients on lymphocyte-modulating agents (e.g. antimeta-bolites and rituximab), which have been shown to severely impair antibody production [6–9].
PWH with CD4+ cell counts less than 200 cells/μl have shown diminished SARS-CoV-2 antibody production after acute infection [23,24], as well as blunted immune responses to multiple vaccine types [11,25–27]. However, although two participants with CD4+ cell counts less than 200 cells/μl demonstrated low T1s (2 and 3 U/ml), both exhibited substantial boosting with a second dose (239 and >250 U/ml).
Limitations of this study include a small, nonrandomized sample, relatively homogeneous in sex and race, and representing PWH with excellent virologic control on ART. These initial results show encouraging immunogenicity and safety of the two-dose mRNA vaccine series, suggesting that all PWH with viral suppression, regardless of CD4+ cell count, may benefit from vaccination. Future studies should include serial antibody sampling as well as integration of cellular immune responses to further characterize the durability and breadth of immune response to COVID-19 vaccination in PWH.
Supplementary Material
Acknowledgements
We acknowledge the following individuals for their assistance with this study: Robin K. Avery, MD, Michael T. Ou, BS, Jennifer D. Motter, MHS, Ross S. Greenberg, BA, and Iulia Barber, BS. All authors contributed to the concept, design, conduct and reporting of the work described in the article.
Conflicts of interest
D.L.S. has the following financial disclosures: consulting and speaking honoraria from Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, Thermo Fisher Scientific. C.M.D. has the following financial disclosures: research grants from GlaxoSmithKline and Abbvie and served on a grant review committee for Gilead Sciences. The other authors have no conflicts of interest.
This research was made possible with generous support of the Ben-Dov family. This work was supported by grant number F32DK124941 (B.J.B.), and K23DK115908 (J.G.W.) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), K24AI144954 (D.L.S.) and U01AI134591 and U01AI138897 (C.M.D., D.L.S.) from the National Institute of Allergy and Infectious Diseases (NIAID), and by a grant from the Transplantation and Immunology Research Network of the American Society of Transplantation (W.A.W.). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
References
- 1.Mirzaei H, McFarland W, Karamouzian M, Sharifi H. COVID-19 among people living with HIV: a systematic review. AIDS Behav 2021; 25:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tesoriero JM, Swain CE, Pierce JL, Zamboni L, Wu M, Holtgrave DR, et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York State. JAMA Netw Open 2021; 4:e2037069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis 2021; 72:340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med 2021; 384:80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 2020; 383:2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruddy JA, Connolly CM, Boyarsky BJ, Werbel WA, Christopher-Stine L, Garonzik-Wang J, et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases (in press). Ann Rheum Dis 2021. doi: 10.1136/annrheumdis-2021-220656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyarsky BJ, Werbel WA, Avery RK, Tobian A, Massie AB, Segev DL, Garonzik-Wang JM. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021; 325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palich R, Veyri M, Marot S, Vozy A, Gligorov J, Maingon P, et al. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann Oncol 2021:S0923–7534(21) 01184–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, El-Qunni AA, et al. Glucocorticoids and B Cell depleting agents substantially impair immunogenicity of mRNA vaccines to SARS-CoV-2. Preprint. medRxiv 2021. doi: 10.1101/2021.04.05.21254656. [DOI] [Google Scholar]
- 10.Kennedy NA, Goodhand JR, Bewshea C, Nice R, Chee D, Lin S, et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut 2021; 70:865–875. [DOI] [PubMed] [Google Scholar]
- 11.Crum-Cianflone NF, Wallace MR. Vaccination in HIV-infected adults. AIDS Patient Care STDS 2014; 28:397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruddy JA, Boyarsky BJ, Werbel WA, Bailey JR, Karaba AH, Garonzik-Wang JM, et al. Safety and antibody response to the first dose of SARS-CoV-2 messenger RNA vaccine in persons with HIV. AIDS 2021. doi: 10.1097/QAD.0000000000002945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins V, Fabros A, Kulasingam V. Quantitative measurement of anti-SARS-CoV-2 antibodies: analytical and clinical evaluation. J Clin Microbiol 2021; 59:e03149–e3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connolly CM, Ruddy JA, Boyarsky BJ, Avery RK, Werbel WA, Segev DL, et al. Safety of the first dose of mRNA SARS-CoV-2 vaccines in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021:annrheumdis-2021–220231. [Google Scholar]
- 18.Ou MT, Boyarsky BJ, Motter JD, Greenberg RS, Teles AT, Ruddy JA, et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation 2021. doi: 10.1097/TP.0000000000003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Resman Rus K, Korva M, Knap N, AvšičŽupanc T, Poljak M. Performance of the rapid high-throughput automated electro-chemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J Clin Virol 2021; 139:104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubio-Acero R, Castelletti N, Fingerle V, Olbrich L, Bakuli A, Wölfel R, et al. In search of the SARS-CoV-2 protection correlate: head-to-head comparison of two quantitative S1 assays in precharacterized oligo-/asymptomatic patients. Infect Dis Ther 2021. doi: 10.1007/s40121-021-00475-x. [DOI] [PubMed] [Google Scholar]
- 21.Mueller T Antibodies against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) in individuals with and without COVID-19 vaccination: a method comparison of two different commercially available serological assays from the same manufacturer. Clin Chim Acta 2021; 518:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavalcanti E, Isgrò MA, Rea D, Di Capua L, Trillò G, Russo L, et al. Vaccination strategy and anti - SARS-CoV-2 S titers in healthcare workers of the INT - IRCCS ‘Fondazione Pascale’ Cancer Center (Naples, Italy). Infect Agent Cancer 2021; 16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mondi A, Cimini E, Colavita F, Cicalini S, Pinnetti C, Matusali G, et al. COVID-19 in people living with HIV: clinical implications of dynamics of the immune response to SARS-CoV-2. J Med Virol 2021; 93:1796–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M, Luo L, Bu H, Xia H. One case of coronavirus disease 2019 (COVID-19) in a patient co-infected by HIV with a low CD4+ T-cell count. Int J Infect Dis 2020; 96:148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vardinon N, Handsher R, Burke M, Zacut V, Yust I. Poliovirus vaccination responses in HIV-infected patients: correlation with T4 cell counts. J Infect Dis 1990; 162:238–241. [DOI] [PubMed] [Google Scholar]
- 26.Kojic EM, Kang M, Cespedes MS, Umbleja T, Godfrey C, Allen RT, et al. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin Infect Dis 2014; 59:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and, Prevention. Vaccination of adults with, HIV. https://www.cdc.gov/vaccines/adults/rec-vac/health-conditions/hiv.html. Published 2 May 2016. [Accessed 3 May 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


