Abstract
Background
Starting in 2018, national death certificates included a new racial classification system that accounts for multiple-race decedents and separates Native Hawaiian and Pacific Islander (NHPI) individuals from Asian individuals. We estimated cancer death rates across updated racial and ethnic categories, sex, and age.
Methods
Age-standardized US cancer mortality rates and rate ratios from 2018 to 2020 among individuals aged 20 years and older were estimated with national death certificate data by race and ethnicity, sex, age, and cancer site.
Results
In 2018, there were approximately 597 000 cancer deaths, 598 000 in 2019, and 601 000 in 2020. Among men, cancer death rates were highest in Black men (298.2 per 100 000; n = 105 632), followed by White (250.8; n = 736 319), American Indian/Alaska Native (AI/AN; 249.2; n = 3376), NHPI (205.6; n = 1080), Latino (177.2; n = 66 167), and Asian (147.9; n = 26 591) men. Among women, Black women had the highest cancer death rates (206.5 per 100 000; n = 104 437), followed by NHPI (192.1; n = 1141), AI/AN (189.9; n = 3239), White (183.0; n = 646 865), Latina (128.4; n = 61 579), and Asian (111.4; n = 26 396) women. The highest death rates by age group occurred among NHPI individuals aged 20-49 years and Black individuals aged 50-69 and 70 years and older. Asian individuals had the lowest cancer death rates across age groups. Compared with Asian individuals, total cancer death rates were 39% higher in NHPI men and 73% higher in NHPI women.
Conclusions
There were striking racial and ethnic disparities in cancer death rates during 2018-2020. Separating NHPI and Asian individuals revealed large differences in cancer mortality between 2 groups that were previously combined in vital statistics data.
In the United States, cancer is the second most common cause of death, accounting for approximately 602 400 deaths in 2020 (1). There are notable racial and ethnic disparities in cancer death rates, driven by differences in cancer incidence and survival, barriers to accessing health care, and other structural factors (2-4). During 2013-2017, cancer incidence rates were highest in White and Black individuals compared with Asian and Pacific Islanders (aggregated group), American Indian/Alaska Native (AI/AN), and Hispanic and Latino individuals (5). Additionally, Black and AI/AN cancer patients had lower 5-year cancer survival rates across most cancers compared with White cancer patients, highlighting racial and ethnic disparities in cancer outcomes (6). As a result, the highest cancer death rates were observed among Black individuals in the United States in 2019 (7). Although reports examine cancer mortality rates in the United States annually, more detailed analyses are needed to capture disparities by cancer site across and within racial and ethnic groups, sex, and age (2,8).
In 1997, the Office of Management and Budget updated federal race classifications to include Native Hawaiian and Pacific Islander (NHPI) individuals as a distinct racial group separate from Asian individuals and a separate category for “more than one race” (9). However, the National Center for Health Statistics (NCHS) did not transition to releasing single race mortality data until 2018 when all states had implemented the new classification on death certificates (10,11). NHPI individuals represent about 0.4% of the US population and have known health disparities that have been masked through the aggregation of their data with the larger Asian population (12-15). We provide a systematic assessment of cancer deaths across racial and ethnic groups using this updated classification system to provide an update on disparities in cancer death rates in the United States and to establish a baseline for future analyses examining trends over time.
Methods
All cancer deaths among those aged 20 years and older in the United States (excluding Puerto Rico or any of the US territories) during 2018-2020 were obtained from the NCHS death certificate data (11). Underlying causes of death were classified based on the International Classification of Disease 10th revision codes. The 2018-2020 population counts were ascertained from the US Census Bureau and grouped by year, self-identified racial and ethnic group, sex, and age group (20-49, 50-69, 70 years and older) (11). Race on death certificates is recorded by a funeral director with information provided by an informant, usually the closest living relative, or based on observation (11). The following mutually exclusive racial and ethnicity categories were used: Hispanic and Latino all races (ie, Latino/a), non-Hispanic AI/AN, non-Hispanic Asian, non-Hispanic Black or African American (ie, Black), non-Hispanic NHPI, non-Hispanic White, and non-Hispanic more than one race (ie, multiracial). Mortality rates for those who identified as multiracial were notably lower than all other groups and thus not presented, as these lower rates are likely due to an under ascertainment of this category on death certificates (16-18). When analyzing the AI/AN population, we restricted to Purchased/Referred Care Delivery Areas counties, where sensitivity of capturing AI/AN race was known to be higher (19,20).
Statistical analyses
Overall, age-specific, and cancer-specific age-standardized death rates and mortality rate ratios (RRs) were compared across racial and ethnic groups. White individuals served as the reference group for rate ratios, as they were the largest racial and ethnic group in the United States. Estimates were further stratified by sex and age group. Cancer rates in NHPI and Asian individuals were also directly compared, as these groups are often aggregated in cancer mortality data. All data analyses were conducted in SEER*stat software version 8.4.0 (21). We considered associations with a P value of less than .05 as statistically significant. Rates based on fewer than 10 deaths were suppressed. All statistical tests were 2-sided.
Results
In 2018, there were approximately 597 000 cancer deaths, 598 000 in 2019, and 601 000 in 2020 among those aged 20 years and older. Though number of cancer deaths increased each year, the rates declined after age standardization from 208.4 to 200.8 per 100 000 person-years.
Cancer mortality in the overall study population
Among men, cancer death rates were highest in Black men (298.2 per 100 000; n = 105 632), followed by White (250.8; n = 736 319), AI/AN (249.2; n = 3376), NHPI (205.6; n = 1080), Latino (177.2; n = 66 167), and Asian (147.9; n = 26 591) men (Table 1, Figure 1). Among women, cancer death rates were highest in Black women (206.5 per 100 000; n = 104 437), followed by NHPI (192.1; n = 1141), AI/AN (189.9; n = 3239), White (183.0; n = 646 865), Latina (128.4; n = 61 579), and Asian (111.4; n = 26 396) women (Table 1, Figure 1). NHPI individuals had the highest cancer death rates among those aged 20-49 years (43.7 per 100 000), whereas Black individuals had the highest cancer death rates among those aged 50-69 and 70 years and older (327.8 and 1070.8 per 100 000, respectively). Asian individuals had the lowest cancer death rates across every age group (15.9, 146.7, and 613.0 per 100 000, respectively). When combined, cancer death rates among Asian and NHPI populations largely reflect cancer death rates in Asian individuals and are not representative of NHPI individuals (Supplementary Table 1, available online).
Table 1.
Age adjusted malignant cancer death rate by race and ethnicity in the United States, aged 20 years or older from 2018 to 2020
| Cancer type | Overall | Men | Rate ratio (95% CI)a | Women | Rate ratio (95% CI)b | 20-49 years | 50-69 years | ≥70 years |
|---|---|---|---|---|---|---|---|---|
| American Indian/Alaska Native individuals | ||||||||
| All cancers | 214.9 | 249.2 | 0.99 (0.96 to 1.03) | 189.9 | 1.04 (1.00 to 1.08)c | 28.7 | 279.6 | 968.6 |
| Oral cavity and pharynx | 2.9 | 4.3 | 0.75 (0.57 to 0.97)c | 1.7 | 0.79 (0.53 to 1.15) | — | 5.6 | 8.4 |
| Esophagus | 5.1 | 8.1 | 0.77 (0.62 to 0.93)c | 2.6 | 1.26 (0.92 to 1.69) | — | 8.1 | 20.9 |
| Stomach | 7.8 | 10.7 | 2.68 (2.23 to 3.19)c | 5.6 | 2.78 (2.23 to 3.42)c | 2.1 | 9.8 | 30.8 |
| Colorectum | 23.5 | 27.9 | 1.30 (1.17 to 1.45)c | 19.8 | 1.30 (1.16 to 1.46)c | 4.9 | 30.7 | 97.0 |
| Liver | 17.6 | 24.8 | 2.10 (1.88 to 2.34)c | 12.0 | 2.33 (2.02 to 2.68)c | 1.0 | 32.2 | 67.3 |
| Pancreas | 14.4 | 16.1 | 0.88 (0.76 to 1.01) | 12.9 | 0.95 (0.83 to 1.09) | 1.4 | 20.4 | 63.7 |
| Lung | 45.2 | 50.1 | 0.84 (0.77 to 0.91)c | 41.5 | 0.94 (0.87 to 1.01) | 1.9 | 57.9 | 225.6 |
| Soft tissue including heart | 1.4 | 1.6 | 0.77 (0.47 to 1.19) | 1.3 | 0.87 (0.53 to 1.34) | 0.6 | 1.8 | 4.8 |
| Breast | 24.8 | — | — | 24.8 | 0.91 (0.82 to 1.00) | 6.9 | 31.3 | 97.1 |
| Cervix | 4.1 | — | — | 4.1 | 1.43 (1.10 to 1.82)c | 2.8 | 7.3 | — |
| Uterus | 6.5 | — | — | 6.5 | 1.00 (0.82 to 1.21) | 1.3 | 9.7 | 25.2 |
| Ovary | 8.3 | — | — | 8.3 | 0.92 (0.77 to 1.09) | 2.1 | 10.6 | 33.2 |
| Prostate | 25.4 | 25.4 | 1.02 (0.90 to 1.16) | — | — | — | 14.8 | 164.3 |
| Bladder | 3.3 | 4.7 | 0.43 (0.32 to 0.57)c | 2.2 | 0.74 (0.51 to 1.03) | — | 2.9 | 18.9 |
| Kidney | 9.2 | 13.2 | 1.79 (1.52 to 2.08)c | 5.9 | 1.92 (1.55 to 2.35)c | 1.1 | 12.7 | 40.3 |
| CNS | 3.5 | 3.8 | 0.45 (0.33 to 0.59)c | 3.2 | 0.59 (0.44 to 0.77)c | 1.4 | 5.0 | 10.2 |
| Non-Hodgkin lymphoma | 6.4 | 8.6 | 0.88 (0.71 to 1.07) | 4.8 | 0.85 (0.67 to 1.07) | 0.7 | 7.3 | 31.8 |
| Myeloma | 4.3 | 5.2 | 1.02 (0.78 to 1.30) | 3.6 | 1.18 (0.89 to 1.53) | — | 4.8 | 21.9 |
| Leukemia | 6.3 | 8.1 | 0.70 (0.57 to 0.84)c | 4.8 | 0.76 (0.60 to 0.95)c | 1.5 | 5.7 | 30.4 |
| Asian individuals | ||||||||
| All cancers | 126.8 | 147.9 | 0.59 (0.58 to 0.60)c | 111.4 | 0.61 (0.60 to 0.62)c | 15.9 | 146.7 | 613.0 |
| Oral cavity and pharynx | 2.8 | 4.3 | 0.74 (0.69 to 0.80)c | 1.6 | 0.75 (0.68 to 0.84)c | 0.6 | 4.3 | 10.3 |
| Esophagus | 2.1 | 3.5 | 0.33 (0.31 to 0.36)c | 1.0 | 0.45 (0.39 to 0.52)c | 0.2 | 2.8 | 9.4 |
| Stomach | 6.0 | 7.6 | 1.90 (1.79 to 2.01)c | 4.8 | 2.36 (2.21 to 2.51)c | 1.0 | 6.6 | 28.7 |
| Colorectum | 12.5 | 15.0 | 0.70 (0.67 to 0.73)c | 10.5 | 0.69 (0.66 to 0.72)c | 2.1 | 15.3 | 55.9 |
| Liver | 11.4 | 17.0 | 1.43 (1.38 to 1.49)c | 7.0 | 1.36 (1.29 to 1.44)c | 1.1 | 14.7 | 53.6 |
| Pancreas | 10.5 | 11.4 | 0.62 (0.59 to 0.65)c | 9.7 | 0.71 (0.68 to 0.75)c | 0.7 | 11.0 | 55.6 |
| Lung | 26.1 | 33.5 | 0.56 (0.55 to 0.58)c | 20.6 | 0.47 (0.45 to 0.48)c | 1.6 | 27.9 | 139.0 |
| Soft tissue including heart | 1.2 | 1.4 | 0.66 (0.58 to 0.74)c | 1.1 | 0.73 (0.64 to 0.83)c | 0.5 | 1.5 | 4.2 |
| Breast | 15.9 | — | — | 15.9 | 0.58 (0.56 to 0.60)c | 4.5 | 25.1 | 51.5 |
| Cervix | 2.1 | — | — | 2.1 | 0.73 (0.67 to 0.80)c | 0.8 | 3.5 | 5.3 |
| Uterus | 4.6 | — | — | 4.6 | 0.70 (0.66 to 0.75)c | 0.7 | 7.7 | 17.1 |
| Ovary | 6.0 | — | — | 6.0 | 0.66 (0.63 to 0.70)c | 1.2 | 10.7 | 19.0 |
| Prostate | 11.6 | 11.6 | 0.47 (0.44 to 0.49)c | — | — | — | 5.3 | 78.5 |
| Bladder | 2.3 | 3.9 | 0.36 (0.33 to 0.38)c | 1.2 | 0.39 (0.34 to 0.44)c | 0.1 | 1.3 | 15.0 |
| Kidney | 2.1 | 3.1 | 0.42 (0.39 to 0.46)c | 1.3 | 0.44 (0.39 to 0.49)c | 0.2 | 2.4 | 10.7 |
| CNS | 3.0 | 3.9 | 0.46 (0.43 to 0.50)c | 2.3 | 0.43 (0.39 to 0.47)c | 1.0 | 4.4 | 10.3 |
| Non-Hodgkin lymphoma | 4.9 | 6.4 | 0.66 (0.62 to 0.70)c | 3.7 | 0.67 (0.63 to 0.72)c | 0.4 | 4.7 | 26.7 |
| Myeloma | 2.0 | 2.6 | 0.49 (0.45 to 0.54)c | 1.6 | 0.54 (0.48 to 0.60)c | 0.1 | 1.9 | 11.7 |
| Leukemia | 4.4 | 5.9 | 0.50 (0.47 to 0.54)c | 3.3 | 0.52 (0.48 to 0.55)c | 0.7 | 4.1 | 22.3 |
| Black individuals | ||||||||
| All cancers | 241.5 | 298.2 | 1.19 (1.18 to 1.20)c | 206.5 | 1.13 (1.12 to 1.14)c | 30.2 | 327.8 | 1070.8 |
| Oral cavity and pharynx | 3.5 | 6.0 | 1.04 (1.00 to 1.09) | 1.7 | 0.80 (0.74 to 0.86)c | 0.5 | 6.4 | 11.7 |
| Esophagus | 4.0 | 6.5 | 0.61 (0.59 to 0.64)c | 2.1 | 1.02 (0.96 to 1.09) | 0.4 | 7.2 | 14.5 |
| Stomach | 6.9 | 9.8 | 2.46 (2.36 to 2.56)c | 4.9 | 2.40 (2.28 to 2.51)c | 1.0 | 8.3 | 31.8 |
| Colorectum | 24.1 | 30.8 | 1.44 (1.41 to 1.47)c | 19.4 | 1.28 (1.25 to 1.31)c | 4.1 | 33.3 | 100.5 |
| Liver | 11.4 | 17.9 | 1.51 (1.47 to 1.55)c | 6.6 | 1.28 (1.23 to 1.33)c | 1.0 | 21.9 | 39.7 |
| Pancreas | 19.3 | 21.8 | 1.19 (1.16 to 1.22)c | 17.4 | 1.28 (1.25 to 1.31)c | 1.5 | 27.3 | 87.7 |
| Lung | 50.2 | 68.7 | 1.15 (1.13 to 1.17)c | 37.6 | 0.85 (0.84 to 0.86)c | 2.5 | 70.5 | 235.7 |
| Soft tissue including heart | 2.1 | 2.1 | 1.02 (0.94 to 1.11) | 2.0 | 1.37 (1.28 to 1.47)c | 0.9 | 2.9 | 6.1 |
| Breast | 38.7 | — | — | 38.7 | 1.41 (1.39 to 1.44)c | 12.1 | 58.7 | 125.3 |
| Cervix | 4.7 | — | — | 4.7 | 1.64 (1.56 to 1.72)c | 2.4 | 7.0 | 10.8 |
| Uterus | 13.1 | — | — | 13.1 | 2.00 (1.95 to 2.06)c | 1.1 | 21.7 | 52.4 |
| Ovary | 8.0 | — | — | 8.0 | 0.88 (0.85 to 0.91)c | 1.1 | 12.9 | 30.5 |
| Prostate | 52.6 | 52.6 | 2.12 (2.08 to 2.16)c | — | — | 0.5 | 38.2 | 328.6 |
| Bladder | 4.7 | 7.3 | 0.66 (0.63 to 0.69)c | 3.1 | 1.02 (0.96 to 1.08) | 0.2 | 4.2 | 26.8 |
| Kidney | 4.6 | 7.1 | 0.96 (0.92 to 1.00)c | 2.9 | 0.94 (0.89 to 0.99)c | 0.7 | 6.1 | 20.1 |
| CNS | 3.8 | 4.7 | 0.55 (0.52 to 0.58)c | 3.1 | 0.57 (0.54 to 0.60)c | 1.2 | 5.6 | 12.4 |
| Non-Hodgkin lymphoma | 5.3 | 7.0 | 0.72 (0.69 to 0.75)c | 4.1 | 0.74 (0.70 to 0.77)c | 1.1 | 6.4 | 23.4 |
| Myeloma | 8.2 | 10.2 | 1.97 (1.89 to 2.05)c | 6.9 | 2.27 (2.18 to 2.36)c | 0.5 | 8.7 | 43.7 |
| Leukemia | 7.1 | 9.3 | 0.80 (0.77 to 0.83)c | 5.7 | 0.89 (0.86 to 0.93)c | 1.5 | 7.6 | 33.1 |
| Latino individuals | ||||||||
| All cancers | 148.5 | 177.2 | 0.71 (0.70 to 0.71)c | 128.4 | 0.70 (0.70 to 0.71)c | 18.9 | 172.6 | 715.0 |
| Oral cavity and pharynx | 2.0 | 3.2 | 0.56 (0.52 to 0.59)c | 1.1 | 0.52 (0.47 to 0.57)c | 0.3 | 2.8 | 9.1 |
| Esophagus | 2.7 | 4.9 | 0.46 (0.44 to 0.48)c | 0.9 | 0.43 (0.39 to 0.48)c | 0.3 | 3.7 | 11.9 |
| Stomach | 6.5 | 8.1 | 2.03 (1.95 to 2.12)c | 5.4 | 2.65 (2.53 to 2.77)c | 1.6 | 7.9 | 27.1 |
| Colorectum | 14.8 | 18.7 | 0.87 (0.85 to 0.90)c | 11.7 | 0.77 (0.75 to 0.79)c | 2.4 | 18.5 | 66.5 |
| Liver | 12.7 | 18.0 | 1.52 (1.48 to 1.56)c | 8.4 | 1.64 (1.58 to 1.70)c | 0.7 | 19.4 | 56.4 |
| Pancreas | 12.4 | 13.6 | 0.74 (0.72 to 0.76)c | 11.3 | 0.84 (0.81 to 0.86)c | 0.8 | 15.0 | 62.2 |
| Lung | 20.8 | 28.0 | 0.47 (0.46 to 0.48)c | 15.5 | 0.35 (0.34 to 0.36)c | 0.9 | 20.7 | 115.4 |
| Soft tissue including heart | 1.5 | 1.6 | 0.77 (0.71 to 0.84)c | 1.4 | 0.92 (0.85 to 1.00)c | 0.6 | 2.0 | 4.4 |
| Breast | 19.0 | — | — | 19.0 | 0.69 (0.68 to 0.71)c | 5.1 | 28.4 | 66.2 |
| Cervix | 3.4 | — | — | 3.4 | 1.19 (1.12 to 1.25)c | 2.0 | 4.8 | 7.2 |
| Uterus | 6.0 | — | — | 6.0 | 0.92 (0.88 to 0.96)c | 0.9 | 9.5 | 23.0 |
| Ovary | 6.7 | — | — | 6.7 | 0.74 (0.71 to 0.76)c | 1.2 | 10.5 | 24.7 |
| Prostate | 20.9 | 20.9 | 0.84 (0.82 to 0.87)c | — | — | 0.1 | 10.8 | 139.8 |
| Bladder | 3.2 | 5.3 | 0.48 (0.46 to 0.51)c | 1.7 | 0.57 (0.53 to 0.61)c | 0.1 | 2.1 | 20.0 |
| Kidney | 4.5 | 6.5 | 0.87 (0.84 to 0.91)c | 2.9 | 0.93 (0.87 to 0.98)c | 0.5 | 5.6 | 21.0 |
| CNS | 4.1 | 4.8 | 0.57 (0.54 to 0.59)c | 3.6 | 0.66 (0.62 to 0.69)c | 1.1 | 5.7 | 15.4 |
| Non-Hodgkin lymphoma | 6.1 | 7.8 | 0.81 (0.77 to 0.84)c | 4.8 | 0.86 (0.82 to 0.90)c | 0.6 | 5.7 | 33.0 |
| Myeloma | 3.6 | 4.3 | 0.84 (0.79 to 0.89)c | 3.0 | 0.98 (0.92 to 1.04) | 0.2 | 3.7 | 19.4 |
| Leukemia | 5.6 | 7.0 | 0.60 (0.58 to 0.63)c | 4.5 | 0.70 (0.67 to 0.74)c | 1.4 | 4.8 | 26.7 |
| Native Hawaiian and Pacific Islander individuals | ||||||||
| All cancers | 197.3 | 205.6 | 0.82 (0.77 to 0.87)c | 192.1 | 1.05 (0.99 to 1.11) | 43.7 | 288.4 | 743.1 |
| Oral cavity and pharynx | 3.7 | 4.7 | 0.82 (0.55 to 1.18) | 2.8 | 1.32 (0.74 to 2.15) | 1.7 | 7.4 | — |
| Esophagus | 2.8 | 4.5 | 0.42 (0.27 to 0.62)c | — | — | — | 4.9 | 9.6 |
| Stomach | 8.7 | 11.2 | 2.81 (2.09 to 3.70)c | 6.7 | 3.32 (2.34 to 4.55)c | 2.3 | 10.4 | 35.8 |
| Colorectum | 14.6 | 16.6 | 0.77 (0.62 to 0.95)c | 12.6 | 0.83 (0.65 to 1.04) | 5.4 | 24.4 | 38.7 |
| Liver | 15.1 | 21.6 | 1.83 (1.51 to 2.20)c | 9.0 | 1.76 (1.30 to 2.32)c | 4.8 | 24.7 | 45.1 |
| Pancreas | 12.2 | 11.8 | 0.64 (0.49 to 0.84)c | 12.5 | 0.92 (0.71 to 1.17) | 1.6 | 17.5 | 51.5 |
| Lung | 38.5 | 47.5 | 0.80 (0.69 to 0.91)c | 30.7 | 0.69 (0.59 to 0.81)c | 3.2 | 53.5 | 175.8 |
| Soft tissue including heart | 2.7 | 1.9 | 0.90 (0.46 to 1.61) | 3.3 | 2.23 (1.36 to 3.46)c | — | 5.4 | — |
| Breast | 33.2 | — | — | 33.2 | 1.21 (1.05 to 1.39)c | 8.2 | 61.0 | 95.8 |
| Cervix | 6.9 | — | — | 6.9 | 2.43 (1.75 to 3.30)c | 4.4 | 8.7 | 15.5 |
| Uterus | 19.5 | — | — | 19.5 | 2.99 (2.47 to 3.58)c | 9.4 | 34.1 | 38.0 |
| Ovary | 7.8 | — | — | 7.8 | 0.86 (0.63 to 1.16) | — | 13.3 | 22.0 |
| Prostate | 23.0 | 23.0 | 0.92 (0.74 to 1.13) | — | — | — | 16.4 | 143.6 |
| Bladder | 4.0 | 6.2 | 0.57 (0.36 to 0.84)c | 2.1 | 0.71 (0.36 to 1.26) | — | — | 24.0 |
| Kidney | 3.7 | 5.5 | 0.74 (0.49 to 1.08) | 2.1 | 0.68 (0.35 to 1.18) | — | 5.9 | 13.9 |
| CNS | 3.8 | 3.9 | 0.46 (0.27 to 0.72)c | 3.7 | 0.69 (0.43 to 1.04) | — | 5.3 | 12.5 |
| Non-Hodgkin lymphoma | 6.8 | 7.2 | 0.74 (0.52 to 1.04) | 6.4 | 1.15 (0.79 to 1.61) | 1.5 | 7.9 | 29.8 |
| Myeloma | 3.8 | 4.4 | 0.86 (0.52 to 1.32) | 3.4 | 1.12 (0.66 to 1.77) | — | 4.9 | 19.2 |
| Leukemia | 7.9 | 9.5 | 0.81 (0.59 to 1.09) | 6.5 | 1.02 (0.71 to 1.42) | 2.3 | 8.8 | 32.4 |
| White individuals | ||||||||
| All cancers | 212.3 | 250.8 | — | 183.0 | — | 23.5 | 255.7 | 1020.3 |
| Oral cavity and pharynx | 3.8 | 5.8 | — | 2.1 | — | 0.5 | 6.3 | 14.7 |
| Esophagus | 6.0 | 10.6 | — | 2.1 | — | 0.6 | 9.4 | 25.0 |
| Stomach | 2.9 | 4.0 | — | 2.0 | — | 0.5 | 3.6 | 12.9 |
| Colorectum | 18.1 | 21.4 | — | 15.2 | — | 3.2 | 22.4 | 80.2 |
| Liver | 8.2 | 11.8 | — | 5.1 | — | 0.6 | 13.2 | 34.3 |
| Pancreas | 15.8 | 18.4 | — | 13.6 | — | 1.2 | 20.1 | 76.7 |
| Lung | 51.1 | 59.7 | — | 44.3 | — | 2.4 | 66.6 | 251.3 |
| Soft tissue including heart | 1.8 | 2.1 | — | 1.5 | — | 0.6 | 2.2 | 6.5 |
| Breast | 27.4 | — | — | 27.4 | — | 6.5 | 37.2 | 106.7 |
| Cervix | 2.9 | — | — | 2.9 | — | 1.9 | 4.2 | 4.6 |
| Uterus | 6.5 | — | — | 6.5 | — | 0.7 | 9.4 | 28.4 |
| Ovary | 9.0 | — | — | 9.0 | — | 1.4 | 12.9 | 37.4 |
| Prostate | 24.8 | 24.8 | — | — | — | 0.2 | 12.8 | 165.8 |
| Bladder | 6.4 | 10.9 | — | 3.0 | — | 0.2 | 4.6 | 39.2 |
| Kidney | 5.1 | 7.4 | — | 3.1 | — | 0.5 | 6.3 | 24.2 |
| CNS | 6.9 | 8.5 | — | 5.4 | — | 2.2 | 10.4 | 22.0 |
| Non-Hodgkin lymphoma | 7.4 | 9.7 | — | 5.6 | — | 0.6 | 6.1 | 42.3 |
| Myeloma | 4.0 | 5.2 | — | 3.1 | — | 0.2 | 3.6 | 22.8 |
| Leukemia | 8.7 | 11.7 | — | 6.3 | — | 1.1 | 6.8 | 48.3 |
Rate ratios compare to White men. “—” signifies statistic not displayed because of fewer than 10 cases or not applicable. CI = confidence Interval; CNS = central nervous system.
Rate ratios compared to White women.
Denotes statistical significance (P < .05).
Figure 1.
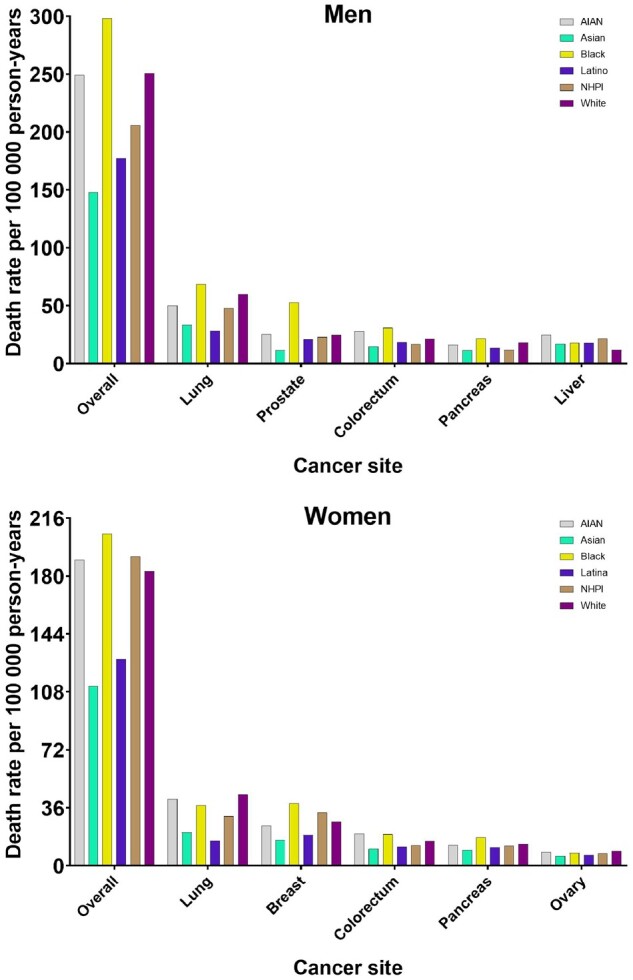
Cancer-specific death rates for overall cancer deaths and the 5 leading causes of cancer deaths by racial and ethnic groups, stratified by sex from 2018 to 2020, among those aged 20 years or older. Bars indicate the cancer death rates. Colors are consistent by racial and ethnic groups; however, scales differ. AI/AN = American Indian/Alaska Native; NHPI = Native Hawaiian and Pacific Islander.
Cancer mortality in AI/AN individuals
During 2018-2020, lung cancer caused the largest number of cancer deaths among AI/AN men overall and among those aged 50-69 and 70 years and older, whereas colorectal cancer was the leading cause of cancer death among AI/AN men aged 20-49 years (Table 1;Supplementary Table 2, available online). Compared with all other racial and ethnic groups, AI/AN men had the highest death rates for liver and kidney cancers (Table 1). Compared with White men, AI/AN men had statistically significantly higher death rates due to cancers of the colorectum, kidney, liver, and stomach (RR = 1.30-2.68; all P <.05) and statistically significantly lower death rates for leukemia and cancers of the bladder, central nervous system (CNS), oral cavity and pharynx, esophagus, and lung (RR = 0.43-0.84; all P <.05).
Among AI/AN women, lung cancer caused the largest number of cancer deaths overall and among those aged 50-69 and 70 years and older, whereas breast cancer caused the largest number of cancer deaths among AI/AN women aged 20-49 years (Table 1;Supplementary Table 2, available online). Compared with all other racial and ethnic groups, AI/AN women had the highest death rates for esophageal, colorectal, liver, and kidney cancers (Table 1). Death rates among AI/AN women were statistically significantly higher than White women for cancer of the colorectum, cervix, kidney, liver, and stomach (RR = 1.30-2.78; all P <.05) and were statistically significantly lower for leukemia (RR = 0.76, 95% confidence interval [CI] = 0.60 to 0.95) and cancers of the CNS (RR = 0.59, 95% CI = 0.44 to 0.77).
Cancer mortality in Asian individuals
During 2018-2020, lung cancer caused the largest number of cancer deaths among Asian men overall and among those aged 50-69 and 70 years and older, and colorectal cancer was the leading cause of cancer death among Asian men aged 20-49 years (Table 1;Supplementary Table 2, available online). Compared with White men, Asian men had lower death rates for nearly all cancers (RR = 0.33-0.74; all P <.05), except for liver (RR = 1.43, 95% CI = 1.38 to 1.49) and stomach cancer (RR = 1.90, 95% CI = 1.79 to 2.01).
Among Asian women, lung cancer caused the largest number of cancer deaths overall and among those aged 70 years and older (Table 1;Supplementary Table 2, available online), and breast cancer caused the largest number of cancer deaths among Asian women aged 20-49 and 50-69 years. Cancer death rates among Asian women were statistically significantly lower than White women for most cancers (RR = 0.39-0.75; all P < .05), with the exception of statistically significantly higher death rates for liver (RR = 1.36, 95% CI = 1.29 to 1.44) and stomach cancers (RR = 2.36, 95% CI = 2.21 to 2.51).
Cancer mortality in Black individuals
During 2018-2020, lung cancer caused the largest number of cancer deaths among Black men overall and among those aged 50-69 and 70 years and older, whereas colorectal cancer was the leading cause of death among Black men aged 20-49 years (Table 1;Supplementary Table 2, available online).
Compared with all other racial and ethnic groups, Black men had the highest death rates for multiple myeloma and cancers of the colorectum, pancreas, lung, prostate, oral cavity and pharynx, and soft tissue including heart. Compared with White men, Black men had statistically significantly elevated death rates because of multiple myeloma and cancers of the lung, pancreas, colorectum, liver, prostate, and stomach (RR = 1.15- 2.46; all P <.05) and statistically significantly lower death rates for leukemia, non-Hodgkin lymphoma (NHL), and cancers of the bladder, esophagus, kidney, and CNS (RR = 0.55-0.96; all P <.05) (Table 1).
Among Black women, breast cancer caused the largest number of cancer deaths overall and among those aged 20-49 and 50-69 years, and lung cancer was the leading cause of cancer death among Black women aged 70 years and older (Table 1;Supplementary Table 2, available online). Compared with all other racial and ethnic groups, Black women had the highest death rates for multiple myeloma and cancers of the pancreas, breast, and bladder (Table 1). Compared with White women, death rates were statistically significantly higher among Black women for multiple myeloma and cancers of the soft tissue including heart, pancreas, colorectum, liver, breast, cervix, uterus, and stomach (RR = 1.28-2.40; all P <.05) and were statistically significantly lower for deaths due to NHL, leukemia, and cancers of the CNS, lung, kidney, oral cavity and pharynx, and ovary (RR = 0.57-0.94; all P <.05).
Cancer mortality in Latino/a individuals
During 2018-2020, lung cancer caused the largest number of cancer deaths among Latino men overall and those aged 70 years and older (Table 1;Supplementary Table 2, available online). Colorectal cancer was the leading cause of cancer death among Latino men aged 20-49 years, and liver and intrahepatic bile duct cancer (ie, liver cancer) was the leading cause of cancer death among Latino men aged 50-69 years. Compared with White men, Latino men had lower death rates for nearly all cancers (RR = 0.46-0.87; all P <.05), except for liver (RR = 1.52, 95% CI = 1.48 to 1.56) and stomach cancers (RR = 2.03, 95% CI = 1.95 to 2.12).
Among Latina women, breast cancer caused the largest number of cancer deaths overall and among those aged 20-49 and 50-69 years (Table 1;Supplementary Table 2, available online), whereas lung cancer was the leading cause of death among those aged 70 years and older. Cancer death rates among Latina women were lower than White women for most cancers (RR = 0.35-0.93; all P <.05), except for liver (RR = 1.64, 95% CI = 1.58 to 1.70), stomach (RR = 2.65, 95% CI = 2.53 to 2.77), and cervical cancers (RR = 1.19, 95% CI = 1.12 to 1.25).
Cancer mortality in NHPI individuals
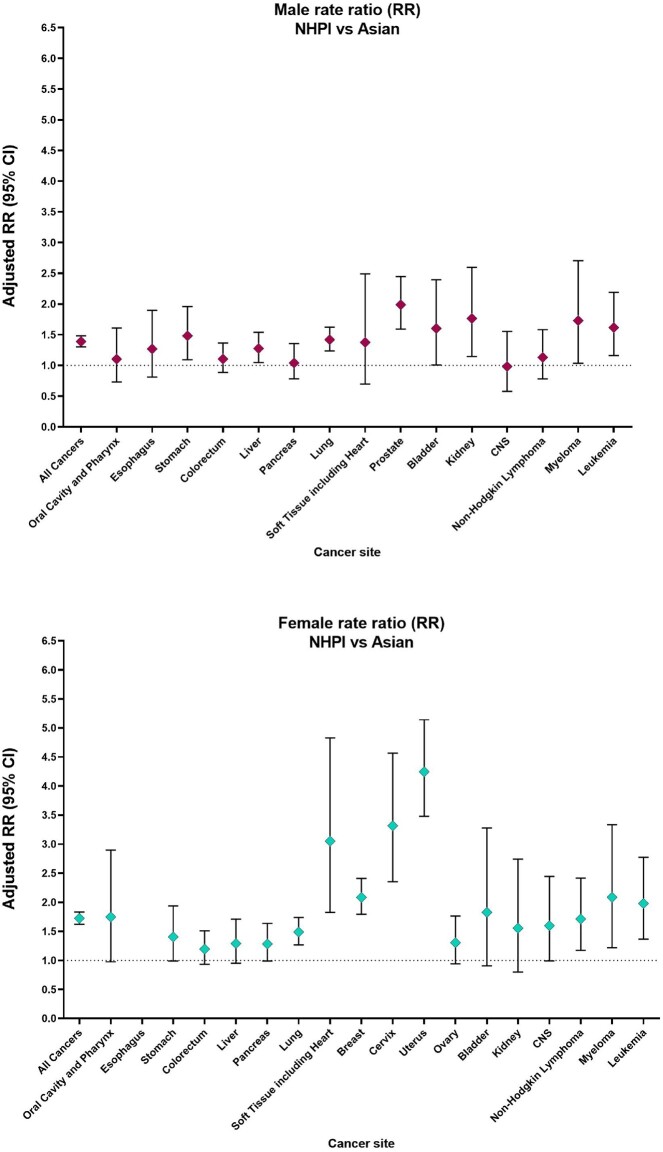
During 2018-2020, lung cancer caused the largest number of cancer deaths among NHPI men overall and among those aged 50-69 and 70 years and older, whereas liver cancer was the leading cause of cancer death among NHPI men aged 20-49 years (Table 1;Supplementary Table 2, available online). Compared with all other racial and ethnic groups, NHPI men had the highest death rates for stomach cancer (Table 1). Compared with White men, NHPI men had statistically significantly higher death rates due to cancers of the liver (RR = 1.83, 95% CI = 1.51 to 2.20) and stomach (RR = 2.81, 95% CI = 2.09 to 3.70) and statistically significantly lower death rates due to cancers of the esophagus, CNS, bladder, pancreas, colorectum, and lung (RR = 0.42-0.80; all P <.05). Relative to Asian men, NHPI men had statistically significantly higher death rates for leukemia, multiple myeloma, and immunoproliferative neoplasms and cancers of the stomach, liver, lung, prostate, bladder, and kidney (RR = 1.28-1.99; all P <.05) (Figure 2).
Figure 2.
Cancer-specific rate ratios for the NHPI population compared with the Asian population stratified by sex from 2018 to 2020, among those aged 20 years or older. The diamonds represent the rate ratio. The error bars represent the 95% confidence interval (CI). CNS = central nervous system; NHPI = Native Hawaiian and Pacific Islander; RR = rate ratio.
Among NHPI women, breast cancer caused the largest number of cancer deaths overall and among those aged 50-69 years (Table 1;Supplementary Table 2, available online). Uterine cancer and lung cancer caused the largest number of deaths among NHPI women aged 20-49 and 70 years and older, respectively. Compared with all other racial and ethnic groups, NHPI women had the highest death rates for NHL, leukemia, and cancers of the oral cavity and pharynx, stomach, soft tissue including heart, cervix, and uterus (Table 1). Compared with White women, cancer death rates among NHPI women were statistically significantly higher for cancers of the stomach, uterus, cervix, soft tissue including heart, liver, and breast (RR = 1.21-3.32; all P <.05) and lower for lung cancer (RR = 0.69, 95% CI = 0.59 to 0.81). Relative to Asian women, NHPI women had statistically significantly elevated death rates for leukemia, NHL, multiple myeloma, and cancers of the breast, cervix, uterus, soft tissue including heart, and lung (RR = 1.49-4.25; all P <.05) (Figure 2).
Cancer mortality in White individuals
During 2018-2020, lung cancer caused the largest number of cancer deaths among White men overall and among those aged 50-69 and 70 years and older, whereas colorectal cancer was the leading cause of cancer death among White men aged 20-49 years (Table 1;Supplementary Table 2, available online). Compared with all other racial and ethnic groups, White men had the highest death rates for NHL, leukemia, and cancers of the esophagus, soft tissue including heart, bladder, and CNS (Table 1).
Among White women, lung cancer caused the largest number of cancer deaths overall and among those aged 50-69 and 70 years and older, and breast cancer was the leading cause of cancer death among White women aged 20-49 years (Table 1;Supplementary Table 2, available online). Compared with all other racial and ethnic groups, White women had the highest death rates for cancers of the CNS, lung, and ovary (Table 1).
Discussion
Our study revealed that the historical aggregation of NHPI and Asian individuals in mortality statistics has masked substantial health disparities among NHPI individuals for decades. In 2018-2020, cancer death rates for NHPI men and women were higher than Asian men and women for many cancers. For example, NHPI women had uterine cancer death rates that exceeded all groups by 50% or more. Additionally, striking racial and ethnic disparities in cancer mortality persisted in the United States across major racial and ethnic groups, with Black men having more than twice the prostate cancer mortality rate of any other group. Cancer death rates for NHPI individuals were the highest of any racial and ethnic group among those aged 20-49 years.
The disaggregation of the NHPI and Asian populations reveals a stark difference in cancer mortality patterns, consistent with studies that have shown disparities in cancer survival (22). Compared with Asian or White individuals, NHPI individuals are known to have lower median household income, lower educational attainment, and a higher percentage of the population living in poverty (12,13,23,24). However, it is important to note that Asian and NHPI populations are each comprised of a diverse range of ethnic groups with vastly different health-care outcomes and socioeconomic status (25,26). We were not able to further disaggregate these populations using the current dataset, though this is an important area for future research.
Cancer death rates are influenced by cancer incidence and survival, which in turn are impacted by risk and protective behaviors, biology, access to care, and other structural factors (2-4). Racial and ethnic disparities can occur at multiple points along the cancer care continuum including differences in exposure to cancer risk factors and access to cancer prevention, early detection, time to treatment, and quality of treatment (3,4,27). Structural racism systematically disadvantages structurally marginalized populations and consequently is an underlying cause of racial and ethnic disparities in health (2,3). For example, the American Cancer Society’s guidelines for cancer prevention focus on modifiable lifestyle factors to lower cancer risk, including obesity and physical activity (28). Obesity rates are high among Black, Latino, AI/AN, and NHPI individuals, who are more likely to live in communities with high rates of economic insecurity, food deserts, and greater barriers to engaging in physical activity (12,29-40). Structurally marginalized populations, especially AI/AN and Latino individuals, have higher percentages of people with limited access to quality care and coverage (4,41-43). Unequal access to health insurance and care has likely influenced higher death rates because of many cancers among Black and NHPI individuals, including breast, colorectal, cervical, and prostate cancers, all screen-detectable cancers (44,45). Additionally, structurally marginalized populations are more likely to receive suboptimal cancer treatment that is inconsistent with recommended clinical practice guidelines and are less likely to be enrolled in clinical trials for new cancer treatments (46-49).
Although Asian and Latino men and women had lower overall cancer death rates than White men and women, liver and stomach cancer death rates were higher. Liver cancer death rates were also elevated in Black, NHPI, and AI/AN men and women. This is partially driven by chronic infection with hepatitis C virus and hepatitis B virus (HBV), which can cause liver cancer (50). Foreign-born Asian individuals from HBV-endemic countries have the highest seroprevalence of HBV, and Black and AI/AN individuals have a high prevalence of hepatitis C virus (50-54). Nonalcoholic fatty liver disease, heavy alcohol consumption, obesity, and diabetes-metabolic diseases also contribute to liver cancer risk (50). For stomach cancer, the prevalence of Helicobacter pylori infection, a known etiological risk factor, is particularly elevated among AI/AN individuals in the southwestern United States, foreign-born Latino individuals, and in Southeast and South Asian countries (55-62).
The main strength of our study is the inclusion of all cancer deaths in the United States during 2018-2020, allowing us to examine disparities in cancer death rates across many cancers. This study examines the updated single race classification recently implemented by NCHS, allowing for the estimation of cancer death rates separately for Asian and NHPI individuals and no longer bridging multiple race individuals into single racial and ethnic categories.
There are limitations to this study that warrant consideration. Demographic data on death certificates are subject to misclassification of race and ethnicity (63). Additionally, there is potential for misclassification of underlying cause of death, which could result from differential reporting of cause of death by race and ethnicity because of racial bias (64). Potential bias introduced by the assignment of race on death certificates by funeral directors is possible also. Further, we chose to compare all racial and ethnic groups with White individuals in the discussion of our results, because this is the largest racial and ethnic group, and we wanted to use a consistent reference group. These comparisons should not imply that cancer rates among White individuals are ideal; indeed, White individuals only have the lowest death rates for liver and stomach cancer deaths. We present age-standardized death rates in the tables so that readers can directly compare any of the groups presented. There are known health disparities within the 6 racial and ethnic groups presented here; however, we were unable to examine cancer death rates in more granular groups based on national heritage or nativity. Finally, it is likely that the COVID-19 pandemic impacted cancer death rates in 2020 due to COVID-19 as a competing cause of death (65).
We have shown the importance of disaggregating Asian and NHPI individuals, as these groups have disparate cancer mortality rates that are hidden when analyzed together (9). Additionally, we demonstrated that racial and ethnic disparities in cancer mortality persisted across racial and ethnic groups in recent years (2,8). Policies aimed at equitable cancer prevention, early detection, and treatment, as well as disaggregation of data for racial and ethnic subpopulations, are needed to address disparities in cancer mortality across racial and ethnic groups.
Supplementary Material
Acknowledgements
The funder did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; but did review the paper prior submission of the manuscript for publication.
The interpretation and reporting of these data are the sole responsibility of the authors.
Results in this manuscript have been presented in part as a poster presentation at the Society for Epidemiologic Research 2022 conference.
Contributor Information
Anika T Haque, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Amy Berrington de González, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA; The Institute of Cancer Research, London, England, UK.
Yingxi Chen, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Emily A Haozous, Southwest Center, Pacific Institute for Research and Evaluation, Albuquerque, NM, USA.
Maki Inoue-Choi, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Wayne R Lawrence, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Jennifer K McGee-Avila, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Anna M Nápoles, Division of Intramural Research, National Institute on Minority Health and Health Disparities, Bethesda, MD, USA.
Eliseo J Pérez-Stable, Division of Intramural Research, National Heart, Lung, and Blood Institute, Bethesda, MD, USA.
Kekoa Taparra, Department of Radiation Oncology, Stanford Cancer Institute, Stanford, CA, USA.
Jacqueline B Vo, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Neal D Freedman, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Meredith S Shiels, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Data availability
The data are publicly available in Centers for Disease Control and Prevention Wide-ranging Online Data for Epidemiologic Research, found at https://wonder.cdc.gov/ucd-icd10-expanded.html.
Author contributions
Anika Tasnim Haque, MPH (Formal analysis; Writing – original draft; Writing – review & Editing), Amy Berrington de González, DPhil (Writing – review & Editing), Yingxi Chen, MD, PhD (Writing—review & editing), Emily A. Haozous, PhD (Writing – review & Editing), Maki Inoue-Choi, PhD (Writing – review & Editing), Wayne R. Lawrence, DrPH (Writing – review & Editing), Jennifer K. McGee-Avila, PhD (Writing – review & Editing), Anna M. Nápoles, PhD (Writing – review & Editing), Eliseo J. Pérez-Stable, MD (Writing – review & Editing), Kekoa Taparra, MD, PhD (Writing – review & Editing), Jacqueline B. Vo, PhD (Writing – review & Editing), Neal D. Freedman, PhD (Writing – review & Editing), and Meredith S. Shiels, PhD (Conceptualization; Formal analysis; Methodology; Supervision; Writing – original draft; Writing – review & Editing).
Funding
This work was supported by the Intramural Research Program of the National Cancer Institute.
Additional funding was provided in part by the Stanford Cancer Institute, an NCI-designated Comprehensive Cancer Center. KT was funded by a Stanford Cancer Institute Women’s Cancer Center Innovation Award and the Stanford Cancer Institute Fellowship Award.
Conflicts of interest
The authors have no potential conflicts of interest to disclose.
References
- 1. Centers for Disease Control and Prevention [CDC]. An update on cancer deaths in the United States. 2022. https://www.cdc.gov/cancer/dcpc/research/update-on-cancer-deaths/index.htm. Accessed February 28, 2022.
- 2. Aizer AA, Wilhite TJ, Chen M-H, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120(10):1532-1539. [DOI] [PubMed] [Google Scholar]
- 3. Williams DR, Priest N, Anderson NB.. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35(4):407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee D-C, Liang H, Shi L.. The convergence of racial and income disparities in health insurance coverage in the United States. Int J Equity Health. 2021;20(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Islami F, Ward EM, Sung H, et al. Annual report to the nation on the status of cancer, part 1: national cancer statistics. J Natl Cancer Inst. 2021;113(12):1648-1669. doi: 10.1093/jnci/djab131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jemal A, Ward EM, Johnson CJ, et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9):djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lawrence WR, McGee-Avila JK, Vo JB, et al. Trends in cancer mortality among black individuals in the US from 1999 to 2019. JAMA Oncol. 2022;8(8):1184. doi: 10.1001/jamaoncol.2022.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. [DOI] [PubMed] [Google Scholar]
- 9. Taparra K, Pellegrin K.. Data aggregation hides Pacific Islander health disparities. Lancet 2022;400(10345):2-3. [DOI] [PubMed] [Google Scholar]
- 10. Heron M. Comparability of race-specific mortality data based on 1977 versus 1997 reporting standards. Natl Vital Stat Rep. 2021;70(3):1-31. [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention Wonder [CDC Wonder]. Underlying cause of death by single race 2018-2019. 2022. https://wonder.cdc.gov/wonder/help/ucd-expanded.html#. Accessed February 13, 2022.
- 12. US Department of Health and Human Services (US) Office of Minority Health. 2022. Profile: Native Hawaiians/Pacific Islanders. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=65. Accessed December 9, 2022.
- 13. Taparra K, Miller RC, Deville C.. Navigating Native Hawaiian and Pacific Islander cancer disparities from a cultural and historical perspective. J Clin Oncol Oncol Pract. 2021;17(3):130-134. [DOI] [PubMed] [Google Scholar]
- 14. Taparra K, Fukui J, Killeen J, et al. Racial and ethnic disparities in rates of invasive second breast cancer among women with ductal carcinoma in situ in Hawai'i. JAMA Netw Open. 2021;4(10):e2128977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taparra K, Dee EC, Dao D, et al. Disaggregation of Asian American and Pacific Islander women with stage 0-II breast cancer unmasks disparities in survival and surgery-to-radiation intervals: a national cancer database analysis from 2004 to 2017. J Clin Oncol Oncology Practice. 2022;18(8):e1255-e1264. doi: 10.1200/op.22.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arias E, Heron M, Hakes J.. The validity of race and Hispanic-origin reporting on death certificates in the United States: an update. National Center for Health Statistics. Vital Health Stat. 2016;2(172):1–21. [PubMed] [Google Scholar]
- 17. Arias E, Xu J, Curtin S, et al. Mortality profile of the non-Hispanic American Indian or Alaska Native population, 2019. Natl Vital Stat Rep. 2021;70(12):1-27. [PubMed] [Google Scholar]
- 18. Campbell ME, Troyer L.. The implications of racial misclassification by observers. Am Sociol Rev. 2007;72(5):750-765. [Google Scholar]
- 19. Espey DK, Jim MA, Richards TB, et al. Methods for improving the quality and completeness of mortality data for American Indians and Alaska Natives. Am J Public Health. 2014;104(suppl 3):S286-S294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson RN, Copeland G, Hayes JM.. Linkages to improve mortality data for American Indians and Alaska Natives: a new model for death reporting? Am J Public Health. 2014;104(S3):S258-S262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Surveillance Research Program. National Cancer Institute SEERStat software, version 8.4.0. 2022. https://seer.cancer.gov/seerstat. Accessed September 16, 2022.
- 22. Taparra K, Qu V, Pollom E.. Disparities in survival and comorbidity burden between Asian and Native Hawaiian and other Pacific Islander patients with cancer. JAMA Netw Open. 2022;5(8):e2226327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Healthcare Quality and Disparities Report Healthcare for Asians and Native Hawaiians/Pacific Islanders. Rockville, MD: Agency for Healthcare Research and Quality: AHRQ Pub. No. 20-0043; 2020.
- 24. Taparra K, Harding M, Deville C.. Healing and health equity for Asian American, Native Hawaiian, and Pacific Islander populations. JAMA. 2021;326(23):2432-2433. [DOI] [PubMed] [Google Scholar]
- 25. Gomez SL, Glaser SL, Horn-Ross PL, et al. Cancer research in Asian American, Native Hawaiian, and Pacific Islander populations: accelerating cancer knowledge by acknowledging and leveraging heterogeneity. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2202-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holland AT, Palaniappan LP.. Problems with the collection and interpretation of Asian-American health data: omission, aggregation, and extrapolation. Ann Epidemiol. 2012;22(6):397-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goding Sauer A, Siegel RL, Jemal A, et al. Current prevalence of major cancer risk factors and screening test use in the United States: disparities by education and race/ethnicity. Cancer Epidemiol Biomarkers Prev. 2019;28(4):629-642. [DOI] [PubMed] [Google Scholar]
- 28. Rock CL, Thomson C, Gansler T, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70(4):245-271. [DOI] [PubMed] [Google Scholar]
- 29. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States. NCHS Data Brief. 2017-2018;2020(360):1-8. [PubMed] [Google Scholar]
- 30. Coleman-Jensen A, Gregory C, Singh A.. Household Food Security in the United States in 2013. USDA-ERS, Economic Research Service. 2014;173. [Google Scholar]
- 31. US Department of Health and Human Services (US) Office of Minority Health. Obesity and American Indians/Alaska Natives. 2020. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=40. Accessed October 9, 2022.
- 32. Zhao G, Hsia J, Vigo-Valentín A, et al. Health-related behavioral risk factors and obesity among American Indians and Alaska Natives of the United States: assessing variations by Indian health service region. Prev Chron Dis. 2022;19:E05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jernigan VBB, Huyser KR, Valdes J, et al. Food insecurity among American Indians and Alaska Natives: a national profile using the current population survey–food security supplement. J Hunger Environ Nutr. 2017;12(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cobb N, Espey D, King J.. Health behaviors and risk factors among American Indians and Alaska Natives, 2000-2010. Am J Public Health. 2014;104(S3):S481-S489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moradi S, Mirzababaei A, Dadfarma A, et al. Food insecurity and adult weight abnormality risk: a systematic review and meta-analysis. Eur J Nutr. 2019;58(1):45-61. [DOI] [PubMed] [Google Scholar]
- 36. Petrelli F, Cortellini A, Indini A, et al. Association of obesity with survival outcomes in patients with cancer. JAMA Netw Open. 2021;4(3):e213520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suglia SF, Shelton RC, Hsiao A, et al. Why the neighborhood social environment is critical in obesity prevention. J Urban Health. 2016;93(1):206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thornton CM, Conway TL, Cain KL, et al. Disparities in pedestrian streetscape environments by income and race/ethnicity. SSM Popul Health. 2016;2:206-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. US Department of Health and Human Services (US) Office of Minority Health. Obesity and Native Hawaiians/Pacific Islanders. 2020. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=85. Accessed October 9, 2022.
- 40. Long CR, Rowland B, McElfish PA, et al. Food security status of Native Hawaiians and Pacific Islanders in the US: analysis of a national survey. J Nutr Educ Behav. 2020;52(8):788-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buchmueller TC, Levy HG.. The ACA’s impact on racial and ethnic disparities in health insurance coverage and access to care. Health Affairs. 2020;39(3):395-402. [DOI] [PubMed] [Google Scholar]
- 42. US Department of Health and Human Services. Issue Brief No. HP-2021-18 Health Insurance Coverage and Access to Care for American Indians and Alaska Natives: Current Trends and Key Challenges. 2021. https://aspe.hhs.gov/reports/health-insurance-coverage-changes-aian. Accessed December 13, 2022.
- 43. US Department of Health and Human Services (US) Office of Minority Health. Profile: American Indian/Alaska Native. 2022. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=62. Accessed December 13, 2022.
- 44. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2019: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2019;69(3):184-210. [DOI] [PubMed] [Google Scholar]
- 45. Jain B, Ng K, Santos PMG, et al. Prostate cancer disparities in risk group at presentation and access to treatment for Asian Americans, Native Hawaiians, and Pacific Islanders: a study with disaggregated ethnic groups. J Clin Oncol Oncol Pract. 2022;18(1):e204-e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gross CP, Smith BD, Wolf E, et al. Racial disparities in cancer therapy. Cancer. 2008;112(4):900-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen L, Li CI.. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24(11):1666-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ooi SL, Martinez ME, Li CI.. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat. 2011;127(3):729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Loree JM, Anand S, Dasari A, et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncology. 2019;5(10):e191870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Welzel TM, Graubard BI, Quraishi S, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108(8):1314-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kruszon-Moran D, Paulose-Ram R, Denniston M, et al. Viral hepatitis among non-Hispanic Asian adults in the United States, 2011-2014. NCHS Data Brief 2015;(225):1-8. [PubMed]
- 52. White DL, Thrift AP, Kanwal F, et al. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology. 2017;152(4):812-820.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. U.S. Department of Health and Human Services. Viral hepatitis national strategic plan: priority populations. 2021. https://www.hhs.gov/hepatitis/viral-hepatitis-national-strategic-plan/priority-populations/index.html. Accessed October 9, 2022.
- 54.Indian Health Service: The Federal Health Program for American Indians and Alaska Natives. 2022. Hepatitis C. https://www.ihs.gov/dccs/hcv/. Accessed October 9, 2022.
- 55. Melkonian SC, Pete D, Jim MA, et al. Gastric cancer among American Indian and Alaska Native populations in the United States, 2005-2016. Am J Gastroenterol. 2020;115(12):1989-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nolen LD, Bruden D, Miernyk K, et al. H. pylori-associated pathologic findings among Alaska Native patients. Int J Circumpolar Health. 2018;77(1):1510715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Harris RB, Brown HE, Begay RL, et al. Helicobacter pylori prevalence and risk factors in three rural indigenous communities of Northern Arizona. Int J Environ Res Public Health. 2022;19(2):797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zali H, Rezaei-Tavirani M, Azodi M.. Gastric cancer: prevention, risk factors and treatment. Gastroenterol Hepatol Bed Bench. 2011;4(4):175-185. [PMC free article] [PubMed] [Google Scholar]
- 59. Kharel S, Bist A, Shrestha S, et al. Helicobacter pylori healthy South Asians. JGH Open. 2020;4(6):1037-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Everhart E, Kruszon‐Moran D, Perez‐Perez GI, et al. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. 2000;181(4):1359-1363. [DOI] [PubMed] [Google Scholar]
- 61. Tsang SH, Avilés-Santa ML, Abnet CC, et al. Seroprevalence and determinants of helicobacter pylori infection in the Hispanic Community Health Study/Study of Latinos. Clin Gastroenterol Hepatol. 2022;20(3):e438-e451. doi: 10.1016/j.cgh.2021.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zaidi SF. Helicobacter pylori associated Asian enigma: does diet deserve distinction? World J Gastrointest Oncol. 2016;8(4):341-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Arias E, Heron M, Xu J.. United States Life Tables, 2012. Natl Vital Stat Rep. 2016;65(8):1-65. [PubMed] [Google Scholar]
- 64. Noymer A, Penner AM, Saperstein A.. Cause of death affects racial classification on death certificates. PLoS One. 2011;6(1):e15812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shiels MS, Haque AT, Haozous EA, et al. Racial and ethnic disparities in excess deaths during the COVID-19 pandemic, March to December 2020. Ann Intern Med. 2021;174(12):1693-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are publicly available in Centers for Disease Control and Prevention Wide-ranging Online Data for Epidemiologic Research, found at https://wonder.cdc.gov/ucd-icd10-expanded.html.