Abstract
Indolylarylsulfones (IASs) are classical HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) with a unique scaffold and possess potent antiviral activity. To address the high cytotoxicity and improve safety profiles of IASs, we introduced various sulfonamide groups linked by alkyl diamine chain to explore the entrance channel of non-nucleoside inhibitors binding pocket. 48 compounds were designed and synthesized to evaluate their anti-HIV-1 activities and reverse transcriptase inhibition activities. Especially, compound R10L4 was endowed with significant inhibitory activity towards wild-type HIV-1 (EC50(WT) = 0.007 μmol/L, SI = 30,930) as well as a panel of single-mutant strains exemplified by L100I (EC50 = 0.017 μmol/L, SI = 13,055), E138K (EC50 = 0.017 μmol/L, SI = 13,123) and Y181C (EC50 = 0.045 μmol/L, SI = 4753) which were superior to Nevirapine and Etravirine. Notably, R10L4 was characterized with significantly reduced cytotoxicity (CC50 = 216.51 μmol/L) and showed no remarkable in vivo toxic effects (acute and subacute toxicity). Moreover, the computer-based docking study was also employed to characterize the binding mode between R10L4 and HIV-1 RT. Additionally, R10L4 presented an acceptable pharmacokinetic profile. Collectively, these results deliver precious insights for next optimization and indicate that the sulfonamide IAS derivatives are promising NNRTIs for further development.
KEY WORDS: HIV-1, NNRTIs, Indolylarylsulfone, Sulfonamide, Cytotoxicity
Graphical abstract
R10L4 exhibited significant inhibitory activity towards wild-type HIV-1 and a panel of single-mutant strains. Notably, R10L4 was characterized with significantly reduced cytotoxicity and showed no remarkable in vivo toxic effects.
1. Introduction
Acquired immunodeficiency syndrome (AIDS) is a chronic infectious disease with serious threat to human health and global economy1,2. Human immunodeficiency virus type 1 (HIV-1), as the pathogen of AIDS, encodes reverse transcriptase (RT) as a key component in its life cycle to conduct the double-stranded DNA synthesis dependent on virus genome RNA3. Non-nucleoside reverse transcriptase inhibitors (NNRTIs) have been widely studied and developed inhibitors targeting RT, taking an important place in highly active anti-retroviral therapy (HAART)4,5. In order to control viral load on a long term, RT inhibitors typically require life-long administration. However, in the extensive clinical application of NNRTIs, severe long-term side effects and unfavorable pharmacokinetic properties reduced patient compliance and clinical effects6,7. Meanwhile, the rapid mutation of HIV and the wide use of current RT inhibitors has led to a variety of drug-resistant strains8. Mutation of certain residues around NNRTIs binding pocket (NNIBP) altered the size, rigidity and electoral property of the binding site, thus impaired ligand binding9,10. All these challenges are forcing the earliest generations of NNRTIs out from HIV clinical therapy4,11. Developing less toxic HIV-1 NNRTIs with high activity towards all drug-resistant strains continues to be an urgent need.
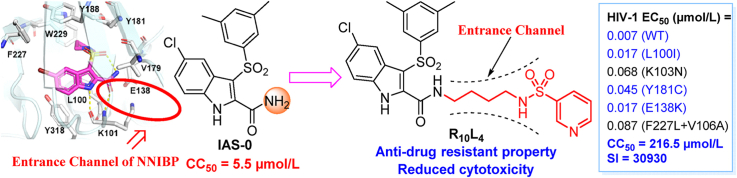
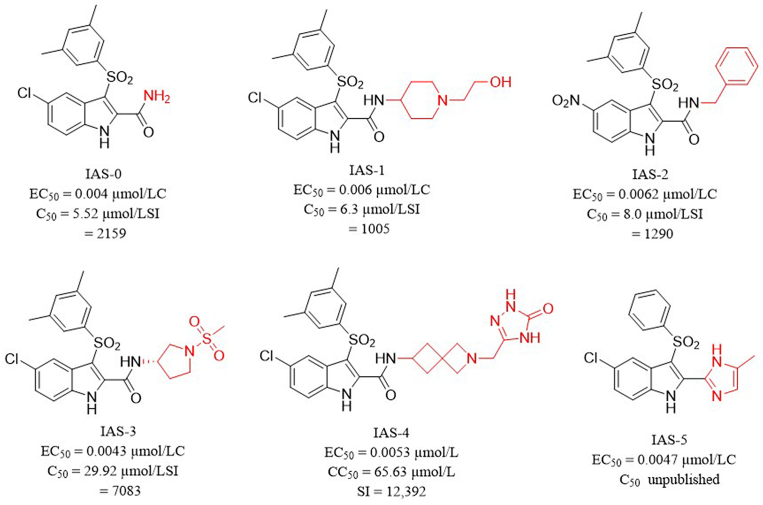
Indolylarylsulfones (IASs) are a class of HIV-1 NNRTIs with unique scaffold and promising antiviral activity12. Compound IAS-0 was proved to inhibit HIV-1 wild-type (WT) and several mutant strains at low-nanomolar EC50 values13. However, a series of issues including high cytotoxicity and poor solubility limited its further development into clinical trials. According to previous structure–activity relationship (SAR) study, the 3,5-dimethyl group on phenylsulfonyl moiety of IAS-0 improved its anti-drug resistant profile, but introduced severe cytotoxicity (CC50 IAS-0 = 5.5 μmol/L)12, which is undesirable for drugs to treat long-term viral infectious diseases. How to reduce the toxicity of IAS derivatives has thus been a challenge in further modifications, but has long been ignored. Among those most active IASs with rationally designed modifications (IAS 1–4), the decrease of toxicity was also not satisfying (Fig. 1)14, 15, 16, 17. Interestingly, our group found that sidearm substitutions containing sulfonamide groups on 2-carboxamide showed a slightly reduced cytotoxicity12. And to enhance water solubility, Merck company replaced the 2-carboxamide group with nitrogen-containing aromatic rings, but ended up with compound IAS-5 with no obvious improvements18.
Figure 1.
Structures, anti-HIV-1 activities and cytotoxicities of some representative IAS derivatives.
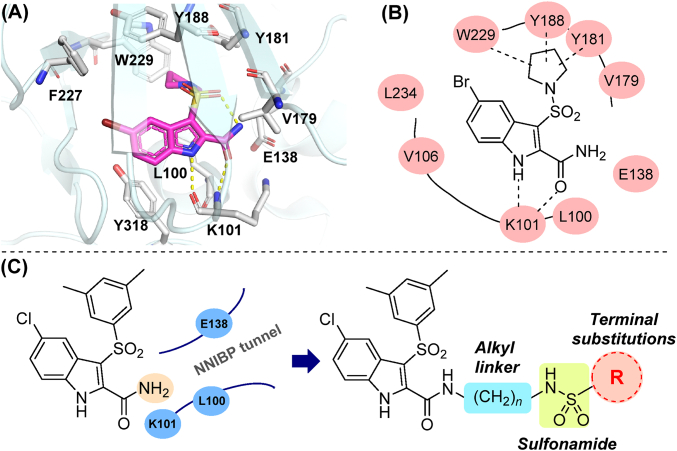
An in-depth study revealed the co-crystal structure of another derivative, IAS-6 and WT HIV-1 RT, demonstrating the “four-points” pharmacophore model distinguishing from most known NNRTIs (Fig. 2A and B)19. Key interactions from four moieties are observed: i) the pyrrolidinyl-1-sulfonyl group gains hydrophobic interactions with the surrounding residues, namely Y181, Y188, F227 and Y318; ii) imnio NH and carbamoyl oxygen attached to indole ring form dual hydrogen bonding with K101 backbone; iii) halogen-substituted phenyl ring of indole fitted into another hydrophobic sub-pocket formed by F227, Y318, etc.; iv) intramolecular H-bond observed between sulfonyl oxygen and carboxamide NH2, thus stabilizing the butterfly-like conformation of NNRTIs. These findings provided an important theoretical foundation for future designing and structural optimization for IAS. To be mentioned, the carboxamide group directs towards the unoccupied entrance of the binding position, a solvent-accessible hydrophilic area surrounded by L100, E138 and V179. This region, referred as the tolerant region II, showed good accommodation for rigid aromatic rings in previous studies20. Thus, the quests on modified IAS compounds have been focused on introducing extended groups on 2-carboxamide site of indole scaffold14, 15, 16, 17. A sidearm with multiple hydrogen bond donors/acceptors could occupy this area to increase ligand affinity and water solubility.
Figure 2.
Binding mode and design strategy of IAS analogs. (A) 3D view of interaction and surrounding residues of IAS-6 and HIV-1 RT (PDB ID: 2RF2). (B) 2D abstract diagram of general binding pockets and regions of IAS derivatives. (C) Design strategy and general formula of target compounds in this work.
Sulfonamides are frequently-used privileged substituents in drug research and development21,22. A large number of structurally novel sulfonamide derivatives have recently been reported to show substantial antiviral activity, as exemplified by the clinically used HIV protease inhibitors, many of the new types of NNRTIs, integrase inhibitors, viral capsid protein allosteric modulators, viral zinc finger protein binders or HIV entry inhibitors23, 24, 25, 26, 27, 28, 29. Sulfonamide motif offers multiple H-bond donors and acceptors, thus enabling additional interactions of ligands with residues around the binding pocket. Moreover, the presence of sulfonamide in small molecules may adjust acidity, increase solubility, improve oral bioavailability and reduce hERG toxicity, due to its polar nature30,31, proving indeed that this versatile moiety may be of great help for modern drug design.
Consequently, with the aim to additionally occupy the second tolerant region of NNIBP, aryl/aliphatic sulfonamide groups were introduced to occupy the entrance region in this work. Terminal substituents were joint with 2-caboxamide by a linear alkyl diamine chain from C2 to C5. Sulfonamides are expected to build up additional interactions with surrounding residues, contributing to improved antiviral activity and reduced cytotoxicity. Highly flexible (CH2)n linker with different length endows the molecules with better adaption to NNIBP tunnel (Fig. 2C). A total of 14 terminal groups and four kinds of linkers were combined to find out the optimal length and structure of this newly introduced pharmacophore. Herein, we depicted the synthesis, antiviral activity evaluation and structure–activity relationships (SARs) discussion of these newly designed compounds. Additionally, the representative candidate, R10L4 (see below), was further evaluated for its in vivo pharmacokinetic profile and toxicity in animal models.
2. Results and discussion
2.1. Chemistry
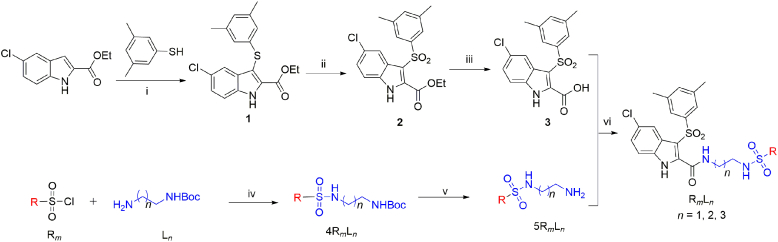
Synthetic routes and protocols of IAS analogues in this study are showed in Scheme 1. In brief, the intermediate 1 was synthesized smoothly starting with the marketable ethyl-5-chloro-1H-indole-2-carboxylate and 3,5-dimethylbenzenethiol according to the developed procedures. The afforded intermediate 2 was treated with m-chloroperoxybenzoic acid (m-CPBA), following the ester hydrolysis under LiOH to give the key intermediate 3. On the other hand, the sulfonyl chlorides (Rm) containing different substituents reacted with diamines (Ln) with different carbon chain lengths protected by a single Boc to prepare the intermediates 4RmLn with different structures. Subsequently, the Boc-NH groups were deprotected by trifluoroacetic acid in dichloromethane to obtain the various intermediates 5RmLn. Finally, the different substituted amines 5RmLn were condensed with the intermediate 3 to synthesize the corresponding target products.
Scheme 1.
Reagents and conditions: (i) 1-(chloromethyl)-4-fluoro-1,4-diazabicyclo [2.2.2]octane-1,4-diium tetrafluoroborate (Selectflour™), acetonitrile (CH3CN), r.t.; (ii) m-CPBA, dichloromethane (DCM), 0 °C; (iii) lithium hydroxide, H2O, tetrahydrofuran (THF), r.t.; (iv) Et3N, DCM, 0 °C–r.t.; (v) CF3COOH, DCM, r.t.; (vi) 1H-1,2,3-triazolo [4,5-b] pyridinium-1-[bis(dimethylamino)-methylene]-3-oxide hexafluorophosphate (HATU), N,N-diisopropylethylamine (DIEA), DCM, 0 °C to r.t.
2.2. Anti-HIV activity evaluation
Anti-HIV activity (WT) of the newly synthesized IAS derivatives were evaluated in MT-4 cells. Three drugs: Nevirapine (NVP), Efavirenz (EFV), Etravirine (ETR) and the lead compound IAS-0 were chosen as positive controls. Additionally, several common clinical single mutant strains (L100I, K103N, Y181C, Y188L) as well as double-mutant strains RES056 (K103N/Y181C) and F227L/V106A were assessed. Table 1, Table 2 displayed the measured properties, including EC50, CC50 and calculated SI (selectivity index, CC50/EC50 ratio) values. Notably, target compounds synthesized in this study showed remarkable potencies towards WT HIV-1 with low micromolar to nanomolar EC50 values, meanwhile having low cytotoxicity (Table 1). Among them, compounds R10L4 (EC50 = 0.0070 μmol/L, SI = 30,930) and R13L2 (EC50 = 0.0041 μmol/L, SI = 41,951) were proved to be the two most potent inhibitors against HIV-1 (IIIB), showing 18- and 32-fold improvement than that of NVP (EC50 = 0.13 μmol/L), respectively. Moreover, both compounds maintained similar potency as EFV (EC50 = 0.0038 μmol/L), ETR (EC50 = 0.0051 μmol/L) and IAS-0 (EC50 = 0.0041 μmol/L). It is worth emphasizing that the cytotoxicity of R10L4 (CC50 = 216.51 μmol/L) and R13L2 (CC50 = 172.00 μmol/L) are significantly decreased compared to that of IAS-0 (CC50 = 5.52 μmol/L), which leads to higher selectivity index, nearly 14 times higher than that of IAS-0. This indicates that the newly introduced terminal sulfonamide groups could significantly reduce the cytotoxicity, which is consistent with our design concept. Moreover, compounds R9L4, R7L4 and R11L4 were also endowed with prominent anti-HIV-1 potencies (EC50 = 0.0075, 0.0086 and 0.0091 μmol/L, respectively) and low cytotoxicity (CC50 = 113.34, 89.33 and 189.54 μmol/L, respectively).
Table 1.
Antiviral activity of target compounds against WT HIV-1 strain.
| Compd. |
n | R | IIIB |
||
|---|---|---|---|---|---|
| EC50 (μmol/L) | CC50 (μmol/L) | SI | |||
| R1L2 | 1 | 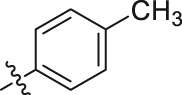 |
0.045 ± 0.007 | 167.86 ± 17.24 | 3787 |
| R1L3 | 2 | 0.036 ± 0.012 | 168.30 ± 23.54 | 4586 | |
| R1L4 | 3 | 0.032 ± 0.012 | 103.03 ± 59.77 | 3232 | |
| R1L5 | 4 | 0.027 ± 0.005 | 113.20 ± 3.74 | 4159 | |
| R2L2 | 1 | 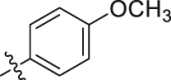 |
0.085 ± 0.043 | 105.8 ± 23.02 | 1233 |
| R2L3 | 2 | 0.037 ± 0.0085 | 162.9 ± 15.3 | 4354 | |
| R2L4 | 3 | 0.036 ± 0.005 | 22.48 ± 9.14 | 615 | |
| R2L5 | 4 | 0.023 ± 0.0081 | 109.8 ± 7.89 | 4772 | |
| R3L2 | 1 | 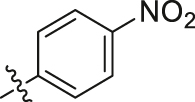 |
0.12 ± 0.029 | >211.84 | >1764 |
| R3L3 | 2 | 0.050 ± 0.023 | 22.99 ± 0.60 | 464 | |
| R3L4 | 3 | 0.028 ± 0.0049 | 104.1 ± 31.44 | 3809 | |
| R3L5 | 4 | 0.027 ± 0.0079 | >197.7 | >7548 | |
| R4L2 | 1 | 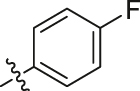 |
0.028 ± 0.0036 | >222.0 | >7921 |
| R4L3 | 2 | 0.038 ± 0.0069 | 34.99 ± 6.97 | 901 | |
| R4L4 | 3 | 0.020 ± 0.0085 | >211.47 | >10,593 | |
| R4L5 | 4 | 0.020 ± 0.0050 | 143.8 ± 13.20 | 7527 | |
| R5L2 | 1 |  |
0.065 ± 0.019 | 23.61 ± 0.58 | 362 |
| R5L3 | 2 | 0.031 ± 0.0034 | 22.56 ± 0.89 | 732 | |
| R5L4 | 3 | 0.017 ± 0.010 | 4.58 ± 3.53 | 279 | |
| R5L5 | 4 | 0.033 ± 0.0082 | >204.20 | >6158 | |
| R6L2 | 1 |  |
0.16 ± 0.027 | 150.98 ± 19.26 | 964 |
| R6L3 | 2 | 0.14 ± 0.013 | 88.08 ± 18.10 | 645 | |
| R6L4 | 3 | 0.077 ± 0.031 | 26.91 ± 16.87 | 347 | |
| R6L5 | 4 | 0.075 ± 0.030 | 37.86 ± 6.32 | 501 | |
| R7L2 | 1 |  |
0.013 ± 0.0048 | 174.80 ± 42.78 | 13,010 |
| R7L3 | 2 | 0.017 ± 0.0067 | >210.77 | >12,913 | |
| R7L4 | 3 | 0.0086 ± 0.00082 | 89.33 ± 17.84 | 10,389 | |
| R7L5 | 4 | 0.010 ± 0.0014 | 114.72 ± 8.89 | 11,132 | |
| R8L2 | 1 |  |
0.10 ± 0.027 | 161.35 ± 50.34 | 1558 |
| R8L3 | 2 | 0.057 ± 0.012 | 120.77 ± 33.45 | 2105 | |
| R8L4 | 3 | 0.045 ± 0.017 | 86.52 ± 11.78 | 1953 | |
| R8L5 | 4 | 0.067 ± 0.027 | 35.37 ± 1.05 | 526 | |
| R9L2 | 1 |  |
0.014 ± 0.0011 | 185.06 ± 8.31 | 13,509 |
| R9L3 | 2 | 0.029 ± 0.0036 | >223.57 | >8047 | |
| R9L4 | 3 | 0.0075 ± 0.0010 | 113.34 ± 26.85 | 15,106 | |
| R9L5 | 4 | 0.015 ± 0.0077 | 128.61 ± 6.85 | 8814 | |
| R10L2 | 1 |  |
0.0073 ± 0.0016 | 23.44 ± 0.75 | 3205 |
| R10L3 | 2 | 0.0080 ± 0.00089 | 55.06 ± 8.07 | 6950 | |
| R10L4 | 3 | 0.0070 ± 0.0021 | 216.51 ± 0.52 | 30,930 | |
| R10L5 | 4 | 0.0076 ± 0.0027 | 41.90 ± 2.18 | 5513 | |
| R11L2 | 1 |  |
0.018 ± 0.0036 | 198.73 ± 8.29 | 11,116 |
| R11L3 | 2 | 0.015 ± 0.0017 | 191.69 ± 4.99 | 12,570 | |
| R11L4 | 3 | 0.0091 ± 0.0025 | 189.54 ± 1.32 | 20,621 | |
| R12L2 | 1 |  |
0.036 ± 0.0036 | 186.90 ± 4.81 | 5151 |
| R12L3 | 2 | 0.035 ± 0.012 | 204.34 ± 10.80 | 5799 | |
| R12L4 | 3 | 0.019 ± 0.0051 | 185.57 ± 12.54 | 10,126 | |
| R13L2 | 1 | 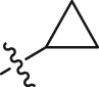 |
0.0041 ± 0.00079 | 172.00 ± 6.91 | 41,951 |
| R14L2 | 1 | 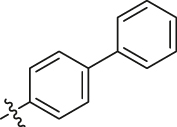 |
0.21 ± 0.048 | 64.35 ± 11.21 | 317 |
| NVP | – | 0.13 ± 0.041 | >15.02 | >114 | |
| EFV | – | 0.0038 ± 0.00063 | >6.34 | >1622 | |
| ETR | – | 0.0051 ± 0.0092 | >4.59 | >900 | |
| IAS-4 | – | 0.0053 ± 0.0014 | 65.63 ± 18.35 | 12,392 | |
| IAS-0 | – | 0.0041 ± 0.0014 | 5.52 ± 0.77 | 2159 | |
Table 2.
Antiviral activity of the target compounds against HIV-1 mutant strains.
| Compd. | EC50 (μmol/L)a |
||||||
|---|---|---|---|---|---|---|---|
| L100I | K103N | Y181C | Y188L | E138K | F227L + V106A | RES056 | |
| R1L2 | 0.68 ± 0.39 | 0.41 ± 0.11 | 0.61 ± 0.13 | 6.42 ± 1.40 | 0.86 ± 0.36 | 1.75 ± 0.20 | 3.38 ± 0.89 |
| R1L3 | 0.54 ± 0.42 | 0.73 ± 0.14 | 1.19 ± 0.3 | 7.14 ± 1.4 | 0.21 ± 0.14 | 1.26 ± 0.38 | 5.97 ± 0.65 |
| R1L4 | 0.16 ± 0.085 | 0.31 ± 0.068 | 0.31 ± 0.068 | 1.52 ± 0.27 | 0.13 ± 0.085 | 0.31 ± 0.034 | >103.03 |
| R1L5 | 0.17 ± 0.017 | 0.63 ± 0.017 | 0.23 ± 0.017 | 2.53 ± 0.18 | 0.18 ± 0.017 | 0.38 ± 0.13 | >113.2 |
| R2L2 | 0.66 ± 0.23 | 0.9 ± 0.38 | 0.85 ± 0.035 | >105.8 | 1.11 ± 0.17 | 1.58 ± 0.45 | >105.8 |
| R2L3 | 0.32 ± 0.00 | 0.66 ± 0.085 | 0.83 ± 0.068 | 5.98 ± 0.31 | 0.25 ± 0.00 | 0.97 ± 0.051 | >162.9 |
| R2L4 | 0.18 ± 0.00 | 0.33 ± 0.033 | 0.33 ± 0.00 | 0.80 ± 0.083 | 0.25 ± 0.05 | 0.28 ± 0.033 | >22.48 |
| R2L5 | 0.094 ± 0.047 | 0.57 ± 0.00 | 0.19 ± 0.016 | 1.28 ± 0.44 | 0.13 ± 0.049 | 0.28 ± 0.065 | >109.8 |
| R3L2 | 0.95 ± 0.085 | 0.59 ± 0.10 | 1.00 ± 0.17 | >211.84 | 1.15 ± 0.00 | 6.17 ± 1.14 | >211.84 |
| R3L3 | 0.35 ± 0.017 | 0.73 ± 0.12 | 1.03 ± 0.050 | 3.29 ± 0.066 | 0.31 ± 0.050 | 1.77 ± 0.033 | 4.92 ± 1.27 |
| R3L4 | 0.13 ± 0.018 | 0.50 ± 0.16 | 0.40 ± 0.11 | 0.87 ± 0.065 | 0.23 ± 0.032 | 0.36 ± 0.032 | 1.88 ± 0.68 |
| R3L5 | 0.13 ± 0.0063 | 0.9 ± 0.079 | 0.30 ± 0.00 | 2.47 ± 0.16 | 0.25 ± 0.032 | 0.54 ± 0.14 | 25.14 ± 0.047 |
| R4L2 | 0.21 ± 0.036 | 0.36 ± 0.16 | 0.37 ± 0.14 | 3.00 ± 0.053 | 0.37 ± 0.11 | 0.83 ± 0.12 | 3.23 ± 0.018 |
| R4L3 | 0.24 ± 0.035 | 0.45 ± 0.10 | 0.69 ± 0.017 | 2.84 ± 0.052 | 0.26 ± 0.017 | 1.04 ± 0.035 | 3.55 ± 0.35 |
| R4L4 | 0.056 ± 0.036 | 0.19 ± 0.051 | 0.20 ± 0.034 | 1.27 ± 0.20 | 0.063 ± 0.003 | 0.29 ± 0.12 | >211.47 |
| R4L5 | 0.074 ± 0.010 | 0.51 ± 0.21 | 0.18 ± 0.017 | 1.67 ± 0.35 | 0.073 ± 0.02 | 0.38 ± 0.017 | >31.17 |
| R5L2 | 0.77 ± 0.035 | 0.84 ± 0.035 | 0.89 ± 0.018 | 3.07 ± 0.12 | 1.26 ± 0.00 | 1.75 ± 0.053 | 3.37 ± 0.21 |
| R5L3 | 0.33 ± 0.034 | 0.92 ± 0.41 | 0.98 ± 0.051 | 3.34 ± 0.017 | 0.27 ± 0.017 | 1.22 ± 0.051 | 4.09 ± 0.00 |
| R5L4 | 0.025 ± 0.002 | 0.067 ± 0.005 | 0.054 ± 0.002 | 0.15 ± 0.010 | 0.032 ± 0.018 | 0.082 ± 0.035 | >4.58 |
| R5L5 | 0.077 ± 0.023 | 0.56 ± 0.13 | 0.21 ± 0.00 | 1.70 ± 0.52 | 0.16 ± 0.016 | 0.34 ± 0.082 | 11.88 ± 4.90 |
| R6L2 | 0.75 ± 0.064 | 0.95 ± 0.16 | 1.01 ± 0.032 | ≥33.74 | 1.27 ± 0.11 | 2.73 ± 0.48 | ≥12.17 |
| R6L3 | 0.78 ± 0.14 | 1.04 ± 0.19 | 1.32 ± 0.19 | 5.26 ± 1.04 | 0.47 ± 0.20 | 1.48 ± 0.11 | >88.08 |
| R6L4 | 0.32 ± 0.15 | 0.69 ± 0.11 | 0.60 ± 0.031 | 1.00 ± 0.15 | 0.78 ± 0.54 | 0.51 ± 0.092 | >26.91 |
| R6L5 | 0.12 ± 0.039 | 0.74 ± 0.090 | 0.35 ± 0.00 | 2.47 ± 0.030 | 0.32 ± 0.015 | 0.68 ± 0.075 | >37.86 |
| R7L2 | 0.17 ± 0.041 | 0.22 ± 0.00 | 0.31 ± 0.16 | 2.66 ± 0.17 | 0.36 ± 0.017 | 1.04 ± 0.35 | 3.09 ± 0.50 |
| R7L3 | 0.098 ± 0.052 | 0.22 ± 0.00 | 0.57 ± 0.25 | 0.19 ± 0.19 | 0.61 ± 0.12 | 2.61 ± 0.12 | 9.00 ± 0.51 |
| R7L4 | 0.074 ± 0.021 | 0.13 ± 0.048 | 0.18 ± 0.016 | 1.53 ± 0.72 | 0.051 ± 0.018 | 0.26 ± 0.00 | >89.33 |
| R7L5 | 0.061 ± 0.0032 | 0.43 ± 0.14 | 0.19 ± 0.00 | 3.14 ± 0.95 | 0.052 ± 0.009 | 0.37 ± 0.11 | >114.72 |
| R8L2 | 0.80 ± 0.094 | 1.18 ± 0.091 | 1.00 ± 0.091 | 6.24 ± 0.65 | 1.20 ± 0.018 | 1.81 ± 0.18 | 4.63 ± 1.54 |
| R8L3 | 0.50 ± 0.14 | 1.03 ± 0.19 | 1.06 ± 0.018 | 3.54 ± 0.34 | 0.28 ± 0.018 | 1.65 ± 0.27 | 5.38 ± 1.29 |
| R8L4 | 0.13 ± 0.059 | 0.47 ± 0.069 | 0.54 ± 0.00 | 1.05 ± 0.035 | 0.19 ± 0.017 | 0.36 ± 0.035 | >86.52 |
| R8L5 | 0.17 ± 0.017 | 1.23 ± 0.22 | 0.73 ± 0.34 | 2.71 ± 0.13 | 0.20 ± 0.051 | 1.26 ± 0.34 | >43.31 |
| R9L2 | 0.26 ± 0.11 | 0.24 ± 0.037 | 0.26 ± 0.073 | 2.81 ± 0.29 | 0.22 ± 0.073 | 1.12 ± 0.35 | 3.14 ± 0.11 |
| R9L3 | 0.18 ± 0.054 | 0.32 ± 0.072 | 0.70 ± 0.27 | 3.08 ± 0.25 | 0.055 ± 0.018 | 0.80 ± 0.25 | 4.76 ± 0.80 |
| R9L4 | 0.028 ± 0.0087 | 0.15 ± 0.045 | 0.15 ± 0.033 | 0.87 ± 0.17 | 0.031 ± 0.007 | 0.28 ± 0.052 | >114.95 |
| R9L5 | 0.044 ± 0.005 | 0.34 ± 0.051 | 0.22 ± 0.017 | 1.46 ± 0.56 | 0.036 ± 0.007 | 0.22 ± 0.085 | >119.33 |
| R10L2 | 0.14 ± 0.046 | 0.29 ± 0.055 | 0.29 ± 0.018 | 2.20 ± 0.40 | 0.12 ± 0.033 | 1.48 ± 0.27 | 4.60 ± 0.31 |
| R10L3 | 0.10 ± 0.032 | 0.20 ± 0.036 | 0.20 ± 0.018 | 2.32 ± 0.11 | 0.043 ± 0.005 | 0.54 ± 0.27 | 7.77 ± 2.32 |
| R10L4 | 0.017 ± 0.002 | 0.068 ± 0.012 | 0.045 ± 0.010 | 0.54 ± 0.087 | 0.017 ± 0.007 | 0.087 ± 0.035 | 5.26 |
| R10L5 | 0.046 ± 0.014 | 0.19 ± 0.034 | 0.11 ± 0.022 | 0.73 ± 0.034 | 0.029 ± 0.012 | 0.14 ± 0.075 | 10.61 ± 6.70 |
| R11L2 | 0.25 ± 0.071 | 0.21 ± 0.053 | 0.28 ± 0.036 | 2.68 ± 0.25 | 0.20 ± 0.036 | 1.23 ± 0.43 | 3.04 ± 0.21 |
| R11L3 | 0.16 ± 0.078 | 0.28 ± 0.035 | 0.61 ± 0.14 | 7.12 ± 0.76 | 0.068 ± 0.002 | 0.88 ± 0.52 | 12.84 ± 10.12 |
| R11L4 | 0.063 ± 0.025 | 0.17 ± 0.017 | 0.20 ± 0.017 | 1.74 ± 0.32 | 0.036 ± 0.005 | 0.29 ± 0.034 | >189.54 |
| R12L2 | 0.46 ± 0.18 | 0.50 ± 0.089 | 0.55 ± 0.089 | 3.30 ± 0.30 | 0.44 ± 0.11 | 2.26 ± 0.55 | 4.94 ± 1.24 |
| R12L3 | 0.26 ± 0.087 | 0.45 ± 0.12 | 0.75 ± 0.21 | 3.38 ± 0.45 | 0.11 ± 0.043 | 0.99 ± 0.14 | 4.73 ± 1.04 |
| R12L4 | 0.063 ± 0.017 | 0.24 ± 0.068 | 0.19 ± 0.034 | 1.45 ± 0.41 | 0.049 ± 0.012 | 0.24 ± 0.034 | >173.28 |
| R13L2 | 0.053 ± 0.022 | 0.11 ± 0.020 | 0.18 ± 0.057 | 1.65 ± 0.39 | 0.055 ± 0.016 | 0.51 ± 0.12 | 32.57 ± 7.96 |
| R14L2 | 0.95 ± 0.097 | 1.19 ± 0.26 | 1.42 ± 0.37 | >31.20 | 0.74 ± 0.097 | 2.99 ± 0.68 | >82.35 |
| NVP | 1.26 ± 0.60 | 5.33 ± 1.07 | >9.52 | >9.52 | 0.095 ± 0.031 | >9.52 | >9.52 |
| EFV | 0.032 ± 0.006 | 0.10 ± 0.019 | 0.007 ± 0.002 | 0.27 ± 0.016 | 0.007 ± 0.001 | 0.27 ± 0.048 | 0.48 ± 0.19 |
| ETR | 0.032 ± 0.028 | 0.015 ± 0.012 | 0.058 ± 0.041 | 0.083 ± 0.053 | 0.037 ± 0.030 | 0.088 ± 0.076 | 0.15 ± 0.12 |
| IAS-4 | 0.047 ± 0.021 | 0.056 ± 0.005 | 0.25 ± 0.018 | 0.56 ± 0.14 | 0.04 ± 0.02 | 0.23 ± 0.070 | 7.45 ± 2.25 |
| IAS-0 | 0.015 ± 0.0086 | 0.017 ± 0.0060 | 0.039 ± 0.0028 | 1.1 ± 0.63 | 0.90 ± 0.065 | 0.047 ± 0.023 | 1.8 ± 0.29 |
EC50: Concentration of compound that decreases 50% HIV-1-induced cytopathy of MT-4 cells.
Preliminary investigation of the SARs suggested that the nature of sulfonamide moiety and the length of the carbon linker will greatly affect the antiviral potency. This can be concluded from the cellular activity data of each group that the optimal carbon chain length is four carbon atoms, and the general rule is C4 ≈ C5 > C3 > C2, exemplified by R3L4 (EC50 = 0.028 μmol/L) ≈ R3L5 (EC50 = 0.027 μmol/L) > R3L3 (EC50 = 0.050 μmol/L) > R3L2 (EC50 = 0.12 μmol/L) and R6L4 (EC50 = 0.077 μmol/L) ≈ R6L5 (EC50 = 0.075 μmol/L) > R6L3 (EC50 = 0.14 μmol/L) > R6L2 (EC50 = 0.16 μmol/L). The lengths of carbon chain determine the relative position of sulfonamide substituents. An ethyl group might be inadequate to support terminal sulfonamide to reach the open area, meanwhile causing repulsive contacts. In contrast, longer carbon chains with higher flexibility will enable larger accessibility to different residues at the edge of pocket.
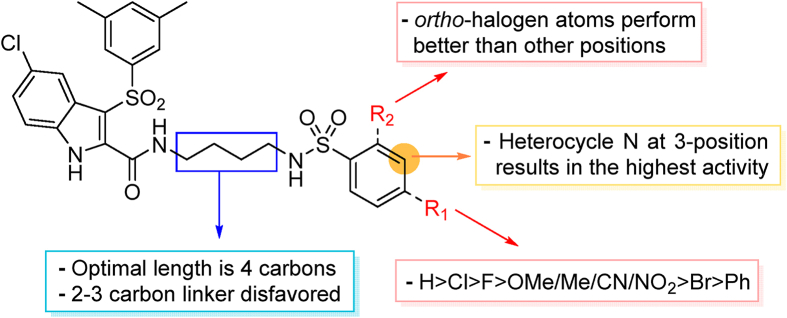
Considering the diverse chemical environment around NNIBP channel, we introduced arylsulfonamides containing lipophilic or hydrophilic groups. Different substitute groups on the para-position of benzenesulfonamide have a great impact on the cellular anti-HIV activity. The detailed SARs are as follows: when the para substituent of benzenesulfonamide is halogen, smaller atomic structure of the substituent lead to better antiviral potency (H (R9L4 = 0.0075 μmol/L) > Cl (R7L4 = 0.0086 μmol/L) > F (R4L4 = 0.020 μmol/L) > Br (R6L4 = 0.077 μmol/L)). In addition, it can be seen from the activity data when the substituent on the benzenesulfonamide is a F atom, the ortho-position seems most beneficial to increase the activity [R11L4 (EC50 = 0.0091 μmol/L) > R4L4 (EC50 = 0.020 μmol/L) ≈ R12L4 (EC50 = 0.019 μmol/L)]. When the para-substituents are strong electron-withdrawing groups (NO2/CN) or electron-donating groups (CH3/OCH3), their activities are no match for most halogen substitutions. Further extending the substituent to benzyl led to compound R14L2 with significantly decreased activity (EC50 = 0.21 μmol/L), suggesting that large groups are unfavorable for the antiviral potency. In brief, less substituent on the benzenesulfonamide will contribute to a prominent antiviral activity (R9L4, EC50 = 0.0075 μmol/L) which demonstrates that the larger groups could prevent inhibitors from entering the NNIBP channel. Moreover, when the benzenesulfonamide is replaced by cyclopropylsulfonamide with a smaller structure, compound R13L2 exhibits the most potency against WT strain (EC50 = 0.0041 μmol/L) which is comparable to that of EFV (EC50 = 0.0038 μmol/L), ETR (EC50 = 0.0051 μmol/L) and IAS-0 (EC50 = 0.0041 μmol/L). It is worth noting that for the aromatic heterocycles, the pyridine sulfonamide seems more favorable than thiophene sulfonamide when comparing the activity of R10L4 (EC50 = 0.007 μmol/L) and R8L4 (EC50 = 0.045 μmol/L). The comprehensive SARs was summarized in Fig. 3. This encouraging improvement in activity might be a consequence of hydrophilic nature of pyridine ring, and additional hydrogen bond between heterocycle N atom and surrounding amino acids. This is confirmed in our molecular docking (see Fig. 4). To our delight, most substituents are conducive to reducing the cytotoxicity, especially the halogen and methyl substituents.
Figure 3.
Schematic overview of SARs of IAS derivatives in this work.
Figure 4.
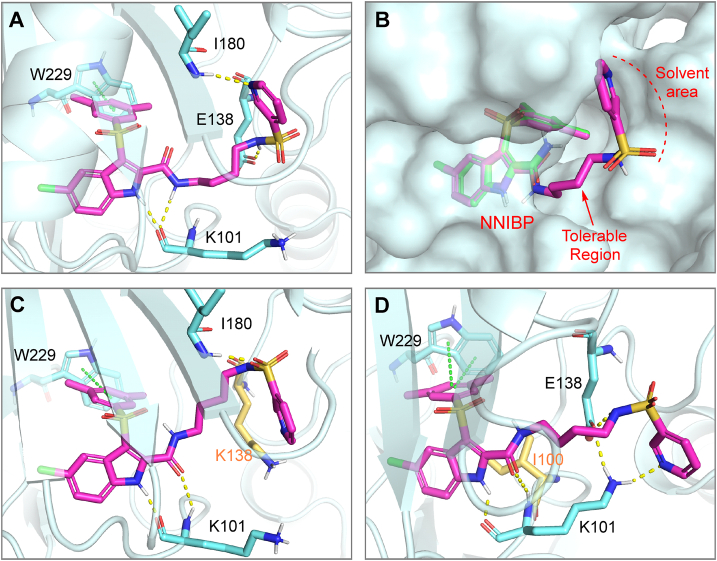
Graphical representation of docking poses and interactions of R10L4 with WT HIV-1 RTs (PDB ID: 6C0N) (A and B), E138K (PDB ID: 6C0L) (C) and L100I (PDB ID: 2OPQ) (D). R10L4 and IAS-0 were shown as sticks with carbon atoms colored in magenta/green. Protein surface and residues were shown in pale cyan. Mutated residues were highlighted by yellow. H-bonds and π–π stackings were represented by yellow/green dashed lines.
As depicted in Table 2, all the synthesized compounds were further assessed for their inhibitory activities against the common clinical single-mutant strains (L100I, K103N, Y181C and E138K) and double-mutant strains F227L + V106A and RES056 (K103N + Y181C). L1001 is selected by EFV, ETR and rilpivirine (RPV), with remarkable resistance towards them. K103N is the most commonly transmitted drug-resistance mutation. Likewise, E138K is mainly seen under RPV treatment. It has moderate resistance to RPV and ETR. Y181C could be selected under a variety of NNRTIs. Y188L is endowed with remarkable resistance towards doravirine (DOR) and EFV. F227L + V106A has 100 folds resistance towards DOR and high-level resistance to EFV32. Their SI and RF (fold-resistance factor, EC50 (mutant strain)/EC50 (WT strain)) were showed in Table 3. Most compounds exhibited moderate to excellent activities to these mutant strains, but slightly reduced compared with that to WT HIV-1 strain. SARs for the inhibition activity of mutant strains are basically in consistent with those of WT strains. Among them, compound R10L4 displayed the most potent inhibitory activity towards commonly occurring mutant strains, especially for L100I, Y181C and E138K (EC50 = 0.017, 0.045 and 0.017 μmol/L, respectively), superior to that of NVP and ETR, similar with EFV. However, these compounds are more sensitive to Y188L change than other mutations. When tyrosine 188 alters to leucine, the loss of key hydrophobic or π–π stacking interaction made most of the compounds inactive. Apparently, for the important NNRTIs-resistant mutant strain E138K, R10L4 exhibited an enhanced inhibitory activity and potent drug resistance (EC50 = 0.017 μmol/L, SI = 13,123, RF = 2.4). Additionally, considering the cytotoxicity, R10L4 possessed a higher selectivity index and safety profiles than the IAS-0, NVP, EFV, and ETR. It is a pity that R13L2, the compound with the best activity against WT strain (EC50 = 0.0041 μmol/L, SI = 41,951), did not show good drug resistance, such as the two most common mutants (EC50 (K103N) = 0.11 μmol/L, RF = 26.8; EC50 (Y181C) = 0.18 μmol/L, RF = 43.9). Moreover, the potency towards double mutant strains RES056 and F227L + V106 were also evaluated. In general, a large part of compounds are rather sensitive to RES056 strain than F227L + V106 mutation. The two most potential compounds R5L4 and R10L4 also exhibited the best inhibitory activities against F227L + V106A, with EC50 values of 0.082 and 0.087 μmol/L, respectively, possessing significantly improved potency than NVP (EC50 > 9.52 μmol/L) and EFV (EC50 = 0.27 μmol/L), and comparable to ETR (EC50 = 0.088 μmol/L). When compared with IAS-4 with similar activity against WT strain (EC50(WT) = 0.0053 μmol/L) in our previous studies15, R10L4 possesses lower toxicity feature and is much less influenced by the mutant strains, exemplified in Table 2, Table 3 From the perspective of anti-drug resistance and cytotoxicity, an apparent advantage of sulfonamide substitution over other groups is thus clearly showed. Meanwhile, further experiments on more multi-mutant strains should also be considered.
Table 3.
SI values of the target compounds.
| Compd. | SI (RF)a |
||||||
|---|---|---|---|---|---|---|---|
| L100I | K103N | Y181C | Y188L | E138K | F227L+ V106A |
RES056 | |
| R1L2 | 247 (15.3) | 404 (9.4) | 274 (13.8) | 26 (144.8) | 197 (19.2) | 96 (39.6) | 50 (76.4) |
| R1L3 | 308 (14.9) | 231 (19.8) | 142 (32.2) | 24 (194.6) | 833 (5.5) | 134 (34.3) | 28 (162.5) |
| R1L4 | 637 (5.1) | 332 (9.7) | 327 (9.9) | 68 (47.4) | 770 (4.2) | 333 (9.7) | <1 (>3232) |
| R1L5 | 679 (6.1) | 177 (23.5) | 491 (8.5) | 45 (93.1) | 593 (7.0) | 301 (13.8) | <1 (>4159.3) |
| R2L2 | 159 (7.8) | 118 (10.5) | 125 (9.8) | 1 (1232.5) | 95 (13.0) | 67 (18.5) | <1 (>1232.5) |
| R2L3 | 494 (8.8) | 246 (17.7) | 195 (22.3) | 27 (159.7) | 636 (6.9) | 169 (25.8) | <1 (>4354.1) |
| R2L4 | 126 (4.9) | 66 (9.3) | 67 (9.1) | 28 (21.9) | 88 (7.0) | 79 (7.8) | <1 (>615.2) |
| R2L5 | 1172 (4.1) | 196 (24.4) | 546 (8.7) | 86 (55.7) | 846 (5.6) | 391 (12.2) | <1 (>4772.2) |
| R3L2 | >224 (7.9) | >358 (4.9) | >212 (8.3) | 1 (1764.0) | >184 (9.6) | >34 (51.3) | <1 (>1764.0) |
| R3L3 | 65 (7.1) | 32 (14.6) | 22 (20.8) | 7 (66.5) | 74 (6.3) | 13 (35.9) | 5 (99.0) |
| R3L4 | 771 (4.9) | 211 (18.1) | 255 (15.0) | 120 (31.8) | 471 (8.1) | 299 (12.7) | 56 (68.4) |
| R3L5 | 1487 (5.1) | 220 (34.3) | 652 (11.6) | 80 (94.2) | 779 (9.7) | 366 (20.6) | >8 (959.4) |
| R4L2 | 1043 (7.6) | 627 (12.6) | 603 (13.1) | 74 (107.4) | 592 (13.4) | 267 (29.6) | 69 (115.4) |
| R4L3 | 143 (6.3) | 77 (11.7) | 50 (18.0) | 12 (73.4) | 136 (6.6) | 34 (26.8) | 10 (91.7) |
| R4L4 | 3765 (2.8) | 1100 (9.6) | 1038 (10.2) | 167 (63.5) | 3420 (3.1) | 732 (14.5) | <1 (>10593.2) |
| R4L5 | 1929 (3.9) | 278 (27.1) | 776 (9.7) | 86 (87.1) | 1996 (3.8) | 371 (20.3) | ≤5 (>1631.1) |
| R5L2 | 30 (11.9) | 28 (13.0) | 26 (13.7) | 8 (47.0) | 19 (19.2) | 13 (27.0) | 7 (51.5) |
| R5L3 | 70 (10.5) | 24 (30.2) | 23 (31.7) | 7 (108.2) | 81 (9.0) | 19 (39.2) | 6 (132.8) |
| R5L4 | 181 (1.5) | 68 (4.1) | 87 (3.2) | 30 (9.2) | 147 (1.9) | 56 (4.9) | <1 (>278.8) |
| R5L5 | 2657 (2.3) | 365 (16.9) | 955 (6.5) | 121 (51.0) | 1315 (4.7) | 585 (10.5) | 17 (358.4) |
| R6L2 | 198 (4.9) | 159 (6.1) | 150 (6.4) | 4 (≥215.6) | 119 (8.1) | 55 (55.5) | ≤12 (>77.7) |
| R6L3 | 112 (5.7) | 85 (7.6) | 67 (9.6) | 17 (38.5) | 188 (3.4) | 60 (10.8) | <1 (>644,7) |
| R6L4 | 85 (4.1) | 39 (8.9) | 45 (7.7) | 27 (12.9) | 35 (10.0) | 54 (6.5) | <1 (>347.4) |
| R6L5 | 316 (1.6) | 51 (9.8) | 108 (4.7) | 15 (32.7) | 119 (4.2) | 55 (9.0) | <1 (>501.4) |
| R7L2 | 1028 (12.7) | 754 (17.3) | 575 (22.6) | 66 (198.1) | 482 (27.0) | 169 (77.0) | 56 (230.4) |
| R7L3 | 2144 (6.0) | 942 (13.7) | 363 (35.5) | 1097 (11.8) | 352 (36.7) | 80 (160.6) | 23 (552.1) |
| R7L4 | 1215 (8.6) | 676 (15.4) | 482 (21.6) | 58 (177.6) | 1730 (6.0) | 333 (31.2) | <1 (10389.1) |
| R7L5 | 1885 (5.9) | 260 (42.9) | 599 (18.6) | 36 (305.4) | 2255 (4.9) | 311 (35.8) | <1 (11132.1) |
| R8L2 | 201 (7.8) | 136 (11.5) | 161 (9.7) | 26 (60.2) | 135 (11.5) | 89 (17.6) | 35 (44.6) |
| R8L3 | 242 (8.7) | 117 (18.0) | 113 (18.6) | 34 (61.7) | 427 (4.9) | 73 (28.7) | 22 (93.9) |
| R8L4 | 658 (3.0) | 186 (10.5) | 161 (12.1) | 82 (23.8) | 437 (4.5) | 243 (8.0) | <1 (>1952.5) |
| R8L5 | 273 (3.0) | 39 (21.3) | 65 (12.7) | 17 (47.4) | 225 (3.7) | 37 (22.1) | <1 (>823.9) |
| R9L2 | 596 (18.7) | 651 (17.1) | 576 (19.3) | 54 (207.7) | 670 (16.6) | 134 (83.2) | 48 (232.8) |
| R9L3 | 1289 (7.9) | 699 (14.6) | 318 (32.2) | 73 (140.7) | 4026 (2.5) | 275 (37.2) | 47 (217.6) |
| R9L4 | 4137 (3.9) | 777 (20.9) | 754 (21.5) | 131 (124.1) | 3660 (4.4) | 408 (39.8) | <1 (16236.9) |
| R9L5 | 2684 (3.6) | 347 (27.8) | 533 (18.1) | 81 (118.3) | 3417 (2.8) | 554 (17.4) | <1 (>9634.3) |
| R10L2 | 171 (14.0) | 81 (29.4) | 81 (29.5) | 11 (216.8) | 200 (11.9) | 16 (145.9) | 5 (453.8) |
| R10L3 | 551 (11.1) | 289 (21.2) | 268 (22.9) | 24 (258.2) | 1284 (4.8) | 102 (59.9) | 7 (864.4) |
| R10L4 | 13,055 (2.4) | 3189 (9.7) | 4753 (6.6) | 408 (76.9) | 13,123 (2.4) | 2493 (12.6) | 41 (757.2) |
| R10L5 | 1480 (6.8) | 353 (28.7) | 638 (15.9) | 93 (108.8) | 2274 (4.5) | 466 (21.7) | 6 (1594.6) |
| R11L2 | 786 (12.1) | 927 (10.3) | 698 (13.6) | 74 (128.3) | 1051 (9.1) | 162 (58.7) | 65 (145.7) |
| R11L3 | 1188 (9.5) | 704 (16.0) | 320 (35.1) | 27 (411.2) | 2908 (3.9) | 218 (51.5) | 15 (741.0) |
| R11L4 | 3022 (5.3) | 1147 (13.8) | 914 (17.4) | 108 (146.5) | 5223 (3.0) | 678 (23.4) | <1 (15876.6) |
| R12L2 | 403 (13.2) | 374 (14.3) | 333 (16.0) | 56 (95.6) | 420 (12.7) | 82 (65.1) | 37 (142.9) |
| R12L3 | >853 (7.9) | >480 (14.0) | >291 (23.1) | >64 (105.3) | >2002 (3.4) | >220 (30.6) | >46 (147.3) |
| R12L4 | 2735 (4.2) | 734 (15.7) | 910 (12.6) | 119 (97.1) | 3553 (3.2) | 712 (16.2) | <1 (>11508.5) |
| R13L2 | 3205 (12.9) | 1503 (26.8) | 972 (43.9) | 104 (347.1) | 3155 (13.4) | 343 (105.6) | 5 (6867.5) |
| R14L2 | 87 (4.9) | 69 (6.2) | 58 (7.3) | 3 (≥161.2) | 112 (3.8) | 28 (15.5) | <1 (>425.6) |
| NVP | >7 (15.2) | >2 (64.0) | 1 (>114.2) | <1 (>114.2) | >100 (1.1) | 1 (>114.2) | <1 (>114.2) |
| EFV | >191 (8.5) | >61 (26.6) | >860 (1.9) | >23 (70.0) | >876 (1.9) | >24 (69.0) | >13 (124.9) |
| ETR | >140 (2.9) | >320 (1.3) | >81 (5.1) | >56 (7.4) | >127 (3.3) | >52 (7.9) | >30 (13.8) |
| IAS-4 | 1396 (8.9) | 1172 (10.6) | 263 (47.2) | 117 (105.7) | 1640 (7.54) | 285 (43.4) | 9 (1405.7) |
| IAS-0 | 279 (7.3) | 131 (15.7) | 97 (21.1) | 3 (593.9) | 377 (5.4) | 54 (≥38.5) | ≤3 (≥832.6) |
RF is the ratio of EC50 (resistant viral strain)/EC50 (wild-type viral strain).
In summary, the most suitable carbon chain length appeared to be is four carbon atoms, and pyridine sulfonamide with hydrogen-bonding receptor was the privileged terminal substituent. It is worth emphasizing that a general decrease in cytotoxicity and improvement of the selectivity index is attained by the introduction of sulfonamide groups, which achieved the primary goal of our design concept.
2.3. HIV-1 RT inhibition assay
For the purpose of directly evaluating target affinity of target compounds, all target compounds along with IAS-0 and EFV as control drugs were selected to test their inhibitory effects against WT HIV-1 RT (Supporting Information Table S1). As shown in Table 4, the representative compounds maintained prominent activity (IC50 = 0.057–0.070 μmol/L) against HIV-1 RT. Excellent enzymatic inhibitory (IC50 = 0.057 μmol/L) was seen in R13L2, one with the best cellular activity. It was comparable to EFV (IC50 = 0.030 μmol/L) but weaker than the lead IAS-0 (IC50 = 0.018 μmol/L). R10L2 (EC50 = 0.0073 μmol/L, IC50 = 0.060 μmol/L) exhibited an inferior cellular activity but with a higher enzymatic inhibitory activity than R10L4 (EC50 = 0.0070 μmol/L, IC50 = 0.070 μmol/L). In comparison, IAS-4 showed an IC50 of 0.17 μmol/L in previous studies under the same method13. Generally, their measured inhibition to RT maintained good consistence with antivirus activity. According to the analysis of above results, we conclude that sulfonamide substituted indolylarylsulfones are typical and effective HIV-1 NNRTIs.
Table 4.
RT inhibitory activity of some selected compounds.
| Compd. | R10L2 | R10L4 | R13L2 | IAS-0 | EFVb |
|---|---|---|---|---|---|
| IC50 (μmol/L)a | 0.060 ± 0.009 | 0.070 ± 0.009 | 0.057 ± 0.012 | 0.018 ± 0.002 | 0.030 ± 0.000 |
IC50: Compound concentration required to inhibit 50% incorporation of biotin-labeled UTP by HIV-1 (WT) RT.
All data were obtained from one batch with the same method.
2.4. Molecular docking study
Molecular docking simulation was conducted to identify the binding pattern between the most potent compound R10L4 (EC50(IIIB) = 0.0070 μmol/L; EC50(L100I) = 0.017 μmol/L; EC50(K103N) = 0.068 μmol/L; EC50(E138K) = 0.017 μmol/L) and HIV-1 RT (wild type, PDB ID: 6C0N; E138K, PDB ID: 6C0L and L100I, PDB ID: 2OPQ). For this purpose, R10L4 and IAS-0 were both chosen as the ligands and the glide module in Schrödinger suite was employed to conduct dockings. Docking results and 3D interactions are visualized in Pymol (www.pymol.org).
As showed in Fig. 4a, R10L4 was well fitted to NNIBP of WT HIV-1 RT with a high docking score up to −11.645, adapting a double-winged conformation without obvious torsions. Newly introduced 4-(pyridine-3-sulfonamido)butyl moiety is well accommodated by a tunnel at the opening of binding site, which is consistent with our anticipation in compound designing. To be noted, the four-carbon direct chain is slightly twisted, thus enabling terminal pyridine-3-sulfonamyl group to build up two hydrogen bonds with E138 and I180. The residual part of R10L4 is well aligned with IAS-0, as the hydrophilic interaction and π–π stacking with surrounding residues are conserved (Fig. 4b). Double hydrogen bonds with the backbone of K101 are also seen in the case of R10L4, but was formed by indole heterocycle NH and 2-carboxamide NH.
Binding mode between R10L4 with E138K and L100I RT mutants was charactered to explain its anti-drug resistance property (Fig. 4c and d). The two mutations exerted no obvious disruptions to the position of indole scaffold, for the interactions towards W229 and K101 remain unchanged. On the other hand, as these mutations reshaped the entry tunnel of NNIBP, significant conformation changes are thus seen in side wing 4-(pyridine-3-sulfonamido)butyl moiety. For E138K model, sulfamide NH no longer interacts with K138, but a close contact with I180 is still viable. Analogously, docking results based on L100I revealed a stable hydrogen bond network among pyridinyl-3-sulfonyl group and sidechains of K101/E138. From a comparison of the results above, the potent inhibition of R10L4 towards mutant strains is mainly contributed by the high flexibility of C4 alkyne linker, and stable interaction of pyridinyl-3-sulfonyl with multiple hydrophilic residues.
2.5. In vivo acute/subacute toxicity assessment
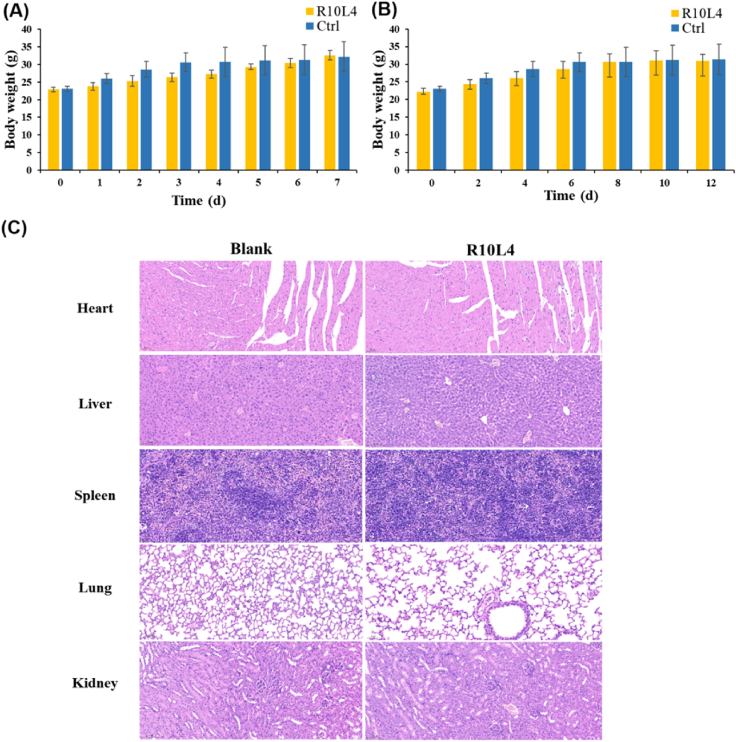
The safety profiles of R10L4 were conducted in SPF Kunming mice. All relative animal experiments in this study were approved by the ethics committee of Cheeloo College of Medicine, Shandong University (Jinan, China). Procedures performed in the studies were guided by the ethical standards of the institution. In both acute toxicity (800 mg/kg, 7 days) and subacute toxicity (50 mg/kg, 12 days) experiments, R10L4 did not lead to any mortality or observable behavior changes. Records of average body weight are presented in Fig. 5a and b. Compared with blank group, only insignificant body weight loss was seen, and results of experiment groups remain identical with that of control groups at the end of 7 or 12 days of administration. At the end of subacute toxicity test, the hematoxylin and eosin-stained slices from vital organs were prepared. Appearance of cells and structures in all organs showed no marked pathological abnormalities after the administration of R10L4, compared with blank vehicle group. All these results are consistent with the low cytotoxicity of R10L4 (CC50 = 216.5 μmol/L), which could be an apparent advantage in its further evaluation and clinical development.
Figure 5.
Visualized results from in vivo toxicity experiment of R10L4. (A) Time courses of body weight in 7-days acute toxicity experiment. (B) Time courses of body weight in 12-days subacute toxicity experiment. (C) Images of organ slices from treated mice in subacute toxicity study. Heart, liver, spleen, lung and kidney were sectioned and stained with hematoxylin and eosin.
2.6. In vivo pharmacokinetics (PK) study
The pharmacokinetic profiles of R10L4 were examined in a Sprague–Dawley rat to evaluate its potential for therapeutic application (Table 5). Six rats were equally divided into two groups, 2 mg/kg i.v. dose and 10 mg/kg oral dose was given. After the i.v. dosing, a modest half-life of 1.61 h, a high clearance rate of 4209 mL/h/kg and an area under the curve (AUC) value of 481 h·ng/mL was observed. Absorption of R10L4 was quick when dosed orally at 10 mg/kg (Tmax = 0.83 h), indicating its high solubility and permeability. R10L4 also showed a favorable oral half-life (t1/2 = 1.78 h), and a maximum concentration (Cmax) up to 80 ng/mL, far beyond its EC50 value (approximately 4.0 ng/mL). The oral bioavailability of R10L4 was 8.5%, which may be inadequate for developing an oral drug. The moderate pharmacokinetic data may also be due to its poor solubility in water, and other efforts should be made on improving the pharmacokinetic features in our laboratory.
Table 5.
Pharmacokinetic parameters of R10L4a.
| Parameter | Unit | i.v.b | p.o.c |
|---|---|---|---|
| t1/2 | h | 1.61 ± 0.04 | 1.78 ± 0.25 |
| Tmax | h | 0.083 ± 0.00 | 0.83 ± 0.15 |
| Cmax | ng/mL | 657 ± 99.2 | 79.9 ± 14.8 |
| AUC0–t | h·ng/mL | 472 ± 63.4 | 199 ± 38.7 |
| AUC0–∞ | h·ng/mL | 481 ± 63.3 | 203 ± 38.4 |
| MRT0–∞ | h | 1.45 ± 0.09 | 2.27 ± 0.05 |
| CL | mL/h/kg | 4209 ± 552 | – |
| F | % | – | 8.5 |
PK parameters (mean ± SD, n = 3).
Dosed intravenously at 2 mg/kg.
Dosed orally at 10 mg/kg.
3. Conclusions
To shed light on the uncharted space of NNIBP tolerant region and reduce the cytotoxicity of IAS-0, alkyne chain linker with different lengths and a variety of sulfonamides containing multiple hydrogen-bonding donors/acceptors were introduced to the 2-carboxamide site of indole scaffold. 48 novel sulfonamide substituted indolylarylsulfones were designed, synthesized and evaluated for antivirus activity. The antiviral results indicated that most of the IASs exhibited potent activity against WT HIV-1, especially R10L4 (EC50 = 0.007 μmol/L, SI = 30,930) and R13L2 (EC50 = 0.004 μmol/L, SI = 41,951), the two most promising inhibitors, were superior to NVP and comparable to ETV and ETR. Additionally, R10L4 maintained the effective potency towards mutant strains with EC50 values of 0.017, 0.068, 0.045, 0.017 and 0.087 μmol/L against L100I, K103N, Y181C, E138K and F227L + V106A strains. The enzyme inhibitory activity assay showed that R10L4 possessed a strong binding affinity for HIV-1 RT (IC50 = 0.07 μmol/L). To be emphasized, the newly designed sulfonamide inhibitor R10L4 exhibited an almost 40-fold decrease in cytotoxicity (CC50 = 216.51 μmol/L) compared to IAS-0 (CC50 = 5.52 μmol/L), which was also confirmed by the acute and subacute toxicity experiments in mice. Furthermore, R10L4 possessed acceptable PK profiles, with moderate clearance and bioavailability (F = 8.5%). Taken altogether, these results confirm our design concept and the sulfonamide IAS derivatives lay a foundation for the development of novel and potent NNRTIs.
4. Experimental
4.1. Chemistry
Solvents, reagents and starting materials of synthesis were provided by commercial suppliers. All reactions were monitored via thin layer chromatography (TLC) and the purifications of compounds were conducted on silica gel columns (200–300 mesh, Qingdao) through column chromatography. A micro melting point apparatus (YRT-3) was used to determine the melting points (m.p.). A G1313A instrument (Agilent, USA) was used to acquire the mass spectra. The NMR spectra were obtained on 400 or 600 MHz spectrometers (Bruker, Switzerland) in DMSO-d6 with TMS as the internal standard. A LTQ Orbitrap XL instrument (Thermo Fisher) was used to record the HRMS spectra. A rapid and simple HPLC method for the purities of representative compounds were established on a Shimadzu HPLC system. HPLC conditions: Agilent ZORBAX, SB-C18 column (250 mm × 4.6 mm × 5 μm). Isocratic elution method: A: methanol (60%); B: water (40%); velocity: 1.0 mL/min; wavelength: 254 nmol/L, temperature, 30 °C; injection volume, 20 μL. The purities of tested compounds are >95%.
4.1.1. Synthetic sequence for the ethyl 5-chloro-3-((3,5-dimethylphenyl)thio)- 1H-indole-2-carboxylate (intermediate 1)
Ethyl 5-chloro-1H-indole-2-carboxylate (4.60 g, 20.57 mmol), 3,5-dimethylbenzenethiol (3.06 g, 22.14 mmol) and Selectflour™ (7.29 g, 20.58 mmol) were dissolved in 100 mL ACN. Stir the reaction at room temperature for 8 h. Upon the completion of reaction, ACN was removed under reduced pressure. To the remaining solid, 60 mL water and 60 mL of DCM were added. Organic phase was collected by a separating funnel, dried over anhydrous Na2SO4, then evaporated under vacuum concentration. The obtained light-brown crude product was recrystallized with 35 mL of methanol and heated to 60 °C under continuous stirring. Precipitants were collected by filtration to yield an off-white solid as purified intermediate 1 (4.6 g). Yield 62%. m.p.: 135–136 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.56 (s, 1H), 7.56 (d, J = 8.9 Hz, 1H), 7.38 (d, J = 2.1 Hz, 1H), 7.35 (dd, J = 8.8, 2.3 Hz, 1H), 6.78 (s, 1H), 6.73 (s, 2H), 4.34 (q, J = 7.0 Hz), 2.15 (s, 6H), 1.25 (t, J = 7.0 Hz, 3H). ESI-MS: m/z 358.12 [M–1]–, C19H18ClNO2S (359.07).
4.1.2. Synthetic sequence for ethyl 5-chloro-3-((3,5-dimethylphenyl)sulfonyl)-1H -indole-2-carboxylate (intermediate 2)
Intermediate 1 (4.6 g) was added to 160 mL of dichloromethane at room temperature. Then add 3-chlorobenzoperoxoic acid (m-CPBA) (6.6 g, 38.25 mmol) to the system. Warm the reaction in a 30 °C water bath until m-CPBA is completely dissolved. After 3 h, the mixture was gradually added to ice water and then washed with saturated Na2S2O3 and NaHCO3 solution sequentially. Dichloromethane was separated, and then concentrated under vacuum concentration. 20 mL ethyl acetate was used to recrystallize the remaining solid. The mixture was heated to 70 °C accompanied with stirring for 30 min. The suspension was cooled down to room temperature. The precipitant was filtered out and vacuum dried to get the target compound 2 (4.7 g). Yield 94%, m.p.: 210–211 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.28 (s, 1H), 8.25 (s, 1H), 7.68–7.58 (m, 3H), 7.45 (d, J = 8.9 Hz, 1H), 7.28 (s, 1H), 4.36 (q, J = 6.6 Hz, 2H), 2.34 (s, 6H), 1.31 (t, J = 6.7 Hz, 3H). ESI-MS: m/z 390.34 [M–1]–. C19H18ClNO4S (391.06).
4.1.3. Synthetic sequence for 5-chloro-3-((3,5-dimethylphenyl)sulfonyl)-1H-indole-2- carboxylic acid (intermediate 3)
Intermediate 2 (4.7 g, mmol) was dissolved in 80 mL of THF under a 0 °C ice bath. Another solution was meanwhile prepared by dissolving LiOH (4.9 g, mmol) into 80 mL of water. The LiOH solution was added into THF solution of intermediate 2 dropwise under a 0 °C ice bath. After 3 h, THF was removed by distillation. 1 mol/L HCl was slowly dropped into the resulting aqueous solution until pH < 3. Gradually precipitated solids were filtered and washed to obtain the intermediate 3 (4.26 g) following the vacuum drying. White solid, Yield 93%, m.p.: 270 °C (decompose). 1H NMR (400 MHz, DMSO-d6) δ 14.24 (s, 1H), 13.11 (s, 1H), 8.24 (s, 1H), 7.62 (s, 2H), 7.55 (d, J = 8.7 Hz, 1H), 7.41 (d, J = 8.8 Hz, 1H), 7.25 (s, 1H), 2.32 (s, 6H). ESI-MS: m/z 362.14 [M–1]–. C17H14ClNO4S (363.03).
4.1.4. General procedure for the preparation of 4RmLn
Amines (L2, L3, L4, L5) (1.0 eq.) were dissolved in dichloromethane, then added with triethylamine (3.0 eq.). Under a 0 °C ice bath, sulfonyl chlorides (1.2 eq.) were dissolved in dichloromethane and dropped into the system and then the mixture was stirred at 0–30 °C for 4–10 h. After the reaction was finished (monitored by TLC), saturated NaHCO3 was added. The separated dichloromethane was washed with saturated NaCl, dried with anhydrous Na2SO4, and removed solvent under vacuum concentration to acquire colorless to light-yellow oils, which were used without further purification.
4.1.5. General procedure for the preparation of intermediates 5RmLn
A solution of 4RmLn series of compounds (1.0 eq.) in dichloromethane was stirred under an ice bath. Trifluoroacetic acid (TFA) (6.0 eq.) was dropped into the mixture and stirred the reaction at 20–30 °C for 5 h. Then remove the organic solvent from the system under reduced pressure. The resulting oils were conducted next step as TFA salts, without further purification.
4.1.6. General procedure for the preparation of final compounds RmLn
Intermediate 3 (0.15 g, 0.41 mmol) and HATU (0.236 g, 0.62 mmol) were suspended in dichloromethane (8 mL) and DMF (2 mL) under a 0 °C ice bath and vigorous stirring. After 30 min, 4RmLn (0.45 mmol) and DIEA (0.16 g, 1.24 mmol) were dumped into the round bottom flask, which was then stirred for 5–20 h at 25–30 °C (monitored by TLC). After the reaction mixture was evaporated, 20 mL of 0.5 mol/L HCl solution was added dropwise to the remaining DMF solution. The resulting precipitant was collected by filtration and recrystallized in a mixture of 4 mL dichloromethane and 1.5 mL methanol. Then filter the suspension to afford the purified target compounds RmLn.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(2-((4-methylphenyl)sulfonamido)ethyl)-1H-indole-2-carboxamide (R1L2). White solid, Yield 57%, m.p.: 208–210 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.95 (s, 1H), 9.00 (t, J = 5.8 Hz, 1H), 7.93 (d, J = 2.0 Hz, 1H), 7.76–7.67 (m, 3H), 7.60 (d, J = 1.5 Hz, 2H), 7.54 (d, J = 8.8 Hz, 1H), 7.42–7.31 (m, 3H), 7.25 (s, 1H), 3.40 (q, J = 6.6 Hz, 2H), 2.97 (p, J = 6.7, 5.3 Hz, 2H), 2.34 (s, 3H), 2.29 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ 159.90, 143.21, 142.94, 139.43, 137.89, 137.26, 135.15, 133.26, 130.12, 127.76, 127.01, 125.66, 125.22, 124.16, 119.42, 115.33, 112.11, 41.96, 39.71, 21.37, 21.18. HRMS: m/z C26H26ClN3O5S2: Calcd. 559.1002, Found 560.1075 [M+H]+.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(3-((4-methylphenyl)sulfonamido)propyl)-1H-indole-2-carboxamide (R1L3). White solid, Yield 62%, m.p.: 194–196 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.00 (s, 3H), 8.94 (t, J = 5.6 Hz, 3H), 7.92 (d, J = 2.1 Hz, 3H), 7.77–7.58 (m, 15H), 7.53 (d, J = 8.8 Hz, 3H), 7.45–7.30 (m, 9H), 7.26 (s, 3H), 3.34 (d, J = 5.0 Hz, 15H), 2.87 (q, J = 7.0 Hz, 9H), 2.69 (s, 2H), 2.33 (d, J = 17.2 Hz, 27H), 1.72 (p, J = 7.1 Hz, 6H). 13C NMR (150 MHz, DMSO-d6) δ 159.79, 143.04, 142.95, 139.40, 138.16, 137.75, 135.09, 133.28, 130.03, 127.68, 126.97, 125.67, 125.09, 124.15, 119.36, 115.28, 111.89, 40.91, 37.57, 29.52, 21.38, 21.21. HRMS: m/z C27H28ClN3O5S2: Calcd. 573.1159, Found 574.1232 [M+H]+.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(4-((4-methylphenyl)sulfonamido)butyl)-1H-indole-2-carboxamide (R1L4). White solid, Yield 45%, m.p.: 194–196 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.01 (s, 1H), 8.95 (t, J = 5.6 Hz, 1H), 7.95 (d, J = 2.0 Hz, 1H), 7.72–7.60 (m, 4H), 7.54 (dd, J = 7.3, 4.9 Hz, 2H), 7.42–7.31 (m, 3H), 7.25 (s, 1H), 3.33–3.27 (m, 2H), 2.77 (p, J = 6.5 Hz, 2H), 2.36 (s, 3H), 2.30 (s, 6H), 1.55 (ddt, J = 21.0, 14.5, 7.5 Hz, 4H). 13C NMR (150 MHz, DMSO-d6) δ 159.59, 143.05, 142.91, 139.42, 138.20, 137.71, 135.12, 133.26, 130.02, 127.70, 126.96, 125.77, 125.11, 124.11, 119.40, 115.29, 111.82, 42.68, 39.38, 26.90, 26.44, 21.38, 21.21. HRMS: m/z C28H30ClN3O5S2: Calcd. 587.1315, Found 588.1388 [M+H]+.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(5-((4-methylphenyl)sulfonamido)pentyl)-1H-indole-2-carboxamide (R1L5). White solid, Yield 58%, m.p.: 144–146 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.98 (s, 1H), 8.91 (t, J = 5.6 Hz, 1H), 7.94 (d, J = 2.1 Hz, 1H), 7.67 (d, J = 8.2 Hz, 2H), 7.62 (d, J = 1.5 Hz, 2H), 7.56–7.46 (m, 2H), 7.41–7.31 (m, 3H), 7.26 (s, 1H), 3.29 (d, J = 6.3 Hz, 2H), 2.73 (q, J = 6.6 Hz, 2H), 2.37 (s, 3H), 2.31 (s, 6H), 1.58–1.29 (m, 6H). 13C NMR (150 MHz, DMSO-d6) δ 159.57, 143.08, 142.90, 139.40, 138.26, 137.81, 135.10, 133.26, 130.02, 127.69, 126.95, 125.77, 125.09, 124.09, 119.40, 115.28, 111.75, 42.93, 39.72, 29.16, 28.77, 23.95, 21.38, 21.21. HRMS: m/z C29H32ClN3O5S2: Calcd. 601.1472, Found 602.1545 [M+H]+.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(2-((4-methoxyphenyl)sulfonamido)ethyl)-1H-indole-2-carboxamide (R2L2). White solid, Yield 70%, m.p.: 218–220 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.96 (s, 1H), 9.01 (t, J = 5.8 Hz, 1H), 7.94 (d, J = 2.1 Hz, 1H), 7.85–7.70 (m, 2H), 7.61 (d, J = 2.6 Hz, 3H), 7.54 (d, J = 8.7 Hz, 1H), 7.35 (dd, J = 8.8, 2.1 Hz, 1H), 7.26 (s, 1H), 7.15–7.04 (m, 2H), 3.81 (s, 3H), 3.40 (q, J = 6.6 Hz, 2H), 2.95 (q, J = 6.6 Hz, 2H), 2.30 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ 162.67, 159.89, 142.94, 139.43, 137.27, 135.15, 133.26, 132.39, 129.15, 127.75, 125.65, 125.21, 124.16, 119.42, 115.33, 114.85, 112.11, 56.06, 41.96, 39.70, 21.19. HRMS: m/z C26H26ClN3O6S2: Calcd. 575.0952, Found 576.1024 [M+H]+. HPLC purity: 95.69%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(3-((4-methoxyphenyl)sulfonamido)propyl)-1H-indole-2-carboxamide (R2L3). White solid, Yield 60%, m.p.: 186–188 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.98 (s, 1H), 8.92 (t, J = 5.6 Hz, 1H), 7.92 (d, J = 2.0 Hz, 1H), 7.78–7.70 (m, 2H), 7.61 (s, 2H), 7.52 (dd, J = 7.3, 3.4 Hz, 2H), 7.34 (dd, J = 8.8, 2.1 Hz, 1H), 7.26 (s, 1H), 7.12–6.99 (m, 2H), 3.80 (s, 3H), 3.38–3.32 (m, 3H), 2.86 (q, J = 6.7 Hz, 2H), 2.30 (s, 6H), 1.71 (p, J = 7.1 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 162.53, 159.78, 143.03, 139.40, 137.75, 135.10, 133.27, 132.69, 129.08, 127.68, 125.66, 125.09, 124.15, 119.36, 115.28, 114.76, 111.90, 56.03, 40.89, 37.59, 29.48, 21.22. HRMS: m/z C27H28ClN3O6S2: Calcd. 589.1108, Found 590.1181 [M+H]+.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(4-((4-methoxyphenyl)sulfonamido)butyl)-1H-indole-2-carboxamide (R2L4). White solid, Yield 55%, m.p.: 182–185 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.97 (s, 1H), 8.91 (t, J = 5.6 Hz, 1H), 7.93 (d, J = 2.0 Hz, 1H), 7.76–7.68 (m, 2H), 7.61 (s, 2H), 7.53 (d, J = 8.7 Hz, 1H), 7.43 (t, J = 5.9 Hz, 1H), 7.34 (dd, J = 8.7, 2.1 Hz, 1H), 7.26 (s, 1H), 7.15–7.03 (m, 2H), 3.81 (s, 3H), 2.76 (q, J = 6.5 Hz, 2H), 2.30 (s, 6H), 1.66–1.44 (m, 4H). 13C NMR (150 MHz, DMSO-d6) δ 162.51, 159.60, 143.06, 139.42, 137.72, 135.12, 133.26, 132.75, 129.07, 127.70, 125.76, 125.11, 124.10, 119.40, 115.29, 114.75, 111.81, 56.05, 42.67, 39.40, 26.87, 26.47, 21.21. HRMS: m/z C28H30ClN3O6S2: Calcd. 603.1265, Found 604.1337 [M+H]+. HPLC purity: 98.56%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(5-((4-methoxyphenyl)sulfonamido)pentyl)-1H-indole-2-carboxamide (R2L5). White solid, Yield 65%, m.p.: 162–166 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.95 (s, 1H), 8.89 (t, J = 5.6 Hz, 1H), 7.93 (d, J = 2.0 Hz, 1H), 7.75–7.67 (m, 2H), 7.60 (s, 2H), 7.53 (d, J = 8.8 Hz, 1H), 7.39 (t, J = 5.8 Hz, 1H), 7.34 (dd, J = 8.8, 2.1 Hz, 1H), 7.26 (s, 1H), 7.13–7.04 (m, 2H), 3.82 (s, 3H), 2.71 (q, J = 6.6 Hz, 2H), 1.57–1.25 (m, 6H). 13C NMR (150 MHz, DMSO-d6) δ 162.50, 159.58, 143.08, 139.40, 137.83, 135.10, 133.26, 132.80, 129.06, 127.69, 125.76, 125.08, 124.09, 119.39, 115.28, 114.75, 111.75, 56.06, 42.92, 39.72, 29.14, 28.78, 23.98, 21.21. HRMS: m/z C29H32ClN3O6S2: Calcd. 617.1421, Found 618.1494 [M+H]+. HPLC purity: 96.89%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(2-((4-nitrophenyl)sulfonamido)ethyl)-1H-indole-2-carboxamide (R3L2). White solid, Yield 65%, m.p.: 235–238 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.95 (s, 1H), 9.03 (t, J = 5.8 Hz, 1H), 8.46–8.34 (m, 2H), 8.19 (t, J = 5.8 Hz, 1H), 8.11–8.03 (m, 2H), 7.93 (d, J = 2.1 Hz, 1H), 7.60 (s, 2H), 7.54 (d, J = 8.8 Hz, 1H), 7.35 (dd, J = 8.7, 2.1 Hz, 1H), 7.25 (s, 1H), 3.43 (q, J = 6.5 Hz, 2H), 3.08 (q, J = 6.5 Hz, 2H), 2.29 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ 159.88, 150.03, 146.42, 142.89, 139.45, 136.97, 135.18, 133.25, 128.54, 127.81, 125.67, 125.30, 125.05, 124.12, 119.44, 115.36, 112.13, 92.55, 84.00, 42.03, 39.70, 21.18. HRMS: m/z C25H23ClN4O7S2: Calcd. 590.0697, Found 591.0769 [M+H]+. HPLC purity: 98.15%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(3-((4-nitrophenyl)sulfonamido)propyl)-1H-indole-2-carboxamide (R3L3). White solid, Yield 80%, m.p.: 174–176 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.90 (s, 1H), 8.86 (t, J = 5.6 Hz, 1H), 8.34–8.25 (m, 2H), 8.04–7.94 (m, 3H), 7.83 (d, J = 2.1 Hz, 1H), 7.52 (d, J = 1.5 Hz, 2H), 7.45 (d, J = 8.7 Hz, 1H), 7.26 (dd, J = 8.7, 2.1 Hz, 1H), 7.17 (s, 1H), 3.28 (q, J = 6.6 Hz, 2H), 2.92 (q, J = 6.9 Hz, 2H), 2.23 (s, 6H), 1.67 (p, J = 7.1 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 159.89, 149.95, 146.64, 142.99, 139.38, 137.83, 135.07, 133.29, 128.51, 127.67, 125.59, 125.07, 124.97, 124.14, 119.32, 115.25, 111.86, 41.00, 37.46, 29.52, 21.21. HRMS: m/z C26H25ClN4O7S2: Calcd. 604.0853, Found 605.0926 [M+H]+.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(4-((4-nitrophenyl)sulfonamido)butyl)-1H-indole-2-carboxamide (R3L4). White solid, Yield 60%, m.p.: 185–188 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.98 (s, 1H), 8.93 (t, J = 5.6 Hz, 1H), 8.44–8.35 (m, 2H), 8.09–8.03 (m, 2H), 8.02 (t, J = 5.8 Hz, 1H), 7.94 (d, J = 2.1 Hz, 1H), 7.61 (d, J = 1.5 Hz, 2H), 7.53 (d, J = 8.7 Hz, 1H), 7.34 (dd, J = 8.7, 2.1 Hz, 1H), 7.25 (s, 1H), 3.31 (d, J = 5.6 Hz, 4H), 2.88 (q, J = 6.5 Hz, 2H), 2.30 (s, 6H), 1.64–1.50 (m, 4H). 13C NMR (150 MHz, DMSO-d6) δ 159.64, 149.98, 146.73, 143.04, 139.41, 137.72, 135.11, 133.27, 128.48, 127.70, 125.74, 125.11, 125.01, 124.10, 119.39, 115.29, 111.81, 42.76, 39.33, 26.97, 26.38, 21.21. HRMS: m/z C27H27ClN4O7S2: Calcd. 618.1010, Found 619.1082 [M+H]+. HPLC purity: 97.12%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(5-((4-nitrophenyl)sulfonamido)pentyl)-1H-indole-2-carboxamide (R3L5). White solid, Yield 67%, m.p.: 154–158 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.89 (s, 1H), 8.84 (t, J = 5.6 Hz, 1H), 8.37–8.28 (m, 2H), 8.01–7.94 (m, 2H), 7.91 (t, J = 5.7 Hz, 1H), 7.86 (d, J = 2.1 Hz, 1H), 7.54 (d, J = 1.6 Hz, 2H), 7.46 (d, J = 8.7 Hz, 1H), 7.26 (dd, J = 8.7, 2.1 Hz, 1H), 7.18 (s, 1H), 3.24–3.20 (m, 2H), 2.76 (q, J = 7.0 Hz, 2H), 2.23 (s, 6H), 1.54–1.19 (m, 6H). 13C NMR (150 MHz, DMSO-d6) δ 159.60, 149.98, 146.76, 143.07, 139.40, 137.82, 135.09, 133.26, 128.48, 127.69, 125.75, 125.08, 125.01, 124.08, 119.38, 115.28, 111.74, 42.99, 29.22, 28.71, 23.86, 21.21. HRMS: m/z C28H29ClN4O7S2: Calcd. 632.1166, Found 633.1239 [M+H]+. HPLC purity: 98.43%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(2-((4-fluorophenyl)sulfonamido)ethyl)-1H-indole-2-carboxamide (R4L2). White solid, Yield 65%, m.p.: 200–203 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.96 (s, 1H), 9.02 (t, J = 5.8 Hz, 1H), 7.94 (d, J = 2.1 Hz, 1H), 7.92–7.87 (m, 2H), 7.83 (t, J = 6.0 Hz, 1H), 7.61 (d, J = 1.6 Hz, 2H), 7.54 (d, J = 8.8 Hz, 1H), 7.47–7.40 (m, 2H), 7.35 (dd, J = 8.8, 2.1 Hz, 1H), 7.25 (s, 1H), 3.48–3.36 (m, 2H), 3.05–2.95 (m, 2H), 2.30 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ 165.46, 163.79, 159.92, 142.94, 139.43, 137.21, 137.17, 137.15, 135.16, 133.26, 130.02, 129.96, 127.77, 125.66, 125.24, 124.15, 119.43, 116.94, 116.79, 115.34, 112.13, 41.97, 21.18. HRMS: m/z C25H23ClFN3O5S2: Calcd. 563.0752, Found 564.0824 [M+H]+. HPLC purity: 95.64%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(3-((4-fluorophenyl)sulfonamido)propyl)-1H-indole-2-carboxamide (R4L3). White solid, Yield 68%, m.p.: 160–162 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.98 (s, 1H), 8.94 (t, J = 5.6 Hz, 1H), 7.92 (d, J = 2.1 Hz, 1H), 7.89–7.84 (m, 2H), 7.73 (t, J = 5.9 Hz, 1H), 7.62 (d, J = 1.5 Hz, 2H), 7.53 (d, J = 8.7 Hz, 1H), 7.45–7.37 (m, 2H), 7.34 (dd, J = 8.7, 2.1 Hz, 1H), 7.25 (s, 1H), 3.45–3.19 (m, 4H), 2.91 (q, J = 6.7 Hz, 2H), 2.31 (s, 6H), 1.73 (p, J = 7.1 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 165.35, 163.69, 159.83, 143.04, 139.39, 137.80, 137.43, 135.09, 133.28, 129.94, 129.88, 127.67, 125.64, 125.08, 124.15, 119.35, 116.82, 116.67, 115.27, 111.88, 40.93, 37.54, 29.50, 21.21. HRMS: m/z C26H25ClFN3O5S2: Calcd. 577.0908, Found 578.0981 [M+H]+.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(4-((4-fluorophenyl)sulfonamido)butyl)-1H-indole-2-carboxamide (R4L4). White solid, Yield 45%, m.p.: 179–180 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.92 (s, 1H), 8.86 (t, J = 5.6 Hz, 1H), 7.87 (d, J = 2.1 Hz, 1H), 7.83–7.72 (m, 2H), 7.59 (t, J = 5.9 Hz, 1H), 7.55 (d, J = 1.6 Hz, 2H), 7.46 (d, J = 8.7 Hz, 1H), 7.38–7.32 (m, 2H), 7.27 (dd, J = 8.7, 2.1 Hz, 1H), 7.18 (s, 1H), 3.23 (t, J = 6.4 Hz, 2H), 2.73 (q, J = 6.5 Hz, 2H), 2.23 (s, 6H), 1.58–1.34 (m, 4H). 13C NMR (150 MHz, DMSO-d6) δ 165.33, 163.66, 159.62, 143.06, 139.41, 137.73, 137.48, 137.46, 135.12, 133.27, 129.93, 129.87, 127.70, 125.76, 125.10, 124.11, 119.40, 116.81, 116.66, 115.29, 111.81, 42.69, 39.36, 26.90, 26.42, 21.21. HRMS: m/z C27H27ClFN3O5S2: Calcd. 591.1065, Found 592.1137 [M+H]+. HPLC purity: 97.17%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(5-((4-fluorophenyl)sulfonamido)pentyl)-1H-indole-2-carboxamide (R4L5). White solid, Yield 56%, m.p.: 159–160 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.98 (s, 1H), 8.92 (t, J = 5.6 Hz, 1H), 7.94 (d, J = 2.1 Hz, 1H), 7.89–7.80 (m, 2H), 7.68–7.58 (m, 3H), 7.53 (d, J = 8.8 Hz, 1H), 7.47–7.38 (m, 2H), 7.34 (dd, J = 8.8, 2.1 Hz, 1H), 7.25 (s, 1H), 3.30 (q, J = 6.7 Hz, 2H), 2.82–2.70 (m, 2H), 2.30 (s, 6H), 1.56–1.30 (m, 6H). 13C NMR (150 MHz, DMSO-d6) δ 165.32, 163.65, 159.59, 143.08, 139.40, 137.84, 137.54, 137.52, 135.10, 133.26, 129.92, 129.86, 127.68, 125.75, 125.08, 124.09, 119.38, 116.81, 116.66, 115.28, 111.74, 42.93, 29.15, 28.76, 23.92, 21.21. HRMS: m/z C28H29ClFN3O5S2: Calcd. 605.1221, Found 606.1294 [M+H]+. HPLC purity: 95.32%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(2-((4-cyanophenyl)sulfonamido)ethyl)-1H-indole-2-carboxamide (R5L2). White solid, Yield 69%, m.p.: 197–200 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.88 (s, 1H), 8.96 (t, J = 5.8 Hz, 1H), 8.04 (t, J = 5.9 Hz, 1H), 8.01–7.96 (m, 2H), 7.94–7.89 (m, 2H), 7.87 (d, J = 2.1 Hz, 1H), 7.53 (d, J = 1.6 Hz, 2H), 7.47 (d, J = 8.8 Hz, 1H), 7.28 (dd, J = 8.8, 2.1 Hz, 1H), 7.18 (s, 1H), 3.39–3.30 (m, 2H), 2.97 (q, J = 6.5 Hz, 2H), 2.22 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ 159.90, 144.93, 142.90, 139.45, 137.06, 135.19, 133.92, 133.25, 127.81, 127.73, 125.67, 125.29, 124.14, 119.44, 118.08, 115.46, 115.36, 112.13, 41.98, 39.69, 21.19. HRMS: m/z C26H23ClN4O5S2: Calcd. 571.0630, Found 571.0871 [M+H]+. HPLC purity: 97.69%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(3-((4-cyanophenyl)sulfonamido)propyl)-1H-indole-2-carboxamide (R5L3). White solid, Yield 65%, m.p.: 207–210 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.99 (s, 1H), 8.94 (t, J = 5.6 Hz, 1H), 8.06–7.99 (m, 3H), 7.99–7.95 (m, 2H), 7.91 (d, J = 2.1 Hz, 1H), 7.61 (d, J = 1.6 Hz, 2H), 7.53 (d, J = 8.8 Hz, 1H), 7.34 (dd, J = 8.7, 2.1 Hz, 1H), 7.26 (s, 1H), 3.35 (q, J = 6.7 Hz, 2H), 2.96 (q, J = 6.9 Hz, 2H), 2.31 (s, 6H), 1.73 (p, J = 7.1 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 159.88, 145.18, 143.02, 139.38, 137.86, 135.09, 133.83, 133.29, 127.69, 127.66, 125.60, 125.07, 124.16, 119.32, 118.16, 115.29, 115.27, 111.87, 40.97, 37.47, 29.55, 21.23. HRMS: m/z C27H25ClN4O5S2: Calcd. 584.0955, Found 585.1028 [M+H]+. HPLC purity: 98.56%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(4-((4-cyanophenyl)sulfonamido)butyl)-1H-indole-2-carboxamide (R5L4). White solid, Yield 51%, m.p.: 175–178 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.99 (s, 2H), 8.94 (t, J = 5.6 Hz, 2H), 8.07 (d, J = 8.4 Hz, 4H), 7.99–7.91 (m, 8H), 7.62 (s, 4H), 7.54 (d, J = 8.7 Hz, 2H), 7.34 (dd, J = 8.7, 2.1 Hz, 2H), 7.26 (s, 2H), 5.76 (s, 1H), 3.33–3.29 (m, 4H), 2.85 (q, J = 6.4 Hz, 4H), 2.51 (t, J = 1.9 Hz, 3H), 2.31 (s, 12H), 1.65–1.44 (m, 9H). 13C NMR (150 MHz, DMSO-d6) δ 159.64, 145.25, 143.04, 139.41, 137.74, 135.12, 133.85, 133.26, 127.70, 127.68, 125.73, 125.11, 124.11, 119.39, 118.18, 115.29, 115.27, 111.81, 55.34, 42.72, 39.33, 26.97, 26.38, 21.22. HRMS: m/z C28H27ClN4O5S2: Calcd. 598.1111, Found 599.1184 [M+H]+.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(5-((4-cyanophenyl)sulfonamido)pentyl)-1H-indole-2-carboxamide (R5L5). White solid, Yield 65%, m.p.: 165–170 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.91 (s, 1H), 8.85 (t, J = 5.6 Hz, 1H), 8.00 (d, J = 8.5 Hz, 2H), 7.91–7.79 (m, 4H), 7.54 (s, 2H), 7.46 (d, J = 8.7 Hz, 1H), 7.26 (dd, J = 8.8, 2.1 Hz, 1H), 7.18 (s, 1H), 3.25–3.19 (m, 2H), 2.73 (q, J = 6.8, 6.3 Hz, 2H), 2.45–2.42 (m, 2H), 2.23 (s, 6H), 1.49–1.24 (m, 6H). 13C NMR (150 MHz, DMSO-d6) δ 159.61, 145.30, 143.07, 139.40, 137.84, 135.10, 133.85, 133.26, 127.67, 125.74, 125.08, 124.10, 119.38, 118.19, 115.28, 115.26, 111.74, 42.96, 29.22, 28.72, 23.85, 21.22. HRMS: m/z C29H29ClN4O5S2: Calcd. 612.1268, Found 613.1341 [M+H]+. HPLC purity: 99.16%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(2-((4-bromophenyl)sulfonamido)ethyl)-1H-indole-2-carboxamide (R6L2). White solid, Yield 64%, m.p.: 220–224 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.95 (s, 1H), 9.02 (tt, J = 5.5, 2.4 Hz, 1H), 7.95 (q, J = 2.2 Hz, 1H), 7.89 (td, J = 6.0, 2.0 Hz, 1H), 7.81 (d, J = 8.5 Hz, 2H), 7.76 (dt, J = 8.5, 1.7 Hz, 2H), 7.61 (s, 2H), 7.55 (dd, J = 8.8, 1.5 Hz, 1H), 7.35 (dd, J = 8.8, 2.1 Hz, 1H), 7.24 (d, J = 3.6 Hz, 1H), 3.46–3.39 (m, 2H), 3.01 (qd, J = 5.9, 3.0 Hz, 2H), 2.29 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ 159.92, 142.96, 140.10, 139.44, 137.17, 135.17, 133.29, 132.82, 129.04, 127.80, 126.77, 125.71, 125.26, 124.15, 119.47, 115.36, 112.16, 41.99, 21.19. HRMS: m/z C25H23BrClN3O5S2: Calcd. 622.9951, Found 624.0024 [M+H]+. HPLC purity: 98.34%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(3-((4-bromophenyl)sulfonamido)propyl)-1H-indole-2-carboxamide (R6L3). White solid, Yield 80%, m.p.: 162–164 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.98 (s, 1H), 8.94 (t, J = 5.6 Hz, 1H), 7.93 (d, J = 2.1 Hz, 1H), 7.82–7.76 (m, 3H), 7.76–7.71 (m, 2H), 7.62 (d, J = 1.6 Hz, 2H), 7.53 (d, J = 8.7 Hz, 1H), 7.34 (dd, J = 8.7, 2.1 Hz, 1H), 7.25 (s, 1H), 3.36 (q, J = 6.7 Hz, 2H), 2.93 (q, J = 6.7 Hz, 2H), 2.31 (s, 6H), 1.74 (p, J = 7.1 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 159.85, 143.05, 140.36, 139.40, 137.79, 135.10, 133.30, 132.72, 128.99, 127.70, 126.56, 125.67, 125.10, 124.16, 119.37, 115.28, 111.91, 40.95, 37.53, 29.53, 21.22. HRMS: m/z C26H25BrClN3O5S2: Calcd. 637.0108, Found 638.0180 [M+H]+. HPLC purity: 96.51%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(4-((4-bromophenyl)sulfonamido)butyl)-1H-indole-2-carboxamide (R6L4). White solid, Yield 82%, m.p.: 186–190 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.98 (s, 1H), 8.93 (t, J = 5.7 Hz, 1H), 7.95 (d, J = 2.1 Hz, 1H), 7.83–7.78 (m, 2H), 7.74 (d, J = 8.6 Hz, 3H), 7.62 (d, J = 1.6 Hz, 2H), 7.54 (d, J = 8.7 Hz, 1H), 7.34 (dd, J = 8.7, 2.1 Hz, 1H), 7.26 (s, 1H), 2.82 (q, J = 6.4 Hz, 2H), 2.31 (s, 6H), 1.63–1.49 (m, 4H). 13C NMR (150 MHz, DMSO-d6) δ 159.63, 143.07, 140.41, 139.43, 137.72, 135.13, 133.28, 132.72, 128.99, 127.72, 126.51, 125.78, 125.12, 124.11, 119.42, 115.30, 111.83, 42.70, 39.36, 26.93, 26.41, 21.22. HRMS: m/z C27H27BrClN3O5S2: Calcd. 651.0264, Found 652.0337 [M+H]+. HPLC purity: 96.29%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(5-((4-bromophenyl)sulfonamido)pentyl)-1H-indole-2-carboxamide (R6L5). White solid, Yield 78%, m.p.: 145–148 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.97 (s, 1H), 8.92 (t, J = 5.6 Hz, 1H), 7.94 (d, J = 2.1 Hz, 1H), 7.82–7.78 (m, 2H), 7.73 (d, J = 2.0 Hz, 1H), 7.72–7.67 (m, 2H), 7.62 (d, J = 1.5 Hz, 2H), 7.54 (d, J = 8.7 Hz, 1H), 7.34 (dd, J = 8.8, 2.1 Hz, 1H), 7.25 (s, 1H), 3.30 (t, J = 6.6 Hz, 5H), 2.80–2.74 (m, 2H), 2.30 (s, 6H), 1.53 (p, J = 7.3 Hz, 2H), 1.44 (h, J = 8.5, 7.8 Hz, 2H), 1.36 (qd, J = 8.5, 7.2, 2.2 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 162.76, 159.60, 143.10, 140.46, 139.41, 137.81, 135.11, 133.28, 132.72, 128.98, 127.71, 126.50, 125.79, 125.10, 124.10, 119.41, 115.29, 111.76, 42.94, 39.70, 36.23, 31.26, 29.19, 28.75, 23.91, 21.22. HRMS: m/z C28H29BrClN3O5S2: Calcd. 665.0421, Found 666.0493 [M+H]+.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(2-((4-chlorophenyl)sulfonamido)ethyl)-1H-indole-2-carboxamide (R7L2). White solid, Yield 77%, m.p.: 217–220 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.89 (s, 2H), 8.96 (t, J = 5.8 Hz, 2H), 7.87 (d, J = 2.1 Hz, 2H), 7.83 (t, J = 5.9 Hz, 2H), 7.76 (d, J = 8.4 Hz, 4H), 7.59 (d, J = 8.6 Hz, 3H), 7.53 (s, 4H), 7.47 (d, J = 8.8 Hz, 2H), 7.27 (dd, J = 8.7, 2.1 Hz, 2H), 7.17 (s, 2H), 3.34 (q, J = 6.6 Hz, 4H), 2.93 (q, J = 6.7 Hz, 4H), 2.22 (s, 12H). 13C NMR (150 MHz, DMSO-d6) δ 159.93, 142.94, 139.65, 139.45, 137.88, 137.18, 135.17, 133.28, 129.88, 128.94, 127.79, 125.68, 125.26, 124.78, 124.16, 119.45, 115.36, 112.14, 41.97, 21.18. HRMS: m/z C25H23Cl2N3O5S2: Calcd. 579.0456, Found 580.0529 [M+H]+. HPLC purity: 98.60%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(3-((4-chlorophenyl)sulfonamido)propyl)-1H-indole-2-carboxamide (R7L3). White solid, Yield 79%, m.p.: 183–186 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.91 (s, 1H), 8.87 (t, J = 5.7 Hz, 1H), 7.85 (d, J = 2.1 Hz, 1H), 7.74 (dd, J = 8.6, 6.6 Hz, 3H), 7.60–7.53 (m, 4H), 7.46 (d, J = 8.7 Hz, 1H), 7.26 (dd, J = 8.8, 2.1 Hz, 1H), 7.17 (s, 1H), 3.29 (q, J = 6.8 Hz, 2H), 2.85 (q, J = 6.7 Hz, 2H), 2.23 (s, 6H), 1.66 (p, J = 7.1 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 159.86, 143.04, 139.92, 139.40, 137.81, 137.67, 135.10, 133.30, 129.78, 128.89, 127.69, 125.65, 125.09, 124.17, 119.36, 115.28, 111.90, 40.94, 37.53, 29.52, 21.22. HRMS: m/z C26H25Cl2N3O5S2: Calcd. 593.0613, Found 594.0685 [M+H]+.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(4-((4-chlorophenyl)sulfonamido)butyl)-1H-indole-2-carboxamide (R7L4). White solid, Yield 75%, m.p.: 196–198 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.99 (s, 1H), 8.94 (t, J = 5.7 Hz, 1H), 7.95 (t, J = 1.8 Hz, 1H), 7.84–7.79 (m, 2H), 7.74 (t, J = 5.8 Hz, 1H), 7.69–7.64 (m, 2H), 7.64–7.61 (m, 2H), 7.54 (d, J = 8.7 Hz, 1H), 7.34 (dd, J = 8.8, 2.1 Hz, 1H), 7.25 (s, 1H), 3.31 (t, J = 6.3 Hz, 2H), 2.30 (s, 6H), 1.63–1.49 (m, 4H). 13C NMR (150 MHz, DMSO-d6) δ 159.64, 143.07, 139.98, 139.43, 137.73, 137.63, 135.13, 133.28, 129.78, 128.88, 127.72, 125.77, 125.12, 124.12, 119.41, 115.30, 111.83, 42.70, 39.36, 26.92, 26.41, 21.21. HRMS: m/z C27H27Cl2N3O5S2: Calcd. 607.0769, Found 608.0842 [M+H]+. HPLC purity: 98.95%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(5-((4-chlorophenyl)sulfonamido)pentyl)-1H-indole-2-carboxamide (R7L5). White solid, Yield 82%, m.p.: 143–146 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.98 (s, 1H), 8.92 (t, J = 5.6 Hz, 1H), 7.95 (d, J = 2.1 Hz, 1H), 7.83–7.78 (m, 2H), 7.73–7.60 (m, 5H), 7.54 (d, J = 8.7 Hz, 1H), 7.34 (dd, J = 8.7, 2.1 Hz, 1H), 7.25 (s, 1H), 3.31 (q, J = 6.7 Hz, 4H), 2.76 (dq, J = 13.4, 6.6 Hz, 2H), 2.30 (s, 5H), 1.53 (p, J = 7.2 Hz, 2H), 1.48–1.31 (m, 5H), 1.26–1.18 (m, 1H). 13C NMR (150 MHz, DMSO-d6) δ 159.61, 143.08, 140.03, 139.41, 137.83, 137.61, 135.11, 133.27, 129.78, 128.88, 128.15, 127.70, 125.77, 125.10, 124.10, 119.40, 115.29, 111.75, 42.94, 42.85, 39.75, 39.69, 39.49, 29.17, 29.02, 28.75, 28.18, 23.90, 23.69, 21.21. HRMS: m/z C28H29Cl2N3O5S2: Calcd. 621.0926, Found 622.0998 [M+H]+.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(2-(thiophene-2-sulfonamido)ethyl)-1H-indole-2-carboxamide (R8L2). White solid, Yield 80%, m.p.: 216–218 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.90 (s, 1H), 8.98 (t, J = 5.8 Hz, 1H), 7.91 (t, J = 5.9 Hz, 1H), 7.87 (dd, J = 5.2, 1.5 Hz, 2H), 7.58–7.54 (m, 3H), 7.47 (d, J = 8.7 Hz, 1H), 7.27 (dd, J = 8.7, 2.1 Hz, 1H), 7.17 (s, 1H), 7.13 (dd, J = 5.0, 3.7 Hz, 1H), 3.37 (q, J = 6.6 Hz, 2H), 3.00 (q, J = 6.6 Hz, 2H), 2.23 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ 159.99, 142.96, 141.60, 139.44, 137.34, 135.16, 133.28, 133.08, 132.10, 128.21, 127.77, 125.65, 125.23, 124.19, 119.43, 115.34, 112.16, 42.19, 39.61, 21.21. HRMS: m/z C23H22ClN3O5S3: Calcd. 551.0410, Found 552.0483 [M+H]+. HPLC purity: 98.02%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(3-(thiophene-2-sulfonamido)propyl)-1H-indole-2-carboxamide (R8L3). White solid, Yield 70%, m.p.: 136–139 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.93 (s, 1H), 8.89 (t, J = 5.7 Hz, 1H), 7.90–7.79 (m, 3H), 7.56 (d, J = 1.6 Hz, 2H), 7.53 (dd, J = 3.7, 1.4 Hz, 1H), 7.46 (d, J = 8.7 Hz, 1H), 7.27 (dd, J = 8.7, 2.1 Hz, 1H), 7.18 (s, 1H), 7.10 (dd, J = 5.0, 3.7 Hz, 1H), 3.33–3.26 (m, 2H), 2.92 (q, J = 6.7 Hz, 2H), 2.82 (s, 1H), 2.23 (s, 6H), 1.69 (p, J = 7.1 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 162.76, 159.85, 143.05, 141.97, 139.41, 137.82, 135.11, 133.29, 132.74, 131.86, 128.10, 127.69, 125.66, 125.10, 124.18, 119.36, 115.29, 111.90, 41.17, 37.58, 36.23, 31.25, 29.42, 21.22. HRMS: m/z C24H24ClN3O5S3: Calcd. 565.0567, Found 566.0639 [M+H]+. HPLC purity: 98.00%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(4-(thiophene-2-sulfonamido)butyl)-1H-indole-2-carboxamide (R8L4). White solid, Yield 76%, m.p.: 188–190 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.93 (s, 1H), 8.88 (t, J = 5.6 Hz, 1H), 7.87 (d, J = 2.1 Hz, 1H), 7.84 (dd, J = 5.0, 1.4 Hz, 1H), 7.75 (t, J = 5.8 Hz, 1H), 7.55 (d, J = 1.6 Hz, 2H), 7.52 (dd, J = 3.7, 1.4 Hz, 1H), 7.46 (d, J = 8.7 Hz, 1H), 7.27 (dd, J = 8.7, 2.1 Hz, 1H), 7.18 (s, 1H), 7.10 (dd, J = 5.0, 3.7 Hz, 1H), 3.25 (t, J = 6.4 Hz, 2H), 2.82 (q, J = 6.4 Hz, 2H), 2.23 (s, 6H), 1.57–1.44 (m, 4H). 13C NMR (150 MHz, DMSO-d6) δ 159.65, 143.06, 142.02, 139.43, 137.77, 135.13, 133.27, 132.70, 132.66, 131.80, 128.09, 127.71, 125.76, 125.12, 124.13, 119.41, 115.30, 111.82, 42.92, 39.38, 26.78, 26.45, 21.22. HRMS: m/z C25H26ClN3O5S3: Calcd. 579.0723, Found 580.0796 [M+H]+. HPLC purity: 95.61%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(5-(thiophene-2-sulfonamido)pentyl)-1H-indole-2-carboxamide (R8L5). White solid, Yield 77%, m.p.: 168–170 °C. 1H NMR (600 MHz, DMSO-d6) δ 13.00 (s, 1H), 8.94 (t, J = 5.7 Hz, 1H), 7.97–7.89 (m, 2H), 7.79 (t, J = 5.8 Hz, 1H), 7.63 (d, J = 1.6 Hz, 2H), 7.58 (dd, J = 3.7, 1.4 Hz, 1H), 7.54 (d, J = 8.7 Hz, 1H), 7.34 (dd, J = 8.7, 2.1 Hz, 1H), 7.25 (s, 1H), 7.18 (dd, J = 5.0, 3.7 Hz, 1H), 3.32 (d, J = 6.4 Hz, 2H), 2.91–2.82 (m, 2H), 2.31 (s, 6H), 1.51 (dp, J = 40.7, 7.2 Hz, 4H), 1.38 (td, J = 8.4, 3.9 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 159.62, 143.09, 142.10, 139.41, 138.77, 137.86, 135.11, 134.72, 133.27, 132.65, 131.77, 128.08, 127.70, 125.76, 125.09, 124.80, 124.11, 120.34, 119.40, 115.29, 111.76, 43.17, 39.72, 29.04, 28.78, 23.96, 21.22. HRMS: m/z C26H28ClN3O5S3: Calcd. 593.0880, Found 594.0952 [M+H]+. HPLC purity: 97.81%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(2-(phenylsulfonamido)ethyl)-1H-indole-2-carboxamide (R9L2). White solid, Yield 76%, m.p.: 228–231 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.89 (s, 1H), 8.95 (t, J = 5.8 Hz, 1H), 7.86 (d, J = 2.1 Hz, 1H), 7.79–7.75 (m, 2H), 7.72 (t, J = 6.0 Hz, 1H), 7.60–7.50 (m, 5H), 7.47 (d, J = 8.7 Hz, 1H), 7.27 (dd, J = 8.7, 2.1 Hz, 1H), 7.17 (s, 1H), 3.34 (q, J = 6.6 Hz, 2H), 2.91 (q, J = 6.7 Hz, 2H), 2.22 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ 159.95, 142.95, 140.75, 139.44, 138.80, 137.31, 135.16, 133.26, 132.96, 129.75, 127.76, 126.95, 125.64, 125.23, 124.79, 124.18, 119.42, 115.34, 112.13, 41.96, 39.72, 21.20. HRMS: m/z C25H24ClN3O5S2: Calcd. 545.0846, Found 546.0919 [M+H]+. HPLC purity: 99.03%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(3-(phenylsulfonamido)propyl)-1H-indole-2-carboxamide (R9L3). White solid, Yield 79%, m.p.: 200–202 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.92 (s, 1H), 8.87 (t, J = 5.6 Hz, 1H), 7.86 (d, J = 2.0 Hz, 1H), 7.77–7.72 (m, 2H), 7.62 (t, J = 5.9 Hz, 1H), 7.57–7.53 (m, 3H), 7.50 (dd, J = 8.2, 6.6 Hz, 2H), 7.46 (d, J = 8.7 Hz, 1H), 7.26 (dd, J = 8.7, 2.1 Hz, 1H), 7.16 (s, 1H), 3.30–3.26 (m, 3H), 2.83 (q, J = 6.7 Hz, 2H), 2.22 (s, 6H), 1.66 (p, J = 7.1 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 159.83, 143.05, 141.05, 139.40, 137.79, 135.10, 133.29, 132.76, 129.64, 127.70, 126.90, 125.67, 125.10, 124.17, 119.37, 115.29, 111.90, 40.94, 37.57, 29.57, 21.21. HRMS: m/z C26H26ClN3O5S2: Calcd. 559.1002, Found 560.1075 [M+H]+. HPLC purity: 98.49%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(4-(phenylsulfonamido)butyl)-1H-indole-2-carboxamide (R9L4). White solid, Yield 83%, m.p.: 186–188 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.92 (s, 1H), 8.86 (t, J = 5.6 Hz, 1H), 7.87 (d, J = 2.1 Hz, 1H), 7.77–7.71 (m, 2H), 7.58–7.49 (m, 6H), 7.46 (d, J = 8.7 Hz, 1H), 7.27 (dd, J = 8.8, 2.1 Hz, 1H), 3.23 (d, J = 6.3 Hz, 2H), 2.73 (q, J = 6.5 Hz, 2H), 2.23 (s, 6H), 1.54–1.41 (m, 4H). 13C NMR (150 MHz, DMSO-d6) δ 159.63, 143.06, 141.06, 139.43, 137.75, 135.13, 133.27, 132.73, 129.63, 127.71, 126.90, 125.76, 125.12, 124.12, 119.40, 115.30, 111.81, 42.69, 39.37, 26.92, 26.42, 21.22. HRMS: m/z C27H28ClN3O5S2: Calcd. 573.1159, Found 574.1232 [M+H]+.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(5-(phenylsulfonamido)pentyl)-1H-indole-2-carboxamide (R9L5). White solid, Yield 77%, m.p.: 197–200 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.91 (s, 1H), 8.84 (t, J = 5.7 Hz, 1H), 7.86 (d, J = 2.1 Hz, 1H), 7.72 (dt, J = 7.0, 1.4 Hz, 2H), 7.59–7.48 (m, 6H), 7.46 (d, J = 8.7 Hz, 1H), 7.27 (dd, J = 8.7, 2.1 Hz, 1H), 7.18 (s, 1H), 3.25–3.19 (m, 2H), 2.71–2.65 (m, 2H), 2.23 (s, 6H), 1.43 (q, J = 7.4 Hz, 2H), 1.35 (h, J = 7.3 Hz, 2H), 1.27 (qd, J = 8.5, 7.2, 2.0 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 159.60, 143.09, 141.14, 139.41, 137.85, 135.11, 133.27, 132.72, 129.62, 127.69, 126.89, 125.76, 125.09, 124.10, 119.39, 115.29, 111.75, 42.94, 39.70, 29.19, 28.76, 23.92, 21.22. HRMS: m/z C28H30ClN3O5S2: Calcd. 587.1315, Found 588.1388 [M+H]+. HPLC purity: 97.04%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(2-(pyridine-3-sulfonamido)ethyl)-1H-indole-2-carboxamide (R10L2). White solid, Yield 76%, m.p.: 216–218 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.97 (s, 1H), 9.06 (t, J = 5.8 Hz, 1H), 9.02–8.98 (m, 1H), 8.82 (dd, J = 4.8, 1.6 Hz, 1H), 8.22 (dt, J = 8.0, 2.0 Hz, 1H), 8.06 (t, J = 5.9 Hz, 1H), 7.94 (d, J = 2.1 Hz, 1H), 7.66 (ddd, J = 8.1, 4.8, 0.9 Hz, 1H), 7.62 (d, J = 1.5 Hz, 2H), 7.55 (d, J = 8.8 Hz, 1H), 7.35 (dd, J = 8.8, 2.1 Hz, 1H), 7.25 (s, 1H), 3.47–3.40 (m, 2H), 3.05 (q, J = 6.6 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 159.97, 153.61, 147.54, 142.93, 139.45, 137.21, 137.14, 135.17, 135.02, 133.27, 127.78, 125.66, 125.25, 124.81, 124.18, 119.43, 115.36, 112.15, 41.95, 39.70, 21.19. HRMS: m/z C24H23ClN4O5S2: Calcd. 546.0798, Found 547.0871 [M+H]+. HPLC purity: 98.64%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(3-(pyridine-3-sulfonamido)propyl)-1H-indole-2-carboxamide (R10L3). White solid, Yield 82%, m.p.: 178–180 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.92 (s, 1H), 8.92–8.85 (m, 2H), 8.73 (dd, J = 4.8, 1.6 Hz, 1H), 8.12 (dt, J = 8.1, 2.0 Hz, 1H), 7.89 (t, J = 5.8 Hz, 1H), 7.85 (d, J = 2.1 Hz, 1H), 7.58–7.52 (m, 3H), 7.45 (d, J = 8.7 Hz, 1H), 7.26 (dd, J = 8.8, 2.1 Hz, 1H), 7.17 (s, 1H), 3.29 (q, J = 6.6 Hz, 2H), 2.90 (q, J = 6.7 Hz, 2H), 2.23 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ 159.88, 153.42, 147.49, 143.05, 139.39, 137.87, 137.41, 135.09, 134.93, 133.29, 127.68, 125.63, 125.08, 124.72, 124.18, 119.35, 115.27, 111.90, 40.93, 37.50, 31.39, 29.59, 21.21, 14.54, 14.38. HRMS: m/z C25H25ClN4O5S2: Calcd. 560.0955, Found 561.1028 [M+H]+. HPLC purity: 99.51%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(4-(pyridine-3-sulfonamido)butyl)-1H-indole-2-carboxamide (R10L4). White solid, Yield 71%, m.p.: 198–200 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.92 (s, 1H), 8.91–8.85 (m, 2H), 8.74 (dd, J = 4.8, 1.6 Hz, 1H), 8.11 (dt, J = 8.1, 2.0 Hz, 1H), 7.87 (d, J = 2.1 Hz, 1H), 7.81 (t, J = 5.8 Hz, 1H), 7.59–7.54 (m, 3H), 7.46 (d, J = 8.7 Hz, 1H), 7.27 (dd, J = 8.7, 2.1 Hz, 1H), 7.17 (s, 1H), 3.26–3.22 (m, 2H), 2.79 (q, J = 6.4 Hz, 2H), 2.23 (s, 6H), 1.56–1.42 (m, 4H). 13C NMR (150 MHz, DMSO-d6) δ 159.66, 153.40, 147.49, 143.06, 139.43, 138.81, 137.76, 137.43, 135.13, 134.93, 133.27, 127.71, 125.75, 125.11, 124.72, 124.13, 119.40, 115.30, 111.82, 42.69, 39.35, 26.96, 26.39, 21.22. HRMS: m/z C26H27ClN4O5S2: Calcd. 574.1111, Found 575.1184 [M+H]+. HPLC purity: 99.43%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(5-(pyridine-3-sulfonamido)pentyl)-1H-indole-2-carboxamide (R10L5). White solid, Yield 74%, m.p.: 178–180 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.92 (s, 1H), 8.90–8.83 (m, 2H), 8.74 (dd, J = 4.8, 1.6 Hz, 1H), 8.10 (dt, J = 8.0, 2.0 Hz, 1H), 7.86 (d, J = 2.1 Hz, 1H), 7.79 (t, J = 5.8 Hz, 1H), 7.57 (ddd, J = 8.1, 4.8, 0.8 Hz, 2H), 7.55 (d, J = 1.6 Hz, 2H), 7.46 (d, J = 8.7 Hz, 1H), 7.27 (dd, J = 8.8, 2.1 Hz, 1H), 7.19–7.16 (m, 1H), 3.23 (q, J = 6.6 Hz, 2H), 2.74 (q, J = 7.0 Hz, 2H), 2.23 (s, 6H), 1.45 (p, J = 7.3 Hz, 2H), 1.37 (p, J = 7.2 Hz, 2H), 1.28 (qd, J = 8.6, 7.3, 2.2 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 159.61, 153.39, 147.48, 143.08, 139.41, 137.85, 137.50, 135.11, 134.92, 133.27, 127.69, 125.75, 125.09, 124.72, 124.11, 119.39, 115.29, 111.75, 42.92, 39.68, 29.21, 28.74, 23.87, 21.21. HRMS: m/z C27H29ClN4O5S2: Calcd. 588.1268, Found 589.1341 [M+H]+. HPLC purity: 98.19%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(2-((2-fluorophenyl)sulfonamido)ethyl)-1H-indole-2-carboxamide (R11L2). White solid, Yield 82%, m.p.: 210–212 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.89 (s, 1H), 8.96 (t, J = 5.8 Hz, 1H), 8.01 (t, J = 5.9 Hz, 1H), 7.86 (d, J = 2.1 Hz, 1H), 7.76 (td, J = 7.6, 1.8 Hz, 1H), 7.65–7.58 (m, 1H), 7.54 (d, J = 1.6 Hz, 2H), 7.47 (d, J = 8.8 Hz, 1H), 7.37 (ddd, J = 10.6, 8.3, 1.1 Hz, 1H), 7.32 (td, J = 7.6, 1.1 Hz, 1H), 7.27 (dd, J = 8.8, 2.1 Hz, 1H), 7.16 (s, 1H), 3.36 (q, J = 6.7 Hz, 2H), 3.04 (q, J = 6.7 Hz, 2H), 2.22 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ 159.96, 159.51, 157.83, 142.96, 139.43, 138.83, 137.30, 135.77, 135.71, 135.15, 134.79, 133.26, 130.10, 128.79, 128.69, 127.77, 125.65, 125.39, 125.37, 125.23, 124.18, 119.43, 117.84, 117.70, 115.33, 112.15, 41.81, 39.86, 21.18. HRMS: m/z C25H23ClFN3O5S2: Calcd. 563.0752, Found 564.0824 [M+H]+. HPLC purity: 99.04%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(3-((2-fluorophenyl)sulfonamido)propyl)-1H-indole-2-carboxamide (R11L3). White solid, Yield 70%, m.p.: 218–220 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.92 (s, 1H), 8.87 (t, J = 5.6 Hz, 1H), 7.92 (t, J = 5.8 Hz, 1H), 7.86 (t, J = 1.6 Hz, 1H), 7.74 (td, J = 7.6, 1.8 Hz, 1H), 7.60 (dddt, J = 11.2, 9.2, 6.1, 3.0 Hz, 1H), 7.55 (d, J = 1.6 Hz, 2H), 7.46 (d, J = 8.8 Hz, 1H), 7.35 (ddd, J = 10.6, 8.3, 1.1 Hz, 1H), 7.32–7.23 (m, 2H), 7.16 (s, 1H), 3.29 (t, J = 6.6 Hz, 2H), 2.95 (q, J = 6.6 Hz, 2H), 2.22 (s, 6H), 1.68 (p, J = 7.1 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 159.84, 159.51, 157.83, 143.04, 139.40, 137.76, 135.58, 135.52, 135.10, 133.29, 130.11, 129.05, 128.95, 127.70, 125.67, 125.29, 125.27, 125.10, 124.16, 119.37, 117.75, 117.62, 115.28, 111.91, 55.34, 40.81, 37.51, 36.23, 31.25, 29.69, 21.20. HRMS: m/z C26H25ClFN3O5S2: Calcd. 577.0908, Found 578.0981 [M+H]+. HPLC purity: 98.44%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(4-((2-fluorophenyl)sulfonamido)butyl)-1H-indole-2-carboxamide (R11L4). Faint yellow solid, Yield 77%, m.p.: 210–212 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.92 (s, 1H), 8.86 (t, J = 5.6 Hz, 1H), 7.89–7.83 (m, 2H), 7.73 (td, J = 7.6, 1.8 Hz, 1H), 7.60 (dddd, J = 8.2, 7.0, 5.0, 1.8 Hz, 1H), 7.55 (d, J = 1.7 Hz, 2H), 7.46 (d, J = 8.7 Hz, 1H), 7.36 (ddd, J = 10.5, 8.3, 1.1 Hz, 1H), 7.32–7.24 (m, 2H), 7.17 (s, 1H), 3.24 (t, J = 6.4 Hz, 2H), 2.85 (q, J = 6.4 Hz, 2H), 2.23 (s, 6H), 1.55–1.42 (m, 4H). 13C NMR (150 MHz, DMSO-d6) δ 159.62, 159.51, 157.83, 143.06, 139.43, 137.72, 135.53, 135.48, 135.13, 133.27, 130.11, 129.09, 129.00, 127.72, 125.77, 125.30, 125.28, 125.12, 124.12, 119.41, 117.74, 117.60, 115.30, 111.82, 42.55, 39.34, 27.01, 26.33, 21.21. HRMS: m/z C27H27ClFN3O5S2: Calcd. 591.1065, Found 592.1137 [M+H]+. HPLC purity: 97.24%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(2-((3-fluorophenyl)sulfonamido)ethyl)-1H-indole-2-carboxamide (R12L2). White solid, Yield 82%, m.p.: 205–208 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.89 (s, 1H), 8.96 (t, J = 5.8 Hz, 1H), 7.86 (t, J = 4.5 Hz, 2H), 7.61 (td, J = 5.2, 4.4, 2.1 Hz, 1H), 7.59 (dd, J = 7.8, 5.1 Hz, 1H), 7.56–7.52 (m, 3H), 7.47 (d, J = 8.8 Hz, 1H), 7.44 (ddt, J = 9.2, 6.3, 1.7 Hz, 1H), 7.28 (dd, J = 8.7, 2.1 Hz, 1H), 7.17 (s, 1H), 3.38–3.32 (m, 2H), 2.95 (dt, J = 7.6, 6.1 Hz, 2H), 2.22 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ 163.11, 161.46, 159.95, 142.94, 142.91, 142.87, 139.44, 137.20, 135.17, 133.27, 132.21, 132.16, 127.78, 125.67, 125.25, 124.17, 123.24, 123.22, 120.22, 120.08, 119.43, 115.35, 114.10, 113.94, 112.15, 41.99, 21.18. HRMS: m/z C25H23ClFN3O5S2: Calcd. 563.0752, Found 564.0824 [M+H]+. HPLC purity: 99.58%.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(3-((3-fluorophenyl)sulfonamido)propyl)-1H-indole-2-carboxamide (R12L3). White solid, Yield 65%, m.p.: 185–188 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.91 (s, 1H), 8.86 (t, J = 5.7 Hz, 1H), 7.85 (d, J = 2.1 Hz, 1H), 7.76 (t, J = 5.9 Hz, 1H), 7.60 (dt, J = 7.8, 1.5 Hz, 1H), 7.58–7.54 (m, 2H), 7.54–7.51 (m, 2H), 7.45 (d, J = 8.8 Hz, 1H), 7.42 (dddd, J = 9.0, 8.0, 2.7, 1.4 Hz, 1H), 7.26 (dd, J = 8.7, 2.1 Hz, 1H), 7.17 (s, 1H), 3.28 (q, J = 6.7 Hz, 2H), 2.87 (q, J = 6.7 Hz, 2H), 2.23 (s, 6H), 1.67 (p, J = 7.1 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 163.08, 161.44, 159.85, 143.24, 143.20, 143.06, 139.39, 137.81, 135.09, 133.29, 132.08, 132.03, 127.68, 125.66, 125.09, 124.16, 123.17, 123.15, 120.01, 119.87, 119.36, 115.28, 114.02, 113.86, 111.91, 40.97, 37.52, 29.57, 21.21. HRMS: m/z C26H25ClFN3O5S2: Calcd. 577.0908, Found 578.0981 [M+H]+.
5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(4-((3-fluorophenyl)sulfonamido)butyl)-1H-indole-2-carboxamide (R12L4). White solid, Yield 72%, m.p.: 196–198 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.93 (s, 1H), 8.88 (t, J = 5.7 Hz, 1H), 7.87 (d, J = 2.1 Hz, 1H), 7.71 (t, J = 5.9 Hz, 1H), 7.61–7.52 (m, 5H), 7.52–7.48 (m, 1H), 7.48–7.39 (m, 3H), 7.27 (dd, J = 8.7, 2.1 Hz, 1H), 7.20–7.16 (m, 1H), 3.24 (q, J = 6.5 Hz, 2H), 2.76 (q, J = 6.4 Hz, 2H), 2.23 (s, 6H), 1.55–1.41 (m, 4H). 13C NMR (150 MHz, DMSO-d6) δ 163.08, 161.44, 159.65, 143.23, 143.19, 143.05, 139.43, 137.76, 135.13, 133.27, 132.09, 132.04, 127.70, 125.74, 125.11, 124.13, 123.18, 123.16, 123.13, 119.99, 119.85, 119.39, 115.30, 114.00, 113.96, 113.84, 113.80, 111.81, 42.72, 42.53, 39.34, 26.93, 26.64, 26.39, 21.21. HRMS: m/z C27H27ClFN3O5S2: Calcd. 591.1065, Found 592.1137 [M+H]+. HPLC purity: 96.41%.
5-Chloro-N-(2-(cyclopropanesulfonamido)ethyl)-3-((3,5-dimethylphenyl)sulfonyl)-1H-indole-2-carboxamide (R13L2). White solid, Yield 66%, m.p.: 210–212 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.02 (t, J = 5.8 Hz, 1H), 7.87 (d, J = 2.1 Hz, 1H), 7.58 (d, J = 1.6 Hz, 2H), 7.48 (d, J = 8.8 Hz, 1H), 7.28 (dd, J = 8.8, 2.1 Hz, 1H), 7.18 (s, 1H), 7.12 (t, J = 6.1 Hz, 1H), 3.46–3.40 (m, 2H), 3.20–3.13 (m, 2H), 2.43 (p, J = 1.9 Hz, 1H), 2.24 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ 160.07, 142.99, 139.44, 137.53, 135.16, 133.30, 127.76, 125.64, 125.21, 124.23, 119.40, 115.35, 112.12, 42.01, 40.59, 40.27, 29.85, 21.21, 5.20. HRMS: m/z C22H24ClN3O5S2: Calcd. 509.0846, Found 510.0919 [M+H]+. HPLC purity: 98.60%.
N-(2-([1,1′-Biphenyl]-4-sulfonamido)ethyl)-5-chloro-3-((3,5-dimethylphenyl)sulfonyl)-1H-indole-2-carboxamide (R14L2). White solid, Yield 74%, m.p.: 250–254 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.89 (s, 1H), 8.97 (t, J = 5.8 Hz, 1H), 7.87 (d, J = 2.1 Hz, 1H), 7.86–7.82 (m, 2H), 7.82–7.75 (m, 3H), 7.64–7.59 (m, 2H), 7.54 (d, J = 1.7 Hz, 2H), 7.45 (d, J = 8.8 Hz, 1H), 7.44–7.39 (m, 2H), 7.38–7.33 (m, 1H), 7.26 (dd, J = 8.7, 2.1 Hz, 1H), 7.14 (s, 1H), 3.42–3.35 (m, 2H), 2.98 (q, J = 6.6 Hz, 2H), 2.19 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ 159.93, 144.51, 142.94, 139.52, 139.44, 138.94, 137.19, 135.16, 133.26, 129.54, 128.92, 127.90, 127.80, 127.68, 127.48, 125.69, 125.25, 124.16, 119.45, 115.34, 112.14, 42.05, 21.17. HRMS: m/z C31H28ClN3O5S2: Calcd. 621.1159, Found 622.1232 [M+H]+.
4.2. In vitro anti-HIV assays
Our previously established assay was conducted to evaluate the cellular activity and cytotoxicity of these target compounds13. The compounds to be measured and drugs as positive references were weighted and prepared into stock solutions of specific concentrations in DMSO. Before testing, the stock solutions were diluted into 5 concentration gradients by purified water, meanwhile three duplicates were set for each concentration. Each sample included the untreated HIV-1 infection and mock-infected cells. HIV-1 WT strain, mutant strains L100I, K103N, Y181C, Y188L, E138K, F227L + V106A and RES056 stock solution (50 μL) at 100–300 CCID50 (50% cell culture infectious dose) or culture medium was added to either the infected or mock-infected wells. Mock-infected groups were established to measure the cytotoxicity target compounds. To each well of the cell culture plate, 100 μL medium, 50 μL MT-4 cell culture medium, and 20 μL HIV-infected MT-4 cell suspension or blank medium were added in sequence. The processed cells were incubated in constant environment for 5 days. MTT solution (20 mL, 5 mg/mL) was added to each well, and the cell culture was centrifuged at 1000 rpm to remove the supernatant after 2 h. The cell viability in mock- and HIV-infected cultures was examined spectrophotometrically with MTT method. The absorbance values were used to calculated the EC50 and CC50 values. SI values were acquired as a ratio between CC50 and EC50.
4.3. HIV-1 RT inhibition assays
The enzymic inhibition assay was ran by a RT kit purchased from Roche. The relevant ELISA method in the process was performed under the guidance of kit protocol. The template/primer complex, nucleotide (dNTPs) and HIV-1 RT was pre-diluted and mixed under the guidance of assay kit. Assay buffers containing desired concentration of each compound were added as well. The mixtures were allowed to incubate at 37 °C, meanwhile gently shaked for 1 h. Then they were transferred to streptavidin-coated microplate and further incubated at 37 °C for another 2 h. Each well was washed 5 times using the assay buffer. After digoxin antibody-peroxidase solution was added, the whole plate was further incubated for 1 h. Subsequently, the washing procedure above was repeated, and followed by adding the ABST peroxide substrate solution. A microplate reader was applied to measure the absorbance value promptly. In the process, the negative control and the blank group were set up. The percentage inhibitory activity of the tested compounds was calculated by Eq. (1):
| Inhibition (%) = [ODnegative–ODtest] / [ODtest–ODblank] | (1) |
The IC50 values represents the concentrations of the inhibitors required to inhibit biotin-dUTP incorporation by 50%.
Acknowledgments
We gratefully acknowledge financial support from Natural Science Foundation of China (81974507), Guangdong Basic and Applied Basic Research Foundation (2021A1515110740, China), China Postdoctoral Science Foundation (2021M702003), Shandong Province Natural Science Foundation for Youths (ZR2022QH036, China), the Foundation for Innovative Research Groups of State Key Laboratory of Microbial Technology (WZCX2021-03, China), Foreign cultural and educational experts Project (GXL20200015001, China), Science Foundation for Outstanding Young Scholars of Shandong Province (ZR2020JQ31, China), the Shandong Provincial Key research and development project (2019JZZY021011, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2023.01.003.
Contributor Information
Christophe Pannecouque, Email: christophe.pannecouque@kuleuven.be.
Peng Zhan, Email: zhanpeng1982@sdu.edu.cn.
Xinyong Liu, Email: xinyongl@sdu.edu.cn.
Author contributions
Xinyong Liu, Peng Zhan, and Yajie Tang as the supervisors conceived the project and supplied the financial support. Shenghua Gao and Yusen Cheng conducted the synthesis of compounds. Letian Song, Mianling Yang and Bing Ye complete the structure confirmation. Shenghua Gao and Letian Song completed the manuscript. Erik De Clercq and Christophe Pannecouque conducted and supervised the activity assay. The molecular docking was conducted by Fabao Zhao and Letian Song. Shenghua Gao designed the pharmacokinetics, acute toxicity and subacute toxicity experiment. Dongwei Kang, Shu Song, and Wei Zhao done the data analysis.
Conflicts of interest
There are no conflicts of interest to declare.
Appendix ASupplementary data
The following is the Supplementary data to this article:
References
- 1.De Clercq E. Antivirals: past, present and future. Biochem Pharmacol. 2013;85:727–744. doi: 10.1016/j.bcp.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Shattock R.J., Warren M., McCormack S., Hankins C.A. AIDS. Turning the tide against HIV. Science. 2011;333:42–43. doi: 10.1126/science.1206399. [DOI] [PubMed] [Google Scholar]
- 3.Cilento M.E., Kirby K.A., Sarafianos S.G. Avoiding drug resistance in HIV reverse transcriptase. Chem Rev. 2021;121:3271–3296. doi: 10.1021/acs.chemrev.0c00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li G., Wang Y., De Clercq E. Approved HIV reverse transcriptase inhibitors in the past decade. Acta Pharm Sin B. 2022;12:1567–1590. doi: 10.1016/j.apsb.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhuang C., Pannecouque C., De Clercq E., Chen F. Development of non-nucleoside reverse transcriptase inhibitors (NNRTIs): our past twenty years. Acta Pharm Sin B. 2020;10:961–978. doi: 10.1016/j.apsb.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrijvers R. Etravirine for the treatment of HIV/AIDS. Expet Opin Pharmacother. 2013;14:1087–1096. doi: 10.1517/14656566.2013.787411. [DOI] [PubMed] [Google Scholar]
- 7.Rai M.A., Pannek S., Fichtenbaum C.J. Emerging reverse transcriptase inhibitors for HIV-1 infection. Expet Opin Emerg Drugs. 2018;23:149–157. doi: 10.1080/14728214.2018.1474202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen R., Quinones-Mateu M.E., Mansky L.M. Drug resistance, virus fitness and HIV-1 mutagenesis. Curr Pharmaceut Des. 2004;10:4065–4070. doi: 10.2174/1381612043382404. [DOI] [PubMed] [Google Scholar]
- 9.Menéndez-Arias L. Mutation rates and intrinsic fidelity of retroviral reverse transcriptases. Viruses. 2009;1:1137–1165. doi: 10.3390/v1031137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menéndez-Arias L. Molecular basis of human immunodeficiency virus drug resistance: an update. Antivir Res. 2010;85:210–231. doi: 10.1016/j.antiviral.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Benedicto A.M., Fuster-Martínez I., Tosca J., Esplugues J.V., Blas-García A., Apostolova N. NNRTI and liver damage: evidence of their association and the mechanisms involved. Cells. 2021;10:1687. doi: 10.3390/cells10071687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Famiglini V., Silvestri R. Indolylarylsulfones, a fascinating story of highly potent human immunodeficiency virus type 1 non-nucleoside reverse transcriptase inhibitors. Antivir Chem Chemother. 2018;26 doi: 10.1177/2040206617753443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silvestri R., De Martino G., La Regina G., Artico M., Massa S., Vargiu L., et al. Novel indolyl aryl sulfones active against HIV-1 carrying NNRTI resistance mutations: synthesis and SAR studies. J Med Chem. 2003;46:2482–2493. doi: 10.1021/jm0211063. [DOI] [PubMed] [Google Scholar]
- 14.Zhao T., Meng Q., Kang D., Ji J., de Clercq E., Pannecouque C., et al. Discovery of novel indolylarylsulfones as potent HIV-1 NNRTIs via structure-guided scaffold morphing. Eur J Med Chem. 2019;182 doi: 10.1016/j.ejmech.2019.111619. [DOI] [PubMed] [Google Scholar]
- 15.Li X., Gao P., Huang B., Zhou Z., Yu Z., Yuan Z., et al. Discovery of novel piperidine-substituted indolylarylsulfones as potent HIV NNRTIs via structure-guided scaffold morphing and fragment rearrangement. Eur J Med Chem. 2017;126:190–201. doi: 10.1016/j.ejmech.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 16.La Regina G., Coluccia A., Brancale A., Piscitelli F., Gatti V., Maga G., et al. Indolylarylsulfones as HIV-1 non-nucleoside reverse transcriptase inhibitors: new cyclic substituents at indole-2-carboxamide. J Med Chem. 2011;54:1587–1598. doi: 10.1021/jm101614j. [DOI] [PubMed] [Google Scholar]
- 17.Gao S., Cheng Y., Song S., Song L., Zhao F., Xu S., et al. Chemical space exploration around indolylarylsulfone scaffold led to a novel class of highly active HIV-1 NNRTIs with spiro structural features. Eur J Med Chem. 2022;238 doi: 10.1016/j.ejmech.2022.114471. [DOI] [PubMed] [Google Scholar]
- 18.Young S.D., Amblard M.C., Brichter S.F., Grey V.E., Tran L.O., Lumma W.C., et al. 2-Heterocyclic indole-3-sulfones as inhibitors of HIV-1 reverse transcriptase. Bioorg Med Chem Lett. 1995;5:491–496. [Google Scholar]
- 19.Zhao Z., Wolkenberg S.E., Sanderson P.E., Lu M., Munshi V., Moyer G., et al. Novel indole-3-sulfonamides as potent HIV non-nucleoside reverse transcriptase inhibitors (NNRTIs) Bioorg Med Chem Lett. 2008;18:554–559. doi: 10.1016/j.bmcl.2007.11.085. [DOI] [PubMed] [Google Scholar]
- 20.Kang D., Ruiz F.X., Sun Y., Feng D., Jing L., Wang Z., et al. 2,4,5-Trisubstituted pyrimidines as potent HIV-1 NNRTIs: rational design, synthesis, activity evaluation, and crystallographic studies. J Med Chem. 2021;64:4239–4256. doi: 10.1021/acs.jmedchem.1c00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arabi A.A. Routes to drug design via bioisosterism of carboxyl and sulfonamide groups. Future Med Chem. 2017;9:2167–2180. doi: 10.4155/fmc-2017-0136. [DOI] [PubMed] [Google Scholar]
- 22.Mustafa M., Winum J.Y. The importance of sulfur-containing motifs in drug design and discovery. Expet Opin Drug Discov. 2022;17:501–512. doi: 10.1080/17460441.2022.2044783. [DOI] [PubMed] [Google Scholar]
- 23.Supuran C.T., Innocenti A., Mastrolorenzo A., Scozzafava A. Antiviral sulfonamide derivatives. Mini Rev Med Chem. 2004;4:189–200. doi: 10.2174/1389557043487402. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y., Lu C., Wang C., Wang R., Liu K., Yang L., et al. Identification of the novel N-phenylbenzenesulfonamide derivatives as potent HIV inhibitors. Chin Chem Lett. 2015;26:243–247. [Google Scholar]
- 25.Han S., Lei Y., Pannecouque C., De Clercq E., Zhuang C., Chen F. Fragment-based discovery of sulfur-containing diarylbenzopyrimidines as novel nonnucleoside reverse transcriptase inhibitors. Chin Chem Lett. 2020;31:764–768. [Google Scholar]
- 26.Gulçin İ., Taslimi P. Sulfonamide inhibitors: a patent review 2013-present. Expert Opin Ther Pat. 2018;28:541–549. doi: 10.1080/13543776.2018.1487400. [DOI] [PubMed] [Google Scholar]
- 27.Zhan P., Liu X., De Clercq E. Recent advances in antiviral activity of benzo/heterothiadiazine dioxide derivatives. Curr Med Chem. 2008;15:1529–1540. doi: 10.2174/092986708784638898. [DOI] [PubMed] [Google Scholar]
- 28.Sun L., Dick A., Meuser M.E., Huang T., Zalloum W.A., Chen C.H., et al. Design, synthesis, and mechanism study of benzenesulfonamide-containing phenylalanine derivatives as novel HIV-1 capsid inhibitors with improved antiviral activities. J Med Chem. 2020;63:4790–4810. doi: 10.1021/acs.jmedchem.0c00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren Y., Ma Y., Cherukupalli S., Tavis J.E., Menéndez-Arias L., Liu X., et al. Discovery and optimization of benzenesulfonamides-based hepatitis B virus capsid modulators via contemporary medicinal chemistry strategies. Eur J Med Chem. 2020;206 doi: 10.1016/j.ejmech.2020.112714. [DOI] [PubMed] [Google Scholar]
- 30.Kang D., Wang Z., Zhang H., Wu G., Zhao T., Zhou Z., et al. Further exploring solvent-exposed tolerant regions of allosteric binding pocket for novel HIV-1 NNRTIs discovery. ACS Med Chem Lett. 2018;9:370–375. doi: 10.1021/acsmedchemlett.8b00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang D., Feng D., Sun Y., Fang Z., Wei F., de Clercq E., et al. Structure-based bioisosterism yields HIV-1 NNRTIs with improved drug-resistance profiles and favorable pharmacokinetic properties. J Med Chem. 2020;63:4837–4848. doi: 10.1021/acs.jmedchem.0c00117. [DOI] [PubMed] [Google Scholar]
- 32.HIV Drug Resistance Database. NNRTI Resistance Notes. Accessed 27 November 2022. Available from: https://hivdb.stanford.edu/dr-summary/resistance-notes/NNRTI/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.