Abstract
The occurrence of obesity has increased across the whole world. Many epidemiological studies have indicated that obesity strongly contributes to the development of cancer, cardiovascular diseases, type 2 diabetes, liver diseases and other disorders, accounting for a heavy burden on the public and on health-care systems every year. Excess energy uptake induces adipocyte hypertrophy, hyperplasia and formation of visceral fat in other non-adipose tissues to evoke cardiovascular disease, liver diseases. Adipose tissue can also secrete adipokines and inflammatory cytokines to affect the local microenvironment, induce insulin resistance, hyperglycemia, and activate associated inflammatory signaling pathways. This further exacerbates the development and progression of obesity-associated diseases. Although some progress in the treatment of obesity has been achieved in preclinical and clinical studies, the progression and pathogenesis of obesity-induced diseases are complex and unclear. We still need to understand their links to better guide the treatment of obesity and associated diseases. In this review, we review the links between obesity and other diseases, with a view to improve the future management and treatment of obesity and its co-morbidities.
KEY WORDS: Obesity, Cardiovascular disease, Liver disease, Insulin resistance, Adipokines, Inflammation, MNK, Lipid accumulation
Graphical abstract
Obesity contributes to the development of cancer, cardiovascular diseases, T2DM, and liver diseases. Understanding their links could provide a better guide for the treatments of obesity and associated diseases.
1. Introduction
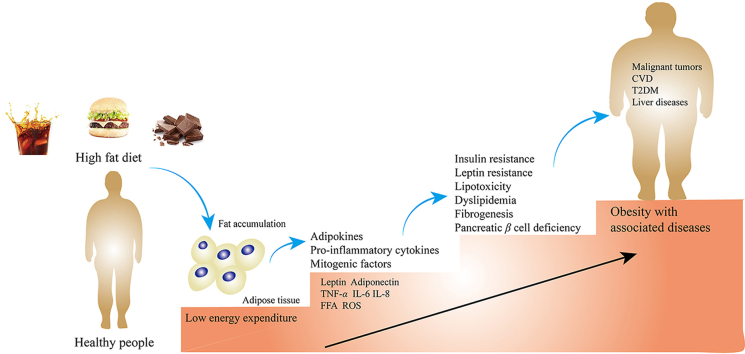
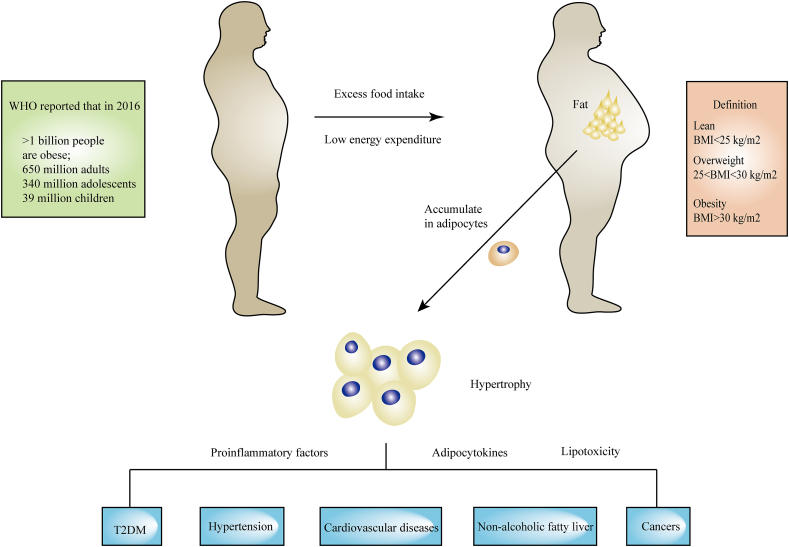
Obesity is defined as abnormal or excessive fat accumulation (World Health Organization, WHO) and has been described as a ‘global pandemic’1, 2, 3. Being overweight or obese are defined by measures of body mass index (BMI)4. A BMI of obesity is over 30 kg/m2 while a BMI of 25–29.9 kg/m2 is defined as overweight5,6. The standard for obesity and overweight are different in certain populations7. For example, for Chinese people, the standard is different from that of WHO. According to the recommended criteria for Chinese people, the categories are defined as follows: underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5–23.9 kg/m2), overweight (BMI 24.0–27.9 kg/m2), and obese (BMI ≥28 kg/m2)8,9. According to a WHO report published in 2021, more than 1 billion people were obese, including 650 million adults, 340 million adolescents and 39 million children based on data gathered in 2016. The global prevalence of being overweight or obese has increased by 27% in adulthood and 47% in childhood during 1980–201310. This number is still increasing, and the WHO estimates that it will increase by approximately 167 million people by 2025 (www.who.int). Obesity is a risk factor for various diseases11,12, notably type 2 diabetes mellitus (T2DM), non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), cardiovascular disease (CVD) and some kinds of cancer13 (Fig. 1). SARS-CoV-2, also called COVID-19, has infected over 598 million people and killed over 6.2 million people worldwide. People with obesity who contracted COVID-19 showed higher levels of hospitalization, ICU admission, and death14, 15, 16, 17. Obesity and related diseases impose a heavy burden on individuals, society, and on the economy, through greater public health costs, morbidity and mortality.
Figure 1.
Overview of the obesity epidemic, obesity definition and obesity-associated diseases.
Obesity mainly develops as an imbalance between caloric intake and energy expenditure18. When energy intake is more than needed, it will be stored as fat and glycogen in subcutaneous adipose tissue (SAT) and organs19,20. Adipose tissue (AT) consists of functionally distinct depots21. White adipose tissue (WAT) is an active endocrine and a major and safe lipid storage organ, whereas brown adipose tissue (BAT) produces heat upon β-adrenergic stimulation or cold exposure, a process known as adaptive thermogenesis22. In humans, WAT can be classified in two main depots: visceral WAT (VAT) and SAT, which have been widely studied for their association with the development of related diseases23. BAT represents merely 1%–2% of fat, but it is vital in maintaining homeostasis and shows beneficial effects on blood glucose24.
People who are overweight or obese has been linked with a low-grade, chronic inflammatory state, which is associated with increased infiltration into AT from the circulation by macrophages of the M1 or ‘classically activated’ phenotype25. These macrophages can be recruited to AT where they secrete inflammatory cytokines (TNF-α, IL-6, IL-8, etc.)26. Along with pro-inflammatory cytokines, anti-inflammatory cytokines (such as IL-4, IL-10, IL-13, IL-19) are secreted from adipocytes; however, their abundance and secretion appear to decrease with weight gain since obesity definitively induces the balance to increase production of more pro-inflammatory adipokines27,28. AT also secretes adipokines (leptin, adiponectin, visfatin and resistin, etc.) and constituents of the extracellular matrix (ECM) to regulate related pathways29,30. Excess accumulation of fat leads to adipose tissue hyperplasia and hypertrophy, which changes the secretome and metabolites released and influences the surrounding microenvironment31,32.
Leptin, one of the most abundant adipokines with proinflammatory properties, increases along with other factors, such as hepatocyte growth factor (HGF)33, plasminogen activator inhibitor-1 (PAI-1), resistin34, TNF-α, IL-1β, IL-6, and monocyte-chemoattractant protein-1 (MCP-1)35,36, while the production of adiponectin is decreased. The inflammatory cytokines released by AT contribute to the progression of the metabolic syndrome (it is well known as a metabolic disorder resulting from obesity which involves glucose intolerance, insulin resistance, central obesity, dyslipidaemia, hypertension and all risk factors for CVD)37 and increased risk for several cancers. Increased free fatty acids (FFAs) in the serum of obese individuals promote the expression of vascular endothelial growth factor-A (VEGF-A) and vimentin by upregulating PPARγ, which is implicated in tumor growth and tumor initiation through cell intrinsic and extrinsic mechanisms38, resulting in insulin resistance39,40 and steatosis in the liver40. Overexpression of TNF-α and leptin can inhibit insulin receptor activation and induce insulin resistance in muscle, liver, islet α-cells and AT, eventually lead to T2DM41. Furthermore, obesity can cause lipid accumulation in non-adipose tissue, such as liver, muscle, pancreas, epicardial and perivascular tissue, among which, the build-up of lipid in epicardial adipose tissue (EAT) and perivascular adipose tissue (PVAT) cause hypoxia and dysfunction of tissue and macrophage infiltration, leading to increases in inflammatory factors associated with CVD42,43.
This review will provide a global overview of the main obesity-associated diseases and associated mechanisms. There is an urgent need to develop advanced approaches to treat these conditions which in turn requires detailed understanding of the molecular mechanisms involved.
2. Obesity and cancer
Obesity is known to be associated with 13 types of cancer44, 45, 46, 47, including breast, uterine, ovarian, esophagal, stomach, colon/rectal, liver, gallbladder, pancreatic, renal, thyroid, and meningeal cancers, as well as multiple myeloma, according to the International Agency for Research on Cancer (IARC) Working Group5,48,49. A 204 meta-analyses revealed that the increased risk of developing cancer for every 5 kg/m2 increase in BMI ranged from 9% for rectal cancer among men to 56% for biliary tract system cancer50. A systematic review and meta-analysis conducted by the World Cancer Research Fund & the American Institute for Cancer Research assessed a cohort of over 9 million men, including 191,000 men with prostate cancer51. They concluded that there was a strong level of evidence indicating an 8%–11% increased risk of advanced prostate cancer and prostate cancer-specific mortality in obese men and found a 6% increased risk of advanced disease per 5% kg/m2 increase in BMI51,52. Additionally, a meta-analysis of 56 observational studies involving more than 7 million individuals and 93,812 colorectal cancer (CRC) cases confirmed that a higher BMI was associated with a higher CRC risk; for every 5% kg/m2 increase in BMI, CRC risk increased by 18%53. There is growing observational evidence that obesity is associated with worse cancer outcomes among individuals with cancer51,54. A meta-analysis of 82 studies involving 213,075 women with breast cancer found a 41% relative increase in all-cause mortality for women with obesity vs those of normal weight55. Similarly, adverse associations of obesity with survival have been reported for endometrial, prostatic, pancreatic56,57, colorectal and ovarian cancers, as well as some hematologic malignancies58. However, being overweight or obese does not always show a positive relationship with cancer, and is actually associated with better outcomes in lung, esophageal and kidney cancer10,59,60.
The association of obesity with cancer is biologically complex61. Many studies have indicated that obesity-related effects on DNA damage and/or repair pathways may be involved in obesity-induced genetic instability due to the formation of reactive oxygen species (ROS)62. Oxidative stress (OS) could be induced by the formation of ROS, and OS promotes the structure modification of carbohydrates, proteins, phospholipids and nucleic acids by the process of oxidation63. Oxidation modification of DNA induces the formation of 8-oxo-dG, which increases genetic instability due to its mutagenic potential64, 65, 66. In obese individuals, excessive accumulation of TG in adipocytes results in enhanced mitochondrial β-oxidation of FFA and increased mitochondrial ROS generation67, 68, 69, which drives the accumulation of genomic damage, reduces the efficacy of DNA repair, and/or enhances the competitiveness of tumor cells by regulating intracellular molecular networks such as the NF-κB, JAK2/STAT3 or PI3K/AKT pathway70. The circulating pro-inflammatory cytokines (TNF-α, IL-1, IL-6 and others) are known to induce the production of ROS71, and this may accelerate the mutational rate of cells and/or interfere with DNA repair mechanisms leading to an increase and accumulation of genetic events63. Furthermore, adipocyte hypertrophy in tissue leads to ischemia and hypoxia, which can cause a greater state of OS and release of ROS to induce mitochondrial dysfunction and damage DNA72. The obesity-associated conditions of hyperglycemia, hyperlipidemia and hyperinsulinemia lead to increased OS and ROS, exacerbating the inflammatory process in obesity.
Moreover, the cellular lipid remodeling induced by obesity impacts multiple facets of physiology relevant to carcinogenesis73. The lipids from neighboring adipocytes stores can be transferred into cancer cells to be used as energy source and promote tumor growth74, which led to the direct transfer of lipids from the adipocytes to the cancer cells and induce lipolysis in the adipocytes and β-oxidation in the cancer cells75. Obesity may drive cancer development through converting fatty acids into protumorigenic signaling lipids (e.g., lysophosphatidic acid, prostaglandins, sphingolipids, phosphatidylinositols) that can then activate cancer cells through paracrine or autocrine interactions to trigger oncogenic responses, including proliferation, tumor growth, immunological responses, motility, invasiveness, and metastasis76. The low-grade inflammation of AT leads to chronic activation of the innate immune system, which speeds the progression of cancer77,78.
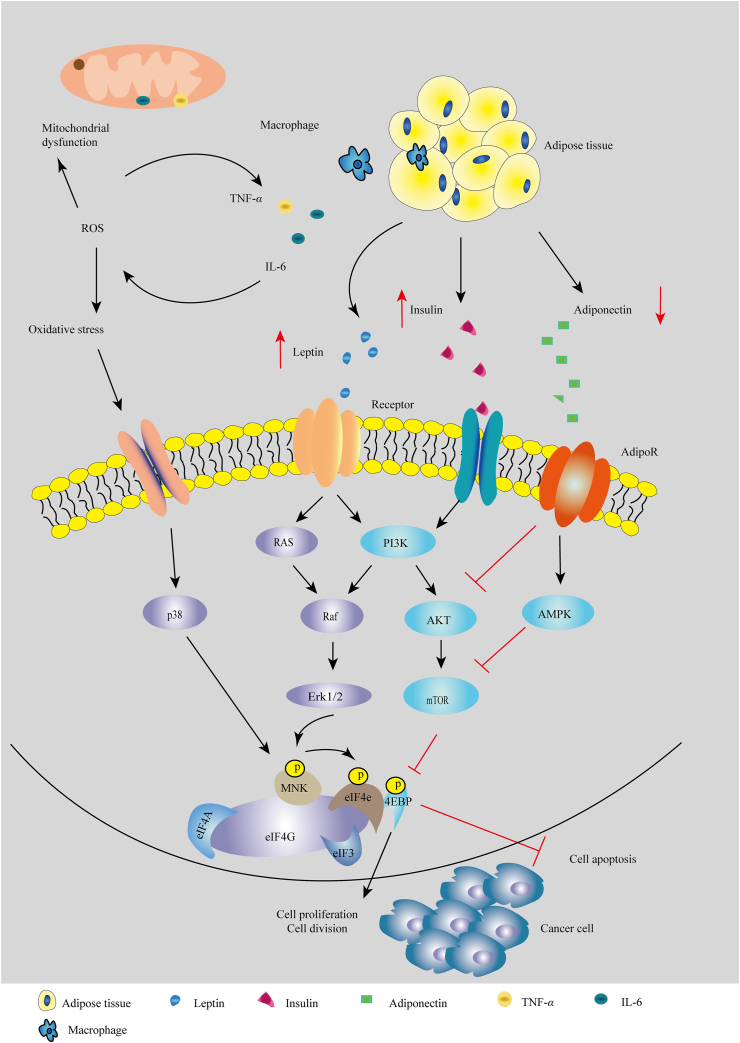
Obesity promotes insulin production and creates a hyperinsulinemic state in vivo, which activates the PI3K/AKT/mTOR and RAS/MAPK signaling pathways, involving in cell proliferation and protein synthesis in cancer cells79,80 (Fig. 2). Specifically, high level of insulin raises the level of insulin-like growth factor (IGF), which is associated with inflammation and impairs the immune system81,82. Increased secretion of IGF-1 promotes mitogenesis and angiogenesis and inhibits apoptosis, facilitating cancer progression83,84. High levels of insulin or hyperinsulinemia are associated with low sex hormone-binding globulin levels and high estrogen bioavailability85, which facilitates the development of hormone-related cancers86,87, such as in breast cancer after menopause88. Estrogen biosynthesis after menopause is induced largely in obese adipose tissue, where it involves the conversion of adrenal androgens into estrogens by aromatase that is increased through the activation of NF-κB pathway and the upregulation of proinflammation cytokines89,90. Further, increased aromatase activity in obese individuals converts testosterone to estradiol resulting in increased concentrations of estradiol91, which may promote prostate cancer growth51.
Figure 2.
Cellular mechanisms linking obesity and cancer. Adipose tissue is enlarged by excess fat and secretes adipokines, such as leptin and insulin that activate the RAS/Erk and PI3K/AKT/mTOR signaling pathways. Adipose tissue also produces proinflammatory cytokines which induce oxidative stress and mitochondrial dysfunction, triggering the p38/MAPK signaling pathway. All these changes promote cancer cell proliferation and cell division and inhibit cell apoptosis.
Additionally, more than 15 adipokines (such as adiponectin, leptin, adipsin, apelin) secreted by AT stimulate cancer cell growth, invasion, angiogenesis, and metastasis54,92.
Leptin plays an important role in the development of obesity and cancer93. It is produced primarily by AT and controls food intake and energy expenditure via a feedback mechanism in the brain94. Leptin has growth-promoting, mitogenic, and antiapoptotic properties in many cancer cells and increases the expression of TNF-α, IL-6 and angiogenic factors95. When leptin circulates in plasma, it binds to its receptors (OB-Rs) and activates Notch signaling to control downstream effector molecules or signaling events; leptin signaling regulates intracellular pathways such as the PI3K/AKT/mTOR, JAK2/STAT3 and ERK/MAPK pathways involved in the control of cell proliferation, differentiation, survival, migration, and invasion96,97. Leptin also has been found to induce phospholipase C γ (PLC-γ), PKC, p38 and nitric oxide (NO), which can activate several genes involved in cell proliferation, including C-FOS, C-JUN, JUNB, EGR-1 and SOCS3, and upregulate the expression angiogenic factors, such as VEGF98,99. Leptin increases estrogen levels through the activation of the aromatase gene expression and aromatase activity to contribute to tumor growth and the development of antiestrogen resistance in obese breast cancer patients100, and enhances the stability of the estrogen receptor alpha (ERα), leading to the maintenance of ERα-dependent transcription in breast cancer cells in the presence of antiestrogens. Several in vitro studies have demonstrated leptin-induced cell invasion and migration in different cancer cells101, for example, leptin stimulated the expression of acetyl-CoA acetyltransferase 2 (ACAT2) through the PI3K/AKT/SREBP2 signaling pathway to enhance the proliferation, migration and invasion of breast cancer cells102 and induce IL-18 expression both in tumor-associated macrophages (TAMs) via NF-κB/NF-κB1 signaling and in breast cancer via PI3K–AKT/ATF-2 signaling to promote the invasion and metastasis of breast cancer cells103. Conversely, leptin also showed a good action in some conditions104. Leptin increased Th1 and suppressed Th2 cytokine production and has been shown to reverse the immunosuppressive effects of acute starvation in mice105; another study found that leptin showed antitumoral functions in human pancreatic cancer cell lines106.
Adiponectin (APN) is also an important adipokine secreted by adipose tissue and is involved in the etiology of some cancers107,108. Obesity is associated with a decreased level of APN in plasma, which activates the insulin pathway and decreases levels of the adipose-derived cytokines IL-6, IL-8 and TNF-α108. Research on the role of APN in tumor growth has provided evidence for both positive and negative influences. In several human studies, adiponectin has been found to be associated with a number of cancer types: its levels are decreased in breast and endometrial cancer but increase in non-small cell lung cancer, prostate, gastric, liver, pancreatic, and hematological cancers, colon cancer, and renal cell carcinoma109, 110, 111, 112, 113. APN shows antitumor effects by affecting some key mechanisms that regulate cell growth114,115, such as inducing the expression of p53 and p21116 and inhibiting the mTOR and PI3K/AKT signaling pathways116,117. Additionally, APN can increase insulin sensitivity118, and the complex synergistic interaction between APN and the IGF system or other various obesity-related biomarkers is believed to induce tumorigenesis and development119. Adiponectin also potently stimulates ceramidase activity by binding with AdipoR1 and AdipoR2, and enhance pro-apoptotic ceramide catabolism leading to formation of its downstream anti-apoptotic metabolite sphingosine-1-phosphate (S1P) independently of AMPK120. However, adiponectin deficiency limited tumor vascularization and significantly reduced tumor growth and angiogenesis in a MMTV polyoma middle T antigen (PyMT) mouse model121. Thus, the role of adiponectin in tumor angiogenesis remains flexible.
Obesity has been shown to increase risk of ‘obesity-related cancers’122. Worsening obesity tends to increase serum concentrations of glucose, insulin, IGF-1, lipids, leptin, estrogen, resistin, and inflammatory cytokines and reduces IGF binding protein and adiponectin levels, each of which has been suggested to contribute to cancer pathogenesis86. Interestingly, recent epidemiological data showed that obesity may be a protective factor for certain cancer types regarding their incidence and mortality, e.g., non-small cell lung cancer (NSCLC) and head and neck cancers59. Potential explanations of the ‘obesity paradox’ (it occurs where the risk of outcome, typically mortality, is significantly reduced for BMI values above this referent, where an increased risk is expected) in cancer patients may include the use of BMI as a measure of general adiposity; study limitations including inadequate adjustment for confounding, selection, stratification and detection biases; confounders such as age, smoking, physical activity, etc.123. We should avoid the misinterpretation that being obese might be ‘good’ or ‘protective’ for cancer patients60.
3. Obesity and cardiovascular diseases
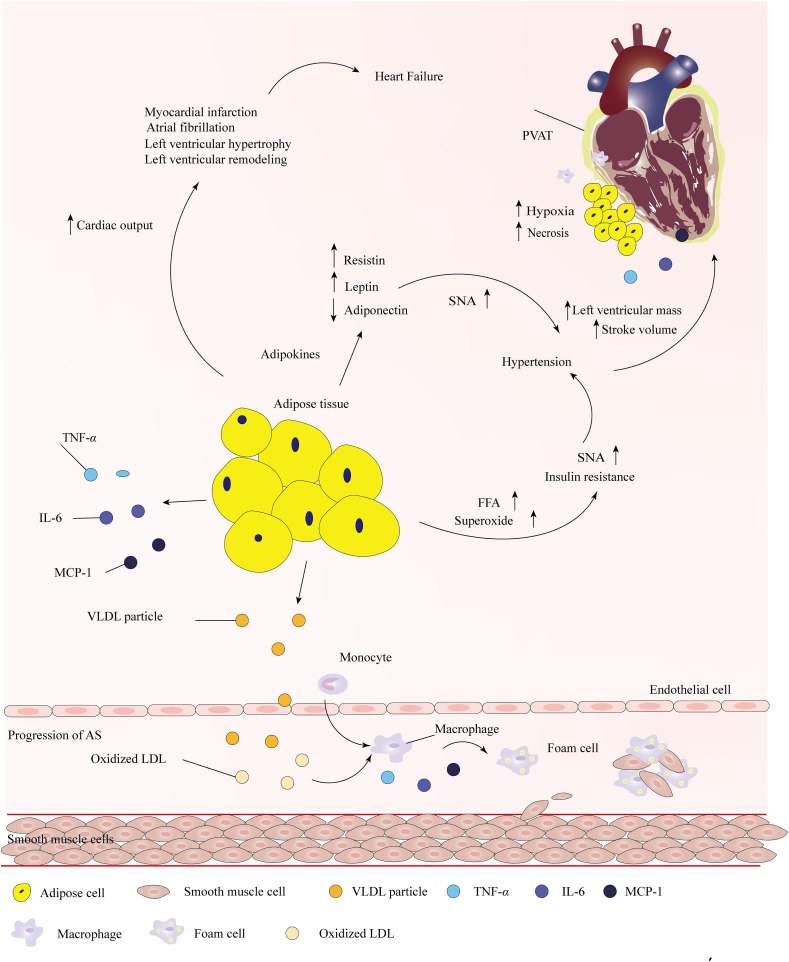
Clinical trials and epidemiological studies have shown that obesity can increase the risk of CVDs124, 125, 126, 127, including coronary artery disease (CAD), heart failure (HF) and atherosclerosis (AS)128. Furthermore, obesity has a strong relationship with other CVD risk factors, such as hypertension (HTN), insulin resistance, and dyslipidemia129. Obesity leads to adipose tissue hypertrophy, dysfunction and inflammation, which ultimately change the structure and function of the cardiovascular system130, including left ventricular (LV) remodeling, greater left ventricular mass, left atrial enlargement, and increased stroke volume131,132, increased total and central blood volume, decreased systemic vascular resistance, cardiac output, LV filling pressure and pulmonary arterial pressure128.
Ectopic deposition of AT in other organs leads to abnormal fat accumulation around the heart, which has been consistently related to CVD risk133. For example, in healthy individuals, cardiomyocytes generate ATP primarily in mitochondria which is associated with ROS production. In patients with obesity and IR, excess FFAs accumulate in cardiomyocytes, which results in increased ROS via fatty acid oxidation and eventually leading to mitochondrial dysfunction134,135. Furthermore, increased myocardial lipid deposition would induce cardiomyopathy owing to cardiac lipotoxicity136. Cardiomyocyte lipid accumulation leads to cell apoptosis and cardiac dysfunction by increasing the synthesis of the pro-apoptotic sphingolipid ceramide, which activates inducible NO synthase137. AT surrounding the major conduit coronary arteries is termed PVAT and has emerged as a major contributor to CVD risk42. Build-up of VAT and PVAT impairs angiogenesis and promotes localized hypoxia and ischemic necrosis, leading to adipocyte death that may stimulate infiltration by activated macrophages thereby causing inflammation in tissues42,138,139.
WAT can be found around major organs and blood vessels in the abdominal cavity and subcutaneously, which is a key determinant of the relative risk for CVD. Brown adipose tissue (BAT) also plays important roles in regulating cardiovascular functions140,141, through attenuating cardiac remodeling and suppressing inflammatory response140. The systemic activation of BAT can quickly induce the utilization of intracellular glycogen and lipid stores, increase uptake of glucose and lipoprotein derived NEFAs and drain remote nutrient stores in liver and WAT, which decreases the risk of CVD. BAT is enriched in mitochondria, and can significantly express uncoupling protein-1 (UCP-1), proliferator-activated receptor gamma coactivator-1α (PGC-1α), PR domain-containing protein 16 (PRDM16), β3-adrenoceptor and other genes related to thermogenesis140,142,143. UCP-1, one of the most important proteins in BAT, uncouples oxidative phosphorylation from ATP production144, ultimately resulting in the generation of heat145,146. Humans with obesity usually have lower BAT content147, while decreased expression of UCP-1 in human EAT is associated with increased adipose tissue oxidative stress and dysfunction and can plausibly alter its communication with neighboring cells of the cardiovascular system148. Moreover, inflammation induced by obesity promote the production of inflammatory cytokines, which are thought to suppress UCP-1 expression in BAT149,150. BAT not only oxidizes fats but can also synthesize and release peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) to assist nitric oxide (NO) to complete vasodilation140,151. BAT also secrets cytokines, such as fibroblast growth factor 21 (FGF-21)152, IL-6153 and vascular endothelial growth factor A (VEGFA), which show protective effects on the cardiovascular system154. For example, FGF21 is shown to have an important protective effect on the cardiac hypertrophy by inducing the expression of PGC-1α and inhibiting the NF-κB pro-inflammatory pathway152; IL-6, whose levels are usually considered an indicator of inflammatory responses155, has a positive function on regulating the glucose homeostasis and the insulin sensitivity of BAT, working together with FGF21156,157; VEGFA expression in BAT leads to increased vessel permeability, promotes TG clearance and provides a autocrine signal to maintain mitochondrial oxidative capacity158. Furthermore, experimental evidence from mice suggests that BAT has a vasoprotective action through an anticontractile effect. For example, protein expression of NADPH oxidase 4 (Nox4) was increased only in BAT, and Nox4-derived hydrogen peroxide from BAT, can induce cGMP-dependent protein kinase G type-1α (PKG-1α) activation, resulting in reduced vascular contractility159. There is some research showing that the activity of BAT declines in obesity160, which results in the insufficient synthesis of PGC-1α and PGC-1α deficiency would impair vasodilation and induce vascular senescence161. This also leads to the elevation of blood pressure, left ventricular hypertrophy with an eccentric remodeling pattern and increased interstitial tissue161. One of the central modes of blood pressure regulation is via the renin-angiotensin aldosterone system (RAAS)145. Angiotensin II is produced from its precursor angiotensinogen by the activation of angiotensin-converting enzymes 1 and 2, and angiotensin-converting enzyme 2 can further induce angiotensin II to generate angiotensins 1–7, which has vasodilatory properties162, 163, 164. Additionally, activation of angiotensin receptor 2 or angiotensin II treatment can induce browning of subcutaneous WAT in vivo with increased UCP-1 expression and oxygen consumption and stimulation of brown precursor differentiation in vitro by increasing PPARγ expression via the ERK1/2, PI3K/AKT and AMPK signaling pathways165,166. Increased sympathetic nerve activation or increased conversion of angiotensin II to angiotensins 1–7 can enhance BAT thermogenesis and WAT lipolysis, which in turn has beneficial effects on blood pressure and attenuates development of CVD166. Moreover, PRDM16, the master regulator of thermogenic AT, interacts with MED1 at brown fat-specific genes to promote gene transcription and stimulates brown adipogenesis by binding to PPARγ167, which is associated with hypertension in humans168.
PVAT is now recognized as an important local regulator of vascular function and dysfunction given its ability to its proximity to the vascular wall and its ability to sense vascular paracrine signals. Signaling pathways in PVAT, such as those using APN, H2S, GLP-1 or pro-inflammatory cytokines, facilitate a range of direct, paracrine effects169,170. Interestingly, PVAT is itself heterogeneous, with its anatomical localization145,171. PVAT surrounding the abdominal aorta and the mesenteric arteries appears to be similar to WAT phenotype in humans and mice, with large lipid droplets and low UCP-1 expressing thermogenic adipocytes. On the other hand, rodent PVAT surrounding the thoracic aorta has a BAT-like phenotype with multilocular adipocytes and similar UCP-1 expression to classical BAT. A study suggests that brown adipocyte-specific aryl hydrocarbon receptor nuclear translocator-like protein 1 (Bmal1) in PVAT in particular is involved in the regulation of angiotensinogen expression and the ensuing increase in angiotensin II, which acts on smooth muscle cells in the vessel walls to regulate vasoactivity and blood pressure172,173.
Adipokines are considered to be a link between obesity and CVD174 (Fig. 3). EAT and PVAT secrete adipokines, such as leptin, adiponectin, resistin, visfatin, and inflammatory cytokines, all of which may modulate vascular tone, smooth muscle cell migration and proliferation, neointimal formation, inflammation and oxidative stress, thereby increasing the risk of CVD factors and CVD175. Resistin serves as an important risk factor for cardiovascular disease by affecting insulin sensitivity and coronary calcification176, 177, 178. Adiponectin regulates vascular steady state by decreasing the activity of C reactive protein (CRP)179 and suppressing the signal mediated by TNF-α and NF-κB180,181. Adiponectin also activates endothelial NO synthase (eNOS) to enhance salt-induced hypertension by activating PI3K/AKT signaling and increasing the production of NO182,183. Obese individuals with low levels of adiponectin show an increased incidence of cardiovascular disease. Leptin inhibits cardiac systole by promoting the production of endothelin-1 (ET-1) and activating NADPH oxidase-mediated pathways184,185.
Figure 3.
Schematic representation of the role of obesity in the promotion of cardiovascular diseases. Adipose tissue hypertrophy and ectopic deposition around the heart and coronary arteries impair angiogenesis, promote local tissue hypoxia and necrosis, and induce left ventricular hypertrophy and remodeling, which contribute to heart failure. Adipokines, free fatty acids (FFAs) and superoxides secreted by adipose tissue activate sympathetic nerve activity to induce hypertension and then increase left ventricular mass, stroke volume and cardiac output. Triglycerides (TG) in ectopic fat is transported by very low-density lipoprotein (VLDL) into the circulation. Low-density lipoprotein (LDL) undergoes oxidation and other modifications to combine with macrophages, forming foam cells, a hallmark of early atherosclerosis (AS) lesions.
Atrial natriuretic peptide (ANP) is primarily secreted from cardiomyocytes186 and is an important hormone with pronounced lipolytic effects on WAT187. ANP induces lipid mobilization and oxidation and enhances insulin sensitivity188. ANP exhibits anti-inflammatory effects by inhibiting proinflammatory cytokines expression and secretion from human AT explants189, such as IL-6 and TNF-α. Considering that AT and systemic inflammation are associated with insulin resistance190. This could be one mechanism by which ANP preserves insulin sensitivity in humans. ANP also plays a critically important role in regulating salt and water reabsorption by inhibiting secretion of vasopressin from the posterior pituitary, which in turn has important effects on blood pressure191. Furthermore, ANP lessens cardiac and pulmonary baroreceptor and chemoreceptor activity, thus reducing sympathetic outflow to the heart. This decrease in sympathetic activity together with the increase in vagal afferent activity result in a reduction in heart rate and cardiac output192. ANP also reduces vascular smooth muscle tone and peripheral vascular resistance193. In obesity, lower concentrations of ANP are seen among those with higher BMI, which has been proposed as an independent risk factor for development of cardiovascular disease, including hypertension194.
Multiple epidemiological studies have demonstrated a strong association between being overweight/obese and HF195, with up to 35%–45% of patients with heart failure being either overweight or obese. People with obesity had a two-fold higher risk of developing heart failure than in those of normal weight; every 1 kg/m2 increment in BMI was found to increase the risk of HF by 5%–7%195,196. The link between obesity and HF is thought to occur through myocardial fibrosis and cardiac stiffening triggering atrial fibrillation (AF) and coronary atherosclerosis and myocardial infarction (MI) resulting in left ventricular remodeling with impaired systolic function197,198. Obesity is likely to increase the risk for AF, including increased cardiac output and development of HTN, by exerting increased preload and afterload on the left ventricle and leading to left ventricular hypertrophy199. Obesity also affects thyroid function, which may predispose to AF and subsequent HF200. AT secrete adipokines and multiple inflammatory cytokines contributing to the development of HF201.
Currently, obesity-associated HTN is a serious risk factor for CVD202. The link between obesity and HTN is influenced by multiple factors203 (Fig. 3). Reabsorption of sodium ions in the renal tubules and vasoconstriction have been observed in obesity. Dysfunctional sodium excretion and renal tubular reabsorption of sodium increase cardiac load capacity and raise blood pressure204,205. Blocking the angiotensin II receptor can significantly reduce the activity of sympathetic nerve activity (SNA) and control blood pressure206. High levels of FFA and superoxide activate SNA along with causing vasoconstriction, high blood pressure and insulin resistance207. Leptin also increases the activity of SNA208,209. Obese patients with hypertension have increased left ventricular mass and have higher stroke volume and cardiac output210.
Atherosclerosis (AS) is the leading cause of CVD, and obesity is a major risk factor for AS211. The accumulation of TG in ectopic fat showed a strong relationship with the development of AS in obese patients212, 213, 214 (Fig. 3). Excess TG is transported by very low-density lipoproteins (VLDL) in the circulation215. Large epidemiological studies confirmed that the elevation of triglyceride-rich lipoproteins (TGRLs) in circulation is atherogenic215,216. These small dense LDL particles easily enter the arterial wall, and they undergo oxidation and other modifications to produce proinflammatory and immunogenicity217,218. Macrophages combine with lipoproteins to become foam cells, a hallmark of early atherosclerotic lesions218, 219, 220. The excessive accumulation of EAT and PVAT can increase inflammatory cytokines138, TNF-α, IL-6 and IL-1β, which in turn promote macrophage migration to AT and increase the number of foam cells. Adiponectin is the most abundant anti-inflammatory and vasculoprotective adipokine secreted by AT221,222. It can induce NO production, suppress proliferation and superoxide generation, and enhance eNOS activity in endothelial cells treated with oxidized low-density lipoprotein223. Adiponectin suppresses the expression of class A macrophage scavenger receptors and consequently reduces foam cell formation, decreases the secretion of proinflammatory cytokines, and limits the initiation of atherosclerotic plaque formation224,225. Increasing adiponectin levels have been suggested as a potential therapeutic target to reduce the AS risk associated with obesity.
Despite the strong relationship between obesity and development of CVDs, a large body of evidence has demonstrated an ‘obesity paradox’ in patients with CVD, where many types of CVD may have a better prognosis in the overweight or obese population compared to their leaner counterparts226. For example, overweight and obese patients have a better short- and intermediate-term prognosis compared with leaner patients with similar degrees of HF after adjustments for confounders227; overweight/obese people with coronary heart disease have a lower risk of total and CVD mortality compared with underweight and normal weight coronary heart disease patients228. Describing the obesity paradox is certainly not to promote being overweight or obese or a suggestion that weight gain is beneficial226, and the balance of data still supports purposeful weight reduction in the prevention and treatment of CV diseases229. It is important to be cautious in judging disease risk only dependent on the BMI.
4. Obesity and T2DM
Obesity is known to be the main risk factor for T2DM230,231, and approximately one-third of obese people develop T2DM. Adults with BMI >35 kg/m2 are 20 times more likely to develop T2DM than those with a BMI between 18.5 and 24.9 kg/m2. Moreover, 80% of T2DM patients are overweight or obese232,233. It is commonly believed that the primary cause of T2DM is obesity-driven insulin resistance in non-adipose tissues, combined with insufficient secretion of insulin by pancreatic β-cells to overcome this resistance234.
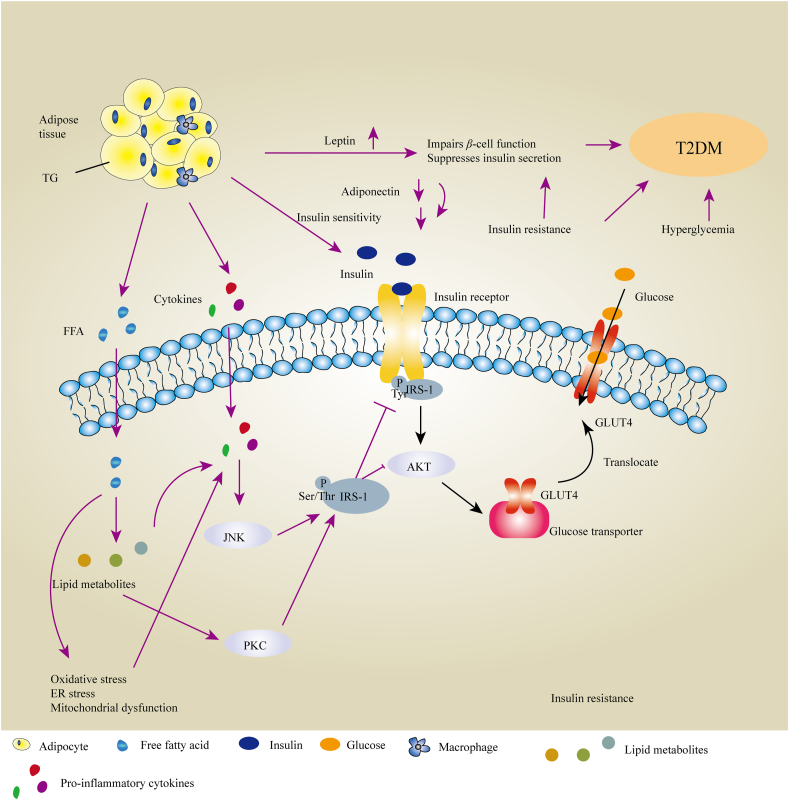
Insulin is an important hormone released by pancreatic β cells, with important physiological roles, including stimulation of the uptake and utilization of glucose, promotion of lipogenesis, inhibition of gluconeogenesis and glycogenolysis, and prevention of protein breakdown and lipolysis235. Insulin binds to cell surface receptors primarily on skeletal muscle, adipose tissue, and liver236, and subsequently promotes receptor autophosphorylation and phosphorylation of insulin receptor substrates (IRSs)237. This process further leads to the activation of AKT (also known as PKB), and recruits glucose transporter 4 (GLUT-4) to the plasma membrane to facilitate glucose uptake into the cell235,238 (Fig. 4). This IRS/PI3K/Akt pathway has an important role in the activation of metabolic, especially biosynthetic processes238.
Figure 4.
Schematic representation of the links between obesity and insulin resistance and their internal mechanisms associated with T2DM. Free fatty acids (FFAs) and their metabolites activate protein kinase C and promote Ser/Thr phosphorylation of insulin receptor substrates 1 (IRS-1), in turn reducing normal Tyr phosphorylation of IRS-1 and impairing the control of the glucose transporter GLUT4, inducing insulin resistance and thus poor glucose tolerance. Proinflammatory cytokines secreted by adipose tissue activate the JNK signaling pathway and indirectly inhibit the translocation of GLUT4 to promote insulin resistance. Elevated leptin and low adiponectin levels, along with insulin resistance, impair β-cell function, suppressing insulin secretion and leading to T2DM. The black line indicates the normal mechanism underlying the insulin-stimulated uptake of glucose involving translocation of the glucose transporter GLUT4 to the plasma membrane. The purple line indicates the defective process(es) in obese individuals.
Insulin resistance (IR) is defined as the inability of insulin target tissues – such as skeletal muscle, liver, and AT – to carry out adequately the physiologic effects (such as glucose uptake and utilization) of circulating insulin239,240. Elevated levels of FFAs are observed in obese individuals. High level of FFAs in the circulation become deposited in insulin-sensitive non-adipose tissues, resulting in lipotoxicity, which is an important cause of insulin resistance241. Lipid metabolites from FFA, such as long-chain fatty acyl CoAs, diacylglycerol (DAG) and ceramides, activate some forms of PKC, the inhibitor of nuclear factor κB kinase b (IKKb), and Jun kinase (JNK), which induce Ser/Thr phosphorylation of IRS-1242,243 and in turn inhibit normal insulin-stimulated tyrosine phosphorylation of IRS-1, resulting in the impairment of insulin signaling235,244,245. Additionally, proinflammatory pathways (for example, NF-κB) are activated, promoting the secretion of proinflammatory cytokines, including TNF-α, MCP-1, and IL-6246. Elevated levels of cytokines activate the JNK signaling pathway and thus inhibit the normal pathway of insulin-stimulated tyrosine phosphorylation of IR and IRS-1, ultimately inhibiting the translocation to the plasma membrane of the insulin-sensitive glucose transporter GLUT-439,247,248 (Fig. 4). FFAs also activate NADPH oxidase and induce ROS production. ROS-induced oxidative stress results in dysregulated production of proinflammatory cytokines and promotes insulin resistance237.
Adipokines can regulate β-cell function and glucose metabolism. APN appears to improve insulin sensitivity by inducing phosphorylation and activation of AMPK and PPARα signaling, promoting phosphorylation (and inhibition) of ACC, and increasing fatty acid oxidation and glucose uptake249, 250, 251. It thus also activates the LKB1/AMPK/TSC1/2 pathway to antagonize inhibition by mTORC1/p70 S6K of insulin signaling252,253 and enhance the ability of insulin to stimulate IRS-1 tyrosine phosphorylation and AKT phosphorylation254,255. Moreover, APN could promote fat storage preferentially in subcutaneous AT rather than in liver or skeletal muscle to reduce VAT mass and inflammation, improving glucose and fat metabolism and enhancing insulin sensitivity180. Obese individuals show lower adiponectin secretion, which impairs insulin sensitivity. In contrast, leptin deficiency causes insulin resistance and T2DM256. Leptin activates the IRS/PI3K pathway to improve insulin sensitivity in peripheral tissues and triggers the translocation of GLUT4 from cytosol to cell surface to increase glucose uptake256, 257, 258. Obesity in humans is usually associated with high circulating leptin levels. However, ample evidence suggests that common forms of obesity are associated with hypothalamic leptin resistance259,260. The potential of leptin monotherapy in obese humans with T2DM in clinical trials have failed to demonstrate therapeutic activity, with no observation of important weight loss or metabolic improvements (insulin sensitization, amelioration of glucose and lipid metabolism)261, 262, 263. Furthermore, leptin has been reported to impair β-cell function and suppress insulin secretion264,265. Leptin could induce β cell hyperpolarization through activating PI3K-dependent PDE3B and the KATP channel to inhibit insulin secretion266,267. VAT regulates metabolic homeostasis and progressive insulin resistance thereby increasing the risk of T2DM268. It also enhances the production and secretion of pro-inflammatory adipokines by these adipose tissues269, which can promote IL-1β-induced apoptosis in β-cells by activating the NF-κB and JAK/STAT signaling pathways270. Insulin-induced hyperlipidemia leads to the accumulation of lipids in β-cells and induces β-cell apoptosis271. Hyperglycemia induced by insulin resistance triggers β-cell apoptosis through glucotoxic effects on β-cells that show adverse effects on insulin secretion272,273.
Here, we will introduce novel targets involved in the process of obesity and T2DM, the MNKs. MNKs (mitogen-activated protein kinase-interacting protein kinases), including MNK1 and MNK2, phosphorylate eIF4E (eukaryotic initiation factor 4E) at Ser209 and then control the translation of certain mRNAs274,275. eIF4E participates in the eukaryotic initiation factor complex 4F, along with the RNA helicase eIF4A and the scaffolding protein eIF4G276. eIF4E directly binds the 5′-cap structure of cytoplasmic mRNAs and plays a crucial role in protein synthesis277. Phosphorylation of eIF4E has been shown to regulate the translation efficiency of some mRNAs, encoding proteins which are involved in tumor development, progression and metastasis278. MNKs are the only kinases that phosphorylate eIF4E at Ser209 in vivo and eIF4E is their only known in vivo substrate MNK double knock-out mice show no overt phenotype under normal vivarium conditions, so appear to be a safe target for disease therapy. Inhibition the activity of MNKs and the phosphorylation of eIF4E has shown good effect on tumor treatment and obesity induced by HFD274.
When MNK1-KO or MNK2-KO mice are fed a high-fat diet, they show different phenotypes279. MNK2-KO mice show less weight gain and improved glucose tolerance compared to control mice given an HFD, as well as better insulin sensitivity and reduced adipose tissue inflammation. HFD-fed MNK1-KO mice show better glucose tolerance and insulin sensitivity. MNK-DKO or hypomorphic eIF4E+/− mice are protected from HFD-induced obesity280,281. Elevated energy expenditure and changes in expression of proteins linked to lipolysis, mitochondrial function, and oxidative metabolism were observed.
In HFD-fed MNK-DKO mice, some genes or proteins showed increased expression, for example, ATGL (adipose triglyceride lipase), HSL (hormone-sensitive lipase), AGPAT9 (1-acylglycerol-3-phosphate O-acyltransferase 9), which are involved in lipid metabolism in AT; ATP6, ND1, ND5, COX1, NRF2, PPARG, PGC1α and YY1 which are involved in oxidative metabolism are also elevated in AT of MNK-DKO mice. These changes may underlie, at least in part, the metabolic phenotype of MNK-DKO mice, which show higher energy expenditure and oxidative metabolism. In turn, this likely contributes to their lower weight gain on an HFD; interestingly, no significant difference in weight gain was seen for WT and MNK-DKO mice on the normal diet. Furthermore, levels of the mRNA for SFRP5, which negatively regulates expression of certain genes involved in mitochondrial function, were found to be lower in AT from MNK-DKO mice.
A subsequent study, using HFD-fed eIF4E+/− mice, employed tandem mass tag labelling–mass spectrometry (TMT–MS) to identify target proteins (and thus potentially mRNAs affected by eIF4E phosphorylation). The levels of a number of proteins (CD36, ELOVL5, LPIN2, APOC3, APOH and PLIN2) involved in fat storage were found to be decreased from the liver of HFD-fed eIF4E+/− mice. eIF4E normally promotes the translation of a set of mRNAs which encode proteins involved in fatty acid oxidation. Moreover, each of two MNK inhibitors was found to inhibit weight gain in HFD mice. ETC-206 and eFT508 prevent weight gain induced by excessive energy consumption (the HFD)280,281. These findings point to a potential new way to prevent weight gain induced by a high-fat diet without causing toxicity owing to the safety of MNKs as drug targets, i.e., the pharmacological inhibition of the MNKs.
5. Obesity and liver diseases
Obesity affects multiple metabolic functions of the liver. It is associated with the development of non-alcoholic fatty liver disease (NAFLD), associated steatosis and inflammation and promotes the progression of several other liver diseases, including hepatitis C and alcoholic liver disease. NAFLD is a common cause of chronic liver disease and has emerged as the most rapidly growing cause of hepatocellular carcinoma (HCC)282, 283, 284, 285. Associated and serious pathological states include liver cell necrosis, liver fibrosis and cirrhosis of the liver283. NAFLD is a complex liver disease that develops from simple steatosis to steatosis with lobular inflammation and cellular injury, which may develop nonalcoholic steatohepatitis (NASH)286. NASH patients have an increased risk of liver fibrosis, liver cirrhosis, and HCC287. A meta-analysis reported that over 40% of NAFLD patients have progressive fibrosis288. NAFLD is a multisystem disease, affecting other organs and regulatory pathways. For example, NAFLD increases risks for T2DM, CVD, and chronic kidney disease (CKD)289. A meta-analysis showed that NAFLD increased overall mortality by 57% mainly from liver-related and CVD causes, and increased the risk of incident T2DM by approximately twofold290. Additionally, increasing attention has also focused on NAFLD-related CKD and a further recent meta-analysis reported that NAFLD was associated with an approximate twofold increased risk of CKD291.
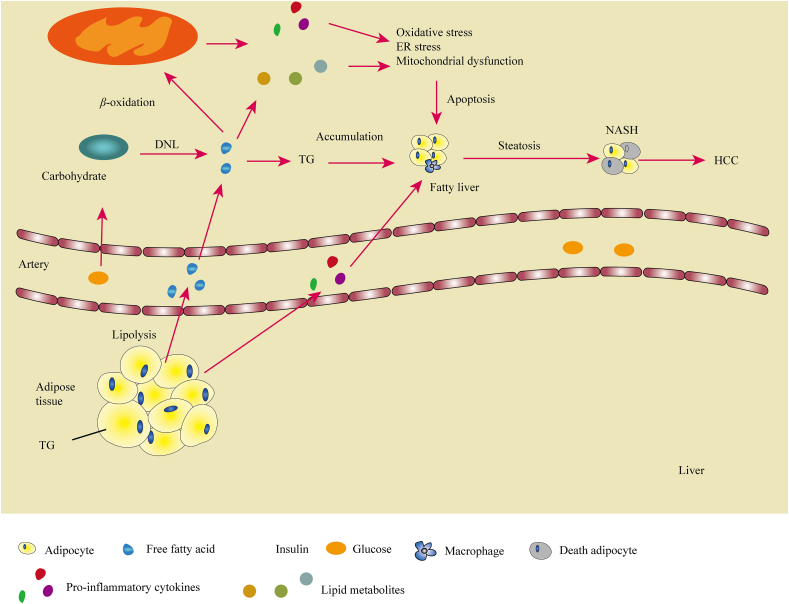
TG accumulation is likely the first step in the pathophysiology of NAFLD and results from an imbalance between TG synthesis and utilization292. In healthy individuals, excess carbohydrate that accumulates in the liver is converted into FFAs by de novo lipogenesis (DNL)293,294; some of these FFAs are esterified into TG in AT for storage, and others are stored in the liver directly293 (Fig. 5). Increased intracellular glucose levels activate the glucose sensor carbohydrate response element-binding protein (ChREBP), which promotes glycolysis and gene expression of DNL genes in the liver295. The newly synthesized TG is mostly packaged into very low-density lipoproteins (VLDL) and exported to AT284. Adipose tissue extracts lipids from VLDL through the action of lipoprotein lipase. In obesity, insulin resistance causes a decreased insulin-dependent inhibition of lipolysis and adipocytes cannot accept TG from VLDL or hydrolyze intracellular TG, which causes more FFAs to be released into the circulation296,297.
Figure 5.
Schematic representation of the links between obesity and liver diseases. Free fatty acids (FFAs) and glucose from the circulation accumulate in the liver and support de novo lipogenesis (DNL) of FAs to form triglycerides (TG), which causes fatty liver. Under the ‘second hit’ of inflammation induced by oxidative stress, ER stress and reactive oxygen species (ROS) cause adipocyte apoptosis, which eventually leads to NASH and sometimes to HCC.
Excess energy intake or limited energy expenditure make redundant fat accumulate in SAT preferentially. Compared with VAT, SAT is more insulin-sensitive and has high avidity for FFAs and TGs, preventing their deposition in non-adipose tissue21,298. Hypertrophic subcutaneous adipocytes have been shown to have a pro-inflammatory gene expression and are associated with greater rates of lipolysis, increased cytokine release, and IR299. When the storage capacity of SAT is exceeded, or when normal lipid metabolism is impaired in obese patients, fat begins to accumulate in other non-adipose tissue organs, which promote the formation of VAT. Each standard deviation increase in subcutaneous AT (SAT) mass decreases the likelihood of IR by 48%, whereas each standard deviation increase of visceral AT (VAT) mass increases likelihood of IR by 80%300. VAT enlarged in the liver contributes to fatty liver.
In states of insulin resistance, insulin continues to support DNL, while its capacity to reduce hepatic gluconeogenesis is impaired in the liver, which upregulates the level of FFA and inhibits FFA β-oxidation, further promoting hepatic fat accumulation301,302. Excess FFA in the liver can be disposed of through oxidation pathways, mainly in mitochondria, but also in peroxisomes and microsomes40,286,303. β-Oxidation of FFAs in mitochondria produces ROS that induce FFA peroxidation and cause damage to mitochondrial DNA and proteins304. VAT in the liver induces hypoxia by affecting vascularization and then promoting adipocyte apoptosis, which increases the infiltration of macrophages into AT and enhances the production of proinflammatory cytokines305,306. These changes increase the risk of NASH292. With the deterioration of obesity, inflammation develops when the influx of FFAs to the liver overwhelms physiologically adaptive mechanisms, leading to ROS formation, ER stress, and hepatocellular dysfunction and injury by lipotoxicity307,308.
The progression to NASH also involves hepatic increased infiltration of different subtypes of immune cells, such as macrophages, Kupffer, dendritic and hepatic stellate cells (HSCs), and the immune cells produce cytokines (such as TGF-β, IL-10) and other factors contributing to inflammation309. Hepatocyte injury followed by inflammation and activation of the innate immune system leads to liver fibrosis mediated by activation of HSCs and to secretion and deposition of extracellular matrix (ECM)308,310,311. In the obese state, hyperglycemia and hyperinsulinemia promote profibrogenic signals in the HSCs312,313, which mediates profibrotic activity, either directly or as a cofactor of TGF-β, a key cytokine mediating the induction and promotion of fibrogenesis314.
NAFLD and NASH are reversible, and not all patients progress to HCC282. A recent report indicated that obesity contributes to NASH and HCC through independent mechanisms. Obesity-induced oxidative stress in the liver inhibits the activity of protein tyrosine phosphatases (PTPs) that results in the activation of STAT-1 and STAT-3 to promote the development of NASH and HCC respectively315,316.
Adipokines secreted by VAT also play important roles in the progression of liver diseases. Adiponectin can enhance insulin sensitivity and inhibit inflammation317,318, oppose fatty acid synthesis and promote mitochondrial β-oxidation by activating AMPK, thereby helping to prevent liver diseases319. Adiponectin lowers intracellular lipid content by two mechanisms to upregulate insulin signaling. First, adiponectin treatment increases the lipoprotein lipase activity in WAT, which may lead to increased uptake of TG into WAT, thus diverting circulating TG away from storage in liver and skeletal muscle320. Second, adiponectin contributes to the activation of AMPK, PPARα and eNOS, which promotes fatty acid oxidation and glucose uptake255. APN activates AMPK through activating the LKB1 and CaMKK pathways, and then AMPK suppression of ACC lowers malonyl-CoA production to increase the oxidation of long chain fatty acids and circumventing insulin stimulated lipid synthesis320. Moreover, AMPK lowers liver G6Pase and PEPCK mRNA expression321 by promoting transducer of regulated cAMP response element–binding protein 2 (TORC2) phosphorylation and blocking its nuclears accumulation to decrease the production of hepatic glucose and plasma glucose322,323. APN increases fatty-acid combustion and energy consumption via PPARα activation which leads to decreased triglyceride content in the liver and skeletal muscle and thus increased insulin sensitivity254. Lipotoxicity can be linked to the abundance of specific lipids and result in NAFLD and insulin resistance. Diacylglycerols (DAGs) and ceramides are the two best-studied mediators of lipid-induced insulin resistance324,325. Ceramides are bioactive sphingolipids produced by the liver that interfere with insulin signaling by activating protein phosphatase 2 (PP2A) and protein kinase C epsilon (PKCε)326, 327, 328, 329, 330. In contrast, plasma membrane sn-1,2-DAGs, the key DAG stereoisomer, impair insulin action via activation of PKCε in liver and subsequently inhibit insulin receptor kinase (IRK, tyrosine kinase) activity331,332. One study has found that 2 weeks of gAcrp30 treatment reversed whole-body insulin resistance in HFD-fed mice by reducing plasma membrane DAG content, resulting in decreased translocation of PKCε to the plasma membrane in liver, leading to increased insulin signaling325. Adiponectin lowers liver ceramide content by activating hepatic ceramidase333 through AdipoR1 and AdipoR2, and stimulates deacylation of ceramides at neutral pH in a dose-dependent manner producing sphingosine, S1P and dihydrosphingosine-1-phosphate, which reduce insulin resistance120.
Leptin exerts a dual action on NAFLD. On one hand, leptin inhibits hepatic de novo lipogenesis, whereas it stimulates fatty acid oxidation, thereby reducing lipid content in livers. On the other hand, leptin upregulates TGF-β1 and other matrix remodeling enzymes to enhance inflammation and hepatic fibrosis334. NAFLD is related to insulin resistance, hepatic steatosis and diabetes and its pathology is adversely affected by obesity335, but not all patients with obesity develop NAFLD336. Despite not having obesity, these individuals often have central adiposity, which predisposes to the metabolic syndrome and is associated with insulin resistance337. Furthermore, some patients with NAFLD are lean. These people belong to the ‘metabolically obese, normal weight’ phenotype338, which refers to individuals who are non-obese, frequently sedentary, and who have impaired insulin sensitivity, increased cardiovascular risk and increased liver lipid levels, the consequence of decreased capacity for storing fat and reduced mitochondrial function in adipose tissue and increased hepatic de novo lipogenesis339.
Although NAFLD is the most common liver diseases impacted by obesity, obesity and hepatic steatosis also influence the development and progression of other forms of liver disease340. Steatosis is frequently seen in individuals with concurrent hepatitis C infection, especially the genotype 3 hepatitis C virus (HCV) infection341. In addition, weight gain and insulin resistance are both associated with progressive fibrosis in chronic HCV infection340. In individuals with chronic hepatitis C, weight gain itself is associated with progressive liver disease. It has been estimated that approximately 20% of individuals infected with HCV are obese. Obesity in these individuals is associated with steatosis and the progression of fibrosis342. HCV proteins have been proven to regulate the host's glucose and lipid metabolism343 through stimulating DNL and increasing the process of lipogenesis340. HCV increases TGF-β1 expression through induction of ROS, and activation of the p38 MAPK, JNK, ERK, and NF-κB pathways344 to promote the development of HCC.
6. Treatment
Obesity and associated diseases have attracted great attention in recent years on account of their widespread and increasing prevalence. Controlling or losing weight to prevent further worsening of obese individuals seems to be an effective method but can be very challenging for people to adhere to in the longer term. For example, people with biopsy-confirmed NASH showed a 5% reduction in BMI has been shown to result in a 25% relative reduction in liver fat345, and people who have weight loss over 10% with T2DM would reduce the end points of CVD by 21%346. The methods of weight loss include increased physical activity, diet changes, bariatric surgery and drug interventions. Dietary modification is central to obesity treatment, which includes nutritional restriction and nutraceutical intervention. Caloric restriction (CR) is a long-term dietary intervention by reducing caloric intake, which represents an effective strategy to reduce weight, influences adipose tissues plasticity and modifies endocrinological function of adipose tissue and skeletal muscle347. Diet intervention and exercise always require a long time (over 6 months) to achieve ideal goals for obese patients, and it is difficult for many people stick to these regimes and continue to lose weight348,349. Bariatric surgery is safe and highly effective in reducing weight, obesity-associated comorbidities, and mortality. Bariatric surgery is appropriate for patients who have a BMI > 40 kg/m2 alone or >35 kg/m2 with comorbidities, or have failed in attempts to diet and exercise, or are free of significant psychological disease350. Bariatric surgery includes gastric banding, sleeve gastrectomy, Roux-en-Y gastric bypass, and/or biliopancreatic diversion with duodenal switch351. Whereas gastric banding and sleeve gastrectomy are purely restrictive in nature, the latter two procedures also result in significant malabsorption352.
Drug treatment can enhance weight loss in the short (phentermine, amfepramone, cathin hydrochloride) and long-term use (sibutramine, fenfluramine, rimonabant and orlistat), although most of these drugs showed adverse cardiovascular effects or addictive potential (Table 1)353. Many reviews have discussed the development of drugs against obesity353, 354, 355. The agents approved by the FDA to treat obesity are involved in regulating mitochondrial function, sympathomimetics, fat absorption, and appetite regulation. However, several drugs have been removed from the market due to adverse effects, only a few are still being used. Phentermin, an agent of sympathomimetics drugs, was approved for short-term use since 1959356. It can cause some side-effects including increased pulse rate and blood pressure, headache and dry mouth. The combination of topiramate with phentermine at low doses showed greater weight loss and fewer side effects by affecting energy metabolism through modulation of GABAergic neurotransmission353,357. The main concern is the risk of oral clefts in infants exposed to topiramate in utero353. Orlistat as a lipase inhibitor to reduce the uptake of dietary fat has been approved as an anti-obesity drug since 1999357. It possesses excellent safety without inducing adverse cardiovascular effects, but has some gastrointestinal side effects. While its effects on weight loss were not obvious358. Bupropion/naltrexone combination is also approved in US for long term weight management by reducing food intake359. Although this combination may increase blood pressure or heart rate, no significant increase in cardiovascular events was found360. In recent years, glucagon-like peptide-1 (GLP-1) receptor agonists are increasingly being used for the treatment of T2DM. Liraglutide and semaglutide have been approved as anti-obesity drugs in 2014 and 2021 respectively353. They can not only reduce glucose concentrations and increase satiety, but also slow gastric emptying and reduce bodyweight in a dose-dependent manner. Both of them are well tolerated and show a low incidence of major adverse cardiovascular events, while the typical GLP-1-related adverse effects including nausea, diarrhea, vomiting and constipation still occur361,362.
Table 1.
Antiobesity drugs on the market.
| Drug | Mechanism | Approval | Side effect |
|---|---|---|---|
| Phentermine | Sympathomimetics agent | 1959 | Palpitations, elevated blood pressure |
| Topiramate/Phentermin | Sympathomimetic/anticonvulsant | 2012 | Depression, suicidal ideation |
| Cathin hydrochloride | Sympathomimetics agent | 1975 | Tachycardia, increase in blood pressure |
| Sibutramine | Sympathomimetics agent | 1997 | Non-fatal myocardial infarction and stroke |
| Fenfluramine | Sympathomimetics agent | 1973 | Cardiac valvular insufficiency |
| Rimonabant | CB1 receptor blocker | 2006 | Depression, suicidal ideation |
| Bupropion/Naltrexone | Opioid receptor antagonist/dopamine and noradrenaline reuptake inhibitor | 2014 | Seizures, palpitations, transient blood pressure elevations |
| Orlistat | Pancreatic lipase inhibitor | 1999 | Gastrointestinal symptoms, liver injury |
| Liraglutide | GLP-1R agonists | 2014 | Nausea/vomiting, diarrhea, gallstones |
| Semaglutide | GLP-1R agonists | 2021 | Nausea/vomiting, diarrhea |
Most obesity-related deaths are due to CVD363, and improving cardiovascular health becomes a primary objective for weight-loss drugs. None of the currently approved anti-obesity drugs have been shown to be effective for primary prevention of CVD or in reducing major adverse cardiovascular events or mortality among patients with obesity356. The exploration of next generation anti-obesity drugs is still ongoing.
7. Conclusions
Obesity remains the most serious risk factor for several cancers, cardiovascular diseases, type 2 diabetes, and liver diseases. Excess uptake of calories leads to accumulation of fat in adipose tissue thereby promoting adipose tissue expansion through adipocyte hypertrophy and hyperplasia. When the available fat exceeds the storage capacity of adipocytes, it will travel through the circulation and form ectopic deposits in other organs, giving rise to visceral fat. Visceral fat can impair vascularization and cause hypoxia, oxidative stress, and ER stress which contribute to the pathogenesis of other associated diseases. Furthermore, excess dietary fats are transformed into free fatty acids to synthesize triglycerides as an energy reserve. High levels of free fatty acids in serum and their metabolites activate the PKC and JNK-1 signaling pathways and change the initial process of ISR-1 to induce insulin resistance that will cause hyperglycemia, T2DM, and liver diseases directly. Adipose tissue, as an endocrine organ, secretes several adipokines, including leptin, adiponectin, and resistin. These adipokines play important roles in regulating the relationship between obesity and associated diseases. For example, elevated leptin activates intracellular signaling, such as the PI3K/AKT/mTOR and ERK/MAPK pathways, including in cancer cells. It impairs β-cell function and suppresses insulin secretion to result in a hyperglycemia state lower levels of adiponectin in obesity decrease insulin sensitivity and fat oxidation. Adipose tissue is in a state of inflammation. VAT secretes some inflammatory cytokines, such as TNF-α, IL-6 and MCP-1, which can activate the NF-κB and JAK/STAT signaling pathways and induce inflammation in the heart and liver. Fat accumulation in non-adipose tissue and inflammation induced by visceral fat are the most important causal factors for obesity-associated diseases. Decreasing the uptake of a high-fat or high-carbohydrate diet and proper physical activity to control body weight and reduce the percent of visceral fat will help people who are overweight or obese lower the risk of other metabolic disorders. In view of the worldwide epidemic of obesity, it is important for us to control body weight and balance our diet. However, many people find it hard to stick to lower food consumption and higher levels of exercise. Therefore, understanding the links between obesity and other diseases and the internal mechanisms that underlie them could help us better prevent and treat obesity and related disorders. Novel therapeutic approaches to this need to be explored and validated.
Acknowledgments
This study was supported by the Natural Science Foundation of China (No. 82073759, China), Qingdao Postdoctoral Science Foundation (No. 862105040014, China), Special funds of Shandong Province for Qingdao National Laboratory of Marine Science and Technology (No. 2022QNLM030003, China), and National Science and Technology Major Project for Significant New Drugs Development (No. 2018ZX09735004, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Changgui Li, Email: lichanggui@medmail.com.cn.
Christopher G. Proud, Email: Christopher.Proud@sahmri.com.
Tao Jiang, Email: jiangtao@ouc.edu.cn.
Author contributions
Xin Jin, Tingting Qiu and Li Li: Writing-Original draft preparation. Rilei Yu, Xiguang Chen, Changgui Li and Christopher G. Proud: Writing-Review & Editing. Tao Jiang: Supervision, Funding acquisition and Writing-Review & Editing. All authors have approved the final article.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Dragano N.R.V., Ferno J., Dieguez C., Lopez M., Milbank E. Recent updates on obesity treatments: available drugs and future directions. Neuroscience. 2020;437:215–239. doi: 10.1016/j.neuroscience.2020.04.034. [DOI] [PubMed] [Google Scholar]
- 2.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 4.Nimptsch K., Konigorski S., Pischon T. Diagnosis of obesity and use of obesity biomarkers in science and clinical medicine. Metabolism. 2019;92:61–70. doi: 10.1016/j.metabol.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Lauby-Secretan B., Scoccianti C., Loomis D., Grosse Y., Bianchini F., Straif K., et al. Body fatness and cancer—viewpoint of the IARC working group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pischon T., Nothlings U., Boeing H. Obesity and cancer. Proc Nutr Soc. 2008;67:128–145. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- 7.Consultation WHOE Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 8.Zeng Q., Li N., Pan X.F., Chen L., Pan A. Clinical management and treatment of obesity in China. Lancet Diabetes Endocrinol. 2021;9:393–405. doi: 10.1016/S2213-8587(21)00047-4. [DOI] [PubMed] [Google Scholar]
- 9.Zhou B.F., Cooperative Meta-Analysis Group of the Working Group on Obesity in China Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96. [PubMed] [Google Scholar]
- 10.Avgerinos K.I., Spyrou N., Mantzoros C.S., Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–135. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Lemieux I., Despres J.P. Metabolic syndrome: past, present and future. Nutrients. 2020;12:3501–3508. doi: 10.3390/nu12113501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bapat S.P., Whitty C., Mowery C.T., Liang Y., Yoo A., Jiang Z., et al. Obesity alters pathology and treatment response in inflammatory disease. Nature. 2022;604:337–342. doi: 10.1038/s41586-022-04536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aleksandrova K., Egea Rodrigues C., Floegel A., Ahrens W. Omics biomarkers in obesity: novel etiological insights and targets for precision prevention. Curr Obes Rep. 2020;9:219–230. doi: 10.1007/s13679-020-00393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrakis D., Margina D., Tsarouhas K., Tekos F., Stan M., Nikitovic D., et al. Obesity—a risk factor for increased COVID19 prevalence, severity and lethality (Review) Mol Med Rep. 2020;22:9–19. doi: 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morais A.H.A., Passos T.S., de Lima Vale S.H., da Silva Maia J.K., Maciel B.L.L. Obesity and the increased risk for COVID-19: mechanisms and nutritional management. Nutr Res Rev. 2021;34:209–221. doi: 10.1017/S095442242000027X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izcovich A., Ragusa M.A., Tortosa F., Lavena Marzio M.A., Agnoletti C., Bengolea A., et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241955. 0241955-0241985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gammone M.A., D'Orazio N. COVID-19 and obesity: overlapping of two pandemics. Obes Facts. 2021;14:579–585. doi: 10.1159/000518386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gadde K.M., Martin C.K., Berthoud H.R., Heymsfield S.B. Obesity: pathophysiology and management. J Am Coll Cardiol. 2018;71:69–84. doi: 10.1016/j.jacc.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas B., Schlinkert P., Mayer P., Eckstein N. Targeting adipose tissue. Diabetol Metab Syndr. 2012;4:43–54. doi: 10.1186/1758-5996-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belaj K.J., Eller P. The fate of fat. Gerontology. 2012;58:120–125. doi: 10.1159/000331798. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim M.M. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 22.Gaspar R.C., Pauli J.R., Shulman G.I., Munoz V.R. An update on brown adipose tissue biology: a discussion of recent findings. Am J Physiol Endocrinol Metab. 2021;320:488–495. doi: 10.1152/ajpendo.00310.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes-Farias M., Fos-Domenech J., Serra D., Herrero L., Sanchez-Infantes D. White adipose tissue dysfunction in obesity and aging. Biochem Pharmacol. 2021;192:114723–114735. doi: 10.1016/j.bcp.2021.114723. [DOI] [PubMed] [Google Scholar]
- 24.Becher T., Palanisamy S., Kramer D.J., Eljalby M., Marx S.J., Wibmer A.G., et al. Brown adipose tissue is associated with cardiometabolic health. Nat Med. 2021;27:58–65. doi: 10.1038/s41591-020-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coenen K.R., Gruen M.L., Chait A., Hasty A.H. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56:564–573. doi: 10.2337/db06-1375. [DOI] [PubMed] [Google Scholar]
- 26.Reilly S.M., Saltiel A.R. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 27.Guzik T.J., Skiba D.S., Touyz R.M., Harrison D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017;113:1009–1023. doi: 10.1093/cvr/cvx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai T., Autieri M.V., Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. 2021;320:375–391. doi: 10.1152/ajpcell.00379.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hermano E., Goldberg R., Rubinstein A.M., Sonnenblick A., Maly B., Nahmias D., et al. Heparanase accelerates obesity-associated breast cancer progression. Cancer Res. 2019;79:5342–5354. doi: 10.1158/0008-5472.CAN-18-4058. [DOI] [PubMed] [Google Scholar]
- 30.Galic S., Oakhill J.S., Steinberg G.R. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Liu X.Z., Pedersen L., Halberg N. Cellular mechanisms linking cancers to obesity. Cell Stress. 2021;5:55–72. doi: 10.15698/cst2021.05.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Habanjar O., Diab-Assaf M., Caldefie-Chezet F., Delort L. The impact of obesity, adipose tissue, and tumor microenvironment on macrophage polarization and metastasis. Biology (Basel) 2022;11:339–365. doi: 10.3390/biology11020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell L.N., Ward J.L., Degawa-Yamauchi M., Bovenkerk J.E., Jones R., Cacucci B.M., et al. Adipose tissue production of hepatocyte growth factor contributes to elevated serum HGF in obesity. Am J Physiol Endocrinol Metab. 2006;291:843–848. doi: 10.1152/ajpendo.00174.2006. [DOI] [PubMed] [Google Scholar]
- 34.Askarpour M., Alizadeh S., Hadi A., Symonds M.E., Miraghajani M., Sheikhi A., et al. Effect of bariatric surgery on the circulating level of adiponectin, chemerin, plasminogen activator inhibitor-1, leptin, resistin, and visfatin: a systematic review and meta-analysis. Horm Metab Res. 2020;52:207–215. doi: 10.1055/a-1129-6785. [DOI] [PubMed] [Google Scholar]
- 35.La Cava A., Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 36.Obradovic M., Sudar-Milovanovic E., Soskic S., Essack M., Arya S., Stewart A.J., et al. Leptin and obesity: role and clinical implication. Front Endocrinol (Lausanne) 2021;12:585887–585901. doi: 10.3389/fendo.2021.585887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckel R.H., Grundy S.M., Zimmet P.Z. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 38.Ha X., Wang J., Chen K., Deng Y., Zhang X., Feng J., et al. Free fatty acids promote the development of prostate cancer by upregulating peroxisome proliferator-activated receptor gamma. Cancer Manag Res. 2020;12:1355–1369. doi: 10.2147/CMAR.S236301. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neuschwander-Tetri B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 41.Niswender K. Diabetes and obesity: therapeutic targeting and risk reduction—a complex interplay. Diabetes Obes Metab. 2010;12:267–287. doi: 10.1111/j.1463-1326.2009.01175.x. [DOI] [PubMed] [Google Scholar]
- 42.Stanek A., Brozyna-Tkaczyk K., Myslinski W. The role of obesity-induced perivascular adipose tissue (PVAT) dysfunction in vascular homeostasis. Nutrients. 2021;13:3842–3861. doi: 10.3390/nu13113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ansaldo A.M., Montecucco F., Sahebkar A., Dallegri F., Carbone F. Epicardial adipose tissue and cardiovascular diseases. Int J Cardiol. 2019;278:254–260. doi: 10.1016/j.ijcard.2018.09.089. [DOI] [PubMed] [Google Scholar]
- 44.Islam S., Zhao Y., Yang T., Liu W. Effect of obesity on several types of cancer. E3S Web Conf. 2021;292 03083-03089. [Google Scholar]
- 45.Franchini F., Palatucci G., Colao A., Ungaro P., Macchia P.E., Nettore I.C. Obesity and thyroid cancer risk: an update. Int J Environ Res Public Health. 2022;19:1116–1131. doi: 10.3390/ijerph19031116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karimi K., Lindgren T.H., Koch C.A., Brodell R.T. Obesity as a risk factor for malignant melanoma and non-melanoma skin cancer. Rev Endocr Metab Disord. 2016;17:389–403. doi: 10.1007/s11154-016-9393-9. [DOI] [PubMed] [Google Scholar]
- 47.Donohoe C.L., Pidgeon G.P., Lysaght J., Reynolds J.V. Obesity and gastrointestinal cancer. Br J Surg. 2010;97:628–642. doi: 10.1002/bjs.7079. [DOI] [PubMed] [Google Scholar]
- 48.Ottaiano A., De Divitiis C., Capozzi M., Avallone A., Pisano C., Pignata S., et al. Obesity and cancer: biological links and treatment implications. Curr Cancer Drug Targets. 2018;18:231–238. doi: 10.2174/1568009617666170330125619. [DOI] [PubMed] [Google Scholar]
- 49.Calle E.E., Thun M.J. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 50.Kyrgiou M., Kalliala I., Markozannes G., Gunter M.J., Paraskevaidis E., Gabra H., et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:477–487. doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson R.L., Taaffe D.R., Newton R.U., Hart N.H., Lyons-Wall P., Galvao D.A. Obesity and prostate cancer: a narrative review. Crit Rev Oncol Hematol. 2022;169:103543–103553. doi: 10.1016/j.critrevonc.2021.103543. [DOI] [PubMed] [Google Scholar]
- 52.Pendyala S., Neff L.M., Suarez-Farinas M., Holt P.R. Diet-induced weight loss reduces colorectal inflammation: implications for colorectal carcinogenesis. Am J Clin Nutr. 2011;93:234–242. doi: 10.3945/ajcn.110.002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breininger S.P., Sabater L., Malcomson F.C., Afshar S., Mann J., Mathers J.C. Obesity and Roux-en-Y gastric bypass drive changes in miR-31 and miR-215 expression in the human rectal mucosa. Int J Obes (Lond) 2022;46:333–341. doi: 10.1038/s41366-021-01005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Franco S., Bianca P., Sardina D.S., Turdo A., Gaggianesi M., Veschi V., et al. Adipose stem cell niche reprograms the colorectal cancer stem cell metastatic machinery. Nat Commun. 2021;12:5006–5022. doi: 10.1038/s41467-021-25333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan D.S.M., Vieira A.R., Aune D., Bandera E.V., Greenwood D.C., McTiernan A., et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rawla P., Thandra K.C., Sunkara T. Pancreatic cancer and obesity: epidemiology, mechanism, and preventive strategies. Clin J Gastroenterol. 2019;12:285–291. doi: 10.1007/s12328-019-00953-3. [DOI] [PubMed] [Google Scholar]
- 57.Pothuraju R., Rachagani S., Junker W.M., Chaudhary S., Saraswathi V., Kaur S., et al. Pancreatic cancer associated with obesity and diabetes: an alternative approach for its targeting. J Exp Clin Cancer Res. 2018;37:319–334. doi: 10.1186/s13046-018-0963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Renehan A.G., Tyson M., Egger M., Heller R.F., Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 59.Trestini I., Carbognin L., Bonaiuto C., Tortora G., Bria E. The obesity paradox in cancer: clinical insights and perspectives. Eat Weight Disord. 2018;23:185–193. doi: 10.1007/s40519-018-0489-y. [DOI] [PubMed] [Google Scholar]
- 60.Lee D.H., Giovannucci E.L. The obesity paradox in cancer: epidemiologic insights and perspectives. Curr Nutr Rep. 2019;8:175–181. doi: 10.1007/s13668-019-00280-6. [DOI] [PubMed] [Google Scholar]
- 61.Gao Y., Chen X., He Q., Gimple R.C., Liao Y., Wang L., et al. Adipocytes promote breast tumorigenesis through TAZ-dependent secretion of resistin. Proc Natl Acad Sci U S A. 2020;117:33295–33304. doi: 10.1073/pnas.2005950117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Setayesh T., Nersesyan A., Misik M., Ferk F., Langie S., Andrade V.M., et al. Impact of obesity and overweight on DNA stability: few facts and many hypotheses. Mutat Res Rev Mutat Res. 2018;777:64–91. doi: 10.1016/j.mrrev.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Cerda C., Sanchez C., Climent B., Vazquez A., Iradi A., El Amrani F., et al. Oxidative stress and DNA damage in obesity-related tumorigenesis. Adv Exp Med Biol. 2014;824:5–17. doi: 10.1007/978-3-319-07320-0_2. [DOI] [PubMed] [Google Scholar]
- 64.Loft S., Danielsen P., Lohr M., Jantzen K., Hemmingsen J.G., Roursgaard M., et al. Urinary excretion of 8-oxo-7,8-dihydroguanine as biomarker of oxidative damage to DNA. Arch Biochem Biophys. 2012;518:142–150. doi: 10.1016/j.abb.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 65.Barregard L., Moller P., Henriksen T., Mistry V., Koppen G., Rossner P., Jr., et al. Human and methodological sources of variability in the measurement of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine. Antioxid Redox Signal. 2013;18:2377–2391. doi: 10.1089/ars.2012.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Collado R., Oliver I., Tormos C., Egea M., Miguel A., Cerda C., et al. Early ROS-mediated DNA damage and oxidative stress biomarkers in monoclonal B lymphocytosis. Cancer Lett. 2012;317:144–149. doi: 10.1016/j.canlet.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 67.Rani V., Deep G., Singh R.K., Palle K., Yadav U.C. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci. 2016;148:183–193. doi: 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Grattagliano I., Palmieri V.O., Portincasa P., Moschetta A., Palasciano G. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem. 2008;19:491–504. doi: 10.1016/j.jnutbio.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 69.Khan N.I., Naz L., Yasmeen G. Obesity: an independent risk factor for systemic oxidative stress. Pak J Pharm Sci. 2006;19:62–65. [PubMed] [Google Scholar]
- 70.Cozzo A.J., Fuller A.M., Makowski L. Contribution of adipose tissue to development of cancer. Compr Physiol. 2017;8:237–282. doi: 10.1002/cphy.c170008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lepetsos P., Papavassiliou K.A., Papavassiliou A.G. Redox and NF-kappaB signaling in osteoarthritis. Free Radic Biol Med. 2019;132:90–100. doi: 10.1016/j.freeradbiomed.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 72.de Mello A.H., Costa A.B., Engel J.D.G., Rezin G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018;192:26–32. doi: 10.1016/j.lfs.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 73.Molendijk J., Robinson H., Djuric Z., Hill M.M. Lipid mechanisms in hallmarks of cancer. Mol Omics. 2020;16:6–18. doi: 10.1039/c9mo00128j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nieman K.M., Kenny H.A., Penicka C.V., Ladanyi A., Buell-Gutbrod R., Zillhardt M.R., et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Louie S.M., Roberts L.S., Nomura D.K. Mechanisms linking obesity and cancer. Biochim Biophys Acta. 2013;1831:1499–1508. doi: 10.1016/j.bbalip.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wymann M.P., Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 77.Rathmell J.C. Obesity, immunity, and cancer. N Engl J Med. 2021;384:1160–1162. doi: 10.1056/NEJMcibr2035081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marcelin G., Gautier E.L., Clement K. Adipose tissue fibrosis in obesity: etiology and challenges. Annu Rev Physiol. 2022;84:135–155. doi: 10.1146/annurev-physiol-060721-092930. [DOI] [PubMed] [Google Scholar]
- 79.Masone S., Velotti N., Savastano S., Filice E., Serao R., Vitiello A., et al. Morbid obesity and thyroid cancer rate. A review of literature. J Clin Med. 2021;10:1894–1903. doi: 10.3390/jcm10091894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang A.M.Y., Wellberg E.A., Kopp J.L., Johnson J.D. Hyperinsulinemia in obesity, inflammation, and cancer. Diabetes Metab J. 2021;45:285–311. doi: 10.4093/dmj.2020.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farag K.I., Makkouk A., Norian L.A. Re-evaluating the effects of obesity on cancer immunotherapy outcomes in renal cancer: what do we really know?. Front Immunol. 2021;12:668494–668509. doi: 10.3389/fimmu.2021.668494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Pergola G., Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546–291557. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adesunloye B.A. Mechanistic insights into the link between obesity and prostate cancer. Int J Mol Sci. 2021;22:3935–3948. doi: 10.3390/ijms22083935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gill E., Sandhu G., Ward D.G., Perks C.M., Bryan R.T. The sirenic links between diabetes, obesity, and bladder cancer. Int J Mol Sci. 2021;22:11150–11164. doi: 10.3390/ijms222011150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wallace I.R., McKinley M.C., Bell P.M., Hunter S.J. Sex hormone binding globulin and insulin resistance. Clin Endocrinol (Oxf) 2013;78:321–329. doi: 10.1111/cen.12086. [DOI] [PubMed] [Google Scholar]
- 86.Perry R.J., Shulman G.I. Mechanistic links between obesity, insulin, and cancer. Trends Cancer. 2020;6:75–78. doi: 10.1016/j.trecan.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ohkuma T., Peters S.A.E., Woodward M. Sex differences in the association between diabetes and cancer: a systematic review and meta-analysis of 121 cohorts including 20 million individuals and one million events. Diabetologia. 2018;61:2140–2154. doi: 10.1007/s00125-018-4664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maguire O.A., Ackerman S.E., Szwed S.K., Maganti A.V., Marchildon F., Huang X., et al. Creatine-mediated crosstalk between adipocytes and cancer cells regulates obesity-driven breast cancer. Cell Metab. 2021;33:499–512. doi: 10.1016/j.cmet.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Picon-Ruiz M., Morata-Tarifa C., Valle-Goffin J.J., Friedman E.R., Slingerland J.M. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378–397. doi: 10.3322/caac.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liedtke S., Schmidt M.E., Vrieling A., Lukanova A., Becker S., Kaaks R., et al. Postmenopausal sex hormones in relation to body fat distribution. Obesity (Silver Spring) 2012;20:1088–1095. doi: 10.1038/oby.2011.383. [DOI] [PubMed] [Google Scholar]
- 91.Allott E.H., Masko E.M., Freedland S.J. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63:800–809. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raucci R., Rusolo F., Sharma A., Colonna G., Castello G., Costantini S. Functional and structural features of adipokine family. Cytokine. 2013;61:1–14. doi: 10.1016/j.cyto.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 93.Benbaibeche H., Bounihi A., Koceir E.A. Leptin level as a biomarker of uncontrolled eating in obesity and overweight. Ir J Med Sci. 2021;190:155–161. doi: 10.1007/s11845-020-02316-1. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Y., Chua S., Jr. Leptin function and regulation. Compr Physiol. 2017;8:351–369. doi: 10.1002/cphy.c160041. [DOI] [PubMed] [Google Scholar]
- 95.Hopkins B.D., Goncalves M.D., Cantley L.C. Obesity and cancer mechanisms: cancer metabolism. J Clin Oncol. 2016;34:4277–4283. doi: 10.1200/JCO.2016.67.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harbuzariu A., Oprea-Ilies G.M., Gonzalez-Perez R.R. The role of notch signaling and leptin-notch crosstalk in pancreatic cancer. Medicines (Basel) 2018;5:68–85. doi: 10.3390/medicines5030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Newman G., Gonzalez-Perez R.R. Leptin-cytokine crosstalk in breast cancer. Mol Cell Endocrinol. 2014;382:570–582. doi: 10.1016/j.mce.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sweeney G. Leptin signalling. Cell Signal. 2002;14:655–663. doi: 10.1016/s0898-6568(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 99.Zabeau L., Lavens D., Peelman F., Eyckerman S., Vandekerckhove J., Tavernier J. The ins and outs of leptin receptor activation. FEBS Lett. 2003;546:45–50. doi: 10.1016/s0014-5793(03)00440-x. [DOI] [PubMed] [Google Scholar]
- 100.Garofalo C., Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207:12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- 101.Ghasemi A., Saeidi J., Azimi-Nejad M., Hashemy S.I. Leptin-induced signaling pathways in cancer cell migration and invasion. Cell Oncol (Dordr) 2019;42:243–260. doi: 10.1007/s13402-019-00428-0. [DOI] [PubMed] [Google Scholar]
- 102.Huang Y., Jin Q., Su M., Ji F., Wang N., Zhong C., et al. Leptin promotes the migration and invasion of breast cancer cells by upregulating ACAT2. Cell Oncol (Dordr) 2017;40:537–547. doi: 10.1007/s13402-017-0342-8. [DOI] [PubMed] [Google Scholar]
- 103.Li K., Wei L., Huang Y., Wu Y., Su M., Pang X., et al. Leptin promotes breast cancer cell migration and invasion via IL-18 expression and secretion. Int J Oncol. 2016;48:2479–2487. doi: 10.3892/ijo.2016.3483. [DOI] [PubMed] [Google Scholar]
- 104.Jimenez-Cortegana C., Lopez-Saavedra A., Sanchez-Jimenez F., Perez-Perez A., Castineiras J., Virizuela-Echaburu J.A., et al. Leptin, both bad and good actor in cancer. Biomolecules. 2021;11:913–929. doi: 10.3390/biom11060913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lord G.M., Matarese G., Howard J.K., Baker R.J., Bloom S.R., Lechler R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 106.Somasundar P., Yu A.K., Vona-Davis L., McFadden D.W. Differential effects of leptin on cancer in vitro. J Surg Res. 2003;113:50–55. doi: 10.1016/s0022-4804(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 107.Pham D.V., Park P.H. Tumor metabolic reprogramming by adipokines as a critical driver of obesity-associated cancer progression. Int J Mol Sci. 2021;22:1444–1466. doi: 10.3390/ijms22031444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kelesidis I., Kelesidis T., Mantzoros C.S. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94:1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nigro E., Scudiero O., Monaco M.L., Palmieri A., Mazzarella G., Costagliola C., et al. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int. 2014;2014:658913–658927. doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ohbuchi Y., Suzuki Y., Hatakeyama I., Nakao Y., Fujito A., Iwasaka T., et al. A lower serum level of middle-molecular-weight adiponectin is a risk factor for endometrial cancer. Int J Clin Oncol. 2014;19:667–673. doi: 10.1007/s10147-013-0603-0. [DOI] [PubMed] [Google Scholar]
- 111.Kerenidi T., Lada M., Tsaroucha A., Georgoulias P., Mystridou P., Gourgoulianis K.I. Clinical significance of serum adipokines levels in lung cancer. Med Oncol. 2013;30:507–515. doi: 10.1007/s12032-013-0507-x. [DOI] [PubMed] [Google Scholar]
- 112.Kosova F., Coskun T., Kaya Y., Kara E., Ari Z. Adipocytokine levels of colon cancer patients before and after treatment. Bratisl Lek Listy. 2013;114:394–397. doi: 10.4149/bll_2013_083. [DOI] [PubMed] [Google Scholar]
- 113.Liao L.M., Schwartz K., Pollak M., Graubard B.I., Li Z., Ruterbusch J., et al. Serum leptin and adiponectin levels and risk of renal cell carcinoma. Obesity (Silver Spring) 2013;21:1478–1485. doi: 10.1002/oby.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Straub L.G., Scherer P.E. Metabolic messengers: adiponectin. Nat Metab. 2019;1:334–339. doi: 10.1038/s42255-019-0041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou Y., Yang Y., Zhou T., Li B., Wang Z. Adiponectin and thyroid cancer: insight into the association between adiponectin and obesity. Aging Dis. 2021;12:597–613. doi: 10.14336/AD.2020.0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Luo Z., Saha A.K., Xiang X., Ruderman N.B. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 117.Di Zazzo E., Polito R., Bartollino S., Nigro E., Porcile C., Bianco A., et al. Adiponectin as link factor between adipose tissue and cancer. Int J Mol Sci. 2019;20:839–852. doi: 10.3390/ijms20040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dalamaga M., Diakopoulos K.N., Mantzoros C.S. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547–594. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 120.Holland W.L., Miller R.A., Wang Z.V., Sun K., Barth B.M., Bui H.H., et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Denzel M.S., Hebbard L.W., Shostak G., Shapiro L., Cardiff R.D., Ranscht B. Adiponectin deficiency limits tumor vascularization in the MMTV-PyV-mT mouse model of mammary cancer. Clin Cancer Res. 2009;15:3256–3264. doi: 10.1158/1078-0432.CCR-08-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Birks S., Peeters A., Backholer K., O'Brien P., Brown W. A systematic review of the impact of weight loss on cancer incidence and mortality. Obes Rev. 2012;13:868–891. doi: 10.1111/j.1467-789X.2012.01010.x. [DOI] [PubMed] [Google Scholar]
- 123.Lennon H., Sperrin M., Badrick E., Renehan A.G. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18:56–64. doi: 10.1007/s11912-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ortega F.B., Lavie C.J., Blair S.N. Obesity and cardiovascular disease. Circ Res. 2016;118:1752–1770. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- 125.Moore K.J., Shah R. Introduction to the obesity, metabolic syndrome, and CVD compendium. Circ Res. 2020;126:1475–1476. doi: 10.1161/CIRCRESAHA.120.317240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.The Emerging Risk Factors C. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Neeland I.J., Poirier P., Despres J.P. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation. 2018;137:1391–1406. doi: 10.1161/CIRCULATIONAHA.117.029617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Koliaki C., Liatis S., Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism. 2019;92:98–107. doi: 10.1016/j.metabol.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 129.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhai A.B., Haddad H. The impact of obesity on heart failure. Curr Opin Cardiol. 2017;32:196–202. doi: 10.1097/HCO.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 131.Cuspidi C., Rescaldani M., Sala C., Grassi G. Left-ventricular hypertrophy and obesity: a systematic review and meta-analysis of echocardiographic studies. J Hypertens. 2014;32:16–25. doi: 10.1097/HJH.0b013e328364fb58. [DOI] [PubMed] [Google Scholar]
- 132.de Simone G., Palmieri V., Bella J.N., Celentano A., Hong Y., Oberman A., et al. Association of left ventricular hypertrophy with metabolic risk factors: the HyperGEN study. J Hypertens. 2002;20:323–331. doi: 10.1097/00004872-200202000-00024. [DOI] [PubMed] [Google Scholar]
- 133.Shimabukuro M., Kozuka C., Taira S., Yabiku K., Dagvasumberel M., Ishida M., et al. Ectopic fat deposition and global cardiometabolic risk: new paradigm in cardiovascular medicine. J Med Invest. 2013;60:1–14. doi: 10.2152/jmi.60.1. [DOI] [PubMed] [Google Scholar]
- 134.Hu Q., Zhang H., Gutierrez Cortes N., Wu D., Wang P., Zhang J., et al. Increased Drp1 acetylation by lipid overload induces cardiomyocyte death and heart dysfunction. Circ Res. 2020;126:456–470. doi: 10.1161/CIRCRESAHA.119.315252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tong M., Saito T., Zhai P., Oka S.I., Mizushima W., Nakamura M., et al. Alternative mitophagy protects the heart against obesity-associated cardiomyopathy. Circ Res. 2021;129:1105–1121. doi: 10.1161/CIRCRESAHA.121.319377. [DOI] [PubMed] [Google Scholar]
- 136.D'Souza K., Nzirorera C., Kienesberger P.C. Lipid metabolism and signaling in cardiac lipotoxicity. Biochim Biophys Acta. 2016;1861:1513–1524. doi: 10.1016/j.bbalip.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 137.Trivedi P.S., Barouch L.A. Cardiomyocyte apoptosis in animal models of obesity. Curr Hypertens Rep. 2008;10:454–460. doi: 10.1007/s11906-008-0085-z. [DOI] [PubMed] [Google Scholar]
- 138.Mancio J., Oikonomou E.K., Antoniades C. Perivascular adipose tissue and coronary atherosclerosis. Heart. 2018;104:1654–1662. doi: 10.1136/heartjnl-2017-312324. [DOI] [PubMed] [Google Scholar]
- 139.Anthony S.R., Guarnieri A.R., Gozdiff A., Helsley R.N., Phillip Owens A., Tranter M. Mechanisms linking adipose tissue inflammation to cardiac hypertrophy and fibrosis. Clin Sci (Lond) 2019;133:2329–2344. doi: 10.1042/CS20190578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen H.J., Meng T., Gao P.J., Ruan C.C. The role of brown adipose tissue dysfunction in the development of cardiovascular disease. Front Endocrinol (Lausanne) 2021;12:652246–652255. doi: 10.3389/fendo.2021.652246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Takx R.A., Ishai A., Truong Q.A., MacNabb M.H., Scherrer-Crosbie M., Tawakol A. Supraclavicular brown adipose tissue 18F-FDG uptake and cardiovascular disease. J Nucl Med. 2016;57:1221–1225. doi: 10.2967/jnumed.115.166025. [DOI] [PubMed] [Google Scholar]
- 142.Seale P., Kajimura S., Yang W., Chin S., Rohas L.M., Uldry M., et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang W., Ishibashi J., Trefely S., Shao M., Cowan A.J., Sakers A., et al. A PRDM16-driven metabolic signal from adipocytes regulates precursor cell fate. Cell Metab. 2019;30:174–189. doi: 10.1016/j.cmet.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bartelt A., Bruns O.T., Reimer R., Hohenberg H., Ittrich H., Peldschus K., et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 145.Koenen M., Hill M.A., Cohen P., Sowers J.R. Obesity, adipose tissue and vascular dysfunction. Circ Res. 2021;128:951–968. doi: 10.1161/CIRCRESAHA.121.318093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Klaus S., Casteilla L., Bouillaud F., Ricquier D. The uncoupling protein UCP: a membraneous mitochondrial ion carrier exclusively expressed in brown adipose tissue. Int J Biochem. 1991;23:791–801. doi: 10.1016/0020-711x(91)90062-r. [DOI] [PubMed] [Google Scholar]
- 147.Franssens B.T., Hoogduin H., Leiner T., van der Graaf Y., Visseren F.L.J. Relation between brown adipose tissue and measures of obesity and metabolic dysfunction in patients with cardiovascular disease. J Magn Reson Imaging. 2017;46:497–504. doi: 10.1002/jmri.25594. [DOI] [PubMed] [Google Scholar]
- 148.Chechi K., Voisine P., Mathieu P., Laplante M., Bonnet S., Picard F., et al. Functional characterization of the Ucp1-associated oxidative phenotype of human epicardial adipose tissue. Sci Rep. 2017;7:15566–15581. doi: 10.1038/s41598-017-15501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chatterjee T.K., Stoll L.L., Denning G.M., Harrelson A., Blomkalns A.L., Idelman G., et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sakamoto T., Nitta T., Maruno K., Yeh Y.S., Kuwata H., Tomita K., et al. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 level in mice. Am J Physiol Endocrinol Metab. 2016;310:E676–E687. doi: 10.1152/ajpendo.00028.2015. [DOI] [PubMed] [Google Scholar]
- 151.Nisoli E., Clementi E., Paolucci C., Cozzi V., Tonello C., Sciorati C., et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 152.Planavila A., Redondo I., Hondares E., Vinciguerra M., Munts C., Iglesias R., et al. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun. 2013;4:2019–2031. doi: 10.1038/ncomms3019. [DOI] [PubMed] [Google Scholar]
- 153.Burysek L., Houstek J. β-Adrenergic stimulation of interleukin-1α and interleukin-6 expression in mouse brown adipocytes. FEBS Lett. 1997;411:83–86. doi: 10.1016/s0014-5793(97)00671-6. [DOI] [PubMed] [Google Scholar]
- 154.Ali Khan A., Hansson J., Weber P., Foehr S., Krijgsveld J., Herzig S., et al. Comparative secretome analyses of primary murine white and brown adipocytes reveal novel adipokines. Mol Cell Proteomics. 2018;17:2358–2370. doi: 10.1074/mcp.RA118.000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kotch C., Barrett D., Teachey D.T. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15:813–822. doi: 10.1080/1744666X.2019.1629904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stanford K.I., Middelbeek R.J., Townsend K.L., An D., Nygaard E.B., Hitchcox K.M., et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Qing H., Desrouleaux R., Israni-Winger K., Mineur Y.S., Fogelman N., Zhang C., et al. Origin and function of stress-induced IL-6 in murine models. Cell. 2020;182:372–387. doi: 10.1016/j.cell.2020.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mahdaviani K., Chess D., Wu Y., Shirihai O., Aprahamian T.R. Autocrine effect of vascular endothelial growth factor-A is essential for mitochondrial function in brown adipocytes. Metabolism. 2016;65:26–35. doi: 10.1016/j.metabol.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Friederich-Persson M., Nguyen Dinh Cat A., Persson P., Montezano A.C., Touyz R.M. Brown adipose tissue regulates small artery function through NADPH oxidase 4-derived hydrogen peroxide and redox-sensitive protein kinase G-1alpha. Arterioscler Thromb Vasc Biol. 2017;37:455–465. doi: 10.1161/ATVBAHA.116.308659. [DOI] [PubMed] [Google Scholar]
- 160.Betz M.J., Enerback S. Human brown adipose tissue: what we have learned so far. Diabetes. 2015;64:2352–2360. doi: 10.2337/db15-0146. [DOI] [PubMed] [Google Scholar]
- 161.Xiong S., Salazar G., Patrushev N., Ma M., Forouzandeh F., Hilenski L., et al. Peroxisome proliferator-activated receptor gamma coactivator-1alpha is a central negative regulator of vascular senescence. Arterioscler Thromb Vasc Biol. 2013;33:988–998. doi: 10.1161/ATVBAHA.112.301019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brosnihan K.B., Li P., Ferrario C.M. Angiotensin-(1–7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension. 1996;27:523–528. doi: 10.1161/01.hyp.27.3.523. [DOI] [PubMed] [Google Scholar]
- 163.Durand M.J., Zinkevich N.S., Riedel M., Gutterman D.D., Nasci V.L., Salato V.K., et al. Vascular actions of angiotensin 1–7 in the human microcirculation: novel role for telomerase. Arterioscler Thromb Vasc Biol. 2016;36:1254–1262. doi: 10.1161/ATVBAHA.116.307518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bujak-Gizycka B., Madej J., Wolkow P.P., Olszanecki R., Drabik L., Rutowski J., et al. Measurement of angiotensin metabolites in organ bath and cell culture experiments by liquid chromatography–electrospray ionization–mass spectrometry (LC–ESI–MS) J Physiol Pharmacol. 2007;58:529–540. [PubMed] [Google Scholar]
- 165.Than A., Xu S., Li R., Leow M.K., Sun L., Chen P. Angiotensin type 2 receptor activation promotes browning of white adipose tissue and brown adipogenesis. Signal Transduct Target Ther. 2017;2:17022–17034. doi: 10.1038/sigtrans.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.de Kloet A.D., Krause E.G., Scott K.A., Foster M.T., Herman J.P., Sakai R.R., et al. Central angiotensin II has catabolic action at white and brown adipose tissue. Am J Physiol Endocrinol Metab. 2011;301:E1081–E1091. doi: 10.1152/ajpendo.00307.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu C., Kraja A.T., Smith J.A., Brody J.A., Franceschini N., Bis J.C., et al. Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat Genet. 2016;48:1162–1170. doi: 10.1038/ng.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Akoumianakis I., Tarun A., Antoniades C. Perivascular adipose tissue as a regulator of vascular disease pathogenesis: identifying novel therapeutic targets. Br J Pharmacol. 2017;174:3411–3424. doi: 10.1111/bph.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Oikonomou E.K., Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol. 2019;16:83–99. doi: 10.1038/s41569-018-0097-6. [DOI] [PubMed] [Google Scholar]
- 171.Qi X.Y., Qu S.L., Xiong W.H., Rom O., Chang L., Jiang Z.S. Perivascular adipose tissue (PVAT) in atherosclerosis: a double-edged sword. Cardiovasc Diabetol. 2018;17:134–154. doi: 10.1186/s12933-018-0777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chang L., Xiong W., Zhao X., Fan Y., Guo Y., Garcia-Barrio M., et al. Bmal1 in perivascular adipose tissue regulates resting-phase blood pressure through transcriptional regulation of angiotensinogen. Circulation. 2018;138:67–79. doi: 10.1161/CIRCULATIONAHA.117.029972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ayala-Lopez N., Thompson J.M., Watts S.W. Perivascular adipose tissue's impact on norepinephrine-induced contraction of mesenteric resistance arteries. Front Physiol. 2017;8:37–51. doi: 10.3389/fphys.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nakamura K., Fuster J.J., Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. 2014;63:250–259. doi: 10.1016/j.jjcc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Koh K.K., Park S.M., Quon M.J. Leptin and cardiovascular disease: response to therapeutic interventions. Circulation. 2008;117:3238–3249. doi: 10.1161/CIRCULATIONAHA.107.741645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Baker J.F., Morales M., Qatanani M., Cucchiara A., Nackos E., Lazar M.A., et al. Resistin levels in lupus and associations with disease-specific measures, insulin resistance, and coronary calcification. J Rheumatol. 2011;38:2369–2375. doi: 10.3899/jrheum.110237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jamaluddin M.S., Weakley S.M., Yao Q., Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165:622–632. doi: 10.1111/j.1476-5381.2011.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Askin L., Abus S., Tanriverdi O. Resistin and cardiovascular disease: a review of the current literature regarding clinical and pathological relationships. Curr Cardiol Rev. 2022;18:70–76. doi: 10.2174/1573403X17666210729101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ouchi N., Kihara S., Funahashi T., Nakamura T., Nishida M., Kumada M., et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 180.Kim J.Y., van de Wall E., Laplante M., Azzara A., Trujillo M.E., Hofmann S.M., et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yamaguchi N., Argueta J.G., Masuhiro Y., Kagishita M., Nonaka K., Saito T., et al. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005;579:6821–6826. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 182.Chen H., Montagnani M., Funahashi T., Shimomura I., Quon M.J. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 183.Ohashi K., Kihara S., Ouchi N., Kumada M., Fujita K., Hiuge A., et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 184.Imerbtham T., Thitiwuthikiat P., Jongjitwimol J., Nuamchit T., Yingchoncharoen T., Siriwittayawan D. Leptin levels are associated with subclinical cardiac dysfunction in obese adolescents. Diabetes Metab Syndr Obes. 2020;13:925–933. doi: 10.2147/DMSO.S245048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Blanca A.J., Ruiz-Armenta M.V., Zambrano S., Salsoso R., Miguel-Carrasco J.L., Fortuno A., et al. Leptin induces oxidative stress through activation of NADPH oxidase in renal tubular cells: antioxidant effect of l-carnitine. J Cell Biochem. 2016;117:2281–2288. doi: 10.1002/jcb.25526. [DOI] [PubMed] [Google Scholar]
- 186.Pandey K.N. Guanylyl cyclase/atrial natriuretic peptide receptor-A: role in the pathophysiology of cardiovascular regulation. Can J Physiol Pharmacol. 2011;89:557–573. doi: 10.1139/y11-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ryden M., Backdahl J., Petrus P., Thorell A., Gao H., Coue M., et al. Impaired atrial natriuretic peptide-mediated lipolysis in obesity. Int J Obes (Lond) 2016;40:714–720. doi: 10.1038/ijo.2015.222. [DOI] [PubMed] [Google Scholar]
- 188.Coue M., Moro C. Natriuretic peptide control of energy balance and glucose homeostasis. Biochimie. 2016;124:84–91. doi: 10.1016/j.biochi.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 189.Moro C., Klimcakova E., Lolmede K., Berlan M., Lafontan M., Stich V., et al. Atrial natriuretic peptide inhibits the production of adipokines and cytokines linked to inflammation and insulin resistance in human subcutaneous adipose tissue. Diabetologia. 2007;50:1038–1047. doi: 10.1007/s00125-007-0614-3. [DOI] [PubMed] [Google Scholar]
- 190.Brestoff J.R., Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161:146–160. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cannone V., Cabassi A., Volpi R., Burnett J.C., Jr. Atrial natriuretic peptide: a molecular target of novel therapeutic approaches to cardio-metabolic disease. Int J Mol Sci. 2019;20:3265–3276. doi: 10.3390/ijms20133265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Volpe M., Carnovali M., Mastromarino V. The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin Sci (Lond) 2016;130:57–77. doi: 10.1042/CS20150469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rubattu S., Calvieri C., Pagliaro B., Volpe M. Atrial natriuretic peptide and regulation of vascular function in hypertension and heart failure: implications for novel therapeutic strategies. J Hypertens. 2013;31:1061–1072. doi: 10.1097/HJH.0b013e32835ed5eb. [DOI] [PubMed] [Google Scholar]
- 194.Januzzi J.L., Jr., Mohebi R. Obesity-mediated disruption of natriuretic peptide–blood pressure rhythms. J Am Coll Cardiol. 2021;77:2304–2306. doi: 10.1016/j.jacc.2021.03.317. [DOI] [PubMed] [Google Scholar]
- 195.Kenchaiah S., Evans J.C., Levy D., Wilson P.W., Benjamin E.J., Larson M.G., et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 196.Jamaly S., Carlsson L., Peltonen M., Jacobson P., Karason K. Surgical obesity treatment and the risk of heart failure. Eur Heart J. 2019;40:2131–2138. doi: 10.1093/eurheartj/ehz295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jamaly S., Carlsson L., Peltonen M., Andersson-Assarsson J.C., Karason K. Heart failure development in obesity: underlying risk factors and mechanistic pathways. ESC Heart Fail. 2021;8:356–367. doi: 10.1002/ehf2.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Horwich T.B., Fonarow G.C., Clark A.L. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2018;61:151–156. doi: 10.1016/j.pcad.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 199.Lavie C.J., Pandey A., Lau D.H., Alpert M.A., Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. 2017;70:2022–2035. doi: 10.1016/j.jacc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 200.Bos D., Bano A., Hofman A., VanderWeele T.J., Kavousi M., Franco O.H., et al. Thyroid function and atrial fibrillation: is there a mediating role for epicardial adipose tissue?. Clin Epidemiol. 2018;10:225–234. doi: 10.2147/CLEP.S149151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bahrami H., Bluemke D.A., Kronmal R., Bertoni A.G., Lloyd-Jones D.M., Shahar E., et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775–1783. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 202.Seravalle G., Grassi G. Obesity and hypertension. Pharmacol Res. 2017;122:1–7. doi: 10.1016/j.phrs.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 203.DeMarco V.G., Aroor A.R., Sowers J.R. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10:364–376. doi: 10.1038/nrendo.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hall J.E., Brands M.W., Henegar J.R. Mechanisms of hypertension and kidney disease in obesity. Ann N Y Acad Sci. 1999;892:91–107. doi: 10.1111/j.1749-6632.1999.tb07788.x. [DOI] [PubMed] [Google Scholar]
- 205.Kassab S., Kato T., Wilkins F.C., Chen R., Hall J.E., Granger J.P. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25:893–897. doi: 10.1161/01.hyp.25.4.893. [DOI] [PubMed] [Google Scholar]
- 206.Sharma A.M. Is there a rationale for angiotensin blockade in the management of obesity hypertension?. Hypertension. 2004;44:12–19. doi: 10.1161/01.HYP.0000132568.71409.a2. [DOI] [PubMed] [Google Scholar]
- 207.Ding L., Kang Y., Dai H.B., Wang F.Z., Zhou H., Gao Q., et al. Adipose afferent reflex is enhanced by TNFalpha in paraventricular nucleus through NADPH oxidase-dependent ROS generation in obesity-related hypertensive rats. J Transl Med. 2019;17:256–269. doi: 10.1186/s12967-019-2006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hall J.E., da Silva A.A., do Carmo J.M., Dubinion J., Hamza S., Munusamy S., et al. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kalil G.Z., Haynes W.G. Sympathetic nervous system in obesity-related hypertension: mechanisms and clinical implications. Hypertens Res. 2012;35:4–16. doi: 10.1038/hr.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Maugeri A., Hruskova J., Jakubik J., Barchitta M., Lo Re O., Kunzova S., et al. Independent effects of hypertension and obesity on left ventricular mass and geometry: evidence from the cardiovision 2030 study. J Clin Med. 2019;8:370–381. doi: 10.3390/jcm8030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Libby P., Buring J.E., Badimon L., Hansson G.K., Deanfield J., Bittencourt M.S., et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56–74. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 212.Nordestgaard B.G., Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 213.Reiner Z. Hypertriglyceridaemia and risk of coronary artery disease. Nat Rev Cardiol. 2017;14:401–411. doi: 10.1038/nrcardio.2017.31. [DOI] [PubMed] [Google Scholar]
- 214.Neeland I.J., Ross R., Després J.P., Matsuzawa Y., Yamashita S., Shai I., et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7:715–725. doi: 10.1016/S2213-8587(19)30084-1. [DOI] [PubMed] [Google Scholar]
- 215.Poledne R., Kralova Lesna I., Cejkova S. Adipose tissue and atherosclerosis. Physiol Res. 2015;64:S395–S402. doi: 10.33549/physiolres.933152. [DOI] [PubMed] [Google Scholar]
- 216.Toth P.P. Triglycerides and atherosclerosis: bringing the association into sharper focus. J Am Coll Cardiol. 2021;77:3042–3045. doi: 10.1016/S0735-1097(21)04397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rocha V.Z., Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 218.Hansson G.K., Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 219.Soehnlein O., Libby P. Targeting inflammation in atherosclerosis—from experimental insights to the clinic. Nat Rev Drug Discov. 2021;20:589–610. doi: 10.1038/s41573-021-00198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Schaftenaar F., Frodermann V., Kuiper J., Lutgens E. Atherosclerosis: the interplay between lipids and immune cells. Curr Opin Lipidol. 2016;27:209–215. doi: 10.1097/MOL.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 221.Katsiki N., Mantzoros C., Mikhailidis D.P. Adiponectin, lipids and atherosclerosis. Curr Opin Lipidol. 2017;28:347–354. doi: 10.1097/MOL.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 222.Behre C.J. Adiponectin, obesity and atherosclerosis. Scand J Clin Lab Invest. 2007;67:449–458. doi: 10.1080/00365510601158717. [DOI] [PubMed] [Google Scholar]
- 223.Lovren F., Teoh H., Verma S. Obesity and atherosclerosis: mechanistic insights. Can J Cardiol. 2015;31:177–183. doi: 10.1016/j.cjca.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 224.Ouchi N., Kihara S., Arita Y., Nishida M., Matsuyama A., Okamoto Y., et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 225.Okamoto Y., Kihara S., Ouchi N., Nishida M., Arita Y., Kumada M., et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 226.Elagizi A., Kachur S., Lavie C.J., Carbone S., Pandey A., Ortega F.B., et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61:142–150. doi: 10.1016/j.pcad.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 227.Lavie C.J., Sharma A., Alpert M.A., De Schutter A., Lopez-Jimenez F., Milani R.V., et al. Update on obesity and obesity paradox in heart failure. Prog Cardiovasc Dis. 2016;58:393–400. doi: 10.1016/j.pcad.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 228.Romero-Corral A., Montori V.M., Somers V.K., Korinek J., Thomas R.J., Allison T.G., et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 229.Lavie C.J., Milani R.V., Ventura H.O. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 230.Bjerregaard L.G., Jensen B.W., Angquist L., Osler M., Sorensen T.I.A., Baker J.L. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N Engl J Med. 2018;378:1302–1312. doi: 10.1056/NEJMoa1713231. [DOI] [PubMed] [Google Scholar]
- 231.Chukir T., Shukla A.P., Saunders K.H., Aronne L.J. Pharmacotherapy for obesity in individuals with type 2 diabetes. Expert Opin Pharmacother. 2018;19:223–231. doi: 10.1080/14656566.2018.1428558. [DOI] [PubMed] [Google Scholar]
- 232.Magkos F., Hjorth M.F., Astrup A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2020;16:545–555. doi: 10.1038/s41574-020-0381-5. [DOI] [PubMed] [Google Scholar]
- 233.Malone J.I., Hansen B.C. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite?. Pediatr Diabetes. 2019;20:5–9. doi: 10.1111/pedi.12787. [DOI] [PubMed] [Google Scholar]
- 234.Rohm T.V., Meier D.T., Olefsky J.M., Donath M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31–55. doi: 10.1016/j.immuni.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tong Y., Xu S., Huang L., Chen C. Obesity and insulin resistance: pathophysiology and treatment. Drug Discov Today. 2022;27:822–830. doi: 10.1016/j.drudis.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 236.Boucher J., Kleinridders A., Kahn C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6:a009191. doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ahmed B., Sultana R., Greene M.W. Adipose tissue and insulin resistance in obese. Biomed Pharmacother. 2021;137:111315–111328. doi: 10.1016/j.biopha.2021.111315. [DOI] [PubMed] [Google Scholar]
- 238.Zhang N., Liu X., Zhuang L., Liu X., Zhao H., Shan Y., et al. Berberine decreases insulin resistance in a PCOS rats by improving GLUT4: dual regulation of the PI3K/AKT and MAPK pathways. Regul Toxicol Pharmacol. 2020;110:104544–104550. doi: 10.1016/j.yrtph.2019.104544. [DOI] [PubMed] [Google Scholar]
- 239.Lebovitz H.E. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S135–S148. doi: 10.1055/s-2001-18576. [DOI] [PubMed] [Google Scholar]
- 240.Lee S.H., Park S.Y., Choi C.S. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. 2022;46:15–37. doi: 10.4093/dmj.2021.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Oh Y.S., Bae G.D., Baek D.J., Park E.Y., Jun H.S. Fatty acid-induced lipotoxicity in pancreatic beta-cells during development of type 2 diabetes. Front Endocrinol (Lausanne) 2018;9:384–394. doi: 10.3389/fendo.2018.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Boden G. Effects of free fatty acids on gluconeogenesis and glycogenolysis. Life Sci. 2003;72:977–988. doi: 10.1016/s0024-3205(02)02350-0. [DOI] [PubMed] [Google Scholar]
- 243.Capurso C., Capurso A. From excess adiposity to insulin resistance: the role of free fatty acids. Vascul Pharmacol. 2012;57:91–97. doi: 10.1016/j.vph.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 244.Muris D.M., Houben A.J., Schram M.T., Stehouwer C.D. Microvascular dysfunction: an emerging pathway in the pathogenesis of obesity-related insulin resistance. Rev Endocr Metab Disord. 2013;14:29–38. doi: 10.1007/s11154-012-9231-7. [DOI] [PubMed] [Google Scholar]
- 245.Martyn J.A., Kaneki M., Yasuhara S. Obesity-induced insulin resistance and hyperglycemia: etiologic factors and molecular mechanisms. Anesthesiology. 2008;109:137–148. doi: 10.1097/ALN.0b013e3181799d45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zand H., Morshedzadeh N., Naghashian F. Signaling pathways linking inflammation to insulin resistance. Diabetes Metab Syndr. 2017;11(Suppl 1):S307–S309. doi: 10.1016/j.dsx.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 247.Bluher M. Adipose tissue inflammation: a cause or consequence of obesity-related insulin resistance?. Clin Sci (Lond) 2016;130:1603–1614. doi: 10.1042/CS20160005. [DOI] [PubMed] [Google Scholar]
- 248.Lauterbach M.A., Wunderlich F.T. Macrophage function in obesity-induced inflammation and insulin resistance. Pflugers Arch. 2017;469:385–396. doi: 10.1007/s00424-017-1955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Fang H., Judd R.L. Adiponectin regulation and function. Compr Physiol. 2018;8:1031–1063. doi: 10.1002/cphy.c170046. [DOI] [PubMed] [Google Scholar]
- 250.Fruebis J., Tsao T.S., Javorschi S., Ebbets-Reed D., Erickson M.R., Yen F.T., et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S., et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 252.Wang C., Mao X., Wang L., Liu M., Wetzel M.D., Guan K.L., et al. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J Biol Chem. 2007;282:7991–7996. doi: 10.1074/jbc.M700098200. [DOI] [PubMed] [Google Scholar]
- 253.Tomas E., Tsao T.S., Saha A.K., Murrey H.E., Zhang Cc C., Itani S.I., et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yadav A., Kataria M.A., Saini V., Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–84. doi: 10.1016/j.cca.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 255.Fasshauer M., Klein J., Kralisch S., Klier M., Lossner U., Bluher M., et al. Growth hormone is a positive regulator of adiponectin receptor 2 in 3T3-L1 adipocytes. FEBS Lett. 2004;558:27–32. doi: 10.1016/S0014-5793(03)01525-4. [DOI] [PubMed] [Google Scholar]
- 256.Morton G.J., Gelling R.W., Niswender K.D., Morrison C.D., Rhodes C.J., Schwartz M.W. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 257.Francisco V., Pino J., Campos-Cabaleiro V., Ruiz-Fernandez C., Mera A., Gonzalez-Gay M.A., et al. Obesity, fat mass and immune system: role for leptin. Front Physiol. 2018;9:640–660. doi: 10.3389/fphys.2018.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Benomar Y., Naour N., Aubourg A., Bailleux V., Gertler A., Djiane J., et al. Insulin and leptin induce Glut4 plasma membrane translocation and glucose uptake in a human neuronal cell line by a phosphatidylinositol 3-kinase-dependent mechanism. Endocrinology. 2006;147:2550–2556. doi: 10.1210/en.2005-1464. [DOI] [PubMed] [Google Scholar]
- 259.Munzberg H., Flier J.S., Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 260.El-Haschimi K., Pierroz D.D., Hileman S.M., Bjorbaek C., Flier J.S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mittendorfer B., Horowitz J.F., DePaoli A.M., McCamish M.A., Patterson B.W., Klein S. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes. 2011;60:1474–1477. doi: 10.2337/db10-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Moon H.S., Matarese G., Brennan A.M., Chamberland J.P., Liu X., Fiorenza C.G., et al. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60:1647–1656. doi: 10.2337/db10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wolsk E., Mygind H., Grondahl T.S., Pedersen B.K., van Hall G. The role of leptin in human lipid and glucose metabolism: the effects of acute recombinant human leptin infusion in young healthy males. Am J Clin Nutr. 2011;94:1533–1544. doi: 10.3945/ajcn.111.012260. [DOI] [PubMed] [Google Scholar]
- 264.Konner A.C., Bruning J.C. Selective insulin and leptin resistance in metabolic disorders. Cell Metab. 2012;16:144–152. doi: 10.1016/j.cmet.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 265.Shintani M., Ogawa Y., Nakao K. Insulin resistance, role of leptin and leptin receptor. Nihon Rinsho. 2000;58:327–332. [PubMed] [Google Scholar]
- 266.Niswender K.D., Magnuson M.A. Obesity and the beta cell: lessons from leptin. J Clin Invest. 2007;117:2753–2756. doi: 10.1172/JCI33528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Morioka T., Asilmaz E., Hu J., Dishinger J.F., Kurpad A.J., Elias C.F., et al. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest. 2007;117:2860–2868. doi: 10.1172/JCI30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Villasante Fricke A.C., Iacobellis G. Epicardial adipose tissue: clinical biomarker of cardio-metabolic risk. Int J Mol Sci. 2019;20:5989–6002. doi: 10.3390/ijms20235989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Liu C., Feng X., Li Q., Wang Y., Li Q., Hua M. Adiponectin, TNF-alpha and inflammatory cytokines and risk of type 2 diabetes: a systematic review and meta-analysis. Cytokine. 2016;86:100–109. doi: 10.1016/j.cyto.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 270.Gurzov E.N., Stanley W.J., Pappas E.G., Thomas H.E., Gough D.J. The JAK/STAT pathway in obesity and diabetes. FEBS J. 2016;283:3002–3015. doi: 10.1111/febs.13709. [DOI] [PubMed] [Google Scholar]
- 271.Pinti M.V., Fink G.K., Hathaway Q.A., Durr A.J., Kunovac A., Hollander J.M. Mitochondrial dysfunction in type 2 diabetes mellitus: an organ-based analysis. Am J Physiol Endocrinol Metab. 2019;316:268–285. doi: 10.1152/ajpendo.00314.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Stumvoll M., Goldstein B.J., van Haeften T.W. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 273.Harmon J.S., Gleason C.E., Tanaka Y., Oseid E.A., Hunter-Berger K.K., Robertson R.P. In vivo prevention of hyperglycemia also prevents glucotoxic effects on PDX-1 and insulin gene expression. Diabetes. 1999;48:1995–2000. doi: 10.2337/diabetes.48.10.1995. [DOI] [PubMed] [Google Scholar]
- 274.Jin X., Yu R., Wang X., Proud C.G., Jiang T. Progress in developing MNK inhibitors. Eur J Med Chem. 2021;219:113420–113436. doi: 10.1016/j.ejmech.2021.113420. [DOI] [PubMed] [Google Scholar]
- 275.Xu W., Kannan S., Verma C.S., Nacro K. Update on the development of MNK inhibitors as therapeutic agents. J Med Chem. 2022;65:983–1007. doi: 10.1021/acs.jmedchem.1c00368. [DOI] [PubMed] [Google Scholar]
- 276.Diab S., Kumarasiri M., Yu M., Teo T., Proud C., Milne R., et al. MAP kinase-interacting kinases—emerging targets against cancer. Chem Biol. 2014;21:441–452. doi: 10.1016/j.chembiol.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 277.Konicek B.W., Dumstorf C.A., Graff J.R. Targeting the eIF4F translation initiation complex for cancer therapy. Cell Cycle. 2008;7:2466–2471. doi: 10.4161/cc.7.16.6464. [DOI] [PubMed] [Google Scholar]
- 278.Lama D., Verma C.S. Deciphering the mechanistic effects of eIF4E phosphorylation on mRNA-cap recognition. Protein Sci. 2019;29:1373–1386. doi: 10.1002/pro.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Moore C.E., Pickford J., Cagampang F.R., Stead R.L., Tian S., Zhao X., et al. MNK1 and MNK2 mediate adverse effects of high-fat feeding in distinct ways. Sci Rep. 2016;6:23476–23491. doi: 10.1038/srep23476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sandeman L.Y., Kang W.X., Wang X., Jensen K.B., Wong D., Bo T., et al. Disabling MNK protein kinases promotes oxidative metabolism and protects against diet-induced obesity. Mol Metab. 2020;42:101054–101069. doi: 10.1016/j.molmet.2020.101054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Conn C.S., Yang H., Tom H.J., Ikeda K., Oses-Prieto J.A., Vu H., et al. The major cap-binding protein eIF4E regulates lipid homeostasis and diet-induced obesity. Nat Metab. 2021;3:244–257. doi: 10.1038/s42255-021-00349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 283.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Mundi M.S., Velapati S., Patel J., Kellogg T.A., Abu Dayyeh B.K., Hurt R.T. Evolution of NAFLD and its management. Nutr Clin Pract. 2020;35:72–84. doi: 10.1002/ncp.10449. [DOI] [PubMed] [Google Scholar]
- 285.White D.L., Kanwal F., El-Serag H.B. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342–1359. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Woo Baidal J.A., Lavine J.E. The intersection of nonalcoholic fatty liver disease and obesity. Sci Transl Med. 2016;8:323–334. doi: 10.1126/scitranslmed.aad8390. [DOI] [PubMed] [Google Scholar]
- 287.Yu J., Shen J., Sun T.T., Zhang X., Wong N. Obesity, insulin resistance, NASH and hepatocellular carcinoma. Semin Cancer Biol. 2013;23:483–491. doi: 10.1016/j.semcancer.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 288.Singh S., Allen A.M., Wang Z., Prokop L.J., Murad M.H., Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Byrne C.D., Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 290.Musso G., Gambino R., Cassader M., Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 291.Musso G., Gambino R., Tabibian J.H., Ekstedt M., Kechagias S., Hamaguchi M., et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001680–e1001706. doi: 10.1371/journal.pmed.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Arab J.P., Arrese M., Trauner M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu Rev Pathol. 2018;13:321–350. doi: 10.1146/annurev-pathol-020117-043617. [DOI] [PubMed] [Google Scholar]
- 293.Duwaerts C.C., Maher J.J. Macronutrients and the adipose–liver axis in obesity and fatty liver. Cell Mol Gastroenterol Hepatol. 2019;7:749–761. doi: 10.1016/j.jcmgh.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Song Z., Xiaoli A.M., Yang F. Regulation and metabolic significance of de novo lipogenesis in adipose tissues. Nutrients. 2018;10:1383–1405. doi: 10.3390/nu10101383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Dentin R., Pegorier J.P., Benhamed F., Foufelle F., Ferre P., Fauveau V., et al. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J Biol Chem. 2004;279:20314–20326. doi: 10.1074/jbc.M312475200. [DOI] [PubMed] [Google Scholar]
- 296.Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 297.Dietrich P., Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28:637–653. doi: 10.1016/j.bpg.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 298.Suarez-Cuenca J.A., De La Pena-Sosa G., De La Vega-Moreno K., Banderas-Lares D.Z., Salamanca-Garcia M., Martinez-Hernandez J.E., et al. Enlarged adipocytes from subcutaneous vs. visceral adipose tissue differentially contribute to metabolic dysfunction and atherogenic risk of patients with obesity. Sci Rep. 2021;11:1831–1842. doi: 10.1038/s41598-021-81289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Azzu V., Vacca M., Virtue S., Allison M., Vidal-Puig A. Adipose tissue–liver cross talk in the control of whole-body metabolism: implications in nonalcoholic fatty liver disease. Gastroenterology. 2020;158:1899–1912. doi: 10.1053/j.gastro.2019.12.054. [DOI] [PubMed] [Google Scholar]
- 300.McLaughlin T., Lamendola C., Liu A., Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–E1760. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Stefan N., Kantartzis K., Haring H.U. Causes and metabolic consequences of fatty liver. Endocr Rev. 2008;29:939–960. doi: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 302.Softic S., Cohen D.E., Kahn C.R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig Dis Sci. 2016;61:1282–1293. doi: 10.1007/s10620-016-4054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ding L., Sun W., Balaz M., He A., Klug M., Wieland S., et al. Peroxisomal beta-oxidation acts as a sensor for intracellular fatty acids and regulates lipolysis. Nat Metab. 2021;3:1648–1661. doi: 10.1038/s42255-021-00489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Koliaki C., Roden M. Hepatic energy metabolism in human diabetes mellitus, obesity and non-alcoholic fatty liver disease. Mol Cell Endocrinol. 2013;379:35–42. doi: 10.1016/j.mce.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 305.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 306.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Hirsova P., Ibrabim S.H., Gores G.J., Malhi H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J Lipid Res. 2016;57:1758–1770. doi: 10.1194/jlr.R066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Koyama Y., Brenner D.A. Liver inflammation and fibrosis. J Clin Invest. 2017;127:55–64. doi: 10.1172/JCI88881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Polyzos S.A., Kountouras J., Mantzoros C.S. Adipose tissue, obesity and non-alcoholic fatty liver disease. Minerva Endocrinol. 2017;42:92–108. doi: 10.23736/S0391-1977.16.02563-3. [DOI] [PubMed] [Google Scholar]
- 310.Makri E., Goulas A., Polyzos S.A. Epidemiology, pathogenesis, diagnosis and emerging treatment of nonalcoholic fatty liver disease. Arch Med Res. 2021;52:25–37. doi: 10.1016/j.arcmed.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 311.Schwabe R.F., Tabas I., Pajvani U.B. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology. 2020;158:1913–1928. doi: 10.1053/j.gastro.2019.11.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Tsuchida T., Friedman S.L. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 313.Paradis V., Perlemuter G., Bonvoust F., Dargere D., Parfait B., Vidaud M., et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–744. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 314.Cai C.X., Buddha H., Castelino-Prabhu S., Zhang Z., Britton R.S., Bacon B.R., et al. Activation of insulin–PI3K/Akt–p70S6K pathway in hepatic stellate cells contributes to fibrosis in nonalcoholic steatohepatitis. Dig Dis Sci. 2017;62:968–978. doi: 10.1007/s10620-017-4470-9. [DOI] [PubMed] [Google Scholar]
- 315.Grohmann M., Wiede F., Dodd G.T., Gurzov E.N., Ooi G.J., Butt T., et al. Obesity drives STAT-1-dependent NASH and STAT-3-dependent HCC. Cell. 2018;175:1289–1306. doi: 10.1016/j.cell.2018.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Knauf U., Tschopp C., Gram H. Negative regulation of protein translation by mitogen-activated protein kinase-interacting kinases 1 and 2. Mol Cell Biol. 2001;21:5500–5511. doi: 10.1128/MCB.21.16.5500-5511.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Masaki T., Chiba S., Tatsukawa H., Yasuda T., Noguchi H., Seike M., et al. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177–184. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- 318.Dong Z., Zhuang Q., Ye X., Ning M., Wu S., Lu L., et al. Adiponectin inhibits NLRP3 inflammasome activation in nonalcoholic steatohepatitis via AMPK–JNK/ErK1/2-NFκB/ROS signaling pathways. Front Med (Lausanne) 2020;7:546445–546456. doi: 10.3389/fmed.2020.546445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Larter C.Z., Farrell G.C. Insulin resistance, adiponectin, cytokines in NASH: which is the best target to treat?. J Hepatol. 2006;44:253–261. doi: 10.1016/j.jhep.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 320.Combs T.P., Marliss E.B. Adiponectin signaling in the liver. Rev Endocr Metab Disord. 2014;15:137–147. doi: 10.1007/s11154-013-9280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Ma Y., Liu D. Hydrodynamic delivery of adiponectin and adiponectin receptor 2 gene blocks high-fat diet-induced obesity and insulin resistance. Gene Ther. 2013;20:846–852. doi: 10.1038/gt.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Miller R.A., Chu Q., Le Lay J., Scherer P.E., Ahima R.S., Kaestner K.H., et al. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1–AMPK signaling. J Clin Invest. 2011;121:2518–2528. doi: 10.1172/JCI45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Koo S.H., Flechner L., Qi L., Zhang X., Screaton R.A., Jeffries S., et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 324.Li X., Zhang D., Vatner D.F., Goedeke L., Hirabara S.M., Zhang Y., et al. Mechanisms by which adiponectin reverses high fat diet-induced insulin resistance in mice. Proc Natl Acad Sci U S A. 2020;117:32584–32593. doi: 10.1073/pnas.1922169117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Petersen M.C., Shulman G.I. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol Sci. 2017;38:649–665. doi: 10.1016/j.tips.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Powell D.J., Hajduch E., Kular G., Hundal H.S. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol Cell Biol. 2003;23:7794–7808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Mukhopadhyay A., Saddoughi S.A., Song P., Sultan I., Ponnusamy S., Senkal C.E., et al. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. FASEB J. 2009;23:751–763. doi: 10.1096/fj.08-120550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Bourbon N.A., Yun J., Berkey D., Wang Y., Kester M. Inhibitory actions of ceramide upon PKC-epsilon/ERK interactions. Am J Physiol Cell Physiol. 2001;280:C1403–C1411. doi: 10.1152/ajpcell.2001.280.6.C1403. [DOI] [PubMed] [Google Scholar]
- 329.Stratford S., Hoehn K.L., Liu F., Summers S.A. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem. 2004;279:36608–36615. doi: 10.1074/jbc.M406499200. [DOI] [PubMed] [Google Scholar]
- 330.Blachnio-Zabielska A.U., Chacinska M., Vendelbo M.H., Zabielski P. The crucial role of C18-Cer in fat-induced skeletal muscle insulin resistance. Cell Physiol Biochem. 2016;40:1207–1220. doi: 10.1159/000453174. [DOI] [PubMed] [Google Scholar]
- 331.Petersen M.C., Madiraju A.K., Gassaway B.M., Marcel M., Nasiri A.R., Butrico G., et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J Clin Invest. 2016;126:4361–4371. doi: 10.1172/JCI86013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Samuel V.T., Liu Z.X., Wang A., Beddow S.A., Geisler J.G., Kahn M., et al. Inhibition of protein kinase cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Villa N.Y., Kupchak B.R., Garitaonandia I., Smith J.L., Alonso E., Alford C., et al. Sphingolipids function as downstream effectors of a fungal PAQR. Mol Pharmacol. 2009;75:866–875. doi: 10.1124/mol.108.049809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Polyzos S.A., Kountouras J., Mantzoros C.S. Leptin in nonalcoholic fatty liver disease: a narrative review. Metabolism. 2015;64:60–78. doi: 10.1016/j.metabol.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 335.Farrell G.C., Larter C.Z. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 336.Marrero J.A. Obesity and liver disease: the new era of liver transplantation. Hepatology. 2019;70:459–461. doi: 10.1002/hep.30854. [DOI] [PubMed] [Google Scholar]
- 337.Corey K.E., Kaplan L.M. Obesity and liver disease: the epidemic of the twenty-first century. Clin Liver Dis. 2014;18:1–18. doi: 10.1016/j.cld.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 338.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 339.Conus F., Rabasa-Lhoret R., Peronnet F. Characteristics of metabolically obese normal-weight (MONW) subjects. Appl Physiol Nutr Metab. 2007;32:4–12. doi: 10.1139/h06-092. [DOI] [PubMed] [Google Scholar]
- 340.Leslie J., Geh D., Elsharkawy A.M., Mann D.A., Vacca M. Metabolic dysfunction and cancer in HCV: shared pathways and mutual interactions. J Hepatol. 2022;77:219–236. doi: 10.1016/j.jhep.2022.01.029. [DOI] [PubMed] [Google Scholar]
- 341.Negro F. Abnormalities of lipid metabolism in hepatitis C virus infection. Gut. 2010;59:1279–1287. doi: 10.1136/gut.2009.192732. [DOI] [PubMed] [Google Scholar]
- 342.Everhart J.E., Lok A.S., Kim H.Y., Morgan T.R., Lindsay K.L., Chung R.T., et al. Weight-related effects on disease progression in the hepatitis C antiviral long-term treatment against cirrhosis trial. Gastroenterology. 2009;137:549–557. doi: 10.1053/j.gastro.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Yamaguchi A., Tazuma S., Nishioka T., Ohishi W., Hyogo H., Nomura S., et al. Hepatitis C virus core protein modulates fatty acid metabolism and thereby causes lipid accumulation in the liver. Dig Dis Sci. 2005;50:1361–1371. doi: 10.1007/s10620-005-2788-1. [DOI] [PubMed] [Google Scholar]
- 344.Lin W., Tsai W.L., Shao R.X., Wu G., Peng L.F., Barlow L.L., et al. Hepatitis C virus regulates transforming growth factor beta1 production through the generation of reactive oxygen species in a nuclear factor kappaB-dependent manner. Gastroenterology. 2010;138:2509–2518. doi: 10.1053/j.gastro.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Patel N.S., Doycheva I., Peterson M.R., Hooker J., Kisselva T., Schnabl B., et al. Effect of weight loss on magnetic resonance imaging estimation of liver fat and volume in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2015;13:561–568. doi: 10.1016/j.cgh.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Clifton P.M., Keogh J.B. Effects of different weight loss approaches on CVD risk. Curr Atheroscler Rep. 2018;20:27. doi: 10.1007/s11883-018-0728-8. [DOI] [PubMed] [Google Scholar]
- 347.Colman R.J., Beasley T.M., Kemnitz J.W., Johnson S.C., Weindruch R., Anderson R.M. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557–3562. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Wing R.R., Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 349.Rejeski W.J., Bray G.A., Chen S.H., Clark J.M., Evans M., Hill J.O., et al. Aging and physical function in type 2 diabetes: 8 years of an intensive lifestyle intervention. J Gerontol A Biol Sci Med Sci. 2015;70:345–353. doi: 10.1093/gerona/glu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Kissler H.J., Settmacher U. Bariatric surgery to treat obesity. Semin Nephrol. 2013;33:75–89. doi: 10.1016/j.semnephrol.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 351.Wolfe B.M., Kvach E., Eckel R.H. Treatment of obesity: weight loss and bariatric surgery. Circ Res. 2016;118:1844–1855. doi: 10.1161/CIRCRESAHA.116.307591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Eldar S., Heneghan H.M., Brethauer S.A., Schauer P.R. Bariatric surgery for treatment of obesity. Int J Obes (Lond) 2011;35(Suppl 3):S16–S21. doi: 10.1038/ijo.2011.142. [DOI] [PubMed] [Google Scholar]
- 353.Muller T.D., Bluher M., Tschop M.H., DiMarchi R.D. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. 2022;21:201–223. doi: 10.1038/s41573-021-00337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yanovski S.Z., Yanovski J.A. Progress in pharmacotherapy for obesity. JAMA. 2021;326:129–130. doi: 10.1001/jama.2021.9486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Chang Y.H., Hung H.Y. Recent advances in natural anti-obesity compounds and derivatives based on in vivo evidence: a mini-review. Eur J Med Chem. 2022;237:114405–114418. doi: 10.1016/j.ejmech.2022.114405. [DOI] [PubMed] [Google Scholar]
- 356.Gadde K.M., Atkins K.D. The limits and challenges of antiobesity pharmacotherapy. Expert Opin Pharmacother. 2020;21:1319–1328. doi: 10.1080/14656566.2020.1748599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Bessesen D.H., Van Gaal L.F. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018;6:237–248. doi: 10.1016/S2213-8587(17)30236-X. [DOI] [PubMed] [Google Scholar]
- 358.Kumar R.B., Aronne L.J. Efficacy comparison of medications approved for chronic weight management. Obesity (Silver Spring) 2015;23(Suppl 1):S4–S7. doi: 10.1002/oby.21093. [DOI] [PubMed] [Google Scholar]
- 359.Apovian C.M., Aronne L., Rubino D., Still C., Wyatt H., Burns C., et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity (Silver Spring) 2013;21:935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Nissen S.E., Wolski K.E., Prcela L., Wadden T., Buse J.B., Bakris G., et al. Effect of naltrexone–bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA. 2016;315:990–1004. doi: 10.1001/jama.2016.1558. [DOI] [PubMed] [Google Scholar]
- 361.Astrup A., Carraro R., Finer N., Harper A., Kunesova M., Lean M.E., et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 2012;36:843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Wilding J.P.H., Batterham R.L., Calanna S., Davies M., Van Gaal L.F., Lingvay I., et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 363.GBD 2015 Obesity Collaborators. Afshin A., Forouzanfar M.H., Reitsma M.B., Sur P., Estep K., et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]