Abstract
Pulmonary arterial hypertension (PAH) is a rare, life‐limiting disease. PAH registries provide real‐world data that complement clinical trial data and inform treatment decisions. The TRIO comprehensive, integrated patient data repository (TRIO CIPDR), is an innovative US repository capturing data on contemporary patients diagnosed with pulmonary hypertension and receiving US Food and Drug Administration‐approved PAH therapies. This repository uniquely combines clinical data from electronic medical records with the ability to track drug‐prescription and drug‐dispensing characteristics, and includes 946 adult patients with PAH (data collected January 2019 to December 2020) enrolled from nine representative US specialist tertiary care centers. Potentially eligible patients were identified based on dispensing data from specialty pharmacies. Hemodynamic and clinical data, as well as dispensing information on prescribed PAH medications, were provided by tertiary centers. At enrollment, 75% of patients were female, 67% were White, median age at PAH diagnosis was 53 years (median time from diagnosis to enrollment was 5 years), and 37% were obese. Comorbidity profiles were as expected for a PAH population, although the proportion with atrial fibrillation (34%) was higher than expected. Overall, 38% of patients had idiopathic PAH and 30% had connective tissue disease‐related PAH. Among 917 patients receiving PAH‐specific therapy, 40% were on monotherapy, 43% on dual therapy, and 17% on triple therapy. Longitudinal data from this repository will allow tracking of the PAH treatment journey in relation to clinical characteristics and outcomes.
Keywords: prescribing data, pulmonary arterial hypertension, real‐world evidence, registry, repository
INTRODUCTION
Randomized controlled clinical trials have provided robust evidence on new treatments that improve outcomes for patients with pulmonary arterial hypertension (PAH). 1 However, due to the rarity, heterogeneity, and life‐limiting nature of PAH, clinical trials can be challenging in this patient population. 2 Consequently, registries have been integral to understanding the disease course and management of patients with PAH by capturing longitudinal data and providing valuable insights into the disease course, management, risk stratification, and outcomes in patients with PAH in the real‐world setting, 3 , 4 , 5 , 6 , 7 , 8 and providing a useful platform for better characterizing PAH (and other rare diseases) for hypothesis generation. In the past 20 years, PAH‐specific therapies have been introduced and recommended in PAH treatment guidelines. 9 Given the evolution of the PAH therapeutic landscape, there is a need to understand how these treatments are used in routine clinical practice and what their impact is on patients with PAH.
The TRIO comprehensive, integrated patient data repository (TRIO CIPDR) is an innovative repository, initiated to capture data on contemporary patients diagnosed with pulmonary hypertension (PH) and treated with US Food and Drug Administration‐approved PAH‐specific therapies. Of note, data for TRIO CIPDR were obtained from Pulmonary Hypertension Association–accredited PH care centers and further integrated with Health Insurance Portability and Accountability Act–compliant secure data on patient outcomes (including hospitalizations and mortality). This is a novel approach in this disease setting, and aims to allow real‐world assessment of the impact of therapies on outcomes, the potential overlap between comorbidities, and the evolving demographics of patients diagnosed with PH and treated with PAH‐specific medications in the contemporary setting. TRIO CIPDR will also provide data that can be analyzed to evaluate adherence to treatment guidelines, and the impact of guideline adherence versus nonadherence on patient outcomes in the routine clinical setting.
Here we describe the development of TRIO CIPDR, the patient population and characteristics of patients in the repository.
METHODS
TRIO CIPDR is a multicenter, observational, retrospective, and prospective repository initiated in January 2019; anticipated enrollment was 1000 patients with PAH in the first year (January 2019 to December 2020). Figure 1 shows an overview of the structure and organization of TRIO CIPDR, and Figure 2 illustrates the steps involved in creating TRIO CIPDR. The repository was set up with guidance from a steering committee of four pulmonologists and two cardiologists who had experience in the design of and/or had previously participated in PH registries, including the Registry to Evaluate Early and Long‐term PAH Disease Management (REVEAL). 10 Data on hemodynamic and clinical parameters, as well as prescription/dispensing of PAH medications, were provided by specialist tertiary care centers and collected by Trio Health, a commercial organization that collects specified data elements and observations directly from medical practices and specialty pharmacies. All data are de‐identified and assigned a unique Trio Health identification number. The specialists participating in TRIO CIPDR are based in the United States of America and treat patients in both academic and community settings.
Figure 1.
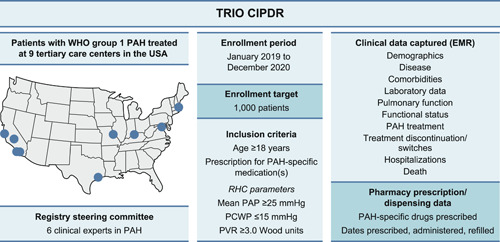
Overview of TRIO CIPDR: patient criteria and data elements. Eleven PHA‐accredited PH care centers initially contributed to CIPDR, however two centers were unable to continue participation due to the impact of COVID‐19. The location of the nine centers contributing data to TRIO CIPDR (as listed in Supporting Information: Table S1) are shown. CIPDR, comprehensive, integrated patient data repository; EMR, electronic medical records; PAH, pulmonary arterial hypertension; PAP, pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; PHA, pulmonary hypertension association; PVR, pulmonary vascular resistance; RHC, right heart catheterization; TRIO CIPDR, TRIO comprehensive, integrated patient data repository; WHO, World Health Organization.
Figure 2.
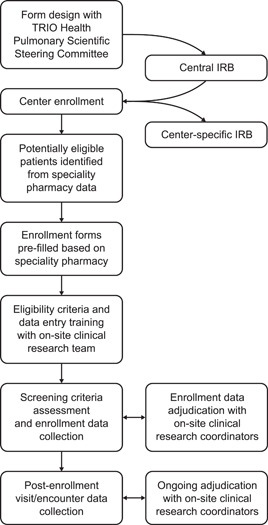
Schematic showing the steps involved in creating TRIO CIPDR. IRB, institutional review board; TRIO CIPDR, TRIO comprehensive, integrated patient data repository.
Centers and participants
Centers were selected based on their treatment of patients with World Health Organization Group 1 PH (PAH) and further input from the CIPDR steering committee (with recommendations based on their expertise in this entity; no specific criteria were predefined for center selection or recommendations). The repository was designed to include a nationwide US cohort, to minimize potential regional bias. Details of participating centers are provided in Figure 1 and in the Supporting Information: Table S1. Central approval was obtained through the Western Institutional Review Board. In addition, all Pulmonary Hypertension Association–accredited centers obtained approval for the TRIO CIPDR protocol from their local institutional review board.
The target sample size for TRIO CIPDR was predefined as 1000 patients with PAH. To facilitate enrollment, specialty pharmacy data corresponding to each center was used to identify patients likely to qualify for the data collection, and to pre‐populate qualification forms. However, no specialty pharmacy data (used for patient eligibility assessment) were captured in the repository itself; data for the repository were taken solely from each center's electronic medical records. Each center reviewed and identified patients who met the TRIO CIPDR inclusion criteria. Qualifying patients had a diagnosis of World Health Organization Group 1 PH (PAH); were ≥18 years; were prescribed PAH‐specific medications; and had documented mean pulmonary arterial pressure ≥25 mmHg, pulmonary capillary wedge pressure ≤15 mmHg, and pulmonary vascular resistance ≥3.0 Wood units at rest, as determined by right heart catheterization (RHC). RHC parameters were aligned with criteria for PAH at the time the repository was established 11 and before recent updates of PH/PAH diagnostic thresholds. 12 Exclusion criteria included diagnosis of World Health Organization PH Group 2, 3, 4, or 5; absence of documentation of hemodynamic and clinical criteria for PAH; participation in a clinical trial; and age <18 years. Only patients with the required demographic, clinical, and hemodynamic data, as well as corresponding drug‐dispensing data, available from January 2015, were included in TRIO CIPDR.
Patients were enrolled in TRIO CIPDR using a pragmatic approach. Patients confirmed by their physician to meet the predefined inclusion criteria were enrolled sequentially in TRIO CIPDR until the predefined sample size (N = 1000) was reached. The site treating physician made the decision about which patients were enrolled and was not required to provide a reason why a patient was not included.
Data collection
TRIO CIPDR holds data on demographics, disease, comorbidities, laboratory data, pulmonary function testing, functional status, PAH treatment, reasons for treatment discontinuation (adverse events, payer/cost issues, death, insufficient efficacy, hospitalization/injury/surgery, leaving the practice, pregnancy, or other reasons), and reasons for treatment switches, hospitalizations, and death, which were collected through Health Insurance Portability and Accountability Act–compliant, secure online forms. To minimize data entry errors, participating centers received training on how to complete online data collection forms and were given ongoing support. Each form contained logic to identify improbable entries. All data were de‐identified before being stored in secure, redundant servers. Engagement with centers, data collection forms, data storage, and data output processes were designed to allow both retrospective and prospective data collection and to facilitate near‐immediate repository expansion through addition of other PAH‐treating centers. Patient enrollment, with recording of clinical and outcome data, was planned during the period of January 2019 and December 2020, with follow‐up data recorded during care encounters continuing through to April 2021. Demographic and clinical data collected at enrollment included age, sex, ethnicity, hemodynamic, and clinical parameters related to PAH, as well as comorbidities. Comorbidity data focused on chronic obstructive pulmonary disease, hypertension, diabetes mellitus, pulmonary embolism, deep vein thrombosis, scleroderma, lupus, rheumatoid arthritis, and renal insufficiency. These data were recorded at the timepoint closest to enrollment; however, no maximum or minimum lookback date was set.
One‐year mortality risk at enrollment was calculated according to the European Society of Cardiology (ESC)/European Respiratory Society (ERS) 2015 risk classification 13 and REVEAL Lite 2 risk calculator. 14 The ESC/ERS 2015 risk classification was calculated using an online risk calculator (https://pahriskcalculatoreu.com), with the scoring methodology (1 = low risk, 2 = intermediate risk, 3 = high risk) adapted from the Swedish PAH Registry. 15 , 16
Parameters collected over time (where available) in the TRIO CIPDR included PAH‐specific medications (including dose, route of administration, start/stop dates, and reasons for discontinuation/switch), insurance type, vital signs, laboratory parameters, additional comorbidities since enrollment, clinical assessment results (including New York Heart Association functional class, 6‐minute walk distance, echocardiogram, RHC, pulmonary function tests, cardiopulmonary exercise test, and computed tomography scan), hospitalizations, and death. All collected data were reviewed with data quality monitoring and adjudication by a Trio Health clinical coordinator in consultation with the person responsible for data entry at each center. Questions not clearly addressable through data review were answered by the site physician.
Statistical analyses
Patient characteristics were summarized as median (interquartile range [IQR]) for continuous values, and as number of patients (percentage) for categorical values.
RESULTS
Patient population
Overall, 3200 patients were identified as potentially eligible for inclusion in TRIO CIPDR, based on specialty pharmacy data indicating they were prescribed a PAH‐specific medication (Figure 3). The target enrollment was 1000 patients, and patients were entered sequentially across the participating centers until the target sample size was achieved. Eleven Pulmonary Hypertension Association–accredited PH care centers initially contributed to CIPDR, although two centers were unable to continue participation due to the impact of COVID‐19. Of those remaining, eight were Pulmonary Hypertension Association–accredited PH care centers (one of which was undergoing accreditation during the enrollment period), and one was a nonaccredited center at the time of the study. All centers were located in the United States of America (Figure 1 and Supporting Information: Table S1). Three centers were responsible for approximately 60% of enrolled subjects.
Figure 3.
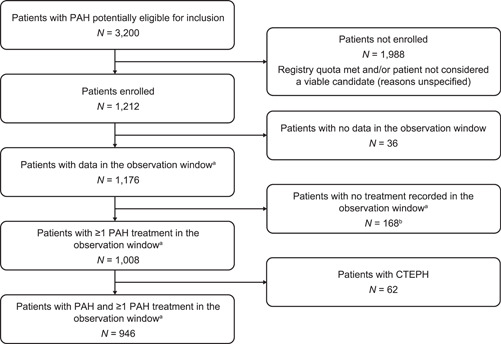
Patient disposition in TRIO CIPDR (enrollment period of January 2019 to October 2020). aJanuary 2019 to December 2020. bThese patients had received PAH treatment based on specialty pharmacy data captured before the observation period, but they did not receive PAH treatment during the observation period. CTEPH, chronic thromboembolic pulmonary hypertension; PAH, pulmonary arterial hypertension; TRIO CIPDR, TRIO comprehensive, integrated patient data repository.
The patient disposition in TRIO CIPDR is shown in Figure 3. Some patients (N = 168) who had received PAH treatment based on specialty pharmacy data captured before the observation period were excluded as they did not receive PAH treatment during the observation period. Some patients potentially eligible for inclusion (N = 1988) were excluded because the repository quota of 1000 patients was met before the center could review the patient, and/or the center deemed the patient not suitable for unspecified reasons―centers were not requested to provide additional detail or rationale for exclusions.
In total, 1008 patients prescribed PAH‐specific medications were initially enrolled. However, 62 of these patients had chronic thromboembolic pulmonary hypertension (CTEPH) and were subsequently excluded. Therefore, 946 patients on PAH‐specific medication and with data in the observation period (January 2019 and December 2020) were included in TRIO CIPDR. Data from 4001 visits were captured from the 946 patients; of these, 2837 were office visits and 1164 were telehealth calls. Patients had a median of four visits (IQR: 3−6) during the observation window, including a median of three in‐office visits (IQR: 2−4) and a median of one televisit (IQR: 0−2). Overall, 71% of visits were in‐person office visits and 29% were televisits.
Demographic characteristics
The demographic characteristics of the patients at enrollment in TRIO CIPDR are shown in Table 1. The majority of patients were female (75%) and White (67%). Median age at PAH diagnosis was 53 years, and median age at enrollment was 60 years. Thirty‐seven percent were categorized as obese (body mass index ≥30 kg/m2) at enrollment. The median time from diagnosis to enrollment was 5 years (IQR: 2−10) with 14% of patients diagnosed within 1 year before enrollment and 29% within 2 years before enrollment. Timelines for PAH diagnosis, enrollment in TRIO CIPDR, and echocardiogram and RHC before enrollment are provided (Supporting Information: Figure S1). For the majority of patients, hemodynamic and PAH parameters were available within 2 years before enrollment (Supporting Information: Table S2). Overall, 80%, 52%, 63%, and 71% of patients had measures for laboratory parameters, pulmonary function tests, RHC, and 6‐minute walk distance, respectively (data not shown).
Table 1.
Patient demographics at enrollment in TRIO CIPDR.
| Characteristic | All enrolled patients |
|---|---|
| (N = 946) | |
| Age at diagnosis, years; median (IQR) | 53 (41−64) |
| (n = 787) | |
| Age at enrollment, years; median (IQR) | 60 (49−71) |
| Age 18−64 years at enrollment, n (%) | 588 (62) |
| Age ≥65 years at enrollment, n (%) | 358 (38) |
| Years from diagnosis to enrollment; median (IQR) | 5 (2−10) |
| (n = 787) | |
| Diagnosed within 1 calendar year before enrollment, n (%) | 110 (14) |
| (n = 787) | |
| Diagnosed within 2 calendar years before enrollment, n (%) | 232 (29) |
| (n = 787) | |
| Female sex, n (%) | 705 (75) |
| Race, n (%) | |
| African−American | 145 (15) |
| Asian | 50 (5) |
| White | 633 (67) |
| Hispanic | 36 (4) |
| Other/unknown | 82 (9) |
| BMI, kg/m2; median (IQR) | 27.6 (23.2−32.6) |
| (n = 995) | |
| BMI categories, n (%) | (n = 993) |
| Underweight: BMI < 18.5 kg/m2 | 33 (4) |
| Normal: BMI 18.5−24.9 kg/m2 | 302 (32) |
| Overweight: BMI 25−29.9 kg/m2 | 250 (27) |
| Obese I: BMI 30−35 kg/m2 | 186 (20) |
| Obese II/III: BMI 35+ kg/m2 | 162 (17) |
| Obese: BMI ≥ 30 kg/m2 | 384 (37) |
| Never smoker, n (%) | 480 (54) |
| (n = 891) |
Abbreviations: BMI, body mass index; IQR, interquartile range; PAH, pulmonary arterial hypertension; TRIO CIPDR, TRIO comprehensive, integrated patient data repository.
Hemodynamic and PAH characteristics
Hemodynamic and PAH clinical parameters at (or closest to) enrollment in TRIO CIPDR are shown in Table 2. The median 6‐minute walk distance was 343 m (IQR: 205−427). Echocardiogram and RHC parameters at enrollment are also detailed in Table 2, and the timings of test conduct relative to enrollment is shown in Supporting Information: Table S2. Median values at enrollment included mean pulmonary arterial pressure 43 mmHg (IQR: 34.0−52.0), mean right atrial pressure 6.0 mmHg (IQR: 3.0−10.0), pulmonary capillary wedge pressure 11.0 mmHg (IQR: 8.0−14.0), cardiac index (Fick) 2.6 L/min (IQR: 2.1−3.1), pulmonary vascular resistance 6.1 Wood units (IQR: 3.8−9.7), and left ventricular ejection fraction 64% (IQR: 59.3−69.0). The most common comorbidities present at enrollment were cardiovascular‐ or pulmonary‐related conditions (Supporting Information: Table S3). One‐third of patients (34%) had atrial fibrillation, 30% had obstructive sleep apnea, and 20% had thyroid disease.
Table 2.
Hemodynamic and PAH clinical parameters at enrollment in TRIO CIPDR.
| Characteristic | All enrolled patients (N = 946) |
|---|---|
| 6MWD (m) | 343 (205−427) |
| (n = 782) | |
| Echocardiogram parameters | |
| LVEF (%) | 64.0 (59.3−69.0) |
| (n = 839) | |
| RVSP (mmHg) | 60.0 (40.0−78.8) |
| (n = 656) | |
| PFT parameters | |
| FVC (%) | 77.0 (63.0−91.0) |
| (n = 707) | |
| FEV1 (%) | 72.0 (57.0−87.0) |
| (n = 709) | |
| FEV1/FVC (% ratio) | 76.0 (69.0−82.0) |
| (n = 685) | |
| DLCO (%) | 50.9 (35.0−65.0) |
| (n = 533) | |
| O2 saturation (%) | 96.0 (92.0−98.0) |
| (n = 478) | |
| RHC parameters | |
| mRAP (mmHg) | 6.0 (3.0−10.0) |
| (n = 946) | |
| mPAP (mmHg) | 43.0 (34.0−52.0) |
| (n = 841) | |
| PCWP (mmHg) | 11.0 (8.0−14.0) |
| (n = 831) | |
| LVEDP (mmHg) | 13.0 (9.8−16.0) |
| (n = 100) | |
| Cardiac output (Fick) (L/min) | 4.8 (3.8−6.0) |
| (n = 713) | |
| Cardiac index (Fick) (L/min) | 2.6 (2.1−3.1) |
| (n = 631) | |
| PVR, Wood units (mmHg/L/min) | 6.1 (3.8−9.7) |
| (n = 786) |
Note: Data are median (IQR).
Abbreviations: 6MWD, six‐minute walk distance; DLCO, diffusing capacity of lung for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IQR, interquartile range; LVEDP, left ventricular end‐diastolic pressure; LVEF, left ventricular ejection fraction; mPAP, mean pulmonary arterial pressure; mRAP, mean right atrial pressure; PAH, pulmonary arterial hypertension; PCWP, pulmonary capillary wedge pressure; PFT, pulmonary function test; PVR, pulmonary vascular resistance; RHC, right heart catheterization; RVSP, right ventricular systolic pressure; TRIO CIPDR, TRIO comprehensive, integrated patient data repository.
The most common PAH etiologies in patients enrolled in TRIO CIPDR were idiopathic PAH (38%) and connective tissue disease‐related PAH (CTD‐PAH; 30%) (Figure 4a). One‐year mortality risk assessment at enrollment was calculated using ESC/ERS 2015 risk classification (Figure 4b); 26% were classified as low risk, 65% as intermediate risk, and 9% as high risk (among 946 patients with available data; with a median 4 variables per score [IQR: 3−4]). Per REVEAL Lite 2, 46% were classified as low risk, 29% as intermediate risk, and 25% as high risk at enrollment (among 842 patients with available data; with a median of 5 variables per score [IQR: 4−5]). New York Heart Association functional class was available for 738 patients, the majority of whom were either New York Heart Association functional class II (47%) or III (41%) at enrollment (Figure 4c).
Figure 4.
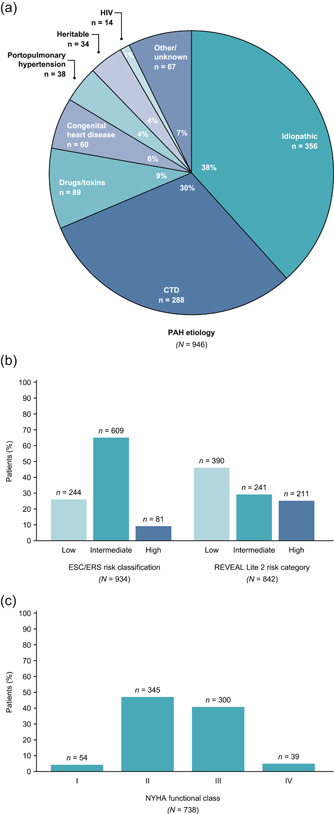
PAH disease characteristics at enrollment in TRIO CIPDR. (a) PAH etiology (note: percentages may not add up to 100 due to rounding); (b) mortality risk classification according to ESC/ERS risk classification and REVEAL Lite 2 risk category; (c) NYHA functional class. ERS, European Respiratory Society; ESC, European Society of Cardiology; HIV, human immunodeficiency virus; NYHA: New York Heart Association; PAH, pulmonary arterial hypertension; REVEAL, Registry to Evaluate Early and Long‐term Pulmonary Arterial Hypertension; TRIO CIPDR, TRIO comprehensive, integrated patient data repository.
Patient characteristics at enrollment by PAH etiology (CTD‐PAH vs. idiopathic PAH) are provided in the Supporting Information: Table S4. Age at diagnosis, at enrollment, and time from diagnosis to enrollment were similar for CTD‐PAH and idiopathic PAH cohorts. Of patients with CTD‐PAH, 23% were African−American, compared with 12% of the idiopathic PAH cohort; 59% of patients with CTD‐PAH and 74% of patients with idiopathic PAH were White. Median body mass index was 25.3 kg/m2 in the CTD‐PAH cohort and 28.5 kg/m2 in the idiopathic PAH cohort. A smaller proportion of patients with CTD‐PAH had a body mass index of ≥30 kg/m2 than those with idiopathic PAH (28% vs. 42%, respectively).
PAH medications at enrollment
At enrollment, patients were prescribed a range of treatments for PAH (Table 3). Of 917 patients receiving PAH‐specific therapy, 40% were on monotherapy, 43% were on double therapy, and 17% were on triple therapy. Of 510 patients receiving an endothelin receptor antagonist, 16% received it as monotherapy, 54% as part of dual therapy, and 30% as part of triple therapy. Of 557 patients taking a phosphodiesterase type 5 inhibitor, 26% did so as monotherapy, 51% as part of dual therapy, and 22% as part of triple therapy. There were 467 patients receiving a prostacyclin pathway agent: 27% as monotherapy, 39% as part of dual therapy, and 33% as part of triple therapy. Of 226 patients receiving treprostinil, the majority (71.7%) were on non‐oral formulations with 10.6% on oral treprostinil and 10.2% on mixed formulation; formulation was not indicated for 7.5% of patients (data not shown). Overall, 97 patients were receiving soluble guanylate cyclase stimulator therapy, with 13% on monotherapy, 48% taking it as part of dual therapy, and 34% as part of triple therapy.
Table 3.
Regimens by drug class at enrollment in TRIO CIPDR (n = 917). a
| Regimen | No of patients | Monotherapy | Dual therapy | Triple therapy | Quadruple therapy |
|---|---|---|---|---|---|
| All regimens | 917 | 365 (40) | 394 (43) | 154 (17) | 4 (>1) |
| Any ERA | 510 | 80 (16) | 273 (54) | 153 (30) | 4 (1) |
| Ambrisentan | 218 | 25 (11) | 121 (56) | 69 (32) | 3 (1) |
| Bosentan | 16 | 5 (31) | 8 (50) | 3 (19) | 0 |
| Macitentan | 283 | 51 (18) | 148 (52) | 83 (29) | 1 (>1) |
| Any PDE5i | 557 | 147 (26) | 284 (51) | 122 (22) | 4 (1) |
| Sildenafil | 206 | 60 (29) | 103 (50) | 43 (21) | 0 |
| Tadalafil | 355 | 88 (25) | 183 (52) | 80 (23) | 4 (1) |
| SGC stimulator | 97 | 13 (13) | 47 (48) | 33 (34) | 4 (4) |
| Riociguat | 97 | 13 (13) | 47 (48) | 33 (34) | 4 (4) |
| Any prostacyclin | 467 | 125 (27) | 184 (39) | 154 (33) | 4 (1) |
| Epoprostenol | 117 | 53 (45) | 44 (38) | 19 (16) | 1 (1) |
| Iloprost | 2 | 1 (50) | 1 (50) | 0 | 0 |
| Selexipag | 143 | 28 (20) | 48 (34) | 66 (46) | 1 (1) |
| Treprostinil | 226 | 47 (21) | 101 (45) | 75 (33) | 3 (1) |
Note: Data in therapy columns are n (%) of patients. The percentages are of total patients for the row. One hundred and forty‐two of 917 patients were receiving more than one drug from the same drug class in the regimen recorded at enrollment.
Abbreviations: ERA, endothelin receptor antagonist; PDE5i, phosphodiesterase 5 inhibitor; SGC, soluble guanylate cyclase; TRIO CIPDR, TRIO comprehensive, integrated patient data repository.
Twenty‐nine of the 946 patients were not on therapy at enrollment.
DISCUSSION
The TRIO CIPDR is a unique real‐world repository capturing the characteristics and management of a group of contemporary patients with PAH in the United States of America. TRIO CIPDR is one of the first new PAH disease registries in the United States of America since the REVEAL registry. 5 Subsequently, there have been new approaches in PAH treatment, including data from studies of macitentan (a dual endothelin receptor antagonist), 17 selexipag (an oral selective prostacyclin receptor agonist), 18 riociguat (a soluble guanylate cyclase stimulator), 19 and upfront combination therapy. 20 , 21 , 22 PAH guidelines have been updated to include these new treatments, as well as recommendations for treatment escalation based on assessment of patients' risk for mortality. 9 , 23 TRIO CIPDR provides an opportunity to monitor how these new treatments have been integrated into routine clinical practice. To our knowledge, since REVEAL, there have been only two other similar registries to the TRIO CIPDR. One of these is the United States Pulmonary Hypertension Scientific Registry (USPHSR), which was established to investigate genetic information, reproductive histories, and environmental exposure data in a contemporary population of PAH patients. USPHSR enrolled 499 patients with Group 1 PAH between 2015 and 2018. 6 , 24 The second registry is the Pulmonary Hypertension Association Registry (PHAR), which was instigated in 2015 to collect data from pediatric and adult patients with PAH or CTEPH (note that TRIO CIPDR does not include pediatric patients or those with CTEPH). PHAR has a longitudinal design, with data capture planned from 3000 patients over a 10‐year timeframe. PHAR data include patient‐reported data and outcomes (including health‐related quality of life measures), as well as data provided by staff at the treating center. 25 The aim of PHAR is to measure patient management process and outcomes, to identify best practice and improve quality of care and health outcomes. 25 As of September 2020, 1361 patients had been enrolled in PHAR, 182 of whom had CTEPH. 26
Here we have reported the design of the TRIO CIPDR and patient characteristics at enrollment. Under the guidance of an independent, expert steering committee, the repository protocol took a new approach compared with that generally used by current and previous PAH registries―by combining prescribing and dispensing data for PAH‐specific medications with clinical data from electronic medical records. There was a stringent process for validating and confirming the Health Insurance Portability and Accountability Act–compliant data in TRIO CIPDR, ensuring data quality and opening opportunities for using the data to support interactions with regulatory health authorities.
Patient characteristics at enrollment in TRIO CIPDR had similarities and differences to REVEAL 5 and the contemporary USPHSR and PHAR registries. 6 , 26 The majority of patients with PAH were female in TRIO CIPDR, REVEAL, USPHSR, and PHAR (75%, 79%, 79%, and 76%, respectively). Patients in TRIO CIPDR had a mean age of 53 years at diagnosis, which is similar to the mean age of patients at diagnosis in REVEAL and USPHSR (mean 50 and 52 years, respectively). Mean age at diagnosis in PHAR was not reported but was 56 years at enrollment. 5 , 6 , 26 In TRIO CIPDR, the median time from diagnosis to enrollment was 5 years, compared with approximately 2 years in REVEAL and 4 years in USPHSR (not reported for PHAR). Reasons for these discrepancies are unclear, but differences in the processes by which patients were defined as diagnosed and enrolled could be a factor. Thirty‐seven percent of patients were obese (body mass index ≥30 kg/m2) at enrollment in TRIO CIPDR (similar to the 33% in REVEAL and 40% in PHAR, but higher than the 21% in USPHSR). Two‐thirds of patients in TRIO CIPDR were White, and 15% were African−American; in USPHSR, 88% were White and 7% were Black, 6 and in PHAR, 77% were White and 14% were Black 26 (not reported in REVEAL 5 ).
The most common PAH etiologies in patients enrolled in TRIO CIPDR were idiopathic PAH (38%) and CTD‐PAH (30%); these are similar proportions to those enrolled in USPHSR (44% and 34%, respectively) 6 and PHAR (41% and 32%, respectively). 26 In REVEAL, 46% of patients had idiopathic PAH and 25% had CTD‐PAH. 5 The distribution of New York Heart Association functional class in TRIO CIPDR was similar to that seen in REVEAL, with 87%−88% of patients in functional class II/III at enrollment in both registries. However, in USPHSR and PHAR, 68% and 57% of patients, respectively, were in functional class II/III at enrollment. 6 , 26 These differences highlight that PAH expertise, treatment, and monitoring are evolving, thereby necessitating the use of prior registries as well as new sources of data to get an accurate picture of the disease landscape.
A benefit of TRIO CIPDR is that it will provide longitudinal data on treatment patterns and outcomes in patients with PAH in a contemporary setting where newer treatments are available, with stronger evidence and recommendations for combination therapy that became available after the completion of most PAH disease registries, including REVEAL. 8 TRIO CIPDR is expected to provide novel insights into potential barriers to treatment access and information on ways economic factors may impact treatment consistency, adherence, and outcomes in patients with PAH (e.g., the potential relationship between increased medication cost and failure to collect dispensed PAH treatments, and the impact of treatment interruptions on outcomes such as hospitalizations). 27
High‐quality real‐world evidence is increasingly being used to provide data on comparative efficacy and real‐world outcomes that support regulatory decision making. 28 A unique feature of TRIO CIPDR is that it was designed to systematically collect Health Insurance Portability and Accountability Act–compliant data on a defined population of patients with PAH (their disease course, complications, and medical care) in a way that complies with the US Food and Drug Administration draft guidance for the development of registries that support regulatory decision making. 29
Study limitations
As with all real‐world datasets, there are limitations concerning the data collected in TRIO CIPDR, including the potential for missing data. Physician participation and patient enrollment in TRIO CIPDR are not truly random; a willingness to participate and pragmatic geographical considerations may have influenced the patient sample (recruitment bias). Additionally, the repository was structured to collect data for hypothesis generation in collaboration with a disease‐specific steering committee. However, participation was optional and aside from meeting the criteria of the screener and having been identified as receiving PAH‐specific medications in the past, no further guidance was provided to centers regarding criteria for patient inclusion. It could be suggested that this leads to selection bias, but it is balanced by the fact that all screeners had the same latitude to determine participants for the study; thus, TRIO CIPDR benefits from capturing clinically validated data from a real‐world population of patients with PAH. There is also no additional information available on patients (approximately 2000) identified as being potentially eligible for inclusion in the repository but who were nevertheless excluded, as centers were not requested to provide their rationale for exclusion. Specifically, concerning the 168 patients excluded because they did not receive PAH treatment during the observation period, information as to the reason(s) they were not treated was not part of the objectives of the study, and thus this information was not collected. In addition, historic data were used for RHC and laboratory parameters; although these data are useful in detailing disease characteristics of this cohort, the large number of patients with only historic data limits the clinical applicability of future analyses utilizing these variables. It is also possible that the patients enrolled may not reflect the general PAH population. However, as PAH is a rare disease and the enrolled patients are being treated at specialist PAH centers, this limitation is inherent in any study of PAH.
CONCLUSIONS
TRIO CIPDR is a contemporary real‐world repository containing data on the demographics, clinical characteristics, treatment, and management of patients with PAH in the United States of America. This unique dataset combines clinical data from electronic medical records with drug‐prescription and drug‐dispensing characteristics in US patients with PAH. We found that the majority of patients had low or intermediate 1‐year mortality risk, and were primarily treated with dual or triple PAH‐specific therapy. Longitudinal data will continue to track the treatment journey in these patients in relation to their clinical characteristics, real‐world prescription usage, and long‐term outcomes.
AUTHOR CONTRIBUTIONS
All authors contributed to the concept and design of the study and the acquisition, analysis, and interpretation of data. All authors contributed to drafting of the manuscript, reviewed and revised the manuscript; and gave final approval for submission. Andrew Frick and Scott Milligan agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, and provided administrative, technical, and material support.
CONFLICTS OF INTEREST STATEMENT
H. W. F. is a speaker for Bayer and a scientific advisory board member for Janssen Pharmaceutical Companies of Johnson & Johnson, Acceleron Pharma, Altavant, and United Therapeutics, and has received research support from Janssen Pharmaceutical Companies of Johnson & Johnson, Eiger, Reata Pharmaceuticals, and United Therapeutics. M. M. C. receives research support from Janssen, Bayer, Medtronic, Liquidia, PhaseBio, United Therapeutics, Altavant, Trio Health Analytics, Acceleron, and Gossamer, and is an advisor for Janssen, Express Scripts, PhaseBio, Altavant, V‐Wave, Gossamer, United Therapeutics, Bayer, Aerovate, and Merck. M. C. is an employee of Janssen Pharmaceuticals US, Inc. R. P. F receives grants and research support from United Therapeutics, Medtronic, and Gossamer Bio, and is scientific medical advisor to Altavant, ShouTi, Liquidia Technologies, Tenax Pharmaceuticals, and Janssen Pharmaceutical Companies of Johnson & Johnson; his institution has received funding from Bayer and Gossamer Bio. A. F. received research support from Janssen Pharmaceutical Companies of Johnson & Johnson, GSK/VIIV, UCB, AbbVie, Horizon, and Takeda. L. L. reports grants and other (speaker for disease state education, advisory board) from Genentech, grants and other (speaker for disease state education) from Boehringer Ingelheim, and grants from Novartis, Celgene, and Bellerophon. S. M. received research support from Janssen Pharmaceutical Companies of Johnson & Johnson, GSK/VIIV, UCB, AbbVie, Horizon, and Takeda. R. O. received grants from AADi, Acceleron, Gossamer Bio, Insmed, Janssen, Liquidia, and United Therapeutics, and honoraria/speaker fees from Acceleron, Gossamer Bio, Insmed, Janssen, United Therapeutics, and V‐Wave. S. P. is an employee of Janssen Pharmaceuticals US, Inc. Y. T. is an employee of Janssen Pharmaceuticals US, Inc. S. D. N. is a consultant and is on the speakers' bureau for Roche‐Genentech and Boehringer Ingelheim, and has received research funding from both companies.
ETHICS STATEMENT
The study protocol was approved by the Western Institutional Review Board and was conducted in accordance with the Declaration of Helsinki, Good Pharmacoepidemiology Practices, and International Conference on Harmonisation Good Clinical Practice (ICH E6). In addition, all Pulmonary Hypertension Association–accredited centers obtained approval for the TRIO CIPDR protocol from their local institutional review board. Patient consent was not required as Trio Health Analytics, who constructed the repository, did not have access to personal health information, and received only existing data.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors thank the centers, physicians, center coordinators, and patients for their contribution to TRIO CIPDR. Medical writing support and editorial assistance were provided by Ify Sargeant, DPhil, and Kathryn Quinn, PhD, on behalf of Twist Medical, and were funded by Actelion Pharmaceuticals US, Inc., a Janssen Pharmaceutical Company of Johnson & Johnson. HWF is the guarantor. Actelion Pharmaceuticals US, Inc., a Janssen Pharmaceutical Company of Johnson & Johnson, sponsored this study and the analysis.
Farber HW, Chakinala MM, Cho M, Frantz RP, Frick A, Lancaster L, Milligan S, Oudiz R, Panjabi S, Tsang Y, Nathan SD. Characteristics of patients with pulmonary arterial hypertension from an innovative, comprehensive real‐world patient data repository. Pulm Circ. 2023;13:e12258. 10.1002/pul2.12258
DATA AVAILABILITY STATEMENT
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. These data were abstracted by each site and compiled and aggregated into a repository, and analyzed by Trio Health Analytics, which were all funded by Janssen. Request for sharing can be sent to Sumeet Panjabi (spanjab1@its.jnj.com).
REFERENCES
- 1. Sitbon O, Gomberg‐Maitland M, Granton J, Lewis MI, Mathai SC, Rainisio M, Stockbridge NL, Wilkins MR, Zamanian RT, Rubin LJ. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chakinala MM, Barst R. From short‐term benefits to long‐term outcomes: the evolution of clinical trials in pulmonary arterial hypertension. Pulm Circ. 2013;3(3):507–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benza RL, Miller DP, Gomberg‐Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long‐term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122(2):164–72. [DOI] [PubMed] [Google Scholar]
- 4. Frost AE, Badesch DB, Barst RJ, Benza RL, Elliott CG, Farber HW, Krichman A, Liou TG, Raskob GE, Wason P, Feldkircher K, Turner M, McGoon MD. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non‐US Contemporary Registries. Chest. 2011;139(1):128–37. [DOI] [PubMed] [Google Scholar]
- 5. Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376–87. [DOI] [PubMed] [Google Scholar]
- 6. Badlam JB, Badesch DB, Austin ED, Benza RL, Chung WK, Farber HW, Feldkircher K, Frost AE, Poms AD, Lutz KA, Pauciulo MW, Yu C, Nichols WC, Elliott CG, Simms R, Fortin T, Safdar Z, Burger CD, Frantz RP, Hill NS, Airhart S, Elwing J, Simon M, White RJ, Robbins IM, Chakinala MM. United States Pulmonary Hypertension Scientific Registry: baseline characteristics. Chest. 2021;159(1):311–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoeper MM, Pausch C, Grünig E, Staehler G, Huscher D, Pittrow D, Olsson KM, Vizza CD, Gall H, Distler O, Opitz C, Gibbs JSR, Delcroix M, Ghofrani HA, Rosenkranz S, Park DH, Ewert R, Kaemmerer H, Lange TJ, Kabitz HJ, Skowasch D, Skride A, Claussen M, Behr J, Milger K, Halank M, Wilkens H, Seyfarth HJ, Held M, Dumitrescu D, Tsangaris I, Vonk‐Noordegraaf A, Ulrich S, Klose H. Temporal trends in pulmonary arterial hypertension: results from the COMPERA registry. Eur Respir J. 2021; 59(6):2102024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Swinnen K, Quarck R, Godinas L, Belge C, Delcroix M. Learning from registries in pulmonary arterial hypertension: pitfalls and recommendations. Eur Respir Rev. 2019;28(154):190050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano‐Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke‐Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S, Schwerzmann M, Dinh‐Xuan AT, Bush A, Abdelhamid M, Aboyans V, Arbustini E, Asteggiano R, Barberà JA, Beghetti M, Čelutkienė J, Cikes M, Condliffe R, de Man F, Falk V, Fauchier L, Gaine S, Galié N, Gin‐Sing W, Granton J, Grünig E, Hassoun PM, Hellemons M, Jaarsma T, Kjellström B, Klok FA, Konradi A, Koskinas KC, Kotecha D, Lang I, Lewis BS, Linhart A, Lip GYH, Løchen ML, Mathioudakis AG, Mindham R, Moledina S, Naeije R, Nielsen JC, Olschewski H, Opitz I, Petersen SE, Prescott E, Rakisheva A, Reis A, Ristić AD, Roche N, Rodrigues R, Selton‐Suty C, Souza R, Swift AJ, Touyz RM, Ulrich S, Wilkins MR, Wort SJ. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–731. [DOI] [PubMed] [Google Scholar]
- 10. McGoon MD, Miller DP. REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev. 2012;21(123):8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, Wilkins MR, Badesch DB. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42–50. [DOI] [PubMed] [Google Scholar]
- 12. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti B, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group . 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119. [DOI] [PubMed] [Google Scholar]
- 14. Benza RL, Kanwar MK, Raina A, Scott JV, Zhao CL, Selej M, Elliott CG, Farber HW. Development and validation of an abridged version of the REVEAL 2.0 risk score calculator, REVEAL Lite 2, for use in patients with pulmonary arterial hypertension. Chest. 2021;159(1):337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kylhammar D, Kjellström B, Hjalmarsson C, Jansson K, Nisell M, Söderberg S, Wikström G, Rådegran G. A comprehensive risk stratification at early follow‐up determines prognosis in pulmonary arterial hypertension. Eur Heart J. 2018;39(47):4175–81. [DOI] [PubMed] [Google Scholar]
- 16. Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, Olsson KM, Meyer K, Vizza CD, Vonk‐Noordegraaf A, Distler O, Opitz C, Gibbs JSR, Delcroix M, Ghofrani HA, Huscher D, Pittrow D, Rosenkranz S, Grünig E. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50(2):1700740. [DOI] [PubMed] [Google Scholar]
- 17. Pulido T, Adzerikho I, Channick RN, Delcroix M, Galiè N, Ghofrani HA, Jansa P, Jing ZC, Le Brun FO, Mehta S, Mittelholzer CM, Perchenet L, Sastry BKS, Sitbon O, Souza R, Torbicki A, Zeng X, Rubin LJ, Simonneau G. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369(9):809–18. [DOI] [PubMed] [Google Scholar]
- 18. Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N, Ghofrani HA, Hoeper MM, Lang IM, Preiss R, Rubin LJ, Di Scala L, Tapson V, Adzerikho I, Liu J, Moiseeva O, Zeng X, Simonneau G, McLaughlin VV. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522–33. [DOI] [PubMed] [Google Scholar]
- 19. Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, Langleben D, Kilama MO, Fritsch A, Neuser D, Rubin LJ. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–40. [DOI] [PubMed] [Google Scholar]
- 20. Kemp K, Savale L, O'Callaghan DS, Jaïs X, Montani D, Humbert M, Simonneau G, Sitbon O. Usefulness of first‐line combination therapy with epoprostenol and bosentan in pulmonary arterial hypertension: an observational study. J Heart Lung Transplant. 2012;31(2):150–8. [DOI] [PubMed] [Google Scholar]
- 21. Sitbon O, Jais X, Savale L, Cottin V, Bergot E, Macari EA, Bouvaist H, Dauphin C, Picard F, Bulifon S, Montani D, Humbert M, Simonneau G. Upfront triple combination therapy in pulmonary arterial hypertension: a pilot study. Eur Respir J. 2014;43(6):1691–7. [DOI] [PubMed] [Google Scholar]
- 22. Galiè N, Barberà JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, Peacock AJ, Simonneau G, Vachiery JL, Grünig E, Oudiz RJ, Vonk‐Noordegraaf A, White RJ, Blair C, Gillies H, Miller KL, Harris JHN, Langley J, Rubin LJ. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373(9):834–44. [DOI] [PubMed] [Google Scholar]
- 23. Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, Preston IR, Pulido T, Safdar Z, Tamura Y, McLaughlin VV. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elliott CG, Austin ED, Badesch D, Badlam J, Benza RL, Chung WK, Farber HW, Feldkircher K, Frost AE, Poms AD, Lutz KA, Pauciulo MW, Yu C, Nichols WC. United States Pulmonary Hypertension Scientific Registry (USPHSR): rationale, design, and clinical implications. Pulm Circ. 2019;9(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gray MP, Kawut SM. The Pulmonary Hypertension Association Registry: rationale, design, and role in quality improvement. Adv Pulmonary Hyper. 2018;16(4):185–8. [Google Scholar]
- 26. Chang KY, Duval S, Badesch DB, Bull TM, Chakinala MM, De Marco T, Frantz RP, Hemnes A, Mathai SC, Rosenzweig EB, Ryan JJ, Thenappan T, Allen R, Bartolome S, Benza R, Cadaret L, Eggert M, Elwing J, Fineman J, Foley R, Ford HJ, Hirsch R, Grinnan J, Ivy DD, Kawut S, Kennedy J, Klinger J, Leary P, Mazimba S, Ramani G, Raina A, Runo J, Swisher J, Varghese N, White RJ, Williamson T, Yung D, Zamanian R, Zwicke D. Mortality in pulmonary arterial hypertension in the modern era: early insights from the Pulmonary Hypertension Association Registry. J Am Heart Assoc. 2022;11(9):e024969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sherman BW, Epstein AJ, Meissner B, Mittal M. Impact of a co‐pay accumulator adjustment program on specialty drug adherence. Am J Manag Care. 2019;25(7):335–40. [PubMed] [Google Scholar]
- 28. Purpura CA, Garry EM, Honig N, Case A, Rassen JA. The role of real‐world evidence in FDA‐approved new drug and biologics license applications. Clin Pharm Ther. 2022;111(1):135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Food and Drug Administration . Real‐world data: assessing Registries to support regulatory decision‐making for drug and biological products: guidance for industry. [Internet]. Food and Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/real-world-data-assessing-registries-support-regulatory-decision-making-drug-and-biological-products [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. These data were abstracted by each site and compiled and aggregated into a repository, and analyzed by Trio Health Analytics, which were all funded by Janssen. Request for sharing can be sent to Sumeet Panjabi (spanjab1@its.jnj.com).


