Abstract
This study aimed to examine the effect of subanesthetic esketamine on postoperative fatigue in patients who underwent laparoscopic colorectal surgery. A total of 62 patients, including 32 in the esketamine group and 30 in the control group, were analysed in this study. Compared with the control group, the patients in the esketamine group had reduced Identity-Consequence Fatigue Scale (ICFS) on the 3rd and 7th days after surgery (P<0.05). There were also significant differences in the Positive and Negative Affect Schedule (PANAS) scale between the two groups. The positive affect scale was higher on postoperative day 3 (POD3), while the negative affect scale was lower on POD3 and postoperative day 7 (POD7) in the esketamine group than in the control group. However, the scores of postoperative hand grip strength, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), Numeric Rating Scale (NRS) and Athens Insomnia Scale (AIS) were not significantly different between the two groups. Furthermore, mediation analysis showed that esketamine played an anti-fatigue role through improving emotional heath. Importantly, no adverse reactions occurred at this dosage of esketamine. Finally, our study suggested that subanesthetic esketamine improved postoperative fatigue, stabilized postoperative mood, reduced intraoperative remifentanil consumption, and promoted postoperative intestinal function recovery without increasing adverse reactions.
Keywords: Esketamine, postoperative fatigue, emotional functioning, laparoscopic colorectal surgery
Introduction
Postoperative fatigue (POF) is one of the most common complications after colorectal cancer surgery, manifested as exhaustion, sleepiness or insomnia, low mood, inability to concentrate, and lack of initiative, which tends to be overlooked. Its pathophysiological mechanism has not been fully elucidated, and few intervention measures have been shown to reduce POF [1-3]. A previous study reported that negative emotion, positive emotion, dysfunction and pain were latent variables; nevertheless, the covariance structure model showed that negative and positive emotions markedly influence POF, while dysfunction and physical pain had no obvious effect on POF [4]. Moreover, the author argued that psychological therapies or drugs might be more effective in reducing the fatigue of patients undergone surgery. However, recent animal and clinical studies have found that POF is associated with abnormal glutamate metabolism in the brain, which may be related to the dysregulation of emotion, suggesting that modulating glutamate delivery may have a potential therapeutic effect on POF [5-7].
Esketamine is the S-enantiomer of ketamine, a mixture of R-ketamine and S-ketamine, with satisfying sedative, analgesic, anti-inflammatory and mood-improving effects. In recent years, a growing number of studies have shown that both subanesthetic doses of ketamine and esketamine have rapid but sustained anti-depression effects [8]. Accumulating evidence suggests that esketamine has a higher binding affinity to NMDA receptors than ketamine does, with fewer side effects [9,10]. In terms of the anti-depression effect, Liu et al. found that pretreatment with S-ketamine can reduce postoperative depression in breast cancer patients more effectively than pretreatment with R-ketamine [11]; therefore, we focused our study on esketamine. As to the anti-fatigue effect, Saligan et al. first described the rapid and sustained anti-fatigue effect of ketamine in patients with treatment-resistant depression, and suggested that the mechanism by which ketamine improves fatigue is related to its anti-depression effects [12]. Another study explored the correlation of ketamine with fatigue as well as depression and found that ketamine improved fatigue in patients with treatment-resistant depression by boosting motivation and mood [13]. Regarding fatigue after surgery, Zhao et al. reported that a subanesthetic dose of ketamine could act as an antifatigue agent due to its anti-inflammatory and neuroprotective effects [14]. However, few studies have explored the effect of esketamine on POF and its functional mechanism. Therefore, this study aimed to assess the effect of the continuous infusion of subanesthetic dose of esketamine on POF in patients undergoing colorectal cancer surgery and explore its effects from both positive and negative emotional aspects as well.
Materials and methods
Study design and patient enrollment
This study was a prospective, double-blind, randomized controlled trial, which was approved by the Ethics Committee of Affiliated Hospital of Xuzhou Medical University (XYFY2022-KL209-01) and was registered in the Chinese Clinical Trial Registry Center (ChiCTR2200056278). The study was performed at the Affiliated Hospital of Xuzhou Medical University from July 2022 to October 2022, and written informed consent was obtained from all participants. The patien inclusion criteria were as follows: (1) scheduled for laparoscopic radical resection for colorectal cancer; (2) 18 to 79 years of age; (3) with BMI between 18.5-28 kg/m2; and (4) with ASA physical status between I-III. The exclusion criteria were as follows: (1) declined to participate in the study; (2) with preoperative fatigue; (3) with history of preoperative radiotherapy and chemotherapy; (4) with severe liver or renal dysfunction; (5) allergic to esketamine; (6) with serious risk of elevated blood pressure or intracranial pressure; (7) with untreated or undertreated hyperthyroidism; and (8) with drug and alcohol dependence.
Randomization and blinding
Stratified block randomization was performed according to sex (male or female) and surgical method (anus retention or not). Every stratum was randomized at a ratio of 1:1 to the esketamine group or control group, in blocks of 4. The randomization scheme was stored in a light-tight sealed envelope that was opened successively according to the inclusion order. The eligible patients were assigned according to the allocation list in the envelope. Identical packaging of Esketamine and saline was prepared by a person who was not involved in the subsequent anesthetic administration or data collection. All investigators as well as participants, data collectors and assessors were unaware of grouping.
Intervention
After patients entered the operating room, intravenous access was established, and ECG, invasive arterial blood pressure and pulse oxygen saturation were monitored. Anesthesia induction was as follows: midazolam (0.03 mg/kg), etomidate (0.3 mg/kg), sufentanil (0.6 μg/kg) and rocuronium (0.6 mg/kg). Before tracheal intubation, esketamine (0.1 mg/kg) was intravenously injected into the patients in the esketamine group at the rate of 0.1 mg/kg·h until the end of surgery. In the control group, the same volume of normal saline was used. Endotracheal intubation was performed after complete muscle relaxation, and then bilateral ultrasound-guided transversus abdominis plane block and internal jugular central veinous catheterization were performed. For anesthesia maintenance, combined inhalation, and intravenous anesthesia with remifentanil 4-18 μg/kg/h, Propofol 2-6 mg/kg/h and 1% sevoflurane was used in each group. Cisatracurium was given intravenously as needed. The bispectral index (BIS) was maintained at 40-60, PETCO2 at 35-45 mmHg, heart rate (HR) and MAP fluctuated within 20% of the baseline, and vasoactive drugs were used when necessary. Remifentanil and propofol intravenous infusions were stopped at the end of the surgery, while sevoflurane was halted 30 minutes earlier. When extubation was ready, the tracheal tube was removed from patients, and the patient-controlled intravenous analgesia, with sufentanil (2 μg/kg) and tropisetron (6 mg) in 100 ml of saline, was performed with an electronic analgesia pump, in which the background infusion rate was 2 ml/h, while the PCA dose was 0.5 ml/time, and the locking time was 15 minutes.
Outcomes
The primary outcome was the Identity-Consequence Fatigue Scale (ICFS) score on postoperative days (POD) 1, 3 and 7, while the secondary outcomes were the Positive and Negative Affect Schedule (PANAS) scale, hand grip strength, Athens Insomnia Scale (AIS) score on POD1, 3, 7. The Numeric Rating Scale (NRS) score on POD1, 2, 3, 7 as well as the levels of serum platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) on POD1 were also monitored as the secondary outcomes. The dosages of propofol and remifentanil, time from intravenous medication withdrawal to extubation, Ramsay score at extubation, length of hospital stay, and adverse reactions were also recorded. The ICFS is a self-report questionnaire that provides a comprehensive evaluation on patient’s POF from five different dimensions, such as fatigue, vigorousness, concentration, energy and daily activity [15]. Each entry is scored on a scale of 1 to 6, with a higher score indicating more fatigue. Due to the limited daily activity of the patients during hospitalization, the activity data were uniformly excluded. Grip strength was used to measure skeletal muscle function. For the PANAS score, 10 positive and 10 negative emotional adjectives were tested, while each adjective has five choices representing a score of 1 to 5. A higher score indicates a higher level of emotional expression.
Sample size
The sample size was calculated based on our pilot study which showed that the estimated effect size was 0.75. With a level of α=0.05, β=0.10, and effect size =0.75, we calculated that 58 patients (29 participants per group) were required by G-power software. Considering a 10% dropout rate, we recruited 64 patients in total.
Statistical analysis
SPSS 26.0 and GraphPad Prism 9.0 were used for all analyses. The data were analyzed with the per protocol principle. In continuous data, normality was tested by the Kolmogorov-Smirnov test. The normally distributed data were represented as the mean ± SD and were examined by two-sample independent t-tests between groups, while the non-normally distributed data were represented by the median and interquartile range (M (IQR)) and were analyzed with the Mann-Whiney U test. The qualitative data were expressed as the frequency. The chi-square test or Fisher’s precision probability test was performed. The analysis of the mediation effect was performed with PROCESS Marco using the Bootstrap method to explore the association among esketamine, emotional functioning, and POF. A repeated-measures ANOVA followed by Bonferroni’s post hoc test was used to determine if the repeated outcomes at each time point differed between the two groups. The significance level was P<0.05 for two-sided tests.
Results
Demographics and baseline characteristics
A total of 64 participants were randomized to either the control or esketamine group, with 32 patients in each group. In the control group, 2 patients were transferred to open surgery due to severe intraperitoneal adhesions. Ultimately, 62 patients including 30 in the control group and 32 in the esketamine group, were analyzed with the per-protocol principle (Figure 1). According to the baseline characteristics (Table 1), there were no statistically significant differences in age, sex, BMI, ASA physical status, educational status, operation history, preoperative hemoglobin and albumin, and TNM stage or pathological type between the two groups (P>0.05).
Figure 1.
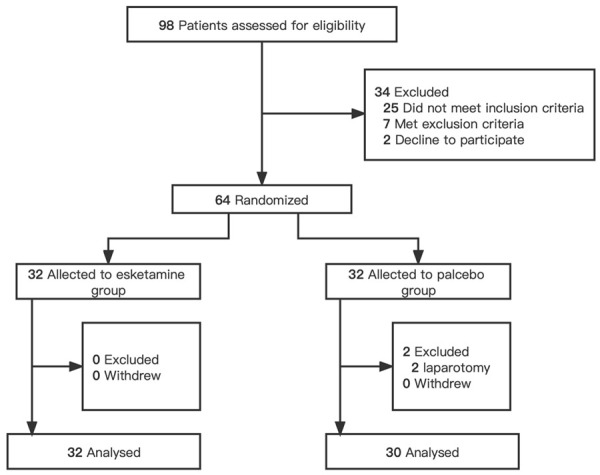
Study Flowchart. Sixty-four participants were randomized. Two patients were excluded from the placebo group, and none were excluded from the esketamine group.
Table 1.
Demographics and baseline characteristics
| Esketamine group (n=32) | Control group (n=30) | P value | |
|---|---|---|---|
| Age (years) | 60.16±7.97 | 62.87±8.37 | 0.196 |
| Sex (n) | 0.829 | ||
| Male | 19 | 17 | |
| Female | 13 | 13 | |
| BMI (kg/m2) | 23.18±3.35 | 23.30±2.30 | 0.871 |
| ASA (n) | 0.591 | ||
| I | 13 | 14 | |
| II | 17 | 16 | |
| III | 2 | 0 | |
| Educational status (n) | 0.832 | ||
| Primary school or below | 12 | 13 | |
| Junior high school | 13 | 10 | |
| Senior high school or above | 7 | 7 | |
| Operation history (n) | 10 | 11 | 0.652 |
| Hemoglobin (g/L) | 116.59±24.17 | 121.53±23.28 | 0.416 |
| Albumin (g/L) | 40.60±4.19 | 40.67±3.56 | 0.947 |
| PSQI score | 4.00 (3.00 to 5.00) | 4.00 (3.00 to 5.00) | 0.258 |
| TNM stage (n) | 0.543 | ||
| I | 3 | 2 | |
| II | 18 | 13 | |
| III | 11 | 15 | |
| Pathological type (n) | 0.492 | ||
| Adenocarcinoma | 30 | 30 | |
| Other | 2 | 0 |
Data are presented as the mean ± SD, median (Q1 to Q3), number; BMI, body mass index; ASA, American Society of Anesthesiologists; PSQI, Pittsburgh Sleep Quality Index.
POF
When we examined the ICFS score, we noticed a significant difference in the group interaction and time (F=10.66, P<0.001); hence, a simple effect was tested. There was no significant difference in ICFS before surgery (F=0.689, P=0.410) or on POD1 (F=2.235, P=0.140) between the two groups. However, the ICFS score of the esketamine group (83.63±9.62) was lower than that of the control group (91.27±8.71) on POD3 (F=10.706, P=0.002) and also on POD7 (esketamine group: 71.56±9.79; control group: 78.27±9.69; F=7.329, P=0.009) (Table 2). Overall, the ICFS scores were increased in both groups after the surgery. As shown in Figure 2, the score peaked on the first day after surgery and then gradually decreased.
Table 2.
Comparison of ICFS scores
| Esketamine group (n=32) | Control group (n=30) | P value | |
|---|---|---|---|
| Preoperative 1 d | 50.47±3.80 | 51.13±3.22 | 0.410 |
| Postoperative 1 d | 97.69±5.18a | 99.60±4.88a | 0.140 |
| Postoperative 3 d | 83.63±9.62a,b | 91.27±8.71a,b | 0.002 |
| Postoperative 7 d | 71.56±9.79a,b,c | 78.27±9.69a,b,c | 0.009 |
Data are presented as the mean ± SD. ICFS, Identity-Consequence Fatigue Scale.
Compared with Preoperative 1 d;
P<0.05.
Compared with Postoperative 1 d;
P<0.05.
Compared with Postoperative 3 d;
P<0.05.
Figure 2.
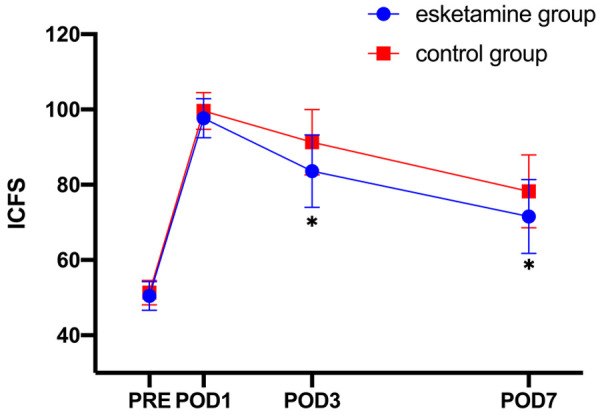
ICFS score at different time points. ICFS, Identity-Consequence Fatigue Scale; PRE, Preoperative; POD, Postoperative Day. A repeated-measures ANOVA followed by Bonferroni’s post hoc test was used. The horizontal axis is the time, and the vertical axis is the ICFS score. The data are expressed as the mean ± SD. *Compared with the control group, P<0.05.
Hand grip strength
As summarized in Table 3, esketamine had no statistically significant effect on hand grip strength on the day before surgery (F=0.023, P=0.879), POD1 (F=0.877, P=0.353), POD3 (F=0.015, P=0.904), and POD7 (F=0.265, P=0.609).
Table 3.
Comparison of hand grip strength
| Esketamine group (n=32) | Control group (n=30) | P value | |
|---|---|---|---|
| Preoperative 1 d | 27.05±7.76 | 27.34±6.81 | 0.879 |
| Postoperative 1 d | 19.50±5.91 | 18.17±5.70 | 0.353 |
| Postoperative 3 d | 22.99±7.45 | 23.20±6.56 | 0.904 |
| Postoperative 7 d | 25.20±7.22 | 26.11±6.62 | 0.609 |
Data are presented as the mean ± SD.
Positive and negative emotion scores
We also found that there was no statistical difference in emotional state between the two groups one day before (positive affect: P=0.985; negative affect: P=0.630) or after surgery (positive affect: P=0.266; negative affect: P=0.543). Nevertheless, compared with the control group, the esketamine group showed increased positive emotion score (F=4.685, P=0.034) but decreased negative emotion score (F=6.391, P=0.014) on POD3. The negative emotion was also significantly ameliorated on POD7 (F=8.006, P=0.006) in the esketamine group, while the positive emotion score presented a trend of improvement but was not significantly different (F=3.203, P=0.079) (Table 4).
Table 4.
Comparison of PANAS scores
| Time | Esketamine group (n=32) | Control group (n=30) | P value | |
|---|---|---|---|---|
| Positive Affect | Preoperative 1 d | 24.88±5.06 | 24.90±5.20 | 0.985 |
| Postoperative 1 d | 11.53±1.02a | 11.23±1.08a | 0.266 | |
| Postoperative 3 d | 21.56±4.56a,b | 19.20±4.00a,b | 0.034 | |
| Postoperative 7 d | 24.81±5.56b,c | 22.40±5.02a,b,c | 0.079 | |
| Negative Affect | Preoperative 1 d | 26.84±3.92 | 26.3±4.90 | 0.630 |
| Postoperative 1 d | 34.34±3.08a | 34.83±3.23a | 0.543 | |
| Postoperative 3 d | 30.28±2.90a,b | 32.67±4.42a | 0.014 | |
| Postoperative 7 d | 28.31±4.00a,b,c | 31.27±4.22a,b,c | 0.006 |
Data are presented as the mean ± SD. The interaction between group and time had statistical significance on positive affect (F=4.391, P=0.026), and a simple effect was tested.
Compared with preoperative 1 d;
P<0.05.
Compared with postoperative 1 d;
P<0.05.
Compared with Postoperative 3 d;
P<0.05.
NLR, PLR, AIS and NRS scores
Our results showed that esketamine had no effect on the scores of postoperative NRS (Fgroup*time=2.475, P=0.067; Fgroup=0.064, P=0.801; Ftime=325.758, P<0.001) and AIS (Fgroup*time=2.199, P=0.115; Fgroup=0.950, P=0.334; Ftime=2225.180, P<0.001). There was also no significant difference on the scores of NLR (P=0.176) and PLR (P=0.346) between the two groups on POD1 (Table 5).
Table 5.
Comparison of AIS score, NRS score, NLR and PLR
| Time | Esketamine group (n=32) | Control group (n=30) | P value | |
|---|---|---|---|---|
| AIS score | Postoperative 1 d | 10.97±1.66 | 11.13±1.22 | 0.660 |
| Postoperative 3 d | 8.22±1.39 | 8.77±1.14 | 0.095 | |
| Postoperative 7 d | 5.84±1.35 | 6.07±1.17 | 0.491 | |
| NRS score | Postoperative 1 d | 3.91±0.59 | 3.90±0.54 | 0.966 |
| Postoperative 2 d | 3.16±0.45 | 3.30±0.60 | 0.258 | |
| Postoperative 3 d | 2.56±0.62 | 2.73±0.58 | 0.269 | |
| Postoperative 7 d | 1.81±0.74 | 1.63±0.71 | 0.337 | |
| NLR | Preoprative | 2.12 (1.58 to 2.62) | 2.21 (1.66 to 2.95) | 0.464 |
| Postoperative 1 d | 10.91 (8.28 to 14.69) | 9.34 (6.40 to 13.00) | 0.176 | |
| PLR | Preoprative | 153.86 (120.28 to 178.88) | 156.18 (124.94 to 182.21) | 0.602 |
| Postoperative 1 d | 274.72±88.83 | 252.40±96.06 | 0.346 |
Data are presented as the mean ± SD, median (Q1 to Q3); AIS, Athens Insomnia Scale; NRS, Numeric Rating Scale; PLR, platelet to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio. NRS score: Fgroup*time=2.475, P=0.067; Fgroup=0.064, P=0.801; Ftime=325.758, P<0.001. AIS score: Fgroup*time=2.199, P=0.115; Fgroup=0.950, P=0.334; Ftime=2225.180, P<0.001.
Adverse events and other indicators
We also assessed the effect of esketamine on other parameters. For example, we found that the esketamine group consumed less remifentanil (P=0.048) and had a significantly shorter first flatus time (P=0.044). However, there were no differences in propofol dosage, extubation time, Ramsay score at extubation, length of postoperative hospital stay, or adverse effects such as nausea, vomiting, fever, dizziness, headache, agitation, nightmare and hallucination (P>0.05) between the two groups (Table 6).
Table 6.
Adverse events and other indicators
| Esketamine group (n=32) | Control group (n=30) | P value | |
|---|---|---|---|
| Propofol dosage (mg) | 479.72±134.68 | 462.47±129.05 | 0.609 |
| Remifentanil dosage (mg) | 1.96±0.68 | 2.28±0.55 | 0.048 |
| BIS | 47.06±2.33 | 47.63±2.79 | 0.384 |
| Blood loss | 80.00 (70.00 to 80.00) | 80.00 (77.50 to 80.00) | 0.231 |
| Operation time (min) | 200.06±45.07 | 198.8±48.00 | 0.915 |
| Awakening time (min) | 11.30±1.89 | 10.93±1.65 | 0.423 |
| Extubation time (min) | 12.38±2.15 | 12.57±2.06 | 0.722 |
| Ramsay score at extubation | 3.00 (2.25 to 3.00) | 3.00 (2.00 to 3.00) | 0.474 |
| Length of postoperative hospital stay (d) | 8.00 (8.00 to 10.00) | 8.0 (7.00 to 10.00) | 0.615 |
| Time to first flatus | 2.00 (2.00 to 3.00) | 3.00 (2.00 to 3.25) | 0.044 |
| Nausea (n) | 18 | 20 | 0.400 |
| Vomiting (n) | 7 | 8 | 0.660 |
| Fever (n) | 1 | 2 | 0.607 |
| Dizziness (n) | 1 | 1 | >0.999 |
| Headache (n) | 0 | 1 | 0.484 |
| Agitation (n) | 2 | 1 | >0.999 |
| Nightmares (n) | 0 | 0 | >0.999 |
| Hallucinations (n) | 0 | 0 | >0.999 |
Data are presented as the mean ± SD, median (Q1 to Q3), number; BIS, bispectral index.
Mediation analysis on POD3
To further investigate the correlation of esketamine with emotional state, and POF, we performed mediation analyses, which determined the importance of the direct, indirect, and overall impacts. A bootstrapped 95% confidence interval (CI) revealed that positive and negative emotions acted as mediators of POF, resulting in the indirect effects of 2.947 and 1.622, respectively. Sixty percent of the total variance in POF was accounted for by the indirect effect of emotional state (Table 7).
Table 7.
Mediating model examination by bootstrap on POD3
| Esketamine→POF | |||
|---|---|---|---|
|
| |||
| Effect | SE | 95% CI | |
| Direct effect | 3.072 | 1.486 | (0.097, 6.048) |
| Indirect effect | 4.569 | 1.919 | (0.926, 8.468) |
| Positive Affect | 2.947 | 1.568 | (0.199, 6.298) |
| Negative Affect | 1.622 | 0.945 | (0.164, 3.776) |
POF, postoperative fatigue; SE, standard error; CI, confidence interval.
Discussion
In this study, we analyzed the effect of the intraoperative use of esketamine in patients undergoing colorectal laparoscopic surgery to relieve POF. We found that a subanesthetic dose of esketamine could improve the ICFS score, reduce the dosage of remifentanil, upregulate positive emotions and downregulate negative emotions. Importantly, no adverse effects were noticed in patients with esketamine administration.
We observed that the ICFS scores were significantly lower in the esketamine group than in the control group on POD3 and POD7, although there was no difference on POD1. One possible explanation is that the POF degree on POD1 in patients with colorectal cancer is so severer that additional interventions, such as esketamine, only added minimal benefits. However, esketamine significantly improved fatigue at days 3 and 7 after surgery, which was consistent with a recent study on colorectal cancer patients with radical laparoscopic surgery, in which patients who received a single dose of intravenous ketamine 0.3 mg/kg before skin incision had reduced POF on POD3, POD5 and POD7 [14]. Nevertheless, Zhao et al. reported that in patients undergoing breast cancer surgery, a low dose of ketamine infusion (bolus of 0.5 mg/kg followed by 0.25 mg/kg/h until the end of surgery) had no effect on the ICFS score at 3 and 7 days after surgery [16], in contrast to our findings. A possible explanation is that the abdominal surgery we evaluated takes longer time as well as involves far more tissue injury and physiological stress than breast cancer surgery does.
POF can also manifest as varying degrees of muscle weakness caused by skeletal muscle oxidative stress and mitochondrial dysfunction after surgery. Hence, grip strength was used to reflect the postoperative skeletal muscle dysfunction. However, we did not observe a significant difference in hand grip strength between the two groups at 1, 3 and 7 days after surgery, suggesting that the low dosage of esketamine might not have a significant effect on muscle fatigue.
In our study, the ICFS scale combined with the grip strength assessment was used to evaluate the mental and peripheral fatigue. Our data showed that esketamine could improve the self-assessment mental fatigue but not the peripheral fatigue. Since the mechanism of POF remains unclear and POF is affected by perioperative factors such as pain, sleep, and mood [17], how esketamine works to improve fatigue after surgery is unknown, despite a report that ketamine exerts its anti-fatigue effect by altering glutamate metabolism, which may be associated with emotion regulation [13,18].
Emotional disorder after colorectal cancer surgery is not only caused by surgical stress but also by concerns about the disease and the treatment efficacy. Esketamine has rapid anti-depression effects in patients with mood disorders, but the perioperative effect of adjuncts used as general anesthetics on postoperative mood remains to be determined. A previous study compared the effects of propofol alone or in combination with low-dose ketamine on postoperative mood and found that the combination therapy could significantly improve postoperative mood [19]. Furthermore, this study suggested that the effect might be related to the antagonizing activity of ketamine against NMDA receptors and its anti-anxiety activity [19]. In our study, the positive emotion score on POD3 was higher in the intervention groups than in the control group, while the negative emotion scores were lower at days 3 and 7 after surgery. Previous studies have suggested that the mechanism by which esketamine regulates mood may be related to the reduction in the activation of negative emotional stimuli in the relevant brain regions that regulates the reward circuits in the limbic system and promotes the release of monoamine neurotransmitters such as dopamine [20,21]. In addition, the long-term effects of ketamine and esketamine have also been investigated [22,23]. For example, Zhao et al. found that the intraoperative administration of ketamine in neurosurgery patients improved the depression symptoms both on the third day after surgery and at the time of discharge [22]. Another study reported that, in cesarean section, the administration of 0.5 mg/kg esketamine after the delivery significantly reduced the incidence of postpartum depression on the 3rd and 30th days after surgery [23]. Nevertheless, how Esketamine improves the long-term mood health remains to be elucidated, although it was suggested that ketamine might cause long-term synaptic changes in the mesolimbic dopamine system [24].
Further mediation analysis on the data of POD3 showed that esketamine played an anti-fatigue role, which was related to the improvement of emotional functioning. Consistent with our findings, Salmon et al. have reported that postoperative emotional disorder is an important factor not only attributing to POF but also aggravating the degree of POF [4,25]. In addition, psychological factors can cause neuroendocrine disorders, including the dysfunction of the HPA axis and SAM system, which is one of the mechanisms of POF [26,17]. Furthermore, postoperative emotional distress will make patients inactive, manifested as a reduction in physical activity, which is one of the measurement dimensions of POF and negatively impacts the quality of recovery [27]. Hence, the improvement in postoperative mood would promote patients to actively cope with surgical trauma and encourage patients to feel energetic again, thereby reducing POF and helping postoperative recovery. Moreover, POF can also aggravate mood disorders, which form a vicious cycle of emotion and fatigue. Hence, Esketamine will help create a positive cycle and have a long-term effect by improving emotional health.
In this study, although subanesthetic esketamine had effects on postoperative emotion and fatigue, no effect was observed on postoperative pain scores, NLR, PLR and AIS score. However, Zhao et al. used a single dose of subanesthetic ketamine in colorectal cancer surgery and found that it had a lasting effect on POF after surgery [14]. The authors proposed that the mechanism was related to the anti-inflammatory action of ketamine, which is different from our observations in this study. One possible explanation is that our study only measured PLR and NLR on POD1 and did not continuously monitor the postoperative inflammation level, which was insufficient to reflect the postoperative inflammatory changes in patients. Alternatively, the effect of esketamine on inflammatory markers may be dose-dependent [28]. Indeed, it has been reported in modified radical mammary surgeries that NLRs were reduced by different doses of S-ketamine (0.25, 0.5, or 0.75 mg/kg/h) in a dose dependent manner [28]. In addition, S-ketamine had no effect on PLR, consistent with our results.
In terms of perioperative analgesia, our study found that esketamine could reduce the intraoperative remifentanil dosage but had no significant effect on the postoperative NRS pain score. The analgesic effect of esketamine is believed to result from blocking NMDA receptors and its binding to μ-opioid receptors, with a relatively short half-life, which could explain the reduction of remifentanil in the esketamine group [29]. However, NRS scores at 1, 2, 3 and 7 days after surgery showed no significant differences between the two groups. In fact, a similar result was also found in a previous study, in which the affective component of pain was significantly improved in the ketamine group after laparoscopic bariatric surgery, and the improvement was relatively durable [30]. The difference in the duration of the effects on emotion and pain by esketamine may be the results of different mechanisms involved. It is also possible that the NRS scale is too general to recognize subtle differences in postoperative pain, and esketamine may improve the emotional component of pain at higher degree.
Few studies have investigated the impact of esketamine on postoperative sleep disorders and the mechanism. Previous studies have shown that Ketamine influences circadian rhythms by controlling clock genes. In addition, a recent study found that 0.3 mg/kg/h perioperative esketamine infusion could reduce the incidence of postoperative sleep disturbance [31-33]. Nevertheless, this study did not find an effect of esketamine on postoperative sleep improvement, which may be due to the different dosages and administration times of esketamine tested in this study. It is worth noting that the time of surgery also affects circadian rhythms and may be a factor attributing to sleep disorders.
There are several limitations in this study. First, since there are many factors affecting POF, the sample size of this study is relatively small, which may affect the reliability of the study results to some extent. However, this study conducted stratified block randomization based on sex and surgical methods, and there was no significant difference in baseline data between the two groups, which somewhat balanced the major influencing factors. Second, in our study, only the per protocol analysis was used, which may partly exaggerate the effect of esketamine. Third, the neurocognitive function of the patients was not assessed in this study, although the assessment of attention dimension in the ICFS reflected the cognitive fatigue of the patients. Lastly, this study did not explore the specific molecular or neural circuit mechanisms of postoperative fatigue, which should be further clarified by neuroimaging and metabolomics in future studies.
Conclusions
In conclusion, we found that the perioperative use of esketamine could reduce POF in patients with colorectal cancer through improving the mental health of the patients.
Acknowledgements
This work was supported by grants from the Sixth “333” Talent Plan of Jiangsu Province and National Natural Science Foundation of China (31100801 and 81200858).
Disclosure of conflict of interest
None.
References
- 1.Rubin GJ, Hardy R, Hotopf M. A systematic review and meta-analysis of the incidence and severity of postoperative fatigue. J Psychosom Res. 2004;57:317–326. doi: 10.1016/S0022-3999(03)00615-9. [DOI] [PubMed] [Google Scholar]
- 2.Rajabiyazdi F, Alam R, Pal A, Montanez J, Law S, Pecorelli N, Watanabe Y, Chiavegato LD, Falconi M, Hirano S, Mayo NE, Lee L, Feldman LS, Fiore JF Jr. Understanding the meaning of recovery to patients undergoing abdominal surgery. JAMA Surg. 2021;156:758–765. doi: 10.1001/jamasurg.2021.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penner IK, Paul F. Fatigue as a symptom or comorbidity of neurological diseases. Nat Rev Neurol. 2017;13:662–675. doi: 10.1038/nrneurol.2017.117. [DOI] [PubMed] [Google Scholar]
- 4.Salmon P, Hall GM. Postoperative fatigue is a component of the emotional response to surgery: results of multivariate analysis. J Psychosom Res. 2001;50:325–335. doi: 10.1016/s0022-3999(01)00209-4. [DOI] [PubMed] [Google Scholar]
- 5.Arya N, Vaish A, Zhao K, Rao H. Neural mechanisms underlying breast cancer related fatigue: a systematic review of neuroimaging studies. Front Neurosci. 2021;15:735945. doi: 10.3389/fnins.2021.735945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Y, Ning H, Jiang X, Yang R, Song D, Yuan H. Metabolomics reveals hippocampal metabolic fluctuations of postoperative fatigue syndrome and anti-fatigue effect of Carthamus tinctorius L extract in rat model. Biomed Chromatogr. 2017;30:1052–1058. doi: 10.1002/bmc.3649. [DOI] [PubMed] [Google Scholar]
- 7.Feng LR, Fernández-Martínez JL, Zaal KJM, deAndrés-Galiana EJ, Wolff BS, Saligan LN. mGluR5 mediates post-radiotherapy fatigue development in cancer patients. Transl Psychiatry. 2018;8:110. doi: 10.1038/s41398-018-0161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowacka A, Borczyk M. Ketamine applications beyond anesthesia: a literature review. Eur J Pharmacol. 2019;860:172547. doi: 10.1016/j.ejphar.2019.172547. [DOI] [PubMed] [Google Scholar]
- 9.Muller J, Pentyala S, Dilger J, Pentyala S. Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther Adv Psychopharmacol. 2016;6:185–192. doi: 10.1177/2045125316631267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Huang J, Yang S, Cui C, Ye L, Wang SY, Yang GP, Pei Q. Pharmacokinetics and safety of esketamine in Chinese patients undergoing painless gastroscopy in comparison with ketamine: a randomized, open-label clinical study. Drug Des Devel Ther. 2019;13:4135–4144. doi: 10.2147/DDDT.S224553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu P, Li P, Li Q, Yan H, Shi X, Liu C, Zhang Y, Peng S. Effect of pretreatment of S-ketamine on postoperative depression for breast cancer patients. J Invest Surg. 2021;34:883–888. doi: 10.1080/08941939.2019.1710626. [DOI] [PubMed] [Google Scholar]
- 12.Saligan LN, Luckenbaugh DA, Slonena EE, Machado-Vieira R, Zarate CA Jr. An assessment of the anti-fatigue effects of ketamine from a double-blind, placebo-controlled, crossover study in bipolar disorder. J Affect Disord. 2016;194:115–119. doi: 10.1016/j.jad.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saligan LN, Farmer C, Ballard ED, Kadriu B, Zarate CA Jr. Disentangling the association of depression on the anti-fatigue effects of ketamine. J Affect Disord. 2019;244:42–45. doi: 10.1016/j.jad.2018.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao L, Zhang H, Cheng H. Effect of a single sub-dose of ketamine on postoperative fatigue syndrome in colorectal cancer patients undergoing radical laparoscopic surgery: a double-blind, pilot study. J Affect Disord. 2022;312:146–151. doi: 10.1016/j.jad.2022.06.029. [DOI] [PubMed] [Google Scholar]
- 15.Paddison JS, Booth RJ, Hill AG, Cameron LD. Comprehensive assessment of peri-operative fatigue: development of the identity-consequence fatigue scale. J Psychosom Res. 2006;60:615–622. doi: 10.1016/j.jpsychores.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Z, Xu Q, Chen Y, Liu C, Zhang F, Han Y, Cao J. The effect of low-dose ketamine on postoperative quality of recovery in patients undergoing breast cancer surgery: a randomised, placebo-controlled trial. Int J Clin Pract. 2021;75:e15010. doi: 10.1111/ijcp.15010. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, Chu S, Gao Y, Ai Q, Liu Y, Li X, Chen N. A narrative review of cancer-related fatigue (CRF) and its possible pathogenesis. Cells. 2019;8:738. doi: 10.3390/cells8070738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nourbakhsh B, Revirajan N, Waubant E. Association between glutamate blockade and fatigue in patients with multiple sclerosis. JAMA Neurol. 2015;72:1374–1375. doi: 10.1001/jamaneurol.2015.2332. [DOI] [PubMed] [Google Scholar]
- 19.Mortero RF, Clark LD, Tolan MM, Metz RJ, Tsueda K, Sheppard RA. The effects of small-dose ketamine on propofol sedation: respiration, postoperative mood, perception, cognition, and pain. Anesth Analg. 2001;92:1465–1469. doi: 10.1097/00000539-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Van Hedger K, Mayo LM, Bershad AK, Madray R, de Wit H. Effects of acute drug administration on emotion: a review of pharmacological MRI studies. Curr Addict Rep. 2021;8:181–193. doi: 10.1007/s40429-021-00362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Argiriadou H, Himmelseher S, Papagiannopoulou P, Georgiou M, Kanakoudis F, Giala M, Kochs E. Improvement of pain treatment after major abdominal surgery by intravenous S+-ketamine. Anesth Analg. 2004;98:1413–8. doi: 10.1213/01.ane.0000111204.31815.2d. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Sun W, Zhang G, Wang A, Lin S, Chan MTV, Peng Y, Wang G, Han R. Ketamine alleviates depressive symptoms in patients undergoing intracranial tumor resection: a randomized controlled trial. Anesth Analg. 2021;133:1588–1597. doi: 10.1213/ANE.0000000000005752. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Xiao M, Sun H, Zhang P. A study on the preventive effect of esketamine on postpartum depression (PPD) after cesarean section. Comput Math Methods Med. 2022;2022:1524198. doi: 10.1155/2022/1524198. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Cardona-Acosta AM, Bolaños-Guzmán CA. Role of the mesolimbic dopamine pathway in the antidepressant effects of ketamine. Neuropharmacology. 2023;225:109374. doi: 10.1016/j.neuropharm.2022.109374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein D, Bennett B, Friedlander M, Davenport T, Hickie I, Lloyd A. Fatigue states after cancer treatment occur both in association with, and independent of, mood disorder: a longitudinal study. BMC Cancer. 2006;6:240. doi: 10.1186/1471-2407-6-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garssen B, Boomsma MF, Beelen RH. Psychological factors in immunomodulation induced by cancer surgery: a review. Biol Psychol. 2010;85:1–13. doi: 10.1016/j.biopsycho.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Poort H, Peters MEWJ, van der Graaf WTA, Nieuwkerk PT, van de Wouw AJ, Nijhuis-van der Sanden MWG, Bleijenberg G, Verhagen CAHHVM, Knoop H. Cognitive behavioral therapy or graded exercise therapy compared with usual care for severe fatigue in patients with advanced cancer during treatment: a randomized controlled trial. Ann Oncol. 2020;31:115–122. doi: 10.1016/j.annonc.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Ma Q, Li W, Li X, Chen X. S-Ketamine attenuates inflammatory effect and modulates the immune response in patients undergoing modified radical mastectomy: a prospective randomized controlled trial. Front Pharmacol. 2023;14:1128924. doi: 10.3389/fphar.2023.1128924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonaventura J, Lam S, Carlton M, Boehm MA, Gomez JL, Solís O, Sánchez-Soto M, Morris PJ, Fredriksson I, Thomas CJ, Sibley DR, Shaham Y, Zarate CA Jr, Michaelides M. Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol Psychiatry. 2021;26:6704–6722. doi: 10.1038/s41380-021-01093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Echevarria GC, Doan L, Ekasumara N, Calvino S, Chae F, Martinez E, Robinson E, Cuff G, Franco L, Muntyan I, Kurian M, Schwack BF, Bedrosian AS, Fielding GA, Ren-Fielding CJ. Effects of a single subanaesthetic dose of ketamine on pain and mood after laparoscopic bariatric surgery: a randomised double-blind placebo controlled study. Eur J Anaesthesiol. 2019;36:16–24. doi: 10.1097/EJA.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 31.Sato S, Bunney B, Mendoza-Viveros L, Bunney W, Borrelli E, Sassone-Corsi P, Orozco-Solis R. Rapid-acting antidepressants and the circadian clock. Neuropsychopharmacology. 2022;47:805–816. doi: 10.1038/s41386-021-01241-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orozco-Solis R, Montellier E, Aguilar-Arnal L, Sato S, Vawter MP, Bunney BG, Bunney WE, Sassone-Corsi P. A circadian genomic signature common to ketamine and sleep deprivation in the anterior cingulate cortex. Biol Psychiatry. 2017;82:351–360. doi: 10.1016/j.biopsych.2017.02.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu D, Wang XM, Yang JJ, Chen S, Yue CB, Hashimoto K, Yang JJ. Effect of intraoperative esketamine infusion on postoperative sleep disturbance after gynecological laparoscopy: a randomized clinical trial. JAMA Netw Open. 2022;5:e2244514. doi: 10.1001/jamanetworkopen.2022.44514. [DOI] [PMC free article] [PubMed] [Google Scholar]


