Abstract
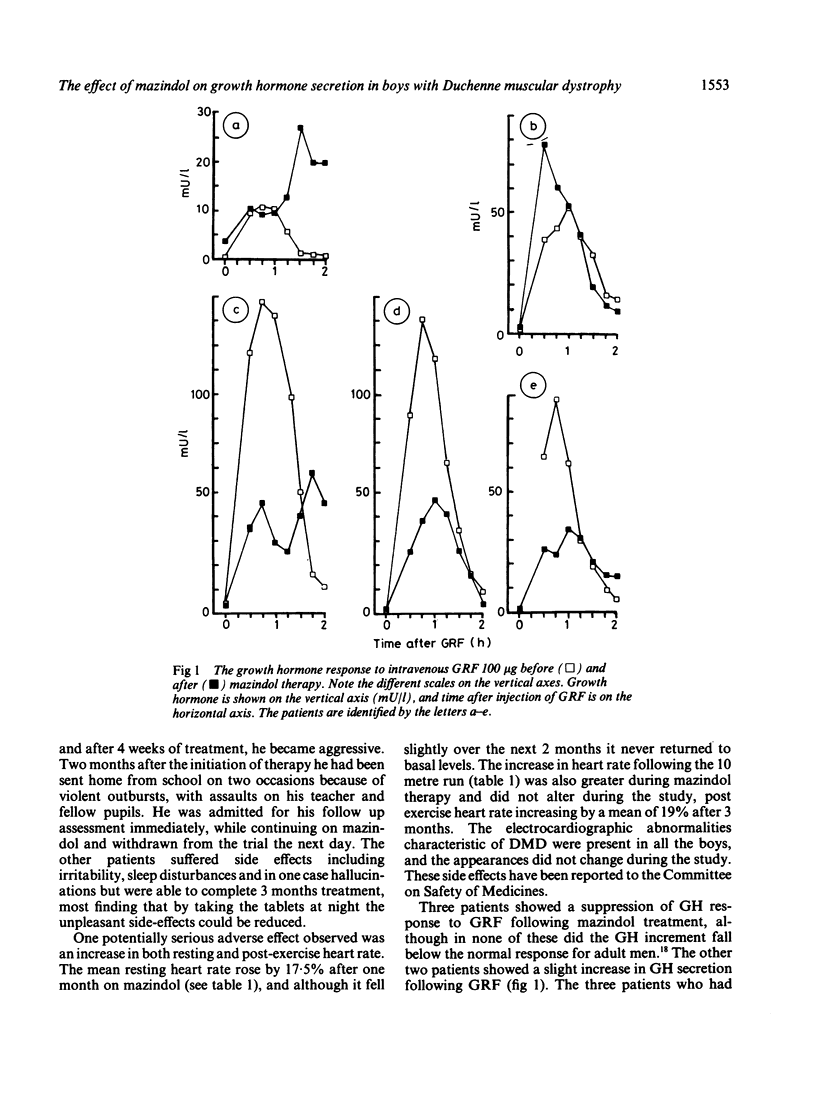
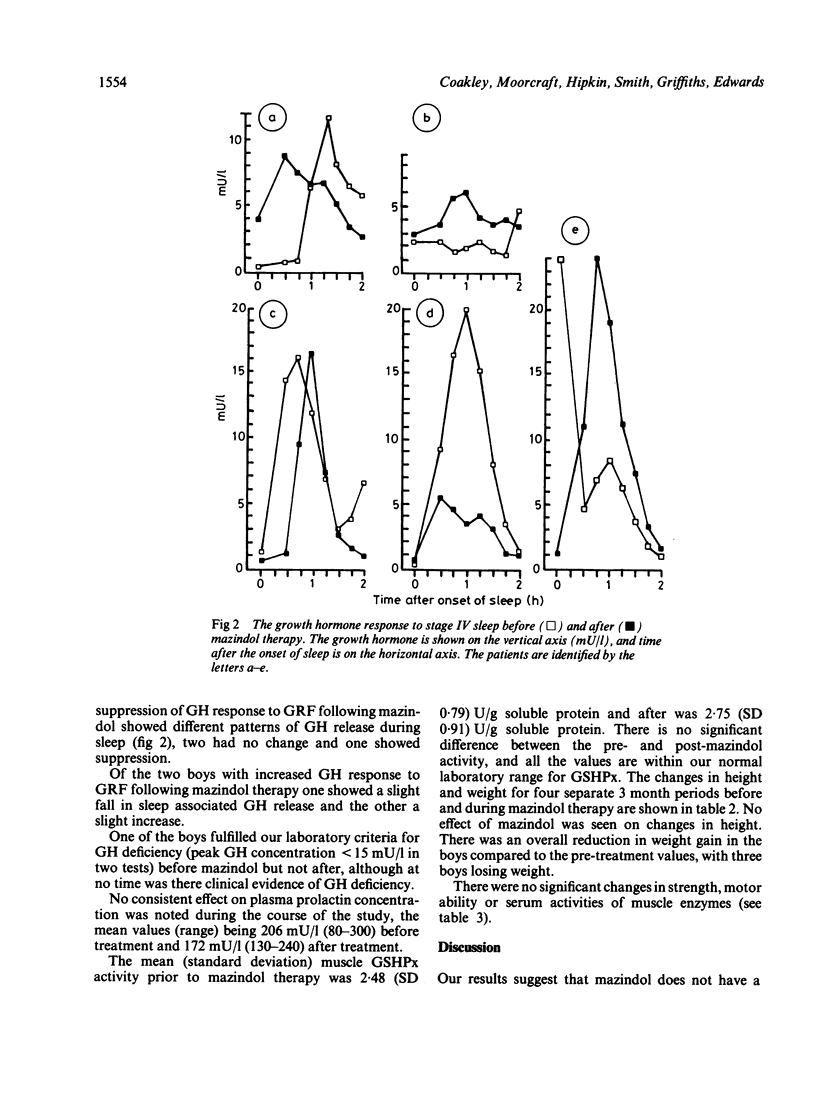
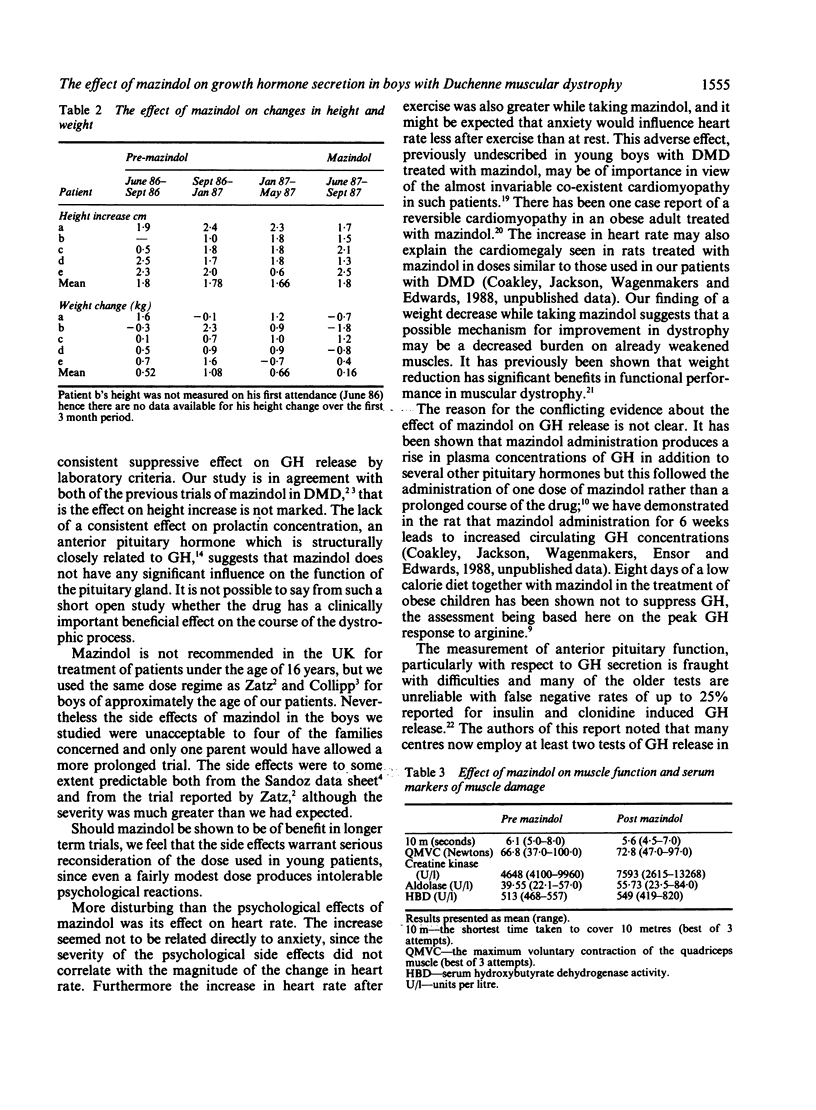
Mazindol has been reported to improve muscle function in Duchenne muscular dystrophy (DMD) by virtue of its growth hormone (GH) suppression. The effects were studied on GH secretion (in response to growth hormone releasing factor and sleep) of mazindol 2 mg daily for 3 months in five boys with DMD. No consistent change was found following mazindol therapy. Adverse effects were noted in all the boys which may preclude long term use of mazindol in DMD.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baritussio A., Enzi G., Rigon G., Molinari M., Inelmen E. M., Crepaldi G. Effect of anorexiant drugs on growth hormone secretion in obese children. Pharmacol Res Commun. 1978 Jun;10(6):529–540. doi: 10.1016/s0031-6989(78)80051-4. [DOI] [PubMed] [Google Scholar]
- Belchetz P. E., Weldon S. F., Davis J. C., Diver M. J., Smith C. S., Harris F. Potential uses of human pancreatic growth hormone-releasing factor 1-44 amide. Clin Endocrinol (Oxf) 1984 Aug;21(2):201–208. doi: 10.1111/j.1365-2265.1984.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Beutler E., Blume K. G., Kaplan J. C., Löhr G. W., Ramot B., Valentine W. N. International Committee for Standardization in Haematology: recommended methods for red-cell enzyme analysis. Br J Haematol. 1977 Feb;35(2):331–340. doi: 10.1111/j.1365-2141.1977.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Chyatte S. B., Rudman D., Patterson J. H., Gerron G. G., O'Beirne I., Barlow J., Jordan A., Shavin J. S. Human growth hormone and estrogens in boys with Duchenne muscular dystrophy. Arch Phys Med Rehabil. 1973 Jun;54(6):248–253. [PubMed] [Google Scholar]
- Collipp P. J., Kelemen J., Chen S. Y., Castro-Magana M., Angulo M., Derenoncourt A. Growth hormone inhibition causes increased selenium levels in Duchenne muscular dystrophy: a possible new approach to therapy. J Med Genet. 1984 Aug;21(4):254–256. doi: 10.1136/jmg.21.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrichson P., Coakley J., Smith P. E., Griffiths R. D., Helliwell T. R., Edwards R. H. Conchotome and needle percutaneous biopsy of skeletal muscle. J Neurol Neurosurg Psychiatry. 1987 Nov;50(11):1461–1467. doi: 10.1136/jnnp.50.11.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. H., Round J. M., Jackson M. J., Griffiths R. D., Lilburn M. F. Weight reduction in boys with muscular dystrophy. Dev Med Child Neurol. 1984 Jun;26(3):384–390. doi: 10.1111/j.1469-8749.1984.tb04457.x. [DOI] [PubMed] [Google Scholar]
- Gebre-Medhin M., Gustavson K. H., Gamstorp I., Plantin L. O. Selenium supplementation in X-linked muscular dystrophy. Effects on erythrocyte and serum selenium and on erythrocyte glutathione peroxidase activity. Acta Paediatr Scand. 1985 Nov;74(6):886–890. doi: 10.1111/j.1651-2227.1985.tb10053.x. [DOI] [PubMed] [Google Scholar]
- Gillis D., Wengrower D., Witztum E., Leitersdorf E. Fenfluramine and mazindol: acute reversible cardiomyopathy associated with their use. Int J Psychiatry Med. 1985;15(2):197–200. doi: 10.2190/yq38-eag3-319r-a8rl. [DOI] [PubMed] [Google Scholar]
- Grossman A., Savage M. O., Besser G. M. Growth hormone releasing hormone. Clin Endocrinol Metab. 1986 Aug;15(3):607–627. doi: 10.1016/s0300-595x(86)80012-3. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Cabbat F. S., Manzino L., Babington R. G., Houlihan W. J. Unexpected differences between mazindol and its homologs on biochemical and behavioral responses. J Pharmacol Exp Ther. 1981 Jun;217(3):745–749. [PubMed] [Google Scholar]
- Jackson M. J., Jones D. A., Edwards R. H. Techniques for studying free radical damage in muscular dystrophy. Med Biol. 1984;62(2):135–138. [PubMed] [Google Scholar]
- King J. M., Price D. A. Sleep-induced growth hormone release--evaluation of a simple test for clinical use. Arch Dis Child. 1983 Mar;58(3):220–222. doi: 10.1136/adc.58.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxley R. T., 3rd, Brooke M. H., Fenichel G. M., Mendell J. R., Griggs R. C., Miller J. P., Province M. A., Patterson V. Clinical investigation in Duchenne dystrophy. VI. Double-blind controlled trial of nifedipine. Muscle Nerve. 1987 Jan;10(1):22–33. doi: 10.1002/mus.880100106. [DOI] [PubMed] [Google Scholar]
- Sekiya K., Okajima T., Kato K., Ibayashi H. Acute effects of mazindol on the secretion of ACTH, beta-lipotropin, beta-endorphin and cortisol in man. Endocrinol Jpn. 1984 Oct;31(5):523–528. doi: 10.1507/endocrj1954.31.523. [DOI] [PubMed] [Google Scholar]
- Smith P. E., Calverley P. M., Edwards R. H., Evans G. A., Campbell E. J. Practical problems in the respiratory care of patients with muscular dystrophy. N Engl J Med. 1987 May 7;316(19):1197–1205. doi: 10.1056/NEJM198705073161906. [DOI] [PubMed] [Google Scholar]
- Zatz M., Betti R. T. Benign Duchenne muscular dystrophy in a patient with growth hormone deficiency: a five years follow-up. Am J Med Genet. 1986 Jul;24(3):567–572. doi: 10.1002/ajmg.1320240323. [DOI] [PubMed] [Google Scholar]
- Zatz M., Betti R. T., Frota-Pessoa O. Treatment of Duchenne muscular dystrophy with growth hormone inhibitors. Am J Med Genet. 1986 Jul;24(3):549–566. doi: 10.1002/ajmg.1320240322. [DOI] [PubMed] [Google Scholar]


