Visual Abstract
Abstract
With growing indications for chimeric antigen receptor (CAR) T-cell therapy, toxicity profiles are evolving. There is an urgent and unmet need of approaches to optimally manage emerging adverse events that extend beyond the standard paradigm of cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome (ICANS). Although management guidelines exist for ICANS, there is little guidance on how to approach patients with neurologic comorbidities, and how to manage rare neurotoxicity presentations, such as CAR T-cell therapy–related cerebral edema, severe motor complications or late-onset neurotoxicity. In this study, we present 3 scenarios of patients treated with CAR T cells who develop unique types of neurotoxicity, and we describe an approach for the evaluation and management based on experience because objective data are limited. The goal of this study is to develop an awareness of emerging and unusual complications, discuss treatment approaches, and help institutions and health care providers establish frameworks to navigate how to best address unusual neurotoxicities to ultimately improve patient outcomes.
With 6 chimeric antigen receptor (CAR) T-cell products approved by the US Food and Drug Administration and many more showing promise in clinical trials, the need for hematologists to be aware of challenging complications and know how to manage them in routine practice is paramount. Introduced by Associate Editor Helen E. Heslop, this How I Treat series highlights several key complications, reviews current understanding of their etiologies, and provides guidance on therapies through discussion of illustrative cases.
Introduction
Neurologic events are a common complication of the chimeric antigen receptor (CAR) T-cell therapy. By expert consensus, a readily recognizable constellation of neurologic clinical features called immune effector cell-associated neurotoxicity syndrome (ICANS) has been defined.1 ICANS consists of an acute, typically monophasic constellation of mental status changes, particularly language disturbance that may progress to more severe manifestations, such as seizures, coma, and cerebral edema.1 Reported risk factors include patient-related factors, such as young age, high tumor burden, and a history of early-onset and/or high-grade cytokine release syndrome (CRS).2,3 The type of costimulatory domain (CD28 vs 4-1BB) in the CAR construct and lymphodepletion regimens have been implicated.4,5 Pretreatment inflammatory markers including interleukin-6 (IL-6), C-reactive protein, and fibrinogen are associated with high ICANS risk.6,7 After the infusion, ICANS is associated with higher peak C-reactive protein; ferritin; proinflammatory cytokines such as interferon-gamma, IL-6, IL-1, IL-10, MCP-1; markers of endothelial activation;7,8 and CAR expansion, compared with the levels before infusion.8, 9, 10, 11, 12, 13 Currently available consensus guidelines and institution-specific interventions14, 15, 16, 17, 18 do not include strategies for managing rarer neurologic complications observed with the commercial products. As new CAR constructs enter clinical trials for different diseases, the neurotoxicity profile of CAR T-cell therapy may continue to evolve.19, 20, 21 In this study, we use 3 unique clinical scenarios of CAR T-cell therapy–related neurotoxicity to illustrate how to approach workup and management.
Case 1
A 6-year-old girl with acute lymphoblastic leukemia (ALL) presents for the consideration of tisagenlecleucel therapy after her third relapse. She has a history of seizures in the setting of sepsis. Her most recent relapse was isolated to the central nervous system (CNS), and she received bridging therapy (including intrathecal methotrexate) to reduce the CNS disease burden. Three weeks before the presentation, she developed a rapid-onset weakness in the left arm and face. Her magnetic resonance imaging (MRI) showed an area of diffusion restriction in the right hemispheric white matter, consistent with methotrexate neurotoxicity. The weakness resolved, but the referring team would have liked further guidance on how to optimize the safety of CAR T-cell therapy.
Neurologic comorbidities in ALL are common. They include CNS disease, chemotherapy, and radiation complications, such as methotrexate toxicity, peripheral neuropathy, seizures, cognitive impairment, and headache.8,22 Although there is mixed evidence on whether neurologic comorbidities increase the overall risk of ICANS,22,23 CAR T-cell treatment often triggers the worsening of chronic conditions. In the context of our patient’s history, we were concerned about breakthrough seizures, so we increased her maintenance dose of levetiracetam. We chose antiseizure medications, such as levetiracetam or lacosamide that could be easily converted to be administered IV. Another concern for our patient was the focal lesion from methotrexate toxicity because the areas of chronic brain injuries (eg, from prior hemorrhage) have been associated with focal inflammation during ICANS.8,22,23
Multiple tools have been developed to stratify the risk of ICANS using clinical markers.6,7,13 None have sufficient sensitivity and specificity at the prelymphodepletion time to guide our decision-making. There is good evidence that CNS leukemia or lymphoma can be successfully targeted by CAR T cells,24, 25, 26 with no increased incidence of ICANS.27 Thus, we recommend that neurologic comorbidities and/or active CNS disease should not preclude eligibility for CAR T-cell therapy. Bridging therapy can be used to decrease the CNS disease burden. Patients with serious preexisting neurologic comorbidities should receive a neurology consultation during their pre-CAR T-cell therapy evaluation to optimize preventive medications and record a baseline neurologic exam (Table 1). We recommend baseline surveillance studies, such as electroencephalography (EEG) and brain MRI, and inpatient admission for observation.
Table 1.
Therapeutic approaches for CAR T-cell therapy–related neurotoxicity
| Therapy | Timing | Comments |
|---|---|---|
| IV corticosteroids | Therapeutic14, 15, 16 | Among patients who received corticosteroids at the onset of grade 3 (ZUMA-1 C 1+2) and earlier for grade 1 NEs (C4), rates of grade ≥3 NEs were 28% and 17%, respectively. |
| IV corticosteroids | Prophylactic28,29 | The incidence of grade ≥3 NEs in ZUMA-1 C6 (13%) was comparable to that of C4 (17%), and both rates were comparably lower than C1+2 (28%). In ZUMA-1 C6, patients received once-daily oral dexamethasone at 10 mg plus C4 management on d 0 (before axi-cel), 1, and 2. The updated follow-up included a few additional NEs and the total incidence of grade ≥3 NEs was 15%. |
| Anakinra | Therapeutic30, 31, 32 | Four of 6 patients who received anakinra for the management of high-grade ICANS experienced clinical benefit.32 In 9 out of additional 14 patients with ICANS, the reduction of peak ICANS could be reached within 1 day after the last anakinra dose.30 Twenty three patients treated with anakinra for steroid-refractory ICANS. CRS/ICANS improvement was observed among 73% of patients; higher response rates in patients receiving higher doses (8 mg/kg per d).33 |
| Anakinra | Prophylactic34 | #NCT04148430: 31 patients received anakinra starting on d 2 or after 2 documented fevers of ≥38.5°C before day 2, whichever time point was earlier. The overall severe ICANS rate was 6%. Other ongoing clinical trials include #NCT04432506, #NCT04359784, #NCT04150913, and #NCT04205838. |
| Tocilizumab | Therapeutic | Approved for use in CRS but is not effective for isolated neurotoxicity and may be associated with worsening neurotoxicity.27 |
| Tocilizumab | Prophylactic35 | Not recommended. ZUMA-1 C3, which incorporated prophylactic tocilizumab on d 2 after axi-cel had grade ≥3 NEs 41% compared with 28% in C1+2.35 |
| Siltuximab | Therapeutic | Ongoing clinical trial #NCT04975555. No data available. |
| Siltuximab | Prophylactic | Ongoing clinical trial #NCT05665725. No data available. |
| Intrathecal therapy | Therapeutic36,37 | 2 cases were treated with a rapid and sustained resolution of ICANS.10 Use with other immunosuppressive agents may be associated with sepsis. |
We provided the references, when available, for using a specific approach as a therapeutic or prophylactic strategy, otherwise, the use of these agents is anecdotal in nature. Please note that ZUMA-1 cohorts do not represent randomized data.
C, cohort; NEs, neurologic events.
Case 1 (continued)
In the neurology consultation, the doctor recommended a repeat MRI before CAR T-cell infusion, the results of which showed resolution of diffusion restriction with residual increased white matter fluid-attenuated inversion recovery (FLAIR), indicative of chronic injury. The neurologic exam showed normal results. On day 4, she developed grade 3 CRS that was successfully treated with tocilizumab and dexamethasone. However, on day 6, she developed confusion, and in the evening, she had a 1-minute seizure that started with shaking of the left arm and evolved into a generalized convulsive seizure. She required intubation and mechanical ventilation because of a persistently low level of consciousness. Report of head computed tomography (CT) was normal, the opening pressure on lumbar puncture was 25 mm Hg, cerebrospinal fluid (CSF) protein was 245 mg/dL, white blood cell count was 33 × 103/μL, and the result of rapid viral polymerase chain reaction was negative. Continuous EEG showed a diffusely slow background but no more seizures. MRI showed T2 hyperintensity and diffusion restriction in the bilateral thalami and leptomeningeal FLAIR hyperintensity. She was treated with methylprednisolone 30 mg/kg and started on anakinra (2 mg/kg every 6 hours). The next morning (day 7), the patient remained comatose without sedation, and the head CT showed possible early cerebral edema. We continued high-dose methylprednisolone and anakinra and added hypertonic saline and a pentobarbital infusion to decrease cerebral energy demand. Head CT on day 9 showed improvement of the cerebral edema, pentobarbital was weaned, and steroids were converted to dexamethasone at a dose of 10 mg twice daily for 3 more days. On day 11, the patient was extubated and within a few days returned to normal neurologic function. She achieved a complete, durable ALL remission.
For patients with severe and/or refractory ICANS, we start high-dose steroids and anakinra with or without siltuximab. We avoid tocilizumab for the treatment of isolated ICANS because it does not appear to reverse neurologic symptoms, and there is concern that it could worsen neurotoxicity.17,38,39 Although high-quality evidence is not yet available, anakinra has become a mainstay therapy for refractory ICANS based on preclinical data and clinical safety experience.30, 31, 32,40 Institution-dependent approaches may favor the use of anakinra for severe ICANS, especially in the pediatric patient population or life-threatening/refractory grade 3 ICANS in the adult setting. For patients with rapidly declining neurologic status and/or signs of increased intracranial pressure, we initiate the treatment of cerebral edema. Imaging findings often lag behind clinical progression in these cases. We model neuroprotective and intracranial pressure-lowering interventions based on standard neurocritical care practice, including normotension, normothermia, normocarbia, aggressive seizure control, raising the head of the bed, hypertonic saline, and, in selected cases, pentobarbital coma. There is no clear role for neurosurgical intervention in the setting of maximal medical therapy. If the patient stabilizes within the first 12 or 24 hours without progressing to herniation, the typical course is monophasic with continued improvement. Continuous EEG monitoring can be very helpful to rule out seizures as EEG background abnormalities closely correlate with ICANS severity.41
In cases of severe ICANS without overt cerebral edema, we have observed that CSF removal is often accompanied by symptomatic improvement. We perform lumbar punctures if it is safe and transfuse platelets if needed. We include opening pressure measurements as well as differential cell counts, protein level measurements, and laboratory tests to rule out infection. Intrathecal immunomodulating therapies, such as corticosteroids and chemotherapy, have been advocated by some groups.36,37 We do not typically use this approach for ICANS because there is little evidence for T-cell proinflammatory cytokine production within the CNS, and CSF cytokine levels largely mirror levels in the blood.42 In addition, a more permeable blood-brain barrier (BBB) during ICANS should allow good drug access from the circulation.43 If there is a concern for cerebral edema or raised intracranial pressure, intrathecal therapy is probably not safe or effective. CSF circulation would be expected to be poor under these conditions, leading to the suboptimal targeting of the neurovascular unit in the brain. Finally, outcomes from 1 case series raise the concern that intrathecal treatments may be associated with grade 5 infection among patients who are also treated with systemic corticosteroids and anakinra.37
Case 2
A 40-year-old woman with diffuse large B-cell lymphoma (DLBCL) was referred for the consideration of axicabtagene ciloleucel (axi-cel). After having progression in bone and lymph nodes with chemotherapy, she was treated with radiotherapy (RT) for symptomatic thoracic epidural disease at T7 6 months before axi-cel, followed by the anti-programmed death-1 (anti-PD1) inhibitor nivolumab for progressive osseous disease. She developed new sacral numbness and pain and was found to have a new disease in her sacrococcygeal bones upon being treated with bridging radiation 1 month before the referral for axi-cel. She was examined by the neurologist for pretreatment, and the results of the neurologic exam were normal, except for a mild sensory loss in the sacrum. A repeat spine MRI was recommended, given her history of the symptomatic epidural disease. The scan showed resolved thoracic epidural disease and very good partial response in the sacrum RT field. The MRI brain report was normal. Will radiation increase her chance of developing ICANS?
Despite the evidence that CD19-CAR T cells are well tolerated with objective responses with no increased neurotoxicity,25,26 in our experience, poor outcomes are common among patients with advanced CNS lymphoma. Therefore, we attempt to limit CNS disease burden before CAR T-cell therapy to increase the chance of disease clearance and reduce toxicity. We pay special attention to lesions that might impair CSF flow as immune-related pseudoprogressions resulting in the transient enlargement of the lesion that could occur from CAR-associated inflammation. This emerging phenomenon has been termed as tumor inflammation–associated neurotoxicity (TIAN).20,44 TIAN is likely mechanistically different from ICANS in that it is directly attributable to focal inflammation at the tumor site, as opposed to the more global brain dysfunctions observed in ICANS.
Bridging therapy may include radiation to the CNS or adjacent structures. Anecdotal evidence suggests that focal injury from radiation or other treatment-related insults may make those areas more vulnerable to ICANS-related inflammation. In a pediatric study, a patient with preexisting radiation injury to the cerebellum developed cerebellar edema upon CD19-CAR T-cell treatment, leading to acute obstructive hydrocephalus requiring temporary CSF diversion.22 In another case report, the CAR T-cell targeting of leukemic infiltrates in the optic nerve and retina of a child led to retinal detachment, likely because of focal inflammation akin to pseudoprogression.45 In larger studies, there did not appear to be any association of bridging therapy use, including RT, with severe CRS or ICANS.46,47 In general, we feel that even a high CNS tumor burden should not preclude eligibility for CAR T-cell therapy. For patients with a history of prior or current CNS involvement, we recommend reimaging areas of the neuroaxis that were sites of disease. A baseline neurologic exam in these situations is important (Table 2). Baseline CSF sampling should be performed if there is proven or suspected active leptomeningeal disease. For patients with high CNS disease burden at the time of CAR infusion, more frequent neurologic checks after the infusion may be warranted for monitoring for TIAN in addition to ICANS.
Table 2.
Neurologic testing in the management of CAR T-cell therapy toxicities
| Before CAR T-cell therapy | From 1 to 30 d after CAR T-cell therapy | 1 mo after CAR–T-cell therapy | |
|---|---|---|---|
| Neurological assessment | YES, baseline mental status, neuro history (Nervous system toxicity from prior therapy, seizure, stroke, migraine, CNS disease, radiation, trauma, andneuropathy). | YES, monitor ICANS, additional attention to handwriting, movement, and personality changes for BCMA-CAR T-cell therapy. High-prolonged CRS increases the risk of ICANS. |
YES, full assessment at 1-3-6 months may be indicated in the case of delayed or prolonged neurotoxicity or in the case of BCMA products to screen for early signs of movement and MNTs. |
| EEG | Generally, not helpful unless a history of epilepsy. | YES, during ICANS to r/o nonconvulsive seizures and to monitor critically ill patients. | YES, consider if any recurrent altered mental status. |
| Neuroimaging | YES, if history of CNS disease in cases of preexisting neurologic injury. | YES, if ICANS occurs. | YES, for any delayed neurologic symptoms. |
| CAR T measurements (blood and CSF) | N/A | Unclear utility in the short-term management. | May be helpful if available. High levels of CAR T cells in blood have been associated with recurrent symptoms/delayed neurotoxicity. The role of CSF CAR measurement is unclear. |
| LP for CSF evaluation | YES, strongly consider if history of CNS disease. | May be diagnostic and therapeutic during ICANS. | May be diagnostic and therapeutic to rule out alternative etiologies in case of new neurologic symptoms. |
N/A, not available; r/o, rule out.
Case 2 (continued)
The patient received axi-cel and, on day +2, developed grade 2 CRS responsive to tocilizumab. On day +5, she developed altered mental status with an immune effector cell encephalopathy (ICE) score of 0. Although she was awake, she was not speaking coherently and followed no commands. She was prescribed dexamethasone at a dose of 10 mg every 6 hours for grade 3 ICANS. Over the next 4 hours, she became rapidly unresponsive to verbal and tactile stimuli (grade 4 ICANS). The neurologic exam revealed that she had focal alterations with right-side neglect, tonic posturing, and left-side stereotyped movements concerning seizures clinically. Head CT report was normal. EEG showed intermittent rhythmic delta activity on the right, with periods of relative attenuation on the left. Because of the clinical concern for nonconvulsive seizures, the levetiracetam dose was increased, and lorazepam and fosphenytoin were added. Her medication profile was reviewed; she was not receiving any seizure-inducing antibiotics. The dexamethasone dose was escalated to 20 mg every 6 hours. A lumbar puncture (LP) was performed that showed that the white blood cell count was 13 × 103/μL, protein level was elevated, and viral polymerase chain reaction reports were negative. The opening pressure was slightly elevated (21 cm H20). Hyponatremia was corrected. She received high-dose thiamine (500 mg) every 8 hours for 72 hours, followed by daily maintenance supplementation. Her mental status rapidly improved 24 hours after the LP, so her treatment was not escalated to pulse methylprednisolone. Brain MRI results showed no complex findings.
As we had done in this scenario, we review the medications of any patient who develops myoclonus or seizure, because certain antibiotics such as imipenem and cefepime carry a risk of excitotoxicity and seizures.48,49 Studies have demonstrated that BBB permeability increases during ICANS,8,12 and this may allow higher concentrations of these excitotoxic antibiotics to reach the CNS, further increasing seizure risk.
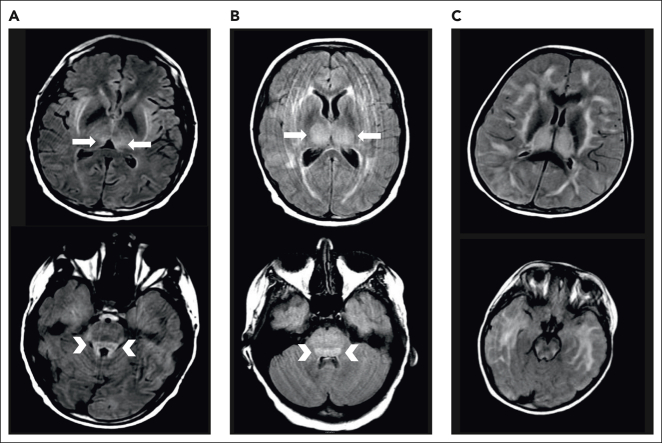
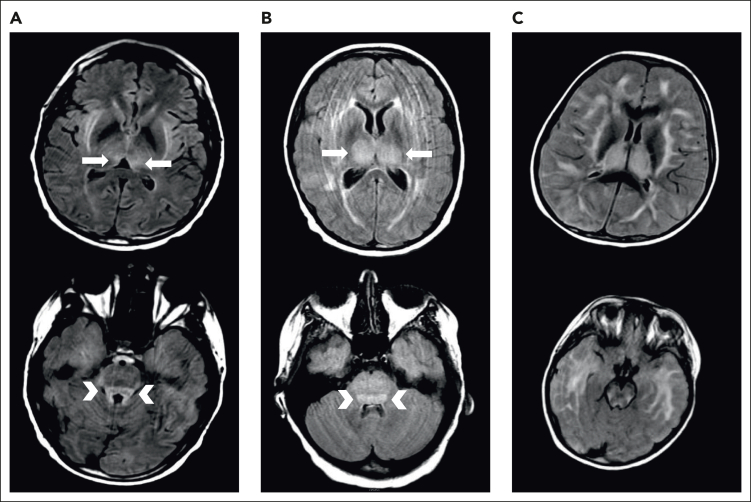
We typically administer thiamine repletion in the setting of severe CRS or ICANS. During severe ICANS, MRI changes resembling those in Wernicke encephalopathy, with T2/FLAIR hyperintensities in the dorsomedial thalami and tectal plate, have been reported12 (Figure 1). We have found abnormally low thiamine levels in hospitalized patients requiring CAR T-cell therapy. Inflammation is thought to be a factor driving thiamine-deficient state, and patients with sepsis are frequently thiamine deficient.50 Thiamine deficiency decreases BBB competence.51 We supplement patients experiencing severe CRS or ICANS with high-dose thiamine for 3 days, followed by daily thiamine until CRS and ICANS resolve.
Figure 1.
Examples of MRI changes during severe ICANS. Brain MRI during severe ICANS may rarely demonstrate symmetric T2/FLAIR hyperintensities in dorsal medial thalami (top; arrows) and tectal plate (bottom; arrowheads) resembling imaging changes associated with Wernicke encephalopathy. (A) MRI images from an adult patient with grade 4 ICANS. (B) MRI images from a pediatric patient with grade 4 ICANS. (C) Images from a pediatric patient with diffuse cerebral edema.
In our experience, hyponatremia can develop before the onset of ICANS, and this should be monitored closely and corrected.52 Cerebral edema and seizures can worsen with or be precipitated by hyponatremia. Although the CSF opening pressure was not elevated, the patient had marked improvement in her mental status the day after the LP was performed. Our opinion is that CSF drainage, even in the setting of near-normal opening pressure, may be helpful in managing ICANS. For patients who have had minimal improvement of ICANS symptoms after starting corticosteroids, we strongly consider performing an LP if safety conditions are met. We acknowledge that there are no published data supporting this approach.
Case 2 (continued)
On day +6, fever and mental status improved. She was oriented to person and more interactive than on previous days. On day +7, she had new paraplegia. She had an L1 sensory level, bilateral, positive Babinski signs, and new urinary retention. Spine MRI performed on day 7 showed diffuse edema of the spinal cord (all levels) with unchanged osseous lesions. She continued high-dose steroids, and high-dose IV immune globulin (IVIG; 0.4G/kg per day ×5 days) was added. Her mental status completely recovered by day 9. She had a repeat spine MRI on day 34 that showed resolved spine edema. Paraplegia gradually improved over 2 months, leading to a gradual tapering of her steroid dose. By 3 weeks, she had improved sphincter control, and by 2 months, she was able to walk with a walker, and the steroids were discontinued. She continues to be in remission of DLBCL after more than 4 years, with ongoing B-cell aplasia. She walks without assistive devices.
A few cases of myelitis after CAR have been reported. Nair et al described 2 patients who developed acute leucoencephalomyelopathy with quadriparesis after treatment with axi-cel.50 One patient with DLBCL developed cerebral edema in addition to spine edema. The patient was treated with corticosteroids for 3 weeks, which resulted in rapid mental status improvement by 2 weeks, radiographic improvement of the brain in 27 days and spine in 6 months, and the ability to ambulate by 6 months. The second patient in this series had primary mediastinal large B-cell lymphoma and developed grade 3 ICANS in addition to quadriparesis, which improved over several weeks after treatment with steroids.53 Another patient developed transverse myelitis with sensory changes, weakness, and urinary retention developing 27 days after receiving axi-cel and had some improvement in symptoms with steroids, IVIG, and plasma exchange.54 Similar to our patient, the patient in this case report had received anti-PD1 as bridging therapy. Immune checkpoint inhibitors can be associated with neurologic adverse events, including longitudinally extensive transverse myelitis,55 although checkpoint blockade has been combined with CAR T-cell therapy with a manageable safety profile.56 Another patient with primary mediastinal large B-cell lymphoma developed myelitis associated with paraplegia on day 6 after CAR T-cell therapy and was treated with siltuximab based on the finding of elevated IL-6 in the CSF. The altered mental status resolved, but the paraplegia did not improve.57 Another case of myelitis was associated with a fatal human herpesvirus 6 infection.58
There is justified concern that high-dose steroids may impair the efficacy of CAR T cells. High doses of steroids initiated early and for prolonged courses were associated with significantly shorter progression-free and overall survival in 1 series of 100 patients who received standard-of-care anti-CD19–CAR T-cell therapy.59 Our patient, despite high-dose steroids initiated early (before day 7) and continued for 2 months, had a complete and durable response. In a pilot retrospective study, IVIG appeared safe to use during CAR T-cell therapy but did not significantly reduce severe ICANS compared with steroids alone.60 IVIG was added to corticosteroids in this case, extrapolating from the treatment for idiopathic inflammatory transverse myelitis, and the patient had near full motor recovery.
Case 3
A 71-year-old man presented with immunoglobulin Gκ multiple myeloma that had progressed after multiple prior lines of therapy and autologous stem cell transplantation. He was treated with idecabtagene vicleucel (Abecma). Within 24 hours of the CAR T-cell infusion, he developed grade 2 CRS that eventually resolved. On day 5, he developed handwriting impairment and subsequently became confused and disoriented (ICE score 6). The neurotoxicity symptoms resolved with standard interventions.
Six months after B-cell maturation antigen (BCMA)–CAR T-cell infusion, he developed seizures, confusion, diminished attention, disorientation, agitation, aphasia, and tremors (grade 3 ICANS) and received steroids. The disease assessment showed a very good partial response. He progressed with obtundation and papilledema and developed cerebral edema. The patient was treated with high-dose steroids and anakinra and supportive care with no success and eventually died of acute respiratory distress syndrome and multiorgan failure.
This case highlights potential delayed toxicity from CAR T-cell therapy. The median time to the onset of neurotoxicity is 5 or 6 days (range, 1-17 days) after the CAR T-cell infusion and may depend on the CAR product and the disease type. Neurotoxicity usually resolves within 4 weeks in CD19 CAR T-cell therapy.17,61 Cohen et al reported that the median time to onset of ICANS with ciltacabtagene autoleucel in patients with myeloma was 8 days (range, 3-12 days), and the median duration was 4 days (range, 1-12 days). However, other CAR T-cell neurotoxicities may develop later after ciltacabtagene autoleucel infusion.62 One extraordinary case report of delayed neurotoxicity describes a 76-year-old woman with DLBCL, who fully recovered after a prolonged course for typical ICANS, then 6 months after CAR T-cell infusion, experienced a recurrence of severe ICANS symptoms. Her condition did not improve after 11 days of treatment with dexamethasone, but she recovered completely after a dose of cyclophosphamide. Her disease response improved from a partial to a complete response during this time, suggesting delayed activation and expansion of her CAR T cells.63 More subtle changes in neurocognitive performance and cognitive domains (ie, attention, executive function, verbal ability, immediate and delayed memory, and visuospatial abilities) have been reported in CAR T-cell therapy recipients up to 1 year after CAR T-cell infusion.64
Symptoms occurring concurrently with CRS tend to be of shorter duration and lower grade (grade 1-2) than neurotoxicity occurring after CRS, which is more commonly grade ≥3 and protracted. Neurologic monitoring, including ICE score and neurologic exam, is recommended for all patients during the first 30 days after CAR infusion but is continued for longer if toxicity is delayed or prolonged. Although the approved BCMA-CAR products are generally associated with lower ICANS rates than CD19 CAR, neurologic monitoring for 3 months or more may be indicated for patients treated with BCMA-CAR even if ICANS has completely resolved. The purpose of this extended monitoring is to screen for early signs of movement and neurocognitive treatment–emergent adverse events (MNTs), rare events that have a later onset than ICANS. In the CARTITUDE-1 trial, the 5 patients who developed MNTs had a median onset of 27 days after infusion (range, 14-108 days) compared with 8 days (range, 3-12 days) for ICANS. These MNTs are characterized by bradykinesia, rigidity, bradyphrenia, flat affect, personality change, apathy, gait disorder, and cognitive impairment. Patients may have normal ICE scores, but handwriting change, specifically micrographia, appears to be an early finding. Risk factors for MNTs include high tumor burden, grade ≥2 CRS after infusion, ICANS after infusion, and high CAR T-cell expansion and persistence. All 5 patients reported were male. This would suggest that a general approach for neurologic monitoring after BCMA-CAR therapy should be a scheduled neurologic assessment at 1-3-6 months after CAR T-cell infusion, with a focus on handwriting, muscle tone, facial expression, and gait. Patients should also be counseled regarding the need to inform their care team if there are any unexpected changes. For instance, unexpected cerebellar toxicities were recently reported in a trial of GPRC5D-specific CAR T cells for patients with multiple myeloma treated with a high dose of CAR T cells.19
The trigger of delayed neurologic events is unknown. In the case of MNTs, distinct pathophysiology involving on-target off-tumor targeting of BCMA in the basal ganglia is hypothesized. More generally, for all CAR products, infections occurring after CAR T-cell infusion may theoretically overactivate and reexpand CAR T cells in vulnerable patients.
Unusual neurologic complications should be anticipated with novel patterns of immune activation as well as new CAR constructs, because on-target off-tumor activity in the CNS is a persistent concern. Possible targeting of BCMA-expressing cells to the basal ganglia was previously reported, although there were additional risk factors including high tumor burden, previous grade ≥2 CRS, previous ICANS, and high CAR –T- cell expansion and persistence.21
General discussion
Although canonical ICANS is well-defined based on diagnostic criteria, patients may present with additional signs and symptoms that require specific management strategies. The first 2 cases address rare edema of the brain or spine occurring after CAR T-cell therapy. Cerebral edema is a rare but potentially fatal neurologic complication of CAR T-cell therapy. In the ROCKET clinical trial evaluating from 19 to 28z CAR T cells in adult patients with B-cell ALL, 5 patients developed fatal cerebral edema leading to the termination of the trial.65 A root cause investigation found that patients who developed cerebral edema were younger than 30 years, had a higher percentage of CD8+ cells in the CAR product, had higher serum levels of IL-15 after the infusion, and had a rapid expansion of CAR T cells peaking within the first week, which was associated with a sharp increase in the blood levels of tumor necrosis factor α (TNFα) and IL-2, compared with those who did not develop cerebral edema. Autopsies of 2 patients revealed a complete breakdown of the BBB but an absence of activated T cells in the CNS. These results suggest that the BBB breakdown and subsequent cerebral edema were probably the result of a surge of serum cytokines rather than the infiltration of CAR T cells into the CNS. The patient who developed leukoencephalomyelopathy in a case report from the ZUMA-1 trial was also found to have a massive expansion of peripheral CAR T cells and prominent features of BBB disruption.53 Our patient had myelopathy and spine edema without cerebral edema but had similar features of early-onset neurotoxicity and excellent response to CAR T-cell therapy despite the high and prolonged dose of steroids used to manage toxicity. Therefore, cerebral or spine edema probably represents an extreme end of the spectrum of pathophysiology underlying the more common ICANS but may have some unique mediators that have not been determined yet.
Although peak CAR T-cell levels in the blood have been demonstrated to be associated with neurotoxicity, most centers do not have the ability to measure CAR T-cell expansion in real time. The ability to do so might help predict patients at risk for severe neurotoxicity, including CNS edema. There may also be value for measuring CAR levels when faced with possible delayed neurotoxicity. CAR levels appear to be abnormally high in these situations as well. Although CD19-specific CAR T-cells can be detected in the CSF of patients with neurotoxicity, they can also be detected in the CSF of patients without neurotoxicity,3,12 and their presence and quantity do not appear to be predictive of neurologic symptoms. The value of measuring CD19 CAR T-cell concentration in CSF remains unclear.
In a recent study, single-cell sequencing analyses demonstrated CD19 expression on human brain mural cells, including pericytes and vascular smooth muscle cells, and it was proposed that an on-target off-tumor effect may contribute to the neurotoxicity observed with CD19 CAR T cells.66 However, it is unclear how to reconcile this hypothesis with the occurrence of ICANS with CAR T cells targeting other antigens such as BCMA, with the limited neurotoxicities observed with some CD19 constructs. For example, patients treated with CD19 CAR T cells containing CD28 and CD3z signaling domains identical to the axi-cel but having different single-chain variable fragment and hinge and transmembrane domains had similar antilymphoma activity but much lower incidence and severity of ICANS (50% severe ICANS compared with 5% severe ICANS).67 This difference was associated with a distinct profile of cytokines (lower) in the reduced ICANS group. That ICANS symptoms are overwhelmingly transient also suggests that they are not so because of on-target off-tumor direct effects from the CAR T-cells.
CANS appears most likely to be a toxic encephalopathy involving proinflammatory cytokines, excitotoxins, activation of vascular endothelium, and disruptions of the BBB. CSF during acute ICANS shows myeloid cytokines and chemokines,12 excitotoxins such as glutamate and quinolinic acid,12 and markers of glial and axonal injury,22,68 suggesting that the activation of microglial or other CNS myeloid cells with resulting CNS injury may play a role.
The last case illustrates delayed toxicities and the need for a thorough workup to rule out other etiologies such as infection or tumor recurrence. BCMA-CAR T cells are associated with lower rates of ICANS, but novel neurotoxicities have been described. Reports of delayed Parkinsonism-like neurologic toxic effects have also been reported in 1% to 5% of patients treated with ciltacabtagene autoleucel (Carvykti)62 and is attributed to the potential expression of BCMA in the basal ganglia.21 Unlike ICANS, these appear to not be readily reversible, even in patients treated with pulse-dose steroid, anakinra, siltuximab, and intrathecal chemotherapy. In addition, some peripheral nerve syndromes have been described, but these appear to be readily reversible with steroids. Other tumor-associated targets in multiple myeloma are currently being studied, some of which have a broader expression outside of plasma cells and warrant careful monitoring.19
Finally, based on our personal observations, rare neurologic toxicities not yet captured in the literature may occur. These include persistent severe postural tremors lasting >2 months after CAR, resembling essential tremor and effectively treated with propranolol; new migraines occurring after ICANS treatment with anakinra, managed with migraine abortive and prophylactic medications; and various peripheral neuropathies treated with steroids. Our approach is to firstly classify the toxicity based on the type with imaging and electrodiagnostic testing and then use trial medication to treat the idiopathic form of the toxicity (ie, acute demyelinating polyneuropathy would be treated with IVIG). Any patient with new mental status changes or CNS lesions arising 2 months after CAR needs neuroimaging LP with CSF analysis to rule out infections (especially JC virus, human herpesvirus 6, and cytomegalovirus) and CNS recurrence of the disease.
As a relatively new treatment, rare and unexpected side effects may occur among patients receiving CAR T-cell therapy. We believe that it is important to investigate the causative mechanisms of CAR-associated neurotoxicity by including careful and systematic monitoring of these side effects in clinical trials as well as continued reporting of such cases through the development of a repository of rare findings that may ultimately increase awareness of rare presentations and advance management strategies for these challenging cases. Additional measures to better understand these events may include CAR measurement in all compartments, blood/CSF sampling/banking, and prospective high-quality–ICANS treatment trials.
Conflict-of-interest disclosure: F.P. received research funding from LRF, Lonza, and NGMBiopharmaceutical. B.D.S. provided consultancy/advisory for Celgene, BMS, Janssen, Legend, Pfizer, and In8bio. J.G. provided consultancy for Johnson & Johnson.
Acknowledgments
The authors acknowledge the efforts of the American Society of Hematology’s subcommittee on emerging gene and cell therapies for their support in prioritizing the need to discuss emergent CAR T-cell toxicities.
Authorship
Contribution: All authors contributed to the content and writing of the manuscript and agreed to the contents of the manuscript before submission.
Footnotes
∗B.D.S. and J.G. equally contributed to this work.
References
- 1.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 2.Karschnia P, Jordan JT, Forst DA, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133(20):2212–2221. doi: 10.1182/blood-2018-12-893396. [DOI] [PubMed] [Google Scholar]
- 3.Gofshteyn JS, Shaw PA, Teachey DT, et al. Neurotoxicity after CTL019 in a pediatric and young adult cohort. Ann Neurol. 2018;84(4):537–546. doi: 10.1002/ana.25315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson CA, Hunter BD, Redd R, et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38(27):3095–3106. doi: 10.1200/JCO.19.02103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol. 2020;38(27):3119–3128. doi: 10.1200/JCO.19.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenbaum U, Dumbrava EI, Biter AB, Haymaker CL, Hong DS. Engineered T-cell receptor T cells for cancer immunotherapy. Cancer Immunol Res. 2021;9(11):1252–1261. doi: 10.1158/2326-6066.CIR-21-0269. [DOI] [PubMed] [Google Scholar]
- 7.Pennisi M, Sanchez-Escamilla M, Flynn JR, et al. Modified EASIX predicts severe cytokine release syndrome and neurotoxicity after chimeric antigen receptor T cells. Blood Adv. 2021;5(17):3397–3406. doi: 10.1182/bloodadvances.2020003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 10.Kochenderfer JN, Somerville RPT, Lu T, et al. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther. 2017;25(10):2245–2253. doi: 10.1016/j.ymthe.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochenderfer JN, Somerville RPT, Lu T, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol. 2017;35(16):1803–1813. doi: 10.1200/JCO.2016.71.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8(8):958–971. doi: 10.1158/2159-8290.CD-17-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin DB, Al Jarrah A, Li K, et al. Clinical predictors of neurotoxicity after chimeric antigen receptor T-cell therapy. JAMA Neurol. 2020;77(12):1536–1542. doi: 10.1001/jamaneurol.2020.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maus MV, Alexander S, Bishop MR, et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. 2020;8(2):e001511. doi: 10.1136/jitc-2020-001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson JA, Schneider BJ, Brahmer J, et al. Management of immunotherapy-related toxicities, version 1.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(4):387–405. doi: 10.6004/jnccn.2022.0020. [DOI] [PubMed] [Google Scholar]
- 16.Santomasso BD, Nastoupil LJ, Adkins S, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J Clin Oncol. 2021;39(35):3978–3992. doi: 10.1200/JCO.21.01992. [DOI] [PubMed] [Google Scholar]
- 17.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mailankody S, Devlin SM, Landa J, et al. GPRC5D-targeted CAR T cells for myeloma. N Engl J Med. 2022;387(13):1196–1206. doi: 10.1056/NEJMoa2209900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majzner RG, Ramakrishna S, Yeom KW, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603(7903):934–941. doi: 10.1038/s41586-022-04489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Oekelen O, Aleman A, Upadhyaya B, et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat Med. 2021;27(12):2099–2103. doi: 10.1038/s41591-021-01564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gust J, Finney OC, Li D, et al. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann Neurol. 2019;86(1):42–54. doi: 10.1002/ana.25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gust J, Ishak GE. Chimeric antigen receptor T-cell neurotoxicity neuroimaging: more than meets the eye. AJNR Am J Neuroradiol. 2019;40(10):E50–E51. doi: 10.3174/ajnr.A6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leahy AB, Newman H, Li Y, et al. CD19-targeted chimeric antigen receptor T-cell therapy for CNS relapsed or refractory acute lymphocytic leukaemia: a post-hoc analysis of pooled data from five clinical trials. Lancet Haematol. 2021;8(10):e711–e722. doi: 10.1016/S2352-3026(21)00238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqi T, Wang X, Blanchard MS, et al. CD19-directed CAR T-cell therapy for treatment of primary CNS lymphoma. Blood Adv. 2021;5(20):4059–4063. doi: 10.1182/bloodadvances.2020004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frigault MJ, Dietrich J, Martinez-Lage M, et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood. 2019;134(11):860–866. doi: 10.1182/blood.2019001694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity associated with CD19-targeted CAR-T cell therapies. CNS Drugs. 2018;32(12):1091–1101. doi: 10.1007/s40263-018-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oluwole OO, Bouabdallah K, Munoz J, et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J Haematol. 2021;194(4):690–700. doi: 10.1111/bjh.17527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oluwole OO, Forcade E, Muñoz Javier, et al. Prophylactic corticosteroid use with axicabtagene ciloleucel (Axi-Cel) in patients (Pts) with relapsed/refractory large b-cell lymphoma (R/R LBCL): one-year follow-up of ZUMA-1 cohort 6 (C6) [abstract] Blood. 2021;138(suppl 1) Abstract 2832. [Google Scholar]
- 30.Wehrli M, Gallagher K, Chen YB, et al. Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS) J Immunother Cancer. 2022;10(1):e003847. doi: 10.1136/jitc-2021-003847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diorio C, Shraim R, Myers R, et al. Comprehensive serum proteome profiling of cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome patients with b-cell all receiving CAR T19. Clin Cancer Res. 2022;28(17):3804–3813. doi: 10.1158/1078-0432.CCR-22-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strati P, Ahmed S, Kebriaei P, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 2020;4(13):3123–3127. doi: 10.1182/bloodadvances.2020002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gazeau N, Barba P, Iacoboni G, et al. Cameron J. Turtle, Jordan Gauthier. safety and efficacy of two anakinra dose regimens for refractory CRS or ICANS after CAR T-cell therapy [abstract] Blood. 2021;138(suppl 1) Abstract 2816. [Google Scholar]
- 34.Park JH, Sauter CS, Palomba ML, et al. A phase II study of prophylactic anakinra to prevent crs and neurotoxicity in patients receiving CD19 CAR T cell therapy for relapsed or refractory lymphoma [abstract] Blood. 2021;138(suppl 1) Abstract 96. [Google Scholar]
- 35.Frederick L, Locke M, Jason R, Westin MD, et al. Phase 1 results from ZUMA-6: axicabtagene ciloleucel (axi-cel; KTE-C19) in combination with atezolizumab for the treatment of patients with refractory diffuse large B cell lymphoma (DLBCL) [abstract] Blood. 2017;130(suppl 1) Abstract 2026. [Google Scholar]
- 36.Shah NN, Johnson BD, Fenske TS, Raj RV, Hari P. Intrathecal chemotherapy for management of steroid-refractory CAR T-cell-associated neurotoxicity syndrome. Blood Adv. 2020;4(10):2119–2122. doi: 10.1182/bloodadvances.2020001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zurko JC, Johnson BD, Aschenbrenner E, et al. Use of early intrathecal therapy to manage high-grade immune effector cell-associated neurotoxicity syndrome. JAMA Oncol. 2022;8(5):773–775. doi: 10.1001/jamaoncol.2022.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neelapu SS, Tummala S, Kebriaei P, et al. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit 'ALL. Nat Rev Clin Oncol. 2018;15(4):218. doi: 10.1038/nrclinonc.2018.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diorio C, Vatsayan A, Talleur AC, et al. Anakinra utilization in refractory pediatric CAR T-cell associated toxicities. Blood Adv. 2022;6(11):3398–3403. doi: 10.1182/bloodadvances.2022006983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gust J, Annesley CE, Gardner RA, Bozarth X. EEG correlates of delirium in children and young adults with CD19-directed CAR T cell treatment-related neurotoxicity. J Clin Neurophysiol. 2021;38(2):135–142. doi: 10.1097/WNP.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gust J, Ponce R, Liles WC, Garden GA, Turtle CJ. Cytokines in CAR T cell-associated neurotoxicity. Front Immunol. 2020;11:577027. doi: 10.3389/fimmu.2020.577027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mount CW, Majzner RG, Sundaresh S, et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat Med. 2018;24(5):572–579. doi: 10.1038/s41591-018-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denton CC, Gange WS, Abdel-Azim H, et al. Bilateral retinal detachment after chimeric antigen receptor T-cell therapy. Blood Adv. 2020;4(10):2158–2162. doi: 10.1182/bloodadvances.2020001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sim AJ, Palm RF, DeLozier KB, et al. MR-guided stereotactic body radiation therapy for intracardiac and pericardial metastases. Clin Transl Radiat Oncol. 2020;25:102–106. doi: 10.1016/j.ctro.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright CM, LaRiviere MJ, Baron JA, et al. bridging radiation therapy before commercial chimeric antigen receptor T-cell therapy for relapsed or refractory aggressive B-cell lymphoma. Int J Radiat Oncol Biol Phys. 2020;108(1):178–188. doi: 10.1016/j.ijrobp.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 48.Payne LE, Gagnon DJ, Riker RR, et al. Cefepime-induced neurotoxicity: a systematic review. Crit Care. 2017;21(1):276. doi: 10.1186/s13054-017-1856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Triplett JD, Lawn ND, Chan J, Dunne JW. Cephalosporin-related neurotoxicity: metabolic encephalopathy or non-convulsive status epilepticus? J Clin Neurosci. 2019;67:163–166. doi: 10.1016/j.jocn.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 50.Costa NA, Gut AL, de Souza Dorna M, et al. Serum thiamine concentration and oxidative stress as predictors of mortality in patients with septic shock. J Crit Care. 2014;29(2):249–252. doi: 10.1016/j.jcrc.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Hazell AS, Butterworth RF. Region-selective permeability of the blood-brain barrier to alpha-aminoisobutyric acid during thiamine deficiency and following its reversal. Metab Brain Dis. 2021;36(2):239–246. doi: 10.1007/s11011-020-00644-w. [DOI] [PubMed] [Google Scholar]
- 52.Dixon BN, Daley RJ, Buie LW, et al. Correlation of IL-6 secretion and hyponatremia with the use of CD19+ chimeric antigen receptor T-cells. Clin Nephrol. 2020;93(1):42–46. doi: 10.5414/CN109872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nair R, Drillet G, Lhomme F, et al. Acute leucoencephalomyelopathy and quadriparesis after CAR T-cell therapy. Haematologica. 2021;106(5):1504–1506. doi: 10.3324/haematol.2020.259952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheikh S, Mokhtari S, Silverman JA, et al. Transverse myelitis after anti-CD19 directed CAR T cell therapy for relapsed large B cell lymphoma. EJHaem. 2022;3(1):223–227. doi: 10.1002/jha2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Picca A, Berzero G, Bihan K, et al. Longitudinally extensive myelitis associated with immune checkpoint inhibitors. Neurol Neuroimmunol Neuroinflamm. 2021;8(3):e967. doi: 10.1212/NXI.0000000000000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chong EA, Melenhorst JJ, Lacey SF, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017;129(8):1039–1041. doi: 10.1182/blood-2016-09-738245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aghajan Y, Yu A, Jacobson CA, et al. Myelopathy because of CAR-T-related neurotoxicity treated with siltuximab. Neurol Clin Pract. 2021;11(6):e944–e946. doi: 10.1212/CPJ.0000000000001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Handley G, Khawaja F, Kondapi DS, et al. Human herpesvirus 6 myelitis after chimeric antigen receptor T-cell therapy. Int J Infect Dis. 2021;112:327–329. doi: 10.1016/j.ijid.2021.09.061. [DOI] [PubMed] [Google Scholar]
- 59.Strati P, Ahmed S, Furqan F, et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood. 2021;137(23):3272–3276. doi: 10.1182/blood.2020008865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mokhtari S, Asquith JM, Bachmeier CA, et al. The use of intravenous immunoglobulin (IVIG) during severe neurotoxicity among the recipients of chimeric antigen receptor T-cell (CAR-T) therapy [abstract] Blood. 2019;134(suppl 1) Abstract 5627. [Google Scholar]
- 61.Papadouli I, Mueller-Berghaus J, Beuneu C, et al. EMA review of axicabtagene ciloleucel (Yescarta) for the treatment of diffuse large B-cell lymphoma. Oncologist. 2020;25(10):894–902. doi: 10.1634/theoncologist.2019-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen AD, Parekh S, Santomasso BD, et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. 2022;12(2):32. doi: 10.1038/s41408-022-00629-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Badar T, Johnson BD, Hamadani M. Delayed neurotoxicity after axicabtagene ciloleucel therapy in relapsed refractory diffuse large B-cell lymphoma. Bone Marrow Transplant. 2021;56(3):683–685. doi: 10.1038/s41409-020-01029-4. [DOI] [PubMed] [Google Scholar]
- 64.Hoogland AI, Barata A, Logue J, et al. Change in neurocognitive performance among patients with non-Hodgkin lymphoma in the first year after chimeric antigen receptor T cell therapy. Transplant Cell Ther. 2022;28(6):305.e1–305.e9. doi: 10.1016/j.jtct.2022.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gilbert MJ. National Harbor; 8-12 November 2017. Severe neurotoxicity in the phase 2 trial of JCAR015 in adult B-ALL (ROCKET Study): analysis of patient, protocol and product attributes. Paper presented at: 32nd Annual SITC Meeting. [Google Scholar]
- 66.Parker KR, Migliorini D, Perkey E, et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T immunotherapies. Cell. 2020;183(1):126–142.e17. doi: 10.1016/j.cell.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brudno JN, Lam N, Vanasse D, et al. Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat Med. 2020;26(2):270–280. doi: 10.1038/s41591-019-0737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gust J, Rawlings-Rhea SD, Wilson AL, et al. GFAP and NfL increase during neurotoxicity from high baseline levels in pediatric CD19-CAR T-cell patients. Blood Adv. 2022;7(6):1001–1010. doi: 10.1182/bloodadvances.2022008119. [DOI] [PMC free article] [PubMed] [Google Scholar]