Abstract
Objective:
To determine whether aggregate measures of occupational exposures are associated with COPD outcomes in the SubPopulations and InteRmediate Outcome Measures In COPD Study cohort.
Methods:
Individuals were assigned to six predetermined exposure hazard categories based on self-reported employment history. Multivariable regression, adjusted for age, gender, race, current smoking status, and smoking pack-years determined the association of such exposures to odds of COPD and morbidity measures. We compared these to the results of a single summary question regarding occupational exposure.
Results:
2772 individuals were included. Some exposure estimates, including ‘gases and vapors’ and ‘dust and fumes’ exposures resulted in associations with effect estimates over two times the estimated effect size when compared to a single summary question.
Conclusions:
Use of occupational hazard categories can identify important associations with COPD morbidity while use of single point measures may underestimate important differences in health risks.
Keywords: Occupational history, COPD, clinical outcomes, observational study, respiratory disease
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a leading cause of death worldwide, affecting an estimated 216 million people every year, and represents an increasing burden for healthcare.1,2 Although cigarette smoking has been identified as the leading causal risk factor of COPD in the United States, occupational exposures to vapors, gases, dusts, and fumes (VGDF) are also associated with an increased risk of development and morbidity of COPD, including increased exacerbations, worse quality of life, and greater computed tomographic (CT) markers of respiratory disease.3–10 The population attributable fraction (PAF) of workplace exposures for COPD was recently reported as 14 percent (%).11
Occupational exposure is often measured using a single question querying a person’s exposure to VGDF, or by a job exposure matrix (JEM), which assigns a low, medium, or high likelihood of VGDF exposures based on self-report of occupation. Although prior research using both VGDF12 and JEM13 supports association between occupational exposures and COPD incidence and morbidity, these measures consolidate the complex nature and multiple hazards of many occupational exposures. VGDF by definition includes compounds of varying chemical and physical characteristics that can impact their inhalation, deposition, and reaction within the lungs. Understanding if the composition of the VGDF exposure affects the odds of COPD or morbidity may offer clues towards the heterogeneity of COPD and could help inform exposure prevention efforts.
To better understand the associations between occupational exposures, mechanisms of disease, and COPD outcomes, there may be value gained in evaluating exposures individually or in more analogous groupings such as gases and vapors, biological/organic dusts or inorganic/mineral dusts. Moreover, determining the association between duration of exposure and clinical and radiographic outcomes may inform clinical practice and identify susceptible subgroups. In the present study, we aim to evaluate the contribution of historical occupational exposure to COPD prevalence and morbidity in the SubPopulations and InteRmediate Outcome Measures In COPD Study (SPIROMICS), a longitudinal cohort designed to identify subpopulations that may benefit from targeted, specialized therapeutic treatments.14 In prior analyses of the same cohort, Paulin et al. examined the effects of occupational exposure assessed using VGDF and JEM questions on COPD morbidity9 and on commuted tomographic imaging characteristics15; our goal is to expand on the use of exposure categories, using gas and particulate classification, specific occupational exposures, and duration of such exposures, and their relationship to COPD.16
METHODS
Study Population
SPIROMICS enrolled approximately 2970 participants from twelve clinical centers across the United States. Participants were enrolled in four strata: 1) non-smoke exposed persons (not included in current analysis); 2) smoke exposed persons (hereafter referred to as smokers) with a history of at least 20 pack/years of exposure, without airways obstruction; 3) smokers with airways obstruction and a forced expiratory volume in one second (FEV1) greater than or equal to 50 percent (≥50%) of the predicted value; and 4) smokers with obstruction and an FEV1 less than 50 percent (<50%) of the predicted value. COPD was defined by airway obstruction with a post-bronchodilator forced expiratory volume in one second over the forced vital capacity (FEV1/FVC) of <0.70. The study design and exclusion criteria have been described in detail previously.14
Data collection
At the baseline visit, SPIROMICS staff collected extensive demographic and clinical data from participants. Morbidity measures included St. George’s Respiratory Questionnaire (SGRQ)17, six-minute walk distance in meters (6MWD)18, dyspnea (modified Medical Research Council questionnaire (mMRC))19, COPD health status (COPD assessment test (CAT))20, and Body-Mass Index, Degree of Airflow Obstruction and Dyspnea, and Exercise Capacity (BODE) Index.21 Current smoking was defined as report of smoking within the last month. Participants reported the total number of exacerbations in the year prior to the enrollment visit and were dichotomized to zero versus one or more exacerbations. Spirometry was performed according to standard procedures.22,23 CT measurements were undertaken at full inspiration and expiration as previously described,24 capturing emphysema (% of total voxels in the field < –950 Hounsfield units at total lung capacity), large-airway disease (measured using Pi10, a measure of airway wall thickness), and small-airway disease (% of total voxels in the field < –856 Hounsfield units at residual volume).15,24 SPIROMICS was approved by Institutional Review Boards at each center and all participants provided written informed consent prior to any data collection.
Occupational Exposure Data
SPIROMICS staff administered a semi-structured occupational questionnaire, which included questions on current and former jobs and ascertained work history through individual dichotomous (yes/no) questions about 22 occupations or trades and 16 specific hazards, as well as free form text fields for longest occupational job, along with approximate number of years exposed to each trade or hazard. Participants were also asked “if they had ever worked and if this job exposed them to VGDF”. Using responses from the baseline occupational questionnaire, two separate exposure definitions were created:
Self-reported ever exposure (yes/no) to VGDF. This dichotomous question ascertained overall potential for exposure even to individuals who may not have worked in any of the industries, occupations, or hazards identified elsewhere in the questionnaire, hereafter referred to as “ever VGDF.”
Assigned exposure category based on participant self-report of ever work in specific trades and/or of ever exposure to specific hazards asked in the questionnaire. We developed exposure categories defined by gases or particulates for our analysis utilizing responses to “ever worked” in the occupation and specific hazard questions from the occupational history questionnaire. Exposure categories were created for “gases and vapors” and “dusts and fumes.” “Dusts and fumes” exposure category was further subdivided into “biological and organic dusts,” “mineral and inorganic dusts and fumes,” “metal dusts and fumes,” and “agricultural dusts.” For example, a person who responded as a welder was placed into exposure categories for “mineral and inorganic dusts and fumes” and “metal dusts and fumes”. Assignments were not exclusive, so an individual could potentially be captured in multiple exposure categories due to reporting of multiple occupational exposures or multiple jobs. Table 1 identifies the occupations and hazards represented in the occupational history questionnaire and presents the exposure category assignments utilized.
Table 1.
Assignment classifications for occupational exposures
| Occupation or Trade Identified in SPIROMICS Questionnaire (n) | Gases and Vapors | Dusts and Fumes | Biological and Organic Dusts | Mineral and Inorganic Dusts and Fumes | Metal Dusts and Fumes | Agricultural Dusts |
| Boilermaker (51) | Yes | Yes | Yes | |||
| Carpenter (318) | Yes | Yes | ||||
| Chemical Worker (120) | Yes | |||||
| Electrician (154) | Yes | Yes | ||||
| Elevator Operator (29) | Yes | |||||
| Insulator (109) | Yes | Yes | Yes | Yes | ||
| Lather (39) | Yes | Yes | Yes | Yes | ||
| Machinist (200) | Yes | Yes | Yes | Yes | ||
| Mechanic (229) | Yes | Yes | Yes | Yes | ||
| Millwright (49) | Yes | Yes | Yes | |||
| Pipefitter (78) | Yes | Yes | Yes | Yes | ||
| Plasterer (120) | Yes | Yes | ||||
| Plumber (126) | Yes | Yes | Yes | Yes | ||
| Sander (175) | Yes | Yes | ||||
| Sheet metal worker (120) | Yes | Yes | Yes | |||
| Steelworker (95) | Yes | Yes | Yes | |||
| Welder (180) | Yes | Yes | Yes | |||
| Pig farmer (40) | Yes | Yes | Yes | Yes | ||
| Rigger (59) | Yes | Yes | Yes | |||
| Roofer (181) | Yes | Yes | Yes | |||
| Painter (300) | Yes | |||||
| Mason (85) | Yes | Yes | ||||
| Specific Vapor, Gas, Dust or Fume (n) | Gases and Vapors | Dusts and Fumes | Biological and Organic Dusts | Mineral and Inorganic Dusts and Fumes | Metal Dusts and Fumes | Agricultural Dusts |
| Irritant gases, such as chlorine or ammonia (469) | Yes | |||||
| Fire, smoke or other combustion products (358) | Yes | Yes | Yes | |||
| Incinerators, boilers or oil refineries (163) | Yes | Yes | Yes | Yes | ||
| Coal dust or powder (113) | Yes | Yes | ||||
| Silica or sand, concrete, cement, or rock dust (384) | Yes | Yes | ||||
| Indoor fuel powered motors, compressors, or engines (366) | Yes | Yes | ||||
| Diesel engine exhaust (404) | Yes | Yes | ||||
| Wheat flour or other grain dusts (104) | Yes | Yes | ||||
| Animal feed fodder (131) | Yes | Yes | Yes | Yes | ||
| Cotton dust or cotton processing (77) | Yes | Yes | Yes | |||
| Wood dust or saw dust (405) | Yes | Yes | ||||
| Cadmium fumes or batteries or silver solder (134) | Yes | Yes | Yes | |||
| Other metal dusts or metal fumes (245) | Yes | Yes | Yes | |||
| Welding or flame cutting (290) | Yes | Yes | Yes | |||
| Fiberglass or other man-made mineral fibers (258) | Yes | Yes | ||||
| Explosives or blasting fumes (104) | Yes | Yes | ||||
| Total Individuals | 1247 | 1291 | 648 | 1084 | 739 | 261 |
A subset of participants (n=2,086) reported the number of years exposed to the 16 specific hazards in addition to years of asbestos exposure. Years of exposure were rounded up to the nearest year, with all reports of < a year rounded to 1 year.
Statistical Analysis
Population and employment demographics were summarized using descriptive statistics. Logistic regression models were used to estimate crude prevalence odds ratios (PORs) and adjusted PORs (aPORs) of having COPD or having an exacerbation in the last year by occupational exposure categories. Using data from the baseline visit, crude and multivariable linear regression models were used to estimate cross-sectional associations between occupational exposure categories and COPD morbidity. In both the crude and multivariable models, the reference category used were individuals who were not included in the occupational exposure hazard category of interest. Models were adjusted a priori for age, gender, race (white vs. non-white), current smoking status, and smoking pack-years. We also included body mass index (BMI; underweight, <18.5 kg/m2; normal weight, 18.5 to <25 kg/m2; overweight, 25 to <30 kg/m2; obese, at least 30 kg/m2) and exam site in our models for CT measures. In a sensitivity analysis, models were adjusted for remaining exposure hazard categories to account for multiple occupational exposures (i.e., models investigating the primary exposure hazard category of gases and vapors were adjusted for dusts and fumes and models investigating the primary exposure hazard category of dusts and fumes, including all subcategories of dusts and fumes, were adjusted for gases and vapors). Results from the different occupational exposures and exposure categories were compared to the ever VGDF. In a separate sensitivity analysis, we merged the assigned exposure categories to form one composite variable representing exposure to any occupational hazard. This dichotomous (yes/no) variable was used in a separate analysis to determine the relationship between assigned exposure to COPD outcomes.
In separate analyses, we assessed effect modification by gender, race, and smoking status of the association between exposure category and our outcomes by including a single interaction term (covariate*exposure) in separate models for each potential effect modifier. Finally, in a separate analysis, crude and multivariable linear regression models were used to estimate the association between years of exposure reported by the participant and COPD status. All analyses were performed with StataIC statistical software, version 15.1 (StataCorp, College Station, TX). Statistical significance was defined as a p-value of <0.05 for main effects and effect modification.25
RESULTS
Study Population Characteristics
General participant demographics are provided in Table 2. Of the 2772 current or former smokers enrolled in SPIROMICS, the average age was 63.5 years old (SD 8.9 years). Individuals with biological and organic dusts exposure were slightly younger with an average age of 61.9 years old (SD 8.9 years). Study participants were between 75–80% white across all exposure categories. All exposure categories had higher % of male participants with the highest being for metal dusts and fumes (82.5% of exposed). Smoking pack-years were 49.3 (SD 26.9) for all participants, and higher for all assigned exposure categories. Over 40% of participants were current smokers in all exposure categories. Among current smokers, mean (SD) pack-years was 46.4 (24.8); former smokers had a mean (SD) of 51.4 (28.1) pack-years. Average values for mMRC, SGRQ, CAT, 6MWD, and BODE Index were similar across all exposure categories.
Table 2.
General population demographics
| Demographic | All Participants (N=2772) |
Gases and Vapors (N=1247) |
Dusts and Fumes (N=1291) |
Biological and Organic Dusts (N=648) |
Mineral and Inorganic Dusts and Fumes (N=1084) |
Metal Dusts and Fumes (N=739) |
Agricultural Dusts (N=261) |
Ever VGDFA (N=1149) |
|---|---|---|---|---|---|---|---|---|
| Age, years (mean, SDB) | 63.5 (8.9) | 62.4 (8.8) | 62.6 (8.8) | 61.9 (8.9) | 62.2 (8.8) | 62.5 (8.6) | 63.1 (9.0) | 62.3 (8.9) |
| WhiteC race (nD, %) | 2123 (76.6) | 956 (76.7) | 1004 (77.8) | 514 (79.3) | 838 (77.3) | 566 (76.6) | 209 (80.1) | 852 (74.2) |
| Male gender (n, %) | 1498 (54.0) | 877 (70.3) | 968 (75.0) | 489 (75.5) | 851 (78.5) | 610 (82.5) | 172 (65.9) | 758 (66.0) |
| Current Smoker (n, %) | 1093 (40.0) | 520 (42.3) | 527 (41.5) | 271 (42.7) | 451 (42.2) | 311 (42.7) | 103 (40.2) | 464 (40.8) |
| Pack years (mean, SD) | 49.3 (26.9) | 50.9 (27.3) | 51.1 (27.5) | 49.8 (25.4) | 51.5 (28.2) | 52.6 (29.5) | 49.9 (27.4) | 50.9 (25.8) |
| BMIE (mean, SD) | 27.9 (5.3) | 28.1 (5.3) | 28.2 (5.2) | 28.0 (5.1) | 28.1 (5.2) | 28.1 (5.3) | 28.1 (5.4) | 28.2 (5.4) |
| Exacerbations in last year (mean, % response) | 0.45 (26.4) | 0.30 (29.8) | 0.28 (28.3) | 0.29 (29.0) | 0.28 (28.3) | 0.29 (28.6) | 0.33 (32.6) | 0.31 (31.1) |
| CATF Score (mean, SD) | 14.1 (8.3) | 15.2 (8.2) | 15.0 (8.2) | 14.7 (7.9) | 15.0 (8.3) | 15.0 (8.5) | 14.9 (7.9) | 15.8 (8.4) |
| mMRCG Score (mean, SD) | 1.08 (1.01) | 1.16 (1.04) | 1.15 (1.05) | 1.13 (1.05) | 1.15 (1.08) | 1.21 (1.14) | 1.21 (1.09) | 1.19 (1.04) |
| SGRQH Score (mean, SD) | 33.6 (20.6) | 36.8 (20.5) | 36.0 (20.6) | 35.9 (20.3) | 36.2 (20.8) | 36.9 (21.1) | 37.1 (20.5) | 37.9 (20.9) |
| Six-minute walk distance (6MWD), meters (mean, SD) | 407.7 (120.6) | 406.9 (120.7) | 405.9 (119.8) | 409.6 (118.3) | 408.3 (122.4) | 407.6 (125.9) | 411.1 (116.8) | 401.1 (123.3) |
| BODEI Index (mean, SD) | 1.53 (1.94) | 1.64 (2.0) | 1.64 (2.0) | 1.61 (2.0) | 1.66 (2.0) | 1.73 (2.0) | 1.65 (2.0) | 1.74 (2.0) |
VGDF = vapors, gases, dusts, and fumes
As exposure categories were created by the study group using responses to trades and specific hazards, it is possible that a participant self-identified as not being exposed to ever VGDF but was still assigned as having exposure. Over 40% of participants (n=1149) responded to “yes” to the single question “Have you ever been exposed to VGDF at work?” Of these 1149 individuals, 248 (22%) were not assigned to an exposure category based on participant self-report of ever work in specific trades and/or of ever exposure to specific hazards asked in the questionnaire. Conversely, 543 out of the 1587 individuals (34%) who did not report ever VGDF exposure were assigned to an exposure category based on their work in specific trades or hazards.
The number of participants who responded to each specific occupational hazard and the subset of these individuals reporting exposure duration to the specific occupational hazard are reported in Table 3. In the subset of participants who reported duration of exposure (n=2,086), those reporting asbestos exposure had the widest range of years exposed, 1–52; while respondents exposed to fire, smoke or other combustion products had the highest average years of exposure 16.7 (SD 13.7).
Table 3.
Specific hazard exposure and exposure duration demographics
| Exposure | All Participants (N=2772) |
Participants Reporting Exposure Duration (N) |
Exposure Duration (Years) |
|
|---|---|---|---|---|
| Mean (SD) | Range* | |||
| Irritant gases, such as chlorine or ammonia | 469 | 203 | 13.3 (12.3) | 1, 51 |
| Wood dust or saw dust | 405 | 187 | 14.2 (13.6) | 0.5, 51 |
| Diesel engine exhaust | 404 | 191 | 2.8 (1.7) | 1, 9 |
| Silica or sand, concrete, cement or rock dust | 384 | 181 | 13.7 (13.3) | 0.4, 51 |
| Indoor fuel powered motors, compressors, or engines | 366 | 173 | 15.8 (14.1) | 1, 51 |
| Fire, smoke or other combustion products | 358 | 163 | 16.7 (13.7) | 1, 50 |
| With asbestos | 353 | 40 | 10.9 (11.1) | 0.2, 52 |
| Welding or flame cutting | 290 | 141 | 13.0 (13.6) | 0.5, 50 |
| Fiberglass or other man-made mineral fibers | 258 | 115 | 12.4 (12.7) | 0.4, 51 |
| Other metal dusts or metal fumes | 245 | 123 | 16.2 (13.5) | 0.5, 50 |
| Incinerators, boilers or oil refineries | 163 | 67 | 12.7 (13.6) | 1, 50 |
| Cadmium fumes or batteries or silver solder | 134 | 63 | 2.9 (1.9) | 1, 9 |
| Animal feed fodder | 131 | 66 | 2.5 (2.1) | 1, 9 |
| Coal dust or powder | 113 | 50 | 12.0 (12.3) | 0.5, 50 |
| Wheat flour or other grain dusts | 104 | 52 | 2.8 (2.0) | 1, 9 |
| Explosives or blasting fumes | 104 | 40 | 3.1 (2.4) | 1, 9 |
| Cotton dust or cotton processing | 77 | 36 | 3.0 (2.0) | 1, 9 |
Note:
All exposure durations were rounded-up to the nearest tenth of a year.
Occupational Exposures and COPD Morbidity Measures
Gases and Vapors
Forty-five % of participants were assigned to the gases and vapors exposure category based on reported trades or specific exposures to gases and vapors. Gases and vapors exposure was significantly associated with increased prevalence odds of COPD (adjusted prevalence odds ratio, (aPOR):1.22; 95% confidence interval (95% CI): 1.02, 1.45; p=0.03). These results were similar to the statistically significant increased prevalence odds among those with ever VGDF exposure (aPOR:1.18; 95%CI: 1.04, 1.35; p=0.01). Conversely, the associations of gas and vapors with other COPD status such as mMRC (β:0.19; 95% CI: 0.11, 0.27), CAT (β:2.23; 95% CI: 1.58, 2.88), and SGRQ (β:6.82; 95% CI: 5.21, 8.44) were almost double the estimated effect size using ever VDGF exposure alone as the exposure metric, values larger than the minimum clinically important difference (MCID) for these outcomes (SGRQ MCID 4 points26; CAT MCID 2 points27). Exposure to gases and vapors was also significantly associated with increases in % emphysema (β:0.95; 95% CI: 0.22, 1.68) and small airways disease (β:2.56; 95% CI: 1.06, 4.06) (Table 4a). In the sensitivity analysis adjusting for multiple exposure hazard categories, results were similar. When examining the relationship between gases and vapors and COPD morbidity, including dusts and fumes exposure as a covariate did not meaningfully change the magnitude of the observed associations, though the relationship was no longer significant for the outcomes of BODE index, 6MWD, % emphysema and small airways disease, and odds of COPD (Supplemental Digital Content Table 1a).
Table 4a. Association of occupational exposures with COPD status.
Association of gas and vapors with COPD status
| Ever VGDF (Yes/No)E (N=1149) |
Gas and Vapors E (N=1247) |
|||
|---|---|---|---|---|
| COPD Status | β (95% CI) | p-valueF | β (95% CI) | p-value |
| BODE Index | 0.18 (0.07, 0.30) | 0.002 | 0.29 (0.13, 0.44) | <0.001 |
| mMRC | 0.08 (0.02, 0.14) | 0.008 | 0.19 (0.11, 0.27) | <0.001 |
| SGRQ | 2.86 (1.67, 4.06) | <0.001 | 6.82 (5.21, 8.44) | <0.001 |
| CAT | 1.04 (0.56, 1.51) | <0.001 | 2.23 (1.58, 2.88) | <0.001 |
| 6MWD | −8.97 (−16.01, −1.93) | 0.01 | −15.25 (−24.88, −5.62) | 0.002 |
| %FEV1 | −3.99 (−5.51, −2.47) | <0.001 | −4.94 (−7.03, −2.85) | <0.001 |
| % EmphysemaA | 0.74 (0.21, 1.27) | 0.006 | 0.95 (0.22, 1.68) | 0.01 |
| Small Airways DiseaseB | 1.46 (0.37, 2.55) | 0.009 | 2.56 (1.06, 4.06) | 0.001 |
| Large Airways DiseaseC | 0.003 (−0.001, 0.007) | 0.10 | 0.002 (−0.004, 0.007) | 0.55 |
| Odds of COPDD | 1.18 (1.04, 1.35) | 0.01 | 1.22 (1.02, 1.45) | 0.03 |
| Odds of exacerbationD | 1.25 (1.10, 1.43) | 0.001 | 1.60 (1.33, 1.93) | <0.001 |
% Emphysema = total voxels in the field less than −950 Hounsfield units at total lung capacity
Small Airways Disease = % of total voxels in the field less than −856 Hounsfield units at residual volume
Large Airways Disease = Pi10, airway wall thickness (mm)
Odds of COPD and Odds of Exacerbations reported as POR.
Adjusted for age, gender, race, current smoking status, and smoking pack-years. BMI and exam site were also included in CT measure models.
p<0.05 indicated in bold
Dusts and Fumes
The dusts and fumes exposure category, determined from our assignments based on specific exposures or from trades where dusts and fumes exposures were common, represented approximately 47 % of individuals in the study population. Dusts and fumes exposures were also significantly associated with all COPD morbidity measures with the exception of prevalence odds of COPD. For example, dusts and fumes exposures resulted in increases of exacerbations (aPOR:1.52; 95% CI: 1.25, 1.84; p<0.001). The largest differences in effect estimates for morbidity measures as compared to the ever VGDF exposure were for 6MWD (β:−21.42; 95% CI: −31.35, −11.49) and SGRQ (β:6.43; 95% CI: 4.75, 8.11), where the effect sizes were more than twice the ever VGDF value and surpassing the MCID for SGRQ. Exposure to dusts and fumes was also significantly associated with increases in CT scan measures of small airways disease (β:2.65; 95% CI: 1.09, 4.20) (Table 4b). In the sensitivity analysis adjusting for multiple exposure hazard categories, results of the models that included gases and vapors as a covariate in the dusts and fumes analysis were largely similar, though the magnitude of the relationships were overall attenuated in many of the subcategories for several outcomes (Supplemental Digital Content Table 1b).” To further examine “dusts and fumes,” additional analysis was undertaken for the four subcategories (organic and biological dusts, inorganic and mineral dusts and fumes, metal dusts and fumes, and agricultural dusts.)
Table 4b.
Association of dusts and fumes and subcategories with COPD status
| Dusts and FumesE (N=1291) |
Organic and Biological DustsE (N=648) |
Inorganic and Mineral Dusts and FumesE (N=1084) |
Metal Dusts and FumesE (N=739) |
Agricultural DustsE (N=261) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COPD Status | β (95% CI) | p-valueF | β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value |
| BODE Index | 0.34 (0.18, 0.50) | <0.001 | 0.17 (−0.01, 0.35) | 0.07 | 0.36 (0.19, 0.52) | <0.001 | 0.40 (0.22, 0.57) | <0.001 | 0.16 (−0.10, 0.41) | 0.23 |
| mMRC | 0.19 (0.11, 0.27) | <0.001 | 0.09 (−0.00, 0.18) | 0.06 | 0.19 (0.11, 0.28) | <0.001 | 0.25 (0.16, 0.34) | <0.001 | 0.17 (0.04, 0.30) | 0.01 |
| SGRQ | 6.43 (4.75, 8.11) | <0.001 | 3.78 (1.88, 5.67) | <0.001 | 6.08 (4.35, 7.81) | <0.001 | 5.95 (4.09, 7.82) | <0.001 | 4.51 (1.86, 7.17) | 0.001 |
| CAT | 2.17 (1.49, 2.84) | <0.001 | 0.85 (0.09, 1.61) | 0.03 | 1.93 (1.24, 2.62) | <0.001 | 1.65 (0.91, 2.40) | <0.001 | 1.02 (−0.04, 2.08) | 0.06 |
| 6MWD | −21.42 (−31.35, −11.49) | <0.001 | −10.65 (−21.78, 0.49) | 0.06 | −18.08 (−28.31, −7.84) | 0.001 | −16.16 (−27.17, −5.15) | 0.004 | −0.68 (−16.29, 14.94) | 0.93 |
| %FEV1 | −4.74 (−6.90, −2.57) | <0.001 | −2.67 (−5.10, −0.25) | 0.03 | −4.27 (−6.49, −2.05) | <0.001 | −3.52 (−5.91, −1.13) | 0.004 | −2.52 (−5.92, 0.88) | 0.15 |
| % EmphysemaA | 0.71 (−0.05, 1.47) | 0.07 | 0.45 (−0.39, 1.30) | 0.29 | 0.61 (−0.17, 1.38) | 0.13 | 0.79 (−0.04, 1.62) | 0.06 | 0.13 (−1.06, 1.33) | 0.83 |
| Small Airways DiseaseB | 2.65 (1.09, 4.20) | 0.001 | 1.86 (0.13, 3.59) | 0.04 | 2.16 (0.56, 3.75) |
0.008
|
1.87 ( 0.16, 3.57) | 0.03 | 0.72 (−1.72, 3.17) | 0.56 |
| Large Airways DiseaseC | 0.004 (−0.001, 0.010) | 0.15 | −0.002 (−0.009, 0.004) | 0.45 | 0.004 (−0.001, 0.010) | 0.13 | 0.005 (−0.001, 0.012) | 0.08 | −0.006 (−0.015, 0.003) | 0.19 |
| Odds of COPDD | 1.19 (0.99, 1.43) | 0.06 | 1.24 (1.00, 1.52) | 0.05 | 1.12 (0.93, 1.36) | 0.23 | 1.14 (0.93, 1.40) | 0.20 | 1.14 (0.85, 1.52) | 0.39 |
| Odds of exacerbationD | 1.52 (1.25, 1.84) | <0.001 | 1.35 (1.09, 1.66) | 0.006 | 1.50 (1.23, 1.83) | <0.001 | 1.46 (1.18, 1.80) | <0.001 | 1.50 (1.13, 1.98) | 0.005 |
% Emphysema = total voxels in the field less than −950 Hounsfield units at total lung capacity
Small Airways Disease = % of total voxels in the field less than −856 Hounsfield units at residual volume
Large Airways Disease = Pi10, airway wall thickness (mm)
Odds of COPD and Odds of Exacerbations reported as POR.
Adjusted for age, gender, race, current smoking status, and smoking pack-years. BMI and exam site were also included in CT measure models.
p<0.05 indicated in bold
Organic and Biological Dusts
Organic and biological dusts exposures were observed in 23% of the participants through their report of trades or specific exposures. Although several morbidity measures were not significant with organic and biological dusts, we observed borderline statistically significant increased prevalence odds of COPD among individuals with reported exposure to organic and biological dusts (aPOR:1.24; 95%CI: 1.00, 1.52; p=0.05) (Table 4b). Increased prevalence odds were observed for increased exacerbation risk (aPOR:1.35; 95% CI: 1.09, 1.66; p=0.006) and increases in SGRQ (β:3.78; 95% CI:1.88, 5.67; p<0.001), CAT (β:0.85; 95% CI: 0.09, 1.61; p=0.03), and %FEV1 (β:−2.67; 95% CI: −5.10, −0.25; p=0.03), although none were appreciably different than effects associated with the ever VGDF exposure. Exposure to organic and biological dusts was also significantly associated with increases in small airways disease (β:1.86; 95% CI: 0.13, 3.59) (Table 4b). Exposures to organic and biological dusts were not associated with BODE Index, mMRC, 6MWD, and % emphysema, unlike the ever VGDF exposure (Table 4b).
Inorganic and Mineral Dusts and Fumes
Analogous to dusts and fumes, we observed statistically significant results for inorganic and mineral dusts and fumes exposures across all morbidity measures with the exception of prevalence odds of COPD. Thirty-nine % of respondents were assigned to the inorganic and mineral dusts and fumes exposure category. For example, CAT results increased by a value of 1.9 (β:1.93; 95% CI: 1.24, 2.62; p<0.001), which approaches the MCID of 2.0.27 The 6MWD difference (β:−18.08; 95% CI: −28.31, −7.84) was twice the ever VGDF value (β:−8.97; 95% CI: −16.01, −1.93). Exposure to inorganic and mineral dusts and fumes was also significantly associated with increases in small airways disease (β:2.16; 95% CI: 0.56, 3.75) (Table 4b).
Metal Dusts and Fumes
Metal dusts and fumes represented a subcategory of both “dusts and fumes” and “mineral and inorganic dusts and fumes.” Despite being a more refined assigned exposure category, 27% of subjects reported exposure to specific metal dusts and fumes or trades involving these exposures, and this exposure category resulted in statistically significant results for COPD morbidity measures comparable to both larger categories of “dusts and fumes” and “inorganic and mineral dusts and fumes.” Metal dusts and fumes were associated with the highest adjusted increase (β:0.40; 95% CI: 0.22, 0.57; p<0.001) for BODE Index. Exposure to metal dusts and fumes was also significantly associated with increases in small airways disease (β:1.87; 95% CI: 0.16, 3.57; p=0.03) (Table 4b).
Agricultural Dusts
Although representing the smallest exposure category with only 261 individuals (9 % of participants) assigned from reports of specific exposures or agricultural trades, agricultural dusts exposures resulted in significant increased prevalence odds of exacerbations (aPOR:1.50; 95% CI: 1.13, 1.98; p=0.005). Agricultural dusts exposures also resulted in statistically significant increases in mMRC scores (β:0.17; 95% CI: 0.04, 0.30; p=0.01) and in statistically significant increase of SGRQ scores (β:4.51; 95% CI: 1.86, 7.17; p=0.001), which is larger than the MCID. Both of these outcome measures were almost double the increases resulting from ever VGDF exposures. Agricultural dust exposures were not associated with significant increases in any of the CT measures (Table 4b).
Composite hazard exposure compared to VGDF
Using the composite assigned exposure variable in the multivariable model resulted in effect sizes that were overall similar though slightly greater than observed when using the ever VGDF variable (Supplemental Digital Content Table 2).
Effect Modification by Current Smoking Status, Race, and Gender
Current smoking status modified the exposure between gases and vapors, dust and fumes, biological and organic dusts, and ever VGDF exposure and COPD status. In general, former (non-current) smokers were more vulnerable to the health effects of occupational exposures as compared to current smokers. There was no significant interaction between mineral and inorganic dusts, metal dusts and fumes, or agricultural dusts and current smoking status. As examples, Figures 1a and 2a show the aPOR for COPD and adjusted difference in BODE Index respectively for current and non-current smokers across the occupational exposure categories.
Figure 1.
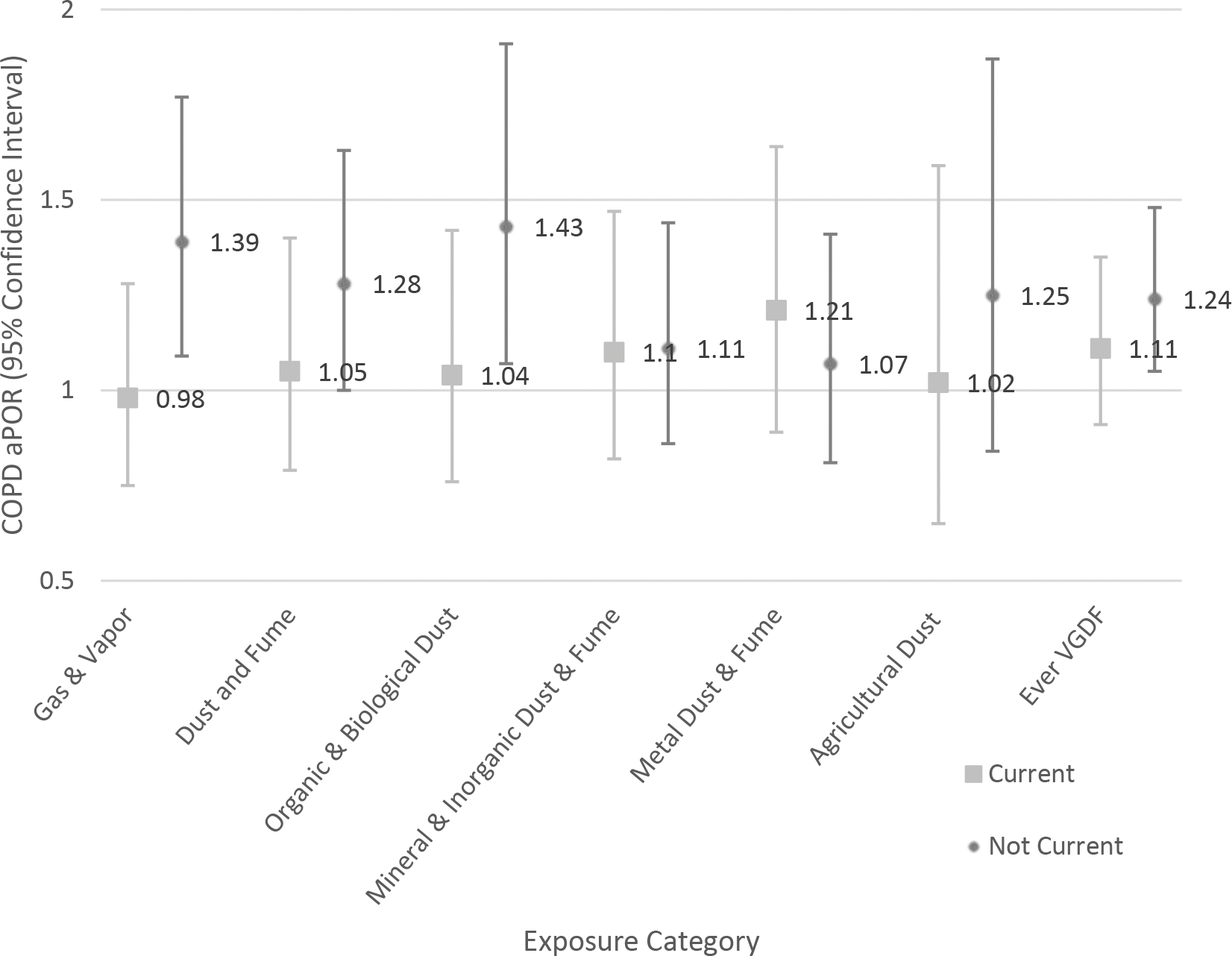
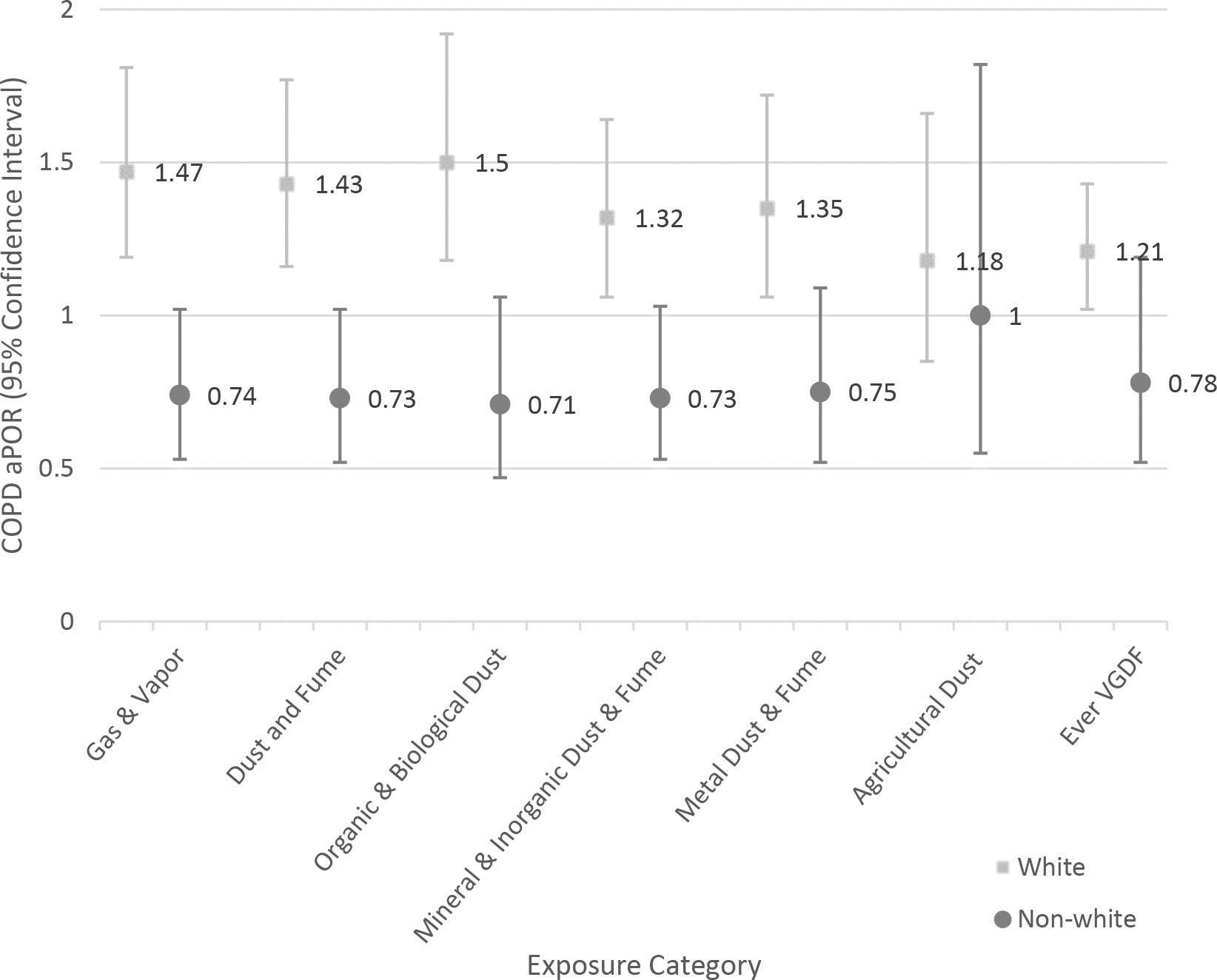
Association of occupational exposures with COPD adjusted prevalence odds ratio (aPOR). Effect modification by cigarette smoke exposure status (A); race (B).
A. Effect modification by cigarette smoke exposure status
B. Effect modification by race
Figure 2.
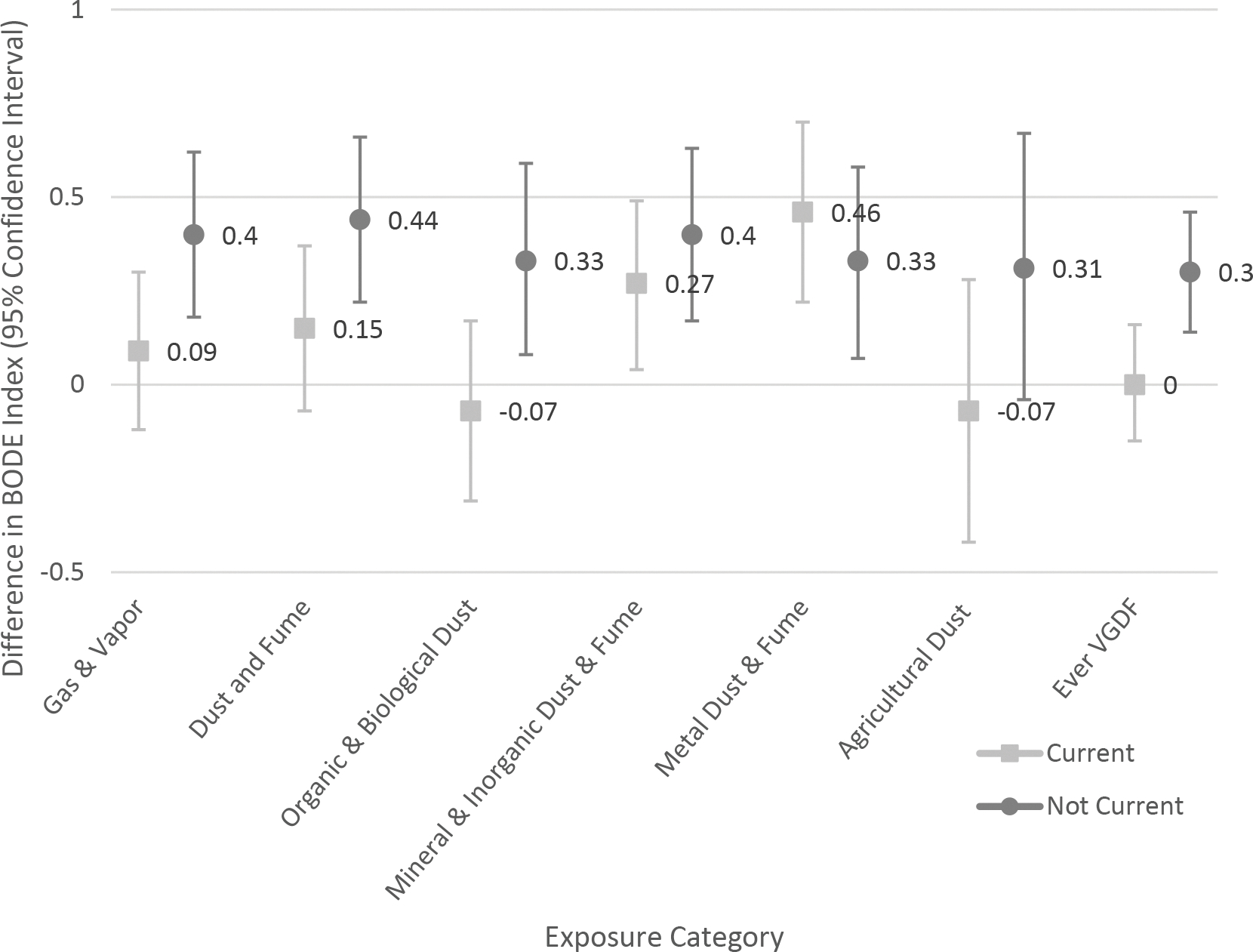
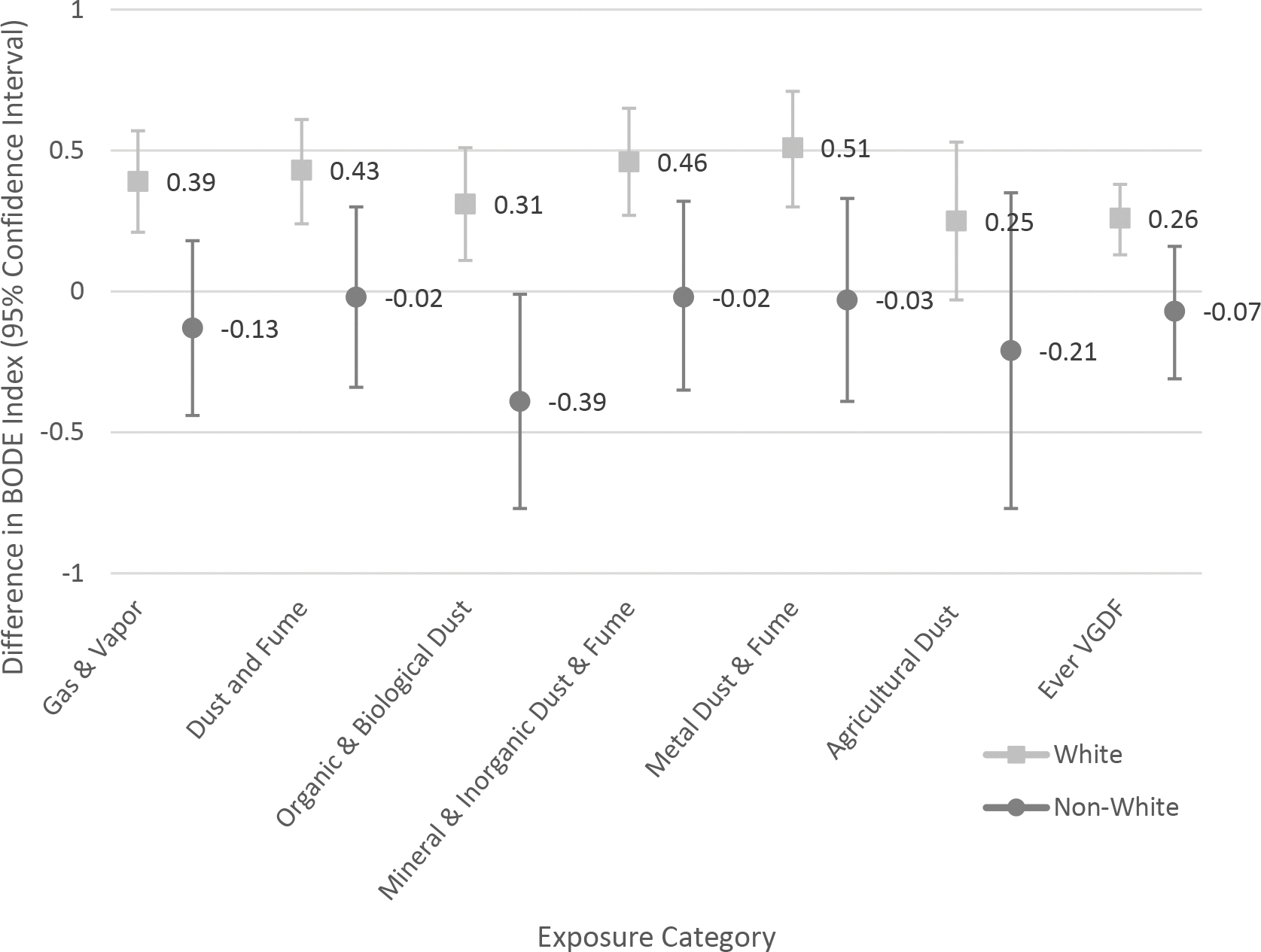
Association of occupational exposures with body-mass index, degree of airflow obstruction and dyspnea, and exercise capacity (BODE) index. Effect modification by cigarette smoke exposure status (A); race (B).
A. Effect modification by cigarette smoke exposure status
B. Effect modification by race
In the main effect model, non-white race was associated with worse COPD outcomes (data not shown). There was evidence of effect modification by race for the exposure categories of gas and vapors, dust and fumes, biological and organic dusts, metal dusts and fumes, and ever VGDF exposure. In general, white participants tended to be more susceptible to the health effects of occupational exposures. There was no significant interaction between race and agricultural dusts. As examples, Figures 1b and 2b show the aPOR for COPD and BODE Index respectively for whites and non-white participants across the exposure categories.
There was no evidence of effect modification by gender (data not shown).
Association of Duration of Exposure and COPD Status
Within the adjusted models, each additional exposure year was significantly associated with increased prevalence odds of COPD for subject reported wood dust or saw dust exposures (aPOR:1.03; 95% CI: 1.00, 1.06; p=0.02). Each additional year of wood dust or saw dust exposure was also significantly associated with decreases in FEV1% predicted (β:−0.29; 95% CI: −0.57, −0.02; p=0.04). Among the CT measures, each additional year of exposure to wood dust resulted in greater small airways disease (β:0.2; 95% CI: 0.0, 0.4; p=0.03). Exposure duration was not statistically significantly related to other outcomes (data not shown).
DISCUSSION
In our study of a large multi-center cohort with extensive clinical phenotyping and detailed occupational exposure questionnaire, we found that several occupational exposure categories were associated with increased respiratory morbidity measures in those with and at risk for COPD. Specific exposure categories of ‘gases and vapors’ and ‘dust and fumes’ exposures resulted in greater impacts on COPD morbidity than the use of self-reported ever VGDF measure. For example, SGRQ values were almost double in several exposure categories (namely gas and vapors) as compared to the ever VGDF, and reductions in 6MWD were close to two times as great for some of the exposure categories (namely dust and fumes) as the ever VGDF. Furthermore, several occupational exposures were associated with morbidity measures with effects > the MCID value (i.e., quality of life, functional status measures), highlighting that these occupational exposures were associated with effects that are clinically meaningful in the lives of patients with and at risk of COPD. We also found that duration of occupational exposure was a valuable predictor of worse COPD morbidity. This suggests that use of summary markers of occupational exposure risk may fail to capture individuals with meaningful occupational exposures and may underestimate the impact of occupational exposures on respiratory status, while querying about exposures on a more granular level allows for capturing variability between exposures in risk and important clinical status.
Our efforts provide further support to existing research of increased risks associated with occupations as reported through examination of several National Health and Nutrition Examination Study (NHANES) cohorts,28–31 as well as specific hazards including silica,32 diesel exhaust,33 biopersistent granular dust,34 and pesticides.35 Our findings for metal dusts and fumes support previously reported evidence of increased morbidity and support the need for additional quantitative exposure studies in these populations. In a case-control study, Kraim-Leleu et al. reported increased COPD OR (aOR: 7.6; 95% CI:4.5,12.9, p<0.001) for metal dusts and fumes from foundry exposure but did not report significant increases for cotton dust from the textile industry.36 Koh et al. also found increased COPD OR (aOR: 3.91; 95% CI: 1.36, 13.33) for welding exposures in a cohort study of shipyard workers.37 Our finding of increased risk for exacerbations from biological dusts further supports Burkes et al. reporting of increased odds and incidence of total and severe exacerbations associated with agricultural occupation in the same cohort.38
The use of CT measures to understand occupational exposure contributions to COPD is relatively novel. Galbán et al. reported using CT measures in the diagnosis of COPD phenotypes and disease progression and recommended their use to assist standard clinical examinations.39 Occupational exposures to dusts and fumes were reported to have greater % emphysema and gas trapping when compared to no exposure in both women and men.40 Previous work by our group reported higher emphysema, greater large airways disease, and greater small airways disease with VGDF exposure.15 As our specific exposures are subsets of the overall ever VGDF exposure, this confirms our analysis.
Current smoking status was an important modifier for morbidity measures with effects generally being larger for former (non-current) smokers, although this interaction was not consistently significant across all COPD morbidity measures, nor across all occupational exposure categories. Individuals who were former smokers may either have had to cease smoking due to health impacts in order to keep working or may have had additional workplace efforts to stop smoking. Conversely, current smokers may have had limited occupational exposures within the same exposure category or may exhibit some resiliency against the health risks of smoke exposure.41,42 We found no interaction with gender; this differs from previous study results reported by Paulin et al.9 and may be due to variations in exposure groupings (“ever” exposure captured in this work vs. “longest job” in the prior work) and definitions of exacerbation status (any exacerbation in this work vs. exacerbations requiring health care utilization in the prior work). While non-white race was associated with worse COPD outcomes in the main model, in the separate interaction analysis, we found that race modified the response between occupational exposure and COPD morbidity, such that whites were more susceptible to the adverse health effects of occupational exposure compared to non-whites. This may be influenced by actual differences in exposures by individuals reporting the same exposures (i.e., carpenter versus a carpenter helper or intensity of employment environment), differences in race demographics across the industries, trades, and occupational exposures represented, selection bias, and/or unmeasured confounding. Further insight into the interactions between race, occupational exposures, and COPD is warranted and beyond the scope of this manuscript..
This study offers two new elements in the approach to examining occupational exposure contributions to COPD. The first is focusing on different exposure classifications and specific hazards versus the more common assessment of exposure to ever VGDF. Associating exposures to occupations and specific tasks where they are understood to occur limits potential recall bias where individuals may not have understood if they had an exposure to VGDF but would remember their occupation and activities. It is also possible that individuals who provide a more granular description of exposure versus ever VGDF may have had more substantial occupational exposures and thus a stronger impact on COPD morbidity. The second, by seeking to understand if exposure duration relates to COPD characteristics, moves away from a dichotomous “exposed or not” variable towards a semi-quantitative analysis. Our results of the exposure year analysis shows that exposure duration may be a helpful predictor of COPD risk and morbidity. This begins to treat occupational exposures in a manner analogous to cigarette exposures, characterized in terms of smoking pack-years. As with smoking cessation and prevention efforts, reductions in occupational exposures are the most direct way to reduce disease prevalence and improve health outcomes. The varying impacts across our exposure categories and hazards suggest that workplace exposure control efforts, in even limited areas, may have benefits towards reducing COPD morbidity. Other study strengths include detailed and objective outcome measures and information on important confounders on smoking status and smoking history. We were also able to examine effect modifiers and explore important considerations for patient care through morbidity measures.
Some limitations must be considered when extrapolating our study results. SPIROMICS is not a population-based study and by design excluded individuals who might have reserved ratio impaired spirometry (PRISm) or other respiratory diseases.43 Further, while it is difficult to generalize results to a non-smoking population, our results suggest that occupational exposures have potential for significant impact on clinical outcomes despite significant smoking histories. Additionally, the use of exposure years is only a semi-quantitative exposure measurement and does not represent airborne hazard concentrations during those years nor inhaled dose. Data on duration of exposure years is limited to a smaller subset of participants who reported information on this variable. In addition, the occupational exposure questionnaires did not seek information regarding use of any personnel protective equipment nor the presence or absence of any other workplace controls that may have impacted these exposures. Finally, knowledge of some of the specific hazard exposures may have been limited or recalled erroneously, especially those that may have occurred decades ago. Further, as the researchers assigned the exposure hazard categories, there is a potential for misclassification as compared to the use of a validated JEM.
In summary, we found that differentiating prior occupational exposure into like categories of gases and vapors and dusts and fumes was associated with increased odds of COPD and worse COPD morbidity. We found prior exposure to gases and vapors and organic and biological dusts was associated with increased odds of COPD, and that prior exposures to gases and vapors, mineral and inorganic dusts and fumes, and metal dusts and fumes resulted in increased COPD morbidity. We identified positive associations between select occupational exposures based on years of exposure and BODE Index, % emphysema, large airways disease, and small airways disease when carefully adjusting for important confounders. The use of more refined exposure measures captured additional individuals and allows for better determination of increased variation in risk and important clinical outcomes. Results from our study allow those in the workplace setting to prioritize exposures for control, as well as increasing the potential of earlier targeted clinical interventions for individuals with these exposures.
Supplementary Material
Supplemental Digital Content Table 1. Association of occupational exposures with COPD status adjusted for reciprocal exposures
Table 1a. Association of gas and vapors with COPD status, additionally adjusted for dusts and fumes exposures
Table 1b. Association of dusts and fumes and subcategories with COPD status, additionally adjusted for gases and vapor exposures
Supplemental Digital Content Table 2. Association of occupational exposures with COPD status
Learning Outcomes.
Discuss how different occupational exposure histories are associated with respiratory morbidity in a population of individuals with and at-risk of developing COPD
Identify specific occupational exposure categories associated with greater COPD morbidity based on an individual’s self-reported occupational history
Describe the impact of additional years of occupational exposures on COPD morbidity based on reported occupational exposure history.
Acknowledgements:
The authors confirm contribution to the paper as follows: study conception and design: JSR, NNH, LMP; data collection: NNH; analysis and interpretation of results: JSR, PSJL, KK, JPB, LQA, NNH, LMP; draft manuscript preparation: JSR. The initial work for this paper was conducted as thesis work by JSR with thesis review LMP, PSJL, KK, JPB, LQA, and NNH. All authors reviewed the results and approved the final version of the manuscript.
Funding:
a Supported by full-time employment with the University of Maryland, Department of the Navy, and Department of Labor, Occupational Safety and Health Administration.
b Supported by the Johns Hopkins University Education and Research Center for Occupational Safety and Health (ERC), which is funded by NIOSH under grant number 5 T42 OH 008428.
c Supported by Cipla, Pulmonx, Teva, Verona, Merck, DevPro, Aerogen, Polarian, UpToDate, Altesa Biopharma, Medscape, NACE, MDBriefcase and Integrity. Received either in kind research support or funds paid to the institution from the Nuvaira, Gala Therapeutics, and Biodesix. Participated in Data Safety Monitoring Boards for Novartis and Medtronic with funds paid to the institution.
e Supported by Konica Minolta and the Continuing Education Alliance.
f Supported by the American Lung Association.
g Supported by the Wake Forest Clinic, Cal site.
h Supported by the Department of Veteran’s Affairs and Partner Therapeutics.
i Supported by Eli Lilly.
j Supported by Verona and grants paid to institution by Teva and Midmark.
SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), grants from the NIH/NHLBI (U01 HL137880 and U24 HL141762), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc.; Chiesi Farmaceutici S.p.A.; Forest Research Institute, Inc.; GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; Novartis Pharmaceuticals Corporation; Nycomed GmbH; ProterixBio; Regeneron Pharmaceuticals, Inc.; Sanofi; Sunovion; Takeda Pharmaceutical Company; and Theravance Biopharma and Mylan.
Footnotes
Conflicts of Interest:
c Stock options received from Meissa Vaccines and Altesa Biopharma.
d Founder and shareholder of VIDA Diagnostics, a company commercializing lung image analysis software developed, in part, at the University of Iowa. Serves as an unpaid member of the Siemens photon counting CT advisory board.
j Additional funding from ongoing consulting to Optimum Patient Care and Becker Pharmaceutical Group, with past consulting to Polarean, American Society Hospital Pharmacists, and Midmark.
Ethical Considerations: SPIROMICS was approved by Institutional Review Boards at each center and all participants provided written informed consent prior to any data collection.
REFERENCES
- 1.World Health Organization. The top 10 causes of death, Fact Sheet N*310. Geneva, Switzerland. 2014. Available at: http://www.who.int/mediacentre/factsheets/fs310/en/ [Google Scholar]
- 2.World Health Organization. Global status report on noncommunicable diseases. Geneva, Switzerland. 2014. Available at: https://www.who.int/nmh/publications/ncd-status-report-2014/en/ [Google Scholar]
- 3.Trupin L, Earnest G, San Pedro M, et al. The occupational burden of chronic obstructive pulmonary disease. European Respiratory Journal. 2003;22:462–9. [DOI] [PubMed] [Google Scholar]
- 4.Blanc PD, Eisner MD, Trupin L, et al. The association between occupational factors and adverse health effects in chronic obstructive disease. Occupational and Environmental Medicine. 2004;61:661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanc PD, Eisner MD, Balmes JR, et al. Exposure to vapors, gas, dust, or fumes: assessment by a single survey item compared to a detailed exposure battery and a job exposure matrix. Am J Ind Med. 2005;48:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanc PD, Eisner MD, Earnest G, et al. Further exploration of the links between occupational exposure and chronic obstructive pulmonary disease. Occupational and Environmental Medicine. 2009; 51:804–810/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harper P, Tashkin DP, Simmons M, et al. for the Lung Health Study Group. Effect of occupational exposures on the decline of lung function in early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:994–1000. 10.1164/rccm.200605-730OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omland O, Wurtz ET, Aasen TB, et al. Occupational chronic obstructive pulmonary disease: a systematic literature review. Scandanavian Journal of Work and Environmental Health. 2014;40:19–35. [DOI] [PubMed] [Google Scholar]
- 9.Paulin LD, Diette GB, Blanc PD, et al. (2015). Occupational Exposures are associated with worse morbidity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lytras T, Kogevinas M, Kromhout H, et al. Occupational exposures and incidence of chronic bronchitis and related symptoms over two decades: the European Community Respiratory Health Survey. Occupational and Environmental Medicine. 2019;76:222–229. [DOI] [PubMed] [Google Scholar]
- 11.Blanc PD, Annesi-Maesano I, Balmes JR, et al. on behalf of the American Thoracic Society and European Respiratory Society (2019). The Occupational Burden of Nonmalignant Respiratory Diseases: An Official American Thoracic Society and European Respiratory Society Statement. Am J Resp Crit Care Med. 2019;199(11):1312–1334. doi: 10.1164/rccm.201904-0717ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryu JY, Lee SY, & Kim DH. Obstructive pulmonary function impairment among Korean male workers exposed to organic solvents, iron oxide dust, and welding fumes. Industrial Health. 2013;51(6):596–602. doi: 10.2486/indhealth.2012-0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadhra S, Kurmi OP, Sadhra SS, Lam KB, & Ayres JG. Occupational COPD and job exposure matrices: a systematic review and meta-analysis. Int J of Chronic Obstructive Pulmonary Disease. 2017;12:725–734. doi: 10.2147/COPD.S125980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couper D, LaVange LM, Han MK, et al. for the SPIROMICS Research Group. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulin LD, Smith BM, Koch A, et al. Occupational Exposures and Computed Tomographic Imaging Characteristics in the SPIROMICS Cohort. ANNALS of American Thoracic Society. 2018;15:1411–1419. doi: 10.1513/AnnalsATS.201802-150OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rous JS. Association of Occupational Exposures and Chronic Obstructive Pulmonary Disease Morbidity. Doctoral thesis. Johns Hopkins Bloomberg School of Public Health, Department of Environmental Health Sciences, Baltimore, MD, March 2020. [Google Scholar]
- 17.Jones PW, Quirk FH, Baveystock CM, & Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. American Review of Respiratory Disease. 1992;145:1321–1327. [DOI] [PubMed] [Google Scholar]
- 18.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. European Respiratory Journal. 2014;44:1428–1446. [DOI] [PubMed] [Google Scholar]
- 19.Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones PW, Harding G, Berry P, et al. (2009). Development and first validation of the COPD Assessment Test. European Respiratory Journal. 2009;34:648–654. [DOI] [PubMed] [Google Scholar]
- 21.Celli BR, Cote CG, Marin JM, et al. The Body-Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity Index in Chronic Obstructive Pulmonary Disease. The New England Journal of Medicine. 2004;350(10):1005–1012. [DOI] [PubMed] [Google Scholar]
- 22.Hankinson JL, Odencrantz JR, & Fedan KB. Spriometric reference values from a smaple of the general U. S. population. Am J Respir Crit Care Med. 1999;159: 179–187. [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson JL, Brusasco V, et al. Standardisation of spirometry. European Respiratory Journal. 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 24.Sieren JP, Newell Jr JD, Barr RG, et al. for the SPIROMICS Research Group. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Resp Crit Care Med. 2016;194:794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvin S Statistical Analysis of Epidemiologic Data. 2004. Oxford University Press. [Google Scholar]
- 26.Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–79. doi: 10.1081/COPD-200050513 [DOI] [PubMed] [Google Scholar]
- 27.Kon SSC, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet. 2014;2(3):195–203. Retrieved from 10.1016/S2213-2600(14)70001-3 [DOI] [PubMed] [Google Scholar]
- 28.Hnzido E, Sullivan PA, Bang KM, & Wagner G. Association between Chronic Obstructive Pulmonary Disease and Employment by Industry and Occupation in the US Population: A Study of Data from the Third National Health and Nutrition Examination Survey. Am J of Epidemiology. 2002;156:738–746. [DOI] [PubMed] [Google Scholar]
- 29.Doney B, Hnizdo E, Syamlal G, et al. Prevalence of chronic obstructive pulmonary disease among US working adults aged 40 to 70 years. National Health Interview Survey data 2004 to 2011. Occupational and Environmental Medicine. 2014;56(10):1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doney B, Kurth L, Halldin CN, & Hnizdo E. Prevalence of airflow obstruction and chronic bronchitis by occupational groups among the U.S. Population aged 40–79 years: NHANES survey data 2007–2010. Am J Resp Crit Care Med. 2015;191.25932660 [Google Scholar]
- 31.Kurth L, Doney B, Halldin CN, Hale J, & Frenck SM. Airflow obstruction among ever-employed U. S. adults aged 18–79 years by industry and occupation: NHANES 2007–2008 to 2011–2012. Am J Ind Med. 2019;62(1):30–42. doi: 10.1002/ajim.22930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hnizdo E, & Vallyathan V. Chronic obstructive pulmonary disease due to occupational exposure to silica dust: a review of epidemiological and pathological evidence. Occupational and Environmental Medicine. 2003;60:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hart JE, Eisen EA, & Laden F. Occupational diesel exhaust exposure as a risk factor for COPD. Current Opinions in Pulmonary Medicine. 2012;18(2):151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruske I, Thiering E, Heinrich J, Huster KM, & Nowak D. Biopersistent Granular Dust and Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. PLOS ONE. 2013;e80977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doust E, Ayres JG, Devereux G, et al. Is pesticide exposure a cause of obstructive airways disease? European Respiratory Review. 2014;23:180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraïm-Leleu M, Lesage FX, Drame M, Lebargy F, & Deschamps F. Occupational Risk Factors for COPD: A Case-Control Study. PLOS ONE. 2016;11(8):e0158719. 10.1371/journal.pone.0158719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koh DH, Kim JI, Kim KH, & Yoo SW. Welding fume exposure and chronic obstructive pulmonary disease in welders. Occupational Medicine. 2015;65(1):72–77. doi: 10.1093/occmed/kqu136 [DOI] [PubMed] [Google Scholar]
- 38.Burkes RM, Gassett AJ, Ceppe AS, et al. for the SPIROMICS Investigators. Rural Residence and COPD Exacerbations: Analysis of SPIROMICS Cohort. Annals of the American Thoracic Society. 2018;15(7): 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galbán CJ, Han MK, Boes JL, et al. Computed tomography–based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nature Medicine. 2012;18(11):1711–1716. doi: 10.1038/nm.2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marchetti N, Garshick E, Kinney GL, et al. & the COPDGene Investigators. Association between Occupational Exposure and Lung Function, Respiratory Symptoms, and High-Resolution Computed Tomography Imaging in COPDGene. Am J Resp Crit Care Med. 2014;190(7):756–762. doi: 10.1164/rccm.201403-0493OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becklake MR & Lalloo UG. The ‘healthy smoker’: a phenomenon of health selection? Respiration. 1990;57(3):137–144. [DOI] [PubMed] [Google Scholar]
- 42.Levine ME & Crimmins EM. Evidence of resiliency among long-lived smokers. Vienna Yearbook of Population Research. 2013;1:205–18. [Google Scholar]
- 43.Wan ES, Castaldi PJ, Cho MH, et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15(1):89. 10.1186/s12931-014-0089-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content Table 1. Association of occupational exposures with COPD status adjusted for reciprocal exposures
Table 1a. Association of gas and vapors with COPD status, additionally adjusted for dusts and fumes exposures
Table 1b. Association of dusts and fumes and subcategories with COPD status, additionally adjusted for gases and vapor exposures
Supplemental Digital Content Table 2. Association of occupational exposures with COPD status


