Abstract
Aim:
Orlistat, an anti-obesity medication, inhibits lipases allowing one third of dietary fat to pass as oil in the stool. Due to the risk of oil and fecal incontinence, acceptance is limited. On the tongue, dietary fat is detected when broken down into long chain fatty acids (LCFA) by lingual lipases resulting in stimulation of neural reward systems. The goal of this study was to investigate the effects of an orlistat mouth rinse on the intake of a high fat meal.
Methods:
A double-blind, balanced order, crossover study was conducted in participants (n=10, BMI 25–30kg/m2) assigned to receive placebo or orlistat (240mg/10cc) prior to a high fat meal. Participants were divided into low- or high-fat consumers based on calories consumed from fat following placebo administration.
Results:
Orlistat mouth rinse decreased total and fat calories consumed during the high fat meal in high-fat consumers, without altering calories consumed in low-fat consumers (p< .05).
Conclusions:
Orlistat decreases LCFA absorption by inhibiting lipases that breakdown triglycerides. Orlistat mouth rinse decreased fat intake in high-fat consumers, suggesting that orlistat inhibited the detection of LCFA from the high fat test meal. Lingual delivery of orlistat is predicted to eliminate the risk of oil incontinence and promote weight loss in individuals who prefer fat.
Keywords: Tongue, Orlistat, Obesity, Fat Sensing
1. Introduction
The lipase inhibitor, orlistat (120mg, 3x/day with meals), was approved by the FDA in 1999 for the treatment of obesity in conjunction with a reduced calorie, 30% fat diet and subsequently approved for over-the-counter use to induce weight loss in adults with an overweight classification1. In a systematic review of the clinical effectiveness of orlistat use for the management of obesity, participants treated with orlistat for one year lost 3.1% more body weight than those treated with placebo2. These effects appear to be dose dependent with a lower dose of orlistat (60mg, 3x/day) leading to lower weight loss (2.4% loss, compared to placebo)2. Modest improvements in blood pressure and glycemia and a disproportionate improvement in cholesterol and steroid metabolism, even in the absence of weight loss, were reported3. Studies in individuals with type 2 diabetes and dyslipidemia also reported weight loss on the higher dose of orlistat2 and longer term studies, with up to four years of orlistat use, reported significant weight loss (2.8% compared to placebo) and a 37% reduction in conversion to diabetes in individuals with impaired glucose tolerance4.
Orlistat acts by inhibiting gastric and pancreatic lipase such that one third of the fat, on a 30% fat diet, passes undigested in the stool. Side effects are associated with oil in the stool and consist of abdominal discomfort, bloating, gaseousness, diarrhea, fecal leakage, fecal incontinence, and steatorrhea. Although not harmful, the popularity and use of orlistat has been limited due to these side effects, which can lead to embarrassment, with only modest weight loss. Furthermore, though not absorbed systemically, orlistat has been associated with rare cases of liver toxicity and pancreatitis5,6 and subsequent cost-effectiveness evaluations determined that orlistat was not cost-effective, on the basis of QUALY assessment, compared to other weight loss strategies7.
In addition to inhibiting pancreatic and gastric lipases, orlistat inhibits lingual lipases8. In the oral cavity, lingual lipase is secreted from Von Ebner glands into the cleft of the circumvallate papillae, which contains approximately 48% of taste buds in humans 9. Lingual lipases are involved in the breakdown of fat and the release of free fatty acids in the proximity of the taste receptor cells. Fat receptors on taste receptors cells, particularly on the circumvallate papillae, detect long chain fatty acids (LCFA)10. The binding of LCFA to lingual fat receptors influences fat preference and intake by initiating neural signals from the tongue to homeostatic and hedonic regions of the brain 11,12. Lingual CD36 has been proposed as the primary fat taste receptor, though other receptors, including G-protein coupled receptor, GPR120, are thought to mediate the orosensory perception of dietary fat 11–16.
Studies investigating the role of lingual fat sensors on fat preference and intake in preclinical and clinical models report individual variability in the orosensory perception of dietary fat, leading to differences in the preference for and sensitivity to fat. Obesity-prone rats exhibit a high preference for fat, but a low lingual sensitivity to fat, suggesting an impairment or desensitization of the fat signal 13,17,18. In humans, orosensory detection of fatty acids is dependent on chain length19, though there is inter-individual variability in the thresholds for LCFA and some studies suggest that there are fatty acid “tasters” and “non-tasters”20. Additionally, studies suggest that individuals considered overweight or obese, based on BMI, have a greater detection threshold and are hyposensitive to the taste of fat21.
The goal of the current pilot study was to determine whether inhibition of lingual lipase with an orlistat mouth rinse, which should decrease the breakdown of triglycerides into fatty acids, would significantly alter consumption of a high fat meal in individuals with an overweight classification. Due to potential individual variability associated with fat taste and the proposal that some individuals are fat tasters and others are non-fat tasters, participants were divided into high-fat and low-fat consumers based on fat calories consumed during the placebo trial.
2. Materials and Methods
2.1. Study Participants
To test the hypothesis that orlistat as a mouth rinse would inhibit lingual lipase and decrease both total food intake and fat intake compared to placebo, ten generally healthy individuals (aged ≥ 18 years) with a body mass index (BMI) between 25 and 30 kg/m2, classified as “overweight”, were recruited from the Pennington Biomedical Research Center database for this pilot trial. Participants that were pregnant, breast feeding, or taking medication known to alter body weight (e.g. systemic glucocorticoids) were excluded. Additional exclusions included a restraint score of 13 or higher on the 3-factor eating questionnaire or taking a medication for which, the dose was not constant for more than a month. The study was approved by the Pennington Biomedical Research Center Institutional Review Board, and all participants signed written informed consent. The trial was registered on ClinicalTrials.gov under the registration number NCT01719419.
2.2. Study Design
A double-blind, placebo-controlled, randomized and balanced crossover study was performed. All participants underwent a screening visit in the Pennington Outpatient Research Clinic, in which they gave their informed consent and completed a medical history questionnaire and 3-factor eating questionnaire22. Anthropometric measurements were performed to determine body mass index (BMI). Participants were scheduled for two meal tests, divided by 30 days (actual: 32 ± 4.9 days, mean ± SDs) in the Pennington Universal Eating Monitor. In menstruating women, study visits were scheduled during the follicular phase of the menstrual cycle.
2.3. Study Protocol
Randomization of the orlistat mouth rinse or placebo mouth rinse testing days were determined via a computer-based algorithm. Participants were asked to restrain from eating and drinking (except for water) after 2100h the night prior to testing days. On test days, participants arrived at the eating laboratory between 1100h and 1300h. Participants were given one of the two mouth rinses. Water was used for the placebo mouth rinse since orlistat dissolves completely in water and is taste-less. Therefore, the two mouth rinses were identical in taste and appearance. A Visual Analogue Scale questionnaire was administered to assess appetite perceptions (i.e. hunger, satiety, satisfaction, and prospective food intake) prior to the start of the test meal. Immediately prior to the test meal, participants were asked to rinse their mouth with 10cc of mouth rinse containing either water (placebo) or 24mg/mL of orlistat, dissolved in water, for 1–2 minutes before spitting out the solution. They were then given access to a high fat meal, containing pieces of bratwurst in an amount larger than they could reasonably eat. Participants were asked to eat to their satisfaction over a 20-minute period. Test meal contents were weighed prior to and following the 20-minute period and total caloric and fat intake were calculated. Immediately following the test meal, participants were again given the visual analog scale to assess their hunger, satiety, and satisfaction.23 The second testing visit was the same as the first, only the mouth rinse was the one that was not given on the first test visit. Participants who had a viral illness or allergy symptoms impeding their sense of smell and taste on the day of the test were rescheduled.
2.4. Statistical Analysis
An initial comparison of the total and fat calorie consumed during the placebo and orlistat mouth rinse conditions was conducted using a paired, within-subjects t-test. Participants were then divided into high-fat consumers (n=5) and low-fat consumers (n=5) using a median split analyses of the total fat calories during the placebo meal. Total caloric intake and calories from fat were analyzed using a mixed ANOVA, with mouth rinse as the repeated measure and high/low fat consumer status as the between subjects factor. Bonferroni post-hoc tests were used. A between-subjects t-test was used to calculate differences in BMI, weight (kg), age, and meal intake (g) between low/high-fat consumers. A Chi-Square was used to determine differences between sex in low/high-fat consumers. Spearman correlations were estimated for BMI and total caloric intake and fat calories consumed the placebo and orlistat conditions. Statistical significance was set at p<.05.
3. Results
3.1. Participants
Nine males and one female (age: 27.4 ± 5.9 years (mean ± SDs)) were screened, enrolled and completed the study. Participants had an average BMI of 27.0 ± 1.8 kg/m2 (mean ± SDs, Table 1). The visual analog scales of hunger and satiety confirmed that the participants were in the same state of hunger and ate to the same level of satiety in each of the cross-over arms of the trial (p=ns). No adverse events were reported.
Table 1.
Descriptive and meal intake data from enrolled participants1.
|
All (n = 10) |
Low-Fat Consumers (n = 5) |
High-Fat Consumers (n = 5) |
p-value |
|
|---|---|---|---|---|
|
| ||||
| Sex, male (%) | 90.0 | 80.0 | 100.0 | 0.999 |
| Age, years | 27.4 ± 5.9 | 26.2 ± 2.2 | 28.6 ± 8.4 | 0.553 |
| Weight (kg) | 80.6 ± 6.7 | 77.6 ± 6.0 | 83.5 ± 6.4 | 0.176 |
| BMI | 27.0 ± 1.8 | 26.1 ± 0.5 | 28.0 ± 2.2 | 0.997 |
| Meal Intake following Placebo Solution | ||||
| Total Meal Intake (g) | 647.0 ± 186.0 | 502.4 ± 147.1 | 791.6 ± 62.8 | < .01 |
| Total Caloric Intake (kcal) | 831.7 ± 350.4 | 540.9 ± 248.4 | 1122.5 ± 56.6 | < .001 |
| Calories from Fat (kcal) | 650.9 ± 273.5 | 424.0 ± 194.3 | 877.8 ± 42.0 | < .001 |
Low-fat and high-fat consumers were divided based on a median split of calories consumed from fat following application of placebo solution. Sex was compared using chi-square. Values are means ± SDs unless otherwise indicated.
3.2. Effect of Orlistat Mouth Rinse on Caloric Intake
Total calories consumed and calories consumed from fat were not significantly altered by the orlistat mouth rinse in the whole cohort of 10 participants (p=ns, Figure 1), Within the entire cohort, pretreatment with the orlistat mouth rinse decreased total caloric intake by 20.1 ± 279.8 kcal and calories from fat by 15.5 ± 219.4 kcal. Additionally, BMI did not significantly correlate with total calories or fat calories consumed during the test meal (p=ns).
Figure 1.
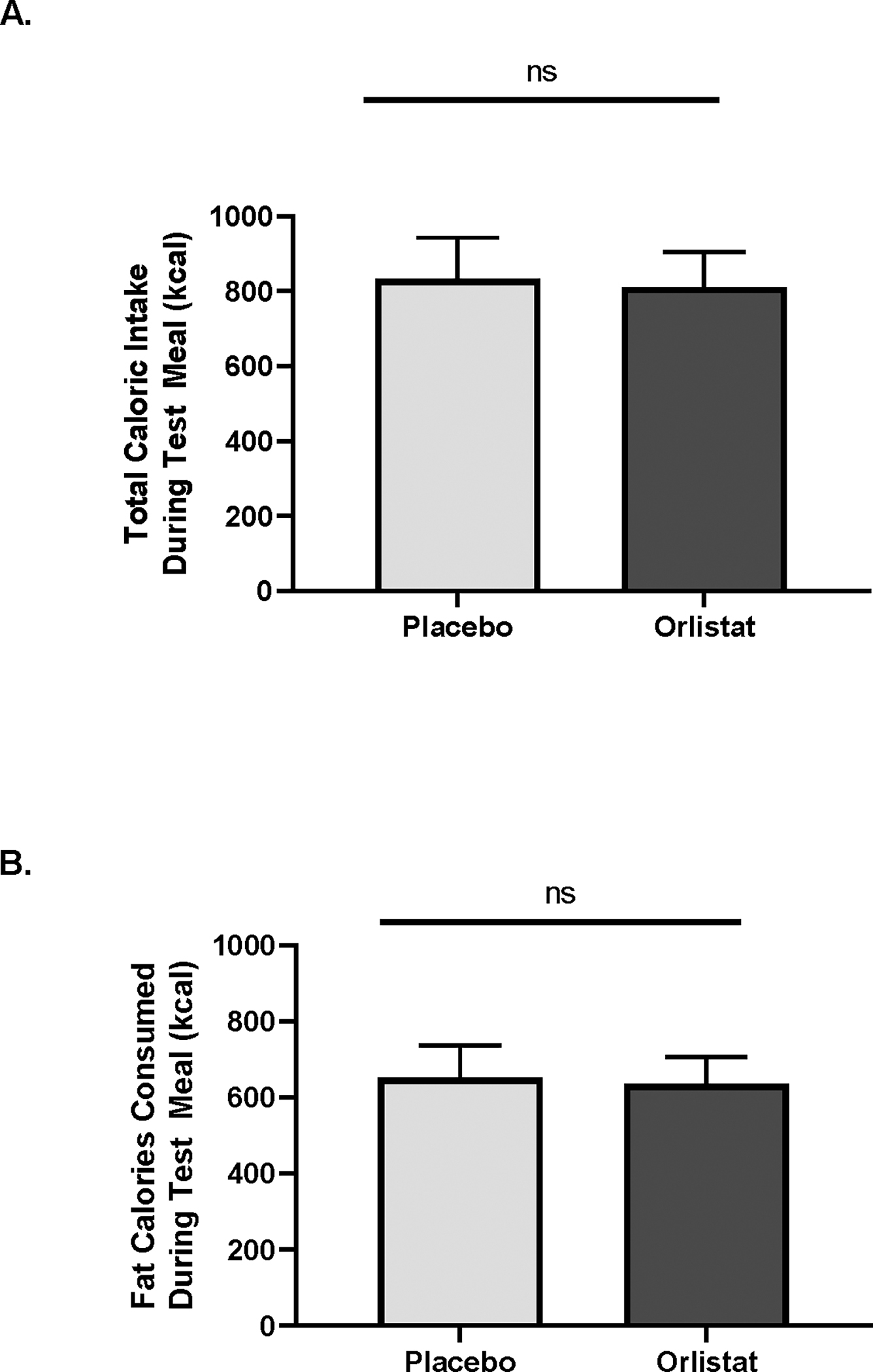
Consumption of a high fat meal was assessed following application of an orlistat mouth rinse. A. Total caloric intake of the test meal was not altered by orlistat, compared to placebo, mouth rinse, when assessed in the full cohort. B. The number of calories from fat during the test meal was not altered by the orlistat mouth rinse in the full cohort. Data is shown as mean α SEM, p=ns.
Participants were divided into low-fat and high-fat consumers based on a median split analysis of the total calories consumed from fat during the placebo trial. Low-fat and high-fat consumers did not differ on age, sex, weight or BMI, however, high-fat consumers consumed more of the test meal in the placebo trial (t = 4.0, p<.01; Table 1). A significant interaction was observed between mouth rinse and high/low-fat consumer status for total caloric intake (F = 7.4, p<.05) and calories from fat (F = 7.3, p<.05) (Figure 2A, B). High-fat consumers consumed more total calories and fat calories from the test meal following the placebo trial, compared to low-fat consumers (p<.05) and the orlistat mouth rinse significantly reduced total caloric intake and fat calories in the high-fat consumers (p<.05), but not in the low-fat consumers. Following orlistat mouth rinse treatment, high-fat consumers reduced their caloric intake by 203.9 ± 201.8 kcal, while low-fat consumers increased their caloric intake by 163.6 ± 225.9 kcal. Additionally, high-fat consumers reduced their fat intake by 159.3 ± 158.5 kcal following orlistat mouth rinse treatment, while low-fat consumers increased their fat intake by 128.3 ± 177.4 kcal.
Figure 2.
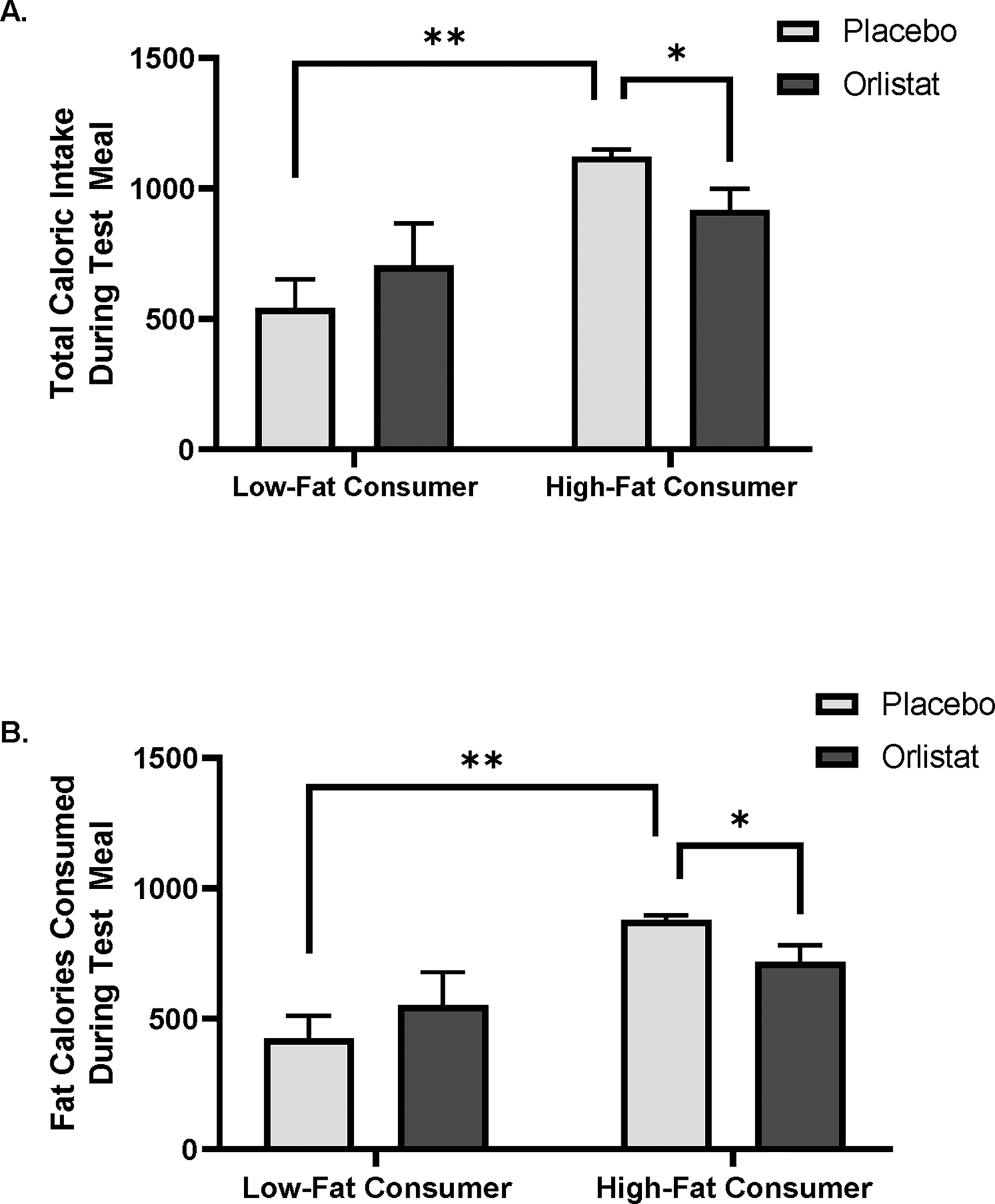
Participants were divided into low- and high-fat consumers based on a medial split analysis from fat calories consumed during the test meal following application of the placebo mouth rinse. A. High-fat consumers consumed more total calories than low-fat consumers and application of orlistat mouth wash decreased total caloric intake in high-fat consumers. B. High-fat consumers consumed more calories from fat during the test meal and orlistat mouth rinse decreased fat calorie intake in high-fat consumers. Data is shown as mean α SEM, * p< .05, **p<.0001.
4. Discussion
The goal of the current pilot study was to determine whether lingual application of the lipase inhibitor, orlistat, via mouth rinse, would reduce calorie intake of a high fat test meal in individuals with an overweight classification. As a lipase inhibitor, application of orlistat to the oral cavity was expected to inhibit lingual lipase, thereby inhibiting the breakdown of triglycerides in the high fat test meal to fatty acids. Since lingual fat taste receptors detect fatty acids, we hypothesized that detection of fatty acids by the fat taste receptors would be diminished following application of the orlistat mouth rinse and fat intake would be decreased. In the current study, as predicted, orlistat mouth rinse significantly decreased total caloric intake and fat calories consumed from the high fat test meal. However, this decrease in caloric intake was only detected in high-fat consumers, suggesting that inherent differences in fat sensing likely mediated the response to the orlistat mouth rinse. Based on these findings, we predict that low-fat consumers have an inherently diminished orosensory perception of fat, while the high-fat consumers have an inherently higher orosensory perception of fat. If low-fat consumers have a decreased oral sensitivity to fat, then further decreasing detection by inhibiting the breakdown of triglycerides to fatty acids by orlistat would have minimal effect.
Based on the findings from the current study, one must consider whether the lingual-mediated reduction in food intake from the orlistat mouth rinse versus the fecal calorie loss via the intestinal tract, from the swallowed orlistat, has a greater potential for weight loss. Orlistat leads to a 2.9% greater weight loss than placebo through fecal calorie loss,24 and Sibutramine, a serotonin and norepinephrine reuptake inhibitor previously used for the treatment of obesity, caused a 4.5% greater weight loss than a placebo by decreasing food intake.24 Sibutramine produced a 14% greater calorie reduction than placebo in a single meal test,25 and in the current pilot study, lingual application of orlistat produced a 18% greater reduction in food intake. The prediction of weight loss from a single meal test is clearly a rough estimate, but the reduction in food intake during a single meal demonstrated by oral sibutramine and lingual orlistat and the higher percentage of weight loss from sibutramine compared to orally swallowed orlistat suggests that the reduction in food intake by lingual orlistat may have a greater potential for weight loss than loss of fecal calories by orally swallowed orlistat.
Orlistat-induced inhibition of lingual lipase may be an important mediator of weight loss due to its ability to alter orosensory processing of dietary fat via lingual fat taste receptors. Taste is an important modulator of fat intake and preference and initiates a cascade of neural events that lead to changes in food intake 26. Taste signaling is a complex process which involves multiple taste receptor cells, activation of afferent nerves and neural signaling between multiple brain regions regulating homeostatic and hedonic eating. Brain regions associated with reward systems in the brain, including the ventral tegmental area, the nucleus accumbens, the pre-frontal cortex, and particularly the orbitofrontal cortex, are influenced by taste. The orbitofrontal cortex integrates sensory modalities such as taste, smell, and vision and is where decisions are made about the foods to eat.27 The reward system is hypoactive in individuals with obesity with higher threshold for oleic acid, and it is postulated that individuals with obesity select more rewarding foods that contain sugar and fat to overcome the hypoactivity of the reward system.28 The hypothalamic hunger centers are more involved in the homeostatic hunger, but the reward system is what allows us to eat dessert after finishing a large meal. The prevalence of obesity has been linked to the increased intake of these highly palatable, high fat foods 29.
Using the physiology of taste and the orosensory perception of fat as modulators of reward to manipulate body weight has not been adequately investigated. Not only is using orlistat to manipulate the preference for fat one example of using taste and the tongue to treat obesity, but there are also other receptors on the tongue that may allow manipulation of body weight in other ways. On the taste bud, Type II cells are taste receptor cells that sense sweet, bitter or umami, while Type III cells are required for the perception of sour taste. These cells activate afferent cranial nerves, thereby sending taste information to hedonic and homeostatic regions of the brain. Taste receptor cells are linked to multiple neurotransmitters and adenosine triphosphate (ATP). ATP in type III cells triggers the release of serotonin, thought to be the primary mediator of fat taste signaling to the brain. Interestingly, gastrointestinal hormones, including glucagon-like peptide-1 (GLP-1), cholecystokinin (CCK), neuropeptide Y (NPY), vasoactive intestinal polypeptide (VIP) and ghrelin, have receptors on taste receptor cells, mainly Type II cells. Leptin receptors on the taste receptor cells are predicted to modulate for the need for higher levels of sugar in the evenings in individuals with obesity due to their high leptin levels11. The hormones and their receptors on the taste receptor cells are summarized in Figure 3.
Figure 3.
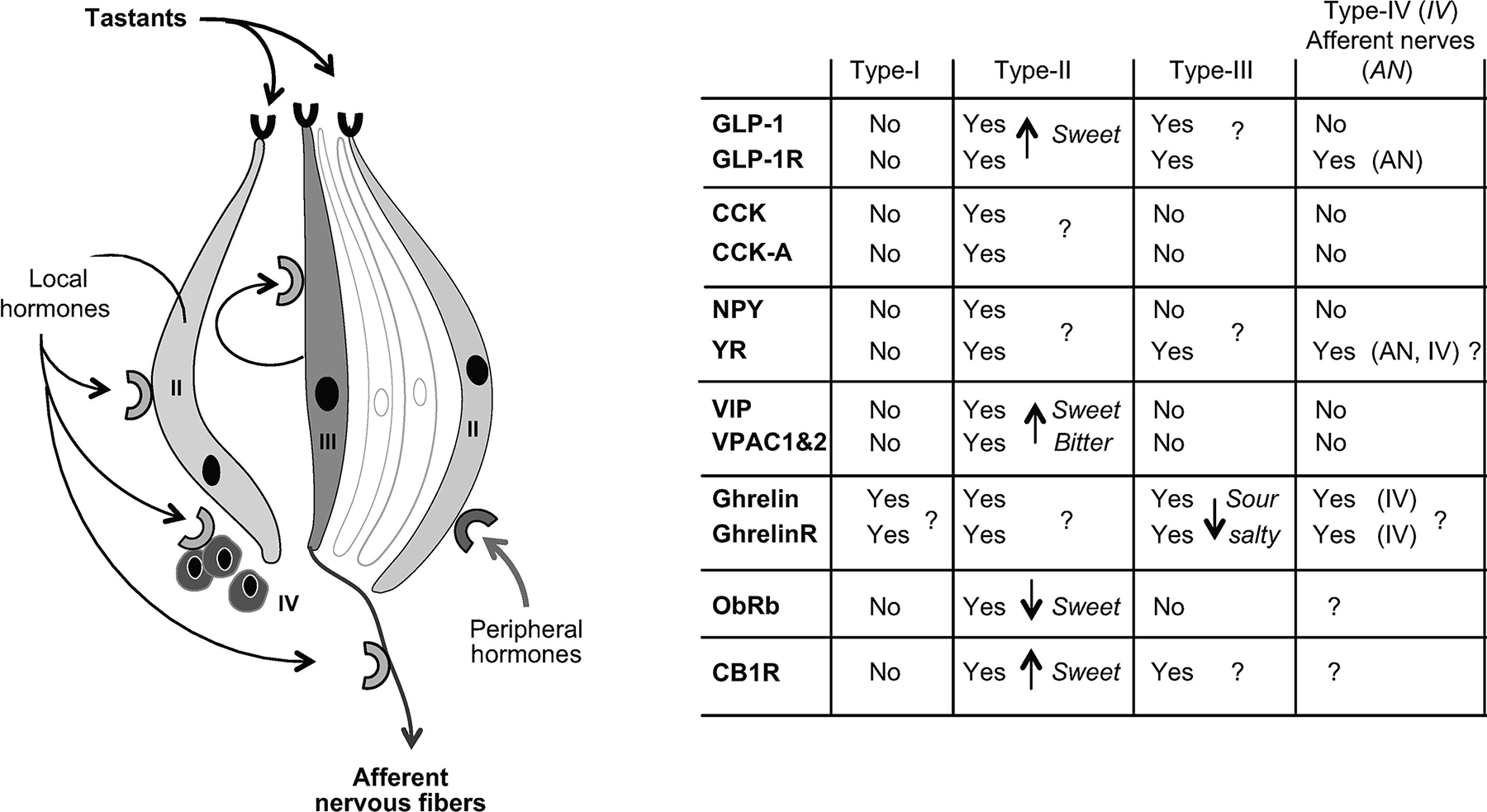
Hormone receptors and their location on taste receptor cells. Hormone location and functions in taste buds. CB1R, cannabinoid receptor type 1; CCK, cholecystokinin; CCK-A, CCK receptor A; GLP-1, glucagon-like peptide 1; GhrelinR; ghrelin receptor; NPY, neuropeptide Y; ObRb, leptin receptor; VIP, vasoactive intestinal peptide; VPAC1&2, VIP receptors; YR, NPY receptor. Reproduced with permission from Besnard et al.11
This pilot trial demonstrated that participants with overweight classification who were in the top 50% of consumers of a high fat meal reduced their calorie intake by 18% and experienced no adverse events when using the orlistat mouth rinse. Orlistat delivered as a mouth rinse, without swallowing, avoided the gastrointestinal side effects reported when orlistat was swallowed. Furthermore, lingual orlistat delivery may increase orlistat-mediated weight loss by acting preferentially on the reward system through taste signaling pathways. Our findings support the use of orlistat mouth rinse as a safer, more efficacious, and tolerable method of delivery for individuals that prefer foods high in fat and who would be at greatest risk of side effects from the traditional method of delivery. It should be noted that there are limitations to the current study, including the small sample size, the limited number of female participants and limiting the study to participants with overweight classification. Additionally, we did not assess inherent differences in fat taste sensitivity between our low- and high-fat consumers and have only investigated a single exposure to the orlistat mouth rinse, which precluded the assessment of long-term changes in caloric intake or body weight.
The findings in the current study reveal potential advantages of the lingual delivery of orlistat for treating obesity and may be extended to other potential obesity treatments by targeting lingual receptor populations (e.g. PYY). Targeting lingual receptors on the tongue to take advantage of the ability of gastrointestinal hormones like GLP-1, PYY, and CCK to reduce food intake may avoid the nausea associated with their systemic use. The reduction in gastrointestinal side effects, including nausea and vomiting, would be an advantage of lingual delivery and potentially increase adherence and ultimately weight loss. Therefore, more studies are needed to fully understand the influence of lingually applied orlistat, or gastrointestinal hormones, on fat preference, caloric intake, and weight loss.
Study Importance.
What is already known about the subject?
Orlistat gives modest weight loss and socially challenging gastrointestinal side effects which has made it unpopular with many patients who have obesity.
Fats containing long-chain fatty acids applied to the tongue in the presence of orlistat decrease the preference for fat normally seen in rodents.
What are the new findings in your manuscript?
Orlistat mouth rinse without swallowing reduces the calorie intake by 18% in the 50% of participants who had the highest intake of the high fat meal without adverse events.
How might your results change the direction of research or focus of clinical practice?
After confirmatory trials and FDA approval, orlistat mouth rinse could be an alternate delivery route with greater safety, efficacy, and tolerability than delivering orlistat through the gastrointestinal tract.
5. Acknowledgements
Supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Unidentified data will be deposited in the Pennington/NORC Repository, an institutional repository that is publicly accessible and independently searchable, as soon as possible and no later than the time of an associated publication or the end of the period of performance (or end of the no-cost extension), whichever comes first. The Pennington/NORC Repository is implementing controls in accordance with the NIH Policy NOT-OD-21-O16 specifically considerations for storing human data.
Footnotes
Disclosure: FLG’s institution receives royalties from licensing a copyright of the Food Craving inventory and shares a small percentage with FLG as one of the creators of the questionnaire. FLG has received consulting fees from the following entities: Altimune, Nutriceutical Corporation, Pfizer, Novmeta Pharma, Jenny Craig, General Nutrition Corporation, Basic Research LLC, Gedeon Richter Pharma, Jazz Pharmaceuticals, and Dr. Reddy’s Laboratories. The following patents were issued and assigned to FLG’s institution: orlistat and geranium oil mouth rinse; synergy of MLR-1023 and menthol; palatable methionine restricted food. The following patents are pending and assigned to FLG’s institution: synergy of albuterol and amylin agonist; synergy of naringenin and beta carotene. FLG participated on the Medpace Board for a registration trial of a drug. FLG owns stock in the following entities: Slim Health Nutrition, Ketogenic Health Systems, Novmeta Pharma, UR Labs, Inc., Rejuvenate Bio, Inc., Energesis Pharmaceuticals, Inc., and Plensat, Inc. Patent(s) issued and assigned to SDP’s institution: orlistat and geranium oil mouth rinse. RD has no conflicts of interest to disclose.
Clinical Trial Registration
This trial was registered on ClinicalTrials.gov under the registration number NCT01719419.
References
- 1.Gila Therapeutics Inc. Gila Therapeutics. [Website]. 2022; https://www.gilatherapeutics.com/. Accessed February 17, 2022.
- 2.O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G. A systematic review of the clinical effectiveness of orlistat used for the management of obesity. Obes Rev. 2004;5(1):51–68. [DOI] [PubMed] [Google Scholar]
- 3.Kwon YJ, Kwon GE, Lee HS, Choi MH, Lee JW. The Effect of Orlistat on Sterol Metabolism in Obese Patients. Front Endocrinol (Lausanne). 2022;13:824269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mancini MC, Halpern A. Orlistat in the prevention of diabetes in the obese patient. Vasc Health Risk Manag. 2008;4(2):325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal AM, Khalili YA. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Orlistat. Orlistat [Internet]. 2020; https://www.ncbi.nlm.nih.gov/books/NBK548898/?report=reader#!po=91.6667. Accessed February 17, 2022.
- 6.Ahmad FA, Mahmud S. Acute pancreatitis following orlistat therapy: report of two cases. JOP. 2010;11(1):61–63. [PubMed] [Google Scholar]
- 7.Finkelstein EA, Kruger E. Meta- and cost-effectiveness analysis of commercial weight loss strategies. Obesity (Silver Spring). 2014;22(9):1942–1951. [DOI] [PubMed] [Google Scholar]
- 8.Gilbertson TA, Khan NA. Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog Lipid Res. 2014;53:82–92. [DOI] [PubMed] [Google Scholar]
- 9.Doyle ME, Premathilake HU, Yao Q, Mazucanti CH, Egan JM. Physiology of the tongue with emphasis on taste transduction. Physiol Rev. 2023;103(2):1193–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiraoka T, Fukuwatari T, Imaizumi M, Fushiki T. Effects of oral stimulation with fats on the cephalic phase of pancreatic enzyme secretion in esophagostomized rats. Physiol Behav. 2003;79(4–5):713–717. [DOI] [PubMed] [Google Scholar]
- 11.Besnard P, Passilly-Degrace P, Khan NA. Taste of Fat: A Sixth Taste Modality? Physiol Rev. 2016;96(1):151–176. [DOI] [PubMed] [Google Scholar]
- 12.Jaime-Lara RB, Brooks BE, Vizioli C, et al. A systematic review of the biological mediators of fat taste and smell. Physiol Rev. 2023;103(1):855–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas Braymer H, Zachary H, Schreiber AL, Primeaux SD. Lingual CD36 and nutritional status differentially regulate fat preference in obesity-prone and obesity-resistant rats. Physiol Behav. 2017;174:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brondel L, Quilliot D, Mouillot T, et al. Taste of Fat and Obesity: Different Hypotheses and Our Point of View. Nutrients. 2022;14(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudet DA, El-Desoky D, Poret JM, Braymer HD, Primeaux SD. Expression of neural markers of gustatory signaling are differentially altered by continuous and intermittent feeding patterns. Physiol Behav. 2019;212:112719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattes RD. Fat taste and lipid metabolism in humans. Physiol Behav. 2005;86:691–697. [DOI] [PubMed] [Google Scholar]
- 17.Chen CS, Bench EM, Allerton TD, Schreiber AL, Arceneaux KP, Primeaux SD. Preference for linoleic acid in obesity-prone and obesity-resistant rats is attenuated by the reduction of CD36 on the tongue. Am J Physiol Regul Integr Comp Physiol. 2013;305(11):R1346–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreiber A, Braymer HD, Primeaux SD. Transection of Gustatory Nerves Differentially Affects Dietary Fat Intake in Obesity-Prone and Obesity-Resistant Rats. Chem Senses. 2020;45(7):541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chale-Rush A, Burgess JR, Mattes RD. Evidence for human orosensory (taste?) sensitivity to free fatty acids. Chem Senses. 2007;32(5):423–431. [DOI] [PubMed] [Google Scholar]
- 20.Kamphuis MM, Saris WH, Westerterp-Plantenga MS. The effect of addition of linoleic acid on food intake regulation in linoleic acid tasters and linoleic acid non-tasters. Br J Nutr. 2003;90(1):199–206. [DOI] [PubMed] [Google Scholar]
- 21.Stewart JE, Seimon RV, Otto B, Keast RS, Clifton PM, Feinle-Bisset C. Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. Am J Clin Nutr. 2011;93(4):703–711. [DOI] [PubMed] [Google Scholar]
- 22.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83. [DOI] [PubMed] [Google Scholar]
- 23.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;142(7):532–546. [DOI] [PubMed] [Google Scholar]
- 25.Taylor A, Fountaine R, Martin C, Mancuso J, Greenway F. Reproducibility of food intake measurements and early detection of efficacy of anorectic drugs [Abstract]. Obesity Research. 2003;11:(Suppl) A96–A97. [Google Scholar]
- 26.Ulven T, Christiansen E. Dietary Fatty Acids and Their Potential for Controlling Metabolic Diseases Through Activation of FFA4/GPR120. Annu Rev Nutr. 2015;35:239–263. [DOI] [PubMed] [Google Scholar]
- 27.Seabrook LT, Borgland SL. The orbitofrontal cortex, food intake and obesity. J Psychiatry Neurosci. 2020;45(5):304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harnischfeger F, Dando R. Obesity-induced taste dysfunction, and its implications for dietary intake. Int J Obes (Lond). 2021;45(8):1644–1655. [DOI] [PubMed] [Google Scholar]
- 29.Cvijanovic N, Feinle-Bisset C, Young RL, Little TJ. Oral and intestinal sweet and fat tasting: impact of receptor polymorphisms and dietary modulation for metabolic disease. Nutr Rev. 2015;73(5):318–334. [DOI] [PubMed] [Google Scholar]


