Abstract
Purpose:
Genetic variants in regions that include the mitochondrial genes TXNRD2 and ME3 are associated with primary open-angle glaucoma (POAG) in genome-wide association studies (GWAS). To assess their clinical impact, we investigated whether TXNRD2 and ME3 genetic risk scores (GRSs) are associated with specific glaucoma phenotypes.
Design:
Cross-sectional study
Participants:
2617 POAG cases and 2634 controls from the NEIGHBORHOOD consortium.
Methods:
All POAG-associated single nucleotide polymorphisms (SNPs) in the TXNRD2 and ME3 loci were identified using GWAS data (p<0.05). Of these, 20 TXNRD2 and 24 ME3 SNPs were selected after adjusting for linkage disequilibrium. The correlation between SNP effect size and gene expression levels was investigated using the Gene-Tissue Expression (GTEx) database. GRSs were constructed for each individual using the unweighted sum of TXNRD2, ME3, and TXNRD2+ME3 combined risk alleles. Age and gender-adjusted odds ratios (ORs) for POAG diagnosis were calculated per decile for each GRS. Additionally, the clinical features of POAG cases in the top 1, 5, and 10% of each GRS were compared to the bottom 1, 5, and 10%, respectively.
Main Outcome Measures:
POAG OR per GRS decile; maximal treated intraocular pressure (IOP) and prevalence of paracentral visual field loss among POAG cases with high vs. low GRSs.
Results:
Increased SNP effect size strongly correlated with higher TXNRD2 and lower ME3 expression levels (r=0.95 and −0.97, respectively, p<0.05 for both). Individuals in decile 10 of TXNRD2+ME3 GRS had the highest odds of POAG diagnosis (OR=1.79 compared to decile 1, p<0.001). POAG cases in the top 1% of TXNRD2 GRS had higher mean maximal treated IOP compared to the bottom 1% (19.9 mmHg vs 15.6 mmHg, adjusted p=0.03). POAG cases in the top 1% of ME3 and TXNRD2+ME3 GRS had a higher prevalence of paracentral field loss compared to the bottom 1% (72.7–88.9% vs 14.3–33.3%; adjusted p=0.03 for both).
Conclusions:
POAG patients with higher TXNRD2 and ME3 GRSs had higher treated IOP and a greater prevalence of paracentral field loss. Functional studies exploring how these variants impact mitochondrial function in glaucoma patients are warranted.
Keywords: glaucoma genetics, genetic risk score, precision medicine, mitochondria
Primary open-angle glaucoma (POAG) is a complex-inherited disease, with multiple genes and environmental risk factors contributing to optic nerve degeneration.1 Recent genome-wide association study (GWAS) meta-analyses have identified 127 genetic loci that are significantly associated with the risk of developing POAG across multiple ethnic groups.2 Interestingly, many of these genes function in molecular pathways that are relevant to POAG pathophysiology, including mitochondrial function, vascular tone, TGF-β signaling and extracellular matrix homeostasis.1–3 These findings suggest that POAG may not be a single disease, but rather, a group of diseases that share a common clinical endpoint of retinal ganglion cell death and associated visual field loss. Further characterization of the different pathways that lead to POAG development will help facilitate precision medicine approaches for individual risk stratification, diagnosis, and targeted treatment.
A genetic risk score (GRS) evaluates the cumulative effect of multiple single nucleotide polymorphisms (SNPs) in a group of genes on disease risk.4 This methodology is useful in diseases with complex inheritance such as POAG where a single gene mutation is not sufficient for disease development. Prior studies have found that individuals with higher POAG GRS have increased disease risk and earlier age at diagnosis compared to those with lower GRS.5,6 However, these studies generated GRSs using genes with several different biological functions. To date, no studies have investigated whether GRSs consisting of POAG-associated genes within a specific biological pathway are associated with distinct clinical phenotypes.
Mitochondrial dysfunction is hypothesized to play a role in POAG pathogenesis, as one of the main sites of injury is the myelin-poor, mitochondrial-rich optic nerve head. High levels of mitochondrial activity lead to the release of reactive oxygen species, which can cause intraocular pressure (IOP)-independent mechanisms of retinal ganglion cell (RGC) injury.7,8 Nicotinamide adenine dinucleotide phosphate (NADPH) is an essential component of the cellular response to reactive oxygen species, as it is required for the production of glutathione, a key cellular antioxidant. Notably, GWAS for POAG have identified 2 genes that may directly influence levels of NADPH: ME3 (malic enzyme 3) catalyzes NADPH production, whereas TXNRD2 (thioredoxin reductase 2) consumes NADPH to maintain redox homeostasis (Figure 1A).9–11 SNPs in the TXNRD2 and ME3 genetic loci may functionally contribute to POAG development and disease severity by decreasing NADPH levels, thereby increasing levels of reactive oxygen species and oxidative stress (Figure 1B).
Figure 1. Roles of the TXNRD2 and ME3 genes in the mitochondria and a possible functional mechanism for disease-associated single nucleotide polymorphisms (SNPs) in primary open-angle glaucoma (POAG) pathogenesis.
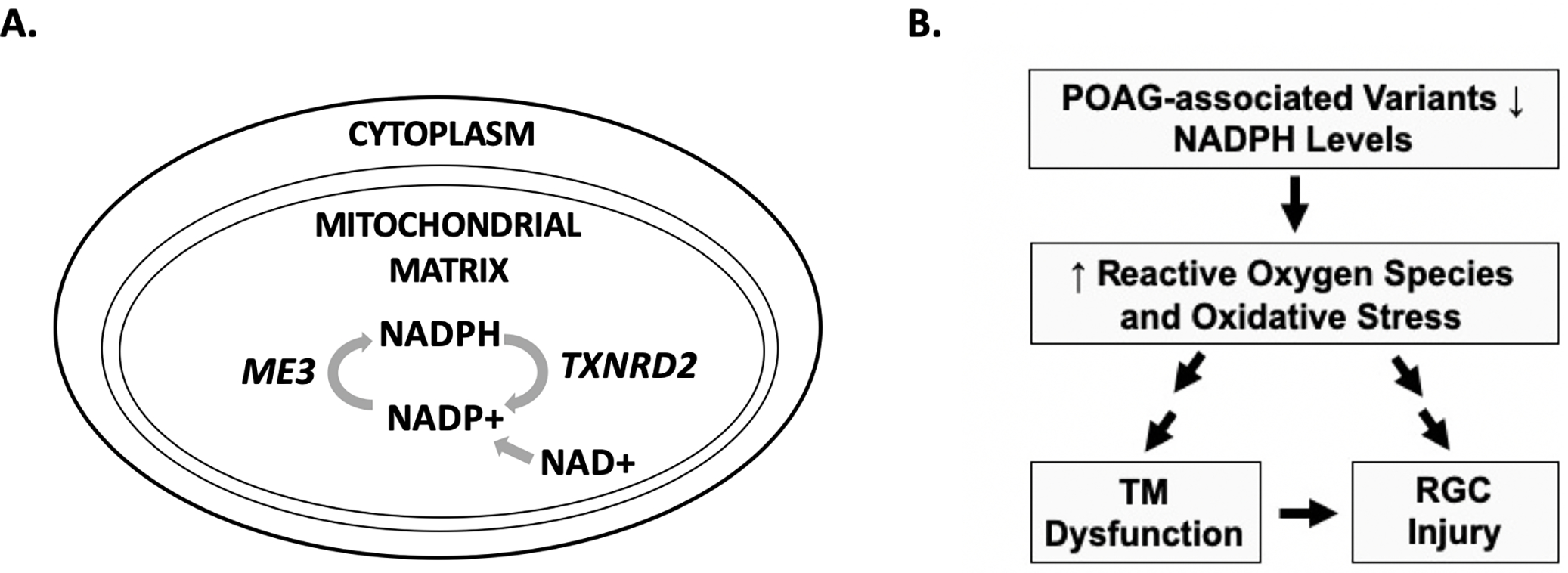
(A) The ME3 gene encodes a protein that catalyzes the production of NADPH from NADP+, while the TXNRD2 gene encodes a protein that consumes NADPH in the mitochondria. (B) POAG-associated SNPs in TXNRD2 and ME3 may lead to lower levels of NADPH, a key mitochondrial antioxidant. This would result in increased reactive oxygen species and oxidative stress. Oxidative stress has been shown to cause dysfunctional aqueous outflow through the trabecular meshwork (TM) and elevated intraocular pressure (IOP). Additionally, mitochondrial dysfunction and oxidative stress can cause toxic injury to retinal ganglion cells (RGCs) independent of IOP.
In this study, we use genetic association, tissue expression and clinical phenotype data to 1) investigate whether TXNRD2 and ME3 SNPs correlate with gene expression changes that would reduce NADPH levels, and 2) assess whether GRSs comprised of TXNRD2 and ME3 SNPs are associated with greater odds of POAG diagnosis as well as specific glaucoma clinical outcomes among individuals enrolled in the NEIGHBORHOOD (National Eye Institute Glaucoma Human Genetics Collaboration Hereditable Overall Operational Database) consortium. These analyses may provide important functional insights into the roles of specific genes and pathways in POAG pathogenesis.
Methods
The study protocol was approved by the institutional review boards of all participating institutions and adhered to the tenets of the Declaration of Helsinki. All subjects provided written informed consent after discussion of the risks, benefits and alternatives to study participation.
Study Participants
The NEIGHBORHOOD consortium study population has been previously described.9,12 In brief, all POAG cases and age/gender-matched controls were enrolled at participating institutions located throughout the United States. All cases and controls were at least 35 years old and were of European-derived or Hispanic ancestry. POAG cases were defined as individuals with open angles on anterior segment examination for whom reliable visual field tests showed characteristic glaucomatous defects localizing to the nerve fiber layer territory; we required visual field defects to be reproduced on a subsequent test or be accompanied by a cup-to-disc ratio (CDR) of 0.7 or more in at least one eye for an individual to be classified as a POAG case. Individuals with clinical signs of a secondary etiology for open-angle glaucoma such as exfoliation syndrome or pigment dispersion syndrome were excluded. Elevated IOP was not required for POAG classification. All controls had IOP less than 21 mmHg (measured in a clinical setting during regular clinic hours), a CDR of less than 0.6, and no family history of glaucoma.
Clinical data that were collected for POAG cases included age at glaucoma diagnosis, known maximal treated IOP, known latest CDR, known maximal visual field mean deviation (MD) and pattern standard deviation (PSD), presence of paracentral visual field loss, and need for glaucoma filtering surgery. Mild disease was defined as visual field MD≥ −6 dB, moderate disease as −12 dB≤MD<−6 dB and severe disease as MD< −12 dB.13 Paracentral visual field loss was classified using the following criteria based on the pattern deviation plot: 1) a cluster of three or more contiguous points in either the superior or inferior paracentral region with retinal sensitivity depression of −5 dB or greater, and 2) no loss in any other zones (Bjerrum, nasal step, or temporal wedge zones).14 Visual fields were assessed manually by two independent graders masked to genotype status.
We also assessed whether GRSs are associated with paracentral visual field loss in the Progression Risk of Glaucoma: RElevant SNPs with Significant Association (PROGRESSA) cohort given the similar definition for visual field scotoma in this dataset.15 Participants in this study were recruited and monitored at multiple public and private ophthalmology practices across Australia between 2012 and 2020. All individuals were over 40 years old at enrollment and >98% were of European-derived ancestry. Individuals who had an optic nerve head appearance suspicious or probable for glaucoma, open angles on gonioscopy and no secondary etiology for elevated IOP were enrolled for longitudinal follow-up. Visual field testing was performed every 6 months or sooner as clinically indicated.
Identification of POAG-associated SNPs in TXNRD2 and ME3
All TXNRD2 and ME3 SNPs nominally associated with POAG risk (p<0.05 and minor allele frequency ≥0.05) were identified using data from the NEIGHBORHOOD consortium GWAS for POAG.9 The genetic loci for SNP identification included the 50kb upstream and 50kb downstream of the 5’ and 3’ gene boundaries, respectively. Linkage disequilibrium clumping was performed to ensure all SNPs were independently associated with disease risk (r2<0.8) and there were no redundant effects on clinical outcome measures in our analyses.16 If two or more SNPs were in linkage disequilibrium (r2>0.8) the most representative SNP was selected using a combination of the following criteria: 1) SNPs with the most complete data available in the GWAS meta-analysis, 2) SNPs that were single nucleotide changes (rather than insertions or deletions), and 3) SNPs with the smallest p-values.
A total of 20 TXNRD2 and 24 ME3 SNPs associated with POAG risk remained for analysis after linkage disequilibrium clumping (Supplementary Table 1).
Correlation of SNP effect size with target gene expression levels
The list of POAG-associated TXNRD2 and ME3 SNPs selected for analysis (Supplementary Table 1) were queried in the Gene-Tissue Expression (GTEx) database to identify SNPs that are expression quantitative trait loci (eQTLs) and may thereby influence expression levels of the genes of interest.17 Given that the GTEx database does not currently include data from ocular tissues, we selected tibial nerve tissue as a surrogate since this is a neuronal tissue and POAG is also a neurodegenerative disorder; this tissue was previously used to identify POAG-associated SNPs that are eQTLs in the NEIGHBORHOOD consortium GWAS.9 The TXNRD2 data were also replicated in a central nervous system (CNS) tissue (brain frontal cortex, BA9); there were no ME3 eQTL data for any CNS tissues in the GTEx database so the ME3 findings were replicated in cultured fibroblasts.
The SNP effect size and tissue expression levels of the corresponding gene were obtained from the GTEx database for each eQTL. The correlation between these two continuous variables was assessed using Pearson’s correlation coefficient (r).
Construction of genetic risk scores (GRSs) and identification of individuals with high and low genetic risk
Quality control, principal components analysis and imputation were previously performed for genotype data in the NEIGHBORHOOD consortium.9 For GRS construction, the alleles of the selected TXNRD2 and ME3 SNPs were first aligned to risk for each individual in the dataset. Given the SNP effect sizes were uniformly low (Supplementary Table 1), unweighted GRSs were constructed by adding the number of risk alleles that a given individual carried for all SNPs of interest, as described previously.4,5 We constructed 3 different GRSs for each individual in the dataset: 1) TXNRD2 SNPs, 2) ME3 SNPs, and 3) TXNRD2+ME3 SNPs combined; we hypothesized that the combined TXNRD2+ME3 GRS may have greater effects on clinical outcomes given a possible combined impact on NAPDH levels (Figure 1A).
To determine whether GRSs affect the odds of POAG diagnosis, univariate analyses were performed to divide the entire cohort of cases and controls into deciles for each GRS. Multivariate logistic regression was used to derive adjusted odds ratios (ORs) of POAG diagnosis for each GRS decile, with decile 1 for that GRS serving as the reference group; age and gender were included as covariates in these analyses.
To determine whether GRSs are associated with specific glaucoma clinical outcomes, univariate analyses were performed to divide the cohort of POAG cases into percentiles for each GRS. The clinical features of POAG cases in the top 1, 5 and 10% for each GRS were then compared to the bottom 1, 5 and 10%, respectively. If data for a clinical feature was missing for a given individual then that person was excluded from that analysis.
Statistical Analysis
Associations between GRSs and continuous clinical variables were assessed using an unpaired two-tailed t-test, while a chi-squared test was used for categorical clinical variables. Adjustment for multiple comparisons was performed using the False Discovery Rate (FDR) method.18 A p-value less than 0.05 was defined as statistically significant.
All statistical analyses were performed in STATA statistical software: release 16 (StataCorp LP, College Station, TX).
Results
Correlation of TXNRD2 and ME3 SNP effect sizes with gene expression levels
To date, no protein-coding variants in TXNRD2 and ME3 have been identified in GWAS for POAG. Rather, all POAG-associated SNPs in these regions are non-coding and may influence disease risk by regulating target gene expression.19 We hypothesized that SNPs associated with higher POAG risk would increase TXNRD2 expression levels and decrease ME3 expression, as these changes could result in lower levels of NADPH and thereby lead to increased oxidative stress and mitochondrial dysfunction (Figure 1).
To test this hypothesis, we first identified all POAG-associated SNPs in the TXNRD2 and ME3 regions that are expression quantitative trait loci (eQTLs). For each eQTL, the SNP effect size and expression levels of the corresponding gene were queried in the Gene-Tissue Expression (GTEx) database in tibial nerve tissue as a surrogate neuronal tissue since GTEx does not currently have publicly available data for ocular tissues.9
In tibial nerve tissue, increasing risk SNP effect size strongly correlated with higher expression levels of TXNRD2 (r=0.95, n=5, p=0.02) and lower levels of ME3 (r= −0.97, n=8, p<0.001) (Figure 2). As seen in Figure 1B, these expression changes could result in lower NADPH levels. We replicated the TXNRD2 findings in brain frontal cortex (BA9) (r=1.0, n=3, p<0.01) (Supplementary Figure 1). The ME3 findings were replicated in cultured fibroblasts (r= −0.99, n=5, p<0.01), as no central nervous system tissues in the GTEx database had eQTL data for ME3 (Supplementary Figure 1).
Figure 2. Correlation of single nucleotide polymorphism (SNP) effect sizes with TXNRD2 and ME3 gene expression levels.
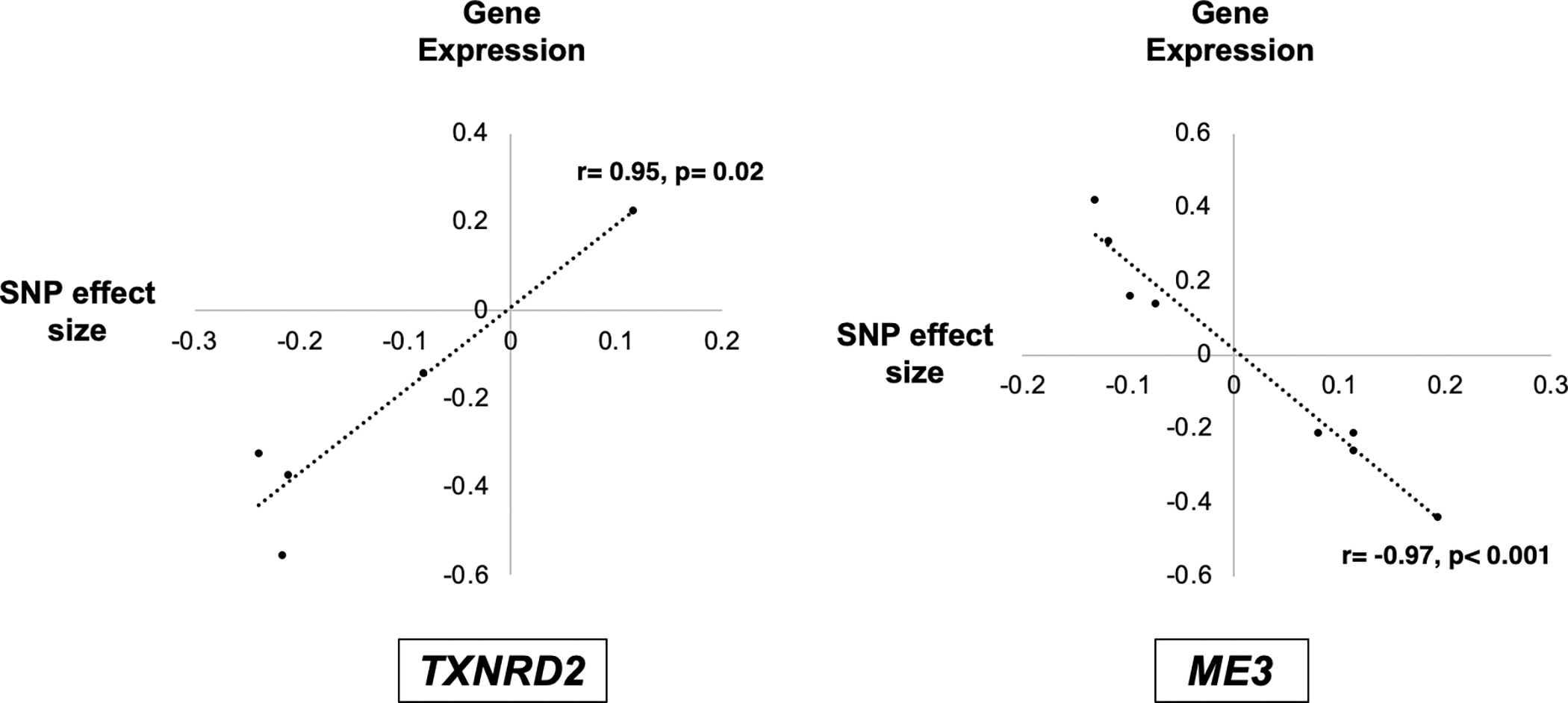
All primary open-angle glaucoma (POAG) associated SNPs in the TXNRD2 and ME3 genetic loci that are expression quantitative trait loci (eQTLs) were identified. For each eQTL, the SNP effect size and expression levels of the corresponding gene were queried in the Gene-Tissue Expression (GTEx) database. Given that the GTEx database does not currently include data from ocular tissues, we selected tibial nerve tissue as a surrogate since this is a neuronal tissue and POAG is also a neurodegenerative disorder. In tibial nerve tissue, a larger SNP risk effect size strongly correlated with higher expression levels of TXNRD2 (r=0.95, n=5, p=0.02) and lower expression levels of ME3 (r= −0.97, n=8, p<0.001). As seen in Figure 1A, these changes could lead to lower levels of NADPH in the mitochondria. Both the TXNRD2 and ME3 findings were replicated in additional tissues in the GTEx database (Supplementary Figure 1).
Demographic and clinical characteristics of NEIGHBORHOOD consortium participants
The predicted effects of TXNRD2 and ME3 SNPs on mitochondrial NADPH levels suggest that these variants could play a functional role in POAG pathogenesis. We next investigated 1) whether individuals with a greater number of disease-associated SNPs in these regions have higher odds of developing POAG, and 2) if POAG patients with a greater number of disease-associated SNPs have clinical outcomes that significantly differ from POAG patients who have a fewer number of SNPs.
A total of 2617 POAG cases and 2634 controls from the NEIGHBORHOOD consortium were included in this study, all of whom were of European-derived or Hispanic ancestry (Table 1). Approximately 50% of cases and controls were female and the mean age of POAG diagnosis was 65.2 years. Controls had a higher mean age at enrollment (68.3 years) compared to POAG cases (p<0.001). Among POAG cases, the mean maximal treated IOP was 18.0 mmHg and the mean CDR was 0.8. Most POAG cases had mild-to-moderate levels of glaucoma damage based on visual field MD criteria13 and 39% had paracentral visual field loss.
Table 1.
Demographic and clinical characteristics of NEIGHBORHOOD consortium study participants.
| Cases | Controls | p-value | |
|---|---|---|---|
| Total number of individuals | 2617 | 2634 | Not performed |
| Female, n (%) | 1369 (52.3%) (n=2617) | 1440 (54.7%) (n=2634) | 0.09 |
| Age at diagnosis (cases) or enrollment (controls) (years), mean ± SD | 65.2 ± 13.3 (n=2459) | 68.3 ± 11.4 (n=2606) | <0.001 |
| Maximal intraocular pressure (mmHg), mean ± SD (treated values for cases) | 18.0 ± 5.7 (n=2337) | 15.2 ± 2.6 (n=2446) | <0.001 |
| Maximal cup-to-disc ratio, mean ± SD | 0.8 ± 0.1 (n=2242) | 0.3 ± 0.1 (n=2391) | <0.001 |
| Visual field mean deviation (dB), mean ± SD | −9.3 ± 7.2 (n=1910) | - | Not performed |
| Visual field pattern standard deviation (dB), mean ± SD | 6.1 ± 3.3 (n=1621) | - | Not performed |
| Prevalence of paracentral visual field loss, n (%) | 353 (38.8%) (n=910) | - | Not performed |
| Need for incisional glaucoma surgery, n (%) | 139 (26.5%) (n=525) | 0 (0%) (n=2634) | Not performed |
POAG= primary open-angle glaucoma; mmHg= millimeters of mercury; dB= decibels; SD= standard deviation; “-“ = visual field testing not performed.
Association of GRSs with clinical outcomes
For each individual in the NEIGHBORHOOD consortium dataset, we constructed a GRS composed of 1) TXNRD2 SNPs, 2) ME3 SNPs, and 3) TXNRD2 and ME3 SNPs; we hypothesized that the TXNRD2+ME3 GRS may have greater effects on clinical outcomes given a possible combined impact on NAPDH levels (Figure 1A). We first calculated age and gender-adjusted odds of POAG diagnosis per decile for each GRS, with decile 1 of that GRS serving as the reference group.
Individuals at higher deciles of each GRS had significantly higher odds of POAG diagnosis and a greater effect was seen in the combined TXNRD2+ME3 GRS compared to the GRS for either gene alone (Table 2). The highest OR was found in decile 10 of the TXNRD2+ME3 GRS (OR=1.79 compared to decile 1, 95% CI: 1.39–2.30, p<0.001).
Table 2.
Age and gender-adjusted odds of POAG diagnosis per decile of GRS among individuals in the NEIGHBORHOOD consortium.
| A. TXNRD2 GRS | B. ME3 GRS | C. TXNRD2 + ME3 GRS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GRS Decile | Odds Ratio | 95% CI | p-value | GRS Decile | Odds Ratio | 95% CI | p-value | GRS Decile | Odds Ratio | 95% CI | p-value |
| 1 | - | - | - | 1 | - | - | - | 1 | - | - | - |
| 2 | 0.83 | 0.65, 1.07 | 0.15 | 2 | 0.98 | 0.76, 1.25 | 0.86 | 2 | 1.17 | 0.91, 1.51 | 0.21 |
| 3 | 1.09 | 0.85, 1.40 | 0.5 | 3 | 1.14 | 0.89, 1.45 | 0.3 | 3 | 1.31 | 1.02, 1.69 | 0.03 |
| 4 | 1.12 | 0.88, 1.44 | 0.36 | 4 | 1.1 | 0.86, 1.41 | 0.43 | 4 | 1.29 | 1.00, 1.66 | 0.047 |
| 5 | 1.1 | 0.86, 1.42 | 0.43 | 5 | 1.33 | 1.01, 1.75 | 0.04 | 5 | 1.05 | 0.82, 1.35 | 0.68 |
| 6 | 1.25 | 0.97, 1.60 | 0.08 | 6 | 1.37 | 1.07, 1.77 | 0.01 | 6 | 1.37 | 1.07, 1.77 | 0.01 |
| 7 | 1.06 | 0.83, 1.36 | 0.66 | 7 | 1.22 | 0.95, 1.55 | 0.12 | 7 | 1.31 | 1.02, 1.68 | 0.04 |
| 8 | 1.41 | 1.10, 1.81 | 0.007 | 8 | 1.27 | 0.99, 1.62 | 0.06 | 8 | 1.67 | 1.30, 2.14 | <0.001 |
| 9 | 1.41 | 1.10, 1.81 | 0.007 | 9 | 1.39 | 1.08, 1.80 | 0.01 | 9 | 1.77 | 1.38, 2.28 | <0.001 |
| 10 | 1.39 | 1.08, 1.78 | 0.01 | 10 | 1.25 | 0.94, 1.67 | 0.13 | 10 | 1.79 | 1.39, 2.30 | <0.001 |
POAG= primary open-angle glaucoma; GRS= genetic risk score; CI= confidence interval
We next investigated whether higher GRSs are associated with specific clinical outcomes. Given the OR for POAG diagnosis did not meaningfully increase until deciles 8 and 9 for TXNRD2 and ME3, respectively, we hypothesized that association with specific clinical outcomes would only be seen at extremes of GRSs. We therefore compared the clinical features of POAG cases in the top 1, 5 and 10% for each GRS to the bottom 1, 5 and 10%, respectively. POAG cases in the top 1% of TXNRD2 GRS had higher mean maximal treated IOP compared to the bottom 1% (19.9 mmHg vs. 15.6 mmHg, adjusted p=0.03) (Table 3A). While POAG cases in the top 1% of ME3 and ME3+TXNRD2 GRSs had higher mean maximal IOP compared to the bottom 1%, these differences were not statistically significant (p>0.05).
Table 3.
Summary of GRS results for specific glaucoma phenotypes among POAG cases in the NEIGHBORHOOD consortium.
| A. Maximal treated intraocular pressure (in mmHg) | |||||
|---|---|---|---|---|---|
| GRS | GRS Percentile | Mean (SD) Top GRS Percentile | Mean (SD) Bottom GRS Percentile | p-value | Adjusted p-value1 |
| TXNRD2 | 1% | 19.9 (7.1) (n=24) | 15.6 (3.9) (n=24) | 0.01 | 0.03 |
| 5% | 17.1 (5.6) (n=118) | 17.0 (4.8) (n=120) | 0.93 | 0.94 | |
| 10% | 17.3 (5.6) (n=241) | 17.8 (5.1) (n=235) | 0.28 | 0.84 | |
| ME3 | 1% | 19.2 (4.5) (n=24) | 17.9 (5.0) (n=24) | 0.36 | 0.43 |
| 5% | 18.2 (5.4) (n=118) | 18.3 (5.9) (n=118) | 0.94 | 0.94 | |
| 10% | 18.4 (5.6) (n=230) | 18.1 (5.9) (n=243) | 0.70 | 0.92 | |
| TXNRD2 + ME3 | 1% | 18.5 (5.1) (n=23) | 16.8 (5.8) (n=26) | 0.28 | 0.42 |
| 5% | 18.1 (5.6) (n=117) | 18.0 (5.5) (n=121) | 0.88 | 0.94 | |
| 10% | 17.8 (5.4) (n=230) | 18.0 (5.3) (n=239) | 0.77 | 0.92 | |
| B. Paracentral visual field loss | |||||
| GRS | GRS Percentile | Prevalence Top GRS Percentile | Prevalence Bottom GRS Percentile | p-value | Adjusted p-value1 |
| TXNRD2 | 1% | 18.2% (n=11) | 20.0% (n=5) | 0.93 | 0.93 |
| 5% | 42.6% (n=47) | 36.6% (n=41) | 0.57 | 0.62 | |
| 10% | 43.2% (n=95) | 42.9% (n=77) | 0.97 | 0.97 | |
| ME3 | 1% | 72.7% (n=11) | 14.3% (n=7) | 0.02 | 0.03 |
| 5% | 51.4% (n=37) | 44.4% (n=54) | 0.52 | 0.62 | |
| 10% | 52.5% (n=80) | 43.3% (n=90) | 0.23 | 0.69 | |
| TXNRD2 + ME3 | 1% | 88.9% (n=9) | 33.3% (n=12) | 0.01 | 0.03 |
| 5% | 41.3% (n=46) | 46.5% (n=43) | 0.62 | 0.62 | |
| 10% | 46.6% (n=88) | 41.9% (n=93) | 0.53 | 0.80 | |
Adjustment for multiple comparisons was performed using the False Discovery Rate (FDR) method
POAG= primary open-angle glaucoma; GRS= genetic risk score; mmHg= millimeters of mercury; SD= standard deviation
Additionally, POAG cases in the top 1% of ME3 GRS had a higher prevalence of paracentral visual field loss compared to the bottom 1% (72.7% vs 14.3%, adjusted p=0.03) (Table 3B). POAG cases in the top 1% of TXNRD2+ME3 GRS also had a significantly higher prevalence of paracentral field loss compared to the bottom 1% (88.9% vs 33.3%, adjusted p=0.03) (Table 3B).
There were no significant associations with GRSs noted for age at diagnosis, latest CDR, visual field mean deviation/pattern standard deviation, or need for glaucoma surgery (Supplementary Table 2). We also assessed the association of GRSs with paracentral visual field loss in the PROGRESSA cohort given the similar definition of visual field scotoma.15 However, while the PROGRESSA data combined with the NEIGHBORHOOD data continued to be significant (Supplemental Table 3), the PROGRESSA data alone did not demonstrate a significant association, possibly due to the smaller sample size (n=816 POAG cases and suspects).
Discussion
GWAS have identified over 120 genetic loci strongly associated with risk for developing POAG.2 A key challenge is characterizing the underlying functional mechanisms and identifying the clinical relevance of these gene variants. Ultimately, this information can be utilized to improve disease diagnosis, prognostication and treatment outcomes.
We have demonstrated that non-coding variants in the mitochondrial genes TXNRD2 and ME3 strongly correlate with target gene expression changes that could lead to lower levels of NADPH, suggesting a potential mechanism for how these genetic variants contribute to POAG pathogenesis. We also utilized the NEIGHBORHOOD consortium dataset to show that individuals with higher GRSs for TXNRD2 and ME3 have greater odds of developing POAG. Moreover, among POAG patients, those with higher GRSs for TXNRD2 and ME3 had higher treated IOP and greater prevalence of paracentral field loss, suggesting that these gene variants are associated with more clinically severe disease with field damage close to fixation.
The significantly higher ORs for POAG diagnosis at higher deciles of GRS and the combined TXNRD2+ME3 GRS compared to either gene alone demonstrates the importance of assessing the cumulative effect of multiple SNPs with small effect sizes on disease risk and disease endophenotypes. We hypothesize that the increased clinical impact with multiple SNPs in these two genes is due to larger effects on target gene expression, which could lead to greater reductions in NADPH levels. Notably, the differences in maximal treated IOP and paracentral visual field loss only became significant when comparing the top vs. bottom 1% of GRSs, suggesting that extremes of GRSs are needed to observe clinically meaningful differences in disease severity. Additionally, while the top 1% of TXNRD2 GRS had higher mean maximal treated IOP and the top 1% of ME3 GRS had a higher prevalence of paracentral visual field loss, the top 1% of TXNRD2+ME3 combined GRS only had a higher prevalence of paracentral field loss and did not have higher mean maximal IOP. We hypothesize this could be due to a threshold level of each individual GRS needed for association with a given phenotype: the individuals in the top 1% of TXNRD2+ME3 combined GRS in our dataset may not have had a GRS for TXNRD2 alone that reached the threshold needed for association with higher IOP.
The association of the TXNRD2 GRS with higher treated IOP is consistent with prior GWAS meta-analyses where SNPs in both TXNRD2 and ME3 were strongly associated with elevated IOP.20 While we also noted higher mean maximal IOP in individuals with high GRS for ME3 and TXNRD2+ME3, these differences were not statistically significant, likely due to the relatively small size of our case-only analyses. Mitochondrial dysfunction in the conventional outflow pathway due to a combination of aging, genetic variants and environmental stressors could lead to elevated IOP and glaucomatous damage. Studies in trabecular meshwork (TM) cells, for instance, show that higher levels of reactive oxygen species and oxidative stress results in aqueous outflow resistance as well as TM cell death.21,22
The association of higher ME3 and TXNRD2+ME3 GRSs with a greater prevalence of paracentral visual field defects was particularly striking and may be explained by the higher mitochondrial requirements of retinal ganglion cells (RGCs) in the papillomacular bundle. The pre-laminar region of the optic nerve is unmyelinated and, as a result, there is a large energy requirement for nerve conduction compared to the myelinated, post-laminar region.23 Thus, levels of mitochondrial activity are particularly high in the pre-laminar region. Given that RGCs in the papillomacular bundle have a smaller cross-sectional area compared to other RGCs, they are already at a physiological disadvantage and are hypothesized to be more susceptible to damage in the setting of mitochondrial dysfunction.23
The results of our study hold important implications for the future of glaucoma clinical management. Patients with high GRSs for TXNRD2 and ME3 had significantly higher rates of paracentral visual field defects in this study, suggesting that they may benefit from closer monitoring and more aggressive treatment to prevent damage close to fixation. Individuals with disease driven by mitochondrial dysfunction may also benefit from targeted therapeutic approaches. For instance, clinical trials suggest that the NAD+ precursor nicotinamide may serve as a neuroprotective strategy POAG.24,25 Given that NAD+ can be converted to NADPH through intracellular enzymatic reactions, individuals who have low levels of NADPH due to disease predisposing genetic variation may be especially likely to benefit from nicotinamide.
Our study does have some limitations. First, this was a cross-sectional study so longitudinal changes or rates of disease progression could not be compared in individuals with high vs. low genetic risk scores. Additionally, the NEIGHBORHOOD consortium is comprised of individuals of Caucasian ancestry and, therefore, the results of this study may not be generalizable to other ethnic populations; both TXNRD2 and ME3 are associated with POAG risk in multi-ethnic GWAS meta-analyses2, but the clinical associations with IOP and paracentral field loss found in this study will need to be confirmed in other ethnic groups.
We hypothesize that mitochondrial dysfunction and reduced levels of NADPH must reach a threshold level for disease manifestation, with age, environmental risk factors and gene variants each impacting this pathway over time. Large prospective clinical studies are needed to identify the threshold levels where high TXNRD2/ME3 GRSs become clinically meaningful and to characterize the role of other genetic and environmental modifiers. Additionally, confirming the predicted effects of TXNRD2/ME3 SNPs on NADPH levels in patient samples and/or cell-based assays is an important future direction to characterize the underlying functional mechanism.
In summary, this is the first study, to our knowledge, to demonstrate that GRSs comprised of POAG-associated genes within a specific biological pathway are associated with distinct glaucoma-related clinical phenotypes. The findings of our work suggest that a subset of POAG may be driven by mitochondrial dysfunction and that individuals with mitochondrial gene variants are at especially high risk of paracentral visual field loss. Further characterization of this pathway and other biological pathways associated with POAG will help facilitate precision medicine approaches for individual risk stratification, diagnosis and targeted treatment.
Supplementary Material
Supplementary Figure 1. Correlation of single nucleotide polymorphism (SNP) effect sizes with TXNRD2 and ME3 gene expression levels in additional tissue types. The findings in Figure 2 were replicated in additional tissues from the GTEx database. A larger SNP effect size strongly correlated with higher expression levels of TXNRD2 in the brain frontal cortex (BA9) (r=1.0, n=3, p<0.01) and lower levels of ME3 in cultured fibroblasts (r= −0.99, n=5, p<0.01) (no central nervous system tissues in the GTEx database had eQTL data available for ME3).
Acknowledgments
We would like to thank all the patients and NEIGHBORHOOD consortium investigators who contributed samples and clinical data to this study.
Financial support:
NIH/NEI R01 EY022305 (JLW), R01 EY015473 (LRP), R01 EY032559 (LRP/JLW); Heed Ophthalmic Foundation/Society of Heed Fellows (IFA); Research to Prevent Blindness Challenge Grant (LRP); The Glaucoma Foundation (LRP). The funding organizations had no role in the design or conduct of this research
Footnotes
Conflict of interest: LRP is a consultant to Eyenovia, Skye Biosciences and Twenty Twenty
Meeting presentation: American Glaucoma Society 2022 Annual Meeting (paper presentation)
References
- 1.Aboobakar IF, Wiggs JL. The genetics of glaucoma: Disease associations, personalised risk assessment and therapeutic opportunities-A review. Clin Exp Ophthalmol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gharahkhani P, Jorgenson E, Hysi P, et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat Commun. 2021;12(1):1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choquet H, Wiggs JL, Khawaja AP. Clinical implications of recent advances in primary open-angle glaucoma genetics. Eye (Lond). 2020;34(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Igo RP Jr., Kinzy TG, Cooke Bailey JN. Genetic Risk Scores. Curr Protoc Hum Genet. 2019;104(1):e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan BJ, Bailey JC, Igo RP Jr., et al. Association of a Primary Open-Angle Glaucoma Genetic Risk Score With Earlier Age at Diagnosis. JAMA Ophthalmol. 2019;137(10):1190–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanon-Moreno V, Ortega-Azorin C, Asensio-Marquez EM, et al. A Multi-Locus Genetic Risk Score for Primary Open-Angle Glaucoma (POAG) Variants Is Associated with POAG Risk in a Mediterranean Population: Inverse Correlations with Plasma Vitamin C and E Concentrations. Int J Mol Sci. 2017;18(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chrysostomou V, Rezania F, Trounce IA, Crowston JG. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr Opin Pharmacol. 2013;13(1):12–15. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J, Wang S, Zhong W, Yang B, Sun L, Zheng Y. Oxidative stress in the trabecular meshwork (Review). Int J Mol Med. 2016;38(4):995–1002. [DOI] [PubMed] [Google Scholar]
- 9.Bailey JN, Loomis SJ, Kang JH, et al. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat Genet. 2016;48(2):189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig JE, Han X, Qassim A, et al. Multitrait analysis of glaucoma identifies new risk loci and enables polygenic prediction of disease susceptibility and progression. Nat Genet. 2020;52(2):160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradshaw PC. Cytoplasmic and Mitochondrial NADPH-Coupled Redox Systems in the Regulation of Aging. Nutrients. 2019;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiggs JL, Hauser MA, Abdrabou W, et al. The NEIGHBOR consortium primary open-angle glaucoma genome-wide association study: rationale, study design, and clinical variables. J Glaucoma. 2013;22(7):517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodapp E, Parrish RK, Anderson DR. Clinical decisions in glaucoma. St. Louis, Mo.: Mosby; 1993. [Google Scholar]
- 14.Loomis SJ, Kang JH, Weinreb RN, et al. Association of CAV1/CAV2 genomic variants with primary open-angle glaucoma overall and by gender and pattern of visual field loss. Ophthalmology. 2014;121(2):508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siggs OM, Qassim A, Han X, et al. Association of High Polygenic Risk With Visual Field Worsening Despite Treatment in Early Primary Open-Angle Glaucoma. JAMA Ophthalmol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40(Database issue):D930–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Consortium GT. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369(6509):1318–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JJ, Roberson PK, Schell MJ. The false discovery rate: a key concept in large-scale genetic studies. Cancer Control. 2010;17(1):58–62. [DOI] [PubMed] [Google Scholar]
- 19.Zhang F, Lupski JR. Non-coding genetic variants in human disease. Hum Mol Genet. 2015;24(R1):R102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khawaja AP, Cooke Bailey JN, Wareham NJ, et al. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat Genet. 2018;50(6):778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamer WD, Acott TS. Current understanding of conventional outflow dysfunction in glaucoma. Curr Opin Ophthalmol. 2012;23(2):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y, Epstein DL, Liton PB. Intralysosomal iron induces lysosomal membrane permeabilization and cathepsin D-mediated cell death in trabecular meshwork cells exposed to oxidative stress. Invest Ophthalmol Vis Sci. 2010;51(12):6483–6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu-Wai-Man P, Griffiths PG, Chinnery PF. Mitochondrial optic neuropathies - disease mechanisms and therapeutic strategies. Prog Retin Eye Res. 2011;30(2):81–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui F, Tang J, Williams PA, et al. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: A crossover randomized clinical trial. Clin Exp Ophthalmol. 2020;48(7):903–914. [DOI] [PubMed] [Google Scholar]
- 25.De Moraes CG, John SWM, Williams PA, Blumberg DM, Cioffi GA, Liebmann JM. Nicotinamide and Pyruvate for Neuroenhancement in Open-Angle Glaucoma: A Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2022;140(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Correlation of single nucleotide polymorphism (SNP) effect sizes with TXNRD2 and ME3 gene expression levels in additional tissue types. The findings in Figure 2 were replicated in additional tissues from the GTEx database. A larger SNP effect size strongly correlated with higher expression levels of TXNRD2 in the brain frontal cortex (BA9) (r=1.0, n=3, p<0.01) and lower levels of ME3 in cultured fibroblasts (r= −0.99, n=5, p<0.01) (no central nervous system tissues in the GTEx database had eQTL data available for ME3).


