Abstract
Cardiac malformations are sporadically diagnosed in domestic species; however, little literature is available for this group of developmental anomalies in goats. We performed a retrospective study to catalog congenital cardiac conditions in goats submitted to the University of California–Davis, Veterinary Medical Teaching Hospital, Anatomic Pathology Autopsy Service. From 2000 to 2021, of 1,886 goat autopsies, 29 cases of cardiac malformations were identified (1.5%). Thirteen were ≤ 2-wk-old, 8 were 1–6-mo-old, and 8 were adults 2–9-y-old. The most common malformations were ventricular septal defect (VSD; 21 of 29), atrial septal defect or persistent foramen ovale (10 of 29), and double-outlet right ventricle (3 of 29). Nine cases had > 1 malformation, typically including a VSD. Conditions that had not been reported in the goat included double-outlet right ventricle (3), tetralogy of Fallot (1), cor triatriatum sinister (1), and mitral valve dysplasia (1). Two adult cases were incidental and not suspected clinically. Cardiac malformations occur not uncommonly in goats and should be considered in a wide age range.
Keywords: autopsy, congenital, goats, heart defects
Congenital cardiac malformations in goats have been reported only rarely. In a study of congenital malformations in 1,092 Saanen and Saanen-cross breed goats, cardiac malformations were not noted. 2 Sporadic case reports exist of ventricular septal defects (VSDs),14,16 tricuspid valve anomaly, 9 hypoplasia of the pulmonary trunk, 17 persistent left cranial vena cava, 15 and Epstein anomaly 12 ; however, the prevalence of congenital cardiac malformations of goats is not available. We describe here congenital cardiac malformations in goats and describe the corresponding gross lesions to aid in the diagnosis of a subset of cardiac malformations seen in this species.
We searched the pathology database of the University of California, Davis–Veterinary Medical Teaching Hospital (UCD-VMTH) for cardiac malformations in the goat for the period from 2000 to 2021. We used combinations of the following keywords: cardiac, heart, anomaly, defect, and malformation (Table 1; Suppl. Table 1). Of 1,886 goats autopsied in this period, 29 cases of congenital heart disease were identified, from 0-d to 9-y-old. Thirteen were ≤ 2-wk-old, 8 were 1–6-mo-old, and 8 were 2–9-y-old. Seventeen were female, 10 were male, and 2 were castrated males. Breeds affected were Toggenburg (7), Nubian (7), Boer (7), Nigerian dwarf (2), mixed-breed (2), Alpine (1), Angora (1), Saanen (1), and Sonnet (1).
Table 1.
Signalment, type of malformation, and cause of death in 29 goats presented to the University of California–Davis, Veterinary Medical Teaching Hospital.
| Case | Age | Sex | Breed | Cardiac malformation | Cause of death |
|---|---|---|---|---|---|
| 1 | 4 d | M | Boer × Nubian | VSD | Atresia ani |
| 2 | 2 wk | M | Toggenburg | VSD | Cardiac |
| 3 | 4 mo | F | Boer | VSD | Cardiac and vertebral malformations |
| 4 | 1d | F | Boer | VSD | Cardiac |
| 5 | 3 mo | F | Nigerian dwarf | VSD | Cardiac, bacterial pneumonia |
| 6 | Adult | F | Mix (unknown) | VSD | Cardiac, dystocia |
| 7 | 5 wk | M | Boer | VSD | Cardiac |
| 8 | 4 y | F | Nubian | VSD | Cardiac |
| 9 | 9 y | CM | Nubian | VSD | Abomasal impaction |
| 10 | 6 y | F | Nubian | VSD | Trauma (dog attack) |
| 11 | 3 y | F | Nubian | VSD (muscular or inlet) | Cardiac |
| 12 | 4 wk | M | Nigerian dwarf | VSD, PFO, PDA | Cardiac |
| 13 | 2 wk | M | Toggenburg | VSD, PFO, PDA | Cardiac |
| 14 | 2 d | F | Toggenburg | VSD, PFO, PDA | Cardiac |
| 15 | 10 d | F | Saanen | VSD, PDA | Cardiac |
| 16 | 2 y | F | Nubian | VSD, PS | Cardiac |
| 17 | 7 d | F | Nubian | DORV with subaortic VSD, PFO or ASD | Cardiac |
| 18 | 14 d | F | Alpine | DORV with subpulmonary VSD, PFO | Cardiac |
| 19 | 4 wk | F | Sonnet | DORV with unspecified VSD | Cardiac |
| 20 | 3 mo | CM | Boer | ASD (ostium secundum defect) | Urolithiasis |
| 21 | 1 d | M | Boer | PFO or ASD | Cardiac |
| 22 | 4 d | M | Toggenburg | Tricuspid atresia with VSD, PFO or ASD, PS, and PDA | Cardiac |
| 23 | Neonatal | F | Boer | Tricuspid atresia without VSD, PFO or ASD | Cardiac |
| 24 | 4 d | F | Toggenburg | Tricuspid valve dysplasia, infundibular VSD, PFO, PDA | Cardiac, bacterial rumenitis |
| 25 | 10 mo | M | Nubian | Mitral valve dysplasia, tricuspid valve dysplasia | Cardiac |
| 26 | 6 mo | F | Toggenburg | Tetralogy of Fallot | Cardiac |
| 27 | 2 y | M | Boer | Cor triatriatum sinister | Urolithiasis |
| 28 | 2 d | F | Toggenburg | Complete atrioventricular canal, common arterial trunk | Cardiac, palatoschisis, sternal agenesis |
| 29 | 14 d | M | Angora | Dilated cardiomyopathy (noninflammatory) | Cardiac |
ASD = atrial septal defect; CM = castrated male; DORV = double-outlet right ventricle; F = female; M = male; PDA = patent ductus arteriosus; PFO = persistent foramen ovale; PS = pulmonic stenosis; VSD = ventricular septal defect.
VSD was the most common defect (21 of 29), followed by atrial septal defect (ASD) or persistent foramen ovale (PFO; 10 of 29), and double-outlet right ventricle (DORV; 3 of 29); we retrieved no goat cases of DORV in a search of Google and PubMed, suggesting that no descriptions of this condition have been reported in goats. Other conditions included tricuspid atresia (2), mitral and/or tricuspid valve dysplasia (2), tetralogy of Fallot (1), cor triatriatum sinister (1), complete atrioventricular canal and common arterial trunk (1), and ventricular dilation of unknown cause (1). Although cardiac malformation was the cause of death or euthanasia in most cases, it was not the main cause of death or euthanasia for 5 cases that included VSDs (3), ASD or PFO (1), and cor triatriatum sinister (1).
The postmortem diagnosis of cardiac malformations is made during gross examination, and hence, careful examination of the heart is required to recognize cardiac anomalies when no relevant history is provided. As expected, goat kids ≤ 2-wk-old were affected most commonly; however, a number of adult goats were also identified. A subset (3 of 8) of adult goats were multiparous and were presented during or after kidding, but not during pregnancy, suggesting that the heart had compensated for the previous pregnancies despite identified malformations.
VSD was the most common single and combined cardiac malformation in our study, as reported in other domestic species.3,7 VSDs typically caused right-sided dilation; identification of the defect is reasonably straightforward based on the identification of a hole that connects the 2 ventricles. In our case series, a right-to-left shunt caused by significant pulmonary hypertension was not identified in any of the patients. Based on location, VSDs can be further characterized into 1) perimembranous, located immediately subjacent to the aortic valve; 2) muscular (or trabecular), located further apical in the interventricular septum; 3) infundibular (or supracristal), located subjacent to the pulmonary valve; and 4) inlet, located behind the atrioventricular valves. Most of the VSDs identified in our cohort were perimembranous. The VSD in case 11 was noted distant from the aortic valve, which suggests muscular or inlet. In the infundibular VSD with tricuspid dysplasia in case 24, the size of the defect did not seem to have clinical significance, especially given that the larger perimembranous VSDs in cases 9 and 10 were not the cause of death or euthanasia.
ASD and PFO were the second most common defects that occurred as a single malformation or in conjunction with other defects. ASDs can also cause right-sided dilation and a shunt reversal, which was not noted in any patients in our series. Typically, a PFO has a residual flap, 10 whereas an ASD is an open defect located most often midway between the atria. A defect above the mitral valve in this location can also resemble other malformations, such as cor triatriatum sinister and supravalvular mitral stenosis. A 3D examination to determine where the defect connects is critical for accurate diagnosis. The most common location of ASD is in the ostium secundum, which occurs along the fossa ovalis where the septum secundum and septum primum overlap (Fig. 1; case 20). 20 Ostium primum ASDs occur immediately above or at the mitral or tricuspid valve, which can form an atrioventricular septal defect. 20 Sinus venosus ASD occurs at the opening of the venae cavae. ASDs can also occur at the coronary sinus. 20 PFOs, in contrast, are generally located higher than ostium secundum defects (cases 21–23). 13
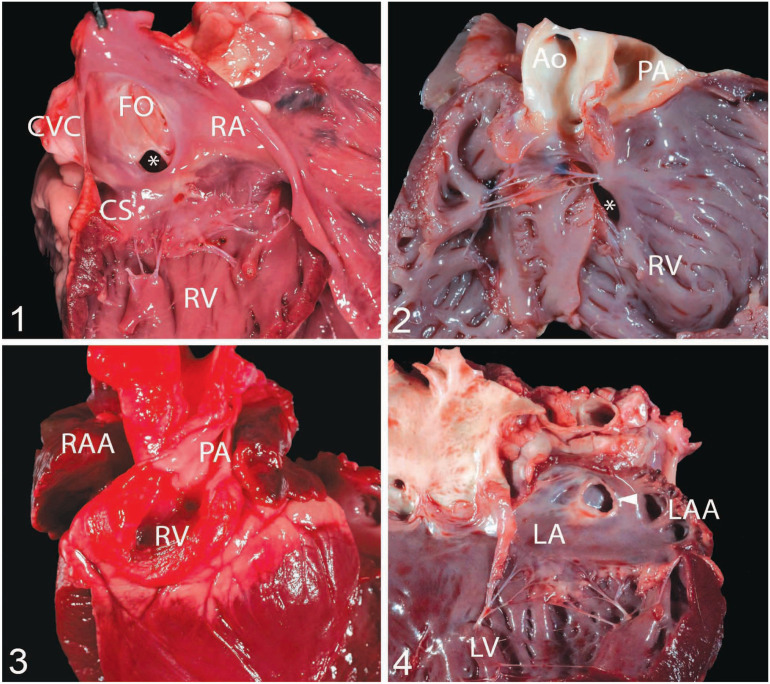
Figures 1–4.
Congenital cardiac malformations identified in our case series from the University of California–Davis, Veterinary Medical Teaching Hospital. Figure 1. Atrial septal defect (ASD; asterisk) of the ostium secundum in case 20, a 3-mo-old, castrated male, Boer goat. The 4-mm defect is located above the tricuspid valve but below the fossa ovale (FO; ostium secundum defect). The coronary sinus (CS) is located at 8 o’clock from the defect. Figure 2. Double-outlet right ventricle in case 17, a 7-d-old, female Nubian kid with a ventricular septal defect (asterisk). This goat also had a patent foramen ovale (not shown). Figure 3. Tricuspid atresia in case 23, a neonatal, female, Boer kid that was cyanotic. The right ventricle (RV) sits at the base of the heart and is a closed chamber. The right atrial appendage (RAA = right auricle) is enlarged and congested. This goat also had a 15-mm ASD. Figure 4. Cor triatriatum sinister in case 27, a 2-y-old, male, Boer goat that was euthanized because of urolithiasis. A single defect composed of a membrane (arrowhead) divides the left atrium (LA) into 2 chambers. The left atrial appendage (LAA = left auricle) is not enlarged. Ao = aorta; CVC = caudal vena cava; LV = left ventricle; PA = pulmonary artery; RA = right atrium; RV = right ventricle.
DORV was identified in cases 17–19. DORV is a serious condition in which both the aorta and pulmonary artery exit from the right ventricle, hence the term “double outlet” (Fig. 2; Suppl. Fig. 1); right-sided dilation is a common external feature. Hearts with DORV have a heterogeneous group of malformations that lead to different clinical presentations. In human medicine, the relationship of the great vessels to the DORV and the location of VSD (subaortic, subpulmonary, noncommitted, or double committed) are important for treatment purposes. 21 DORV has been characterized in a subset of dogs and cats, with 1 dog alive at 53-mo-old. 4
Tricuspid atresia is a condition in which the tricuspid (right atrioventricular) valve fails to form and hence causes separation of the right atrium and ventricle (Fig. 3). 19 The size of the affected right chambers depends on the presence of other defects that allow inflow and outflow of blood. VSDs and pulmonary stenosis or atresia are common malformations that dictate the subtype. Other malformations such as patent ductus arteriosus and PFO can also be seen concurrently. Dissection from both the venae cavae and the pulmonary artery is the most logical way to confirm tricuspid atresia grossly.
Tetralogy of Fallot is characterized by pulmonary stenosis, VSD, overriding aorta, and right ventricular hypertrophy, and has been reported in the goat. 8 Age at presentation depends on the severity of the malformations.
Cor triatriatum sinister is a rare congenital heart defect caused by abnormal pulmonary vein incorporation into the left atrium, creating an anomalous fibromuscular membrane that divides the left atrium into 2 chambers (Fig. 4). 1 Grossly, left atrial enlargement, excluding the left auricle, and pulmonary edema are most apparent. The low (apical) left atrial chamber communicates with the left ventricle via the mitral valve; the high chamber directly receives pulmonary venous inflow. In veterinary species, this condition is most often described in the cat. 18
Atrioventricular valve dysplasia can affect one or multiple leaflets, and clinical severity depends on the amount of reflux caused by the malformed valves (Suppl. Figs. 2, 3). When tricuspid dysplasia is noted, care should be taken to examine the location of the valve because apical malpositioning of the valve will support the diagnosis of Epstein anomaly, which has been reported in the goat. 12
Complete atrioventricular canal with common arterial trunk occurs when the septum does not form because of failure of fusion of the endocardial cushions, 11 which leads to a single ventricle, single atrium, and single arterial trunk that later branches into the aorta and pulmonary artery. Externally, the heart is enlarged with abnormal major vessels at the base. Clinical signs are generally the result of pulmonary circulation overload and congestive heart failure. In people, atrioventricular canal defects are typically associated with a syndromic abnormality. 11 Case 28 also had palatoschisis and sternal bone agenesis, both of which can lead to respiratory distress. These malformations collectively led to the clinical signs of weakness with open-mouth breathing and euthanasia when 2-d-old.
Neonatal dilated cardiomyopathy, with or without myocarditis, is an idiopathic condition that can have a viral, metabolic, and/or genetic etiology. 6 In people, coxsackievirus or adenovirus have been implicated as well as neonatal hypocalcemia. 5 No external causation was identified in case 29, hence a genetic origin is presumed.
A diversity of congenital cardiac malformations, some of which were incidental findings, have been identified in goats presented to the UCD-VMTH. We recommend a thorough cardiac examination in a goat autopsy, as with other species, with or without a history of cardiac disease, to better understand the prevalence, types of malformation, and clinical significance of these lesions.
Supplemental Material
Supplemental material, sj-pdf-1-vdi-10.1177_10406387231171568 for A retrospective study of congenital cardiac malformations in 29 goats by Christine Haake, Samantha L. Kovacs and Eunju April Choi in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank the past and present University of California–Davis clinicians who submitted these cases and the anatomic pathology residents and faculty involved in the autopsies for the documentation and diagnoses of these congenital heart diseases. Special thanks go to Drs. Megan McCarthy (Fig. 1), Talia Wong (Fig. 2), Natalia Vapniarsky-Arzi (Fig. 3), Kristine Vu (Fig. 4), Elizabeth Rose (Suppl. Fig. 1), and Sai Fingerhood (Suppl. Figs. 2, 3) for taking and uploading images to the UC Davis pathology database.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Samantha L. Kovacs  https://orcid.org/0000-0001-8147-3472
https://orcid.org/0000-0001-8147-3472
Eunju April Choi  https://orcid.org/0000-0002-5183-3980
https://orcid.org/0000-0002-5183-3980
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Christine Haake, Veterinary Medical Teaching Hospital, School of Veterinary Medicine, University of California–Davis, Davis, CA, USA; Current address: Washington Animal Disease Diagnostic Laboratory, Department of Veterinary Microbiology and Pathology, Washington State University, Pullman, WA, USA.
Samantha L. Kovacs, Veterinary Medical Teaching Hospital, School of Veterinary Medicine, University of California–Davis, Davis, CA, USA
Eunju April Choi, Department of Pathology, Microbiology and Immunology, School of Veterinary Medicine, University of California–Davis, Davis, CA, USA.
References
- 1.Al Kindi HN, et al. Cor triatriatum sinister (divided left atrium): histopathologic features and clinical management. Ann Thorac Surg 2020;110:1380–1386. [DOI] [PubMed] [Google Scholar]
- 2.Basrur PK.Congenital abnormalities of the goat. Vet Clin North Am Food Anim Pract 1993;9:183–202. [DOI] [PubMed] [Google Scholar]
- 3.Buczinski S, et al. Ventricular septal defects in cattle: a retrospective study of 25 cases. Can Vet J 2006;47:246–252. [PMC free article] [PubMed] [Google Scholar]
- 4.Chetboul V, et al. The variety of phenotypes behind ‘double outlet right ventricle’: clinical and imaging presentations in four dogs and a cat. J Vet Cardiol 2020;31:51–60. [DOI] [PubMed] [Google Scholar]
- 5.Chou P-C, et al. Life-threatening dilated cardiomyopathy induced by late-onset neonatal hypocalcemia. Pediatr Neonatol 2016;57:535–538. [DOI] [PubMed] [Google Scholar]
- 6.Daubeney PEF, et al. Clinical features and outcomes of childhood dilated cardiomyopathy: results from a national population-based study. Circulation 2006;114:2671–2678. [DOI] [PubMed] [Google Scholar]
- 7.De Lange L, et al. Prevalence and characteristics of ventricular septal defects in a non-racehorse equine population (2008–2019). J Vet Intern Med 2021;35:1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floriano DA, et al. Anesthesia case of the month. J Am Vet Med Assoc 2021;258:1341–1344. [DOI] [PubMed] [Google Scholar]
- 9.Gardner SY, et al. Echocardiographic diagnosis of an anomaly of the tricuspid valve in a male pygmy goat. J Am Vet Med Assoc 1992;200:521–523. [PubMed] [Google Scholar]
- 10.Kheiwa A, et al. Patent foramen ovale and atrial septal defect. Echocardiography 2020;37:2172–2184. [DOI] [PubMed] [Google Scholar]
- 11.Kim JS, et al. Development of the myocardium of the atrioventricular canal and the vestibular spine in the human heart. Circ Res 2001;88:395–402. [DOI] [PubMed] [Google Scholar]
- 12.Laus F, et al. Congenital cardiac defect in a pygmy goat (Capra hircus). Turkish J Vet Anim Sci 2011;35:471–475. [Google Scholar]
- 13.Naqvi N, et al. Anatomy of the atrial septum and interatrial communications. J Thorac Dis 2018;10(Suppl 24):S2837–S2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parry BW, et al. Ventricular septal defects in three familially-related female Saanen goats. Aust Vet J 1982;59:72–76. [DOI] [PubMed] [Google Scholar]
- 15.Ranjan R, et al. Diagnostic imaging and pacemaker implantation in a domestic goat with persistent left cranial vena cava. J Vet Cardiol 2014;16:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scarratt WK, et al. Ventricular septal defects in two goats. Cornell Vet 1984;74:136–145. [PubMed] [Google Scholar]
- 17.Schrama HJ, et al. Congenital hypoplasia of the pulmonary trunk without a ventricular septal defect in a goat lamb. Vet Q 1982;4:186–189. [DOI] [PubMed] [Google Scholar]
- 18.Stern JA, et al. Hybrid cutting balloon dilatation for treatment of cor triatriatum sinister in a cat. J Vet Cardiol 2013;15:205–210. [DOI] [PubMed] [Google Scholar]
- 19.Sumal AS, et al. Tricuspid atresia: where are we now? J Card Surg 2020;35:1609–1617. [DOI] [PubMed] [Google Scholar]
- 20.Webb G, Gatzoulis MA.Atrial septal defects in the adult: recent progress and overview. Circulation 2006;114:1645–1653. [DOI] [PubMed] [Google Scholar]
- 21.Yim D, et al. Essential modifiers of double outlet right ventricle: revisit with endocardial surface images and 3-dimensional print models. Circ Cardiovasc Imaging 2018;11:e006891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vdi-10.1177_10406387231171568 for A retrospective study of congenital cardiac malformations in 29 goats by Christine Haake, Samantha L. Kovacs and Eunju April Choi in Journal of Veterinary Diagnostic Investigation