Abstract
Background
Pleural cytology is currently used to assess targetable mutations in patients with advanced lung adenocarcinoma. However, it is fraught with low diagnostic yield.
Research Question
Can pleural cell-free DNA (cfDNA) be used to assess targetable mutations in lung adenocarcinoma patients with malignant pleural effusions (MPE)?
Study Design and Methods
Patients with lung adenocarcinoma MPE were recruited prospectively between January 2017 and September 2021. Oncogenic mutations were assessed by treating providers using pleural fluid cytology or lung cancer biopsies. Pleural and plasma cfDNA were used to assess the mutations using next-generation sequencing (NGS).
Results
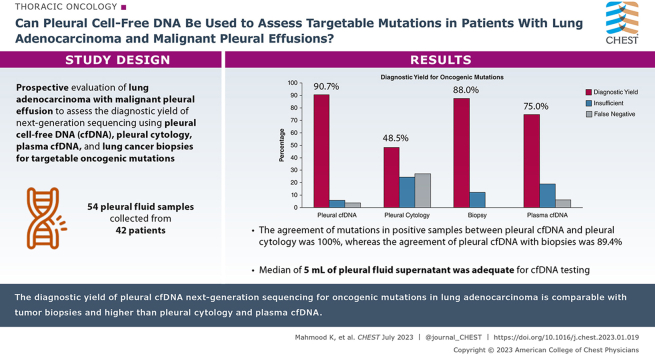
Fifty-four pleural fluid samples were collected from 42 patients. The diagnostic yield to detect oncogenic mutations for pleural cfDNA, pleural cytology, biopsy, and plasma cfDNA was 49/54 (90.7%), 16/33 (48.5%), 22/25 (88%), and 24/32 (75%), respectively, P < .001. The agreement of mutations in positive samples between pleural cfDNA and pleural cytology was 100%, whereas the agreement of pleural cfDNA with biopsies was 89.4%. The median concentration (interquartile range) of pleural cfDNA was higher than plasma: 28,444 (4,957-67,051) vs 2,966.5 (2,167-5,025) copies of amplifiable DNA per mL, P < .01. Median of 5 mL (interquartile range, 4.5-5) of pleural fluid supernatant was adequate for cfDNA testing.
Interpretation
The diagnostic yield of pleural cfDNA NGS for oncogenic mutations in lung adenocarcinoma patients is comparable to tumor biopsies and higher than pleural cytology and plasma cfDNA. The pleural cfDNA can be longitudinally collected, can be readily incorporated in clinical workflow, and may decrease the need for additional biopsies.
Key Words: Cell-free DNA, lung adenocarcinoma, malignant pleural effusion, mutations
Graphical Abstract
Take-home Points.
Study Question: Can pleural cell-free DNA (cfDNA) be used to assess targetable mutations in lung adenocarcinoma patients with malignant pleural effusions?
Results: In a prospective study of lung adenocarcinoma patients with pleural effusions, the diagnostic yield of next-generation sequencing to detect oncogenic mutations in pleural cfDNA was higher compared with pleural fluid cytology and plasma cfDNA but comparable to lung cancer biopsies.
Interpretation: These findings suggest that pleural cfDNA can be prioritized to detect oncogenic mutations in lung adenocarcinoma patients.
Lung cancer is the leading cause of cancer mortality worldwide, accounting for 2.2 million new cases and 1.8 million deaths in 2020.1 Approximately 40% of patients with lung cancer develop malignant pleural effusions (MPE), which are associated with a poor prognosis and a median survival of 6 months.2,3 The use of tyrosine kinase inhibitors has been shown to improve the prognosis of lung adenocarcinoma patients with targetable mutations, even with MPE.4, 5, 6 However, the availability of optimal samples for the identification of mutations has been the Achilles’ heel of targeted therapy, because many samples fail to provide adequate quantity of tumor cells.7,8 For molecular analysis, lung biopsies are generally considered the standard of care but require invasive procedures that can be associated with significant complications.9
Pleural fluid cytology is currently used to diagnose cancer and detect targetable, oncogenic mutations.4,7 The pleural fluid is centrifuged and malignant cells are pelleted into a cell block and assessed by polymerase chain reaction (PCR) or next-generation sequencing (NGS) to characterize the mutational profile. Unfortunately, this strategy has a limited yield of 54% to 71% in different studies.7,8 Data are emerging about PCR or NGS testing on cell-free DNA (cfDNA) from the pleural fluid supernatant for detection of mutations in Asian and European patient cohorts.10,11 We hypothesized that pleural cfDNA will have a better yield compared with pleural cytology to diagnose targetable mutations in lung adenocarcinoma patients in a multiracial US cohort.
Study Design and Methods
Patients
Fifty-four pleural fluid samples were prospectively collected from 42 consecutive patients with lung adenocarcinoma who presented for pleural effusion drainage between January 1, 2017, and September 1, 2021. Informed consent was obtained from all patients enrolled, and the study was approved by the institutional review board (Pro00074697). Samples from pleural effusions were obtained by thoracentesis or drainage of indwelling pleural catheters.
The patients underwent cytological testing for detection of malignancy and oncogenic mutation analysis as clinically indicated by the treating providers. Some of these patients underwent biopsies of the lung tumors, as directed by clinical need, usually at different times than the pleural fluid sampling. Oncogenic mutations analysis was performed by targeted PCR or NGS, per standard clinical workflow as previously described,7 at the different times of collection using separate assays. When targeted PCR was performed, fluorescence in situ hybridization testing was done to evaluate ALK and ROS1 rearrangements.
NGS was performed for clinical testing on pleural cytology and lung cancer biopsies, using either an institutional 50-gene panel or a commercial 324-gene panel (FoundationOne CDx, Foundation Medicine) at the discretion of treating physicians. At the institutional laboratory, formalin-fixed, paraffin-embedded pleural cytology or cancer biopsy slides were examined under microscope, and approximately 10% of tumor cells in total cells were required for DNA extraction.7 The area with the most tumor cells was macrodissected, and DNA was extracted using Pinpoint Slide DNA Isolation System (Zymo Research).7 Afterward, NGS was performed on the Ion Torrent platform (Thermo Fisher Scientific). At the Foundation Medicine, DNA was extracted from formalin-fixed, paraffin-embedded cytological or biopsy specimens by KingFisher Flex Magnetic Particle Processor (Thermo Fisher Scientific). Afterward, whole-genome shotgun library construction, hybridization-based capture, and sequencing was performed, using the Illumina HiSeq 4000 platform (Illumina, Inc).
Programmed death-ligand 1 (PD-L1) expression on tumor cells in pleural cytology and cancer biopsy specimens was also determined by immunohistochemistry using Dako PD-L1 IHC 22C3 pharmDx Kit (Agilent). PD-L1 expression as the percentage of tumor cells showing membrane staining or tumor proportion score was classified as negative if < 1%, low when 1%-49%, and high when ≥ 50%.
cfDNA Isolation and Quantification from Pleural Fluid and Plasma
To isolate cfDNA from pleural fluid, the pleural effusion was initially centrifuged at 350g, and the supernatant was removed and centrifuged again at 2,500g. The double-spin supernatant was frozen at −80 °C until analysis. The cfDNA was isolated from the supernatant by QIAamp Circulating Nucleic Acid Kit (QIAGEN), using the manufacturer’s protocol. The concentration of cfDNA in copies per milliliter was determined by amplification of a 108-bp region of ribonuclease P/MRP subunit p30 (RPP30) gene as previously described.12 The use of the RPP30 quantification method also allows us to assess the quality of the cfDNA.13 This method is more accurate because it overcomes the issues with indirect methods, which simply measure the amount of DNA in ng/mL, some of which may consist of degraded DNA.13,14 In addition, the cfDNA fragment lengths between 108 bp and 2,000 bp were assessed to determine the genomic DNA contamination, which usually consists of large DNA fragments.15 The number of mutant molecules per milliliter pleural fluid or plasma also was assessed, which is not influenced by genomic DNA contamination. The mutant and nonmutant DNA molecules were directly measured by sequencing. In addition to pleural fluid, peripheral blood was collected in ethylenediamine tetra-acetic acid vacutainers, and double-spin, platelet-poor ethylenediamine tetra-acetic acid plasma was isolated and stored at −80 ° C until analysis.
Next-Generation Sequencing of Pleural and Plasma cfDNA
The sequencing of cfDNA from pleural fluid supernatant and plasma was performed using the InvisionFirst-Lung platform (Inivata) to target 37 oncogenic genes, including single-nucleotide variants, insertions and deletions, copy number amplifications, and structural rearrangements (gene fusions), as shown in e-Table 1. Initial targeted PCR was performed spanning the relevant portions of the listed genes, including common hotspots and fusion break points. After DNA cleanup, a second PCR was performed to add sequencing adapters and barcodes, allowing for the samples to be pooled and sequenced on the Illumina NextSeq platform (Illumina).12 For cfDNA, the assay has a limit of detection of ≥ 90% for single-nucleotide variants, insertions and deletions, and gene fusions at variant allele fraction (VAF) of ≥ 0.25% and a high rate of detection at lower VAF while maintaining high specificity of 99.9997% per base.16 The median depth at which a mutation is called has been shown to be at approximately 69,000×.16
Interpretation of Results
The diagnostic yield of a specimen to detect oncogenic mutations was defined as the ability to obtain a positive or true negative test for a mutation in total number of specimens tested. True negative was defined as when the test for mutation was negative in all available corresponding specimens from the same patient. False negative was defined as when the test was negative in a specimen type but positive in other corresponding specimens. Insufficient result was noted when the specimen could not be processed for the mutation testing because of poor quality. All the positive tests were assumed to be true positives and cross-referenced between all corresponding specimen types.
Statistical Analysis
Descriptive statistics were used to summarize demographic and clinical characteristics of the patients. Continuous variables were described as median and interquartile range (IQR). Categorical variables were summarized using percentages. Wilcoxon rank-sum test was used to compare continuous variables, and χ2 or Fisher exact tests were used to compare categorical variables. Kappa statistics were calculated to measure the agreement between pleural cfDNA and pleural cytology, biopsy, and plasma cfDNA. A two-sided P-value of .05 or less was considered statistically significant, with the exception of examining differences in reported mutations by various specimens. For later, we implemented a Bonferroni correction, which changed our alpha level to 0.0071 (to account for seven independent tests). All analyses were conducted using SAS version 9.4 (SAS Institute Inc).
Results
Fifty-four pleural effusion samples were collected from 42 patients. The demographics of the patients and fluid characteristics are shown in Table 1. The median age (IQR) of patients was 67.5 (62-78) years, and 45.2% were female. There were 73.8% White and 21.4% Black patients. Patients with former and current combustible tobacco use were 54.8% and 4.8%, respectively. Thirty-six patients had lung cancer biopsies available for comparison, most of which were obtained via bronchoscopy (58.4%) and were collected at a median (IQR) of 189 (50-666) days from the pleural fluid drainage. However, only 25 of these lung cancer biopsy specimens were further tested for oncogenic mutations by the treating providers. PD-L1 expression was determined by treating clinicians in four pleural cytology specimens; it was high in two of four (50%), low in one of four (25%), and the specimen was inadequate in one of four (25%) samples. PD-L1 expression was assessed in 19 lung cancer biopsy specimens; it was high in five of 19 (26.3%), low in eight of 19 (42.1%), and negative in six of 19 (31.5%). Indwelling pleural catheters were placed in 57.1% patients. Therapies included tyrosine kinase inhibitors (35.7%), chemotherapy (61.9%), immunotherapy (45.2%), radiation (47.6%), and surgery (4.8%); many patients received two or more therapies as decided by the treating physicians. Pleural effusions were exudative in 95.8% of the tested samples.
Table 1.
Demographics and Characteristics of Patients
| Characteristics | Patients (N = 42) |
|---|---|
| Age, median (IQR), y | 67.5 (62-78) |
| Sex female, No. (%) | 19 (45.2) |
| Race, No. (%) | |
| Black | 9 (21.4) |
| Asian | 1 (2.4) |
| White | 31 (73.8) |
| Native Hawaiian | 1 (2.4) |
| Combustible tobacco use status, No. (%) | |
| Current | 2 (4.8) |
| Former | 23 (54.8) |
| None | 17 (40.5) |
| Lung cancer biopsies, No. (%) | Total = 36 |
| Surgical | 7 (19.4) |
| Bronchoscopic forceps | 11 (30.6) |
| EBUS-TBNA | 10 (27.8) |
| Radiology-guided needle biopsy | 8 (22.2) |
| IPC placement, No. (%) | 24 (57.1) |
| Oncologic treatment,a No. (%) | |
| Tyrosine kinase inhibitor | 15 (35.7) |
| Chemotherapy | 26 (61.9) |
| Immunotherapy | 19 (45.2) |
| Radiation | 20 (47.6) |
| Surgery | 2 (4.8) |
| Pleural fluid cytology consistent with lung adenocarcinoma | Total = 46b |
| Positive, No. (%) | 34 (73.9) |
| Negative, No. (%) | 12 (26.1) |
| Pleural fluid characteristics | Total = 48b |
| Exudative, No. (%) | 46 (95.8) |
| Lactate dehydrogenase, U/L, median (IQR) | 227 (125-462) |
| Protein, g/dL, median (IQR) | 3.8 (3.4-4.4) |
| Glucose, mg/dL, median (IQR) | 97 (79-114) |
| pH, median (IQR) | 7.44 (7.40-7.50) |
EBUS-TBNA = Endobronchial ultrasound-guided transbronchial fine needle aspiration; IPC = indwelling pleural catheter; IQR = interquartile range; U = Units.
Patients received multiple therapies by the treating providers.
Some repeat pleural samples did not have cytology or chemistry checked.
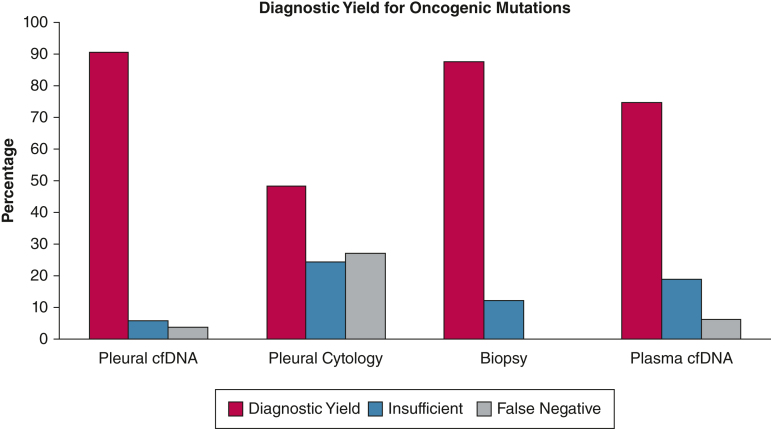
The diagnostic yield of pleural cfDNA, pleural cytology, biopsy, and plasma cfDNA to detect mutations was 49 of 54 (90.7%), 16 of 33 (48.5%), 22 of 25 (88%), and 24 of 32 (75%), respectively, P < .001 (Table 2, Fig 1). We noted variable false-negative and insufficient results across the various specimen types. Two (3.7%) pleural cfDNA results were false negative, but the biopsy or plasma cfDNA were positive for mutations. Nine (27.3%) pleural cytology specimens were false negative. In seven of these nine specimens, the pleural cytology was negative for malignancy, but pleural cfDNA was able to detect mutations. Two (6.2%) plasma cfDNA were false negative as well. The mutation testing was attempted but could not be performed because of insufficient quality of pleural cfDNA, pleural cytology, biopsy, and plasma cfDNA specimens in 5.5%, 24.2%, 12% and 18.7%, respectively. Overall, the actionable mutations (EGFR, KRAS G12C, BRAF V600E, ERBB2, ALK, and ROS1 rearrangements) for which tyrosine kinase inhibitor therapy was available were missed by pleural cfDNA, pleural cytology, biopsy, and plasma cfDNA in one of 54 (1.8%), eight of 33 (24.2%), two of 25 (8%), and eight of 32 (25%) specimens, respectively.
Table 2.
Oncogenic Mutation Testing Results in Different Specimens
| Testing Results | Pleural cfDNA (N = 54) | Pleural Cytology (N = 33) | Biopsy (N = 25) | Plasma cfDNA (N = 32) | P Value for Difference |
|---|---|---|---|---|---|
| Diagnostic yield,a No. (%) | 49 (90.7) | 16 (48.5) | 22 (88) | 24 (75) | < .001 |
| Positive | 43 (79.6) | 9 (27.2) | 20 (80) | 19 (59.3) | |
| True negative | 6 (11.1) | 7 (21.2) | 2 (8) | 5 (15.6) | |
| False negative | 2 (3.7) | 9 (27.3)b | 0 | 2 (6.2) | |
| Insufficient | 3 (5.5) | 8 (24.2) | 3 (12) | 6 (18.7) | |
| Not checked,c,d No. (%) | 0 | 21 (38.3) | 11 (20.3) | 0 | NA |
| No samples,c,e No. (%) | 0 | 0 | 18 (33.3) | 22 (40.7) | NA |
cfDNA = cell-free DNA.
Diagnostic yield: defined as ability to obtain a positive or true negative test for an oncogenic mutation in total No. of specimens tested: Positive + true negative specimens/total specimens tested (%).
Includes seven specimens with negative cytology for malignant cells but positive pleural cell-free DNA testing for mutations.
Denominator is 54 samples, which is the total pleural fluid samples collected.
Not checked. Samples available but mutation testing not done as per decision of the treating providers.
No samples. Samples were not obtained clinically.
Figure 1.
Diagnostic yield of pleural cell-free DNA, pleural cytology, biopsies, and plasma cell-free DNA. cfDNA = cell-free DNA.
Pleural cfDNA and pleural cytology agreed in all nine patients who had positive results for mutations in both specimen types (Table 3). Pleural cfDNA and biopsy agreed in 17 of 19 (89.4%) specimens that had positive results for mutations. In the two disagreeing specimens, biopsy in the first patient showed TP53 mutation, whereas the pleural cfDNA had EGFR Ex19Del; biopsy from the second patient identified BRAF G469V while pleural cfDNA detected NRAS. Pleural cfDNA and plasma cfDNA agreed in 16 of 17 positive specimen pairs (94.1%). In the disagreeing specimens, pleural cfDNA had EGFR E709A mutation but plasma had TP53 mutation. We also calculated the overall agreement between pleural cfDNA and other specimen types when both the specimen types were available for comparison and accounted for all the positive, negative, false-negative, and insufficient results. The overall agreement was 51.5% (kappa statistic = 0.11; P = .44) between pleural cfDNA and pleural cytology, 76% (kappa statistic = 0.32; P = .21) between pleural cfDNA and biopsy and 68.8% (kappa statistic = 0.25; P = .06) between pleural cfDNA and plasma cfDNA.
Table 3.
Agreement of Pleural cfDNA for Mutations With Pleural Cytology, Biopsy, and Plasma cfDNA
| Testing Modality | Agreement of Pleural cfDNA in Samples With Positive Mutations | Overall Agreement of Pleural cfDNA | Overall Kappa Statistic of Agreement of Pleural cfDNA Kappa (95% CI) |
Overall P Value for Agreement of Pleural cfDNA |
|---|---|---|---|---|
| Pleural cytology, n/N (%) | 9/9 (100) | 17/33 (51.5) | 0.11 (−0.16, 0.39) | .44 |
| Biopsy, n/N (%) | 17/19 (89.4) | 19/25 (76) | 0.32 (−0.01, 0.65) | .21 |
| Plasma cfDNA, n/N (%) | 16/17 (94.1) | 22/32 (68.8) | 0.25 (−0.20, 0.70) | .06 |
cfDNA = cell-free DNA; n = concordant samples.
The median (IQR) volume of supernatant from pleural fluid and plasma used for cfDNA testing was 5 mL (4.5-5) and 1.5 mL (1-2), respectively. The initial input of cfDNA to construct the libraries was different in different samples and calculated as copies of amplifiable DNA/mL, as shown in Table 4 and normalized to 1 mL pleural fluid or plasma volume. The median (IQR) concentration of cfDNA in pleural fluid vs plasma was 28,444 (4,957-67,051) copies/mL vs 2,966.5 (2,167-5,025) copies/mL, P < .01 (Table 4). The median (IQR) number of mutant molecules per milliliter in pleural fluid vs plasma when both specimens were positive was also different between the two compartments, 2,507 (157-42,732) vs 26 (4-221), P < .01, respectively (Table 4). Higher median (IQR) VAF percentage was observed in pleural fluid compared with plasma, 19.14 (0.71-50.9) vs 0.47 (0.13-5.56), P < .01 (Table 4).
Table 4.
Comparison of cfDNA in Pleural Fluid and Plasma
| Measurement | Pleural Fluid | Plasma | P Value for Difference |
|---|---|---|---|
| Concentration of cfDNA– Copies of amplifiable DNA/mL, Median (IQR) |
28,444 (4,957-67,051) N = 53 |
2,966.5 (2,167-5,025) N = 26 |
< .01 |
| No. of mutant molecules per mL N = 15a Median (IQR) |
2,507 (157-42,732) | 26 (4-221) | < .01 |
| Variant allele fraction % N = 15a Median (IQR) |
19.14 (0.71-50.9) | 0.47 (0.13-5.56) | < .01 |
cfDNA = cell-free DNA; IQR = interquartile range.
Matched patients with positive primary mutations in both pleural fluid and plasma.
The frequency of different mutations detected by pleural cfDNA, pleural cytology, cancer biopsy, and plasma cfDNA is shown in Table 5 and Figure 2. More unique mutations and heterogeneity were detected in pleural cfDNA, compared with pleural cytology, biopsy, and plasma cfDNA. Total number of mutations detected in pleural cfDNA, pleural cytology, biopsy, and plasma cfDNA were 98, 12, 25, and 29, respectively.
Table 5.
Frequency of Oncogenic Mutations Detected by Pleural cfDNA, Pleural Cytology, Biopsy, and Plasma cfDNA
| Mutations, No. (%)a | Pleural cfDNA Mutations |
Pleural Cytology Mutations |
Biopsy Mutations |
Plasma cfDNA Mutations |
P Value for Difference |
|---|---|---|---|---|---|
| Total mutations detected | 98 | 12 | 25 | 29 | |
| EGFR | 19 (19.4) | 7 (58.3) | 8 (32) | 3 (10.3) | .0068 |
| Exon 19 Deletion | 6 (6.1) | 2 (16.7) | 3 (12) | 0 | |
| L858R | 4 (4) | 3 (25) | 2 (8) | 1 (3.5) | |
| Exon 20 Insertion | 1 (1) | 1 (8.3) | 1 (4) | 0 | |
| E709A | 1 (1) | 0 | 1 (4) | 0 | |
| G719A | 1 (1) | 0 | 0 | 0 | |
| T790M | 2 (2) | 1 (8.3) | 1 (4) | 2 (6.9) | |
| EGFR amplification | 2 (2) | 0 | 0 | 0 | |
| V742I | 1 (1) | 0 | 0 | 0 | |
| V834A | 1 (1) | 0 | 0 | 0 | |
| KRAS | 11 (11.2) | 0 | 5 (20) | 5 (17.2) | .32 |
| G12A | 2 (2) | 0 | 2 (8) | 0 | |
| G12C | 4 (4) | 0 | 1 (4) | 3 (10.3) | |
| G12D | 1 (1) | 0 | 0 | 1 (3.5) | |
| G12V | 2 (2) | 0 | 2 (8) | 0 | |
| G13C | 1 (1) | 0 | 0 | 1 (3.5) | |
| G13D | 1 (1) | 0 | 0 | 0 | |
| BRAF | 8 (8.1) | 0 | 4 (16) | 2 (6.9) | .45 |
| V600E | 7 (7.1) | 0 | 2 (8) | 1 (3.5) | |
| G469A | 1 (1) | 0 | 1 (4) | 1 (3.5) | |
| G469V | 0 | 0 | 1 (4) | 0 | |
| EML-ALK fusion | 3 (3.1) | 2 (16.7) | 1 (4) | 0 | .10 |
| ERBB2 | 4 (4.1) | 1 (8.3) | 2 (8) | 2 (6.9) | .61 |
| TP53 | 25 (25.5) | 2 (16.7) | 3 (12) | 8 (27.6) | .24 |
| STK11 | 6 (6.1) | 0 | 1 (4) | 3 (10.3) | .67 |
| NRAS | 1 (1) | 0 | 0 | 0 | |
| HRAS | 1 (1) | 0 | 0 | 0 | |
| IDH1 | 2 (2) | 0 | 0 | 1 (3.5) | |
| IDH2 | 1 (1) | 0 | 0 | 1 (3.5) | |
| PIK3CA | 3 (3.1) | 0 | 0 | 0 | |
| U2AF1 | 4 (4.1) | 0 | 0 | 1 (3.5) | |
| CDKN2A | 2 (2) | 0 | 0 | 1 (3.5) | |
| CTNNB1 | 2 (2) | 0 | 0 | 0 | |
| CCND1 | 0 | 0 | 1 (4.0) | 0 | |
| MET | 3 (3.1) | 0 | 0 | 0 | |
| ROS1 | 1 (1) | 0 | 0 | 1 (3.4) | |
| GATA3 | 1 (1) | 0 | 0 | 0 | |
| KIT | 0 | 0 | 0 | 1 (3.5) | |
| MYC | 1 (1) | 0 | 0 | 0 |
cfDNA = cell-free DNA.
Percentage of total mutations in the specimen type.
Figure 2.
Frequency of oncogenic mutations detected by pleural cfDNA, pleural cytology, biopsies, and plasma cfDNA. cfDNA = cell-free DNA.
Discussion
To our knowledge, this is the first prospective study that assesses the diagnostic yield of cfDNA for oncogenic mutations from pleural fluid and compares results with the clinically available specimens in a multiracial US cohort. The pleural cfDNA had a higher diagnostic yield, especially for actionable mutations, compared with pleural cytology and plasma cfDNA, whereas the yield was similar to cancer biopsy specimens. Pleural fluid also had higher concentrations of cfDNA, mutant molecules, and VAF compared with plasma cfDNA.
The inadequacy of biological specimens to identify targetable mutations is well known.17, 18, 19 The current standard of care to assess targetable genetic mutations in pleural fluid of lung adenocarcinoma patients is PCR or NGS, using the malignant cells in pleural fluid sediment. The diagnostic yield of pleural fluid cytology for detection of malignancy in lung adenocarcinoma patients is 80.2%, and it increases to 85% with a second thoracentesis.4 In another study of 56 patients with MPE and positive cytology for lung adenocarcinoma, pleural cytology could detect targetable mutations in approximately 71.4% of patients.7 In this study, the yield of tumor biopsies for targetable mutations was 85.7%. Carter and colleagues8 could evaluate targetable mutations in only 27 (54%) of 50 adenocarcinoma pleural cytology specimens. If we consider the “missed opportunity” of undiagnosed malignancy in pleural effusions and then undetected mutations in confirmed MPE, the yield of pleural cytology for mutations becomes suboptimal. In the current study, we demonstrated that the pleural cfDNA can diagnose oncogenic mutations with a yield of 90.7%, and misses less targetable mutations compared with other specimens.
Reports have been made of using pleural cfDNA to assess different genetic mutations in patients with lung adenocarcinoma using PCR. Kimura and colleagues20 used pleural cfDNA to detect EGFR mutation using PCR in a cohort of 43 Japanese patients with non-small cell lung cancer and were able to detect the mutation in 11 patients.20 Lin and colleagues21 found EGFR mutations using PCR amplification in 15 of 36 patients in pleural cfDNA and 14 of 36 cell pellets.21 Li and colleagues10 showed that the sensitivity of digital droplet PCR for detection of EGFR mutations in pleural cfDNA is higher compared with direct sequencing or amplification-refractory mutation system.10 Mokanszki and colleagues11 assessed targetable mutations in 36 patients with lung adenocarcinoma MPE. EGFR, KRAS, and BRAF mutations were detected in 14 patients using cfDNA from pleural fluid supernatant using a reverse-hybridization assay.11 In a subset of 13 patients who had testing done on pleural fluid sediment and plasma, the yield of pleural cfDNA was 12 of 13, plasma cfDNA was 5 of 13, and pleural cell-block was 0 of 13.11 Chen and colleagues22 showed that the sensitivity of pleural cfDNA for detection of EGFR, KRAS, and HER2 using PCR was 81.5% when compared with tissue biopsies.22 Hummelink and colleagues23 were able to assess EGFR and KRAS mutations in the supernatant cfDNA of 35 of 44 (79.5%) MPE samples from 37 patients with non-small cell lung, colon, and appendiceal cancer using droplet digital PCR.23 Our work with pleural cfDNA analysis using NGS extends the published literature.
NGS has emerged as a powerful high-throughput genomic profiling tool to assess the molecular landscape of cancer.24 In a study with 42 different body fluid specimens, including 12 MPE from Italian and Spanish centers, Villatoro and colleagues25 showed that body fluid cfDNA PCR or NGS had better yield for mutation detection, along with higher concentration and allele frequency compared with plasma.25 In 63 patients from China with non-small cell lung cancer, Tong and colleagues26 assessed the yield of cfDNA for mutation detection, using NGS in pleural fluid supernatant, sediment, and plasma. In the patients with matched tissue biopsy, the sensitivity of cfDNA for mutation detection from supernatant, sediment, and plasma was 93%, 90%, and 63%, respectively. The concentration of cfDNA was higher in supernatant compared with sediment or plasma, and exhibited more unique mutational profiles compared with the other samples. In another Asian cohort of 77 patients with MPE using NGS, no difference was seen in the mutation detection rate and maximum allele frequency between supernatant cfDNA and precipitate.27 Our findings are in line with the published literature, even with a more racially diverse cohort.
In our study using NGS, the pleural cfDNA had a diagnostic yield of 90.7%, which was higher than pleural cytology, biopsy, and plasma cfDNA of 48.4%, 88%, and 72.7%, respectively. In patients with positive mutations, we found complete agreement of pleural cfDNA with pleural cytology, 85% agreement with biopsy, and 94.1% agreement with plasma cfDNA. The overall agreement or kappa statistic of pleural cfDNA with other specimens was low when accounting for false-negative and insufficient samples. We speculate that some disagreement of pleural cfDNA, and biopsies or plasma can be related to a difference in pleural vs primary tumor mutation profiles, dynamics of different tissue compartments, or timing of pleural fluid drainage vs biopsies.
We also observed that cfDNA mutational analyses can be performed on almost all the patients with only 5 mL of supernatant from MPE. In a previous study, Abouzgheib and colleagues28 showed that 50 mL pleural fluid is sufficient to ascertain malignancy. Other studies demonstrated that 50 to 100 mL pleural fluid is also sufficient to assess genetic mutations based on cytological methods.7,8 Our observation that only 5 mL pleural supernatant may be adequate to detect mutations using NGS is a key finding because this limited volume can be easily incorporated into the current workflow using standard collection procedures.
We also observed that pleural fluid had a higher concentration of cfDNA, mutant molecules, and VAF compared with plasma cfDNA. This may be attributable to direct shedding of cfDNA from the tumor studding the pleura and decreased dilution or removal of cfDNA from pleural fluid compared with plasma.29 The higher cfDNA concentration in pleural fluid vs plasma may have improved the diagnostic yield of pleural cfDNA to detect mutations. Another important finding of our study is that pleural cfDNA has more unique mutations, which may reflect on variable molecular profiles of different compartments in the same patient. Thus, pleural fluid may allow a more comprehensive molecular profiling and clonal heterogeneity assessment.29
The strengths of our study include a prospective design in a racially diverse cohort and comparison of pleural cfDNA NGS with clinical diagnostic modalities. The limitations of the study include small sample size from a single center, and adequate plasma volumes were not available from many patients. The clinical assessment of oncogenic mutations was heterogeneous using institutional and commercial NGS assays, and not performed in all the pleural cytology and cancer biopsy specimens because one of the two specimen types was generally used to assess mutations. In addition, we did not determine the CD274 (PD-L1) gene amplification using cfDNA in pleural fluid supernatant or plasma as a predictor of response to immunotherapy and future studies should be performed to assess the CD274 gene amplification.
Interpretation
Our findings indicate that the yield of pleural cfDNA NGS to detect oncogenic mutations in lung adenocarcinoma is comparable to that of tumor biopsies and higher than pleural cytology and plasma cfDNA. Pleural fluid also has higher concentration of cfDNA, VAF, and genetic heterogeneity compared with plasma. Although future studies are needed to confirm the results, the routine use of pleural cfDNA may improve clinical genotyping of lung adenocarcinoma.
Funding/Support
The work was supported by US Department of Defense, Lung Cancer Research Program, award number W81XWH-20-1-0280. Inivata (Research Triangle Park, NC) performed next-generation sequencing on the research specimens.
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: G. J. is an employee of Inivata, Inc. None declared (K. M., P. J., J. M. C., S. W., X. W., M. M. W., C. X. G., M. D., S. L. S., J. S., S. J. A., A. B. N.).
Acknowledgments
Authorcontributions: K.M. had full access to all of the data in the study and takes responsibility for the content, integrity of the data and the accuracy of the data analysis. K. M.: conceptualization, methodology, validation, investigation, data curation, writing- original draft, writing review & editing, visualization, supervision, project administration, funding acquisition. P. J.: methodology, validation, investigation, data curation, writing review & editing, visualization. J. M. C.: conceptualization, methodology, writing review & editing. Steven Wolf: formal analysis, writing review & editing, visualization. X. W.: formal analysis, writing review & editing, visualization. M. M. W.: conceptualization, methodology, investigation, writing review & editing. C. X. G.: conceptualization, methodology, investigation, writing review & editing. M. D.: conceptualization, methodology, investigation, writing review & editing. S. L. S.: conceptualization, methodology, investigation, writing review & editing. J. S.: investigation, writing review & editing, project administration. G. J.: conceptualization, methodology, validation, investigation, data curation, writing review & editing, project administration. S. J. A.: conceptualization, methodology, writing review & editing. A. N.: conceptualization, methodology, validation, investigation, resources, writing review & editing, supervision, project administration.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We are grateful to our patients and families for their grit and courage in fighting lung cancer and participating in the research.
Additional information: The e-Table is available online under “Supplementary Data.”
Supplementary Data
References
- 1.World Health Organization Cancer Key Facts 2020. https://www.who.int/news-room/fact-sheets/detail/cancer World Health Organization website.
- 2.Morgensztern D., Waqar S., Subramanian J., Trinkaus K., Govindan R. Prognostic impact of malignant pleural effusion at presentation in patients with metastatic non-small-cell lung cancer. J Thorac Oncol. 2012;7(10):1485–1489. doi: 10.1097/JTO.0b013e318267223a. [DOI] [PubMed] [Google Scholar]
- 3.Porcel J.M., Gasol A., Bielsa S., Civit C., Light R.W., Salud A. Clinical features and survival of lung cancer patients with pleural effusions. Respirology. 2015;20(4):654–659. doi: 10.1111/resp.12496. [DOI] [PubMed] [Google Scholar]
- 4.Dorry M., Davidson K., Dash R., et al. Pleural effusions associated with squamous cell lung carcinoma have a low diagnostic yield and a poor prognosis. Transl Lung Cancer Res. 2021;10(6):2500–2508. doi: 10.21037/tlcr-21-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mok T.S., Wu Y.L., Thongprasert S., et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 6.Zhou C., Wu Y.L., Chen G., et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 7.DeMaio A., Clarke J.M., Dash R., et al. Yield of malignant pleural effusion for detection of oncogenic driver mutations in lung adenocarcinoma. J Bronchol Interv Pulmonol. 2019;26(2):96–101. doi: 10.1097/LBR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 8.Carter J., Miller J.A., Feller-Kopman D., Ettinger D., Sidransky D., Maleki Z. Molecular profiling of malignant pleural effusion in metastatic non-small-cell lung carcinoma: the effect of preanalytical factors. Ann Am Thorac Soc. 2017;14(7):1169–1176. doi: 10.1513/AnnalsATS.201609-709OC. [DOI] [PubMed] [Google Scholar]
- 9.Heerink W.J., de Bock G.H., de Jonge G.J., Groen H.J., Vliegenthart R., Oudkerk M. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol. 2017;27(1):138–148. doi: 10.1007/s00330-016-4357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X., Liu Y., Shi W., et al. Droplet digital PCR improved the EGFR mutation diagnosis with pleural fluid samples in non-small-cell lung cancer patients. Clin Chim Acta. 2017;471:177–184. doi: 10.1016/j.cca.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Mokanszki A., Badon E.S., Monus A., Toth L., Bittner N., Mehes G. Cell-free DNA from pleural effusion samples: is it right for molecular testing in lung adenocarcinoma? Pathol Oncol Res. 2021;27 doi: 10.3389/pore.2021.613071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gale D., Lawson A.R.J., Howarth K., et al. Development of a highly sensitive liquid biopsy platform to detect clinically-relevant cancer mutations at low allele fractions in cell-free DNA. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0194630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J., Yu S., Wang Y., Zhu J., Zhang Z. New insights into the role of ribonuclease P protein subunit p30 from tumor to internal reference. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.1018279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbisier P., Pinheiro L., Mazoua S., et al. DNA copy number concentration measured by digital and droplet digital quantitative PCR using certified reference materials. Anal Bioanal Chem. 2015;407(7):1831–1840. doi: 10.1007/s00216-015-8458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alcaide M., Cheung M., Hillman J., et al. Evaluating the quantity, quality and size distribution of cell-free DNA by multiplex droplet digital PCR. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-69432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plagnol V., Woodhouse S., Howarth K., et al. Analytical validation of a next generation sequencing liquid biopsy assay for high sensitivity broad molecular profiling. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0193802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kris M.G., Johnson B.E., Berry L.D., et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cecchini M.J., Yi E.S. Liquid biopsy is a valuable tool in the diagnosis and management of lung cancer. J Thorac Dis. 2020;12(11):7048–7056. doi: 10.21037/jtd.2020.04.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durin L., Pradines A., Basset C., et al. Liquid biopsy of non-plasma body fluids in non-small cell lung cancer: look closer to the tumor. Cells. 2020;9(11):2486. doi: 10.3390/cells9112486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura H., Fujiwara Y., Sone T., et al. EGFR mutation status in tumour-derived DNA from pleural effusion fluid is a practical basis for predicting the response to gefitinib. Br J Cancer. 2006;95(10):1390–1395. doi: 10.1038/sj.bjc.6603428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin J., Gu Y., Du R., Deng M., Lu Y., Ding Y. Detection of EGFR mutation in supernatant, cell pellets of pleural effusion and tumor tissues from non-small cell lung cancer patients by high resolution melting analysis and sequencing. Int J Clin Exp Pathol. 2014;7(12):8813–8822. [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X., Li K., Liu Z., et al. Multigene PCR using both cfDNA and cfRNA in the supernatant of pleural effusion achieves accurate and rapid detection of mutations and fusions of driver genes in patients with advanced NSCLC. Cancer Med. 2021;10(7):2286–2292. doi: 10.1002/cam4.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hummelink K., Muller M., Linders T.C., et al. Cell-free DNA in the supernatant of pleural effusion can be used to detect driver and resistance mutations, and can guide tyrosine kinase inhibitor treatment decisions. ERJ Open Res. 2019;5(1):00016–2019. doi: 10.1183/23120541.00016-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvayrac O., Pradines A., Pons E., Mazieres J., Guibert N. Molecular biomarkers for lung adenocarcinoma. Eur Respir J. 2017;49(4) doi: 10.1183/13993003.01734-2016. [DOI] [PubMed] [Google Scholar]
- 25.Villatoro S., Mayo-de-Las-Casas C., Jordana-Ariza N., et al. Prospective detection of mutations in cerebrospinal fluid, pleural effusion, and ascites of advanced cancer patients to guide treatment decisions. Mol Oncol. 2019;13(12):2633–2645. doi: 10.1002/1878-0261.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong L., Ding N., Tong X., et al. Tumor-derived DNA from pleural effusion supernatant as a promising alternative to tumor tissue in genomic profiling of advanced lung cancer. Theranostics. 2019;9(19):5532–5541. doi: 10.7150/thno.34070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J., Li X., Wang W., Shao Y., Zhang Y., Song Z. Gene alterations in paired supernatants and precipitates from malignant pleural effusions of non-squamous non-small cell lung cancer. Transl Oncol. 2020;13(8) doi: 10.1016/j.tranon.2020.100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abouzgheib W., Bartter T., Dagher H., Pratter M., Klump W. A prospective study of the volume of pleural fluid required for accurate diagnosis of malignant pleural effusion. Chest. 2009;135(4):999–1001. doi: 10.1378/chest.08-2002. [DOI] [PubMed] [Google Scholar]
- 29.Ponti G., Manfredini M., Tomasi A. Non-blood sources of cell-free DNA for cancer molecular profiling in clinical pathology and oncology. Crit Rev Oncol Hematol. 2019;141:36–42. doi: 10.1016/j.critrevonc.2019.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.