Abstract
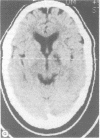
Clinical disorders of memory are believed to occur from the dysfunction of either the mesial temporal lobe, the mesial thalamus, or the basal forebrain. Fibre tract damage at the level of the fornix has only inconsistently produced amnesia. A patient is reported who suffered a cerebrovascular accident involving the posterior limb of the left internal capsule that resulted in a persistent and severe disorder of verbal memory. The inferior extent of the lesion effectively disconnected the mesial thalamus from the amygdala and the frontal cortex by disrupting the ventral amygdalofugal and thalamic-frontal pathways as they course through the diencephalon. This case demonstrates that an isolated lesion may cause memory loss without involvement of traditional structures associated with memory and may explain memory disturbances in other white matter disease such as multiple sclerosis and lacunar state.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggleton J. P., Mishkin M. Projections of the amygdala to the thalamus in the cynomolgus monkey. J Comp Neurol. 1984 Jan 1;222(1):56–68. doi: 10.1002/cne.902220106. [DOI] [PubMed] [Google Scholar]
- Aggleton J. P., Mishkin M. Visual recognition impairment following medial thalamic lesions in monkeys. Neuropsychologia. 1983;21(3):189–197. doi: 10.1016/0028-3932(83)90037-4. [DOI] [PubMed] [Google Scholar]
- Becker J. T., Olton D. S., Anderson C. A., Breitinger E. R. Cognitive mapping in rats: the role of the hippocampal and frontal system in retention and reversal. Behav Brain Res. 1981 Jul;3(1):1–22. doi: 10.1016/0166-4328(81)90025-5. [DOI] [PubMed] [Google Scholar]
- Benson D. F., Marsden C. D., Meadows J. C. The amnesic syndrome of posterior cerebral artery occlusion. Acta Neurol Scand. 1974;50(2):133–145. doi: 10.1111/j.1600-0404.1974.tb02767.x. [DOI] [PubMed] [Google Scholar]
- Choi D., Sudarsky L., Schachter S., Biber M., Burke P. Medial thalamic hemorrhage with amnesia. Arch Neurol. 1983 Oct;40(10):611–613. doi: 10.1001/archneur.1983.04050090047006. [DOI] [PubMed] [Google Scholar]
- Cummings J. L., Tomiyasu U., Read S., Benson D. F. Amnesia with hippocampal lesions after cardiopulmonary arrest. Neurology. 1984 May;34(5):679–681. doi: 10.1212/wnl.34.5.679. [DOI] [PubMed] [Google Scholar]
- DRACHMAN D. A., ADAMS R. D. Herpes simplex and acute inclusion-body encephalitis. Arch Neurol. 1962 Jul;7:45–63. doi: 10.1001/archneur.1962.04210010051005. [DOI] [PubMed] [Google Scholar]
- Damasio A. R., Damasio H. The anatomic basis of pure alexia. Neurology. 1983 Dec;33(12):1573–1583. doi: 10.1212/wnl.33.12.1573. [DOI] [PubMed] [Google Scholar]
- Damasio A. R., Graff-Radford N. R., Eslinger P. J., Damasio H., Kassell N. Amnesia following basal forebrain lesions. Arch Neurol. 1985 Mar;42(3):263–271. doi: 10.1001/archneur.1985.04060030081013. [DOI] [PubMed] [Google Scholar]
- Divac I. Magnocellular nuclei of the basal forebrain project to neocortex, brain stem, and olfactory bulb. Review of some functional correlates. Brain Res. 1975 Aug 15;93(3):385–398. doi: 10.1016/0006-8993(75)90178-x. [DOI] [PubMed] [Google Scholar]
- Graff-Radford N. R., Eslinger P. J., Damasio A. R., Yamada T. Nonhemorrhagic infarction of the thalamus: behavioral, anatomic, and physiologic correlates. Neurology. 1984 Jan;34(1):14–23. doi: 10.1212/wnl.34.1.14. [DOI] [PubMed] [Google Scholar]
- Heilman K. M., Sypert G. W. Korsakoff's syndrome resulting from bilateral fornix lesions. Neurology. 1977 May;27(5):490–493. doi: 10.1212/wnl.27.5.490. [DOI] [PubMed] [Google Scholar]
- Horel J. A. The neuroanatomy of amnesia. A critique of the hippocampal memory hypothesis. Brain. 1978 Sep;101(3):403–445. doi: 10.1093/brain/101.3.403. [DOI] [PubMed] [Google Scholar]
- Krettek J. E., Price J. L. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977 Jan 15;171(2):157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Mahut H., Zola-Morgan S., Moss M. Hippocampal resections impair associative learning and recognition memory in the monkey. J Neurosci. 1982 Sep;2(9):1214–1220. doi: 10.1523/JNEUROSCI.02-09-01214.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W. G., Warrington E. K., Weiskrantz L. Memory disorder in Korsakoff's psychosis: a neuropathological and neuropsychological investigation of two cases. Brain. 1979 Dec;102(4):749–783. doi: 10.1093/brain/102.4.749. [DOI] [PubMed] [Google Scholar]
- Markowitsch H. J. Thalamic mediodorsal nucleus and memory: a critical evaluation of studies in animals and man. Neurosci Biobehav Rev. 1982 Fall;6(3):351–380. doi: 10.1016/0149-7634(82)90046-x. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M., Mufson E. J., Levey A. I., Wainer B. H. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983 Feb 20;214(2):170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M., Mufson E. J. Neural inputs into the nucleus basalis of the substantia innominata (Ch4) in the rhesus monkey. Brain. 1984 Mar;107(Pt 1):253–274. doi: 10.1093/brain/107.1.253. [DOI] [PubMed] [Google Scholar]
- Mishkin M. A memory system in the monkey. Philos Trans R Soc Lond B Biol Sci. 1982 Jun 25;298(1089):83–95. doi: 10.1098/rstb.1982.0074. [DOI] [PubMed] [Google Scholar]
- Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978 May 25;273(5660):297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- Robertson R. T., Kaitz S. S. Thalamic connections with limbic cortex. I. Thalamocortical projections. J Comp Neurol. 1981 Jan 20;195(3):501–525. doi: 10.1002/cne.901950308. [DOI] [PubMed] [Google Scholar]
- Rogers J. D., Brogan D., Mirra S. S. The nucleus basalis of Meynert in neurological disease: a quantitative morphological study. Ann Neurol. 1985 Feb;17(2):163–170. doi: 10.1002/ana.410170210. [DOI] [PubMed] [Google Scholar]
- SCOVILLE W. B., MILNER B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957 Feb;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Structural organization of nonspecific thalamic nuclei and their projection toward cortex. Brain Res. 1967 Sep;6(1):60–94. doi: 10.1016/0006-8993(67)90183-7. [DOI] [PubMed] [Google Scholar]
- Skinner J. E., Lindsley D. B. Electrophysiological and behavioral effects of blockade of the nonspecific thalamo-cortical system. Brain Res. 1967 Sep;6(1):95–118. doi: 10.1016/0006-8993(67)90184-9. [DOI] [PubMed] [Google Scholar]
- Speedie L. J., Heilman K. M. Amnestic disturbance following infarction of the left dorsomedial nucleus of the thalamus. Neuropsychologia. 1982;20(5):597–604. doi: 10.1016/0028-3932(82)90033-1. [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J., Price D. L., Clark A. W., Coyle J. T., DeLong M. R. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981 Aug;10(2):122–126. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- Woolsey R. M., Nelson J. S. Asymptomatic destruction of the fornix in man. Arch Neurol. 1975 Aug;32(8):566–568. doi: 10.1001/archneur.1975.00490500086012. [DOI] [PubMed] [Google Scholar]