Abstract
The evolution of nest site use and nest architecture in the non-avian ancestors of birds remains poorly understood because nest structures do not preserve well as fossils. Nevertheless, the evidence suggests that the earliest dinosaurs probably buried eggs below ground and covered them with soil so that heat from the substrate fuelled embryo development, while some later dinosaurs laid partially exposed clutches where adults incubated them and protected them from predators and parasites. The nests of euornithine birds—the precursors to modern birds—were probably partially open and the neornithine birds—or modern birds—were probably the first to build fully exposed nests. The shift towards smaller, open cup nests has been accompanied by shifts in reproductive traits, with female birds having one functioning ovary in contrast to the two ovaries of crocodilians and many non-avian dinosaurs. The evolutionary trend among extant birds and their ancestors has been toward the evolution of greater cognitive abilities to construct in a wider diversity of sites and providing more care for significantly fewer, increasingly altricial, offspring. The highly derived passerines reflect this pattern with many species building small, architecturally complex nests in open sites and investing significant care into altricial young.
This article is part of the theme issue ‘The evolutionary ecology of nests: a cross-taxon approach’.
Keywords: birds, crocodilians, dinosaurs, evolution, nest architecture, nest sites
1. Introduction
There are over 10 000 extant species of birds worldwide and they use a variety of nest designs and nest sites [1]. Nests are structures built with the express purpose of containing eggs and/or offspring, while nest sites refer to the location of nests [2]. Here we review the evolution of nest site selection and nest architecture by extant birds and their extinct ancestors, while also considering the evolution of modern birds themselves. However, our understanding of the use of nest sites and nest architecture by ancient birds and their non-avian ancestors is incomplete because nests and their substrates do not preserve well as fossils [3].
Despite an increasing number of fossilized clutches of dinosaur eggs being found [4–6], the fossil record of nest remains is sparse, particularly for early dinosaurs such as sauropodomorphs (figure 1). Based on clutch forms and eggshell porosity, sauropods primarily buried their eggs beneath the ground [7]. As their nests were filled with muddy flood plain deposits and, thus, in conditions not conductive to fossilization, discernable traces of their nests are only rarely preserved [8,9]. Pedogenic and diagenetic processes probably destroyed the nests of the earliest dinosaurs, yet our understanding has recently increased because of the discovery of new fossils and the development of new analytical approaches [10–12].
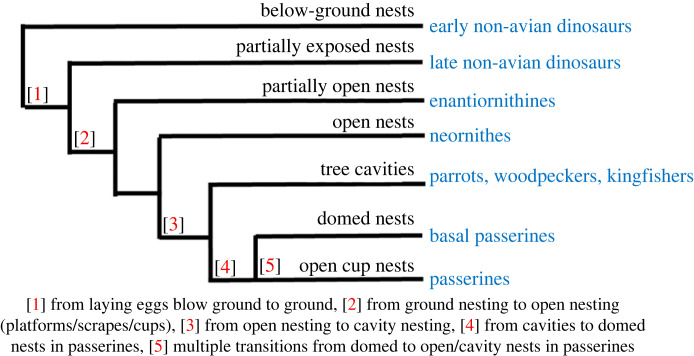
Figure 1.
The evolution of nest characteristics in birds and their non-avian ancestors.
Extant birds (clade Avialae) fall within Theropoda, the clade that includes all carnivorous dinosaurs, with small species such as Troodon and Velociraptor and large species such as Allosaurus and Tyrannosaurus [12]. Birds are hypothesized to be derived from within the less inclusive clade Pennaraptora, representing birds and their close relatives the troodontids, dromaeosaurids and oviraptorosaurs [12,13]. It is thought that birds (avialians) evolved from pennaraptorans approximately 165–150 Ma during the Jurassic period and had begun to diversify by the Early Cretaceous, as confirmed by 120–130 Myr old fossils found in China [13,14]. Enantiornithines were the dominant avialian group during the Cretaceous as modern birds, members of the clade Neornithes, largely represent a post-Cretaceous radiation [12]. Enantiornithines were decimated during the mass extinction event at the end of the Cretaceous period, as were the non-avian dinosaurs, but the neornithine survivors rapidly diversified and there are now over 10 000 bird species worldwide [12].
2. The evolution of nests and nest site use by the ancestors of modern birds
(a) . Early non-avian dinosaurs
Our understanding of the reproductive biology of the earliest dinosaurs remains relatively poor [15,16], yet the ecological context of fossil remains provide important insights into both the nesting sites and also the dispersion of nest sites. Saurischians are thought to be the earliest known clade of dinosaurs, and are thought to have laid unpigmented white eggs [17] below ground, covering them with substrate so that eggs were incubated by environmental heat sources such as soil moisture and thermoradiance, without any heat from incubating parents [7,8,11]. Massospondylus, a genus of prosauropod dinosaur from the Early Jurassic period, suggest that they deposited a single layer of tightly packed eggs below ground [8,9,18]. Meanwhile, the fossilized remains of thousands of the eggs of sauropods, relatives of prosauropods within the clade Sauropodmorpha [19], were found distributed within little over 1 km2 at Auca Mahuevo, Argentina, suggesting that hundreds of individuals nested colonially in that area [20]. Titanosaur sauropod clutches of Europe [7] and India [4] were buried underground. There is evidence that some sauropods repeatedly nested at a site with a peculiarly warm hydrothermal geology, which would have also facilitated incubation [21]. Such behaviours can still be found in the extant megapodes, although the behaviour is a convergent trait evolved from ancestors which used body heat for incubation [22]. For example, Australian brush-turkeys (Alectura lathami) bury their eggs in mounds of decomposing leaves, which provide the heat source for incubation [23], and Polynesian megapodes (Megapodius pritchardii) select nest sites which provide geothermal warmth for incubation [24].
The subterranean development of eggs of the earliest dinosaurs had important implications for nest architecture, egg characteristics, sex determination and parental care. Nests were simple scrapes or holes in the ground and the eggs were probably laid ‘en masse’ in one ovideposition event, similar to modern reptiles, before being covered with substrate. The eggs were subspherical and had modular ornamentation that enabled air to flow easily between eggs within the nests and the eggshells were perforated by many pore canals as a compensation for the high moisture content within nests [11]. After controlling for egg size, Deeming [25] showed that compared to the eggs of extant birds, dinosaur eggs are thought to have had relatively thicker eggshells, with more pores which would have meant that water vapour conductance was significantly higher relative to shell size and thickness. The absence of parental incubation meant that dinosaur eggs were not turned, while all extant birds, except for megapodes and the three-banded courser [26], turn them on a regular basis.
The eggs of the earliest dinosaurs were probably white [17,27] because they were buried and neither were exposed to potentially harmful UV light, nor were they visible to conspecifics or predators [10,28]. It is probable that any role of the parents after egg laying was limited to the protection of the nest site and the benefits of group defence may explain why sauropods were colonial nesters [20]. We are unaware of any studies which quantify the predation rates of the nests of non-avian dinosaurs, yet it is intuitive that predators did prey upon the eggs of dinosaurs, whether they were buried or not. Ruxton et al. [29] estimated that the eggs of sauropods took 65–82 days to hatch and suggested that the small egg sizes and clutch sizes of sauropods, based on their enormous body sizes, were a result of life-history trade-offs with high nest predation rates. The eggs of later dinosaurs were, however, coloured and maculated, presumably following selection pressure for camouflage against predation, as in modern birds. This evolution of coloured eggs is associated with the later dinosaurs leaving their eggs unburied and incubating the clutch directly, so that oviraptors probably sat on dark blue eggs which are better camouflaged than plain white eggs [17,27].
Nesting traces from Auca Mahuevo may indicate that these clutches were incubated subaerially [7,30,31], as eggs from this site exhibit significantly lower porosity than those from Europe [32]. The fossil evidence suggests that dinosaurs within the clade Pennaraptora began to lay their eggs closer to ground level so the eggs were partially exposed thereby representing an important shift in nest sites by the avian ancestors [10,33]. The shift to nesting fully above ground was gradual because taphonomic evidence suggests that these dinosaur nesters, such as oviraptorosaurs and troodontids (Pennaraptora), were probably the first dinosaurs to incubate their eggs [34]. The shift from completely burying eggs below ground to having partially or fully exposed eggs is covered in more detail by Hogan & Varricchio [10].
(b) . Late non-avian dinosaurs
The shift to partially exposed eggs is hypothesized to have been associated with changes in parental care [34]. The discovery of oviraptorosaur and troodontid skeletons positioned atop their eggs [33] indicates that they were incubating in a similar way to modern birds (Neornithes). However, it is thought that oviraptorid eggs were paired and arranged sub-horizontally up to three layers deep and, as the parents did not appear to have rotated them, it seems improbable that parents could have effectively incubated all the eggs at once through contact body heat [35]. Oviraptorids may represent an intermediate phylogenetic precursor to the incubation seen in extant birds [11] or a divergent behaviour [35]. The increase in parental care among these dinosaurs may have contributed to the evolutionary radiation of pennaraptoran theropods [10].
The increasing amount of care provided by parents for their offspring is encapsulated by the transition from parents guarding their buried eggs through to them incubating eggs and providing direct warmth for the embryos as well as protection against predators. The discovery of a few well-preserved nests in Montana, North America, is therefore notable. The fossilized nests and eggs of either a dromaesaurid or a caenagnathid (Ornithoraptora) in the Two Medicine Formation from the late Cretaceous period indicate that the nests were in a sparsely vegetated area of freshly deposited sand, implying it was close to an active river channel [36]. A Troodon nest in the same rock formation indicates a bowl-shaped depression with a distinct rim which contained a tightly packed clutch of 24 eggs. The size and shape of the nest, relatively tight clutch arrangement, and low organic carbon content of the overlying mudstone suggests that the eggs were incubated by the parents and not by environmental heat sources [37].
Troodon (Paraves) provides a crucial evolutionary link between their earlier crocodilian sister taxa in the Archosauria and their later avian relatives (Avialae) as while Troodon maintained partially buried eggs, probably without egg rotation similarly to crocodilians, their open nests, partially exposed eggs and parental incubation were similar to some extant birds (Neornithes: [37,38]). The more compact clutches of both troodontids and some enatiornithines suggest an intermediate role for the former and the persistence of buried eggs from non-avian dinosaurs thru Mesozoic birds [33], as is outlined more fully in Hogan & Varricchio [10]. Modelling of Troodon incubation suggests that contact incubating partially buried eggs would still confer an energetic advantage and provides ‘evidence for a possible evolutionary path from guarding behaviour to thermoregulatory contact incubation’ of modern birds [39].
The location and architecture of nests were probably influenced by clutch sizes, which appear to have declined over time from non-dinosaur archosaurs, through non-avian dinosaurs and on to extant birds [12]. This pattern is probably due, at least in part, to more basal dinosaurs having two functioning ovaries, consistent with modern crocodilians [11,40]. By contrast, evidence for pennaraptoran theropods suggests they retained two ovaries and oviducts but that they functioned in an avian fashion. Important specimens include clutches exhibiting egg-pairing and gravid oviraptorosaurs associated with two eggs [40,41]. Modern birds have one functional ovary while crocodilians, sister taxa of the other extant archosaurs, have retained two ovaries. Fossil discoveries of some of the earliest birds (Avialae) in the form of the long bony-tailed Jeholornis and two enantiornithine birds, in rock formations from the Early Cretaceous period at Jehol Biota in China, show they had one functioning ovary [42]. Thus, the shift from two ovaries to one occurred close to the origin of flight. This may represent an exaptation [43] as eggs represent payloads that impact flight performance [44].
As derived theropod dinosaurs began to incubate partially exposed eggs, they also had pigmented eggs which were laid during several visits to nests presumably over several days. Wiemann et al. [27] used high-resolution Raman microspectroscopy to show that although ornithischian and sauropod eggs were white, the later dinosaurs laid coloured and maculated eggs. These pennaraptoran theropods retained two ovaries each functioning in an avian-like manner, thus an adult would produce two eggs per day or greater intervals [40] so that, for illustration, a clutch of 30 eggs was laid over a minimum period of 15 days. The later theropod dinosaurs are hypothesized to only have begun to incubate eggs after the final eggs were laid, so that clutches hatched synchronously [37,45].
The shift to incubating partially exposed eggs is hypothesized to have resulted in dinosaurs providing increasing amounts of care for offspring. Several adult oviraptorid fossils (e.g. Maniraptora) in Mongolia and China were preserved lying on top of clutches of eggs and thus are interpreted to have been incubating eggs similarly to extant birds [46–48]. Although Mesozoic birds (Enantiornithes and Ornithuromorpha) were probably too heavy to contact incubate their eggs ([49]; but see [50]), evidence suggests that non-avian ancestors provided increasing amounts of care for their precocial offspring. Dial [51] hypothesized that shifts in nest elevation, architectural complexity and parental care were associated with decreasing clutch size and increasing altricial ontogeny. The evolution of increasingly sophisticated patterns of parental care was driven by the mutual reinforcement of different components of parental care and offspring behaviours; the evolution of food provisioning caused or enabled parents to select safer nest sites and also resulted in increased levels of sibling competition, which further selected for increased provisioning in response to offspring begging displays [52]. Nesting in increasingly safer nest sites was, therefore, associated with increasing altricial care and offspring begging behaviours.
(c) . Enantiornithines
The enantiornithines were the dominant Avialae during the Cretaceous period, and were contemporary with the dinosaurs. They are now extinct, but during the Mesozoic era, they were the most abundant and diverse group. Virtually all of the enantiornithines had clawed fingers on their wings and retained teeth but otherwise, had a similar morphology to modern birds [12]. They are also commonly resolved as the sister to the Ornithuromorpha, the clade within which all living birds are placed [53]. Our knowledge of their nests is poor [54], although several fossils provide useful insights [33,50].
First, fossils from Argentina show that enantiornithine birds nested among sand dunes, close to an ephemeral water course in an arid landscape, and the eggs were laid either singly or sometimes in pairs and were half-buried in sand with their sharp end pointing downwards which is hypothesized to have precluded egg turning (figure 2, [55]). Mongolian specimens also show eggs placed on their end within substrates either singly as in Argentina or in clutches, with two nests preserved with poorly intact adults atop [33,50]. Second, fossils of enantiornithine birds found in Romania suggest that they nested colonially [56]. There has, nevertheless, been disagreement over the nesting sites of perhaps the most famous Paravian, Archaeopteryx. Although there are no known eggs for Archaeopteryx, some argue that they laid eggs below ground where they would have been incubated by environmental heat sources similarly to those used by crocodilians [57], others argue that they nested above ground [58].
Figure 2.
The fossilized remains of in situ eggs of Mesozoic birds (Neornithes) from the Late Cretaceous in Patagonia, Argentina. Reproduced with permission from Fernández et al. [55].
Fossil evidence suggests that enantiornithine birds buried their eggs in substrate [54] which means it was the primitive nest sites of birds and that nesting free of substrate was a derived characteristic that evolved in the euornithines (figure 1). No fossils of the nests of early ornithuromorphs have been found [54] but they were probably simple scrapes lined with plant material, with such nests still being used by some of the basal neornithine birds such as galloanserines and paleognaths [1]. Fossil evidence suggests that enantiornithe birds provided some primitive forms of care to their offspring and although Varricchio & Barta [50] assumed that as they sat on their partially exposed eggs, they may have been guarding their eggs as opposed to incubating them. The egg shapes of the enantiornithines and ornithuromorphs also differed: enantiornithine birds laid narrow, elongated eggs that resembled the eggs of the non-avian theropod dinosaurs, whereas ornithurine birds laid eggs that were comparatively larger and rounder [54].
Despite the presence of enantiornithine birds, non-avian dinosaurs were the dominant vertebrate group during the Cretaceous period although their fate took a dramatic downturn during the mass extinction event that occurred at the end of the Cretaceous period, some 66 Ma [12]. It is widely agreed that some form of catastrophic event occurred although there is disagreement over what the event was and the severity of its effect because one school of thought suggests that dinosaurs and archaic birds were declining well before the mass extinction event [59]. There is, however, a consensus that the mass extinction event was caused by the Chicxulub asteroid which caused rapid and catastrophic changes in environmental conditions via the inducement of earthquakes, wildfires, tsunamis and acid rainfall [12]. There is also a consensus that neither dinosaurs nor the archaic birds were declining prior to the mass extinction event and pertinently, Longrich et al. [50] provided evidence of a diverse avifauna, represented by enantiornithes, ichthyornithes, hesperornithes and an Apsaravis-like bird, in the fossil record of western North America shortly before the mass extinction event occurred. As none of these groups are known to have survived into the Paleogene, then they probably perished very rapidly during the Chicxulub asteroid impact. The enantiornithines were the dominant bird group during the Cretaceous period but, after they had been decimated during the mass extinction event alongside the dinosaurs, the neornithes, which are considered to be the first modern birds, radiated and filled the ecological spaces that the enantiornithine birds left behind [12,59].
(d) . Neornithes
Neornithes, known widely as modern birds, are the most recent common ancestor of all living birds (class Aves) and their descendants. They are usually divided into two superorders; the Paleognathae which consists of flightless ratites and tinamous, and the Neognathae which contains all other birds [54,60]. It is hypothesized that, like early neornithine birds nested free of sediment and in more open locations in contrast to either the enantiornithine birds or non-avian dinosaurs and it is proposed that their relatively open nest sites associated with increased parental care helped them survive the mass extinction event [33,54]. It has also been suggested that nesting above ground meant that during the periods of rapid environmental changes in the aftermath of the Chicxulub asteroid, neornithine parents could flexibly alter incubation patterns and thus maintain the viability of their embryos to a much greater extent than could the enantiornithine birds and dinosaurs, both of which had eggs variably incubated by environmental heat sources [54]. Neornithe birds are also thought to have provided increased amounts of care to hatched offspring, which is highly likely to have increased their progeny's per capita survival [54].
It has been suggested that neornithine birds were the earliest groups to lay eggs which required regular turning for successful development [3,54]. Egg turning is thought to have evolved in conjunction with an increase in protein-rich albumen in eggs, because regular egg turning is required to prevent the albumen inside eggs from stratifying which would limit water and protein uptake by embryos [54]. Current support for this comes from the extant megapodes, which bury their eggs below ground and so do not turn them, but also have very low amounts of albumen within their eggs. Irrespective of the amount of albumen in eggs, megapodes are some of the only extant birds with ‘superprecocial' chicks that receive no further care after the chicks leave the nest and, as the offspring of enantiornithine birds were superprecocial [61,62], then it is likely to have been the plesiomorphic pattern of offspring development in Neornithes. With the further exception for young obligate brood parasitic black-headed ducks Heteronetta atricapilla, which receive no care from their foster parents, all other extant birds provide at least some care for their hatched offspring [63].
In summary, the early dinosaurs (Saurischians) laid and buried relatively large clutches of white eggs which were incubated by environmental heat sources [11,12]. Birds are the only extant tetrapods that both lay their eggs above ground and incubate the eggs themselves, thus suggesting that nesting above ground evolved relatively late within the ornithuromorpha, the clade that includes modern birds [10,33]. It has been suggested that the shift to nesting above-ground resulted in an increase in the relative volume of albumen within eggs [64] and the beginnings of offspring being increasingly dependent on their parents, as seen in altricial offspring. Further, it is probable that the early ornithuromorphs laid eggs in nests built on the ground, outside of burrows, and began to protect their offspring from adverse weather conditions and predators [54]. It is also probable that the neornithine birds were the first to lay their eggs in fully exposed nest sites and provide care for their offspring as is widespread in extant birds [12]. There has, therefore, been a trend in dinosaurs and ancestral birds towards nest sites becoming progressively more open, and free of sediment, and parents laying pigmented eggs and providing increasing amounts of care for their offspring, which has continued among the extant birds.
3. The evolution of nest site use and nest architecture by extant birds
The evolution of nest site use among extant birds has received relatively little attention and, until relatively recently, our understanding was based on studies that have focused on single families of birds. Although several review articles have described aspects of the evolution of nest site selection by extant passerines [65–67], they have largely consisted of interesting, but ultimately anecdotal, observations and so our understanding of the evolution of nest sites by extant birds has remained relatively poor.
Our understanding of the evolution of nest site uses, nest structures, and methods of material attachment in all of the 242 avian families was greatly enhanced by a landmark study across the world's bird families (figure 3, [1]). In the families of extant birds, 60% nest in trees, 20% nest in non-tree vegetation and the remaining 20% nest on the ground, in river banks or on cliffs. In terms of nest structure, meanwhile, cup nests are by far the most common, while domed nests, platform nests and nests in tree holes are less common. Finally, 80% of families attach their nests to the substrate via basal attachment, with the three other attachment types, namely lateral, horizontally forked and pensile each being used by less than 10% of families [1].
Figure 3.
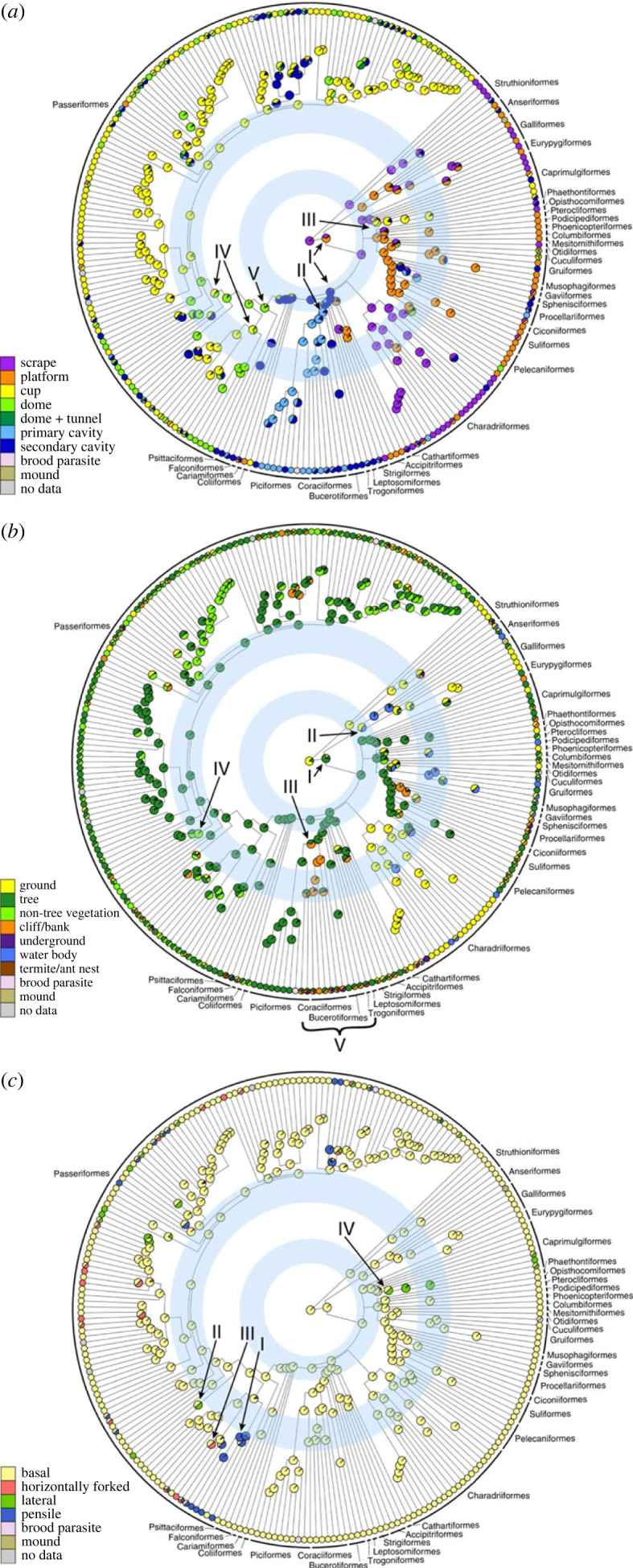
The phylogenetic distribution of (a) nest structure, (b) nest site and (c) nest attachment among extant bird families. Filled coloured circles at the tips and nodes of the trees show nest character states in extant families and their ancestors, respectively; circles filled with multiple colours show families or ancestral taxa with multiple character states; and blue rings indicate the two major adaptive radiations in extant birds. Reproduced with permission from Fang et al. [1].
Fang et al. [1] also showed that the use of nest sites in trees evolved quite early in extant birds, while the use of nest sites in non-tree vegetation, on cliffs and on a variety of water bodies evolved later. The trend for passerines to nest in non-tree vegetation is one of the most important evolutionary transitions in extant birds, with coevolutionary analyses showing that nesting in non-tree vegetation came after the appearance of cup nests. Ancestral state reconstruction techniques showed that extant birds began nesting in trees, on water bodies, on cliffs and in riverbanks after cavity and platform nests evolved from scrape nests. Further, although scrape nests are always located on the ground, scrape or non-scrape nests and nests either on the ground or above the ground are traits that evolved independently of each other [1].
(a) . Hole nests in the ground
A handful of species of megapodes, such as the Australian brush-turkey (Alectura lathami), build mounds in which to incubate eggs via environmental heat sources [24], which is similar to the early non-avian dinosaurs. The evolution of hole nests in the ground for species other than megapodes has received little research attention but they probably protect the occupants from strong winds [66].
(b) . Tree cavities
Nests in tree holes, meanwhile, provide the occupants with protection from wind and rainfall, while also having lower nest predation rates than species nesting outside of holes [66], as was shown in passerines in Arizona, North America [68]. Nests in holes suffer relatively low predation rates and are protected from adverse weather conditions. While primary cavity nesting species such as woodpeckers are able to excavate their own holes, secondary cavity nesting species such as chickadees and tits are incapable of excavating their own holes and are, therefore, reliant upon holes either excavated by primary cavity nesting species or created by tree damage or decay [69]. The distribution of secondary cavity nesting species can, therefore, be reliant upon the presence of primary cavity nesters in some habitats and, for example, there are very few secondary cavity nesting species in Australia, New Zealand or New Guinea where woodpeckers are absent [70]. By contrast, holes caused by tree damage or decay are more common in older European forests, and the availability of unused holes suggests that while nest site availability is not always limiting [71,72], there can be both intraspecific and interspecific competition for hole-nesting sites in some habitats [73,74]. A disadvantage of hole nesting, however, is that incubating birds may be trapped in the nest by a predator either remaining at the entrance or being small enough to enter the hole, thereby preventing adult escape [66]. This may explain why extant birds have shifted from hole nesting to open nesting over time.
(c) . Domed nests
In terms of nest architecture, domed nests are the most similar to cavity nests because they both have small entrance holes and are protected on all sides. Some species whose ancestors nested in tree holes may have shifted to nest in more open sites using domed nests. For example, a study of the African lovebird genus Agapornis showed that the shift from nesting inside tree holes to building domed nests in cavities was derived from the parrots lining their tree hole nests and progressively building more complex nest structures [75]. Elsewhere, the shift from nesting inside tree holes to constructing vegetative nests outside of tree holes has occurred at least three times during the evolution of the synallaxine and furnariine ovenbirds and was probably adaptive because it served as an ecological release that enabled them to exploit a wider variety of breeding habitats [76,77].
Domed nests provide birds with similar, but perhaps lower, levels of protection from adverse weather conditions in comparison to cavities, because domed nest walls are relatively insubstantial when compared to the protection provided by tree trunks. However, the birds are able to build such domed nests in a much wider variety of locations [78]. Domed nests have been identified as an ancestral nest type in passerine birds [77,79,80] because basal passerine families such as the New Zealand wrens (Acanthisittidae) build enclosed nests in crevices and the broadbills (Eurylaimidae) construct domed nests with side entrances [79]. Furthermore, the suboscine ovenbirds (Furnariidae) build a diversity of nest types and in two lineages the species building primitive tree hole nests have evolved to build more complex domed nests [77] that offer considerable flexibility because domed nests can be built wherever the parents wish to build them [66].
The primary function of domed nests has been debated. One suggestion is that they reduce the risk of predation in comparison to open cup nests and, in support of this idea, a comparative analysis of the Old-World babblers (Timaliidae) showed that species building domed nests bred closer to the ground than species building cup-shaped nests [81]. It was argued that the evolution of domed nests was dependent on the transition to nesting either on or close to the ground and that the roof provided greater protection against the increased level of predation risk on the ground [81]. Nevertheless, field studies have shown that some species with domed nests suffer high levels of nest predation, with 72% of long-tailed tit (Aegithalos caudatus) nests predated annually [82], although that study did not assess the distribution of the height of lost nests above ground and so the pattern may not be universal. Meanwhile, comparative studies of tropical and temperate passerine birds showed that species with domed and non-domed nests had similar predation rates [83,84], thus suggesting that the primary function of domed nests is not to minimize predation risk.
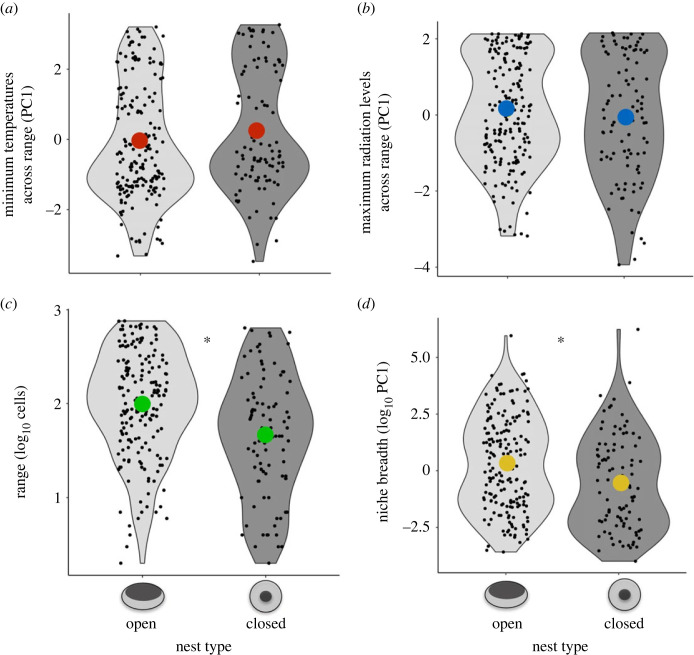
An alternative idea is that the architecture of birds' nests has evolved in response to environmental conditions [85]. Species building domed nests were found to more commonly occur in arid than in non-arid regions of Australia [86], although another study of Australian birds found no such pattern [87]. Instead, species building domed nests were found to have smaller distributions than species building non-domed nests, suggesting that domed nests were lost through evolutionary time as birds in Australia expanded to occupy less arid regions (figure 4, [87]). Moreover, the domed nests of sharp-tailed sparrows (Ammodramus caudacutus) helped prevent their eggs being lost when flooding events occurred on saltmarshes [88], providing further support for the idea that domed nests serve primarily to create optimal nest microclimates and structures rather than a cover against predation. However, it is prudent to consider that the costs and benefits of domed nests may differ between species, and geographical regions, and further studies that simultaneously quantify nest predation rates and the extent to which domed nests protect birds from adverse weather conditions are needed to determine their function/s [89].
Figure 4.
Australian passerines with open and closed nests are distributed across ranges with similar (a) minimum temperatures and (b) maximum radiation levels, but species with open nests have (c) larger ranges and (d) broader niches than species with domed nests. Note that the black dots represent raw data values and the coloured dots represent average values per group. Reproduced with permission from Medina [87].
(d) . Open cup nests
Open cup nests are the other major nest type in extant birds, and they evolved from domed nests within passerine birds [80]. Passerines rapidly evolved and expanded during the Cenozoic period possibly because they could build open cup nests in a wide variety of nesting sites, and they also had smaller body sizes and strong flying capabilities [66,67]. The majority of plant species also went extinct during the mass extinction event between the Cretaceous and Paleogene eras but when vegetation began to recolonize the planet, plants with shrub-like structures rapidly became available as both nesting sites and a source of nest materials for passerine birds which were rapidly diversifying at the same time. Newly evolved plants thus provided a plethora of new potential nesting sites for passerine birds for both domed and open cup nests and may have permitted passerines to use previously occupied ecological space and thus not compete with Piciform and Coraciiform birds that nest in cavities in trees that were probably limited in availability [68]. Specifically, passerine birds had evolved small body sizes and could select a wide variety of nest sites, which is hypothesized to have enabled them to occupy new niches. The piciform and coraciiform birds that were larger hole-nesting species required large, somewhat decayed trees which were likely to have been in relatively limited supply, while passerines could occupy essentially unlimited elevated nesting sites from small shrubs to tree tops [66,67]. Open cup nests are therefore considered to be the most adaptable nest type, and having adaptable nest types is a trait that is argued to be a key innovation which enabled passerines to diversify [67]. The offspring of birds have evolved to become increasingly altricial over time and in the passerines, their offspring are born naked, blind and helpless and are therefore utterly dependent on their parents during the early stages of their lives. The open cup nests that are so prevalent in passerines [1] may also be best able to provide a location in which to raise altricial offspring relative to enclosed nests [66,67] and this possibility requires further research attention.
Meanwhile, studies show that open cup nests evolved from burrow nests excavated in substrate in swallow species [90] and that species of Old-World babblers evolved to construct open nests higher off the ground than species with domed nests [81]. In turn, patterns of allometric scaling suggest that the provision of structural support, as opposed to environmental conditions, determine nest architecture in Australian passerines [91]. However, a study of 36 species of Australian passerine species showed that they adaptively vary their use of insulating materials in their nests in relation to spatial variation in rainfall. Specifically, birds inhabiting warm climates used poorly insulating materials in regions with high rainfall but not in regions of low rainfall, while birds inhabiting cool climates use well insulating materials regardless of the amount of rainfall, so that the composition of nest material mitigates spatial variation in weather conditions throughout Australia [92]. Open cup nests therefore provide more exposed conditions for parents and offspring but open cup nesting species protect themselves from adverse environmental conditions by adaptively using materials that provide insulation.
It may have been thought likely that nest architecture, nest sites, and the method of nest attachment may have evolved in parallel with each other, yet they appear to have evolved independently of each other [1]. Basal attachment is the most common form of attachment, presumably because it is the easiest way of supporting nests and works with gravity, although other forms of attachment evolved because they only evolved in lineages with domed or cup nests [1]. In Melophagoidea, there is a link between body size and method of attachment, with larger species being less likely to have suspended nests [87]. The evolution of domed or cup-shaped nests could have driven the evolution of non-basal methods of attachment or vice versa, but coevolutionary analyses showed that the evolution of domed or cup-shaped nests preceded the evolution of non-basal methods of attachment rather than vice versa [1]. Finally, methods of nest attachment in avian families were more similar to distantly, rather than closely, related families, suggesting that nest attachment methods are highly conserved [1].
The studies above have generally focused on single families of birds and, while informative, interspecific studies involving more species from across the avian phylogeny are needed. In the past decade or so, studies have used data from hundreds and sometimes thousands of species from entire continents or globally to examine the evolution of nest sites or nest architecture in relation to the sex of the building parent/s [93], egg characteristics [94,95], host use by brood parasites [96], conservation threats [97] and ecological success [98]. The landmark study by Fang et al. [1] examined the evolution of nest sites, nests structures, and methods of material attachment in all of the 242 avian families and therefore provided unparalleled insights into the evolution of nest sites and nest architecture in extant birds. Nevertheless, we need even more data on the nest sites and nest architecture of the world's bird species, because we are lacking sufficient descriptions for many species from South America and Africa, and this leaves important gaps in phylogenetically controlled comparative analyses. Meanwhile, it is also important to consider that while such comparative studies have proven insightful, they are also open to bias because, for example, different studies may produce quantitatively different results because of variation in the interpretation of the raw data collected from online data sources.
4. Conclusion
Non-avian dinosaurs transitioned from incubating eggs fully buried to partially buried, conditions maintained in enantiornithines, the dominant birds of the Mesozoic. Modern birds evolved to build smaller and more elaborate but still open cup-shaped nests in a greater variety of nest sites, which has been accompanied by an increasing amount of care being provided for fewer, more altricial offspring. In particular, nests changed substantially when the Enantiornithes went extinct and the earliest birds (Neornithes) exploited and thus filled the niches they left behind. Prior to the end-Cretaceous mass extinction event, most neornithine nests are hypothesized to have been scrape or platform nests, and the earliest modern birds evolved to build more complex nest structures than ever seen before, which in turn enabled them to use nesting sites that included trees, shrubs, cliffs, on water bodies and in river banks [1]. It also included the use of cavities and the transition from cavities to domed nest in ancestral passerines. Passerine birds, in particular, then took advantage of newly evolved plants to build small open cup nests in increasingly exposed locations, which may have enabled passerines to use previously unoccupied ecological space and thus not compete with parrots (Psittaciiform), kingfishers (Coraciiform) and woodpecker (Piciform) which mostly nest in cavities in trees that were probably limited in availability. Accordingly, in the passerines, there have been multiple transitions from domed nests to open cup nests and cavity nests.
Acknowledgements
We thank Cassie Stoddard, Susan Healy, and an anonymous reviewer for helpful comments that improved the manuscript.
Data accessibility
This article contains no additional data.
Authors' contributions
M.C.M.: conceptualization, writing—original draft; I.M., B.W.T., I.R.H., D.J.V. and M.E.H.: conceptualization, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare no conflicts of interest.
Funding
We received no funding for this study.
References
- 1.Fang Y-T, Tuanmu M-N, Hung C-M. 2018. Asynchronous evolution of interdependent nest characters across the avian phylogeny. Nat. Commun. 9, 1863. ( 10.1038/s41467-018-04265-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansell MH. 2005. Animal architecture. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Deeming DC. 2015. The fossil record and evolution of avian egg nesting and incubation. In Nests, eggs, and incubation: new ideas about avian reproduction (eds Deeming DC, Reynolds SJ), pp. 8-15. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Dhiman H, Verma V, Singh LR, Miglani V, Jha DK, Sanyal P, Tandon SK, Prasad GVR. 2023. New Late Cretaceous titanosaur sauropod dinosaur egg clutches from lower Narmada Valley, India: palaeobiology and taphonomy. PLoS ONE 18, e0278242. ( 10.1371/journal.pone.0278242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norell MA, Clark JM, Demberelyin D, Rhinchen B, Chiappe LM, Davidson AR, McKenna MC, Altangerel P, Novacek MJ. 1994. A theropod dinosaur embryo and the affinities of the Flaming Cliffs dinosaur eggs. Science 266, 779-782. ( 10.1126/science.266.5186.779) [DOI] [PubMed] [Google Scholar]
- 6.Varricchio DJ, Jackson FD. 2004. Two eggs sunny-side up: reproductive physiology in the dinosaur Troodon formosus. In Feathered dragons: studies on the transition from dinosaurs to birds (eds Currie PJ, Koppelhus EB, Shugar MA, Wright JL), pp. 215-233. Bloomington, IN: Indiana University Press. [Google Scholar]
- 7.Sander DPM, Peitz C, Jackson FD, Chiappe LM. 2008. Upper Cretaceous titanosaur nesting sites and their implications for sauropod dinosaur reproductive biology. Palaeontogr. Abt. A. 284, 69-107. ( 10.1127/pala/284/2008/69) [DOI] [Google Scholar]
- 8.Reisz RR, Evans DC, Roberts EM, Sues HD, Yates AM. 2012. Oldest known dinosaurian nesting site and reproductive biology of the Early Jurassic sauropodomorph Massospondylus. Proc. Natl Acad. Sci. USA 109, 2428-2433. ( 10.1073/pnas.1109385109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reisz RR, et al. 2013. Embryology of Early Jurassic dinosaur from China with evidence of preserved organic remains. Nature 496, 210-214. ( 10.1038/nature11978) [DOI] [PubMed] [Google Scholar]
- 10.Hogan JD, Varricchio DJ. 2023. Chthonic severance: dinosaur eggs of the Mesozoic, the significance of partially buried eggs, and contact incubation precursors. Phil. Trans. R. Soc. B 378, 20220144. ( 10.1098/rstb.2022.0144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grellet-Tinner G, Chiappe L, Norell M, Bottjer D. 2006. Dinosaur eggs and nesting behaviours: a paleobiological investigation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 232, 294-321. ( 10.1016/j.palaeo.2005.10.029) [DOI] [Google Scholar]
- 12.Brusatte SL, O'Connor JK, Jarvis ED. 2015. The origin and diversification of birds. Curr. Biol. 25, R888-R898. ( 10.1016/j.cub.2015.08.003) [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Zhou Z, Dudley R, Mackem S, Chuong C-M, Erickson GM, Varricchio DJ. 2014. An integrative approach to understanding bird origins. Science 346, 1253293. ( 10.1126/science.125329) [DOI] [PubMed] [Google Scholar]
- 14.Zhou Z, Barrett PM, Hilton J. 2003. An exceptionally preserved Lower Cretaceus ecosystem. Nature 421, 807-814. ( 10.1038/nature01420) [DOI] [PubMed] [Google Scholar]
- 15.Grellet-Tinner G, Chiappe LM. 2004. Dinosaur eggs and nesting: implications for understanding the origin of birds. In Feathered dragons: studies on the transition from dinosaurs to birds (eds Currie PJ, Koppelhus EB, Shugar MA, Wright JL), pp. 185-214. Bloomington, IN: Indiana University Press. [Google Scholar]
- 16.Upchurch P. 1995. The evolutionary history of sauropod dinosaurs precursors. Phil. Trans. R. Soc. Lond. B 349, 365-390. ( 10.1098/rstb.1995.0125) [DOI] [Google Scholar]
- 17.Wiemann J, Yang T-R, Sander PNN, Schneider M, Engeser M, Kath-Schorr S, Müller CE, Sander PM. 2015. The blue-green eggs of dinosaurs: how fossil metabolites provide insights into the evolution of bird reproduction. PeerJ 3, e1323. ( 10.7717/peerj.3706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varricchio DJ, Jackson FD. 2003. Origins of avian reproduction: answers and questions from dinosaurs. Palaeovertebrata 32, 149-170. [Google Scholar]
- 19.Upchurch P, Barrett PM, Dodson P. 2004. Sauropoda. In The Dinosauria: second edition (eds Dodson P, Weishampel B, Osmólska H), pp. 259-322. Berkeley, CA: University of California Press. [Google Scholar]
- 20.Chiappe LM, Coria RA, Dingus L, Jackson F, Chinsamy A, Fox M. 1998. Sauropod dinosaur embryos from the late Cretaceous of Patagonia. Nature 396, 258-261. ( 10.1038/24370) [DOI] [Google Scholar]
- 21.Grellet-Trinner G, Fiorelli LE. 2010. A new Argentinian nesting site showing neosauropod dinosaur reproduction in a Cretaceous hydrothermal environment. Nat. Commun. 1, 32. ( 10.1038/ncomms1031) [DOI] [PubMed] [Google Scholar]
- 22.Dekker RWRJ, Brom TG. 1992. Megapode phylogeny and the interpretation of incubation strategies. Zool. Verh. 278, 19-31. [Google Scholar]
- 23.Eiby YA, Booth DT. 2008. Embryonic thermal tolerance and temperature variation in mounds of the Australian brush-turkey (Alectura lathami). Auk 125, 594-599. ( 10.1525/auk.2008.07083) [DOI] [Google Scholar]
- 24.Göth A, Booth DT. 2005. Temperature-dependent sex ratio in a bird. Biol. Lett. 1, 31-33. ( 10.1098/rspb.2008.0954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deeming DC. 2006. Ultrastructural and functional morphology of eggshells supports the idea that dinosaur eggs were incubated buried in a substrate. Palaeontology 49, 171-185. ( 10.1111/j.1475-4983.2005.00536.x) [DOI] [Google Scholar]
- 26.Kemp AC, Maclean GL. 1973. Nesting of the three-banded courser. Ostrich 44, 82-83. [Google Scholar]
- 27.Wiemann J, Yang T-R, Norell MA. 2018. Dinosaur egg colour had a single evolutionary origin. Nature 563, 555-558. ( 10.1038/s41586-018-0646-5) [DOI] [PubMed] [Google Scholar]
- 28.Lahti DC, Ardia DR. 2016. Shedding light on bird egg color: pigment as parasol and the dark car effect. Am. Nat. 187, 547-563. ( 10.1086/685780) [DOI] [PubMed] [Google Scholar]
- 29.Ruxton GD, Birchard GF, Deeming DC. 2014. Incubation time as an important influence on egg production and distribution into clutches for sauropod dinosaurs. Paleobiology 40, 323-330. ( 10.1666/13028) [DOI] [Google Scholar]
- 30.Chiappe LM, Schmitt JG, Jackson FD, Garrido A, Dingus L, Grellet-Tinner G. 2004. Nest structure for sauropods: sedimentary criteria for recognition of dinosaur nesting traces. Palaios 19, 89-95. [Google Scholar]
- 31.Jackson FD, Garrido A, Schmitt JG, Chiappe LM, Dingus L, Loope DB. 2004. Abnormal, multilayered titanosaur (Dinosauria: Sauropoda) eggs from in situ clutches at the Auca Mahuevo locality, Neuquén Province, Argentina. J. Vertebr. Paleontol. 24, 913-922. ( 10.1671/0272-4634(2004)024[0913:AMTDSE]2.0.CO;2) [DOI] [Google Scholar]
- 32.Vila B, Galobart A, Oms O, Poza B, Bravo AM. 2010. Assessing the nesting strategies of Late Cretaceous titanosaurs: 3-D clutch geometry from a new megaloolithid eggsite. Lethaia 43, 197-208. ( 10.1111/j.1502-3931.2009.00183.x) [DOI] [Google Scholar]
- 33.Varricchio DJ, Jackson FD. 2016. Reproduction in Mesozoic birds and evolution of the modern avian reproductive mode. Auk 133, 654-684. ( 10.1642/AUK-15-216.1) [DOI] [Google Scholar]
- 34.Tanaka K, Zelenitsky DK, Therrien F. 2015. Eggshell porosity provides insight on evolution of nesting in dinosaurs. PLoS ONE 10, e0142829. ( 10.1371/journal.pone.0142829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang T-R, Wiemann J, Xu L, Cheng Y-N, Wu X-C, Sander PM. 2019. Reconstruction of oviraptorid clutches illuminates their unique nesting biology. Acta Palaeontol. Pol. 64, 581-596. ( 10.4202/app.00497.2018) [DOI] [Google Scholar]
- 36.Zelenitsky DK, Therrien F. 2008. Unique maniraptoran egg clutch from the Upper Cretaceous Two Medicine Formation of Montana reveals theropod nesting behaviour. Palaeontology 51, 1253-1259. ( 10.1111/j.1475-4983.2008.00815.x) [DOI] [Google Scholar]
- 37.Varricchio DJ, Jackson F, Trueman CN. 1999. A nesting trace with eggs for the Cretaceous theropod dinosaur Troodon formosus. J. Vertebr. Paleontol. 19, 91-100. [Google Scholar]
- 38.Varricchio DJ, Moore JR, Erickson GM, Norell MA, Jackson FD, Borkowski JJ. 2008. Avian paternal care had dinosaur origin. Science 322, 1826-1828. ( 10.1126/science.116324) [DOI] [PubMed] [Google Scholar]
- 39.Hogan JD, Varricchio DJ. 2021. Do paleontologists dream of electric dinosaurs? Investigating the presumed inefficiency of dinosaurs contact incubating partially buried eggs. Paleobiology 47, 101-114. ( 10.1017/pab.2020.49) [DOI] [Google Scholar]
- 40.Varricchio DJ, Jackson F, Borkowski JJ, Horner JR. 1997. Nest and egg clutches of the dinosaur Troodon formosus and the evolution of avian reproductive traits. Nature 385, 247-250. ( 10.1038/385247a0) [DOI] [Google Scholar]
- 41.Sato T, Cheng YN, Wu D, Zelenitsky DK, Hsiao Y. 2005. A pair of shelled eggs inside a female dinosaur. Science 308, 375. ( 10.1126/science.111057) [DOI] [PubMed] [Google Scholar]
- 42.Zheng X, O'Connor J, Huchzermeyer F, Wang X, Wang Y, Wang M, Zhou Z. 2013. Preservation of ovarian follicles reveals early evolution of avian reproductive behaviour. Nature 495, 507-511. ( 10.1038/nature11985) [DOI] [PubMed] [Google Scholar]
- 43.Gould SJ, Vrba ES. 1982. Exaptation—a missing term in the science of form. Paleobiology 8, 4-15. ( 10.1017/S0094837300004310) [DOI] [Google Scholar]
- 44.Lee SJ, Witter MS, Cuthill IC, Goldsmith AR. 1996. Reduction in escape performance as a cost of reproduction in gravid starlings, Sturnus vulgaris. Proc. R. Soc. Lond. B 263, 619-623. ( 10.1098/rspb.1996.0093) [DOI] [Google Scholar]
- 45.Prum RO. 2002. Why ornithologists should care about the theropod origin of birds. Auk 119, 1-17. ( 10.1093/auk/119.1.1) [DOI] [Google Scholar]
- 46.Clark JM, Norell MA, Chiappe LM. 1999. An oviraptorid skeleton from the Late Cretaceous of Ukhaa Tolgod, Mongolia, preserved in an avianlike brooding position over an oviraptorid nest. Am. Mus. Novitates 3265, 1-36. [Google Scholar]
- 47.Dong ZM, Currie PJ. 1996. On the discovery of an oviraptorid skeleton on a nest of eggs at Bayan Mandahu, Inner Mongolia, People's Republic of China. Can. J. Earth Sci. 33, 631-636. ( 10.1139/e96-046) [DOI] [Google Scholar]
- 48.Norell MA, Clark JM, Chiappe LM, Dashzeveg D. 1995. A nesting dinosaur. Nature 378, 774-776. ( 10.1038/378774a0) [DOI] [Google Scholar]
- 49.Deeming DC, Mayr G. 2018. Pelvis morphology suggests that Early Mesozoic birds were too heavy to contact incubate their eggs. J. Evol. Biol. 31, 701-709. ( 10.1111/jeb.13256) [DOI] [PubMed] [Google Scholar]
- 50.Varricchio DJ, Barta DE. 2015. Revisiting Sabath's ‘Larger Avian Eggs’ from the Gobi Cretaceous. Acta. Palaeontol. Pol. 60, 11-25. ( 10.4202/app.00085.2014) [DOI] [Google Scholar]
- 51.Dial KP. 2003. Evolution of avian locomotion: correlates of flight style, locomotor modules, nesting biology, body size, development, and the origin of flapping flight. Auk 120, 941-952. ( 10.1642/0004-8038(2003)120[0941:EOALCO]2.0.CO;2) [DOI] [Google Scholar]
- 52.Gardner A, Smiseth PT. 2011. Evolution of parental care driven by mutual reinforcement of parental food provisioning and sibling competition. Proc. R. Soc. B 278, 196-203. ( 10.1098/rspb.2010.1171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang M, O'Connor JK, Zhao T, Pan Y, Zheng X, Wang X, Zhou Z. 2021. An Early Cretaceous enantiornithine bird with a pintail. Curr. Biol. 31, 4845-4852. ( 10.1016/j.cub.2021.08.044) [DOI] [PubMed] [Google Scholar]
- 54.Mayr G. 2017. Evolution of avian breeding strategies and its relation to the habitat preferences of Mesozoic birds. Evol. Ecol. 31, 131-141. ( 10.1007/s10682-016-9872-1) [DOI] [Google Scholar]
- 55.Fernández MS, García RA, Fiorelli L, Scolaro A, Salvador RB, Cotaro CN, Kaiser GW, Dyke GJ. 2013. A large accumulation of avian eggs from the Late Cretaceous of Patagonia (Argentina) reveals a novel nesting strategy in Mesozoic birds. PLoS ONE 8, e61030. ( 10.1371/journal.pone.0061030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dyke G, Vremir M, Kaiser G, Naish D. 2012. A drowned Mesozoic bird breeding colony from the Late Cretaceous of Transylvania. Naturwissenschaften 99, 435-442. ( 10.1007/s00114-012-0917-1) [DOI] [PubMed] [Google Scholar]
- 57.Stephan B. 1987. Urvögel: archeopterygiformes. Wittenberg, Germany: A. Ziemsen. [Google Scholar]
- 58.Wellnhofer P. 2009. Archaeopteryx: the icon of evolution. Munich, Germany: Verlag Dr. Friedrich Pfeil. [Google Scholar]
- 59.Longrich NR, Tokaryk T, Field DJ. 2011. Mass extinction of birds at the Cretaceous-Paleogene (K-Pg) boundary. Proc. Natl Acad. Sci. USA 108, 15 253-15 257. ( 10.1073/pnas.11103951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dyke GJ, van Tuinen M. 2004. The evolutionary radiation of modern birds (Neornithes): reconciling molecules, morphology and the fossil record. Zool. J. Linn. Soc. 141, 153-177. ( 10.1111/j.1096-3642.2004.00118.x) [DOI] [Google Scholar]
- 61.Elżanowski A. 1981. Embryonic bird skeletons from the Late Cretaceous of Mongolia. Palaeontol. Pol. 42, 147-179. [Google Scholar]
- 62.Zhou Z, Zhang F. 2004. A precocial avian embryo from the Lower Cretaceous of China. Science 306, 653. ( 10.1126/science.110000) [DOI] [PubMed] [Google Scholar]
- 63.Mock DW. 2022. Parental care in birds. Curr. Biol. 32, 1132-1136. [DOI] [PubMed] [Google Scholar]
- 64.Deeming DC. 2002. Patterns and significance of egg turning. In Avian incubation: behaviour, environment, and evolution (ed. Deeming DC), pp. 161-178. Oxford, UK: Oxford University Press. [Google Scholar]
- 65.Collias NE. 1964. The evolution of nests and nest-building in birds. Am. Zool. 4, 175-190. [Google Scholar]
- 66.Collias NE. 1997. On the origin and evolution of nest building by passerine birds. Condor 99, 253-270. ( 10.2307/1369932) [DOI] [Google Scholar]
- 67.Collias NE, Collias EC. 1984. Nest building and bird behaviour. Princeton, NJ: Princeton University Press. [Google Scholar]
- 68.Martin TE, Li P. 1992. Life history traits of open- vs. cavity-nesting birds. Ecology 73, 579-592. ( 10.2307/1940764) [DOI] [Google Scholar]
- 69.Christman BJ, Dhondt AA. 1997. Nest predation in black-capped chickadees: how safe are cavity nests? Auk 114, 769-773. ( 10.2307/4089299) [DOI] [Google Scholar]
- 70.Cockle KL, Martin K, Wesołowski T. 2011. Woodpeckers, decay, and the future of cavity-nesting vertebrate communities worldwide. Front. Ecol. Environ. 9, 377-382. ( 10.1890/110013) [DOI] [Google Scholar]
- 71.Bai M-L, Wichmann F, Mühlenberg M. 2003. The abundance of tree holes and their utilization by hole-nesting birds in a primeval boreal forest of Mongolia. Acta Ornithol. 38, 95-102. ( 10.3161/068.038.0205) [DOI] [Google Scholar]
- 72.Carlson A, Sandström U, Olsson K. 1998. Availability and use of natural tree holes by cavity nesting birds in a Swedish deciduous forest. Ardea 86, 109-119. [Google Scholar]
- 73.Alerstam ND, Högstedt G. 1981. Evolution of hole-nesting birds. Ornis Scand. 12, 188-193. [Google Scholar]
- 74.von Haartman L. 1957. Adaptations in hole-nesting birds. Evolution 11, 339-347. [Google Scholar]
- 75.Eberhard JR. 1998. Evolution of nest-building behaviour in Agapornis parrots. Auk 115, 455-464. ( 10.2307/4089204) [DOI] [Google Scholar]
- 76.Irestedt M, Fjeldså F, Ericson PGP. 2006. Evolution of the ovenbird-woodcreeper assemblage (Aves: Furnariidae)—major shifts in nest architecture and adaptive radiation. J. Avian Biol. 37, 260-272. ( 10.1111/j.2006.0908-8857.03612.x) [DOI] [Google Scholar]
- 77.Zyskowski K, Prum RO. 1999. Phylogenetic analysis of the nest architecture of neotropical oven birds (Furnariidae). Auk 116, 891-911. ( 10.2307/4089670) [DOI] [Google Scholar]
- 78.Newton I. 1994. The role of nest sites in limiting the numbers of hole-nesting birds: a review. Biol. Conserv. 70, 265-276. ( 10.1016/0006-3207(94)90172-4) [DOI] [Google Scholar]
- 79.Prum RO. 1993. Phylogeny, biogeography and evolution of the broadbills (Eurylaimidae) and asities (Philepittidae) based on morphology. Auk 110, 304-324. ( 10.1093/auk/110.2.304) [DOI] [Google Scholar]
- 80.Price JJ, Griffiths SC. 2017. Open cup nests evolved from roofed nests in the early passerines. Proc. R. Soc. B 284, 20162708. ( 10.1098/rspb.2016.2708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hall ZJ, Street SE, Auty S, Healy SD. 2015. The coevolution of building nests on the ground and domed nests in Timaliidae. Auk 132, 584-593. ( 10.1642/AUK-15-23.1) [DOI] [Google Scholar]
- 82.Hatchwell BJ, Sharp SP, Beckerman AP, Meade J. 2013. Ecological and demographic correlates of helping behaviour in a cooperatively breeding bird. J. Anim. Ecol. 82, 486-494. ( 10.1111/1365-2656.12017) [DOI] [PubMed] [Google Scholar]
- 83.Martin TE, Boyce AJ, Fierro-Calderón K, Mitchell AE, Armstad CE, Mouton JC, Bin Soudi EE. 2017. Enclosed nests may provide greater thermal than nest predation benefits compared with open nests across latitudes. Funct. Ecol. 31, 1231-1240. ( 10.1111/1365-2435.12819) [DOI] [Google Scholar]
- 84.Unzeta M, Martin TE, Sol D. 2020. Daily nest predation rates decrease with body size in passerine birds. Am. Nat. 196, 743-754. ( 10.1086/711413) [DOI] [PubMed] [Google Scholar]
- 85.Perez DM, Gardner JL, Medina I. 2020. Climate as an evolutionary driver of nest morphology in birds: a review. Front. Ecol. Evol. 8, 566018. ( 10.3389/fevo.2020.566018) [DOI] [Google Scholar]
- 86.Duursma DE, Gallagher RV, Price JJ, Griffiths SC. 2018. Variation in avian egg shape and nest structure is explained by climatic conditions. Sci. Rep. 8, 4141. ( 10.1038/s41598-018-22436-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Medina I. 2019. The role of the environment in the evolution of nest shape in Australian passerines. Sci. Rep. 9, 157. ( 10.1038/s41598-019-41948-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Humphries S, Elphick CS, Gjerdrum C, Rubega M. 2007. Testing the function of the domed nests of saltmarsh sharp-tailed sparrows. J. Field Ornithol. 78, 152-158. ( 10.1111/j.1557-9263.2007.00098.x) [DOI] [Google Scholar]
- 89.Mainwaring MC, Hartley IR, Lambrechts MM, Deeming DC. 2014. The design and function of birds' nests. Ecol. Evol. 4, 3909-3928. ( 10.1002/ece3.1054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Winkler DW, Sheldon FH. 1993. Evolution of nest construction in swallows (Hirundinidae)—a molecular phylogenetic perspective. Proc. Natl Acad. Sci. USA 90, 5705-5707. ( 10.1073/pnas.90.12.5705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heenan CB, Seymour RS. 2011. Structural support, not insulation, is the primary driver for avian cup-shaped nest design. Proc. R. Soc. B 278, 2924-2929. ( 10.1098/rspb.2010.2798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heenan CB, Goodman BA, White CR. 2015. The influence of climate on avian nest construction across large geographical scales. Glob. Ecol. Biogeogr. 24, 1203-1211. ( 10.1111/geb.12378) [DOI] [Google Scholar]
- 93.Mainwaring MC, Nagy J, Hauber ME. 2021. Sex-specific contributions to nest building in birds. Behav. Ecol. 32, 1075-1085. ( 10.1093/beheco/arab035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nagy J, Hauber ME, Hartley IR, Mainwaring MC. 2019. Correlated evolution of nest and egg characteristics in birds. Anim. Behav. 158, 211-225. ( 10.1016/j.anbehav.2019.10.015) [DOI] [Google Scholar]
- 95.Stoddard MC, Yong EH, Akkaynak D, Sheard C, Tobias J, Mahadevan L. 2017. Avian egg shape: form, function and evolution. Science 6344, 1249-1254. ( 10.1126/science.aaj1945) [DOI] [PubMed] [Google Scholar]
- 96.Antonson ND, Rubenstein DR, Hauber ME, Botero CA. 2020. Ecological uncertainty favours the diversification of host use in avian brood parasites. Nat. Commun. 11, 4185. ( 10.1038/s41467-020-18038-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tobias JA, Pigot AL. 2019. Integrating behaviour and ecology into global biodiversity conservation strategies. Phil. Trans. R. Soc. B 374, 20190012. ( 10.1098/rstb.2019.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Medina I, Perez DM, Afonso Silva AC, Cally J, León C, Maliet O, Quintero I. 2022. Nest architecture is linked with ecological success in songbirds. Ecol. Lett. 25, 1365-1375. ( 10.1111/ele.13998) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article contains no additional data.