ABSTRACT
A growing literature supports a protective association between vaccines targeting an array of pathogens (e.g., influenza, pneumococcus, herpes zoster) and the risk of Alzheimer disease (AD). This article discusses the potential underlying mechanisms for this apparent protective effect of immunizations against infectious pathogens on the risk of AD; explores the basic and pharmacoepidemiologic evidence for this association, with particular attention paid to important methodological variations among the epidemiologic studies; and reviews the remaining uncertainties regarding the effects of anti-pathogen vaccines on Alzheimer disease and all-cause dementia, with recommendations for future directions to address those uncertainties.
KEYWORDS: Vaccines, influenza vaccines, diphtheria-tetanus-acellular pertussis vaccines, pneumococcal vaccines, innate immunity, trained immunity, Alzheimer disease, dementia, neuroimmunomodulation, pharmacoepidemiology
Late-onset Alzheimer disease (AD), which is typically defined as AD with symptom onset at the age of 65 or older and which constitutes a majority of dementia cases, is a pathobiologically complex disease with multiple contributing factors, including patients’ endogenous attributes (e.g., age, genetics), exogenous influences (e.g., environmental pollutants, exposures to pathogenic microbes), and the interactions thereof (e.g., epigenetics, lifestyle, development of infectious diseases and other comorbidities).1,2
For all patients with AD, the neuroimmune axis inevitably has a crucial role in disease pathophysiology as the immune system responds to the hallmark pathological aggregates of AD.3–5 Similarly, the immune system appears to contribute to AD pathogenesis in some patients through processes such as a chronic, low-grade, sterile inflammation known as “inflammaging.”6,7 Importantly, many lines of evidence have demonstrated that infections have a negative influence on the development or progression of AD in a subset of patients,8–11 perhaps through causing a further increase in proinflammatory immunologic activity.
Vaccinations also activate the immune system, of course, which is how they promote immunity to pathogens. As a result, it is also possible that they would promote AD pathology, thereby worsening it. It is somewhat surprising, then, that a growing body of literature supports a protective effect of immunizations, which lie at the intersection of infectious disease and immunology, on the risk of AD and of all-cause dementia more broadly. Vaccinations against numerous pathogens have been associated with a decreased risk of AD and all-cause dementia, including those targeted by the Centers for Disease Control and Prevention (CDC)’s schedule of routine immunizations for older adults (e.g., influenza, pneumococcus, tetanus and diphtheria, herpes zoster) and several other pathogens (e.g., tuberculosis, typhoid).12–14
The mechanism(s) underlying the apparent effect of nonspecific vaccines (i.e., those not specifically designed to elicit an immune response to AD neuropathology) remains unclear, but several non–mutually-exclusive mechanisms are consistent with the existing literature: reduced risk of infectious diseases, many of which are associated with an increased risk of AD15,16; enhanced efficiency of immune-mediated clearance of AD histopathology (e.g., removal of amyloid-β plaques)17–19; and/or a modulation of the immune system’s response to existing AD histopathology such that inflammatory damage to nearby normal brain parenchyma is dampened (i.e., “collateral damage control”).20,21
Longitudinal biomarker studies of late-onset AD have shown that a preclinical phase of the neuropathological hallmarks of AD (e.g., formation of extracellular amyloid plaques and intraneuronal neurofibrillary tangles, and neuroinflammatory changes) precedes the onset of cognitive decline by 10–20 years.1,4 It remains unclear when during the AD pathophysiologic timeline that nonspecific vaccines exert their effect. Because the literature on immunizations and risk of AD thus far consists primarily of retrospective epidemiologic studies with only a handful of basic science studies, it is also unclear how vaccines exert their effects on AD. However, as mentioned above, there are at least a few hypothetical mechanisms underlying the apparent effect of nonspecific vaccinations on AD risk (Figure 1). Importantly, the pathophysiologic targets of these hypothetical mechanisms span the preclinical phase of AD and continue into the clinical stages of the disease1,4; therefore, the period during which nonspecific vaccination could plausibly influence the development of AD is as expansive as the decades-long preclinical phase itself.
Figure 1.
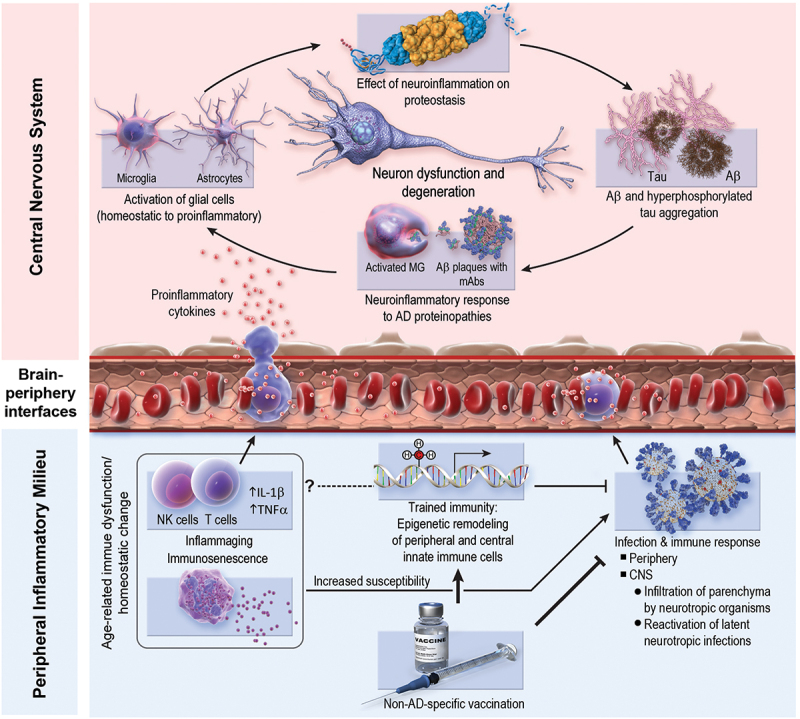
Possible mechanisms underlying the protective effect of non–AD-specific vaccines on AD pathobiology. Bottom right: vaccines may reduce AD risk by mitigating infectious burden, including neurotropic infections (e.g., herpes zoster) and peripheral (i.e., non-CNS) infections. Peripheral infections can influence central neuroinflammation via stimulation of pro-inflammatory immune responses, which can modulate the central immune milieu. Bottom left: vaccine-induced trained immunity (i.e., epigenetic remodeling of innate immune cells) may reduce AD risk by providing non–pathogen-specific protection from infections. Moreover, trained immunity may ameliorate homeostatic imbalances associated with aging (e.g., inflammaging, immunosenescence). This modulation of peripheral and central immune milieus may result in enhanced efficiency of immune-mediated clearance of AD histopathology (e.g., amyloid-β plaques), resolution of AD-induced neuroinflammation, and/or modulation of the immune system’s response to existing AD histopathology such that inflammatory damage to nearby non-pathologic brain parenchyma is dampened (i.e., “collateral damage control”). One possible mechanism that is not pictured above and is likely unique to the influenza vaccine is possible antigenic cross-reactivity between the fusion domain of influenza hemagglutinin and the C-terminus of Aβ1–42 monomers in lipidic environments.17 Aβ, amyloid- β; AD, Alzheimer disease; CNS, central nervous system; IL, interleukin; MG, microglia; NK, natural killer; TNF, tumor necrosis factor.
Epidemiologic studies provide indirect support for short- and long-term mechanisms underlying the effect of nonspecific vaccines on AD risk. The minimum age among most population-based studies of the association between vaccination and risk of AD and/or all-cause has ranged from 60 to 65 years old, with median follow-up periods ranging from 3 to 10 years.12,13 The brevity of these follow-up periods relative to the 10–20-years preclinical phase of AD, in conjunction with the positive findings in the majority of these studies, supports an effect of nonspecific vaccination on the later processes, such as the neuroinflammatory response to existing pathologic aggregates. Moreover, a recent study of the risk of neurodegenerative disorders after various infections supports the existence of both long-term and short-term effects of vaccination on AD risk as well as our suspicion that short-term effects may predominate in the association between vaccines and AD risk. In the study by Levine and colleagues, for example, the risk of AD increased 1, 5, 10, and 15 years after cases of influenza or pneumonia, but the effect was greatest at the 1-year lag period.15
Animal studies may provide greater mechanistic insight into the effects of vaccines on AD pathophysiology. Prior studies using the APP/PS1 mouse model of AD have demonstrated that Bacillus Calmette-Guérin (BCG) vaccination of mice that have AD neuropathology but are not yet symptomatic (the equivalent of the preclinical stage of AD in humans) ameliorates hippocampal dendritic spine pathology, increases expression of cerebral neurotrophic factors, increases serum and cerebral levels of interferon-γ and IL-4, and decreases serum and cerebral levels of several pro-inflammatory cytokines, including TNF-α and IL-1β.19 In older APP/PS1 mice with cognitive decline, similar changes to those seen in pre-symptomatic mice were observed after BCG vaccination, but with two noteworthy differences. First, in sham-treated APP/PS1 mice with cognitive decline, peripheral Foxp3+ Treg activity was significantly increased compared to wild-type, age-matched mice; however, among the APP/PS1 demented mice who received the BCG vaccination, this difference in peripheral Treg activity was not observed. Secondly, BCG vaccination was associated with enhanced recruitment of “inflammation-resolving monocytes” from the periphery to intracerebral sites of amyloid-β plaque pathology.20,21 Interestingly, these BCG-induced changes were associated with a reversal of cognitive impairment such that the task performance of BCG-inoculated mice did not differ significantly from that of age-matched wild-type mice. However, there was no significant difference in the hippocampal and cortical amyloid-β plaque burdens of BCG-treated and sham-treated APP/PS1 mice, nor in the concentrations of soluble Aβ1–40 and Aβ1–42 in the brain or serum of BCG-treated and sham-treated APP/PS1 mice.21
Similar to BCG vaccination, serial influenza vaccination in pre-symptomatic APP/PS1 mice results in antagonization of the peripheral overactivity of Treg cells and mitigation of the cognitive decline seen in sham-treated APP/PS1 mice. However, unlike BCG, influenza vaccination was associated with increased recruitment of microglia to the vicinity of amyloid-β plaques, increased microglial phagocytosis of amyloid-β plaques, and decreased cerebral amyloid-β plaque burden.18,19 These studies provide useful insights into the potential mechanisms underlying the effects of vaccination on AD risk and progression, highlighting both the commonalities (e.g., alteration in peripheral Treg activity, peripheral-CNS immune crosstalk, and involvement of both innate and adaptive immune cells) and the differences (e.g., effect on amyloid-β plaque burden) in the possible mechanisms by which various nonspecific vaccines may influence AD pathology.
Methododological differences
Although the literature thus far overwhelmingly supports a protective effect of nonspecific vaccines on AD risk,12,13 the recent publication of a study with opposing findings in the Journal of Infectious Disease (JID) highlights the need to consider potential differences in the methods used in studies on this topic.22 Indeed, pharmacoepidemiologic studies using routinely collected health data (e.g., administrative data, electronic health record data) to investigate the effect of vaccination on dementia risk have employed a wide range of population databases and methodologies. Here we will note some potentially important differences among datasets and study designs.
Analytical design
Unlike randomized controlled trials, the inferential strength for assessing causality in observational longitudinal studies is limited by the inability to randomly assign treatment. Random treatment assignment theoretically ensures that exposure status – such as whether an individual receives an influenza vaccine – is not confounded by measured or unmeasured baseline characteristics. Through appropriate study design and covariate selection, observational studies can minimize confounding due to measured baseline characteristics. For example, quasi-randomization methods such as propensity score matching (PSM) can minimize confounding from measured confounders by producing exposed and unexposed groups with similar distributions of baseline characteristics. However, cohort designs using PSM typically suffer from other biases, such as immortal time bias.23
Different study designs often carry different biases. For example, a recent JID study found an increased risk of dementia after influenza or pneumococcal vaccination. It used a nested case–control design with matching by the outcome, age, sex, race, database (the study used two separate datasets), year of cohort entry, and duration of follow-up.22 Because the study’s design matched on the outcome, the selected sample had significant differences in baseline characteristics between the exposed and unexposed groups. Such differences may only be partially mitigated by inclusion in the subsequent regression model (the use of which also entails the possibility of model misspecification); hence, confounding by measured covariates remains a concern.24 Moreover, the rate of data missingness in that study was significantly higher for baseline characteristics in the unexposed group, suggestive of underlying information bias.25,26
Regardless of the specific study design, however, no observational study can eliminate the risk of confounding due to unmeasured characteristics. It is critical, therefore, that researchers routinely provide estimates of robustness to unmeasured confounding (e.g., E-values).27
Lag between exposure and AD
The induction (“lag”) period between vaccination and incident AD, or the minimum amount of time that a vaccination must precede a diagnosis of incident AD in order to be counted as a relevant exposure, is another point of concern with significant variability in the literature. Causality requires that cause precede the posited consequence, so vaccinations must precede incident AD diagnoses by at least the smallest temporal unit available (typically a day in most longitudinal retrospective databases) to be causally relevant. Definitions of a meaningful induction period longer than the minimum temporal separation required for causality are ideally predicated on the study hypothesis (or hypotheses). However, as noted in the introduction, the literature is thus far consistent with (or is at least not falsifiable of) multiple mechanistic hypotheses (Figure 1), and the mechanism(s) may vary by the targeted pathogen and by vaccine technology (e.g., live-attenuated versus inactivated, presence of an adjuvant). Moreover, the AD-related pathophysiologic targets (e.g., protein aggregates, neuroinflammation) of these hypothesized mechanisms span much (if not all) of the preclinical phase of AD, and many of these pathophysiologic processes continue well into the symptomatic stages of clinical AD.1,4 Therefore, in the absence of a clear biological basis for hypothesizing a specific induction period, one conservative approach is to use the smallest temporal unit available. Assuming the true induction time for the effect of nonspecific vaccines on AD risk is greater than 1 day, the use of a 1-day induction time would cause the incidence rate measured among the vaccinated to be an average rate reflecting periods of exposure effect and periods without exposure effect, thereby “diluting” the effect estimate and biasing the analysis toward the null.28
Healthy-vaccinee bias
Beyond the overall study design, the set of covariates accounted for in pharmacoepidemiologic studies of vaccines and AD risk is particularly important given the possibility of confounding by indication or secondary to the healthy-vaccinee bias. The healthy-vaccinee bias refers to the notion that individuals who practice healthy lifestyles associated with lower risks of dementia (e.g., frequent exercise) may also be more likely to obtain the routine vaccines recommended by medical professionals. In confounding by indication, conditions that affect AD risk (e.g., hypertension, diabetes) are also associated with the likelihood of vaccination. For example, because chronic diseases associated with increased AD risk (e.g., hypertension, diabetes) are also associated with greater morbidity and mortality from vaccine-preventable diseases (e.g., influenza or pneumococcal infection), physicians may more strongly and persistently recommend routine immunizations to patients with these chronic diseases. In light of these potential biases, studies using routinely collected health data to investigate an association between vaccination and dementia risk should strongly consider inclusion of variables related to lifestyle and socioeconomic condition (e.g., exercise habits, healthcare access), health-care utilization habits (e.g., number of routine well-being exams, overall number of health-care encounters), and comorbidities relevant to dementia risk and/or likelihood of vaccination. This recommendation is particularly salient for studies comparing vaccinated and unvaccinated patients.
Importantly, in a study under review, our group compared the risk of AD among people who received adjuvanted or high-dose influenza vaccines with the risk of AD among those who received non-adjuvanted, standard-dose (NASD) influenza vaccines. We hypothesized that adjuvanted and high-dose vaccines, through mechanisms such as greater protection against influenza infection, would be associated with a lower risk of AD compared to NASD influenza vaccines. Indeed, we found preliminarily that influenza vaccines with an adjuvant or higher antigen dosage were associated with decreased risk of AD compared to NASD influenza vaccines. This study design is notable in that all patients were vaccinated, i.e. it compares vaccine recipients with vaccine recipients. Presumably, the comparison of two vaccinated groups – rather than a vaccinated group with an unvaccinated group – greatly reduces the possibility of confounding due to unmeasured characteristics. As a result, this forthcoming study will be important in (1) suggesting a lower AD risk after receipt of a high-dose or adjuvanted influenza vaccine than after an NASD influenza vaccine and (2) using a design that is less susceptible to many of the potential limitations (such as confounding by indication and the healthy-vaccinee bias) among the prior studies of nonspecific vaccines and AD or all-cause dementia.
Next steps
Although the preponderance of evidence supports a protective effect of several routine vaccines on AD risk, the presence of contradictory findings in the pharmacoepidemiologic literature on this topic necessitates further research. For future observational studies on this topic, the concept of triangulation provides a useful framework for the planning and integration of studies into the existing corpus.29 It seems prudent that studies would clearly identify the key sources and expected direction of bias given the study data and design, and estimates of these biases should be provided whenever possible. For example, in cohort studies that are susceptible to immortal time bias and that estimate effect sizes as functions of outcome frequencies (e.g., as risk ratios), the distribution of at-risk time for members of each exposure level should be reported. More specifically, in closed cohort studies, at-risk time for a given exposed individual may be measured from that person’s date of exposure, rather than the fixed start date of the follow-up period. Similarly, pharmacoepidemiologic studies using routinely collected health data should also pay careful attention to right-censoring because, unlike randomized controlled trials or some prospective cohort studies, there is no common duration from the start of follow-up until which all individuals are followed; therefore, for individuals who do not develop the outcome of interest (e.g., all-cause dementia or AD-specific dementia) or the competing-risk event (e.g., death) during the follow-up period, the end of a given person’s at-risk period might be best defined as the date of that person’s last encounter in the study period, not as the fixed end date of the study period.
Another avenue in the path toward triangulation is the application of differing methodologies to the same or similar databases, or vice versa. Thus far, the literature on vaccines and AD risk consists of many different methodologies applied to different databases; there are few instances, if any, in which independent research groups have studied the same database. The comparison of multiple methodologies, ideally with countervailing strengths and biases, to similar substrates would help to elucidate the extent to which differing methodologies account for variations in the literature. With regard to robust methodologies that have heretofore been absent from the literature on vaccines and AD risk, the growing field of target-trial emulation offers a promising approach for designing and reporting observational studies of routinely collected health data that attempt to answer causal questions.30 Applications such as sequential target-trial emulation are particularly attractive as they can reduce the risk of selection bias and avert common issues in traditional observational studies such as immortal time bias.31
Equally important as study design in pharmacoepidemiologic research is the data source itself, particularly given the significant differences in the kinds and qualities of information collected in the two main types of routinely collected health data, electronic health record (EHR) data and administrative data.32 Given the differential strengths of EHR-based and claims-based data, datasets comprised of linked administrative and EHR data are particularly appealing in overcoming the disadvantages encountered when using each data type independently. Whatever the design or dataset used, adherence to standard reporting guidelines for pharmacoepidemiologic studies (e.g., RECORD-PE) must remain an unwavering norm.33
Many questions in the field of vaccines and their apparent effect on AD remain ripe for investigation, with ample space for contribution from both clinicoepidemiological research and basic science studies. What is the effect of a lifetime of annual influenza vaccinations? Do routine vaccines interact – synergistically, antagonistically – in their effect on AD risk, and does subsequent (or prior) exposure to the targeted pathogen modify the effect on AD pathophysiology? For patients with symptomatic AD, does receipt of routine vaccines influence the rate of pathologic and/or symptomatic progression? Moreover, how do patient characteristics such as age, sex, genotype, and microbiome influence the association between routine vaccines and AD risk and/or progression? Does the effect differ significantly by the antigen dosage, presence, and type of adjuvant, or vaccine technology (e.g., live-attenuated versus inactivated viruses, conjugated versus unconjugated, mRNA versus viral vector)? And does vaccination delay AD onset or prevent it?
While questions remain, the preponderance of evidence at various levels of investigation – from petri dish, to murine models, to large pharmacoepidemiologic studies – supports the existence of a beneficial effect of nonspecific vaccinations on AD risk. This effect underscores the role of inflammation as a central pathologic process and therapeutic target in AD. Moreover, the infrastructures needed to take advantage of this effect are already common, as most of these vaccines are included in routine immunization schedules and, unlike many of the emerging AD therapies, do not require infusion suites or other costly equipment. Looking ahead, as we accrue additional evidence, the field must remain open-minded. We appreciate the laudable ethos conveyed by the subtitle of an editorial commentary in JID regarding the recent study that found a deleterious association between influenza or pneumococcal vaccination and AD risk: “Science can be messy but eventually leads to truths.”34 Future research may come to different conclusions. It would be particularly noteworthy if newer, more robust designs, such as sequential-trial emulation, reliably demonstrated a different outcome. Regardless, years of data demonstrating routine vaccines’ safety and the clear benefits of protection against infectious diseases with potentially significant morbidity and mortality, especially among older adults, already provide compelling motivation for obtaining the routine vaccines recommended by the CDC. Hopefully, better understanding of the mechanisms, applications, and limits of the effects of nonspecific vaccines on the risk and nuances of neurodegeneration will provide us with another very important tool in the arsenal against AD.
Conflict of Interest
Xiaoqian Jiang is funded by Christopher Sarofim Family Professorship, the CPRIT RR180012 award, UT Stars award, and NIH grants R01GM114612 and U01TR002062. Paul E. Schulz is funded by the McCord Family Professorship in Neurology, the Umphrey Family Professorship in Neurodegenerative Disorder, multiple NIH grants, several foundation grants, and contracts with multiple pharmaceutical companies related to the performance of clinical trials; he serves as a consultant and speaker for Eli Lilly, Biogen, and Acadia Pharmaceuticals.
References
- 1.2023 Alzheimer disease facts and figures. Alzheimer & Dementia. 2023.
- 2.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396(10248):413–6. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jorfi M, Maaser-Hecker A, Tanzi RE.. The neuroimmune axis of Alzheimer disease. Genome Med. 2023;15(1):6. doi: 10.1186/s13073-023-01155-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179(2):312–39. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pluchino S, Willis C. Intrinsic antiviral immunity drives neurodegeneration in Alzheimer disease. J Clin Invest. 2020;130(4):1622–4. doi: 10.1172/JCI135906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lima MN, Barbosa-Silva MC, Maron-Gutierrez T. Microglial priming in infections and its risk to neurodegenerative diseases. Front Cell Neurosci. 2022;16:878987. doi: 10.3389/fncel.2022.878987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franceschi C, Salvioli S, Garagnani P, de Eguileor M, Monti D, Capri M. Immunobiography and the heterogeneity of immune responses in the elderly: a focus on inflammaging and trained immunity. Front Immunol. 2017;8:982. doi: 10.3389/fimmu.2017.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itzhaki RF. Overwhelming evidence for a major role for herpes simplex virus type 1 (HSV1) in Alzheimer disease (AD); underwhelming evidence against. Vaccines. 2021;9(6):679. doi: 10.3390/vaccines9060679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ou YN, Zhu JX, Hou XH, Shen XN, Xu W, Dong Q, Tan L, Yu J-T. Associations of infectious agents with Alzheimer disease: a systematic review and meta-analysis. J Alzheimers Dis. 2020;75(1):299–309. doi: 10.3233/JAD-191337. [DOI] [PubMed] [Google Scholar]
- 10.Itzhaki RF, Golde TE, Heneka MT, Readhead B. Do infections have a role in the pathogenesis of Alzheimer disease? Nat Rev Neurol. 2020;16(4):193–7. doi: 10.1038/s41582-020-0323-9. [DOI] [PubMed] [Google Scholar]
- 11.Muzambi R, Bhaskaran K, Smeeth L, Brayne C, Chaturvedi N, Warren-Gash C. Assessment of common infections and incident dementia using UK primary and secondary care data: a historical cohort study. Lancet Healthy Longev. 2021;2(7):e426–35. doi: 10.1016/S2666-7568(21)00118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Yang H, He S, Xia T, Chen D, Zhou Y, Liu J, Liu M, Sun Z. Adult vaccination as a protective factor for dementia: a meta-analysis and systematic review of population-based observational studies. Front Immunol. 2022;13. doi: 10.3389/fimmu.2022.872542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukhbinder AS, Ling Y, Hasan O, Jiang X, Kim Y, Phelps KN, Schmandt RE, Amran A, Coburn R, Ramesh S, et al. Risk of Alzheimer disease following influenza vaccination: a claims-based cohort study using propensity score matching. J Alzheimers Dis. 2022;88(3):1061–74. doi: 10.3233/JAD-220361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klinger D, Hill BL, Barda N, Halperin E, Gofrit ON, Greenblatt CL, Rappoport N, Linial M, Bercovier H. Bladder cancer immunotherapy by BCG is associated with a significantly reduced risk of Alzheimer disease and Parkinson’s disease. Vaccines. 2021;9(5):491. doi: 10.3390/vaccines9050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine KS, Leonard HL, Blauwendraat C, Iwaki H, Johnson N, Bandres-Ciga S, Ferrucci L, Faghri F, Singleton AB, Nalls MA. Virus exposure and neurodegenerative disease risk across national biobanks. Neuron. 2023;111(7):1086–93.e2. doi: 10.1016/j.neuron.2022.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piekut T, Hurła M, Banaszek N, Szejn P, Dorszewska J, Kozubski W, Prendecki M. Infectious agents and Alzheimer disease. JIN. 2022;21(2). doi: 10.31083/j.jin2102073. [DOI] [PubMed] [Google Scholar]
- 17.Crescenzi O, Tomaselli S, Guerrini R, Salvadori S, D’Ursi AM, Temussi PA, Picone D. Solution structure of the Alzheimer amyloid β-peptide (1-42) in an apolar microenvironment. Eur J Biochem. 2002;269(22):5642–8. doi: 10.1046/j.1432-1033.2002.03271.x. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, He Z, Xing Z, Zuo Z, Yuan L, Wu Y, Jiang M, Qi F, Yao Z. Influenza vaccination in early Alzheimer disease rescues amyloidosis and ameliorates cognitive deficits in APP/PS1 mice by inhibiting regulatory T cells. J Neuroinflammation. 2020;17(1):65. doi: 10.1186/s12974-020-01741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Wang X, Wang ZH, Lin Z, Yang J, Chen J, Wang R, Ye W, Li Y, Wu Y, et al. Changes in dendritic complexity and spine morphology following BCG immunization in APP/PS1 mice. Hum Vaccin Immunother. 2022;18(6):2121568. doi: 10.1080/21645515.2022.2121568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuo Z, Qi F, Xing Z, Yuan L, Yang Y, He Z, Zhou L, Yao Z. Bacille Calmette-Guérin attenuates vascular amyloid pathology and maximizes synaptic preservation in APP/PS1 mice following active amyloid-β immunotherapy. Neurobiol Aging. 2021;101:94–108. doi: 10.1016/j.neurobiolaging.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Zuo Z, Qi F, Yang J, Wang X, Wu Y, Wen Y, Yuan Q, Zou J, Guo K, Yao ZB. Immunization with Bacillus Calmette-Guérin (BCG) alleviates neuroinflammation and cognitive deficits in APP/PS1 mice via the recruitment of inflammation-resolving monocytes to the brain. Neurobiol Dis. 2017;101:27–39. doi: 10.1016/j.nbd.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Douros A, Ante Z, Suissa S, Brassard P. Common vaccines and the risk of incident dementia: a population-based cohort study. J Infect Dis. 2022. doi: 10.1093/infdis/jiac484. [DOI] [PubMed] [Google Scholar]
- 23.Yadav K, Lewis RJ. Immortal time bias in observational studies. JAMA. 2021;325(7):686–7. doi: 10.1001/jama.2020.9151. [DOI] [PubMed] [Google Scholar]
- 24.Amoah J, Stuart EA, Cosgrove SE, Harris AD, Han JH, Lautenbach E, Tamma PD. Comparing propensity score methods versus traditional regression analysis for the evaluation of observational data: a case study evaluating the treatment of gram-negative bloodstream infections. Clin Infect Dis. 2020;71:e497–505. doi: 10.1093/cid/ciaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bankhead CR, Spencer EA, D N. Information bias . Sackett catalogue of biases 2019. 2020.
- 26.Douros A, Santella C, Dell’Aniello S, Azoulay L, Renoux C, Suissa S, Brassard P. Infectious disease burden and the risk of Alzheimer disease: a population-based study. J Alzheimers Dis. 2021;81(1):329–38. doi: 10.3233/JAD-201534. [DOI] [PubMed] [Google Scholar]
- 27.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602–3. doi: 10.1001/jama.2018.21554. [DOI] [PubMed] [Google Scholar]
- 28.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 29.Lawlor DA, Tilling K, Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol. 2017;45:1866–86. doi: 10.1093/ije/dyw314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available: table 1. Am J Epidemiol. 2016;183(8):758–64. doi: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickerman BA, García-Albéniz X, Logan RW, Denaxas S, Hernán MA. Avoidable flaws in observational analyses: an application to statins and cancer. Nat Med. 2019;25(10):1601–6. doi: 10.1038/s41591-019-0597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–37. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Langan SM, Schmidt SA, Wing K, Ehrenstein V, Nicholls SG, Filion KB, Klungel O, Petersen I, Sorensen HT, Dixon WG, et al. The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE). BMJ. 2018;363:k3532. doi: 10.1136/bmj.k3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salmon DA, Black S, Didierlaurent AM, Moulton LH. Commentary on “Common vaccines and the risk of dementia: a population-based cohort study”: science can be messy but eventually leads to truths. J Infect Dis. 2022. doi: 10.1093/infdis/jiac487. [DOI] [PubMed] [Google Scholar]


