Abstract
Chimeric antigen receptor (CAR) T-cells serve to overcome chemotherapeutic resistance and have been proven to be highly effective in B-cell hematologic malignancies. Although initial use has been in patients with multiply relapsed/refractory disease, as CAR T-cells are used earlier in the treatment paradigm, it will be important to explore implications of this novel therapy on cancer late-effects. We sought to assess the current framework for considerations of fertility surrounding CAR T-cell use and identify opportunities for education and future research. To assess current practice patterns regarding post-CAR T-cell fertility, peri-CAR T-cell fertility guidance, utilization of fertility preservation surrounding CAR T-cell administration and identify future areas of research, a cross-sectional survey assessing practice patterns regarding fertility counseling and outcomes surrounding CAR T-cell therapy was distributed electronically to approximately 300 Center for International Blood and Marrow Transplant Research medical centers treating patients with CAR T-cell therapy in the United States and internationally between October 12 and November 2, 2021. One medical provider was asked to complete the study survey on behalf of their institution. We received 96 survey responses, of which 66 centers utilized CAR T-cells and provided at least partial responses that were used for the primary analysis. Centers were varied in demographics, experience in administering CAR T-cells, and aspects of patients receiving CAR T-cells. Eighteen centers exclusively treated pediatric patients, and patients at these centers were more likely to be treated for B-cell acute lymphoblastic leukemia. Seven pregnancies and 5 live births after CAR T-cells were reported from 6 centers (1 pediatric-only). Most centers had no established guidelines in place regarding fertility preservation in the peri-CAR T-cell period or regarding recommendations for avoiding pregnancy/fathering a child after receiving CAR T-cells. Areas for future research were elicited from responding centers and categorized into 3 broad themes, including: standardized peri-CAR T-cell fertility guidelines; long-term fertility outcomes after CAR T-cell therapy; impact of CAR T-cells on a developing fetus; and determining the relevance of studying fertility in patients who receive CAR T-cells. We identified a high degree of variability in peri-CAR T-cell guidance on avoidance of pregnancy/fathering a child, as well as a wide-range of practices surrounding referral for fertility preservation, the latter of which may be likely due to the fact that patients receiving CAR T-cells in the present era are likely multiply relapsed/refractory. In summary, this is the first report of several live-births following CAR T-cells, which highlights the important need for further research in CAR T-cell therapy and fertility, with a host of novel research questions identified.
Keywords: Fertility preservation, CAR T-cells, Cellular therapy, Immunotherapy
INTRODUCTION
Chimeric antigen receptor (CAR) T-cell based immunotherapeutic strategies for treatment of relapsed/refractory B-cell malignancies have been highly effective, and multiple unique CAR T-cell constructs are approved for children and adult patients across a host of relapsed/refractory B-cell malignancies [1–8]. Based on harnessing the immune system to induce tumor killing, acute toxicities related to CAR T-cell expansion are well established and include a systemic inflammatory response, generally referred to as cytokine release syndrome and sequelae thereof [9]. Longer-term effects of CAR T-cells, however, are not well known, and longitudinal follow-up is ongoing [10,11]. One potential advantage of immunotherapy is that it is hypothesized to spare long-term toxicities associated with cytotoxic therapies, such as infertility.
Fertility is a major concern for both males and females undergoing cancer treatment, and particularly for children and adolescents/young adults (AYA) who have most of their reproductive years ahead of them [12–17]. Because >80% of children and AYA with cancer are expected to survive beyond 5 years [18,19], an increasing emphasis has been placed on offering options for fertility preservation such as oocyte cryopreservation, ovarian tissue cryopreservation, and sperm cryopreservation to patients with cancer to enhance posttreatment quality-of-life [20]. It has been shown that cumulative exposure to alkylating chemotherapy, radiation, and hematopoietic stem cell transplantation (HSCT) are key risk factors for infertility after cancer therapy, thus consideration of fertility preservation before therapy, or earlier in the treatment course, is preferred [21,22].
Because CAR T-cells have primarily been used in patients with multiply relapsed/refractory disease and the full potential of CAR T-cells is still being realized, fertility considerations have not been at the forefront. However, because CAR T-cell therapy may potentially reduce exposure to gonadotoxic therapies as it moves earlier into the treatment plan, and because these genetically modified T-cells may persist long term [23], prospective studies to specifically identify the impact of CAR T-cells on gonadal function are greatly needed.
Inspired by a young adult treated for B-cell acute lymphoblastic leukemia (ALL) with CAR T-cells at our institution who subsequently proceeded to father a healthy child after infusion (without receiving any interim therapy or HSCT) and in consideration of a complete dearth of information on CAR T-cells and fertility, we sought to ascertain practice patterns regarding approach to fertility and fertility preservation in the peri-CAR T-cell period and to obtain information regarding early experiences with post-CAR T-cell fertility and gonadal function.
METHODS
Design and sample
A cross-sectional survey assessing practice patterns regarding fertility and CAR T-cell therapy, alongside fertility outcomes, was distributed electronically between October 12 and November 2, 2021, to center directors at approximately 300 unique centers (of which approximately 200 are in the United States) treating patients with HSCT or CAR T-cell therapy. One medical provider was asked to complete the study survey on behalf of their institution on practices in fertility preservation counseling and reproductive outcomes following CAR T-cell therapy. An announcement of the anonymous survey, with an initial email and letter of introduction to explain the purpose and scope of the study, was distributed with permission on the Center for International Blood and Marrow Transplant Research (CIBMTR) Listserv, with 2 reminders during the timeframe when the survey was open. The Office of Human Subjects Research Protections at the National Institutes of Health determined that the survey format and content did not qualify as human subjects research and therefore was Institutional Review Board exempt.
Measures
Because no measure assessing fertility in the post-CAR setting currently exists, a survey instrument was developed that consisted of questions designed by a collaborative, interdisciplinary study team (2 pediatric oncology physicians, 1 reproductive endocrinology and infertility specialist, 1 pediatric/adolescent gynecology physician, 1 social worker, 1 research nurse) according to the Tailored Method of Survey Design [24]. The survey was independently reviewed, piloted, revised, and repiloted by the interdisciplinary team before administration on SurveyMonkey. Respondents were screened with 2 required questions confirming both (1) agreement to participate and (2) that the center treats patients with CAR T-cell therapy. If the response to either question was “no,” the survey ended, and no further responses were collected.
Subsequent questions were all optional. This allowed participants the opportunity to choose to leave questions unanswered if they did not wish to respond or did not know the answer, leading to a different number of respondents for each question. Questions were categorized by the following topics: CAR T-cell therapy treatment practices and patient population demographics, pre-CAR fertility considerations, availability of fertility preservation, post-CAR fertility recommendations and outcomes, and post-CAR fertility referrals. The final survey instrument (available as a Supplementary Appendix) was comprised of 66 multiple-choice and 1 open-ended question: “What research questions do you think are important to consider in terms of fertility following CAR T-cell therapy?” Additionally, several questions had an open-ended option where respondents were prompted to explain their answer.
Data collection and analysis
An encrypted SurveyMonkey questionnaire format was used for online data collection. The analyses were descriptive and univariate in nature, and data are reported as percentages of respondents endorsing each item. The study team used counts for categorical variable responses. For missing responses due to skip logic in the survey or for nonresponse, the number of respondents was used as the denominator (actual n). Percentages for responses are presented.
Open-ended responses were analyzed by content analysis. Themes were identified, discussed, and agreed upon by 3 independent coders (J.A.L., J.Y.M., N.N.S.). A coding dictionary was created. The responses were then independently coded and checked for accuracy by the 2 investigators who met regularly to review the coding process [25]. Discrepancies in coding were reviewed and resolved by the team.
RESULTS
Basic Respondent and CAR T-cell infusion Demographics
Of approximately 300 centers contacted we received 96 survey responses, among which 66 centers reported treating patients with CAR T-cells and had at least some response data that could be used for analysis. Representation, when provided, spanned across 24 states (Figure 1A), with 2018 representing the median year that most centers first implemented CAR T-cell therapy at their institution (Figure 1B). The number of average infusions/year (as an approximate average over the past 3 years) varied (Figure 1C), with most centers reporting at least 10 infusions/year. Additionally, the majority of reporting centers reported treating children and young adults (Figure 1D). The most prevalent indication for CAR T-cell infusion at most centers was for non-Hodgkin lymphoma (Figure 1E). A minority of centers reported that most infusions were for ALL or multiple myeloma. Corresponding to the emergence of commercially available CAR T-cell constructs, a majority of centers reported that most of their patients received a commercial CAR T-cell construct (Figure 1F) as opposed to an investigational product on a clinical trial.
Figure 1.

Basic characteristics of responding centers. (A) U.S. States represented among survey respondents along with number of responses from the state. (B) Number of centers with first CAR T-cell infusion in a given year. (C) Approximate number of CAR T-cell infusions per center (averaged over prior 3 years). (D) Centers focusing on pediatric CAR T-cell infusions versus adults versus a mixture. A center was considered to be “pediatric” if the oldest age in the range of ages treated was 35 or lower. A center was considered to be “adult only” if the youngest age in the range of ages was over 18. Centers meeting neither criteria were considered to be “mixed.” (E) Number of centers with specific diseases comprising the majority of indications (at least 50%) for CAR T-cell infusion. “Multiple” denotes centers where no single disease constituted at least 50% of indications for CAR T-cell infusion. MM indicates multiple myeloma; NHL, non-Hodgkin lymphoma. (F) Centers by the percentage of infusions which were a commercial CAR T-cell product (as opposed to an investigational product administered on a clinical trial).
CAR T-cell infusion recipient demographics
Because patient attributes and prior therapies inform fertility considerations, we evaluated pre-existing factors that might impact fertility of current CAR T-cell recipients. Because pubertal status impacts gonadotoxicity risk, it is important to note that at most centers a distinct majority of patients (both males and females) were postpubertal (Figures 2A, 2B). Furthermore, because prior therapies inform fertility considerations, we evaluated prior therapies that CAR T-cell recipients might have received. HSCT, either autologous or allogeneic, was frequently used in prior CAR T-cell recipients (Supplemental Figures S1A, S1B). The 18 centers that exclusively treated children and AYA patients notably had a higher percentage of patients who were prepubertal, a larger fraction of patients who had received prior allogeneic HSCT, and a substantially higher proportion of patients treated for ALL compared to our overall results (Supplemental Figures S1C–G).
Figure 2.

Factors affecting fertility and family planning after CAR T-cell infusion. Centers where a majority (>50%) of (A) male and (B) female patients are either prepubertal or postpubertal. For unclear reasons, a minority of centers reported neither prepubertal nor postpubertal patients as being a majority population. (C) Cyclophosphamide-equivalent dose given for lymphodepletion (LD) by centers. (D) Guidelines to avoid fathering a child after CAR T-cell infusion. (E) Guidelines to avoid pregnancy after CAR T-cell infusion. (F) Frequency of discussing fertility preservation before CAR T-cell infusion. (G) Peri-CAR T-cell referral patterns for fertility counseling. (H) Proportion of centers with a fertility preservation program. (I) Proportion of centers where fertility preservation procedures are covered by insurance.
Peri-CAR T-cell lymphodepletion
Among 64 centers reporting on lymphodepletion use, 62 (96.9%) centers gave >75% of their patients a lymphodepletion regimen that incorporated cyclophosphamide. Of note, the doses were low with all reporting administering <2000 mg/m2 cyclophosphamide, which is, on its own, considered low risk for gonadotoxicity in both males and females [26] (Figure 2C).
Peri-CAR T-cell fertility guidance
Because no literature exists on the impact of CAR T-cells on gonadal function, on future fertility, or on a developing fetus, we inquired into centers’ practices of counseling regarding becoming pregnant or fathering a child after CAR T-cell therapy. Importantly, 25 centers (41.7% of respondents to the question on females, 42.4% of respondents to the question on males) stated that they give no recommendation at all; and avoiding pregnancy/fathering a child for 6 to 12 months after CAR T-cells was the most common recommendation, when any guidance was given at all (Figures 2D, 2E). Additionally, a minority of centers stated that they “always” discuss fertility preservation prior to CAR T-cell therapy, and nearly half of centers reported “rarely” or “never” doing so (Figure 2F). Importantly, given that the majority of centers are using commercial CAR T-cell constructs as their primary experience with CAR T-cells, there is no standardized guidance available for any approved construct (Supplemental Table S1).
Additionally, most centers reported lacking guidelines and few routinely monitor laboratory surrogates of fertility (Supplemental Figures 2A, 2B). We similarly asked how frequently patients are referred to a reproductive endocrinology and infertility specialist or urologist and found that a distinct minority (<25%) are referred at most centers, or that the respondent was unsure (Supplemental Figures S2C, S2D). Of the centers that do follow laboratory surrogates of fertility, there was some consensus. All 9 centers that responded regarding females reported after follicle-stimulating hormone, luteinizing hormone, and estradiol in females, and 6/9 also followed anti-Mullerian hormone. Similarly, all 8 centers responding for males reported following follicle-stimulating hormone and luteinizing hormone in males, and 7/8 respondents reported following testosterone (Supplemental Figures S2E, S2F). Labs were monitored annually among all respondents.
Pregnancy outcomes after CAR T-cell Therapy
Because we were inspired to do this analysis based on a report of one of our long-term survivors fathering a child after CAR T-cell therapy, we asked what other centers’ experiences had been in regard to patients conceiving or fathering children after CAR T-cell therapy. Of 61 respondents, 6 centers (9.8%) reported 7 total pregnancies (4 female patients who became pregnant, and 3 male patients whose partners conceived), with 5 confirmed live births after CAR T-cell therapy and 2 with unknown outcomes (Table 1). There were no reported pregnancy complications or report of any spontaneous or elective abortions. Seven centers reported referring 13 patients for assisted reproductive techniques after CAR T-cell therapy. Of those patients, reported outcomes included 2 pregnancies with live births from patients’ own eggs and 1 successful embryo cryopreservation. Use of donor egg and donor sperm was reported from the same center, without a successful pregnancy reported. Two centers reported patients starting a family through other means such as adoption or surrogacy.
Table 1.
Pregnancy Outcomes After CAR T-Cells (Total Respondents, n = 61)
| Center No. | Age Range Treated | No. of Patients Pregnant/ Fathering After CAR | No. Known Live Births After CAR | Assisted Reproductive Technique? | Specific CAR Received | Underlying Malignancy | Earliest Time Point After CAR T-Cells Pregnancy Occurred |
|---|---|---|---|---|---|---|---|
| Female CAR T-cell recipients | |||||||
| 50 | Pediatric | 2 | 2 | Yes (both, used own egg) | “In house” | ALL | 1–2 years |
| 59 | Adult | 1 | 1 | No | Unknown | DLBCL | 2–5 years |
| 77 | Both | 1 | 1 | No | Axicabtagene ciloleucel | DLBCL | 2–5 years |
| Male CAR T-cell recipients | |||||||
| 45 | Adult | 1 | 1 | No | Axicabtagene ciloleucel | DLBCL | 6–12 months |
| 63 | Both | 1 | Unknown | No | CD19/22 CAR | B-ALL | 1–2 years |
| 76 | Both | 1 | Unknown | No | CD19/CD22 bispecific | ALL | 2–5 years |
B-ALL indicates B-cell ALL; DLBCL, diffuse large B-cell lymphoma.
Pre-CAR fertility preservation practices and availability
Although most patients currently receiving CAR T-cells are multiply relapsed/refractory and may not be candidates for fertility preservation, we anticipate that this may not always be the case. Thus we sought to understand availability of fertility preservation programs and practices at centers that participated in this survey, to understand what resources might be available and anticipate what challenges there might be. Slightly more than half of respondents reporting referring for fertility preservation services, with a slight predilection for referring before CAR T-cell therapy rather than after (Figure 2G). Importantly, 75% of centers reported availability of a fertility preservation program (Figure 2H). Nearly half of centers reported at least some fertility preservation procedures being covered by insurance in their state, although “all” procedures were covered in <10% of responding centers, and there was a significant knowledge gap where 25% of respondents did not know whether fertility preservation procedures are covered by insurance in their state (Figure 2I).
For the 26 respondents (roughly 40%) who said their center does not refer to fertility preservation procedures, 25 provided reasons for nonreferral (Table 2). Among the non-mutually exclusive reasons provided (meaning that respondents could select multiple reasons for nonreferral), “Fertility preservation is not an option due to prior therapy,” “Patient already had fertility preservation,” and “There is not time to allow for fertility preservation due to active/progressive disease” were the 3 most frequent reasons given and were each reported by roughly 50% of respondents. Pediatric centers did not appreciably differ in terms of whether they routinely address fertility in pre-CAR discussions.
Table 2.
Reasons for Fertility Preservation Nonreferral After CAR T-Cells
| Answer choices (not mutually exclusive) | Responses (n = 25) |
|---|---|
| Patients are only referred for CAR and primary oncology care is elsewhere | 6 (24%) |
| Patient already had fertility preservation | 13 (52%) |
| Fertility preservation is not an option due to prior therapy | 14 (56%) |
| Fertility preservation is not an option due to age (postmenopausal) | 11 (44%) |
| Fertility preservation is not an option due to lack of access to ovarian tissue and/or testicular tissue cryopreservation | 5 (20%) |
| It’s something we do not routinely think about as part of the pre-CAR evaluation | 8 (32%) |
| There is not time to allow for fertility preservation due to active/progressive disease | 12 (48%) |
| The cost of fertility preservation is prohibitive, or would not be covered by insurance | 6 (24%) |
| Patient declined for another reason | 0 (0%) |
| Other (please specify) | 5 (20%) |
We also assessed which fertility preservation methods are available at CAR T-cell centers (Figure 3). As expected, the vast majority (84%) of 66 centers offer sperm and oocyte cryopreservation, because these are the most well-established fertility preservation procedures. Ovarian tissue cryopreservation, which is no longer considered experimental, was offered by 59% of centers, and 38% of centers offered invasive sperm extraction techniques. Testicular tissue cryopreservation, which remains experimental, was offered by 30% of centers, and nearly one quarter of centers (22%) reported being unsure of which procedures were available.
Figure 3.
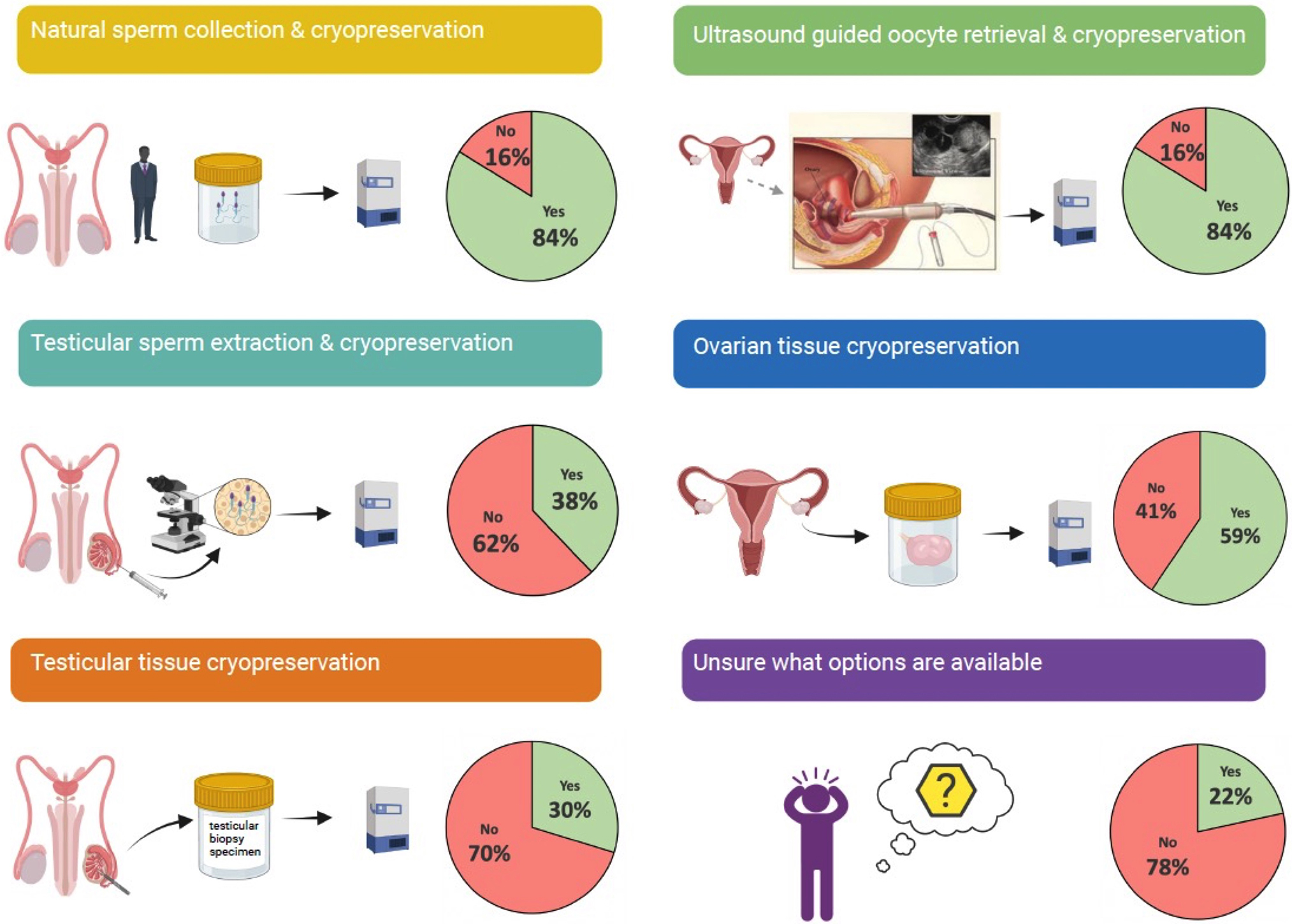
Fertility preservation procedures available at CAR T-cell centers. Brief graphical description of 5 most common fertility preservation procedures and the frequency with which they are available at CAR T-cell centers.
Areas for future research
The final question was an open-ended question asking respondents what research questions are important to consider in terms of fertility after CAR T-cell therapy. We received responses from 31/66 centers (47%), and of the 31 responses 13 questioned the clinical relevance or ability to conduct fertility research in such a heavily pretreated population. From the remaining 18 responses, 3 overarching themes surrounding CAR T-cells and fertility research were identified and included the following major areas: (1) Need for standardized fertility guidance in the peri-CAR T-cell setting; (2) Need to collect long-term outcomes on fertility post CAR T-cells; and (3) Need to understand impact of circulating CAR T-cells on a developing fetus. Separating each free text response into these 3 themes, a number of important items were identified as topics for future research. Questions/comments felt by the authors to be of highest interest to the field and proposed suggestions are highlighted in Table 3.
Table 3.
Future Research: CAR T-Cells and Fertility
| Theme | Specific question/comments | Suggestions for future |
|---|---|---|
| Standardized peri-CAR T-cell fertility guidance | • This is an important issue for younger patients • Identifying role for pre-CAR T-cell fertility assessment • Timing of pregnancy/fathering a child after CAR T-cell therapy • Does CRS have an impact on fertility? • Insurance coverage |
Discussion within existing working groups and committees to create expert consensus guidelines for fertility preservation in CAR T-cell recipients. |
| Long-term outcomes | • Long-term data on fertility status • Fertility monitoring after single lymphodepletion therapy • What percentage get pregnant post CAR T-cells; pregnancy success |
Establish multicenter collaborative to collect prospective fertility data from patients who receive CAR T-cell therapy and their eventual offspring, and compare to outcomes from patients who receive traditional therapies (i.e., chemotherapy, radiation, and stem cell transplantation). |
| Impact of CAR T-cells on developing fetus | • Are CAR T-cells passed through the placenta causing B-cell aplasia in the baby? • Outcomes of pregnancies/live births after CAR T-cells |
DISCUSSION
CAR T-cell therapies have revolutionized the field and are undoubtedly here to stay, making it all that more imperative to understand how this new treatment paradigm will impact long-term outcomes [10,27], beyond the typical metrics of overall survival. Given the curative potential of CAR T-cells alongside the critical importance of fertility considerations for long-term quality of life [20], our goal was to identify current practice patterns regarding fertility considerations and where guidelines and future research are needed. By sparing the need for additional gonadotoxic therapy and potentially facilitating preserved fertility, this is particularly relevant in children and AYA where earlier utilization of CAR T-cells is being explored and where future fertility and puberty considerations are paramount. This preliminary analysis, which provides both the first report on live-births in patients after CAR T-cell therapy and an overview of current practices, lays the foundation for future research regarding CAR T-cells and fertility.
Importantly, despite the relevance of the question and the unknown risks of CAR T-cell therapy on future fertility, this survey revealed that a substantial fraction of centers provided no counseling surrounding the question of duration of avoidance of becoming pregnant or fathering a child following CAR T-cell therapy. Although this is likely due to the current utilization of CAR T-cells as salvage therapy—with patients presenting with relapsed/refractory disease and an urgent need to get to the next line of therapy—we anticipate (or hope) that this may not always be the case, especially as the efficacy of CAR Tcells continues to improve and children and young adults can achieve long-term durable remission [1,28,29]. Additionally, as we identify the best timing for CAR T-cell utilization—which may be much earlier in the treatment course than what is currently seen—issues of fertility will become pressing. To systematically incorporate considerations of fertility in the peri CAR T-cell setting, we provide an algorithm for when these discussions should ideally take place (Figure 4). We are hopeful that through continued collaborations a consensus algorithm could be created which may foster a foundation for future research.
Figure 4.

Proposed algorithm to consider fertility preservation discussions with patients receiving CAR T-cell therapy.
In this regard, although most centers did use lymphodepleting chemotherapy before CAR T-cell infusion, cyclophosphamide equivalent doses were in ranges where fertility could be preserved, an important consideration because patients may potentially be receiving fewer lines of therapy before proceeding to CAR T-cells and present with preserved fertility. Accordingly, the need for guidelines and/or standardized approach to peri-CAR T-cell fertility considerations was thus highlighted as one of the main themes emerging from this work. It should be noted that relevance of the topic was also raised as a question, particularly since fertility preservation may not be feasible in currently referred patients where prior gonadotoxic therapy may be a substantial confounder and long-term survival may be limited. However, this should not dissuade our colleagues from investing efforts on this critically important topic—especially because the field is striving toward improving on efforts in adoptive cell therapy to optimally leverage this potent approach for long-term cure.
To further emphasize the critical need for research on fertility in the peri-CAR T-cell setting, we also provide the first report of live births in patients after CAR T-cells—which is both remarkable in these heavily pre-treated patients and illustrative of the critical need to have discussions surrounding fertility with patients. These conversations will both provide hope that starting a family “after cancer” may be possible and also ensure that appropriate guidance toward effective forms of contraception is provided and that infertility is not assumed. Survey respondents further raised questions about the need to study the long-term impacts of CAR T-cell on fertility, including the potential for transgenerational transfer of CAR T-cells and possible impact on a developing fetus. Cosgrove and colleagues recently published their hypothesis and approaches to study this particular concern, raising also the issue of lack of knowledge and information [30]. Beyond the considerations of maternal transfer of T-cells, outstanding questions remain of impact of CAR T-cells and of cytokine release syndrome on long-term fertility—which without robust data are difficult to study. A few reports from male patients who received immune checkpoint inhibition suggest that fertility, as assessed by sperm analysis, may be affected by immunotherapy [31,32], supporting a need to study these issues in those receiving CAR T-cell therapy.
Importantly, most CAR T-cell centers have fertility preservation programs in place. This pre-existing infrastructure enables access to experts in fertility preservation for both our patients receiving CAR T-cells and our immunotherapy providers and presents an opportunity to collaborate on this new avenue of oncofertility considerations [33,34]. The natural development of peri-CAR T-cell guidelines, monitoring of fertility parameters and consultation with reproductive endocrinology and infertility, pediatric gynecology, and urology should be incorporated into future efforts. Important considerations should also include discussions regarding timing of fertility preservation. For instance, for patients who may be using CAR T-cells for remission induction prior to more intensive therapies (e.g., myeloablative HSCT), considering post CAR T-cell fertility preservation before HSCT may be an opportune time to leverage a remission status while planning for HSCT. Moreover, this may be particularly important for patients with hematologic malignancies who often do not have the opportunity to undergo initial pretreatment fertility preservation given the need to urgently start therapy and where there are concerns for ovarian tissue involvement by malignancy [35] (which could potentially be eradicated by CAR T-cells given the ability for CAR T-cells to eradicate extramedullary disease [36]).
Limitations to this report are self-evident—as an exploratory survey to try to understand the current landscape of practices surrounding fertility across CIBMTR centers, our report provides preliminary insights into the centers who responded (representing approximately one third of centers that the survey was sent to, and of those only about two thirds of those centers use CAR T-cells, or overall approximately 20% of centers that received the survey). Respondents were unable to provide data on critical patient-level details (such as CED levels and pelvic/testicular radiation exposure prior to CAR T, as well as gonadal failure before and after treatment) that would be useful to understand local practices. Furthermore, it is possible that the single respondent from an institution may not be familiar with all of the institution’s practices. Nonetheless, this effort serves as a platform to raise these issues and understand what future efforts could entail.
In conclusion, fertility considerations are among the most important issues that cancer survivors struggle with. Although one hypothesized benefit of immunotherapy is that it may spare some of the infertility caused by gonadotoxic chemotherapy and radiation therapy, this is understudied, and there was previously no information on the topic of fertility after CAR T-cells. Because CAR T-cells are increasingly being used earlier in treatment with the goal of extending long-term disease-free survival, while sparing the need for additional standard therapy, understanding long-term toxicities and quality of life-related factors such as the impact of these therapies on fertility become imperative. Our report serves to provide a current overview of the landscape with the goal to draw attention to the need to develop uniform guidelines as a next step and establish a framework to study this as a research aim of patients receiving CAR T-cells. This critical next step will serve to improve patient outcomes and quality of life, a necessary aim alongside the rapid expansion of this novel immunotherapeutic approach.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Amanda Gilsinger, and Drs. Marcelo Pasquini and Bronwen Shaw from the CIBMTR for their support in distribution of this survey to CIBMTR centers.
The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Financial disclosure:
Supported in part by the Center for Cancer Research, Intramural Research Program, National Cancer Institute (ZIA BC 011823, N.N.S) and the National Institute of Child Health and Human Development (NIH ZIA# HD008985, J.Y.M and V.G.L).
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jtct.2022.06.002.
REFERENCES
- 1.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 3.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson CA, Chavez JC, Sehgal AR, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23:91–103. [DOI] [PubMed] [Google Scholar]
- 5.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396 (10254):839–852. [DOI] [PubMed] [Google Scholar]
- 8.Munshi NC, Anderson LD Jr., Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384:705–716. [DOI] [PubMed] [Google Scholar]
- 9.Maus MV, Alexander S, Bishop MR, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. Dec 2020;8(2): e001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shalabi H, Gust J, Taraseviciute A, et al. Beyond the storm—subacute toxicities and late effects in children receiving CAR T cells. Nat Rev Clin Oncol. 2021;18:363–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cordeiro A, Bezerra ED, Hirayama AV, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant. 2020;26:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow EJ, Kamineni A, Daling JR, et al. Reproductive outcomes in male childhood cancer survivors: a linked cancer-birth registry analysis. Arch Pediatr Adolesc Med. 2009;163:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gnaneswaran S, Deans R, Cohn RJ. Reproductive late effects in female survivors of childhood cancer. Obstet Gynecol Int. 2012;2012: 564794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudson MM. Reproductive outcomes for survivors of childhood cancer. Obstet Gynecol. 2010;116:1171–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel V, Jones P, Judd A, Senko V, Altieri G, Pettee D. Recollection of fertility discussion in adolescent and young adult oncology patients: a single-institution study. J Adolesc Young Adult Oncol. 2020;9:72–77. [DOI] [PubMed] [Google Scholar]
- 16.Benedict C, Thom B, Friedman DN, Pottenger E, Raghunathan N, Kelvin JF. Fertility information needs and concerns post-treatment contribute to lowered quality of life among young adult female cancer survivors. Support Care Cancer. 2018;26:2209–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi G, Kicinski M, Suciu S, et al. Fertility status among long-term childhood acute lymphoblastic leukaemia survivors enrolled between 1971 and 1998 in EORTC CLG studies: results of the 58 Late Adverse Effects study. Hum Reprod. 2021;37:44–53. [DOI] [PubMed] [Google Scholar]
- 18.Close AG, Dreyzin A, Miller KD, Seynnaeve BKN, Rapkin LB. Adolescent and young adult oncology-past, present, and future. CA Cancer J Clin. 2019;69:485–496. [DOI] [PubMed] [Google Scholar]
- 19.van der Meer DJ, Karim-Kos HE, van der Mark M, et al. Incidence, survival, and mortality trends of cancers diagnosed in adolescents and young adults (15–39 years): a population-based study in the Netherlands 1990–2016. Cancers (Basel). 2020;12:3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022–1033. [DOI] [PubMed] [Google Scholar]
- 21.Meacham LR, Burns K, Orwig KE, Levine J. Standardizing risk assessment for treatment-related gonadal insufficiency and infertility in childhood adolescent and young adult cancer: the Pediatric Initiative Network Risk Stratification System. J Adolesc Young Adult Oncol. 2020;9:662–666. [DOI] [PubMed] [Google Scholar]
- 22.Green DM, Liu W, Kutteh WH, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. Lancet Oncol. 2014;15:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melenhorst JJ, Chen GM, Wang M, et al. Decade-long leukaemia remissions with persistence of CD4(+) CAR T cells. Nature. Feb 2022;602(7897):503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dillman D SJ, Christian L. Internet, Mail, and Mixed-Mode Surveys: The Tailored Design Method. Hoboken, NJ: John Wiley & Sons, Inc; 2009. [Google Scholar]
- 25.MacQueen KM, McLellan E, Kay K, Milstein B. Codebook development for team-based qualitative analysis. CAM J. 1998;10:31–36. [Google Scholar]
- 26.Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2677–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Callahan C, Barry A, Fooks-Parker S, Smith L, Baniewicz D, Hobbie W. Pediatric survivorship: considerations following CAR T-cell therapy. Clin J Oncol Nurs. 2019;23:35–41. [DOI] [PubMed] [Google Scholar]
- 28.Schultz LM, Baggott C, Prabhu S, et al. Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: a Pediatric Real-World Chimeric Antigen Receptor Consortium report. J Clin Oncol. 2022;40:945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers RM, Taraseviciute A, Steinberg SM, et al. Blinatumomab nonresponse and high-disease burden are associated with inferior outcomes after CD19-CAR for B-ALL. J Clin Oncol. 2022;40:932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cosgrove C, Dellacecca ER, van den Berg JH, et al. Transgenerational transfer of gene-modified T cells. J Immunother Cancer. 2019;7:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scovell JM, Benz K, Samarska I, et al. Association of impaired spermatogenesis with the use of immune checkpoint inhibitors in patients with metastatic melanoma. JAMA Oncol. 2020;6:1297–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabinowitz MJ, Kohn TP, Pena VN, Samarska IV, Matoso A, Herati AS. Onset of azoospermia in man treated with ipilimumab/nivolumab for BRAF negative metastatic melanoma. Urol Case Rep. 2021;34: 101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moravek MB, Appiah LC, Anazodo A, et al. Development of a pediatric fertility preservation program: a report from the Pediatric Initiative Network of the Oncofertility Consortium. J Adolesc Health. 2019;64:563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodruff TK, Ataman-Millhouse L, Acharya KS, et al. A View from the past into our collective future: the oncofertility consortium vision statement. J Assist Reprod Genet. Jan 2021;38:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zver T, Frontczak S, Poirot C, et al. Minimal residual disease detection by multicolor flow cytometry in cryopreserved ovarian tissue from leukemia patients. J Ovarian Res. 2022;15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holland EM, Yates B, Ling A, et al. Characterization of extramedullary disease in B-ALL and response to CAR T-cell therapy. Blood Adv. 2022;6:2167–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


