Abstract
Epidermal growth factor (EGF) is a growth factor that plays a pivotal role in wound healing and maintaining tissue homeostasis by regulating cell survival, proliferation, migration, and differentiation. Exogenous administration of bioidentical human recombinant epidermal growth factor (rhEGF) has been known to promote skin wound healing, although rhEGF is increasingly being used in drug delivery systems and nanotechnology. However, despite considerable attention being focused on the potential clinical applications of rhEGF in several dermatological conditions beyond wound healing, the number of studies still remains relatively low. Herein, we conducted a literature search of PubMed/Medline and Google Scholar databases to retrieve published literature related to rhEGF and summarised the effects of rhEGF in the treatment of various wound types, radiotherapy or chemotherapy‐related skin reactions, atopic dermatitis, skin aging, and post‐inflammatory hyperpigmentation.
Keywords: cosmetics, dermatology, epidermal growth factor, wound
1. INTRODUCTION
Epidermal growth factor (EGF) is a well‐known mitogenic polypeptide that was first isolated from the submaxillary gland of mice by Stanley Cohen in the early 1960 seconds. 1 EGF exerts mitogenic effects by binding to its membrane receptor (epidermal growth factor receptor [EGFR]), which can then regulate cell growth, proliferation, differentiation, and survival, among other biological effects. 1 EGFRs are expressed in a variety of human tissues including most epithelial tissues, fibroblasts, and endothelial cells, meaning that EGF plays a key role in wound healing and maintaining tissue integrity. 2
Advances in recombinant DNA technology have enabled the production of human recombinant EGF (rhEGF) in large quantities. Consequently, in‐depth studies of rhEGF and the healing of surgical wounds, burn wounds, and diabetic foot ulcers have been carried out. 3 However, because of its short half‐life, large molecular weight, and lack of efficient formulation, 4 , 5 , 6 delivering rhEGF transdermally poses a significant clinical challenge. With the recent development of drug delivery systems and nanotechnologies stabilised the rhEGF, therefore the use of rhEGF in the dermatologic field has also been resurgent. 7 , 8 , 9 , 10 , 11 In addition, research has shown that EGF does not appear to be involved in the malignant transformation of wound bed cells and does not initiate tumorigenesis, thereby increasing its clinical usefulness. 12 , 13 Hence, this narrative review aims to highlight the benefits of using rhEGF in the treatment of dermatological pathologies including wounds (eg, traumatic, surgical), acneiform disorders, dermatitis, and hair loss, and also discusses potential aesthetic applications.
2. WOUND HEALING AND SCAR PREVENTION
Wound healing is a highly complex process of inflammation, cellular proliferation, differentiation, and remodelling, and involves multiple cytokines and growth factors including EGF. 14 As such, modulating this process is challenging, so numerous studies have attempted to investigate the role of rhEGF in treating acute or chronic wounds. rhEGF is known to facilitate wound healing by promoting re‐epithelialisation, and angiogenesis, and can stimulate myofibroblast activation and proliferation via the PI3K/AKT and ERK/MAPK signalling pathways (Figure 1). 15 , 16 In a rat model, EGF was also shown to protect fibroblasts from oxidative stress by scavenging already formed toxic oxidation products during the acute wound healing phase. 17
FIGURE 1.
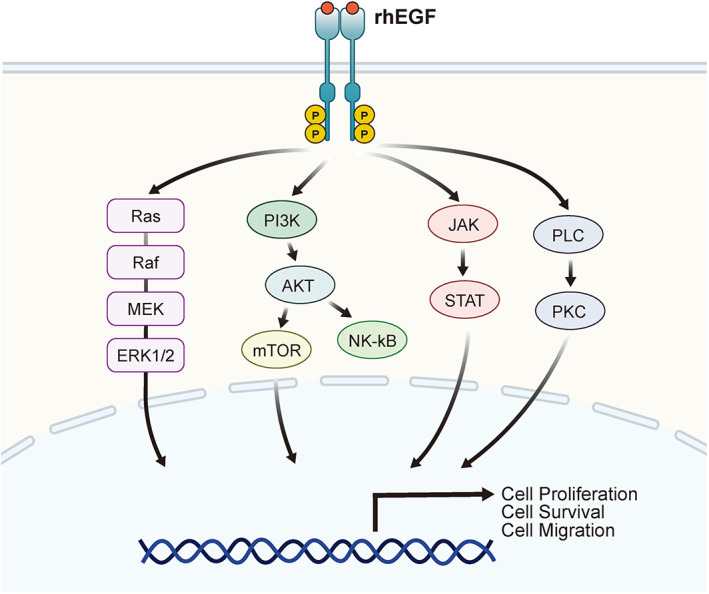
Schematic view of intracellular signalling pathways involved in rhEGF function
Several clinical trials evaluating the effects of topical applications of rhEGF on diabetic foot ulcers, including a phase 3 trial conducted in Korea, showed that rhEGF significantly improved wound healing by reducing the wound healing time. 18 , 19 , 20 rhEGF was also shown to be beneficial in treating pressure sores, second‐degree burns, and chronic, non‐healing traumatic wounds in a small number of case series and small clinical trials, including our own clinical experiences (Figure 2). 21 , 22 , 23 However, topical rhEGF ointment was difficult to apply to open wounds because of the wound exudate and because rhEGF is degraded by biofilm proteases. 24 Drug delivery systems such as polymeric nanoparticles and biomedical scaffolds have been developed and have improved the bioavailability of rhEGF. 25
FIGURE 2.
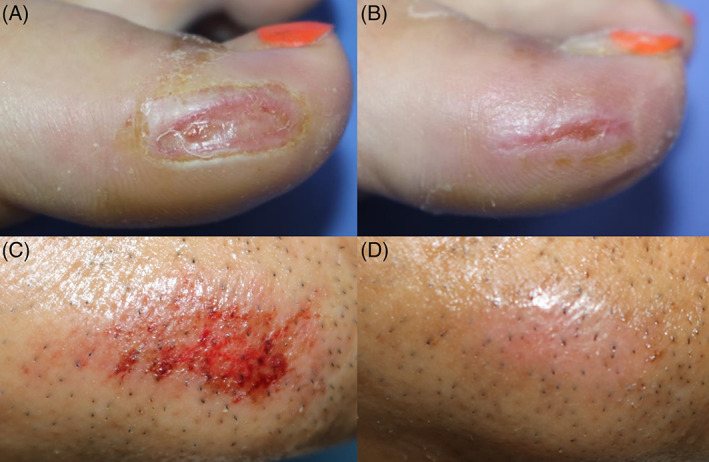
(A) Clinical photographs of a chronic and recalcitrant traumatic wound in a 35‐year‐old female before, and (B) after two weeks of treatment with rhEGF ointment. (C) Clinical photographs of a traumatic abrasion wound in a 62‐year‐old male before, and (D) after three days of treatment with rhEGF ointment
Additional studies have linked rhEGF with the treatment of surgical wounds. Suh et al. reported that rhEGF ointment successfully treated the excisional wound of keratoacanthoma without a primary closure, which took less time than normal healing. 26 Additionally, Shin et al. demonstrated that rhEGF significantly improved scar pliability and thickness in thyroidectomy scars after four weeks, compared with the control group. 27
Kao et al. conducted a study with 60 Taiwanese women 28 regarding microencapsulated rhEGF on the cutaneous scar after a caesarean section. Their results showed that scar vascularity, pigmentation, elasticity, and the Vancouver Scar Scale (VSS) total score after nine months were all significantly lower in the rhEGF group than in the silicone gel group (control group). Ryu et al. reported that rhEGF ointment decreased the length and area of excision scars and also showed similar short‐term cosmetic results—as reflected in the melanin index and the erythema index—to mupirocin ointment. 29 Their results indicate that rhEGF may reduce antibiotic resistance by reducing the use of topical antibiotics after clean wound surgeries. 29 Although well‐designed controlled trial data are still relatively scarce, the use of rhEGF in scar prevention is expected to become more prevalent in future.
3. CHEMOTHERAPY AND RADIATION‐RELATED SKIN REACTIONS
Radiation therapy can cause skin injuries such as erythema, itching, blisters, desquamation, and non‐healing ulcers, which often interrupts the radiotherapy treatment schedule for cancer patients. Therefore, the effectiveness of rhEGF in the treatment of radiation dermatitis has also been studied.
Hong et al. first reported a case of a chest ulcer caused by radiation successfully treated with rhEGF ointment, which was refractory to conservative wound dressings and debridement. 30 They also showed that rhEGF accelerated the proliferation of radiation‐induced fibroblasts and keratinocytes in vitro, 31 and demonstrated that the systemic injection of rhEGF ameliorated radiation‐induced mucosal damage in a mouse model. 31 The topical application of rhEGF was shown to accelerate wound healing and prevent the recurrence of radiodermatitis in a mouse model. 32 In a separate study, Kong et al. evaluated the topical application of rhEGF‐containing cream in 20 breast cancer patients and found that the severity of radiation dermatitis decreased significantly compared with supportive skin care. 33
A large multicentre observational study of 1172 patients who received more than 50 Gy of radiotherapy showed that rhEGF‐containing cream assisted in the prevention or alleviation of radiation dermatitis without any severe adverse events. 34
Several EGFR inhibitors were developed as chemotherapeutic agents once the role that EGF played in tumorigenesis was confirmed. 35 EGFR inhibition can interrupt normal keratinocyte growth and migration, resulting in epidermal thinning, xerosis, and acneiform eruption, which may adversely impact the patient's quality of life. 35 In human epidermal keratinocytes, rhEGF was shown to activate EGFR signalling and attenuate the expression of pro‐inflammatory cytokines such as IL‐1, IL‐8, and TNF‐α, which were induced by EGFR inhibitors. 36 Kim et al. also demonstrated that rhEGF treatment normalises the proliferation and differentiation of keratinocytes in 3D‐cultured human skin tissue, as well as in the skin lesions of patients with EGFR inhibitor‐related skin toxicities. 36 Furthermore, a pilot phase 3 trial of rhEGF for treating EGFR inhibitor‐induced skin toxicities showed that rhEGF ointment significantly improved skin lesions and patients’ quality of life when compared with a placebo. 37
There have been concerns, recently, that rhEGF may be involved in tumorigenesis; however, a recent literature review shows that there is no evidence that topical rhEGF preparation stimulates cancer cell proliferation. 13
4. ACNE
Acne is one of the most common inflammatory skin conditions and affects approximately 80%–90% of adolescents. Occasionally, it can lead to scarring, which can be unsightly. Both EGF and the EGFR pathway are known to affect commensal and pathogenic bacteria associated with acne pathogenesis, such as Cutibacterium acnes (C. acnes) and Staphylococcus epidermidis. 38 , 39 Recently, studies have showed the therapeutic efficacy of rhEGF on acne inflammation and acne scarring.
An in vitro study by Kim et al. showed that rhEGF inhibits the mRNA expression of pro‐inflammatory cytokines including IL‐1α, IL‐8, and TNF‐α induced by C. acnes in cultured human keratinocytes. 40 The C. acnes‐induced expression of toll‐like receptor 2 (TLR2) and nuclear factor kappa B (NF‐κB) activity were also reduced by rhEGF. Interestingly, they also found that the inhibition of EGFR by gefitinib attenuated the inhibitory effect of rhEGF on the expression of pro‐inflammatory cytokines as well as TLR2 and NF‐κB activity.
Kim et al. also performed a split‐face study evaluating the efficacy of rhEGF‐containing cream in 20 Korean patients with mild‐to‐moderate acne. 41 After six weeks of application, the rhEGF‐treated side showed a significant reduction in inflammatory and non‐inflammatory acne lesions, sebum output, and overall acne severity compared with the vehicle‐treated side. Additionally, rhEGF‐containing cream was well‐tolerated and no significant adverse events were reported.
During a 12‐week trial, Ronald et al. demonstrated the beneficial effects of topical rhEGF in acne scarring by showing how the mean investigator global assessment (IGA) score and the Goodman grade—a qualitative scar grading system for acne scarring—improved with rhEGF treatment. 42 , 43 , 44 More recently, a split‐face study of 36 patients with mild‐to‐moderate acne demonstrated that topical application of rhEGF significantly reduced the acne lesions and scar counts, with decreased IGA and ECCA scar grades. 45
Additionally, rhEGF increased the expression of TGF‐β1, elastin, and collagen type 1 and 3, and decreased keratin 16, NF‐κB p65, IL‐1α, and IL‐8 expression, which implies that rhEGF has anti‐inflammatory properties. 45 Collectively, these studies suggest that rhEGF may be a possible therapeutic adjuvant for both acne and acne scarring.
5. ATOPIC DERMATITIS
In contrast to the aforementioned studies regarding the efficacy of rhEGF in wound healing or radiation dermatitis, studies on the efficacy of rhEGF in atopic dermatitis (AD) have mostly been conducted in vitro. In a study by Zhang et al., EGF treatment in an AD‐like mice model significantly attenuated transepidermal water loss (TEWL), epidermal thickness, AD inflammation, and total and allergen‐specific immunoglobulin E (IgE) levels induced by cutaneous allergen rechallenge. 46 More specifically, EGF suppressed allergen‐induced expression of IL‐17A, CXCL1, CXCL2, neutrophil accumulation, and IL‐6 production in atopic skin. Based on these findings, the authors suggested that EGF played a protective role in AD by modulating Th17 responses in the skin.46 Similarly, Kim et al. reported that topical application of EGF improved skin lesion severity, skin thickness, epidermal hyperplasia, scratching behaviours, serum total IgE level, and TEWL in a 2,4‐dinitrochlorobenzene (DNCB)‐induced AD mice model. 47 They also reported that EGF increased the expression of skin barrier‐related proteins including filaggrin, involucrin, loricrin, occluding, and zonula occludens‐1. In addition, EGF attenuated the expression of protease‐activated receptor‐2 (PAR‐2) and thymic stromal lymphopoietin (TSLP), which plays a key role in pruritus and Th2 immune response in AD. Choi et al. also demonstrated that topical EGF application suppressed S. aureus‐induced inflammation in human epidermal keratinocytes and DNCB‐induced AD mice models. 48 These findings suggest that by regulating skin barrier function and immune response, rhEGF may be an adjuvant therapeutic option in AD cases.
Nevertheless, the mechanisms underlying the protective effects of rhEGF are not fully understood and clinical data are insufficient. Further research is necessary to verify the therapeutic efficacy and mechanism of rhEGF in AD.
6. HAIR LOSS
The exact role of EGF on the development and growth of hair follicles has yet to be fully elucidated and there is still controversy about whether rhEGF can reduce hair loss. An older paper by Philpott et al. showed that EGF stimulated hair elongation and DNA synthesis in the outer root sheath (ORS) and was also able to induce an artificial catagen‐like effect in isolated human hair follicles. 49 Zhang et al. reported similar results using a mink model, showing that EGF promotes the proliferation, and migration of ORS cells and induces the catagen phase. 50 They also suggested that EGF activates the Wnt/β‐catenin signalling pathway, the most important signalling pathway that regulates hair regeneration. 50 , 51 A more recent study by Bai et al. found that EGF induces the proliferation of hair follicle‐derived mesenchymal stem cells through the ERK and AKT signalling pathways. 52 By contrast, however, Mak et al. showed that the continuous expression of EGF in transgenic mice arrested follicular growth and development during the final stage of hair follicle morphogenesis. 53 Moreover, EGF has been shown to inhibit hair shaft elongation and change the morphology to catagen growth patterns by suppressing mitotic regulators including RCC2 and Statmin1. 54 , 55 El‐Refai et al. also reported that EGF negatively impacts hair follicle growth and may be linked to the pathogenesis of alopecia areata. 56
Additionally, a recent paper by Paek et al. showed that topical application of rhEGF promoted primary hair recovery through the dystrophic anagen pathway in a mouse model of cyclophosphamide‐induced alopecia. 57 As the evidence for this is currently weak, further research will be necessary to understand the exact roles of the EGF in hair follicle morphogenesis and cycling, as well as their therapeutic potential for reducing hair loss.
7. COSMETIC APPLICATIONS
rhEGF has also been studied in aesthetic applications. In vitro studies have shown that rhEGF promotes the migration and contractility of aged fibroblasts and increases both hyaluronic acid and collagen synthesis. 58 , 59 Based on this experimental background, several preclinical and clinical studies on the effect of rhEGF on photoaged skin have been published. For example, Shin et al. demonstrated that EGF‐containing hyaluronic acid filler induces type 1 and 3 collagen production and downregulates the expression of MMP‐9. 60 Schouest et al. showed that a three‐month topical application of EGF‐containing serum significantly improved brown pigmentation, skin texture, pore size, and wrinkles in 29 females with photoaged skin.61 Ha et al. reported that a four‐week application of micro‐spicule containing EGF cream was effective for the clinical improvement of periocular wrinkles in 20 Korean women. 62 Additionally, a newly developed hyaluronic acid‐based microneedle patch containing rhEGF significantly improved the appearance of wrinkles compared with a microneedle patch alone. 63 However, these studies are underpowered because of their small sample size and lack of a control group. Therefore, the clinical efficacy of rhEGF in improving the appearance of photoaged skin remains unclear.
Recent studies have demonstrated that rhEGF is beneficial for treating laser‐induced post‐inflammatory hyperpigmentation (PIH). Yun et al. reported that rhEGF alleviates melanogenesis in a PIH model by using laser‐treated keratinocyte‐conditioned culture media. 64 In their study, human melanocytes responded to EGF via ERK signalling, without any alterations in cell proliferation. 64 They also suggested that EGF may regulate the action of pro‐inflammatory cytokines generated by laser‐irradiated keratinocytes including PGE2, which can stimulate melanogenesis. 52 Based on these results, a few clinical studies have attempted to investigate the therapeutic effects of rhEGF on laser‐induced PIH. Park et al. conducted a randomised controlled trial, with a control group, to evaluate the effect of rhEGF‐containing cream after the 532‐nm Q‐switched neodymium‐doped yttrium aluminium garnet (Nd:YAG) laser irradiation of 25 Korean patients with senile lentigines. 65 The incidence of PIH and melanin index was significantly lower in the EGF‐containing cream group than in the control group. Kim et al. recently conducted a similar study evaluating the effect of EGF‐containing ointment for treating solar lentigines as an adjuvant to Q‐switched 532 Nd:YAG laser. 66 Techapichetvanich et al. also performed a split‐face study comparing the efficacy of rhEGF ointment after fractional ablative CO2 resurfacing with petrolatum ointment in 19 Thai patients. 67 Although not statistically significant, topical rhEGF ointment did slightly lower the risk of PIH compared with the control group (52.6% vs. 57.9%). However, when analysing the efficacy of the laser‐induced wound healing process, there was no significant difference in the duration of scab shedding, the duration of post‐laser erythema, or transepidermal water loss compared with the control.
Taken together, these studies indicate the therapeutic potential of rhEGF in the prevention of laser‐induced PIH, although further robust clinical studies are needed to further explore the potential benefits of rhEGF.
8. CONCLUSION
The extant literature demonstrates the therapeutic potential of rhEGF in dermatological conditions including various wounds, acne, radiation‐induced dermatitis, skin aging, and laser‐induced hyperpigmentation (Table 1). We expect this review article to provide a deeper insight into rhEGF for dermatologists and hope that further research will be conducted to elucidate the precise mechanisms underlying EGF signalling in various skin conditions.
TABLE 1.
Overview of applications of EGF in dermatological fields
| Indication | Mechanism of action | Preclinical/clinical model | Clinical function |
|---|---|---|---|
| Wound/scar | Acute full thickness wound/Rat 15 |
|
|
| Diabetic foot ulcers/Human 19 , 20 | |||
| Pressure ulcers/Human 21 |
|
||
| Ulcers, surgical or traumatic wounds, burns, and scars/Human 22 |
|
||
| Second‐degree burns/Human 23 |
|
||
| Excisional wound of keratoacanthoma without a primary closure/Human 26 |
|
||
| Surgical scars after thyroidectomy/Human 27 |
|
||
| Surgical scars after Caesarean section/Human 28 |
|
||
| Surgical wounds after cutaneous resection/Human 29 |
|
||
| Radiation dermatitis | Radiation‐induced oral mucosal dermatitis/Mouse 31 |
|
|
| Radiation dermatitis/Mouse 32 |
|
||
| Breast cancer with postoperative radiation treatment/Human 33 |
|
||
| Malignancies with radiation treatment/Human 34 |
|
||
| EGFRI‐related skin toxicity |
|
EGFRI‐related adverse skin events/Human 37 |
|
| Acne and acne scars | Mild‐to‐moderate acne vulgaris/Human 41 |
|
|
| Atrophic acne scarring/Human 42 , 43 |
|
||
| Acne and acne scarring/Human 44 |
|
||
| Atopic dermatitis | AD/Mouse 46 , 47 , 48 | ||
| Hair loss | Positive effects
|
Chemotherapy‐induced alopecia/Mouse 57 |
|
| Negative effects | Alopecia Areata/Human 56 |
|
|
| Cosmetic application | Photodamaged aging skin/Human 61 |
|
|
| Periocular wrinkles/Human 62 |
|
||
| Aging face/Human 63 |
|
||
|
Senile lentigines/Human 65 Solar lentigines/Human 66 |
|||
| Post‐laser resurfacing wound/Human 67 |
|
Abbreviations: AD, atopic dermatitis; ECCA, échelle d'évaluation clinique des cicatrices d'acné; C. acnes, Cutibacterium acnes; EGFRI, epidermal growth factor receptor inhibitor; IGA, Investigator global assessment; Nd:YAG, neodymium‐doped yttrium aluminium garnet; PIH, post‐inflammatory hyperpigmentation; rhEGF, recombinant human epidermal growth factor; S. aureus, Staphylococcus aureus; SGA, scar global assessment; VSS, vancouver scar scale.
AUTHOR CONTRIBUTIONS
Sun Hye Shin: Original draft, investigation. Young Gue Koh: Validation, investigation. Woo Geon Lee: Validation, investigation. Joon Seok: Conceptualisation, methodology, review, and editing of the manuscript. Kui Young Park: Conceptualisation, methodology, review, and editing of the manuscript.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Shin SH, Koh YG, Lee WG, Seok J, Park KY. The use of epidermal growth factor in dermatological practice. Int Wound J. 2023;20(6):2414‐2423. doi: 10.1111/iwj.14075
DATA AVAILABILITY STATEMENT
Data sharing not applicable ‐ no new data generated.
REFERENCES
- 1. Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new‐born animal. J Biol Chem. 1962;237:1555‐1562. [PubMed] [Google Scholar]
- 2. Wenczak BA, Lynch JB, Nanney LB. Epidermal growth factor receptor distribution in burn wounds. Implications for growth factor‐mediated repair. J Clin Invest. 1992;90(6):2392‐2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang S, Geng Z, Ma K, Sun X, Fu X. Efficacy of topical recombinant human epidermal growth factor for treatment of diabetic foot ulcer: a systematic review and meta‐analysis. Int J Low Extrem Wounds. 2016;15(2):120‐125. [DOI] [PubMed] [Google Scholar]
- 4. Dogan S, Demirer S, Kepenekci I, et al. Epidermal growth factor‐containing wound closure enhances wound healing in non‐diabetic and diabetic rats. Int Wound J. 2009;6(2):107‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krishnamurthy R, Manning MC. The stability factor: importance in formulation development. Curr Pharm Biotechnol. 2002;3(4):361‐371. [DOI] [PubMed] [Google Scholar]
- 6. Ogiso H, Ishitani R, Nureki O, et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110(6):775‐787. [DOI] [PubMed] [Google Scholar]
- 7. Choi SM, Lee KM, Kim HJ, et al. Effects of structurally stabilized EGF and bFGF on wound healing in type I and type II diabetic mice. Acta Biomater. 2018;66:325‐334. [DOI] [PubMed] [Google Scholar]
- 8. Li S, Liu Y, Huang Z, Kou Y, Hu A. Efficacy and safety of nano‐silver dressings combined with recombinant human epidermal growth factor for deep second‐degree burns: a meta‐analysis. Burns. 2021;47(3):643‐653. [DOI] [PubMed] [Google Scholar]
- 9. Koppa Raghu P, Bansal KK, Thakor P, et al. Evolution of nanotechnology in delivering drugs to eyes, skin and wounds via topical route. Pharmaceuticals (Basel). 2020;13(8):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi JH, Nam SH, Song YS, et al. Treatment with low‐temperature atmospheric pressure plasma enhances cutaneous delivery of epidermal growth factor by regulating E‐cadherin‐mediated cell junctions. Arch Dermatol Res. 2014;306(7):635‐643. [DOI] [PubMed] [Google Scholar]
- 11. Kim J, Jang JH, Lee JH, et al. Enhanced topical delivery of small hydrophilic or lipophilic active agents and epidermal growth factor by fractional radiofrequency microporation. Pharm Res. 2012;29(7):2017‐2029. [DOI] [PubMed] [Google Scholar]
- 12. Berlanga‐Acosta J, Gavilondo‐Cowley J, Barco‐Herrera DG, Martín‐Machado J, Guillén‐Nieto GE. Epidermal growth factor (EGF) and platelet‐derived growth factor (PDGF) as tissue healing agents: clarifying concerns about their possible role in malignant transformation and tumor progression. Journal of Carcinogenesis & Mutagenesis. 2011;02:1‐14. [Google Scholar]
- 13. Berlanga‐Acosta J, Gavilondo‐Cowley J, Lopez‐Saura P, et al. Epidermal growth factor in clinical practice ‐ a review of its biological actions, clinical indications and safety implications. Int Wound J. 2009;6(5):331‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kwon Y‐B, Kim H‐W, Roh D‐H, et al. Topical application of epidermal growth factor accelerates wound healing by myofibroblast proliferation and collagen synthesis in rat. J Vet Sci. 2006;7(2):105‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laato M, Kahari VM, Niinikoski J, Vuorio E. Epidermal growth factor increases collagen production in granulation tissue by stimulation of fibroblast proliferation and not by activation of procollagen genes. Biochem J. 1987;247(2):385‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalay Z, Cevher SC. Oxidant and antioxidant events during epidermal growth factor therapy to cutaneous wound healing in rats. Int Wound J. 2012;9(4):362‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gomez‐Villa R, Aguilar‐Rebolledo F, Lozano‐Platonoff A, et al. Efficacy of intralesional recombinant human epidermal growth factor in diabetic foot ulcers in Mexican patients: a randomized double‐blinded controlled trial. Wound Repair Regen. 2014;22(4):497‐503. [DOI] [PubMed] [Google Scholar]
- 19. Park KH, Han SH, Hong JP, et al. Topical epidermal growth factor spray for the treatment of chronic diabetic foot ulcers: a phase III multicenter, double‐blind, randomized, placebo‐controlled trial. Diabetes Res Clin Pract. 2018;142:335‐344. [DOI] [PubMed] [Google Scholar]
- 20. Singla S, Garg R, Kumar A, Gill C. Efficacy of topical application of beta urogastrone (recombinant human epidermal growth factor) in Wagner's grade 1 and 2 diabetic foot ulcers: comparative analysis of 50 patients. J Nat Sci Biol Med. 2014;5(2):273‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cho KH, Kim YJ. Therapeutic effect of the recombinant human epidermal growth factor (rhEGF) in pressure ulcer. Journal of the Korean Academy of Rehabilitation Medicine. 2010;34(3):253‐258. [Google Scholar]
- 22. Esquirol‐Caussa J, Herrero‐Vila E. Human recombinant epidermal growth factor in skin lesions: 77 cases in EPItelizando project. J Dermatolog Treat. 2019;30(1):96‐101. [DOI] [PubMed] [Google Scholar]
- 23. Guo X, Tan M, Guo L, Xiong A, Li Y, He X. Clinical study on repair of burn wounds of degree II with recombinant human epidermal growth factor in elderly patients. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24(4):462‐464. [PubMed] [Google Scholar]
- 24. Kim H, Kong WH, Seong KY, et al. Hyaluronate‐epidermal growth factor conjugate for skin wound healing and regeneration. Biomacromolecules. 2016;17(11):3694‐3705. [DOI] [PubMed] [Google Scholar]
- 25. Chu Y, Yu D, Wang P, Xu J, Li D, Ding M. Nanotechnology promotes the full‐thickness diabetic wound healing effect of recombinant human epidermal growth factor in diabetic rats. Wound Repair Regen. 2010;18(5):499‐505. [DOI] [PubMed] [Google Scholar]
- 26. Suh DW, Lew BL, Sim WY. Using recombinant human epidermal growth factor for the successful treatment of an excisional wound without a primary closure. Dermatol Surg. 2014;40(6):706‐708. [DOI] [PubMed] [Google Scholar]
- 27. Shin JU, Kang SW, Jeong JJ, Nam KH, Chung WY, Lee JH. Effect of recombinant human epidermal growth factor on cutaneous scar quality in thyroidectomy patients. J Dermatolog Treat. 2015;26(2):159‐164. [DOI] [PubMed] [Google Scholar]
- 28. Kao CC, Huang SY, Chiang CH, Lin CH, Chang TC. Microencapsulated rhEGF to facilitate epithelial healing and prevent scar formation of cesarean wound: a randomized controlled trial. Taiwan J Obstet Gynecol. 2021;60(3):468‐473. [DOI] [PubMed] [Google Scholar]
- 29. Ryu SI, Kim KE, Jeong JY, Park JH, Moon HR, Kim IH. Effect of the recombinant human epidermal growth factor ointment on cutaneous surgical wounds compared to antibiotic ointment. Ann Dermatol. 2021;33(6):549‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee SW, Moon SY, Kim YH, Hong JP. The use of recombinant human epidermal growth factor to promote healing for chronic radiation ulcer. Int Wound J. 2007;4(3):216‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ryu SH, Moon SY, Yang YJ, et al. Recombinant human epidermal growth factor accelerates the proliferation of irradiated human fibroblasts and keratinocytes in vitro and in vivo. J Radiat Res. 2009;50(6):545‐552. [DOI] [PubMed] [Google Scholar]
- 32. Ryu SH, Kim YH, Lee SW, Hong JP. The preventive effect of recombinant human growth factor (rhEGF) on the recurrence of radiodermatitis. J Radiat Res. 2010;51(5):511‐517. [DOI] [PubMed] [Google Scholar]
- 33. Kong M, Hong SE. Topical use of recombinant human epidermal growth factor (EGF)‐based cream to prevent radiation dermatitis in breast cancer patients: a single‐blind randomized preliminary study. Asian Pac J Cancer Prev. 2013;14(8):4859‐4864. [DOI] [PubMed] [Google Scholar]
- 34. Kang HC, Ahn SD, Choi DH, Kang MK, Chung WK, Wu HG. The safety and efficacy of EGF‐based cream for the prevention of radiotherapy‐induced skin injury: results from a multicenter observational study. Radiat Oncol J. 2014;32(3):156‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6(10):803‐812. [DOI] [PubMed] [Google Scholar]
- 36. Kim JM, Ji JH, Kim YS, et al. rhEGF treatment improves EGFR inhibitor‐induced skin barrier and immune defects. Cancers (Basel). 2020;12(11):3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim YS, Ji JH, Oh SY, et al. A randomized controlled trial of epidermal growth factor ointment for treating epidermal growth factor receptor inhibitor‐induced skin toxicities. Oncologist. 2020;25(1):e186‐e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Satoh TK, Mellett M, Meier‐Schiesser B, et al. IL‐36gamma drives skin toxicity induced by EGFR/MEK inhibition and commensal Cutibacterium acnes. J Clin Invest. 2020;130(3):1417‐1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park K, Ommori R, Imoto K, Asada H. Epidermal growth factor receptor inhibitors selectively inhibit the expressions of human beta‐defensins induced by Staphylococcus epidermidis. J Dermatol Sci. 2014;75(2):94‐99. [DOI] [PubMed] [Google Scholar]
- 40. Kim JM, Choo JE, Lee HJ, Kim KN, Chang SE. Epidermal growth factor attenuated the expression of inflammatory cytokines in human epidermal keratinocyte exposed to Propionibacterium acnes. Ann Dermatol. 2018;30(1):54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim HK, Yeo IK, Li K, Kim BJ, Kim MN, Hong CK. Topical epidermal growth factor for the improvement of acne lesions: a randomized, double‐blinded, placebo‐controlled, split‐face trial. Int J Dermatol. 2014;53(8):1031‐1036. [DOI] [PubMed] [Google Scholar]
- 42. Seidel R, Moy RL. Improvement in atrophic acne scars using topical synthetic epidermal growth factor (EGF) serum: a pilot study. J Drugs Dermatol. 2015;14(9):1005‐1010. [PubMed] [Google Scholar]
- 43. Stoddard MA, Herrmann J, Moy L, Moy R. Improvement of atrophic acne scars in skin of color using topical synthetic epidermal growth factor (EGF) serum: a pilot study. J Drugs Dermatol. 2017;16(4):322‐326. [PubMed] [Google Scholar]
- 44. Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32(12):1458‐1466. [DOI] [PubMed] [Google Scholar]
- 45. Kim DH, Yang JH, Cho SI, et al. Clinical and histological effects of topical epidermal growth factor on acne and acne scars. Dermatology. 2022;238(5):837‐845. [DOI] [PubMed] [Google Scholar]
- 46. Zhang Z, Xiao C, Gibson AM, Bass SA, Khurana Hershey GK. EGFR signaling blunts allergen‐induced IL‐6 production and Th17 responses in the skin and attenuates development and relapse of atopic dermatitis. J Immunol. 2014;192(3):859‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim YJ, Choi MJ, Bak DH, et al. Topical administration of EGF suppresses immune response and protects skin barrier in DNCB‐induced atopic dermatitis in NC/Nga mice. Sci Rep. 2018;8(1):11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Choi SY, Lee YJ, Kim JM, Kang HJ, Cho SH, Chang SE. Epidermal growth factor relieves inflammatory signals in Staphylococcus aureus‐treated human epidermal keratinocytes and atopic dermatitis‐like skin lesions in Nc/Nga mice. Biomed Res Int. 2018;2018:9439182‐9439189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Philpott MP, Kealey T. Effects of EGF on the morphology and patterns of DNA synthesis in isolated human hair follicles. J Invest Dermatol. 1994;102(2):186‐191. [DOI] [PubMed] [Google Scholar]
- 50. Zhang H, Nan W, Wang S, et al. Epidermal growth factor promotes proliferation and migration of follicular outer root sheath cells via Wnt/beta‐catenin signaling. Cell Physiol Biochem. 2016;39(1):360‐370. [DOI] [PubMed] [Google Scholar]
- 51. Reddy S, Andl T, Bagasra A, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107(1–2):69‐82. [DOI] [PubMed] [Google Scholar]
- 52. Bai T, Liu F, Zou F, et al. Epidermal growth factor induces proliferation of hair follicle‐derived mesenchymal stem cells through epidermal growth factor receptor‐mediated activation of ERK and AKT signaling pathways associated with upregulation of cyclin D1 and downregulation of p16. Stem Cells Dev. 2017;26(2):113‐122. [DOI] [PubMed] [Google Scholar]
- 53. Mak KK, Chan SY. Epidermal growth factor as a biologic switch in hair growth cycle. J Biol Chem. 2003;278(28):26120‐26126. [DOI] [PubMed] [Google Scholar]
- 54. Bichsel KJ, Hammiller B, Trempus CS, Li Y, Hansen LA. The epidermal growth factor receptor decreases Stathmin 1 and triggers catagen entry in the mouse. Exp Dermatol. 2016;25(4):275‐281. [DOI] [PubMed] [Google Scholar]
- 55. Richardson GD, Bazzi H, Fantauzzo KA, et al. KGF and EGF signalling block hair follicle induction and promote interfollicular epidermal fate in developing mouse skin. Development. 2009;136(13):2153‐2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. El‐Refai AM, Elhabak DM, Khashaba RA. More is not always better in hair growth factors. Epidermal growth factor: hair growth factor involved in alopecia areata pathogenesis. Int J Trichology. 2020;12(4):182‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Paik SH, Yoon JS, Ryu HH, et al. Pretreatment of epidermal growth factor promotes primary hair recovery via the dystrophic anagen pathway after chemotherapy‐induced alopecia. Exp Dermatol. 2013;22(7):496‐499. [DOI] [PubMed] [Google Scholar]
- 58. Kim D, Kim SY, Mun SK, Rhee S, Kim BJ. Epidermal growth factor improves the migration and contractility of aged fibroblasts cultured on 3D collagen matrices. Int J Mol Med. 2015;35(4):1017‐1025. [DOI] [PubMed] [Google Scholar]
- 59. Jeon YJ, Kim YH, Jeon YJ, et al. Increased synthesis of hyaluronic acid by enhanced penetration of CTP‐EGF recombinant in human keratinocytes. J Cosmet Dermatol. 2019;18:1539‐1545. [DOI] [PubMed] [Google Scholar]
- 60. Shin SH, Roh YJ, Jin SC, et al. Rheological properties and preclinical data of novel hyaluronic acid filler containing epidermal growth factor. Exp Dermatol. 2022;31:1685‐1692. [DOI] [PubMed] [Google Scholar]
- 61. Schouest JM, Luu TK, Moy RL. Improved texture and appearance of human facial skin after daily topical application of barley produced, synthetic, human‐like epidermal growth factor (EGF) serum. J Drugs Dermatol. 2012;11(5):613‐620. [PubMed] [Google Scholar]
- 62. Ha JM, Lim CA, Han K, et al. The effect of micro‐spicule containing epidermal growth factor on periocular wrinkles. Ann Dermatol. 2017;29(2):187‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. An JH, Lee HJ, Yoon MS, Kim DH. Anti‐wrinkle efficacy of cross‐linked hyaluronic acid‐based microneedle patch with acetyl Hexapeptide‐8 and epidermal growth factor on Korean skin. Ann Dermatol. 2019;31(3):263‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yun WJ, Bang SH, Min KH, Kim SW, Lee MW, Chang SE. Epidermal growth factor and epidermal growth factor signaling attenuate laser‐induced melanogenesis. Dermatol Surg. 2013;39(12):1903‐1911. [DOI] [PubMed] [Google Scholar]
- 65. Park GH, do Rhee Y, Moon HR, et al. Effect of an epidermal growth factor‐containing cream on postinflammatory hyperpigmentation after Q‐switched 532‐nm neodymium‐doped yttrium aluminum garnet laser treatment. Dermatol Surg. 2015;41(1):131‐135. [DOI] [PubMed] [Google Scholar]
- 66. Kim HO, Kim HR, Kim JC, et al. A Randomized Controlled Trial on the Effectiveness of Epidermal Growth Factor‐Containing Ointment on the Treatment of Solar Lentigines as Adjuvant Therapy. Vol 57. Kaunas: Medicina; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Techapichetvanich T, Wanitphakdeedecha R, Iamphonrat T, et al. The effects of recombinant human epidermal growth factor containing ointment on wound healing and post inflammatory hyperpigmentation prevention after fractional ablative skin resurfacing: a split‐face randomized controlled study. J Cosmet Dermatol. 2018;17(5):756‐761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable ‐ no new data generated.


