Abstract
Background
Low back pain (LBP) is a common reason individuals seek healthcare. Nonpharmacologic management (NPM) is often recommended as a primary intervention, and earlier use of NPM for LBP shows positive clinical outcomes. Our purpose was to evaluate how timing of engagement in NPM for LBP affects downstream LBP visits during the first year.
Methods
This study was a secondary analysis of an observational cohort study of national electronic health record data. Patients entering the Musculoskeletal Diagnosis/Complementary and Integrative Health Cohort with LBP from October 1, 2016 to September 30, 2017 were included. Exclusive patient groups were defined by engagement in NPM within 30 days of entry (“very early NPM”), between 31 and 90 days (“early NPM”), or not within the first 90 days (“no NPM”). The outcome was time, in days, to the final LBP follow-up after 90 days and within the first year. Cox proportional hazards regression was used to model time to final follow up, controlling for additional demographic and clinical covariables.
Results
The study population included 44,175 patients, with 16.7% engaging in very early NPM and 13.1% in early NPM. Patients with very early NPM (5.2 visits, SD=4.5) or early NPM (5.7 visits, SD=4.6) had a higher mean number of LBP visits within the first year than those not receiving NPM in the first 90 days (3.2 visits, SD = 2.5). The very early NPM (HR=1.50, 95% CI: 1.46–1.54; median=48 days, IQR=97) and early NPM (HR=1.27, 95% CI: 1.23–1.30; median=88 days, IQR=92) had a significantly shorter time to final follow-up than the no NPM group (median=109 days, IQR=150).
Conclusions
Veterans Health Administration patients receiving NPM for LBP within the first 90 days after initially seeking care demonstrate a significantly faster time to final follow-up visit within the first year compared to those who do not.
Keywords: Low back pain, Musculoskeletal pain/therapy, Nonpharmacological management, Veterans Health, Health services research, Healthcare utilization
Introduction
Low back pain (LBP) is highly prevalent and among the most common reasons people seek healthcare [1], especially in the Veterans Health Administration (VHA) [2]. Evidence-based nonpharmacological management (NPM) for pain is a primary recommendation for LBP care [3,4].
There is growing knowledge about the use and timing of initiation of NPM for LBP and its effects on system-level health services outcomes or patient-level clinical outcomes. Early use of NPM by patients with LBP shows favorable clinical outcomes [5]. There is mixed quality evidence of early physical therapy initiation favoring decreased healthcare costs and utilization [6]. Early use of chiropractic care or physical therapy has been found to be associated with reduced opioid use (including long-term opioid use) [7,8]. Limited evidence exists in other clinical disciplines commonly providing NPM for LBP.
The VHA is an ideal venue to study use of NPM for LBP given system-wide adoption and availability [9] and its encouraged use in its Stepped Care Model [10,11]. Further, the VHA has overcome many barriers to NPM uptake that may remain present in other health systems [12]. More broadly, VHA offers nationally-distributed healthcare delivery system for a diverse, and often understudied, patient population using a robust integrated electronic health record (EHR) to facilitate generalizable observational health services research [13].
We assessed how the timing of engagement in NPM affects downstream follow-up for patients newly receiving treatment for LBP in the VHA. We sought to identify the proportion of patients receiving NPM in the first 90 days and the effect of such care on time to final LBP follow-up, hypothesizing that earlier engagement leads to reduced time to final follow-up.
Methods
This observational cohort study used EHR data from the Musculoskeletal Diagnosis/Complementary and Integrative Health (MSD/CIH) Cohort [2]. Study reporting was informed by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [14]. This study was approved by the VA Connecticut Healthcare System Institutional Review Board.
We assessed care received by VHA patients at VHA facilities only. Patients with a cohort index outpatient visit for LBP (based on ICD-10 diagnoses) from October 1, 2016 to September 30, 2017 were included in order to accrue sufficient follow-up time for NPM use and outcomes. We defined new LBP as a new initiation of healthcare for a LBP-related diagnosis among patients who had at least 1 year without any visit with a LBP diagnosis before the index LBP diagnosis date. To increase the likelihood that the index visit represented the start of an episode of care rather than an incidental finding or continuation of a prior episode, included patients had at least one follow-up visit for LBP in the year after the index date.
The exposure was the timing of engagement in NPM following LBP index visit, defined by at least 1 visit to a clinic commonly providing NPM, including physical therapy, chiropractic care, mental health, occupational therapy, and/or “other CIH treatments” (eg, acupuncture, yoga, tai chi). Exclusive exposure groups were defined as “very early NPM” (≤30 days after index) or “early NPM” (31–90 days after index) based on the first visit to any of those clinics. The comparison group did not receive NPM within 90 days of index.
The outcome was time, in days, from the index visit to the final follow-up visit with a LBP diagnosis after 90 days and within the first year (90 days < t ≤ 365 days). We recognized a potential source of bias that may be present is the overlap in the exposure period and outcome observation window during the first 90 days after index. The outcome distribution in the early NPM group is also right-shifted relative to the other 2 groups due to the requirement of at least 1 follow-up visit occurring after 30 days. Given that outcomes would be known for all participants, we did not pursue left truncation to exclude participants with a final follow-up prior to 90 days as it can yield an unstable, less precise effect estimate [15]. Instead to handle these completely left-censored events while avoiding potential overestimation of the possible effect, minimum value imputation was used to adjust the outcome event time to day 90.5 (immediately prior to the beginning of the outcome observation window) for participants with an outcome at less than 90 days.
Cox proportional hazards regression was used to model the effect of very early or early NPM on the time to final LBP follow-up visit. Statistical analyses were completed in RStudio (Boston, MA) using R version 3.6.3 (R Core Team, Vienna, Austria) [16] and the "survival” package [17].
Additional demographic and clinical covariables were included in the inferred model. Demographic variables included age at index LBP visit, sex, and race/ethnicity. Race/ethnicity was based on EHR data from veteran self-report at VHA registration and grouped into non-Hispanic White, non-Hispanic Black, Hispanic, or “other race/ethnicity” based on the most frequently reported race. Other race/ethnicity included responses of American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, Two or more Races, Unknown by patient, Declined to answer, or Missing.
Pain intensity was included by measuring the maximum 11-point numerical rating scale (0–10) score on the date of the index LBP visit and categorizing into no pain (NRS = 0), mild pain (NRS = 1–3), moderate pain (NRS = 4–6), severe pain (NRS = 7–10), or missing pain score [18]. Smoking status was categorized as “never smoker”, “former smoker”, “current smoker”, or missing smoking status based on EHR data collected from clinical reminders and text entries [19]. Veteran service-connected disability percentage is a marker of veteran compensation for injuries or illnesses attributable to or worsened by military service and is also used to determine access to VHA healthcare services and copay exemptions [20]. Service-connected disability percentage at index was categorized into “less than 50% service-connected disability”, “50-100% service-connected disability”, or missing. Body mass index (BMI) was calculated based on height and weight values recorded in the EHR and categorized into “not obese” (BMI < 30.0 kg/m2), “obese” (BMI ≥ 30.0 kg/m2), or missing [21]. Opioid use (excluding methadone or tramadol use) was identified as a binary variable based on any prescription filled within 30 days of cohort index.
Charlson Comorbidity Index (CCI) [22,23] was calculated at index for all veterans based on the presence of International Classification of Diseases codes appearing in their medical records and categorized into four groups (CCI = 0, CCI = 1, CCI = 2, CCI = 3 or greater). The presence of medical comorbidities including post-traumatic stress disorder, alcohol use disorder, drug use disorder, and neck pain were identified based on diagnosis codes in the medical record, and individuals with missing comorbidity data were excluded from the multivariable analysis.
Results
There were 44,175 patients meeting the inclusion criteria (Table 1), with 16.7% engaging in very early NPM and 13.1% in early NPM. Patients with very early or early NPM most frequently had at least one visit to physical therapy (10,679 patients), followed by chiropractic care (1,635 patients), mental health (889 patients), occupational therapy (300 patients), and other CIH clinics (254 patients). Patients with very early NPM (5.2 visits, SD = 4.5) or early NPM (5.7 visits, SD = 4.6) had a higher mean number of LBP visits within the first year than those not receiving NPM in the first 90 days (3.2 visits, SD = 2.5). The median time to final LBP follow-up was 48 days in the very early NPM group (IQR = 97), 88 days in the early NPM group (IQR = 92), and 109 days in the group not receiving NPM in the first 90 days (IQR = 150).
Table 1.
Patient demographics and clinical characteristics for individuals receiving very early NPM (≤30 days after index), early NPM (31–90 days after index), or no NPM within 90 days of index.
| Very early NPM (N=7,393) | Early NPM (N=5,774) | No NPM (N=31,008) | Overall (N=44,175) | |
|---|---|---|---|---|
| Days to Final Follow-Up, median [IQR] | 48 [97] | 88 [92] | 109 [150] | 94 [146] |
| Final Follow-up after 90 days (%) | 31.8 | 48.1 | 57.8 | 52.2 |
| Age*(y), median [IQR] | 47.0 [32.0] | 44.0 [28.0] | 47.0 [29.0] | 46.0 [30.0] |
| Female Sex (%) | 11.6 | 13.8 | 11.2 | 11.6 |
| Race/Ethnicity | ||||
| White (%) | 63.9 | 59.0 | 60.9 | 61.2 |
| Black (%) | 17.7 | 20.6 | 19.5 | 19.3 |
| Hispanic (%) | 9.4 | 10.8 | 10.1 | 10.0 |
| Other/Unknown (%) | 9.0 | 9.7 | 9.6 | 9.5 |
| NRS Pain Score, median [IQR]† | 5 [5] | 5 [5] | 5 [5] | 5 [5] |
| No Pain (NRS = 0) (%) | 14.0 | 16.8 | 18.8 | 17.7 |
| Mild Pain (NRS = 1–3) (%) | 14.3 | 16.3 | 14.8 | 14.9 |
| Moderate Pain (NRS = 4–6) (%) | 27.3 | 33.1 | 29.8 | 29.8 |
| Severe Pain (NRS = 7–10) (%) | 20.2 | 25.6 | 25.7 | 24.8 |
| BMI (kg/m2), mean (SD)‡ | 30.1 (±5.6) | 30.2 (±5.6) | 30.1 (±5.7) | 30.1 (±5.7) |
| Not obese (BMI < 30 kg/m2) (%) | 51.4 | 51.6 | 51.5 | 51.5 |
| Obese (BMI ≥ 30 kg/m2) (%) | 44.9 | 45.3 | 44.8 | 44.9 |
| Smoking Status§ | ||||
| Current (%) | 31.9 | 32.3 | 34.9 | 34.1 |
| Former (%) | 24.3 | 20.5 | 20.9 | 21.4 |
| Never (%) | 38.2 | 41.5 | 38.1 | 38.5 |
| Service-Connected Disabilityǁ | ||||
| < 50% (%) | 25.7 | 25.6 | 23.8 | 24.3 |
| ≥ 50% (%) | 36.5 | 39.6 | 38.5 | 38.3 |
| Charlson Comorbidity Index | ||||
| CCI = 0 (%) | 70.4 | 74.6 | 70.4 | 71.0 |
| CCI = 1 (%) | 15.9 | 14.1 | 16.0 | 15.8 |
| CCI = 2 (%) | 5.8 | 5.0 | 5.9 | 5.7 |
| CCI ≥ 3 (%) | 7.9 | 6.2 | 7.7 | 7.6 |
| Post-traumatic Stress Disorder (%)¶ | 17.5 | 19.2 | 17.2 | 17.5 |
| Alcohol Use Disorder (%)¶ | 6.4 | 5.5 | 6.0 | 6.0 |
| Drug Use Disorder (%)¶ | 3.3 | 3.4 | 3.0 | 3.1 |
| Neck Pain (%) | 7.4 | 8.6 | 8.2 | 8.1 |
| Opioid Prescription (%)♯ | 2.6 | 2.8 | 3.7 | 3.4 |
BMI = Body Mass Index
Age as of Cohort Index
5,618 missing
1,600 missing
2,650 missing
16,490 missing
21 missing
filled within 30 days of cohort entry
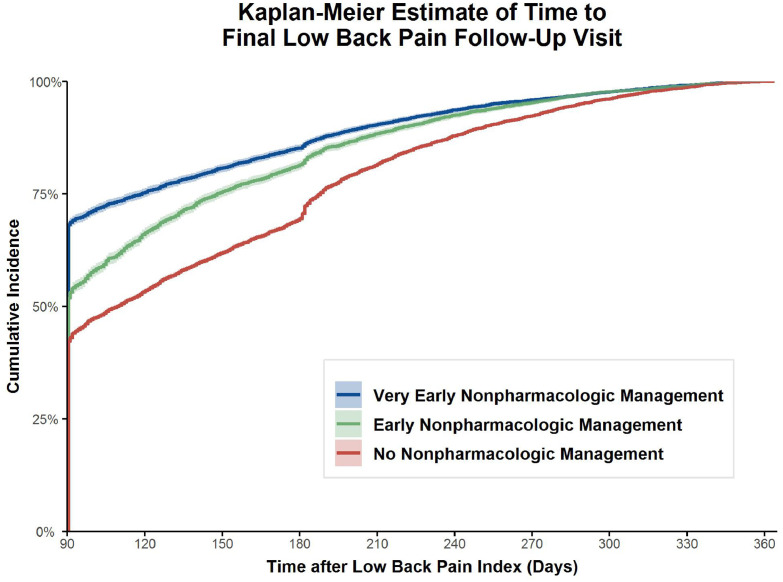
The univariable Kaplan-Meier estimate, with 95% confidence intervals, of the cumulative incidence of the time to final follow-up after 90 days by group is presented in Fig. 1. The very early NPM (HR = 1.52, 95% CI: 1.48–1.56) and early NPM (HR = 1.27, 95% CI: 1.23–1.30) groups had a significantly shorter time to final follow-up than the comparison group.
Fig. 1.
Kaplan-Meier estimated cumulative incidence of the time to final low back pain follow-up visit within 1 year of cohort index based on engagement in very early or early nonpharmacologic management compared to no nonpharmacologic management within the first 90 days.
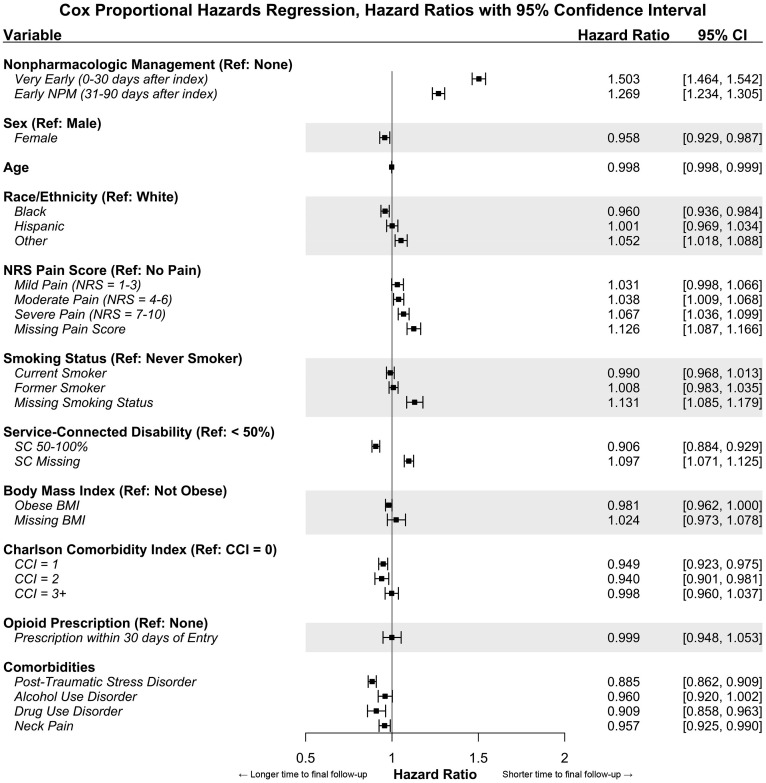
In the multivariable Cox proportional hazards regression model, 21 cases were excluded due to missing comorbidity data (n=44,154 cases included). Fig. 2 shows the adjusted hazard ratios with 95% confidence intervals for very early or early NPM and the included covariables. The very early NPM (HR = 1.50, 95% CI: 1.46–1.54) and early NPM (HR = 1.27, 95% CI: 1.23–1.30) groups had a significantly shorter time to final follow-up than those without NPM in the first 90 days when controlling for potential confounders.
Fig. 2.
Multivariable Cox proportional hazards regression model of time to final low back pain follow-up visit within 1 year of cohort index. Hazard ratios greater than 1 indicate a shorter time to final low back pain follow-up, while those less than 1 indicate a longer time to final low back pain follow-up.
Discussion
This is the first report on timing of NPM use impacting downstream use of healthcare services for new LBP treatment episodes in VHA. We found that the time to final follow-up for LBP within one year was significantly shorter for those who engaged in very early or early NPM compared to those who did not. When controlling for additional patient variables in the multivariable regression model, the effect of very early or early NPM on the time to final follow-up outcome was not substantially attenuated. Other predictor variables that were included in the multivariable model may be related to the time to final LBP follow-up with a statistically significant effect. However, they are unlikely to be confounding or moderating the identified relationship between very early or early NPM and the time to final LBP follow-up outcome.
It was also interesting that the very early NPM and early NPM groups had a greater average number of visits in the first year than those who did not receive NPM, despite having a significantly faster time to final follow up visit. This increased service use during a condensed portion of the observation period may promote decreased service use in subsequent time periods as a result of more “successful” early treatment. Clinical practice guidelines for LBP frequently recommend NPM as a primary intervention, citing consistent evidence of favorable patient outcomes. Care inconsistent with clinical practice guidelines has been identified as a risk factor for transition from acute to chronic LBP (defined as LBP present for greater than 3 months) [24]. Though our outcome was not progression to chronic LBP, we suspect potentially overlapping mechanisms affecting continued LBP care in our study and progression to chronic LBP. It's plausible that individuals who experience chronification may continue to receive ongoing LBP care and that interventions known to reduce likelihood of chronification, such as early NPM, may also affect the frequency and/or duration of follow up care after newly seeking healthcare for LBP.
We defined NPM exposure as any visit to one or more of a group of clinics but did not assess specific NPM services offered during individual visits. Given that providers in these NPM clinics often employ multimodal care strategies, features of individual clinic visits may be contributing to the association found between early NPM and time to final LBP follow up. For example, receiving physical therapy was much more common than receiving any other NPM, likely due to it being ubiquitous across the VHA compared to other services of limited availability. There may be specific features of physical therapy care that are largely driving our results. Additionally, in the absence of evidence recommending priority engagement in any specific NPM clinics, we suspect patients are consulted to these clinics based on patient preference and/or individual referring provider clinical decision making, consistent with the VHA stepped care model for pain [10]. Yet it is possible that improvements in sequencing the use of given NPM clinics could yield better patient and system outcomes. Future work should evaluate the potential effects of engaging in specific clinics, and the timing of that engagement, on downstream follow up care.
While we found that controlling for additional potential confounding variables did not attenuate the estimated effect of early or very early NPM use on time to final follow up, we believe the associations identified are not yet sufficient to define a causal relationship. We recognize additional patient or facility factors may influence time to final follow-up, such as disease severity or wait times to visits, which were not included in the model.
We identified many relationships between included covariables and the time to final follow up visit that were significant, but most had only a small strength of association. While possible that these reached statistical significance primarily because of the large sample size of our cohort, we propose potential explanations for select variables of interest.
Females had a significantly longer time to final LBP follow up than males. This could be due to an increased use of outpatient healthcare services by females in general [25] and for musculoskeletal pain [26], and/or different pain experiences in males and females potentially attributable to a variety of biopsychosocial factors [27]. Non-Hispanic Black individuals had a significantly longer time to final LBP follow up and individuals in the “other race/ethnicity” group had a shorter time to final LBP follow up compared to non-Hispanic White individuals. Prior work studying racial and ethnic differences in LBP care in non-VHA settings has found slightly worse improvement in LBP-related function and less spine-related healthcare utilization in Black and Hispanic populations compared to Whites and non-Hispanics [28], which may relate to our findings of a longer time to final follow up.
Patients with increasingly more severe baseline pain scores had progressively stronger associations with a faster time to final follow up. It is possible that this reflects an increased attention of individuals with more intense LBP to seek care earlier, which may be a positive trend given that higher pain intensity has been found to be a prognostic indicator of increased risk of progression to chronic LBP [29].
We suspect individuals who may be considered “more complex” patients may either necessitate additional care or are higher healthcare utilizers in general [30], therefore expecting a slower time to final LBP follow up visit. This may include patients with higher VHA service-connected disability ratings and patients with other comorbid medical conditions – including post-traumatic stress disorder, drug use disorder, and concurrent neck pain. Patients with a CCI score of 1 or 2 had a significantly longer time to final LBP follow up compared to those with a CCI score of 0. However, it was interesting that we found individuals with a CCI score of 3 or greater, who are considered relatively more complex than those with lower CCI scores, did not demonstrate a significant association with time to final follow up in either direction. While surprising that the most comorbidly complex patients in our population did not show a progressively increased time to final LBP follow-up as expected, we suspect this may be due to limitations in using the CCI as a proxy to reflect medical complexity rather than the true effect of comorbidity and complexity. The CCI is most frequently used to evaluate risk for mortality and morbidity, and it has been shown that while the CCI can be used to predict overall healthcare use, other indices may perform better as a comorbidity adjustment [31].
We recognize several limitations to this study. While our outcome was time to final VHA LBP visit within one year of index as a representation of healthcare service use, this should not be interpreted as a proxy for improvement or resolution of LBP. There are many reasons why an individual may start or stop receiving VHA care for LBP, including improvement of their LBP condition or transition to receiving VHA community care or non-VHA care. Receiving or not receiving healthcare for LBP also does not necessarily reflect the presence or absence of LBP, especially in the context of encouraged self-management and the episodic nature of LBP as a recurring and remitting condition (including the possibility of multiple episodes within the course of a single year). We also only included care received within one year after index to reflect a “new” episode of LBP, but this may not account for continued LBP care occurring after one year for the same episode. Future work, including prospective studies, should further examine the impacts of timing of NPM use on follow up care and other health services outcomes (such as total healthcare service use for LBP within a period after onset) and markers of pain intensity and function and other patient clinical outcomes (such as validated patient reported outcome measure scores).
Conclusion
Overall, this study provides favorable evidence that earlier use of NPM for LBP may be beneficial in shortening the duration of follow-up care. Future work should consider mechanisms by which this may occur to optimize LBP care.
Declarations of Competing Interests
The authors have no conflicts of interest, financial or otherwise, to disclose.
Acknowledgments
Funding
The authors report funding from the National Center for Complementary & Integrative Health of the National Institutes of Health under Award Number K08AT011570 (Coleman, PI), the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development IIR-16-262 (Goulet, PI) and CIN-13-407, and the Palmer College Foundation (Lisi, PI). This work was completed with resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT.
Acknowledgments
The contents of this manuscript represent the view of the authors and do not necessarily reflect the position or policy of the U.S. Department of Veterans Affairs, the National Institutes of Health, nor the United States Government.
Footnotes
FDA device/drug status: Not applicable.
Author disclosures: BC: Grant: NCCIH (F, Paid directly to institution), NCMIC Foundation (B, Paid directly to institution). AL: Grant: Palmer College Foundation (F, Paid directly to institution). EA: Nothing to disclose. TR: Nothing to disclose. JG: VA Health Services Research and Development (I, Paid directly to institution).
Ethics Approval: This study was approved by the Institutional Review Board of the VA Connecticut Healthcare System.
Availability of Data and Materials: To maximize protection security of Veterans’ data while making these data available to researchers, the US Department of Veterans Affairs (VA) developed the VA Informatics and Computing Infrastructure (VINCI). VA researchers must log onto VINCI via a secure gateway or virtual private network connection (VPN) and use a virtual workspace on VINCI to access and analyze VA data. By VA Office of Research and Development policy, VINCI does not allow the transfer of any patient-level data out of its secure environment without special permission. Researchers who are not VA employees must be vetted and receive “without compensation” (WOC) employee status to gain access to VINCI. All analyses performed for this study took place on the VINCI platform. For questions about data access, contact study lead, Dr. Brian C. Coleman (Brian.Coleman2@va.gov) or the VA Office of Research and Development (VHACOORDRegulatory@va.gov).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.xnsj.2023.100233.
Appendix. Supplementary materials
References
- 1.St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88(1):56–67. doi: 10.1016/j.mayocp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. Pain. 2016;157(8):1696–1703. doi: 10.1097/j.pain.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qaseem A, Wilt TJ, McLean RM, Forciea MA. Clinical Guidelines Committee of the American College of P. noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514–530. doi: 10.7326/M16-2367. [DOI] [PubMed] [Google Scholar]
- 4.Tick H, Nielsen A, Pelletier KR, et al. Evidence-based nonpharmacologic strategies for comprehensive pain care: the consortium pain task force white paper. Explore (NY) 2018;14(3):177–211. doi: 10.1016/j.explore.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Fritz JM, Cleland JA, Brennan GP. Does adherence to the guideline recommendation for active treatments improve the quality of care for patients with acute low back pain delivered by physical therapists? Med Care. 2007;45(10):973–980. doi: 10.1097/MLR.0b013e318070c6cd. [DOI] [PubMed] [Google Scholar]
- 6.Ojha HA, Wyrsta NJ, Davenport TE, Egan WE, Gellhorn AC. Timing of physical therapy initiation for nonsurgical management of musculoskeletal disorders and effects on patient outcomes: a systematic review. J Orthop Sports Phys Ther. 2016;46(2):56–70. doi: 10.2519/jospt.2016.6138. [DOI] [PubMed] [Google Scholar]
- 7.Acharya M, Chopra D, Smith AM, Fritz JM, Martin BC. Associations between early chiropractic care and physical therapy on subsequent opioid use among persons with low back pain in Arkansas. J Chiropr Med. 2022;21(2):67–76. doi: 10.1016/j.jcm.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazis LE, Ameli O, Rothendler J, et al. Observational retrospective study of the association of initial healthcare provider for new-onset low back pain with early and long-term opioid use. BMJ Open. 2019;9(9) doi: 10.1136/bmjopen-2018-028633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannes ZL, Stohl M, Fink DS, et al. Non-pharmacological treatment for chronic pain in US veterans treated within the veterans health administration: implications for expansion in US healthcare systems. J Gen Intern Med. 2022;37(15):3937–3946. doi: 10.1007/s11606-021-07370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerns RD, Philip EJ, Lee AW, Rosenberger PH. Implementation of the veterans health administration national pain management strategy. Transl Behav Med. 2011;1(4):635–643. doi: 10.1007/s13142-011-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Department of Veterans Affairs . US Department of Veterans Affairs; Washington, D.C: 2009. VA Directive 2009-053 - Pain Management. [Google Scholar]
- 12.Becker WC, Dorflinger L, Edmond SN, Islam L, Heapy AA, Fraenkel L. Barriers and facilitators to use of non-pharmacological treatments in chronic pain. BMC Fam Pract. 2017;18(1):41. doi: 10.1186/s12875-017-0608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Justice AC, Erdos J, Brandt C, Conigliaro J, Tierney W, Bryant K. The veterans affairs healthcare system: a unique laboratory for observational and interventional research. Med Care. 2006;44(8 Suppl 2):S7–12. doi: 10.1097/01.mlr.0000228027.80012.c5. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Cain KC, Harlow SD, Little RJ, et al. Bias due to left truncation and left censoring in longitudinal studies of developmental and disease processes. Am J Epidemiol. 2011;173(9):1078–1084. doi: 10.1093/aje/kwq481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2022. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 17.Therneau T. A Package for Survival Analysis in R. R package version 3.4-0. Accessed March 14, 2023. https://CRAN.R-project.org/package=survival.
- 18.Goulet JL, Brandt C, Crystal S, et al. Agreement between electronic medical record-based and self-administered pain numeric rating scale: clinical and research implications. Med Care. 2013;51(3):245–250. doi: 10.1097/MLR.0b013e318277f1ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the veteran's affairs health factors dataset, an electronic data source. Nicotine Tob Res. 2011;13(12):1233–1239. doi: 10.1093/ntr/ntr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen MI, Martino S, Sellinger J, Lazar CM, Fenton BT, Mattocks K. Access to pain care from compensation clinics: a relational coordination perspective. Fed Pract. 2020;37(7):336–342. [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Healthy Weight: About Adult BMI. Centers for Disease Control and Prevention. Published 2017. Accessed March 14, 2023. https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html.
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 24.Stevans JM, Delitto A, Khoja SS, et al. Risk factors associated with transition from acute to chronic low back pain in US patients seeking primary care. JAMA Netw Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2020.37371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schappert SM, Burt CW. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001-02. Vital Health Stat. 2006;13(159):1–66. [PubMed] [Google Scholar]
- 26.Wijnhoven HA, de Vet HC, Picavet HS. Sex differences in consequences of musculoskeletal pain. Spine (Phila Pa 1976) 2007;32(12):1360–1367. doi: 10.1097/BRS.0b013e31805931fd. [DOI] [PubMed] [Google Scholar]
- 27.Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and pain perception - part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain. 2012;153(3):619–635. doi: 10.1016/j.pain.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 28.Milani CJ, Rundell SD, Jarvik JG, et al. Associations of race and ethnicity with patient-reported outcomes and health care utilization among older adults initiating a new episode of care for back pain. Spine (Phila Pa 1976) 2018;43(14):1007–1017. doi: 10.1097/BRS.0000000000002499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nieminen LK, Pyysalo LM, Kankaanpaa MJ. Prognostic factors for pain chronicity in low back pain: a systematic review. Pain Rep. 2021;6(1):e919. doi: 10.1097/PR9.0000000000000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wammes JJG, van der Wees PJ, Tanke MAC, Westert GP, Jeurissen PPT. Systematic review of high-cost patients' characteristics and healthcare utilisation. BMJ Open. 2018;8(9) doi: 10.1136/bmjopen-2018-023113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dominick KL, Dudley TK, Coffman CJ, Bosworth HB. Comparison of three comorbidity measures for predicting health service use in patients with osteoarthritis. Arthritis Rheum. 2005;53(5):666–672. doi: 10.1002/art.21440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.