Abstract
Hypertension is a primary modifiable risk factor for CVD, whereby even small reductions in blood pressure (BP) can decrease risk for CVD events. Modification of dietary patterns is an established, nonpharmacologic approach for the prevention and management of hypertension. Legumes are a prevailing component of dietary patterns associated with lower BP in observational research, but there is a need to understand the effects of legume consumption on BP. This study aimed to synthesize evidence from randomized controlled trials (RCTs) for the effects of non-oil seed legume consumption on systolic blood pressure (SBP) and diastolic blood pressure (DBP) (PROSPERO registration: CRD42021237732). We searched CINAHL, Cochrane, Medline, and PubMed scientific databases from inception through November 2022. A random-effects meta-analysis was conducted to assess the mean differences (MDs) for each outcome variable between legume-based and comparator diets. This review included 16 RCTs and 1092 participants. Studies ranged in duration (4–52 wk), participant age (17–75 y), and weekly legume dose (450–3150 g) in whole or powdered form. No significant overall effect between legume consumption and BP amelioration was observed in the meta-analysis (SBP—MD: −1.06 mm Hg; 95% CI: −2.57, 0.4410 mm Hg; I2 = 45%; DBP—MD: −0.48 mm Hg; 95% CI: −1.06, 0.10 mm Hg; I2 = 0%). The certainty of evidence was determined as low for SBP and DBP. Significant subgroup differences in SBP were found when studies were grouped according to participant BMI, with SBP reduction found for participants with overweight/obese BMI (MD −2.79 mm Hg, 95% CI: −4.68, −0.90 mm Hg). There is a need for large, high-quality trials to clearly define the benefits and mechanisms of legume consumption in BP management. Consideration of the relevance in individuals with obesity, overweight, and hypertension may also be warranted.
This trial was registered at PROSPERO as CRD42021237732.
Keywords: legume, blood pressure, hypertension, systematic reviews, meta-analysis, dietary pulses, dietary guidelines
Statement of Significance.
Legumes are a prevailing component of healthy dietary patterns shown to ameliorate blood pressure. To our knowledge, this is the most recent and comprehensive review to synthesize and critically evaluate this aggregate body of evidence, with consideration of subgroup populations, intervention dose, form, and duration and highlights the future research needed in individuals with obesity, overweight, and hypertension.
Introduction
Hypertension [systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg] is considered the primary modifiable, metabolic risk factor for CVD [1]. Elevated blood pressure (BP) accounts for almost a quarter of the population-attributable fraction for CVD and mortality in countries worldwide, regardless of the income level [2]. Hypertension attributes to 54% of all stroke incidences and 47% of all coronary artery disease (CAD) [3]. Studies show that a reduction in BP can rapidly decrease CAD risk by a quarter, notwithstanding the presence or absence of existing CVD or elevated BP [4]. A 10-mm Hg decrease in SBP or 5-mm Hg decrease in DBP correlates with a reduction in both stroke (30%) and heart failure (25%) risks [4]. In normotensive individuals, even a 2-mm Hg decrease in normal SBP is associated with a risk reduction for stroke (10%) and ischemic heart disease (IHD) (7%) [5,6]. Considering its effect on health, exacerbated by increasing prevalence with age and obesity [7], elevated BP has emerged as a principal area of interest for the health of populations and a leading contributor to the global burden of diseases [5,8].
Although there are numerous pharmacologic treatments for hypertension, there is substantial evidence supporting the modification of dietary patterns and specific nutritional elements for the prevention and management of BP [9,10]. Indeed, the dietary approach may not only be more accessible and affordable than pharmacologic interventions but also potentially have far reaching health benefits associated with optimal BP control [6,11]. For instance, the Mediterranean diet and DASH diet are low in energy density, rich in fruits, vegetables, low-fat dairy, legumes, and nuts, and significantly lower in sodium content in comparison with a Western diet [12]. These dietary characteristics are paralleled by the increased concentrations of protective nutrients such as fiber, magnesium, and potassium [[12], [13], [14]]. The BP-lowering effects of such diets have been elucidated as an effective nutritional strategy for hypertension [12,15], shown to reduce SBP by 7.1 mm Hg [16] and DBP by 2.6 mm Hg [12], and may be further enhanced by macronutrient manipulation, such as the substitution of carbohydrates for protein [17]. Similarly, the New Nordic Diet, a plant-based diet that promotes the consumption of whole grains, legumes, fruits, and vegetables, has been shown to significantly decrease BP (SBP by 5.1 mm Hg and DBP by 3.2 mm Hg) [18,19].
To inform dietary guidelines and other dietary strategies, it is valuable to understand the key components of these diets and their potential for BP management. As a prevailing component of healthy dietary patterns, legumes are a rich source of nutrients that are associated with the amelioration of high BP [[20], [21], [22]], including lower GI carbohydrate, potassium, magnesium, folate, polyphenols, unsaturated fatty acid [22,23], and dietary fiber (soluble, insoluble, and resistant starch) concentrations [24]. Evidence indicates that a 17-g daily increase in dietary fiber correlates with a significant reduction in total BP (1.15 mm Hg in SBP and 1.65 mm Hg in DBP) [25]. Furthermore, legumes are a rich source of plant protein with a unique amino acid profile assumed to impart significant BP-lowering effects, such as vasodilation, owing to their high arginine content [26,27] and angiotensin-converting enzyme inhibitory activity [28]. A secondary analysis of data from the National Health and Nutrition Examination Survey 1999–2002 determined that regular consumption of a variety of legumes provided consumers with a favorable nutrient intake and improved satiety, concurrent with a reduced risk for obesity, hypertension, and hypertension-related disorders such as CVD and CAD [29].
The findings of a recent systematic review and meta-analysis of prospective cohort studies evaluating the association between legumes and cardiometabolic diseases, supports the promotion of increased legume consumption for the prevention of such conditions. However, the study concluded that the available evidence is, at best, weak and the pivotal significance of the effects of legume intake on BP, inconclusive [30].
This review builds on a previous systematic review and meta-analysis of controlled feeding trials, which evaluated 8 randomized controlled trials (RCTs), published between 2009 and 2012, examining the effects of non-oil seed (NOS) legumes on BP [31]. This previous review concluded that although there was significant evidence for the promotion of legume consumption in the amelioration of elevated BP, there were a multitude of limitations to the included studies, highlighting a need for superior quality, large-scale trials to consolidate these findings [31]. There are numerous critical challenges that undermine the translatability of dietary intervention findings into clinical practice, such as study design, methodology, high clinical heterogeneity, inadequacy of outcome measures, and low adherence rates [32]. Therefore, this study aimed to examine all available evidence from RCTs on the effects of the consumption of NOS legumes on BP and to describe the certainty of evidence base. This may serve to provide evidence for the development of future dietary guidelines. To our knowledge, this is the most recent review to synthesize and critically evaluate this aggregate body of evidence, with consideration to population subgroups, intervention dose, form, and duration.
Methods
Study protocol
This systematic review and meta-analyses followed the Cochrane Handbook for Systematic Reviews of Interventions guidelines [33] and is reported in accordance with the PRISMA guidelines [34]. The protocol was preregistered in the International Prospective Register of Systematic Reviews (PROSPERO) [35] (https://www.crd.york.ac.uk/prospero; registration number CRD42021237732.
A systematic search of 4 scientific databases CINAHL (through EBSCO), Medline (through EBSCO), PubMed, and Cochrane CENTRAL was conducted from their inception to 3 November, 2022, to identify published interventions that examined the effect of NOS legume consumption on BP in adults. Free-text search terms and relevant controlled vocabulary terms that related to legumes and BP were used (lupin OR legume OR bean OR lentil OR chickpea OR mung OR pea OR non-oil seed legumes OR lens culinaris OR cicer arietinum OR garbanzo OR phaseolus vulgaris) AND (blood pressure OR diastolic pressure OR pulse pressure OR systolic pressure OR hypertension OR arterial pressure OR aortic pressure OR aortic tension OR arterial tension). Full search terms are reported in Supplemental Table 1.
Inclusion criteria
Eligible studies were required to meet the inclusion criteria as follows: 1) RCTs (parallel and crossover) that investigated the effects of NOS legume consumption on BP; 2) human participants (males and/or females) aged ≥18 y; 3) legumes consumed in whole form (sprouted, cooked, and raw) or powders/flours where all components of the legume are contained; 4) intervention duration of ≥3 weeks, considered the minimum period required for evaluating the effect of an intervention [36]; 5) measured BP (SBP and DBP); and 6) published in English.
Exclusion criteria
Exclusion criteria were applied as follows: 1) intervention involves oil seed legumes (soy and peanut) or the isolated components of legumes (fiber, protein, and isoflavones) owing to differences in their physicochemical properties, nutritional profile, and potential for confounding effects on BP; 2) pre-existing renal disease (chronic kidney disease stages 3–4); and 3) trials where consumption of legumes could not be isolated as the intervention, within the context of healthy dietary patterns such as Mediterranean or DASH diets. There were no restrictions regarding other pre-existing conditions, BMI, or ethnic background. Although a previous systematic review was conducted through to 2012, no restriction was set on the date of publication to ensure all relevant studies could be synthesized in this review.
Study selection
All identified articles were exported to Covidence (Covidence System Review Software; 2019; Veritas) for screening and full-text review of articles. After the removal of duplicates, screening based on title and abstract was conducted by 2 independent authors (GLR and EJB), against the predefined eligibility criteria. In the case that an abstract was not available or insufficient, the full text was retrieved and examined to enable the reviewer to decide on the article’s eligibility. Remaining articles were progressed for full-text review, conducted independently by 2 authors (GLR and EPN), for the analysis against eligibility criteria. Articles were further assessed for duplication across study population, and where trial results had been reported across multiple studies, only those reporting eligible outcomes were included. In addition, the reference lists of eligible articles were reviewed for potentially relevant articles. Conflicts regarding inclusion/exclusion of an article were resolved through consensus.
Data extraction
Data extraction was performed by a single author (GLR) in consultation with the research team and tabulated as follows: author, published year, country, and funding; study design (crossover/parallel and blinding) and analysis (intention-to-treat); duration; primary outcome; sample size and attrition; inclusion criteria; population (sex and age); details of intervention (legume type, form, and dose) and comparator diets; dietary assessments used; and BP measurement. Where legume dose was reported as a serve or cup, these values were converted to grams per week. Attempts to contact study authors were made to seek clarification or where data were missing from the publication.
Quality assessment and risk of bias
The methodologic quality of each included studies was independently assessed by 2 authors (GLR and EJB), using version 2 of the Cochrane risk of bias tool for randomized trials (RoB2.0) [37]. Final assessments were based on consensus between the authors.
Statistical analysis
A meta-analysis was conducted on each outcome variable (SBP and DBP) using RevMan5 [Review Manager (RevMan); Computer program; version 5.4.1; The Cochrane Collaboration, 2020]. The effect size was reported as the mean difference (MD) and 95% CIs. Data were extracted as mean change and SD where available. The final mean values and corresponding SDs were used where change was not available. Where SDs were not reported, the values were derived from the 95% CIs [[38], [39], [40]] and standard error [[41], [42], [43]] using the RevMan calculator [44]. In the case that a study included multiple legume intervention groups, these intervention groups were combined using the RevMan calculator. Owing to the broad variability in study methodology, random-effects models were applied to calculate the pooled effect size. Crossover trials were initially included in the meta-analysis, in the same way as parallel trials, by comparing measurements from the intervention periods with the control periods. Although this approach results in a unit-of-analysis error, it is considered a conservative approach [33]. In addition, sensitivity analyses were conducted using a paired analysis of crossover trials with a range of correlation coefficients (0.25, 0.5, and 0.75), to explore whether crossover studies were underweighted. Finally, subgroup analyses were conducted to explore the effect of legume consumption on BP in crossover trials compared with that in parallel trials. Statistical significance was set at P ≤ 0.05, and between-study heterogeneity was assessed using I2 and categorized according to Cochrane guidelines: low heterogeneity (I2 = 0% to 40%); moderate heterogeneity (I2 = 30% to 60%); substantial heterogeneity (I2 = 50% to 90%); and considerable heterogeneity (I2 = 75% to 100%) [33].
Sources of heterogeneity were explored by sensitivity and subgroup analyses. Sensitivity analyses (leave-one-out method) were performed to estimate the influence of each individual study on the overall pooled effect by omitting 1 study at a time with a recalculation of the summary of estimates. To investigate sources of heterogeneity, post hoc subgroup analyses were undertaken for study design (parallel/crossover), participant characteristics (sex and age), underlying health status [overweight/obese, type 2 diabetes mellitus (T2DM)], dietary intervention (hypocaloric and legume type and form, dose, and duration). Publication bias was investigated by the visual inspection of funnel plots and formal testing with the Egger's test [45].
Certainty assessment
The certainty of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach [46]. Evidence was graded by 2 independent authors (GLR and KL). The body of evidence of RCTs was graded as high level of evidence by default and downgraded based on the following 5 prespecified criteria: 1) risk of bias (ROB) (assessed by Cochrane RoB2.0 [37]); 2) inconsistency of results (substantial, unexplained and within-study heterogeneity); 3) indirectness of evidence (external validity and limited generalizability); 4) imprecision (small sample size and wide CIs); and 5) publication bias (significant evidence of small study effects).
Results
Study selection
Figure 1 presents the PRISMA diagram of the full systematic search and selection of literature. Of the 4825 records identified from databases and manual searches, 2231 duplicates were removed. Of the 2594 records retrieved for title and abstract screening, 2562 were excluded due to failure to meet the eligibility criteria. A full-text review was conducted on 32 records, of which a further 16 studies were excluded due to failure to meet the eligibility criteria. Finally, 16 studies were included in the systematic review and meta-analysis.
FIGURE 1.
Screening and selection of randomized controlled trials on legume intake and blood pressure.
Study characteristics
Table 1 provides a summary of study characteristics. Studies were conducted in Canada (38%) [39, [47], [48], [49], [50], [51]], Iran (31%) [[41], [42], [43],52,53], Australia (19%) [38,40,54], and Spain (13%) [55,56]. Among the trials, 5 studies applied a crossover design [41,43,47,51,54], whereas the remaining used a parallel design (n = 11; 69%) [[38], [39], [40],42,[48], [49], [50],52,53,55,56], and 5 studies incorporated some degree of blinding [38,47,49,51,54]. Study duration ranged from 4 [51] to 52 weeks [38], and most commonly, studies had an 8-week duration (n = 7; 44%) [41,47,50,52,[54], [55], [56]]. Nine studies used the intention-to-treat (ITT) approach to address bias in the original analysis [[38], [39], [40], [41],43,47,49,53,54], whereas the remaining studies did not detail participant numbers in the final analyses. All but 2 studies [42,56] explained the method of BP measurement used, with most using sphygmomanometer. Ten studies measured BP after a period of rest (range: 5–15 min) [39,41,43,47,48,50,[52], [53], [54], [55]], and 8 studies reported BP as a mean of 2 or more measures (range: duplicate to quadruplicate) [39,41,48,[50], [51], [52], [53], [54]]. Of the 16 included studies, BP was reported as the primary outcome variable in only 2 studies [40,53].
TABLE 1.
Study characteristics of the included randomized controlled trials examining the effect of legume intake on blood pressure
| First author (year), country, funding | Study design (analysis) | Study duration (wk) | Primary outcome | Sample size (attrition) | Inclusion criteria | Sex, age1 (y) | Intervention |
Comparator diet | Dietary assessment | BP measure | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet | Type, form | Dose (g/wk) | ||||||||||
| Abete et al. (2009) [55], Spain, Government, University | Parallel (not reported) | 8 | Weight loss |
N = 182 I: 8; C: 10 (0%) |
Male, OB | 0% F 38 ± 7 |
Hypocaloric + high legume intake | Mixed4, Whole5 | 400–6006 | Hypocaloric + animal protein. Lean meats, eggs, skimmed dairy. Omit legumes, fatty fish. Hypocaloric: −30% EER |
3-d weighted FR (weeks 1 and 7); weekly session with dietician | Sphyg, seated before 5 min |
| One serve legumes, 4 d/wk. Avoid fatty fish, decrease animal protein. Hypocaloric: −30% EER | ||||||||||||
| Other diet treatments3 Hypocaloric diet with fatty fish | ||||||||||||
| Hypocaloric high-protein diet | ||||||||||||
| Abeysekara et al. (2012) [47], Canada, Govt., Saskatchewan Pulse Growers | Crossover, SB, 4-wk washout (ITT analysis) | 8 | LDL-C | N = 87 (24%) | ≥50 y, inactive | 65% F7 | Legume-enriched diet | Mixed4 | 1750 | Usual diet | 110-item FFQ weeks 0 and 8; daily food log | Seated before 5 min |
| 59.7 ± 6.3 | 2 serves (250 g wet weight) of legumes daily as per supplied snacks, salads, soups, and meals. Rotated legume type | Whole5 | ||||||||||
| Alizadeh et al. (2014) [42], Iran, University | Parallel (not reported) | 6 | MetS | N = 34 | 20–50 y, female, OB | 100% F | Hypocaloric diet enriched with legumes; Substitute meat with 2 serves (1 cup) legumes daily. Hypocaloric: −500 kcal/d | Mixed4 | 16808 | Hypocaloric diet without legumes (HDWL) | 3-d FR (weeks 0, 3, and 6); weekly sessions with a nutritionist | Not reported |
| I: 17; C: 17 (19%) | 36.1 ± 1.4 | Whole5 | Omit legumes. Increase animal protein 2 serves (60 g)/d. Hypocaloric: −500 kcal/d | |||||||||
| Belski (2011) [38], Australia, Govt | Parallel, DB (ITT analysis) | 52 | Weight loss |
N = 93 I: 46; C: 47 (29%) |
20–71 y, OW/OB | 44% F7 46.6 ± 9.7 |
Energy-restricted diet with lupin flour enriched | Lupin kernel flour9 | 35010 | Energy-restricted diet with wheat flour | Fortnightly sessions with dietician for initial 12 wk. Weekly 3-d FR | Sphyg, mean 24-h ABPM |
| Substitute products with supplied lupin kernel–enriched (25% to 40% lupin flour) breads, biscuits, pasta. | Substitute usual products with supplied whole-wheat flour breads, biscuits, pasta | |||||||||||
| Hypocaloric: −35% EER (12 wk) | Hypocaloric: −35% EER (12 wk) | |||||||||||
| Gravel et al. (2010) [48], Canada, Pulse Canada | Parallel (ITT analysis) | 16 | MetS | N = 114 | 30–65 y, female, ≥2 metabolic risk factors | 100% F | Legume-based meals | Mixed4 | 57011 | Legume-free meals | Intake checklist, nutrient analysis; 3-d FR (weeks 0, 8, and 16) | Rested before 5 min, mean of 3 |
| I: 60; C: 54 (14%) | 47.5 ± 17.5 | 5 legume-based meals (750 mL)/wk, ad libitum. Supplied meals isocalorically matched to control meals | Whole5 | 5 legume-free meals/wk, ad libitum | ||||||||
| Hassanzadeh-Rostami et al. (2019) [52], Iran, University | Parallel (not reported) | 8 | Cardio-MetS |
N = 43 I: 20; C: 23 (14%) |
40–65 y, T2DM | 72% F7 52.5 ± 12.5 |
Legume-based weight maintenance diet | Mixed4 Whole5 |
45013 | Red meat-based weight maintenance diet5 Two serves (60 g) red meat, 3 d/wk. Nonintervention days: eat chicken or fish, omit red meat, legumes |
Weekly phone session; monthly visit; daily food checklist; 24-h FR (weeks 0, 4, and 8) | Sphyg, seated before 15 min, mean of 2 |
| Replace meat with 2 serves (1 cup) legumes, 3 d/wk. | ||||||||||||
| Nonintervention days: eat chicken or fish, omit red meat, legumes | ||||||||||||
| Other diet treatment3 | ||||||||||||
| Soybean-based weight maintenance diet | ||||||||||||
| Hermsdorff et al. (2011) [56], Spain, Govt., University | Parallel (not reported) | 8 | Plasma CRP |
N = 30 I: 15; C: 15 (0%) |
OW/OB | 43% F7 36.0 ± 8 |
Legume-based energy-restricted diet (n = 15) | Mixed4 Whole5 |
640–94014 | Legume-free energy-restricted diet (n = 15) | 3-d weighed FR (weeks 0 and 8); weekly dietician session | As per WHO criteria [86] |
| 7-d legume-based menu with 4 serves legumes/wk. | 7-d legume-free menu. | |||||||||||
| Hypocaloric: −30% EER/d | Hypocaloric: −30% EER/d | |||||||||||
| Hosseinpour-Niazi (2015) [41], Iran, University | Crossover,4-wk washout (ITT analysis) | 8 | LDL-C | N = 31 (22%) | 40–75 y, T2DM, OW | 77% F7 | Legume-based, energy-reduced, TLC15 diet | Mixed4 | 45013 | Legume-free energy-reduced, TLC15 diet | Weekly 3-d FR and dietician sessions | Sphyg, seated before 15 min, mean of 2 |
| 58.1 ± 6.0 | TLC diet, replace 2 serves red meat with variety of legumes (1 cup) on 3 d/wk | Whole5 | Omit legumes | |||||||||
| Hosseinpour-Niazi (2022) [53], Iran, University | Parallel (ITT analysis) | 16 | Blood pressure |
N = 300 I: 150; C: 150 (5%) |
30–65 y, T2DM, OW/OB | 57% F 7 55.4 ± 7.0 |
Legume-based DASH diet | Mixed4 Whole5 |
>550–62516 | Traditional DASH diet Hypocaloric: −500 kcal/d |
Fortnightly 3-d FR by dietician | Sphyg, rested before 15 min, mean of 2 |
| DASH diet, substitute 1 serve red meat with 1 serve legumes ≥5 d/wk, reduce 1 serve bread on those days. | ||||||||||||
| Hypocaloric: −500 kcal/d | ||||||||||||
| Jenkins et al. (2012) [39], Canada, PURENet, Saskatchewan Pulse Growers | Parallel (ITT analysis) | 12 | HbA1c | N = 121 | T2DM, HbA1c: 6.5% to 8.5% | 50% F7 | Low-GI legume-based diet | Mixed4 | 1330 | High wheat fiber diet | 7-d FR (weeks 0, 8, 10, and 12) | Sphyg, seated, mean of 3 |
| I: 60; C: 61 (8%) | 59.5 ± 9.0 | Implement food checklist (15 g carbohydrate portions), consume 1 cup (190 g) legumes daily | Whole5 | Whole-wheat carbohydrate foods (cereals, breads, and brown rice) | ||||||||
| Kazemi et al. (2018) [49], Canada, Govt., University, Saskatchewan Pulse Growers | Parallel, SB (ITT analysis) | 16 | Insulin resistance | N = 61 | 18–35 y, female, PCOS | 100% F | Low-GI legume-based diet | Mixed4 | 1260–3150 | TLC15 diet | 24-h diet recall (weeks 0 and 16) | Sphyg and stethoscope |
| I: 31; C: 30 (36%) | 26.5 ± 8.5 | TLC diet for breakfast, snacks. Supplied legume-based meals (soups, salads, and mains) for lunch, dinner daily. 90–225 g17 legumes/meal | Whole5 | Lean meat, poultry, and low-fat milk as protein sources. Omit legumes | ||||||||
| Lee et al. (2009) [40], Australia, not reported | Parallel (ITT analysis) | 16 | Blood pressure | N = 74 | 20–70 y, OW/OB | 65% F | Lupin kernel flour-enriched bread–based diet Substitute 15% to 20% daily energy intake with supplied lupin kernel–enriched flour bread (4 × 40 g slice) |
Lupin kernel flour9 | 94218 | White bread–based diet | Daily FR assessed by dietician fortnightly; modified FFQ (weeks 0 and 16) | Sphyg, mean 24-ABPM |
| I: 37; C: 37 (16%) | 57.9 ± 8.0 | Substitute 15% to 20% daily energy intake with supplied white bread (4 × 40 g slice) | ||||||||||
| Mollard et al. (2012) [50], Canada, Govt. | Parallel (not reported) | 8 | Risk factors for MetS |
N = 40 I: 19; C: 21 (9%) |
35–55 y, OW/OB | 72% F7 45 ± 10 |
Legume-enriched ad libitum diet | Mixed4 Whole5 |
89619 | Dietary counseling to reduce energy intake Individualized hypocaloric (−500 kcal/d) diet, portion control, reduced fat, sugar, alcohol. Increased fruit, vegetables | FR (weeks 1, 4, and 8); log of gastrointestinal discomfort | Rested before 5 min, mean of 2 |
| 5 supplied legume-based meals (salad, soup, and side dish) included into usual weekly diet, ad libitum. | ||||||||||||
| 1 cup legumes/meal. | ||||||||||||
| Hypocaloric: none | ||||||||||||
| Saraf-Bank et al. (2016) [43], Iran, University | Crossover, 2-wk washout (ITT analysis) | 6 | Serum lipid profile | N = 26 (0%) | First-degree relatives with diabetes | 54% F7 | Legume-enriched diet | Pinto bean, lentil Whole5 |
69320 | Usual diet | 24-h FR, weeks 0, 2, 4, and 6 | Sphyg, seated before for 5 min |
| 50 ± 6.6 | Four packages (240 g) legumes/wk ad libitum as part of usual diet + recommendations to improve diet and lifestyle | Recommendations to improve diet and lifestyle | ||||||||||
| Veenstra et al. (2010) [51], Canada, Pulse Canada, Saskatchewan Pulse Growers | Crossover, DB, PC, 4-wk washout (not reported) | 4 | Gastrointestinal function | N = 19 (26%) | 19–40 y, male, BMI 20–30 kg/m2 | 0% F | Dehydrated legume powder | Mixed4 | 70021 | Dehydrated potato flake | Daily food diary, return empty treatment packages | Mean of 2 |
| 28.1 ± 5.9 | Rehydrate legume powder (dw:100 g), incorporate into usual diet/foods, consume as a single serve | Spray-dried powder | Rehydrate potato flake (dw: 50 g/d), incorporate into usual diet/foods, consume as a single serve | |||||||||
| Ward et al. (2020) [54], Australia, RPH research foundation | Crossover, DB, 8-wk washout (ITT analysis) | 8 | Glycaemic control | N = 17 (23%) | 40–70 y, T2DM (HbA1c ≤9%), BMI 18–35 kg/m2 | 36% F | Energy-matched lupin kernel–enriched foods | Lupin kernel flour9 | 315 | Energy-matched wheat-based foods | 7-d FR for study duration; FFQ at the end of each diet treatment | A&D digital monitor, rested before 5 min, mean of 4 (mane, nocte) |
| 58.0 ± 6.6 | Substitute 20% daily energy intake with study foods at breakfast, lunch daily, and dinner 3 d/wk (cereal, pasta, and bread) equivalent to ∼45 g lupin kernel/d | Substitute 20% daily energy intake with study foods at breakfast, lunch daily, and dinner 3 d/wk (cereal, pasta, and bread) | ||||||||||
ABPM, ambulatory blood pressure monitoring; BP, blood pressure; C, control; DB, double blind; DW, dry weight; EER, estimated energy requirement; FR, food record; Govt., government; I, intervention; ITT, intention-to-treat; MetS, metabolic syndrome; OB, obesity; OW, overweight; PC, placebo controlled; PCOS, polycystic ovarian syndrome; SB, single blind; SBP, systolic blood pressure; Sphyg, sphygmomanometer; T2DM, type 2 diabetes mellitus; TLC, therapeutic lifestyle change.
12Value refers to the total number of participants in the control and nonsoy legume groups. Participants in soy legume group have been excluded for irrelevance (total participants across 3 diet treatments, n = 75).
Age is presented as mean ± SD.
Value refers to the total number of participants in the control and legume-based groups. Participants in other diet groups were excluded for irrelevance (total participants across 4 diet treatments, n = 35).
Cointerventions have been listed; however, they were not included in the comparison or analysis in this review.
The term mixed encompassed a variety of legumes, where the study authors did not specify.
The term whole encompassed legumes in the following forms: whole, cooked, or canned and incorporated directly into meal/diet.
Dose provided in serves and converted to grams, as derived from an estimation provided by study authors [55], where 1 serve is equivalent to 100–150 g cooked legumes. Value is the approximated average, over a 1-wk period (4 serves/wk).
Final percentage of female participants analyzed at the end point.
Dose provided in cups and converted to grams, as derived from an estimation provided by study authors [42], where 1 cup is equivalent to 240 g cooked legumes. Value is approximated over a 1-wk period (7 cups/wk).
The term flour refers to cooked and dehydrated legumes incorporated into flour for use in baked goods.
Value is an average weekly dose derived from an estimation provided by study authors [38] as >50 g lupin kernel/d (7 d/wk).
Dose provided in milliliters and converted to grams, as derived from an estimation provided by study authors [87] where 1 mL is equivalent to 0.76 g cooked legumes. Value is approximated over a 1-wk period (5 meals/wk).
Dose provided as a serve of legumes consumed as a meat substitute, where 1 serve or 1 cup was equivalent to 150 g [88].
Dose range provided as 160–235 g per serve in accordance with individual’s daily energy intake. Value is approximated over a 1-wk period (4 serves/wk).
The National Cholesterol Education Program recommends the TLC diet to reduce cardiometabolic risk factors [89].
Dose provided in serves converted to grams, as derived from an estimation provided by study authors [53], where 1 serve is equivalent to 110–125 g cooked legumes. Value is approximated over a 1-wk period (5 serves/wk).
Kazemi et al. [49] based the quantity of specific legumes, included in each meal, on values shown to reduce insulin, blood glucose, and lipid concentrations in previous studies.
Daily value derived from the study by Jayalath et al. [31] and calculated as a weekly sum.
Dose provided as a cup measure. Weekly gram value was derived from an estimation provided by the study authors [50] where 5 cups were equivalent to 896 g cooked legumes.
Dose provided as raw legumes (65 g/pack) and converted into cooked grams where 1 g raw legumes was equivalent to 2.66 g cooked legumes [56].
Value refers to the dry weight of spray-dried, powdered legumes (100 g/d), being calculated as a weekly dose.
Participant characteristics
Sixteen studies involved 1288 randomly assigned participants, of which 1092 participants were accounted for at the end point. Sample sizes ranged from 17 [54] to 300 [53] participants. Participant ages ranged from 18 to 75 y, and most studies included both males and females; 3 recruited only females [42,48,49], whereas 2 recruited only males [51,55]. Participant health status included overweight/obesity (n = 8; 50%) [38,[40], [41], [42],50,53,55,56], T2DM (n = 5; 31%) [39,41,[52], [53], [54]]; T2DM and overweight/obesity (n = 2) [41,53]; and healthy status (n = 2) [43,51].
Dietary interventions
The median duration of interventions was 8 weeks (range: 4–16 wk) with the exception of 1 study that continued for 52 weeks [38]. Although most of the interventions incorporated varied whole, cooked legumes, with a median weekly dosage of 700 g and a mean dosage of 1028 g (range: 450–3150 g), 3 studies used lupin kernel–enriched flour with a mean dose of 529 g (range: 30–924 g) [38,40,54]. One study tested the effect of 700 g/wk of 3 different dehydrated powdered legumes (chickpea, lentil, and green pea) against dehydrated potato flakes in the comparator diet [51] (Table 1). Most studies incorporated isocaloric foods or diets across both arms of the dietary treatment [[38], [39], [40], [41], [42],48,52,[54], [55], [56]], and 5 included energy restriction (hypocaloric) as a component of both dietary treatment groups [42,50,53,55,56]. A single study compared an ad libitum legume-based diet against a hypocaloric diet [50]. Three studies assessed the effects of enriching healthy dietary patterns with legumes, such as the therapeutic lifestyle change (TLC) diet [41,49] and DASH diet [53]. Six studies required participants to consume provided meals [38,40,[47], [48], [49],54], whereas 2 studies incorporated legumes into the usual diet [43,47] (Table 1).
ROB in individual studies
Overall, the ROB ranged from low to some concerns across the 16 included studies. Seven studies had a low ROB [38,43,47,49,51,53,54], with remaining 9 assessed as having some concerns [[39], [40], [41], [42],48,50,52,55,56], primarily owing to a lack of information for Domain 5, the analyses of results in accordance with prespecified analysis [37]. The Cochrane RoB2.0 assessment for each study (Supplemental Figure 1 and Supplemental Table 2) and the ROB graph (Supplemental Figure 2) are available in the Supplementary Material.
Effect of legume consumption on BP
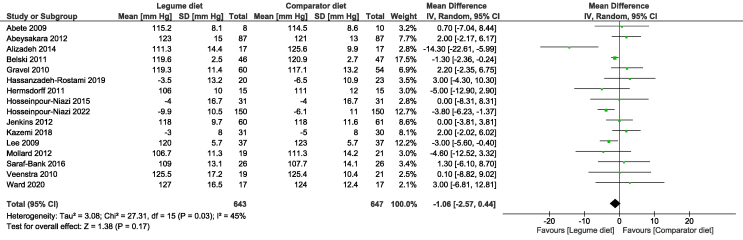
The meta-analysis of the included studies revealed that, relative to the comparator diet, legume consumption showed small reductions in both SBP (MD: −1.06 mm Hg; 95% CI: −2.57, 0.44) (Figure 2) or DBP (MD: −0.48 mm Hg; 95% CI: −1.06, 0.10) (Figure 3), although they did not reach a statistical significance. There was evidence of significant moderate heterogeneity in the analysis of SBP (I2 = 45%) and low, nonsignificant heterogeneity for DBP (I2 = 0%).
FIGURE 2.
Forest plot showing the mean difference and 95% CIs of the overall effect of legume intake on systolic blood pressure in 16 randomized controlled trials, pooled by using random-effects model.
FIGURE 3.
Forest plot showing the mean difference and 95% CIs of the overall effect of legume intake on diastolic blood pressure in 15 randomized controlled trials, pooled by using random-effects model.
Sensitivity and sensitivity analysis
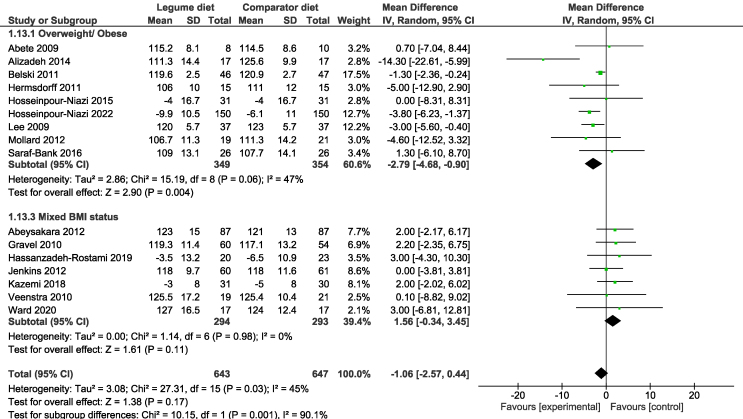
Sensitivity analyses of crossover trials, using correlation coefficients of 0.25, 0.5, and 0.75, found overall similar results to the primary analysis (Supplemental Table 3). Subgroup analyses were conducted examining study design, duration, legume type, form, dosage, participant sex, age, and health status. Significant subgroup differences in SBP were found when studies were grouped according to participant BMI (I2 = 90.1%), with reductions in SBP only found for participants with overweight/obese BMI (MD: −2.79 mm Hg; 95% CI: −4.68, −0.90) (Figure 4). Sensitivity analyses did not modify the effect or heterogeneity for pooled estimates for treatment effects on SBP nor DBP. The results of subgroup analyses, including test for subgroup differences, are summarized in Table 2.
FIGURE 4.
Forest plot showing the mean difference and 95% CIs of the subgroup analysis for the effect of legume intake on blood pressure in overweight, obese individuals, pooled by using the random-effects meta-analysis.
TABLE 2.
The results of subgroup analyses according to key participant and study characteristics
| Subgroup category | Subgroup | Systolic blood pressure |
Diastolic blood pressure |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of studies | No. of participants | Effect estimate MD (95% CI) (mm Hg) | Test for subgroup differences | No. of studies | No. of participants | Effect estimate MD (95% CI) (mm Hg) | Test for subgroup differences | ||
| Study design | Parallel | 11 | 928 | −1.63 (−3.40, 0.14) | χ2 = 3.17, df = 1; (P = 0.07), I2 = 68.5% | 10 | 888 | −0.63 (−1.25, 0.00) | χ2 = 2.54, df = 1; (P = 0.11), I2 = 60.6% |
| Crossover | 5 | 362 | 1.51 (−1.46, 4.48) | 5 | 362 | 0.94 (−0.88, 2.77) | |||
| Study blinding | With blinding | 5 | 402 | −0.08 (−1.88, 1.71) | χ2 = 1.61, df = 1; (P = 0.20), I2 = 37.8% | 5 | 402 | −0.63 (−2.18, 0.93) | χ2 = 0.60, df = 1; (P = 0.44), I2 = 0% |
| Without blinding | 11 | 888 | −1.92 (−4.11, 0.27) | 10 | 848 | 0.09 (−0.84, 1.03) | |||
| Study duration (wk) | 1–4 | 1 | 40 | 0.10 (−8.82, 9.02) | χ2 = 0.14, df = 3; (P = 0.99), I2 = 0% | 1 | 40 | −1.10 (−5.99, 3.79) | χ2 = 4.89, df = 3; (P = 0.18), I2 = 38.6% |
| 5–8 | 9 | 487 | −1.28 (−4.75, 2.19) | 8 | 447 | 0.97 (−0.69, 2.63) | |||
| 9–16 | 5 | 670 | −0.98 (−3.42, 1.45) | 5 | 670 | −0.20 (−1.17, 0.78) | |||
| >16 | 1 | 93 | −1.30 (−2.36, 0.24) | 1 | 93 | −1.00 (−1.81, −0.19) | |||
| Sample size | n < 50 | 9 | 353 | −1.76 (−5.32, 1.80) | χ2 = 0.18, df = 1; (P = 0.67), I2 = 0% | 8 | 313 | −0.03 (−2.03, 1.98) | χ2 = 0.07, df = 1; (P = 0.79), I2 = 0% |
| n ≥ 50 | 7 | 937 | −0.91 (−2.51, 0.69) | 7 | 937 | −0.32 (−1.23, 0.59) | |||
| Participant sex | Males and females | 11 | 1023 | −1.52 (−2.75, −0.29) | χ2 = 0.48, df = 2; (P = 0.79), I2 = 0% | 10 | 983 | −0.24 (−0.96, 0.49) | χ2 = 1.72, df = 2; (P = 0.42), I2 = 0% |
| Females only | 3 | 209 | −2.47 (−10.32, 5.38) | 3 | 209 | −1.67 (−3.74, 0.40) | |||
| Males only | 2 | 58 | 0.44 (−5.40, 6.29) | 2 | 58 | 0.23 (−3.74, 4.20) | |||
| Participant age | <40 y | 3 | 135 | −3.72 (−13.59, 6.16) | χ2 = 0.29, df = 2; (P = 0.86), I2 = 0% | 3 | 135 | −2.49 (−5.07, 0.10) | χ2 = 7.54, df = 2; (P = 0.02), I2 = 73.5% |
| ≥40 y | 8 | 860 | −0.98 (−2.97, 1.00) | 8 | 860 | 0.47 (−0.47, 1.41) | |||
| Mixed ages | 5 | 295 | −1.21 (−2.22, −0.19) | 4 | 255 | −0.93 (−1.70, −0.16) | |||
| BMI status | Overweight/obese | 9 | 703 | −2.79 (−4.68, −0.90) | χ2 = 10.15, df = 1; (P = 0.001), I2 = 90.1% | 8 | 663 | −0.56 (−1.20, 0.09) | χ2 = 0.19, df = 1; (P = 0.67), I2 = 0% |
| Mixed BMI | 7 | 587 | 1.56 (−0.34, 3.45) | 7 | 587 | −0.19 (−1.72, 1.34) | |||
| Diabetes status | With diabetes | 5 | 560 | −1.00 (−3.85, 1.85) | χ2 = 0.11, df = 2; (P = 0.95), I2 = 0% | 5 | 560 | −0.13 (−1.50, 1.24) | χ2 = 0.46, df = 2; (P = 0.79), I2 = 0% |
| Without diabetes | 6 | 369 | −1.39 (−2.39, −0.38) | 6 | 369 | −0.65 (−1.34, 0.04) | |||
| Unspecified | 5 | 361 | −1.90 (−6.95, 3.15) | 4 | 321 | −0.38 (−3.22, 2.46) | |||
| Legume type | Lupin, flour | 3 | 201 | −1.57 (−2.81, −0.34) | χ2 = 0.34, df = 1; (P = 0.56), I2 = 0% | 3 | 201 | −0.46 (−1.64, 0.72) | χ2 = 0.21, df = 1; (P = 0.65), I2 = 0% |
| Mixed, whole | 13 | 1089 | −0.82 (−3.04, 1.39) | 12 | 1049 | −0.10 (−1.09, 0.88) | |||
| Legume dose, weekly (g) | ≤450 | 4 | 232 | −1.15 (−2.18, −0.11) | χ2 = 1.17, df = 2; (P = 0.56), I2 = 0% | 4 | 232 | −0.91 (−1.70, −0.11) | χ2 = 2.66, df = 2; (P = 0.26), I2 = 24.8% |
| >450 to <1000 | 8 | 668 | −2.26 (−4.00, −0.52) | 7 | 628 | 0.14 (−0.85, 1.14) | |||
| ≥1000 g | 4 | 390 | −1.36 (−6.27, 3.55) | 4 | 390 | −0.79 (−3.37, 1.79) | |||
| Intake frequency | 1–4 d/wk | 5 | 205 | 0.14 (−3.31, 3.59) | χ2 = 0.51, df = 1; (P = 0.47), I2 = 0% | 5 | 205 | 1.05 (−1.55, 3.66) | χ2 = 1.22, df = 1; (P = 0.27), I2 = 17.8% |
| ≥5 d/wk | 11 | 1085 | −1.27 (−3.04, 0.49) | 10 | 1045 | −0.47 (−1.18, 0.24) | |||
| Dietary feature | Hypocaloric | 6 | 515 | −3.61 (−6.43, −0.79) | χ2 = 5.02, df = 1; (P = 0.03), I2 = 80.1% | 5 | 575 | −0.84 (−1.56, −0.12) | χ2 = 2.83, df = 1; (P = 0.09), I2 = 64.6% |
| Usual diet | 10 | 775 | 0.03 (−1.45, 1.50) | 10 | 775 | 0.21 (−0.78, 1.19) | |||
Publication bias
The visual inspection of funnel plots and results for the Egger test did not indicate small study effects for SBP (bias: 0.211; 95% CI: −0.932, 1.354) (Supplemental Figure 3) or DBP (bias: 0.333; 95% CI: −0.553, 1.218) (Supplemental Figure 4).
Certainty of evidence
In accordance with GRADE guidelines [46], the certainty of evidence was determined as low for both SBP and DBP, owing to the downgrade for ROB and imprecision (Supplemental Table 4).
Discussion
This review of 16 RCTs (1092 participants) extends the findings of the previous review [31] by an additional 8 studies. In this review, the certainty of evidence was determined as low for SBP and DBP. The evidence suggests that legumes consumption results in little to no difference in BP outcomes (SBP or DBP) [57], although there was some evidence of a reduction in SBP in participants who were overweight or obese. However, our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect [58].
Significant moderate heterogeneity was observed only for SBP (Figure 2). These findings are in contrast to the previous review [31], which found that legume consumption significantly reduced SBP (MD:−2.25 mm Hg; 95% CI: −4.22, −0.28), although DBP was nonsignificantly reduced (MD: −0.74 mm Hg; 95% CI: −1.74, 0.31). In addition, the previous review [31] observed significant between-study heterogeneity for both SBP (I2 = 73%) and DBP (I2 = 58%).
Notably, half (8/16) of the studies included in this review reported that legume consumption had a significant ameliorating effect on ≥1 component of BP. Evidence suggests that study design and duration influence BP reductions in nutrition interventions [59]. Dong et al. [59] posit that a 12-wk study duration is associated with significant BP reductions. Furthermore, in crossover trials, the short duration of washout periods may not be sufficient to eliminate residual effects on potential BP reductions, thus suggesting that parallel trials may be better suited to such interventions. In this review, most of the interventions (63%) included a study duration of <12 wk, although no significant differences between the study durations were observed in our subgroup analysis.
A 2022 review of the population-based study, European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk [60], found that higher legume intake was associated with significantly lower odds of hypertension in all participants (43% reduced risk) and particularly in women (68% reduced risk) [61], warranting dietary guidance to increase daily legume intake to ≥55 g as a means to reducing the burden of hypertension and CVD [61]. In this review, all included studies, except 1 [54], used higher weekly doses, than those recommended in the EPIC-Norfolk study [60]; however, our subgroup analysis did not demonstrate significant differences between the doses. Nutritionally, legumes are equally rich in protein and dietary fiber [62], both of which are considered important components of the BP-lowering effects of legumes [63,64]. Daily consumption of 12 g dietary fiber is associated with a 1-mm Hg decrease in SBP and 1.2-mm Hg decrease in DBP in individuals with normotensive or slightly elevated BP and further magnified in hypertensive individuals aged >40 y [65]. In consideration of the complementary role of fiber in the diet, a study assessed the BP-ameliorating effects of a high-fiber, high-protein diet compared with those of a high-protein diet. The findings demonstrated an additional 4.7 mm Hg reduction in SBP and an additional 1 mm Hg reduction in DBP [63]. Analogously, in this review, Lee et al. [40] increased the daily fiber intake by 13 g and demonstrated a 3-mm Hg reduction in SBP in the lupin-enriched intervention diet. These results equate to a lower risk of both stroke and IHD (10% and 7%, respectively) [5,6]. It is worth noting that the nutritional composition of legumes broadly varies between the legume types [66], and the effects of cooking methods further alter protein, fiber, and micronutrient content [67]. Clinical heterogeneity was observed across the included studies, suggesting future studies are warranted to scrutinize the BP-lowering potential of legumes based on the duration, dose, and specific legume types, whereas considering the methods by which legumes are prepared and cooked [32].
Excess central adiposity and obesity are major risk factors for hypertension, accounting for <65% of primary hypertension cases [68]. Excessive weight gain is positively associated with elevated BP, evidenced by a 4-mm Hg increase in SBP for each 4.5-kg increase in weight [69]. Individuals in the highest BMI quartile demonstrate significantly higher SBP (16 mm Hg) and DBP (9 mm Hg) than those in the lower quartile [69]. Obesity-related hypertension represents an expanding health concern affecting health care systems, worldwide [70]. As part of a therapeutic approach, dietary modification to include foods rich in fiber, lean protein, and antioxidants, whereas restricting saturated fats and sodium, is considered useful in the management of obesity and the potential mechanisms of obesity-related hypertension [71,72]. We observed a significant reduction in SBP (−2.8 mm Hg) in our subgroup analysis of individuals with overweight/obese BMI; although this finding may be clinically significant in reducing risk for stroke and IHD, it is, by nature, entirely observational and must be interpreted with caution [33].
Energy-restricted diets associated with weight loss, have been shown to rapidly decrease BP, particularly SBP, because DBP reduction may take longer [9,29,73]. Studies posit that a modest weight loss of 3.5 kg in normotensive individuals is accompanied by a reduction in SBP (5.8 mm Hg) and DBP (3.2 mm Hg) [74,75]. Although legumes are effective for weight management in energy-restricted diets, owing to their satiety-inducing components [55], our subgroup analyses of studies incorporating a hypocaloric dietary component did not suggest a differing effect compared with studies incorporating legumes into the usual diet.
Dietary patterns involve complex biochemical interactions that synergistically and antagonistically affect health [76]. Dietary guidelines advocate diet modification as a primary factor for the prevention and management of hypertension. Indeed, nuts, legumes, and wholegrains are the food groups shown to be most effective in the primary prevention of metabolic disturbances and hypertension [77,78]. Nevertheless, the emphasis is on healthy dietary patterns over individual nutrients or foods, and consumption of these food groups, in addition to fruits and vegetables, paralleled by avoidance of foods high in saturated fat and sodium [9,16], are well represented in dietary patterns shown to ameliorate BP, such as the DASH, Mediterranean, and TLC diets [9,79]. The BP-reducing effects of such diets may be further enhanced with dietary counseling and provided meals, particularly in older hypertensive adults [80]. Included in our meta-analysis, Hosseinpour-Niazi et al. [53] implemented a DASH diet enriched with legumes and found higher legume intake (>96 g/d) was associated with a significant reduction in SBP. Furthermore, of the 2 studies that implemented a TLC diet, Kazemi et al. [54] provided participants with legume-based meals and reported a significant reduction in DBP; however, caution in interpreting these findings is warranted given the small sample size and limited generalizability.
It should be noted that studies included in this review did not investigate the effect of legume intake on BP in individuals with hypertension, potentially masking the effects of legume consumtpion on BP. This is particularly pertinent given that other dietary interventions for BP, such as sodium reductions, have been found to be more effective in individuals with hypertension. This may partly explain the greater reduction in BP in participants with overweight/obese BMI status. Although the studies included in this review did not have diagnosed hypertension, individuals with overweight/obese BMI status may be at an increased CVD risk and, therefore, experience a greater response to legume consumption [81].
As highlighted, there are several plausible explanations for why our findings did not reflect the epidemiological data, and to this point, we acknowledge several limitations to the current review. In RCTs, the primary outcome is closely correlated with the study design. Commonly, sample size calculations and reproducibility estimates are based on the primary outcome [82]. Alshamsi et al. [82] highlight the importance of building the study around a clinically relevant primary outcome to reduce bias; however, in this review, only 2 (12%) included studies defined BP as a primary outcome.
Dietary adherence to the diets was not assessed by biochemical markers; instead, studies used self-reported food records and 24-h recalls. These methods are prone to underreporting and random and systematic errors [83,84]. RCTs provide a high internal validity; however, translation of findings into clinical practice are commonly undermined by the complex nature of dietary interventions and challenges such as collinearity between dietary components, diverse dietary behaviors and food culture, and multitargeted interventional effects [32]. Nevertheless, there are several strengths to this study. First, this review provides a comprehensive account of the effect of legume consumption on BP based on the pooled evidence from 16 RCTs conducted across a range of geographical locations. In addition, the varied participant characteristics such as age, sex, baseline health status, and ethnicity may provide a degree of external validity and generalizability to our findings. Second, in comparison with the previous review [31], this meta-analyses included an additional 8 RCTs, enhancing the sample size and power to detect differences in the effect. Finally, potential sources of heterogeneity were explored through sensitivity and subgroup analyses, contributing to the robustness of our study.
Future research should focus on innovative study design with careful consideration for targeted population, definition of primary outcome, larger sample sizes, longer study duration, and focused efforts to address limitations such as adherence, blinding, and randomization [32,85].
In conclusion, this review systematically assessed the evidence from intervention studies investigating the effect of legume intake on BP. The certainty of evidence was deemed low for SBP and DBP. Legume intake did not have a significant overall effect on BP; however, significant subgroup differences in SBP were found when studies were grouped according to participant BMI, with reductions in SBP found only for individuals with overweight and obese BMI status. To improve the certainty of evidence, future research should focus on large-scale intervention trials, exploring differences in legume types, doses, and duration when considered in the context of healthy dietary patterns such as DASH and Mediterranean diets. Consideration of the relevance in individuals with obesity, overweight, and hypertension may also be warranted.
Acknowledgments
The authors’ responsibilities were as follows: GLR, EJB, KL, EPN: contributed to the conceptualization and design of review, methodology and formal analysis, review and editing of the final content; GLR: wrote the original draft; and all authors: read and approved the final manuscript.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request.
Author disclosures
The authors report no conflicts of interest.
Funding
The authors reported no funding received for this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2023.04.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Unger T., Borghi C., Charchar F., Khan N.A., Poulter N.R., Prabhakaran D., et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/hypertensionaha.120.15026. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S., Joseph P., Rangarajan S., Islam S., Mente A., Hystad P., et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808. doi: 10.1016/s0140-6736(19)32008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C.Y., Hu H.Y., Chou Y.J., Huang N., Chou Y.C., Li C.P. High blood pressure and all-cause and cardiovascular disease mortalities in community-dwelling older adults. Med (Baltim). 2015;94(47) doi: 10.1097/md.0000000000002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Law M.R., Morris J.K., Wald N.J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy S.T., Loehr L.R., Butler K.R., Chakladar S., Chang P.P., Folsom A.R., et al. Reducing the blood pressure–related burden of cardiovascular disease: impact of achievable improvements in blood pressure prevention and control. J. Am. Heart Assoc. 2015;4(10) doi: 10.1161/jaha.115.002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewington S., Clarke R., Qizilbash N., Peto R., Collins R. Prospective Studies Collaboration, Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 7.Boledovičová M., Hendl J., Lišková L., Slamková A., Matoulek M., Stránská Z., et al. Blood pressure relation to body composition and age: analysis of a nurse-led investigation and consultation program. Med. Sci. Monit. 2013;19:612–617. doi: 10.12659/msm.883984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher N.D.L., Curfman G. Hypertension—a public health challenge of global proportions. JAMA. 2018;320(17):1757–1759. doi: 10.1001/jama.2018.16760. [DOI] [PubMed] [Google Scholar]
- 9.Schwingshackl L., Schwedhelm C., Hoffmann G., Knüppel S., Iqbal K., Andriolo V., et al. Food groups and risk of hypertension: a systematic review and dose-response meta-analysis of prospective studies. Adv. Nutr. 2017;8(6):793–803. doi: 10.3945/an.117.017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazzano L.A., Green T., Harrison T.N., Reynolds K. Dietary approaches to prevent hypertension. Curr. Hypertens. Rep. 2013;15(6):694–702. doi: 10.1007/s11906-013-0390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feyh A., Bracero L., Lakhani H.V., Santhanam P., Shapiro J.I., Khitan Z., et al. Role of dietary components in modulating hypertension. J. Clin. Exp. Cardiolog. 2016;7(4):433. doi: 10.4172/2155-9880.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiavaroli L., Viguiliouk E., Nishi S.K., Blanco Mejia S., Rahelić D., Kahleová H., et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11(2):338. doi: 10.3390/nu11020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez León E.E., Henríquez P., Serra-Majem L. Mediterranean diet and metabolic syndrome: a cross-sectional study in the Canary Islands. Public Health Nutr. 2006;9(8A):1089–1098. doi: 10.1017/s1368980007668487. [DOI] [PubMed] [Google Scholar]
- 14.Burch J., Thapa B. How does a Mediterranean-style diet compare with a low-fat diet for the primary prevention of cardiovascular disease (CVD)? Cochrane Clinical Answers. 2019 doi: 10.1002/cca.2536. [DOI] [Google Scholar]
- 15.Bensaaud A., Seery S., Gibson I., Jones J., Flaherty G., McEvoy J.W., et al. Dietary Approaches to Stop Hypertension (DASH) for the primary and secondary prevention of cardiovascular diseases. Cochrane Database Syst. Rev. 2020;9:CD013729. doi: 10.1002/14651858.CD013729. [DOI] [Google Scholar]
- 16.Sacks F.M., Svetkey L.P., Vollmer W.M., Appel L.J., Bray G.A., Harsha D., et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. N. Engl. J. Med. 2001;344(1):3–10. doi: 10.1056/nejm200101043440101. [DOI] [PubMed] [Google Scholar]
- 17.Appel L.J., Sacks F.M., Carey V.J., Obarzanek E., Swain J.F., Miller E.R., et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294(19):2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 18.Poulsen S.K., Due A., Jordy A.B., Kiens B., Stark K.D., Stender S., et al. Health effect of the New Nordic Diet in adults with increased waist circumference: a 6-mo randomized controlled trial. Am. J. Clin. Nutr. 2014;99(1):35–45. doi: 10.3945/ajcn.113.069393. [DOI] [PubMed] [Google Scholar]
- 19.Adamsson V., Reumark A., Fredriksson I.B., Hammarström E., Vessby B., Johansson G., et al. Effects of a healthy Nordic diet on cardiovascular risk factors in hypercholesterolaemic subjects: a randomized controlled trial (NORDIET) J. Intern. Med. 2011;269(2):150–159. doi: 10.1111/j.1365-2796.2010.02290.x. [DOI] [PubMed] [Google Scholar]
- 20.Ascherio A., Rimm E.B., Giovannucci E.L., Colditz G.A., Rosner B., Willett W.C., et al. A prospective study of nutritional factors and hypertension among US men. Circulation. 1992;86(5):1475–1484. doi: 10.1161/01.cir.86.5.1475. [DOI] [PubMed] [Google Scholar]
- 21.Lukus P.K., Doma K.M., Duncan A.M. The role of pulses in cardiovascular disease risk for adults with diabetes. Am. J. Lifestyle Med. 2020;14(6):571–584. doi: 10.1177/1559827620916698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon J.A., Fong J., Bernert J.T. Serum fatty acids and blood pressure. Hypertension. 1996;27(2):303–307. doi: 10.1161/01.HYP.27.2.303. [DOI] [PubMed] [Google Scholar]
- 23.Caprioli G., Giusti F., Ballini R., Sagratini G., Vila-Donat P., Vittori S., et al. Lipid nutritional value of legumes: evaluation of different extraction methods and determination of fatty acid composition. Food Chem. 2016;192:965–971. doi: 10.1016/j.foodchem.2015.07.102. [DOI] [PubMed] [Google Scholar]
- 24.Clemente A., Olias R. Beneficial effects of legumes in gut health. Curr. Opin. Food Sci. 2017;14:32–36. doi: 10.1016/j.cofs.2017.01.005. [DOI] [Google Scholar]
- 25.Whelton S.P., Hyre A.D., Pedersen B., Yi Y., Whelton P.K., He J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J. Hypertens. 2005;23(3):475–481. doi: 10.1097/01.hjh.0000160199.51158.cf. [DOI] [PubMed] [Google Scholar]
- 26.Vasdev S., Stuckless J. Antihypertensive effects of dietary protein and its mechanism. Int. J. Angiol. 2010;19(1):e7–e20. doi: 10.1055/s-0031-1278362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siani A., Pagano E., Iacone R., Iacoviello L., Scopacasa F., Strazzullo P. Blood pressure and metabolic changes during dietary L-arginine supplementation in humans. Am. J. Hypertens. 2000;13(5 Pt 1):547–551. doi: 10.1016/s0895-7061(99)00233-2. [DOI] [PubMed] [Google Scholar]
- 28.Boschin G., Scigliuolo G.M., Resta D., Arnoldi A. ACE-inhibitory activity of enzymatic protein hydrolysates from lupin and other legumes. Food Chem. 2014;145:34–40. doi: 10.1016/j.foodchem.2013.07.076. [DOI] [PubMed] [Google Scholar]
- 29.Papanikolaou Y., Fulgoni V.L. Bean consumption is associated with greater nutrient intake, reduced systolic blood pressure, lower body weight, and a smaller waist circumference in adults: results from the National Health and Nutrition Examination Survey 1999-2002. J. Am. Coll. Nutr. 2008;27(5):569–576. doi: 10.1080/07315724.2008.10719740. [DOI] [PubMed] [Google Scholar]
- 30.Viguiliouk E., Glenn A.J., Nishi S.K., Chiavaroli L., Seider M., Khan T., et al. Associations between dietary pulses alone or with other legumes and cardiometabolic disease outcomes: an umbrella review and updated systematic review and meta-analysis of prospective cohort studies. Adv. Nutr. 2019;10(Suppl 4):S308–S319. doi: 10.1093/advances/nmz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jayalath V.H., De Souza R.J., Sievenpiper J.L., Ha V., Chiavaroli L., Mirrahimi A., et al. Effect of dietary pulses on blood pressure: a systematic review and meta-analysis of controlled feeding trials. Am. J. Hypertens. 2014;27(1):56–64. doi: 10.1093/ajh/hpt155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mirmiran P., Bahadoran Z., Gaeini Z. Common limitations and challenges of dietary clinical trials for translation into clinical practices. Int. J. Endocrinol. Metab. 2021;19(3) doi: 10.5812/ijem.108170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook for Systematic Reviews of Interventions. 2022. www.training.cochrane.org/handbook Cochrane. [updated February 2022, cited 2022 Dec 03]. Available from: [Google Scholar]
- 34.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Booth A., Clarke M., Dooley G., Ghersi D., Moher D., Petticrew M., et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst. Rev. 2012;1(1):2. doi: 10.1186/2046-4053-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Center for Food Safety and Applied Nutrition, Evidence-Based Review for Scientific Evaluation of Health Claims. US Food and Drug Administration; 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-evidence-based-review-system-scientific-evaluation-health-claims [Internet] [cited 6 April, 2021]. Available from: [Google Scholar]
- 37.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 38.Belski R., Mori T.A., Puddey I.B., Sipsas S., Woodman R.J., Ackland T.R., et al. Effects of lupin-enriched foods on body composition and cardiovascular disease risk factors: a 12-month randomized controlled weight loss trial. Int. J. Obes. (Lond). 2011;35(6):810–819. doi: 10.1038/ijo.2010.213. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins D.J.A., Kendall C.W.C., Augustin L.S.A., Mitchell S., Sahye-Pudaruth S., Blanco Mejia S., et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: a randomized controlled trial. Arch. Intern. Med. 2012;172(21):1653–1660. doi: 10.1001/2013.jamainternmed.70. [DOI] [PubMed] [Google Scholar]
- 40.Lee Y.P., Mori T.A., Puddey I.B., Sipsas S., Ackland T.R., Beilin L.J., et al. Effects of lupin kernel flour–enriched bread on blood pressure: a controlled intervention study. Am. J. Clin. Nutr. 2009;89(3):766–772. doi: 10.3945/ajcn.2008.26708. [DOI] [PubMed] [Google Scholar]
- 41.Hosseinpour-Niazi S., Mirmiran P., Hedayati M., Azizi F. Substitution of red meat with legumes in the therapeutic lifestyle change diet based on dietary advice improves cardiometabolic risk factors in overweight type 2 diabetes patients: a cross-over randomized clinical trial. Eur. J. Clin. Nutr. 2015;69(5):592–597. doi: 10.1038/ejcn.2014.228. [DOI] [PubMed] [Google Scholar]
- 42.Alizadeh M., Gharaaghaji R., Gargari B.P. The effects of legumes on metabolic features, insulin resistance and hepatic function tests in women with central obesity: a randomized controlled trial. Int. J. Prev. Med. 2014;5(6):710–720. [PMC free article] [PubMed] [Google Scholar]
- 43.Saraf-Bank S., Esmaillzadeh A., Faghihimani E., Azadbakht L. Effects of legume-enriched diet on cardiometabolic risk factors among individuals at risk for diabetes: a crossover study. J. Am. Coll. Nutr. 2016;35(1):31–40. doi: 10.1080/07315724.2014.931262. [DOI] [PubMed] [Google Scholar]
- 44.Drahota A., Beller E. RevMan Calculator [Internet] Cochrane Training. 2022 https://training.cochrane.org/resource/revman-calculator [cited 2 Dec 3. Available from: [Google Scholar]
- 45.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The GRADE Working Group . In: GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Schünemann H., Brozek J., Guyatt G., Oxman A., editors. The GRADE Working Group; 2013. www.guidelinedevelopment.org/handbook Available from. [Google Scholar]
- 47.Abeysekara S., Chilibeck P.D., Vatanparast H., Zello G.A. A pulse-based diet is effective for reducing total and LDL-cholesterol in older adults. Br. J. Nutr. 2012;108(S 1):S103–S110. doi: 10.1017/s0007114512000748. [DOI] [PubMed] [Google Scholar]
- 48.Gravel K., Lemieux S., Asselin G., Dufresne A., Lemay A., Forest J.-C., Dodin S. Effects of pulse consumption in women presenting components of the metabolic syndrome: a randomized controlled trial. Mediterr. J. Nutr. Metab. 2010;3(2):143–151. doi: 10.1007/s12349-010-0009-8. [DOI] [Google Scholar]
- 49.Kazemi M., McBreairty L.E., Chizen D.R., Pierson R.A., Chilibeck P.D., Zello G.A. A comparison of a pulse-based diet and the therapeutic lifestyle changes diet in combination with exercise and health counselling on the cardio-metabolic risk profile in women with polycystic ovary syndrome: a randomized controlled trial. Nutrients. 2018;10(10):1387. doi: 10.3390/nu10101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mollard R.C., Luhovyy B.L., Panahi S., Nunez M., Hanley A., Anderson G.H. Regular consumption of pulses for 8 weeks reduces metabolic syndrome risk factors in overweight and obese adults. Br. J. Nutr. 2012;108(S 1):S111–S122. doi: 10.1017/s0007114512000712. [DOI] [PubMed] [Google Scholar]
- 51.Veenstra J.M., Duncan A.M., Cryne C.N., Deschambault B.R., Boye J.I., Benali M., et al. Effect of pulse consumption on perceived flatulence and gastrointestinal function in healthy males. Food Res. Int. 2010;43(2):553–559. doi: 10.1016/j.foodres.2009.07.029. [DOI] [Google Scholar]
- 52.Hassanzadeh-Rostami Z., Hemmatdar Z., Pishdad G.R., Faghih S. Moderate consumption of red meat, compared to soy or non-soy legume, has no adverse effect on cardio-metabolic factors in patients with type 2 diabetes. Exp. Clin. Endocrinol. Diabetes. 2021;129(6):429–437. doi: 10.1055/a-0929-6287. [DOI] [PubMed] [Google Scholar]
- 53.Hosseinpour-Niazi S., Hadaegh F., Mirmiran P., Daneshpour M.S., Mahdavi M., Azizi F. Effect of legumes in energy reduced Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure among overweight and obese type 2 diabetic patients: a randomized controlled trial. Diabetol. Metab. Syndr. 2022;14(1):72. doi: 10.1186/s13098-022-00841-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ward N.C., Mori T.A., Beilin L.J., Johnson S., Williams C., Gan S.K., et al. The effect of regular consumption of lupin-containing foods on glycaemic control and blood pressure in people with type 2 diabetes mellitus. Food Funct. 2020;11(1):741–747. doi: 10.1039/c9fo01778j. [DOI] [PubMed] [Google Scholar]
- 55.Abete I., Parra D., Martinez J.A. Legume-, fish-, or high-protein-based hypocaloric diets: effects on weight loss and mitochondrial oxidation in obese men. J. Med. Food. 2009;12(1):100–108. doi: 10.1089/jmf.2007.0700. [DOI] [PubMed] [Google Scholar]
- 56.Hermsdorff H.H.M., Zulet M.Á., Abete I., Martínez J.A. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur. J. Nutr. 2011;50(1):61–69. doi: 10.1007/s00394-010-0115-x. [DOI] [PubMed] [Google Scholar]
- 57.Santesso N., Glenton C., Dahm P., Garner P., Akl E.A., Alper B., et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J. Clin. Epidemiol. 2020;119:126–135. doi: 10.1016/j.jclinepi.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 58.Guidelines for Guidelines: Assessing Certainty of Evidence [Internet]. NHMRC [updated 6 September, 2019; cited 2022 Dec 03] Available from: https://nhmrc.gov.au/guidelinesforguidelines/develop/assessing-certainty-evidence.
- 59.Dong J.Y., Tong X., Wu Z.W., Xun P.C., He K., Qin L.Q. Effect of soya protein on blood pressure: a meta-analysis of randomised controlled trials. Br. J. Nutr. 2011;106(3):317–326. doi: 10.1017/s0007114511000262. [DOI] [PubMed] [Google Scholar]
- 60.The EPIC-Norfolk Study. MRC Epidemiology Unit [Internet]. University of Cambridge [cited 2022 Nov 15]. Available from: https://www.epic-norfolk.org.uk/.
- 61.Hartley M., Fyfe C.L., Wareham N.J., Khaw K.-T., Johnstone A.M., Myint P.K. Association between legume consumption and risk of hypertension in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Nutrients. 2022;14(16):3363. doi: 10.3390/nu14163363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kouris-Blazos A., Belski R. Health benefits of legumes and pulses with a focus on Australian sweet lupins, Asia Pac. J. Clin. Nutr. 2016;25(1):1–17. doi: 10.6133/apjcn.2016.25.1.23. [DOI] [PubMed] [Google Scholar]
- 63.Burke V., Hodgson J.M., Beilin L.J., Giangiulioi N., Rogers P., Puddey I.B. Dietary protein and soluble fiber reduce ambulatory blood pressure in treated hypertensives. Hypertension. 2001;38(4):821–826. doi: 10.1161/hy1001.092614. [DOI] [PubMed] [Google Scholar]
- 64.Lee Y.P., Puddey I.B., Hodgson J.M. Protein, fibre and blood pressure: potential benefit of legumes. Clin. Exp. Pharmacol. Physiol. 2008;35(4):473–476. doi: 10.1111/j.1440-1681.2008.04899.x. [DOI] [PubMed] [Google Scholar]
- 65.Streppel M.T., Arends L.R., van ’t Veer P., Grobbee D.E., Geleijnse J.M. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch. Intern. Med. 2005;165(2):150–156. doi: 10.1001/archinte.165.2.150. [DOI] [PubMed] [Google Scholar]
- 66.Sánchez-Chino X., Jiménez-Martínez C., Dávila-Ortiz G., Álvarez-González I., Madrigal-Bujaidar E. Nutrient and nonnutrient components of legumes, and its chemopreventive activity: a review. Nutr. Cancer. 2015;67(3):401–410. doi: 10.1080/01635581.2015.1004729. [DOI] [PubMed] [Google Scholar]
- 67.Margier M., Georgé S., Hafnaoui N., Remond D., Nowicki M., Du Chaffaut L., et al. Nutritional composition and bioactive content of legumes: characterization of pulses frequently consumed in france and effect of the cooking method. Nutrients. 2018;10(11):1668. doi: 10.3390/nu10111668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shariq O.A., McKenzie T.J. Obesity-related hypertension: a review of pathophysiology, management, and the role of metabolic surgery. Gland Surg. 2020;9(1):80–93. doi: 10.21037/gs.2019.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aronow W.S. Association of obesity with hypertension. Ann. Transl. Med. 2017;5(17):350. doi: 10.21037/atm.2017.06.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hall M.E., Cohen J.B., Ard J.D., Egan B.M., Hall J.E., Lavie C.J., et al. Weight-loss strategies for prevention and treatment of hypertension: a scientific statement from the American Heart Association. Hypertension. 2021;78(5):e 38–e50. doi: 10.1161/hyp.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 71.Jiang S.-Z., Lu W., Zong X.-F., Ruan H.-Y., Liu Y. Obesity and hypertension. Exp. Ther. Med. 2016;12(4):2395–2399. doi: 10.3892/etm.2016.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rebello C.J., Greenway F.L., Finley J.W. A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes. Rev. 2014;15(5):392–407. doi: 10.1111/obr.12144. [DOI] [PubMed] [Google Scholar]
- 73.Nicoll R., Henein M.Y. Caloric restriction and its effect on blood pressure, heart rate variability and arterial stiffness and dilatation: a review of the evidence. Int. J. Mol. Sci. 2018;19(3):751. doi: 10.3390/ijms19030751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He J., Whelton P.K., Appel L.J., Charleston J., Klag M.J. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension. 2000;35(2):544–549. doi: 10.1161/01.hyp.35.2.544. [DOI] [PubMed] [Google Scholar]
- 75.Jensen M.D., Ryan D.H., Donato K.A., Apovian C.M., Ard J.D., Comuzzie A.G. Executive summary: guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. Obesity (Silver Spring). 2014;22(S2):S5–S39. doi: 10.1002/oby.20821. [DOI] [PubMed] [Google Scholar]
- 76.Tapsell L.C., Neale E.P., Satija A., Hu F.B. Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv. Nutr. 2016;7(3):445–454. doi: 10.3945/an.115.011718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwingshackl L., Hoffmann G., Iqbal K., Schwedhelm C., Boeing H. Food groups and intermediate disease markers: a systematic review and network meta-analysis of randomized trials. Am. J. Clin. Nutr. 2018;108(3):576–586. doi: 10.1093/ajcn/nqy151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mohammadifard N., Salehi-Abargouei A., Salas-Salvadó J., Guasch-Ferré M., Humphries K., Sarrafzadegan N. The effect of tree nut, peanut, and soy nut consumption on blood pressure: a systematic review and meta-analysis of randomized controlled clinical trials. Am. J. Clin. Nutr. 2015;101(5):966–982. doi: 10.3945/ajcn.114.091595. [DOI] [PubMed] [Google Scholar]
- 79.Watson K., Jamerson K. Therapeutic lifestyle changes for hypertension and cardiovascular risk reduction. J. Clin. Hypertens (Greenwich). 2003;5(1):32–37. doi: 10.1111/j.1524-6175.2003.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ozemek C., Laddu D.R., Arena R., Lavie C.J. The role of diet for prevention and management of hypertension. Curr. Opin. Cardiol. 2018;33(4):388–393. doi: 10.1097/HCO.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 81.Graudal N.A., Hubeck-Graudal T., Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst. Rev. 2020;12:CD004022. doi: 10.1002/14651858.CD004022.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alshamsi M., Mehta J., Nibali L. Study design and primary outcome in randomized controlled trials in periodontology. A systematic review. J. Clin. Periodontol. 2021;48(6):859–866. doi: 10.1111/jcpe.13443. [DOI] [PubMed] [Google Scholar]
- 83.McCarney R., Warner J., Iliffe S., Van Haselen R., Griffin M., Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med. Res. Methodol. 2007;7(1):30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thompson F.E., Kirkpatrick S.I., Subar A.F., Reedy J., Schap T.E., Wilson M.M., et al. The National Cancer Institute’s dietary assessment primer: a resource for diet research. J. Acad. Nutr. Diet. 2015;115(12):1986–1995. doi: 10.1016/j.jand.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weaver C.M., Miller J.W. Challenges in conducting clinical nutrition research. Nutr. Rev. 2017;75(7):491–499. doi: 10.1093/nutrit/nux026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep 894:1–253. [PubMed]
- 87.Gravel K., Lemieux S., Asselin G., Lemay A., West G., Forest J.C., et al. Does legumes consumption in a real life context can improve components of metabolic syndrome? A randomized controlled trial. J. Diabetes. 2009;1:A277. doi: 10.1111/j.1753-0407.2009.00020.x. [DOI] [Google Scholar]
- 88.Australian Dietary Guidelines Canberra, Australia. National Health and Medical Research Council. 2013 https://www.eatforhealth.gov.au/guidelines/australian-dietary-guidelines-1-5 [Internet] [cited 2022 Nov 23]. Available from: [Google Scholar]
- 89.Expert Panel On Detection Evaluation, and Treatment Of High Blood Cholesterol in Adults, Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request.