Abstract
Within the organism, the liver is the main organ responsible for metabolic homeostasis and xenobiotic transformation. To maintain an adequate liver weight-to-bodyweight ratio, this organ has an extraordinary regenerative capacity and is able to respond to an acute insult or partial hepatectomy. Maintenance of hepatic homeostasis is crucial for the proper functioning of the liver, and in this context, adequate nutrition with macro- and micronutrient intake is mandatory. Among all known macro-minerals, magnesium has a key role in energy metabolism and in metabolic and signaling pathways that maintain liver function and physiology throughout its life span. In the present review, the cation is reported as a potential key molecule during embryogenesis, liver regeneration, and aging. The exact role of the cation during liver formation and regeneration is not fully understood due to its unclear role in the activation and inhibition of those processes, and further research in a developmental context is needed. As individuals age, they may develop hypomagnesemia, a condition that aggravates the characteristic alterations. Additionally, risk of developing liver pathologies increases with age, and hypomagnesemia may be a contributing factor. Therefore, magnesium loss must be prevented by adequate intake of magnesium-rich foods such as seeds, nuts, spinach, or rice to prevent age-related hepatic alterations and contribute to the maintenance of hepatic homeostasis. Since magnesium-rich sources include a variety of foods, a varied and balanced diet can meet both macronutrient and micronutrient needs.
Keywords: magnesium, embryogenesis, liver regeneration, liver disease, aging, hypomagnesemia, nutritional intake
Statement of Significance.
Hypomagnesemia may contribute to age-related hepatic alterations, and an adequate intake of magnesium-rich foods might prevent such alterations and contribute to the maintenance of hepatic homeostasis. Further research in specific developmental and regenerative contexts is required to fully understand the role of magnesium, and novel magnesium labeling methods may be helpful.
Introduction
The liver is the main organ responsible for maintaining nutrient homeostasis in the organism. It ensures a constant supply of oxidizable substrates. During food intake, the liver builds up energy stores in the form of glycogen and triglycerides, which are later released during fasting in the form of glucose and ketone bodies [1]. A complex network of signals regulating metabolic pathways controls this transition from feeding to fasting and ensures an adequate supply of nutrients at the precise moment. Moreover, the liver plays a key role in the transformation of exogenous compounds as it contains a variety of xenobiotic biotransforming enzymes involved in oxidation, reduction, hydrolysis, and conjugation [2]. Often exposed to toxic insults and perturbations that may alter its correct functioning, the regeneration capacity of the liver is another remarkable characteristic of this organ. After an acute injury or partial hepatectomy (PH) and during chronic liver injury, the liver accounts for a vast number of regenerative mechanisms that ensure a correct liver-to-bodyweight ratio that guarantees the correct function of the liver [3].
Maintaining homeostasis is crucial for the correct development of liver functions, with an adequate balance of macro- and micronutrients ensuring the aforementioned processes. In this context, magnesium has appeared as a key compound for such processes, as it has been associated with liver diseases [4] or related comorbidities [5]. Magnesium, Mg2+ in its free form, is the most abundant divalent cation in the cell and the fourth most abundant element in the body, with concentrations ranging from 5 to 20 nM. It is required for the correct activity of many enzymes related to energy, such as ATP-involving reactions and the metabolism of nucleic acids, and plays a key role as a second messenger [6]. Therefore, maintaining homeostasis is crucial to avoiding imbalances in these processes. Magnesium levels in the organism are determined by the ratio between its secretion and intake or reabsorption. Bones act as a reservoir (>50% of magnesium in the organism), the kidney regulates its excretion, and the gut modulates the uptake of the cation (from a daily 300 mg intake, 24%–75% is absorbed) [7]. Due to the fact that hydrated magnesium increases its ratio 400-fold, the action of several membrane proteins is essential for its transport across cell membranes [8]. In this context, several proteins involved in the transport of magnesium out of and into the cell have been characterized, including cyclin M family (CNNM 1–4) [9], solute carrier 41A, magnesium transporter subtype 1 [10], membrane magnesium transporter 1 [11], transient receptor protein melastatin 6/7 [12] or mitochondrial RNA Splicing 2 [13].
The present work reviews the role of magnesium in liver physiology during its life span. As outlined in this work, magnesium homeostasis is involved in both the development of the liver during embryogenesis and its proper function and regenerative capacity. Additionally, physiological changes during aging affect magnesium homeostasis, and in the meantime, magnesium homeostasis affects the aforementioned processes, creating a vicious cycle that promotes both aging and hypomagnesemia.
Magnesium during embryogenesis
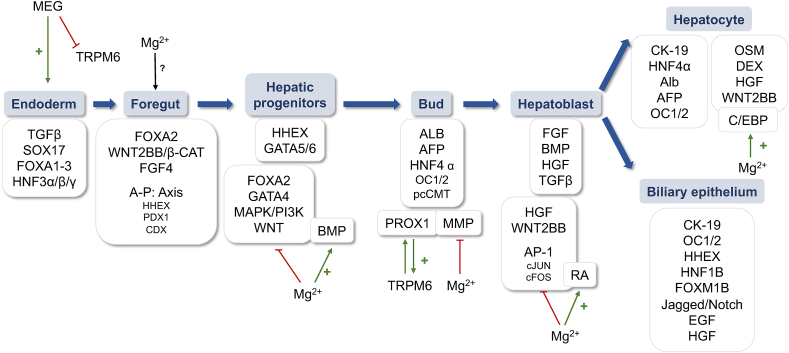
During liver development in embryogenesis, complex regulation of specific signaling pathways acts in the transition between different phases. The final differentiation of the cells into hepatocytes and biliary epithelial cells is preceded by the formation of the zygote for the latter endoderm, foregut, and hepatoblast formation [14]. The process and its relationship to magnesium homeostasis are described in detail below (Figure 1).
FIGURE 1.
Schematic representation of liver development, the main signaling pathways involved, and the contribution of magnesium (Mg2+) in each step of the process. Abbreviations list: AFP, α-fetoprotein; ALB, albumin; AP-1, activating protein-1; BMP, bone morphogenic rotein; CDX, caudal type homeobox; CK-19, keratin-19; DEX, dextranase precursor; EGF, epidermal growth factor; FGF4, fibroblast growth factor 4; FOXA1-3, forkhead Box A1-3; FOXM1B, forkhead box M1B; HGF, hepatic growth factor; HHEX, hematopoietically-expressed homeobox; HNF1β, hepatocyte nuclear factor- 1beta; HNF3α/β/γ, hepatocyte nuclear factor 3α/β/γ; HNF4α, hepatocyte nuclear factor 4 alpha; MAPK, mitogen-activated protein kinases; MEG, mesendogen; MMP, matrix metalloproteinases; PPMA1A, protein phosphatase magnesium-dependent 1A; pcCMT, prenylcysteine carboxylmethyltransferase; RA, retinoic acid; SOX17, SRY-box transcription factor 17; TRPM6/7: transient receptor protein melastatin 6/7.
During endoderm formation, the action of transforming growth factor β (TGFβ) together with SRY-box transcription factor 17, forkhead Box A1–3, (FOXA1–3) and hepatocyte nuclear factor 3α/β/γ is crucial [15]. The inhibitor mesendogen (MEG), targeting the magnesiotropic protein called transient receptor potential cation channel subfamily M6 (TRPM6), modulates the mesoderm and definitive endoderm differentiation of human embryonic stem cells (hESCs), altering magnesium homeostasis [16]. This is because low intracellular magnesium concentrations promote mesoendoderm differentiation through a Nodal/TGFβ/Activin A/WNT axis that inhibits neuroectoderm formation in pluripotent hESCs [16].
It has also been reported that FOXA2 signaling is essential for foregut formation [16], which is characterized by the formation of a differential gradient of WNT/β-catenin (β-cat) and fibroblast growth factor 4 (FGF4) [15]. In such a gradient, the antero-posterior axis is formed, with different transcription factors involved in each part: the hematopoietically-expressed homeobox (HHEX) in the foregut, the pancreatic and duodenal homeobox 1 in the midgut, and the caudal type homeobox for the posterior endoderm. The role of magnesium in these processes has not yet been investigated.
In the formation of hepatic progenitors, the binding of FOXA2 and HHEX, together with GATA4-6-binding proteins, to the enhancer elements of the ALBUMIN (ALB) gene is important [17]. GATA4 expression is increased during magnesium deficiency [18], whereas magnesium enhances the activity of other proteins involved in the formation of hepatic progenitors, such as bone morphogenic protein (BMP) [19,20]. Together with FOXA2, HHEX, and GATA4- 6, the role of mitogen-activated protein kinases (MAPK)/phosphatidyl-inositol-3-kinase (PI3K), and WNT2BB are relevant [21]. Similarly, studies on the roles of magnesium in such processes have found that its deficiency promotes PI3K/MAPK [22], whereas WNT signaling is inhibited by magnesium [23].
Once the ALB gene is already expressed, liver bud morphogenesis takes place, accompanied by the expression of α-fetoprotein (AFP) and hepatocyte nuclear factor 4 alpha [24]. In this regard, prospero homeobox 1 (PROX1), outer capsid protein 1/2 (OC1/2), matrix metalloproteinases (MMPs), and prenylcysteine carboxylmethyltransferase are crucial for hepatoblast delamination [25]. The relationship between these pathways and magnesium is controversial because, although deletion of PROX1 reduces the expression of TRPM6 and induces hypomagnesemia [26], the expression of MMP is decreased when magnesium is added [27]. Thus, further research is required to characterize the involvement of magnesium in bud morphogenesis.
When the liver bud forms, several mesenchymal signals are involved in its growth. Hepatic growth factor (HGF), BMP, WNT, TGFβ, and retinoic acid (RA) promote hepatoblast migration, proliferation, and survival. Apart from the relevance of magnesium redistribution by RA, which promotes liver bud differentiation [28], magnesium has been reported to inhibit HGF [29] and WNT [23] signaling. Meanwhile, magnesium plays a role in hepatoblast proliferation and survival by negatively regulating TNF [30] and reducing the expression of activating protein-1 (AP-1) components such as cJUN or cFOS [31].
For hepatoblast differentiation, keratin-19 (CK-19) accompanies HNF4α, albumin, AFP, OC1/2, and several other signals that act in the differentiation of hepatoblastS into immature hepatocytes and immature biliary epithelial cells (iBEC). CCAAT-enhanced-binding proteins (C/EBP) promote further differentiation of immature hepatocytes, with magnesium playing a key role in C/EBP-mediated action by promoting its binding to DNA [32]. C/EBP also acts in final hepatocyte differentiation together with oncostatin M (OSM), dextranase precursor (DEX), HGF, WNT, and HNF4. In the case of iBEC differentiation, the action of CK-19, OC1/2, HHEX, hepatocyte nuclear factor-1 beta, and forkhead box M1B (FOXM1B) is required, as is the combination of Jagged/Notch, epidermal growth factor (EGF), HGF, and CK-19 [33].
Additionally, Günther et al. [34] studied the effect of magnesium injection on fetal development and found that magnesium remains extracellularly in the liver precursor, alters hormone secretion, and modulates the activity of regulatory enzymes that promote liver formation. Given this finding and the effect of RA on the distribution of magnesium to promote differentiation [28], it is reasonable to speculate that the site at which magnesium remains during liver development may play a role that needs to be further elucidated. The development of methods to label magnesium [9,35], including in specific organelles such as mitochondria [9], may allow more comprehensive research on the cation and its location in liver formation during embryogenesis.
Moreover, Komiya et al. [38] have elegantly reviewed the action of magnesium transporters. Although some of the above pathways link decreased magnesium levels to the promotion of endoderm, foregut, and bud formation, both TRPM6 and TRPM7 are essential for early development, and TRPM7 in particular for gastrulation during vertebrate embryogenesis [36]. In the context of magnesium’s role as a second messenger, the cation interacts with calcium and is essential for phospholipase Cγ1 (PLCγ1), which is responsible for the development of the immune response and the activation of c-Jun N-terminal kinase (JNK). Moreover, the cation is essential for the production of reactive oxygen species (ROS) and the action of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which are essential for embryonic development [37]. Magnesium induces ROS and JNK activation, enhancing WNT for actin polymerization, which promotes cell migration and thus convergent extension movement and neural tube closure [33].
Overall, there is limited research on the cation and its role during embryogenesis and at each stage of the process: endoderm, foregut, hepatic progenitors, bud, hepatoblast, and cell differentiation to hepatocytes and biliary epithelium. In addition, there is controversy about the exact role of the cation in promoting or inhibiting each step during embryogenesis. On the one hand, the TRPM6 inhibitor MEG has been reported to promote endoderm formation [16], whereas magnesium inhibits many signaling pathways: PI3K/MAPK and WNT during hepatic progenitor cells [22,23], PROX1 and MMP during bud morphogenesis [26,27], and AP-1 components during hepatoblast formation [31]. To the contrary, magnesium has been reported to exert an enhancing effect on BMP during liver progenitor cell formation [19,20] and C/EBP during hepatoblast formation [32]. Moreover, both TRPM6 and TRPM7 are essential for early development [38], whereas the role of the cation as a second messenger in stimulating PLCγ1 and JNK activity, ROS production, and NADPH generation is essential for liver development [37]. In the study where magnesium is injected and fetal development is evaluated, the results correlate with those indicating RA-mediated magnesium distribution [34]. Herein, Günther et al. characterized an effect of magnesium on modulating hormone content over fetal development as the cation remained extracellular.
Based on previous information, magnesium appears to play a key role in liver development, but 2 facts need to be considered to support this hypothesis: 1) Most studies on the effect of magnesium in signaling pathways have not been performed in a developmental context, and 2) the effect of magnesium distribution needs further elucidation. The development of new labeling methods, such as the 1 developed by Bucella’s group [9,35], may contribute to new outcomes in this field.
Magnesium in liver regeneration
The size of the liver is adjusted to the needs of the body in a ratio called "hepatostat," a homeostatic liver weight-to-bodyweight ratio that must be maintained for optimal performance [39]. To maintain it, the liver shows a unique regenerative capacity when there is a massive loss of hepatocytes and liver function. The complex mechanisms involved in this unique hepatic process include a variety of regenerative pathways that are likely tightly regulated by intracellular magnesium levels [40].
Liver regeneration occurs in 2 different ways: 1) PH, in which ≤two-thirds of the liver is removed and the remaining hepatocytes proliferate to compensate for the loss of liver tissue; and 2) the regenerative model triggered by an insult such as toxins or viral infection, in which the hepatocytes are damaged and the tissue is replaced by stem cell differentiation [41]. Depending on the size of the lost tissue and the proliferative capacity of the cellular compartments, both processes may occur in different proportions. Furthermore, the importance of regulating the regenerative capacity should be emphasized since the chronic loss of hepatocytes is associated with regenerative efforts characterized by the continuous proliferation of hepatocytes and often has adverse consequences such as the development of cirrhosis or liver cancer [39]. Hepatic progenitor cells, called oval cells because of their shape, are able to proliferate and give rise to mature hepatocytes that repopulate the organ [41]. Some studies consider them to be equivalent to hepatic stem cells, although other studies suggest that they originate from other sources in the body, particularly hematopoietic stem cells from bone marrow [42]. Despite their unknown origin, they have been extensively studied, and it is known that the transcriptional activity of nuclear factor kappa-B (NF-κB) and signal transducer and activator of transcription 3 (STAT3) is required for the activation of these cells [43]. In this process, magnesium may play a role as a repressor of signal transduction by inhibiting the action of both transcription factors [44,45]. In addition, magnesium deficiency has been reported to enhance the transcriptional remodeling of stem cells [18].
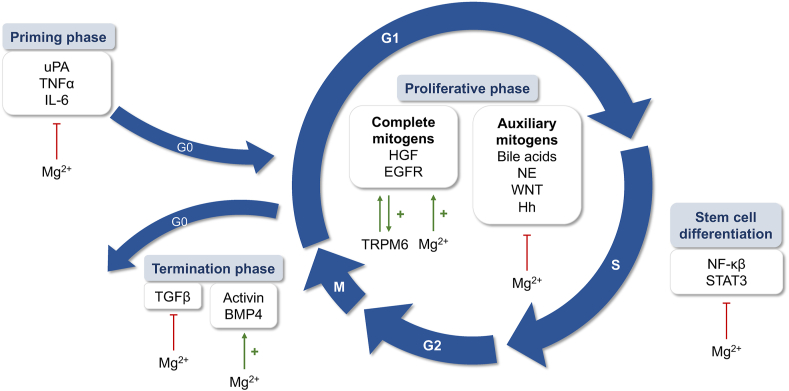
Partial resection of the liver is a common procedure in patients with hepatic neoplasms or hepatocellular carcinomas (HCC). During PH, ≤70% of the liver is removed, after which the organ regenerates within a few weeks and fully regains its original mass [46]. The overall process of liver regeneration involves 3 phases: the priming phase, in which liver cells enter the cell cycle to prepare for replication; the proliferation phase, in which cells undergo a series of cycles of cell division and expansion; and the termination phase, in which negative regulators stop the regeneration process and prevent overgrowth of liver tissue [47] (Figure 2).
FIGURE 2.
Schematic representation of liver generation and the contribution of magnesium (Mg2+) in each step of the cell cycle, with the main pathways involved.
The priming phase occurs in the early period of 0–5 h after PH and consists of the preparation of liver cells to enter the cell cycle through the mediation of cytokines and growth factors [48]. Several signaling pathways are initiated shortly after hepatectomy, of which the increase in urokinase activity is the earliest documented. Urokinase-type plasminogen activator’s (uPA) activity increases rapidly within 1 minute after PH [49], processing plasminogen to plasmin, which stimulates MMPs to trigger extracellular matrix (ECM) degradation and allowing hepatocyte proliferation [50]. Although MMPs are known for their antifibrotic effects due to their ability to degrade ECM proteins, it has been recognized that they can also exert pro-fibrotic effects, which is essential for proper ECM homeostasis [51]. Despite not being studied in the liver, magnesium has been described as inhibiting the production of MMPs in both cardiac fibroblasts and vascular smooth muscle cells [27,52], highlighting its role in limiting protease activity and thus limiting fibrosis development. In addition, uPA is also a key molecule associated with the activation of HGF during liver regeneration [53], which is sequestered in the ECM of a healthy liver and released into plasma after PH [54]. Urokinase activity also appears to be inhibited, at least in part, by magnesium [55]. The role of this element in this priming phase of ECM proteolysis has not yet been studied; however, its inhibitory effect on uPA, the main protease during regeneration initiation, suggests that decreased intracellular magnesium levels may be the key to activation of this early phase.
ECM remodeling leads to a rapid and profound change in the gene expression pattern of the hepatocytes, which simultaneously prepare for mitosis. Hepatocytes are stable cells and rarely divide, as they are quiescent in the G0 phase of the cell cycle. So, upon physical injuries, such as resection, the remaining hepatocytes must transition from the G0 to the G1 of the cell cycle to start proliferating and restoring their functional mass [56]. The re-entry into the cell cycle is mainly triggered by cytokines and growth factors released by inflammatory cells. The most studied proinflammatory cytokines are TNFα and IL-6, which are mainly released by Kupffer cells (KC) [57]. Magnesium plays an important role in maintaining host regulatory mechanisms during inflammation. Its deficiency maintains an activated state of immune cells with increased plasma levels of IL-6 and TNFα [58,59], which is detrimental to the liver and might ultimately lead to the development of liver tumors [60]. Therefore, despite an initial drop in magnesium levels after the first insult, it is essential to increase intracellular magnesium concentrations to control the effects of these proinflammatory cytokines and allow cells to progress through the cell cycle.
During the proliferative phase, hepatocytes proceed to mitosis beyond the restriction point [61]. The signaling pathways involved in this process can be divided into 2 main categories [62]: 1) complete mitogens and 2) auxiliary mitogens. The first group is able to induce liver enlargement in vivo when administered to intact nonoperated animals, and hepatocytes were grown in serum-free media in vitro by activating secondary gene responses to stimulate DNA synthesis and cell proliferation. This group includes HGF and epidermal growth factor receptor (EGFR), and its associated ligands, EGF, transforming growth factor alpha (TGF-α), amphiregulin, and heparin-binding EGF-like growth factor [63]. EGFR activation mediates its downstream effects via numerous signaling cascades. Among others, EGFR signaling triggers a redistribution of TRPM6 from the endomembrane to the plasma membrane, resulting in a regulatory mechanism for Mg2+ reabsorption [64]. This might be essential for hepatocyte proliferation, as an increase in intracellular magnesium has been described in the G1 and S phases of the cell cycle, correlating with an increase in protein synthesis and the onset of DNA synthesis [65]. EGFR-mediated magnesium homeostasis is further supported by the observation that patients treated with cetuximab, a monoclonal antibody directed against EGFR, develop hypomagnesemia [66].
In the absence of the second category of extracellular signals, auxiliary mitogens, liver regeneration is delayed but not interrupted [63]. This group includes bile acids [67], norepinephrine [68], WNT/β-cat [69], and hedgehog signaling pathways [70]. Unlike mitogens, these nonmitogenic signals have been reported to have a suppressive response to magnesium, as the cation has been shown to reduce bile acid-induced cell proliferation [71,72] and inhibit the release of norepinephrine [73]. Moreover, norepinephrine elicits marked magnesium efflux from liver cells [74], indicating a negative reciprocal regulation between them. Both the Wnt/β-cat [23,38] and Hedgehog signaling pathways [75] are also suppressed by magnesium.
In the final phase, hepatic cells cease proliferation under the control of negative factors to return the organ to its exact size. The termination phase of liver regeneration has not been studied as thoroughly as the initiation phase. However, it is known that the number of hepatocytes at the end of this process is higher than at the beginning, so apoptosis is necessary to correct this [76].
The best-known antiproliferative factors are TGF-β and its related family members. Elevated TGF-β levels in the liver are notable in the initial stages of regeneration, and after PH, a temporal wave of TGF-β increase progresses from the periportal to the perivenous regions. This is immediately followed by a wave of hepatocyte proliferation [77]. Similarly, TGF-β also acts as an apoptosis inducer by binding to its receptors [78]. As mentioned earlier, low levels of intracellular magnesium stimulate TGF-β signaling [79]. It has also been shown that the downregulation of TRPM6 expression leads to the activation of this pathway [80]. Although TGF-β is essential for the termination of regeneration, it is also an important pro-fibrotic factor, such that magnesium as its inhibitor exerts a direct effect on hepatic fibrosis [81]. Other primary members of the TGF-β family implicated in liver regeneration as negative regulators are activins and BMPs. Activin A is produced by hepatocytes and has an autocrine effect that induces both hepatocyte growth arrest and apoptosis [82]. Among BMPs, different subunits exhibit different effects; for example, BMP7 promotes hepatocyte proliferation, whereas BMP4 suppresses it [83]. It has been shown that the expression of BMP and activin A receptors is lower in the liver of Mg-deficient rats, which would prolong the proliferation phase of hepatocytes according to PH [84].
All these studies suggest that magnesium plays an important role in liver regeneration, especially in the proliferative phase by advancing hepatocytes in the cell cycle. Indeed, magnesium may have a similar biological effect as in embryogenesis, although some of the pathways studied have not yet been characterized in a regenerative context. Remarkably, low intracellular magnesium concentrations appear to occur more frequently early in the process, when the insult has happened, whereas higher intracellular magnesium concentrations are more likely to occur during the proliferation and the arrest phases. Considering the role of magnesium in modulating DNA stability and energy metabolic pathways, elucidating the timing of the aforementioned transition from low to high liver magnesium levels may be of interest.
Aging and magnesium alterations: trigger or consequence?
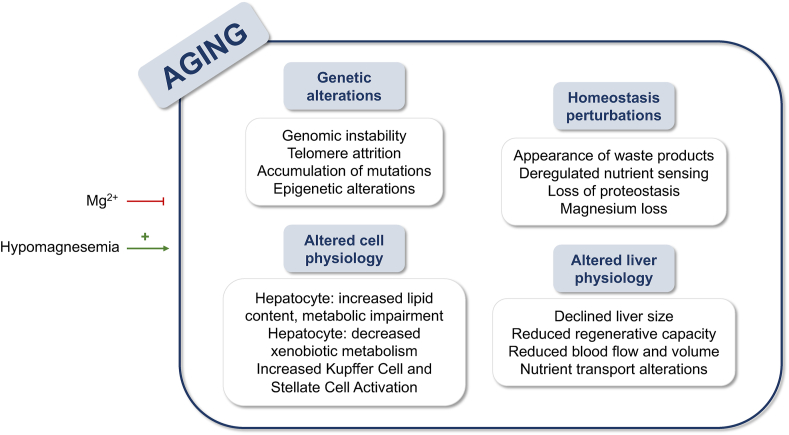
Aging is an inevitable process defined as the accumulation of deleterious changes in cells and tissues over time. Such changes have various effects on the physiology of the system and organs, and several theories explain this process on evolutionary, systems biology, molecular, and cellular bases [85]. Of importance is the role of the cation magnesium, whose contribution to the phenomena that occur has been reported by several authors and reviewed in the present manuscript (Figure 3).
FIGURE 3.
Summary of the mechanisms involved in aging, with the effect of hypomagnesemia as a contributor and the supplementation of Mg2+ as an inhibitor.
During the aging process, changes occur in the genome and the structure of the genetic material, leading to impaired liver function. In this context, genomic instability, and the accumulation of mutations is an important aspect promoted by magnesium deficiency. Hypomagnesemia leads to DNA mutations that contribute to aging and cancer development, even increasing the prevalence and risk within a few years [4]. Magnesium also regulates telomere structure, as the enzyme telomerase, which is responsible for preventing telomere degradation that occurs with aging, is dependent on magnesium [86]. In the work of Rowe [87], life extension by correction of magnesium deficiency is suggested; as they observe, a decrease in intracellular magnesium levels might lead to an increase in catecholamines and loss of cardiovascular functioning. Other genetic changes related to epigenomics have also been reported to occur during aging, with magnesium being a possible contributor. The cation plays a role in DNA assembly, chromatin folding, phase separation, and cell differentiation [88], as well as stabilizing DNA interaction with histones in the formation of nucleosomes [89]. Age-related hypomagnesemia may affect all these processes.
Among other age-related changes unrelated to the genome, loss of proteostasis and nutrient homeostasis and the appearance of waste products (ROS or lipofuscin) have also been pointed out [85]. Deregulation of nutrient perception due to neuroendocrine changes has been reported [90], and supplementation with oral magnesium can reverse such age-related neuroendocrine changes in humans [91]. The accumulation of free radicals characteristic of aging can also be reduced by magnesium supplementation, as the cation reduces the presence of inflammation and ROS [92]. Otherwise, loss of magnesium increases substance P secretion, leading to stimulation of leukocytes in the bone marrow, which may injure the liver via NF-κB secretion [93]. In the context of waste product accumulation, excess lipofuscin increases the production of ROS and decreases cell survival [94].
Regarding liver function, with age there are physiological changes that alter the normal functioning of the liver, whose size is reduced due to a decreased hepatic regenerative capacity [95], accompanied by decreased blood flow and volume with a decreased albumin content [96]. In this context, the essential role of magnesium in the formation of the ATPase complex [97] may contribute to these changes. The inefficient hepatic blood transfer that occurs with aging exacerbates the reduction in liver size, and the transport of lipoproteins and carbohydrates has also been reported to be altered. Magnesium has been described to correlate inversely with the quality of circulating carbohydrates [98], and a relationship between the cation and insulin secretion and insulin resistance (IR) has been recently studied by our group [5]. Almost 50% of patients with type 2 diabetes mellitus (T2DM) suffer from hypomagnesemia, mainly caused by low intake and increased urinary loss of magnesium [99]. Interestingly, increased intake of this cation is linked to lower body mass index, waist circumference, and serum glucose levels in patients [100].
Hepatocyte physiology is also altered; polyploidy and accumulation of dense bodies [95], together with increased lipid content [101] and impaired metabolic pathways such as glycolysis [102], β-oxidation [103], autophagy, energy sensing, and circadian rhythms [104], are characteristic of aged hepatocytes. In this context, our group has reported that magnesium modulates the lipid content of the liver [5], and its role as a cofactor in energy-processing enzymes may prevent age-related energetic impairment. Similarly, magnesium plays a role in preventing the activation of KCs [105] and the production of alpha-smooth muscle actin by hepatic stellate cells (HSC) [106]. Both KCs [107] and HSCs [108] activation has been reported to be altered with aging, resulting in an increased risk of fibrosis development. Aging also implies a decreased hepatic capacity in the metabolization of xenobiotics mediated by cytochrome P450 (CYP) [109]. Remarkably, isoforms of CYP require magnesium as a cofactor, and when they are unable to metabolize a drug due to hypomagnesemia, it may accumulate and lead to increased toxicity, increasing risk of cross-reactivity with other structurally similar compounds [110]. Magnesium also protects against other age-related hallmarks such as ROS-mediated mitochondrial dysfunction [111] and endoplasmic reticulum (ER) stress [5], whereas hypomagnesemia accelerates cellular senescence [112].
Having established that physiological changes during aging can be exacerbated by hypomagnesemia, the mechanisms underlying magnesium loss need to be elucidated. Aging is a risk factor for magnesium deficit [113], and there is an age-related decline in intracellular magnesium levels [114], accompanied by inadequate intake by the elderly, according to the NHANES III survey [115]. Similarly, with age, there is decreased reabsorption of the cation in the renal loop of Henle and increased urine loss due to changes in vitamin D [116]. Moreover, in old age, the absorption of various drugs is increased, although the ability of the liver to metabolize them decreases, enhancing their urinary secretion and contributing to hypomagnesemia [117]. The occurrence of other pathologies such as IR, diabetes, acetaminophen-induced liver injury [40], alcohol intake, or stroke also decreases magnesium reabsorption.
Aging and liver diseases
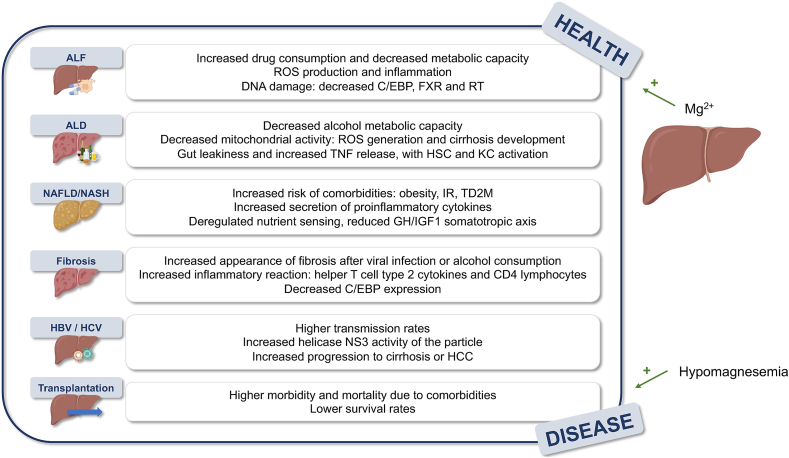
Over the years, the liver becomes more susceptible to the development of pathologies. Since it is responsible for the metabolism of compounds and xenobiotics as well as the elimination of certain toxic metabolites produced in the organism, the accumulation of impairments can lead to the development of chronic pathologies that are aggravated by aging (Figure 4). The following is an overview of the role of magnesium in these changes.
FIGURE 4.
Summary of age-derived aggravations of liver pathologies where Mg2+ has a preventive effect and hypomagnesemia increases risk of development. Abbreviations list: ALD, alcoholic liver disease; ALF, acute liver failure; C/EBP, CCAAT-enhanced-binding proteins; FXR, farnesoid R receptor; HBV, hepatitis B; HCC, hepatocellular carcinoma; HSC, hepatic stellate cell; IR, insulin resistance; KC, Kupffer cells; NASH, NAFLD encompasses a spectrum of diseases ranging from steatosis to steatohepatitis; NAFLD, nonlcoholic fatty liver disease; ROS, reactive oxygen species; RT, reverse telomerase; T2DM, type 2 diabetes mellitus.
The previously mentioned decrease in the ability to metabolize drugs increases risk of developing acute liver failure (ALF), which is exacerbated by the increased consumption of drugs that is characteristic of aging. In this regard, increased ROS production and inflammation occur during aging, aggravating liver injury under toxic exogenous compounds [118], with damage to DNA causing inadequate repair capacity [119] and decreased expression of C/EBP, farnesoid R receptor, and reverse telomerase [120]. Hypomagnesemia occurring during aging may contribute to the amplification of these phenomena, as magnesium not only contributes to the regulation of oxidative metabolism and the production of ROS [37] but is also essential for genomic stability [121] and improves C/EBP-DNA binding [32].
The prevalence of alcohol-related liver complications increases with age, as ∼79% of elderly patients with alcoholic liver disease (ALD) have cirrhosis, 40% of ALD cirrhotic patients develop hepatitis, and 15%–25% of hepatitis patients die [122]. Similar to drug metabolism, the activity of enzymes that metabolize alcohol decreases with age [123], which, together with increased drug intake, leads to an exacerbated pathology. Decreased mitochondrial activity promotes the formation of ROS and the development of cirrhosis [123], whereas gut leakiness boosts the generation of TNF, favoring the exacerbation of ALD by activated KCs [105], a process accelerated by hypomagnesemia [106]. In a vicious cycle, alcohol consumption increases magnesium loss with enhanced urinary excretion [4], and as a result, the effect of toll-like receptor 4 (TLR4) worsens ALD, activating KCs that secrete TNF, interleukin 1 (IL1), TGFβ, and IL6, which activate HSCs to release TNF and ROS [124].
Similar to ALD, the prevalence of nonalcoholic fatty liver disease (NAFLD) is increased in the elderly population [125]. NAFLD encompasses a spectrum of diseases ranging from steatosis to steatohepatitis (NASH) and fibrosis to HCC [125]. Although the prevalence of NAFLD is estimated to be ∼25% worldwide, it increases by ≤35% in patients ≥65 y of age [126]. In our study, we demonstrated an inverse association between hepatocyte magnesium content and lipid accumulation in the development of NASH and showed that silencing the magnesium effluxer CNNM4 ameliorates NASH by increasing intracellular magnesium. This leads to a reduction in ER stress, oxidative stress, steatosis, and fibrosis [9].
Aging is often accompanied by other comorbidities, such as obesity, which causes IR and secretion of proinflammatory cytokines leading to the development of metabolic syndrome and T2DM [127]. This is due to the aforementioned nutrient sensing, in which the effect of the somatotropic axis of growth hormone (GH) and insulin growth factor 1 is reduced [127]. Remarkably, magnesium has been reported to have an effect on GH [128] and its supplementation reverses neuro-endocrine changes in humans [91].
Risk of fibrosis development also increases with age. Although fibrosis is the response to chronic liver injury, disorders have been reported to occur with an increased tendency in elderly patients with viral infections [129] or excessive alcohol consumption [130]. Moreover, there is an increased inflammatory response of CD4 lymphocytes and macrophages expressing helper T-cell type 2 cytokines [131], whereas magnesium supplementation affects cytokine secretion by decreasing proinflammatory IL5 and IL13 and increasing anti-inflammatory interferon γ (IFNγ) [132]. In murine models of carbon tetrachloride intoxication, which are commonly used to study fibrosis [133], there is an increased fibrotic response in older mice with decreased C/EBP expression [131], similar to drug-induced liver injury (DILI), in which magnesium homeostasis dysregulation leads to ROS production and ER stress [40].
Although the prevalence of viral hepatitis has almost disappeared with the appearance of the drug sofosbuvir, an antiviral drug for the treatment of chronic hepatitis C, there are still some patients suffering from either B or C viral infection, with elderly patients having a higher transmission rate [134] and patients ≤65 y of age having a lower sustained viral response [135]. In this aspect, magnesium contributes to the prevention of viral infection by directly binding to the NS3 helicase and downregulating the capacity of the viral particle [136]. As mentioned earlier, the progression of viral hepatitis to fibrosis is lower in patients ≤40 y of age [129], and in them, hypomagnesemia worsens the cirrhotic response [4]. In addition, magnesium may also prevent the increased development of HCC due to viral infection, which is exacerbated by age, as the cation prevents protein phosphatase magnesium-dependent 1A and blocks TGFβ-dependent SMAD2/3 dephosphorylation, preventing the transcription of several genes required for HCC [79].
Finally, aging affects liver physiology during liver transplantation. This is a life-saving procedure for patients with end-stage liver disease. However, the shortage of organ donors significantly affects transplantation rates, which meet <10% of global demand [36]. The need for liver transplantation increases with age; however, high morbidity and mortality due to comorbidities are frequently observed in elderly people. In recipients ≥60 y, survival rates range from 90% to 64% in men and from 70% to 59% in women [137]. Supplementation with magnesium before transplantation has shown improved prognosis by attenuating the ischemia-reperfusion injury [33], representing a new approach to ameliorating the procedure.
In conclusion, magnesium plays a key role in the prevention of aging and the increased prevalence of liver pathologies, with hypomagnesemia indicating a possible contribution to the associated changes. Hypomagnesemia leads to mutations in DNA [4], characteristic of aging, and to the elevation of catecholamines [87], which actually promote hypomagnesemia, leading to a vicious cycle. Magnesium also prevents the loss of proteostasis and nutrient sensing that occur during aging. Moreover, the inevitable loss of magnesium that occurs during aging may contribute to the observed increased prevalence of liver pathologies in elderly patients. Magnesium generally plays a role in regulating ROS production, preventing the development and progression of pathologies such as ALF, ALD, and DILI caused by acetaminophenoverdose [40], or the occurrence of fibrosis and cirrhosis in NAFLD. The role of the cation in modulating the immune response may lead to hypomagnesemia to enhance fibrosis during aging by regulating the inflammatory response by CD4 lymphocytes [131]. Regarding liver pathologies with viral etiology, age-related hypomagnesemia may contribute to the increased prevalence of HBV and HCV in elderly patients because the cation prevents the activity of helicase NS3 and downregulates viral transmission [129,134]. Thus, the progression of all pathologies to HCC, whose risk is increased in certain pathologies such as HBV, HCV, or NAFLD, is increased under hypomagnesemic conditions, which are more common in the elderly [79].
Magnesium nutritional data
Magnesium is an essential micronutrient in the daily human diet due to its role in various biological processes and metabolic pathways. Several studies have shown that increased magnesium intake is associated with a reduction in the development and progression of pathologies such as NAFLD, metabolic syndrome, and type 2 diabetes [4,138,139]. Moreover, magnesium deficiency is also associated with disease progression and a higher risk of liver-related mortality in ALD [140].
Magnesium deficiency is also associated with oxidative stress in different tissues and organs [141]. Martin and colleagues observed that a deficiency of this cation negatively affects hepatocyte survival through oxidative stress-induced apoptosis. However, these authors did not observe a reduction in apoptosis by magnesium supplementation [140]. Other authors have indicated that magnesium deficiency is related to an acceleration of cellular senescence, making magnesium supplementation a beneficial approach to preventing aging [142].
Finally, according to the NHANES III survey, magnesium intake in the general population is inadequate, contributing to hypomagnesemia and reducing the protective role magnesium plays in our organism. Maintaining an adequate diet consuming magnesium-rich sources, as summarized in Table 1 [143], may help in the prevention of age-related hepatic alterations and, in the meantime, in the maintenance of hepatic homeostasis to avoid the development of liver pathologies. As shown in the table, magnesium-rich sources include a variety of foods, so a varied and balanced diet can meet both macronutrient and micronutrient needs.
TABLE 1.
Major dietary sources of magnesium. USDA Food Data Central database. Daily values were calculated using daily values published by the FDA.
| Source | mg magnesium per 100 g serving |
|---|---|
| Hemps seeds | 700 |
| Pumpkin seeds | 535 |
| Flax seeds | 392 |
| Brazil nuts | 376 |
| Boiled spinach | 122 |
| Whole wheat bread | 66 |
| Rice | 66 |
| Kidney beans | 55 |
| Baked potato | 43 |
| Raisins | 36 |
| Avocado | 34 |
| Farmed Atlantic Salmon | 30 |
| Cooked halibut | 30 |
| Chicken breast | 30 |
| Beef | 30 |
| Broccoli | 19 |
| Yogur | 19 |
| White rice | 16 |
| Milk | 11 |
| Apple | 9 |
| Raw carrots | 7 |
In conclusion, overall, maintaining homeostasis of magnesium, the most abundant divalent cation in the cell, is essential for the proper functioning of the liver and the entire organism. In this work, we have reviewed the potential role of magnesium in embryogenesis, liver regeneration, and aging. The cation seems to play a similar role during embryogenesis and liver regeneration, but further research in specific developmental and regenerative contexts is required to fully understand it, and novel magnesium labeling methods may be helpful. As individuals age, risk of developing hypomagnesemia increases, a condition that exacerbates age-related alterations. This condition may also contribute to the development of liver pathologies. Therefore, to prevent age-related hepatic alterations and contribute to the maintenance of hepatic homeostasis, magnesium loss must be prevented by adequate intake of magnesium-rich foods such as seeds, nuts, spinach, or rice.
Acknowledgments
We thank Ministerio de Ciencia e Innovación, Programa Retos-Colaboración RTC2019-007125-1 (for J.S. and M.L.M.-C.); Instituto de Salud Carlos III: Proyectos de Investigación en Salud DTS20/00138 (for J.S. and M.L.M.-C.); CIBERehd: EHD21TRF01/2022 (to M.L.M.-C.); Departamento de Industria del Gobierno Vasco (for M.L.M.-C.); Ministerio de Ciencia, Innovación y Universidades MICINN: PID2020-117116RB-I00 integrado en el Plan Estatal de Investigación Cientifica y Técnica y Innovación, cofinanciado con Fondos FEDER (for M.L.M.-C); BIOEF (Basque Foundation for Innovation and Health Research); Asociación Española contra el Cáncer (AECC) (to M.L.M.-C.); Fundación Científica de la Asociación Española Contra el Cancer (AECC Scientific Foundation) Rare Tumor Calls 2017 (for M.L.M.); La Caixa Foundation Program (for M.L.M.); Ministerio de Ciencia, Innovación y universidades PID2019- 109055RB-I00 (L.A.M.-C.); Spanish Ministry of Economy and Competitiveness Grants BFU2013-47531-R and BFU2016-77408-R (L.A.M.-C.) and the FIGHT- CNNM2 project from the EJP RD Joint Transnational Call (JTC2019) (Ref. AC19/00073) (for L.A.M.-C); CIBERehd was funded by the Instituto de Salud Carlos III and Cofunded by FEDER funds.
Author disclosures
The authors report no conflicts of interest.
Contributor Information
Naroa Goikoetxea-Usandizaga, Email: ngoikoetxea@cicbiogune.es.
María Luz Martínez-Chantar, Email: mlmartinez@cicbiogune.es.
References
- 1.van den Berghe G. The role of the liver in metabolic homeostasis: implications for inborn errors of metabolism. J. Inherit. Metab. Dis. 1991;14(4):407–420. doi: 10.1007/BF01797914. [DOI] [PubMed] [Google Scholar]
- 2.Grant D.M. Detoxification pathways in the liver. J. Inherit. Metab. Dis. 1991;14(4):421–430. doi: 10.1007/BF01797915. [DOI] [PubMed] [Google Scholar]
- 3.Michalopoulos G.K., Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021;18(1):40–55. doi: 10.1038/s41575-020-0342-4. [DOI] [PubMed] [Google Scholar]
- 4.Liu M., Yang H., Mao Y. Magnesium and liver disease. Ann. Transl. Med. 2019;7(20):578. doi: 10.21037/atm.2019.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simón J., Delgado T.C., Martinez-Cruz L.A., Martínez-Chantar M.L. Magnesium, little known but possibly relevant: a link between NASH and related comorbidities. Biomedicines. 2021;9(2):125. doi: 10.3390/biomedicines9020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Baaij J.H.F., Hoenderop J.G.J., Bindels R.J.M. Magnesium in man: implications for health and disease. Physiol. Rev. 2015;95(1):1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 7.Alexander R.T., Hoenderop J.G., Bindels R.J. Molecular determinants of magnesium homeostasis: insights from human disease. J. Am. Soc. Nephrol. 2008;19(8):1451–1458. doi: 10.1681/ASN.2008010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jahnen-dechent W., Ketteler M. Magnesium basics. Clin. Kidney J. 2012;5(Suppl. 1):i3–i14. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simón J., Goikoetxea-Usandizaga N., Serrano-Maciá M., Fernández-Ramos D., Sáenz de Urturi D., Gruskos J.J., et al. Magnesium accumulation upon cyclin M4 silencing activates microsomal triglyceride transfer protein improving NASH. J. Hepatol. 2021;75(1):34–45. doi: 10.1016/j.jhep.2021.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goytain A., Quamme G.A. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. B.M.C. Genomics. 2005;6:48. doi: 10.1186/1471-2164-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quamme G.A. Molecular identification of ancient and modern mammalian magnesium transporters. Am. J. Physiol. Cell Physiol. 2010;298(3):C407–C429. doi: 10.1152/ajpcell.00124.2009. https://doi.org/10.038/ng889. [DOI] [PubMed] [Google Scholar]
- 12.Schlingmann K.P., Weber S., Peters M., Niemann Nejsum L., Vitzthum H., Klingel K., et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 2002;31(2):166–170. doi: 10.1152/ajpcell.00124.2009. [DOI] [PubMed] [Google Scholar]
- 13.Kolisek M., Zsurka G., Samaj J., Weghuber J., Schweyen R.J., Schweigel M. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. E.M.B.O. J. 2003;22(6):1235–1244. doi: 10.1093/emboj/cdg122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordillo M., Evans T., Gouon-Evans V. Orchestrating liver development. Development. 2015;142(12):2094–2108. doi: 10.1242/dev.114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zorn A.M., Wells J.M. Molecular basis of vertebrate endoderm development. Int. Rev. Cytol. 2007;259:49–111. doi: 10.1016/S0074-7696(06)59002-3. [DOI] [PubMed] [Google Scholar]
- 16.Geng Y., Feng B. Mesendogen, a novel inhibitor of TRPM6, promotes mesoderm and definitive endoderm differentiation of human embryonic stem cells through alteration of magnesium homeostasis. Heliyon. 2015;1(4) doi: 10.1016/j.heliyon.2015.e00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bossard P., Zaret K.S. Repressive and restrictive mesodermal interactions with gut endoderm: possible relation to Meckel’s diverticulum. Development. 2000;127(22):4915–4923. doi: 10.1242/dev.127.22.4915. [DOI] [PubMed] [Google Scholar]
- 18.Sargenti A., Castiglioni S., Olivi E., Bianchi F., Cazzaniga A., Farruggia G., et al. Magnesium deprivation potentiates human mesenchymal stem cell transcriptional remodeling. Int. J. Mol. Sci. 2018;19(5):1410. doi: 10.3390/ijms19051410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding S., Zhang J., Tian Y., Huang B., Yuan Y., Liu C. Magnesium modification up-regulates the bioactivity of bone morphogenetic Protein-2 upon calcium phosphate cement via enhanced BMP receptor recognition and Smad signaling pathway. Colloids Surf. B Biointerfaces. 2016;145:140–151. doi: 10.1016/j.colsurfb.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda-Taira S. Hepatic induction in the avian embryo: specificity of reactive endoderm and inductive mesoderm. J. Embryol. Exp. Morphol. 1981;63:111–125. doi: 10.1242/dev.63.1.111. [DOI] [PubMed] [Google Scholar]
- 21.Calmont A., Wandzioch E., Tremblay K.D., Minowada G., Kaestner K.H., Martin G.R., et al. An FGF Response pathway that mediates hepatic gene induction in embryonic endoderm cells. Dev. Cell. 2006;11(3):339–348. doi: 10.1016/j.devcel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Yang Z., Wang J., Altura B.T., Altura B.M. Extracellular magnesium deficiency induces contraction of arterial muscle: role of PI3-kinases and MAPK signaling pathways. Pflugers Arch. 2000;439(3):240–247. doi: 10.1007/s004249900179. [DOI] [PubMed] [Google Scholar]
- 23.Montes de Oca A., Guerrero F., Martinez-Moreno J.M., Madueño J.A., Herencia C., Peralta A., et al. Magnesium inhibits Wnt/β-catenin activity and reverses the osteogenic transformation of vascular smooth muscle cells. PLOS ONE. 2014;9(2) doi: 10.1371/journal.pone.0089525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bort R., Signore M., Tremblay K., Martinez Barbera J.P.M., Zaret K.S. Hex homeobox gene controls the transition of the endoderm to a pseudostratified, cell emergent epithelium for liver bud development. Dev. Biol. 2006;290(1):44–56. doi: 10.1016/j.ydbio.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Burke Z., Oliver G. Prox1 is an early specific marker for the developing liver and pancreas in the mammalian foregut endoderm. Mech. Dev. 2002;118(1-2):147–155. doi: 10.1016/s0925-4773(02)00240-x. [DOI] [PubMed] [Google Scholar]
- 26.Schnoz C., Moser S., Kratschmar D.V., Odermatt A., Loffing-Cueni D., Loffing J. Deletion of the transcription factor Prox-1 specifically in the renal distal convoluted tubule causes hypomagnesemia via reduced expression of TRPM6 and NCC. Pflugers Arch. 2021;473(1):79–93. doi: 10.1007/s00424-020-02491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue H., Lee J.D., Shimizu H., Uzui H., Mitsuke Y., Ueda T. Effects of magnesium on the production of extracellular matrix metalloproteinases in cultured rat vascular smooth muscle cells. Atherosclerosis. 2003;166(2):271–277. doi: 10.1016/s0021-9150(02)00390-8. [DOI] [PubMed] [Google Scholar]
- 28.Di Francesco A., Desnoyer R.W., Covacci V., Wolf F.I., Romani A., Cittadini A., et al. Changes in magnesium content and subcellular distribution during retinoic acid-induced differentiation of HL60 cells. Arch. Biochem. Biophys. 1998;360(2):149–157. doi: 10.1006/abbi.1998.0937. [DOI] [PubMed] [Google Scholar]
- 29.Wright T.G., Tsai J., Jia Z., Elliott B.E. Inhibition by copper(II) binding of hepatocyte growth factor (HGF) interaction with its receptor met and blockade of HGF/Met function. J. Biol. Chem. 2004;279(31):32499–32506. doi: 10.1074/jbc.M405043200. [DOI] [PubMed] [Google Scholar]
- 30.Yu X., Guan P.P., Zhu D., Liang Y.Y., Wang T., Wang Z.Y., et al. Magnesium ions inhibit the expression of tumor necrosis factor α and the activity of γ-secretase in a β-amyloid protein-dependent mechanism in APP/PS1 transgenic mice. Front. Mol. Neurosci. 2018;11:172. doi: 10.3389/fnmol.2018.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C.-H., Tsai P.-S., Huang C.-J. Magnesium sulfate inhibits activator protein-1 upregulation in endotoxin-activated murine macrophages. Tzu Chi Med. J. 2010;22(4):177–183. doi: 10.1016/S1016-3190(10)60068-7. [DOI] [Google Scholar]
- 32.Moll J.R., Acharya A., Gal J., Mir A.A., Vinson C. Magnesium is required for specific DNA binding of the CREB B-ZIP domain. Nucleic Acids Res. 2002;30(5):1240–1246. doi: 10.1093/nar/30.5.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J.E., Jeon J.P., Choi J.H., Lee S.H., Ryu K.H., et al. The effects of magnesium pretreatment on reperfusion injury during living donor liver transplantation. Korean J. Anesthesiol. 2011;60(6):408–415. doi: 10.4097/kjae.2011.60.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Günther T., Vormann J., Merker H.J. Effect of magnesium injection on foetal development. J. Clin. Chem. Clin. Biochem. 1984;22(7):473–478. doi: 10.1515/cclm.1984.22.7.473. [DOI] [PubMed] [Google Scholar]
- 35.Gruskos J.J., Zhang G., Buccella D. Visualizing compartmentalized cellular Mg2+ on demand with small-molecule fluorescent sensors. J. Am. Chem. Soc. 2016;138(44):14639–14649. doi: 10.1021/jacs.6b07927. [DOI] [PubMed] [Google Scholar]
- 36.Moon D.B., Lee S.G. Liver transplantation. Gut Liver. 2009;3(3):145–165. doi: 10.5009/gnl.2009.3.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi T., Kaneda M., Kakinuma K. Essential requirement of magnesium ion for optimal activity of the NADPH oxidase of Guinea Pig polymorphonuclear leukocytes. Biochem. Biophys. Res. Commun. 1983;115(1):261–267. doi: 10.1016/0006-291x(83)90998-1. [DOI] [PubMed] [Google Scholar]
- 38.Komiya Y., Su L.T., Chen H.C., Habas R., Runnels L.W. Magnesium and embryonic development. Magnes. Res. 2014;27(1):1–8. doi: 10.1684/mrh.2014.0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michalopoulos G.K. Hepatostat: liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65(4):1384–1392. doi: 10.1002/hep.28988. [DOI] [PubMed] [Google Scholar]
- 40.González-Recio I., Simón J., Goikoetxea-Usandizaga N., Serrano-Maciá M., Mercado-Gómez M., Rodríguez-Agudo R., et al. Restoring cellular magnesium balance through cyclin M4 protects against acetaminophen-induced liver damage. Nat. Commun. 2022;13(1):6816. doi: 10.1038/s41467-022-34262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evarts R.P., Nagy P., Nakatsukasa H., Marsden E., Thorgeirsson S.S. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989;49(6):1541–1547. [PubMed] [Google Scholar]
- 42.Lagasse E., Connors H., Al-Dhalimy M., Reitsma M., Dohse M., Osborne L., et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000;6(11):1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 43.Sánchez A., Factor V.M., Schroeder I.S., Nagy P., Thorgeirsson S.S. Activation of NF-kappaB and STAT3 in rat oval cells during 2-acetylaminofluorene/partial hepatectomy-induced liver regeneration. Hepatology. 2004;39(2):376–385. doi: 10.1002/hep.20040. [DOI] [PubMed] [Google Scholar]
- 44.Zhao X.J., Yang Y.Z., Zheng Y.J., Wang S.C., Gu H.M., Pan Y., et al. Magnesium isoglycyrrhizinate blocks fructose-induced hepatic NF-κB/NLRP3 inflammasome activation and lipid metabolism disorder. Eur. J. Pharmacol. 2017;809:141–150. doi: 10.1016/j.ejphar.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 45.Tang G.H., Yang H.Y., Zhang J.C., Ren J.J., Sang X.T., Lu X., et al. Magnesium isoglycyrrhizinate inhibits inflammatory response through STAT3 pathway to protect remnant liver function. World J. Gastroenterol. 2015;21(43):12370–12380. doi: 10.3748/wjg.v21.i43.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fausto N., Campbell J.S., Riehle K.J. Liver regeneration. Hepatology. 2006;43(2):S45–S53. doi: 10.1002/hep.20969. Suppl. 1. [DOI] [PubMed] [Google Scholar]
- 47.Tao Y., Wang M., Chen E., Tang H. Liver regeneration: analysis of the main relevant signaling molecules. Mediators Inflamm. 2017:4256352. doi: 10.1155/2017/4256352. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michalopoulos G.K. Liver regeneration. J. Cell. Physiol. 2007;213(2):286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim T.H., Mars W.M., Stolz D.B., Petersen B.E., Michalopoulos G.K. Extracellular matrix remodeling at the early stages of liver regeneration in the rat. Hepatology. 1997;26(4):896–904. doi: 10.1002/hep.510260415. [DOI] [PubMed] [Google Scholar]
- 50.Kim T.H., Mars W.M., Stolz D.B., Michalopoulos G.K. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology. 2000;31(1):75–82. doi: 10.1002/hep.510310114. [DOI] [PubMed] [Google Scholar]
- 51.Duarte S., Baber J., Fujii T., Coito A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015;44–46:147–156. doi: 10.1016/j.matbio.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yue H., Uzui H., Lee J.D., Shimizu H., Ueda T. Effects of magnesium on matrix metalloproteinase-2 production in cultured rat cardiac fibroblasts. Basic Res. Cardiol. 2004;99(4):257–263. doi: 10.1007/s00395-004-0472-9. [DOI] [PubMed] [Google Scholar]
- 53.Naldini L., Tamagnone L., Vigna E., Sachs M., Hartmann G., Birchmeier W., et al. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. E.M.B.O. J. 1992;11(13):4825–4833. doi: 10.1002/j.1460-2075.1992.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masumoto A., Yamamoto N. Sequestration of a hepatocyte growth factor in extracellular matrix in normal adult rat liver. Biochem. Biophys. Res. Commun. 1991;174(1):90–95. doi: 10.1016/0006-291x(91)90489-t. [DOI] [PubMed] [Google Scholar]
- 55.van Aswegen C.H., Dirksen van Sckalckwyk J.C.D., du Toit P.J., Verster L., Franz R.C., du Plessis D.J. The effect of calcium and magnesium ions on urinary urokinase and sialidase activity. Urol. Res. 1992;20(1):41–44. doi: 10.1007/BF00294333. [DOI] [PubMed] [Google Scholar]
- 56.Fabris G., Dumortier O., Pisani D.F., Gautier N., Van Obberghen E. Amino acid-induced regulation of hepatocyte growth: possible role of drosha. Cell Death Dis. 2019;10(8):566. doi: 10.1038/s41419-019-1779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karin M., Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529(7586):307–315. doi: 10.1038/nature17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malpuech-Brugère C., Nowacki W., Daveau M., Gueux E., Linard C., Rock E., et al. Inflammatory response following acute magnesium deficiency in the rat. Biochim. Biophys. Acta. 2000;1501(2–3):91–98. doi: 10.1016/s0925-4439(00)00018-1. [DOI] [PubMed] [Google Scholar]
- 59.Malpuech-Brugère C., Nowacki W., Rock E., Gueux E., Mazur A., Rayssiguier Y. Enhanced tumor necrosis factor-α production following endotoxin challenge in rats is an early event during magnesium deficiency. Biochim. Biophys. Acta. 1999;1453(1):35–40. doi: 10.1016/s0925-4439(98)00081-7. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt-Arras D., Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. J. Hepatol. 2016;64(6):1403–1415. doi: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Kang L.I., Mars W.M., Michalopoulos G.K. Signals and cells involved in regulating liver regeneration. Cells. 2012;1(4):1261–1292. doi: 10.3390/cells1041261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michalopoulos G.K. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am. J. Pathol. 2010;176(1):2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Apte U., Limaye P.B., Michalopoulos G.K. Liver Regeneration: Basic Mechanisms, Relevant Models and Clinical Applications. Academic Press; Cambridge: 2015. Chapter 5, Extracellular signals involved in liver regeneration: direct and auxiliary mitogens; pp. 65–75. [Google Scholar]
- 64.Thebault S., Alexander R.T., Tiel Groenestege W.M.T., Hoenderop J.G., Bindels R.J. EGF increases TRPM6 activity and surface expression. J. Am. Soc. Nephrol. 2009;20(1):78–85. doi: 10.1681/ASN.2008030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maier J.A. Encyclopedia of Metalloproteins. Springer; New York: 2013. Magnesium and cell cycle; pp. 1227–1232. [Google Scholar]
- 66.Pietropaolo G., Pugliese D., Armuzzi A., Guidi L., Gasbarrini A., Rapaccini G.L., et al. Magnesium absorption in intestinal cells: evidence of cross-talk between Egf and Trpm6 and novel implications for cetuximab therapy. Nutrients. 2020;12(11):1–11. doi: 10.3390/nu12113277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang W., Ma K., Zhang J., Qatanani M., Cuvillier J., Liu J., et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312(5771):233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 68.Oben J.A., Roskams T., Yang S., Lin H., Sinelli N., Torbenson M., et al. Hepatic fibrogenesis requires sympathetic neurotransmitters. Gut. 2004;53(3):438–445. doi: 10.1136/gut.2003.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monga S.P.S., Pediaditakis P., Mule K., Stolz D.B., Michalopoulos G.K. Changes in Wnt/β-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33(5):1098–1109. doi: 10.1053/jhep.2001.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ochoa B., Syn W.K., Delgado I., Karaca G.F., Jung Y., Wang J., et al. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology. 2010;51(5):1712–1723. doi: 10.1002/hep.23525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eshraghi T., Eidi A., Mortazavi P., Asghari A., Tavangar S.M. Magnesium protects against bile duct ligation-induced liver injury in male Wistar rats. Magnes. Res. 2015;28(1):32–45. doi: 10.1684/mrh.2015.0380. [DOI] [PubMed] [Google Scholar]
- 72.Wang A., Yoshimi N., Tanaka T., Mori H. The inhibitory effect of magnesium hydroxide on the bile acid-induced cell proliferation of colon epithelium in rats with comparison to the action of calcium lactate. Carcinogenesis. 1994;15(11):2661–2663. doi: 10.1093/carcin/15.11.2661. [DOI] [PubMed] [Google Scholar]
- 73.Shimosawa T., Takano K., Ando K., Fujita T. Magnesium inhibits norepinephrine release by blocking N-type calcium channels at peripheral sympathetic nerve endings. Hypertension. 2004;44(6):897–902. doi: 10.1161/01.HYP.0000146536.68208.84. [DOI] [PubMed] [Google Scholar]
- 74.Romani A., Scarpa A. Norepinephrine evokes a marked Mg2+ efflux from liver cells. F.E.B.S. Lett. 1990;269(1):37–40. doi: 10.1016/0014-5793(90)81113-3. [DOI] [PubMed] [Google Scholar]
- 75.Lu C., Xu W., Shao J., Zhang F., Chen A., Zheng S. Blockade of hedgehog pathway is required for the protective effects of magnesium isoglycyrrhizinate against ethanol-induced hepatocyte steatosis and apoptosis. I.U.B.M.B. Life. 2017;69(7):540–552. doi: 10.1002/iub.1639. [DOI] [PubMed] [Google Scholar]
- 76.Sakamoto T., Liu Z., Murase N., Ezure T., Yokomuro S., Poli V., et al. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology. 1999;29(2):403–411. doi: 10.1002/hep.510290244. [DOI] [PubMed] [Google Scholar]
- 77.Jirtle R.L., Carr B.I., Scott C.D. Modulation of insulin-like growth factor-II/mannose 6-phosphate receptors and transforming growth factor-Β1 during liver regeneration. J. Biol. Chem. 1991;266(33):22444–22450. doi: 10.1016/S0021-9258(18)54592-0. [DOI] [PubMed] [Google Scholar]
- 78.Romero-Gallo J., Sozmen E.G., Chytil A., Russell W.E., Whitehead R., Parks W.T., et al. Inactivation of TGF-β signaling in hepatocytes results in an increased proliferative response after partial hepatectomy. Oncogene. 2005;24(18):3028–3041. doi: 10.1038/sj.onc.1208475. [DOI] [PubMed] [Google Scholar]
- 79.Liu Y., Xu Y., Ma H., Wang B., Xu L., Zhang H., et al. Hepatitis B virus X protein amplifies TGF-β promotion on HCC motility through down-regulating PPM1a. Oncotarget. 2016;7(22):33125–33135. doi: 10.18632/oncotarget.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu H.Y., Wu J.L., Ni Z.L. Overexpression of microRNA-202-3p protects against myocardial ischemia-reperfusion injury through activation of TGF-β1/Smads signaling pathway by targeting TRPM6. Cell Cycle. 2019;18(5):621–637. doi: 10.1080/15384101.2019.1580494. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Tee J.K., Peng F., Tan Y.L., Yu B., Ho H.K. Magnesium isoglycyrrhizinate ameliorates fibrosis and disrupts TGF-β-mediated SMAD pathway in activated hepatic stellate cell line LX2. Front. Pharmacol. 2018;9:1018. doi: 10.3389/fphar.2018.01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takamura K., Tsuchida K., Miyake H., Tashiro S., Sugino H. Activin and activin receptor expression changes in liver regeneration in rat. J. Surg. Res. 2005;126(1):3–11. doi: 10.1016/j.jss.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 83.Do N., Zhao R., Ray K., Ho K., Dib M., Ren X., et al. BMP4 is a novel paracrine inhibitor of liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303(11):G1220–G1227. doi: 10.1152/ajpgi.00105.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ishizaki N., Kotani M., Funaba M., Matsui T. Hepcidin expression in the liver of rats fed a magnesium-deficient diet. Br. J. Nutr. 2011;106(8):1169–1172. doi: 10.1017/S0007114511001553. [DOI] [PubMed] [Google Scholar]
- 85.Jayanthi P., Joshua E., Ranganathan K. Ageing and its implications. J. Oral Maxillofac. Pathol. 2010;14(2):48–51. doi: 10.4103/0973-029X.72500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maguire D., Neytchev O., Talwar D., McMillan D., Shiels P.G. Telomere homeostasis: interplay with magnesium. Int. J. Mol. Sci. 2018;19(1):157. doi: 10.3390/ijms19010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rowe W.J. Correcting magnesium deficiencies may prolong life. Clin. Interv. Aging. 2012;7:51–54. doi: 10.2147/CIA.S28768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ohyama T. New aspects of magnesium function: a key regulator in nucleosome self-assembly, chromatin folding and phase separation. Int. J. Mol. Sci. 2019;20(17):4232. doi: 10.3390/ijms20174232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takaya J., Iharada A., Okihana H., Kaneko K. Magnesium deficiency in pregnant rats alters methylation of specific cytosines in the hepatic hydroxysteroid dehydrogenase-2 promoter of the offspring. Epigenetics. 2011;6(5):573–578. doi: 10.4161/epi.6.5.15220. [DOI] [PubMed] [Google Scholar]
- 90.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Held K., Antonijevic I.A., Künzel H., Uhr M., Wetter T.C., Golly I.C., et al. Oral Mg(2+) supplementation reverses age-related neuroendocrine and sleep EEG changes in humans. Pharmacopsychiatry. 2002;35(4):135–143. doi: 10.1055/s-2002-33195. [DOI] [PubMed] [Google Scholar]
- 92.Garcia L.A., DeJong S.C., Martin S.M., Smith R.S., Buettner G.R., Kerber R.E. Magnesium reduces free radicals in an in vivo coronary occlusion-reperfusion model. J. Am. Coll. Cardiol. 1998;32(2):536–539. doi: 10.1016/s0735-1097(98)00231-9. [DOI] [PubMed] [Google Scholar]
- 93.Liu X., Green R.M. Endoplasmic reticulum stress and liver diseases. Liver Res. 2019;3(1):55–64. doi: 10.1016/j.livres.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Höhn A., Grune T. Lipofuscin: formation, effects and role of macroautophagy. Redox Biol. 2013;1(1):140–144. doi: 10.1016/j.redox.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schmucker D.L. Age-related changes in liver structure and function: implications for disease. Exp. Gerontol. 2005;40(8-9):650–659. doi: 10.1016/j.exger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 96.Tietz N.W., Shuey D.F., Wekstein D.R. Laboratory values in fit aging individuals--sexagenarians through centenarians. Clin. Chem. 1992;38(6):1167–1185. doi: 10.1093/clinchem/38.6.1167. [DOI] [PubMed] [Google Scholar]
- 97.Yamanaka R., Tabata S., Shindo Y., Hotta K., Suzuki K., Soga T., et al. Mitochondrial Mg(2+) homeostasis decides cellular energy metabolism and vulnerability to stress. Sci. Rep. 2016;6:30027. doi: 10.1038/srep30027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hruby A., Guasch-Ferré M., Bhupathiraju S.N., Manson J.E., Willett W.C., McKeown N.M., et al. Magnesium intake, quality of carbohydrates, and risk of type 2 diabetes: results from three U.S. cohorts. Diabetes Care. 2017;40(12):1695–1702. doi: 10.2337/dc17-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Piuri G., Zocchi M., Della Porta M., Ficara V., Manoni M., Zuccotti G.V., et al. Magnesium in obesity, metabolic syndrome, and type 2 diabetes. Nutrients. 2021;13(2):160–195. doi: 10.3390/nu13020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Castellanos-Gutiérrez A., Sánchez-Pimienta T.G., Carriquiry A., da Costa T.H.M., Ariza A.C. Higher dietary magnesium intake is associated with lower body mass index, waist circumference and serum glucose in Mexican adults. Nutr. J. 2018;17(1):114. doi: 10.1186/s12937-018-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ghiraldini F.G., Crispim A.C.V., Mello M.L.S. Effects of hyperglycemia and aging on nuclear sirtuins and DNA damage of mouse hepatocytes. Mol. Biol. Cell. 2013;24(15):2467–2476. doi: 10.1091/mbc.E13-04-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aravinthan A. Cellular senescence: a hitchhiker’s guide. Hum. Cell. 2015;28(2):51–64. doi: 10.1007/s13577-015-0110-x. [DOI] [PubMed] [Google Scholar]
- 103.Ogrodnik M., Miwa S., Tchkonia T., Tiniakos D., Wilson C.L., Lahat A., et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 2017;8:15691. doi: 10.1038/ncomms15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Madrigal-Matute J., Cuervo A.M. Regulation of liver metabolism by autophagy. Gastroenterology. 2016;150(2):328–339. doi: 10.1053/j.gastro.2015.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shigematsu M., Tomonaga S., Shimokawa F., Murakami M., Imamura T., Matsui T., et al. Regulatory responses of hepatocytes, macrophages and vascular endothelial cells to magnesium deficiency. J. Nutr. Biochem. 2018;56:35–47. doi: 10.1016/j.jnutbio.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 106.Sui M., Jiang X., Chen J., Yang H., Zhu Y. Magnesium isoglycyrrhizinate ameliorates liver fibrosis and hepatic stellate cell activation by regulating ferroptosis signaling pathway. Biomed. Pharmacother. 2018;106:125–133. doi: 10.1016/j.biopha.2018.06.060. [DOI] [PubMed] [Google Scholar]
- 107.Hilmer S.N., Cogger V.C., Le Couteur D.G. Basal activity of Kupffer cells increases with old age. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62(9):973–978. doi: 10.1093/gerona/62.9.973. [DOI] [PubMed] [Google Scholar]
- 108.Warren A., Cogger V.C., Fraser R., Deleve L.D., McCuskey R.S., Le Couteur D.G. The effects of old age on hepatic stellate Cells. Curr. Gerontol. Geriatr. Res. 2011;2011:439835. doi: 10.1155/2011/439835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhutto A., Morley J.E. The clinical significance of gastrointestinal changes with aging. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11(5):651–660. doi: 10.1097/MCO.0b013e32830b5d37. [DOI] [PubMed] [Google Scholar]
- 110.Mansmann H.C. Consider magnesium homeostasis: III: Cytochrome P450 enzymes and drug toxicity, Pediatr. Asthma Allergy Immunol. 1994;8(1):7–28. doi: 10.1089/pai.1994.8.7. [DOI] [Google Scholar]
- 111.Kubota T., Shindo Y., Tokuno K., Komatsu H., Ogawa H., Kudo S., et al. Mitochondria are intracellular magnesium stores: investigation by simultaneous fluorescent imagings in PC12 cells. Biochim. Biophys. Acta. 2005;1744(1):19–28. doi: 10.1016/j.bbamcr.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 112.Killilea D.W., Maier J.A.M. A connection between magnesium deficiency and aging: new insights from cellular studies. Magnes. Res. 2008;21(2):77–82. [PMC free article] [PubMed] [Google Scholar]
- 113.Rayssiguier Y., Durlach J., Gueux E., Rock E., Mazur A. Magnesium and ageing. I. Experimental data: importance of oxidative damage. Magnes. Res. 1993;6(4):369–378. [PubMed] [Google Scholar]
- 114.Barbagallo M., Veronese N., Dominguez L.J. Magnesium in aging, health and diseases. Nutrients. 2021;13(2):1–20. doi: 10.3390/nu13020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cowan A.E., Jun S., Tooze J.A., Eicher-Miller H.A., Dodd K.W., Gahche J.J., et al. Total usual micronutrient intakes compared to the dietary reference intakes among U.S. Adults by food security status. Nutrients. 2019;12(1):1–11. doi: 10.3390/nu12010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Unwin R.J., Capasso G., Shirley D.G. An overview of divalent cation and citrate handling by the kidney. Nephron Physiol. 2004;98(2):15–20. doi: 10.1159/000080259. [DOI] [PubMed] [Google Scholar]
- 117.Gröber U. Magnesium and drugs. Int. J. Mol. Sci. 2019;20(9):2094. doi: 10.3390/ijms20092094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stine J.G., Sateesh P., Lewis J.H. Drug-induced liver injury in the elderly. Curr. Gastroenterol. Rep. 2013;15(1):299. doi: 10.1007/s11894-012-0299-8. [DOI] [PubMed] [Google Scholar]
- 119.Guedj A., Geiger-Maor A., Galun E., Benyamini H., Nevo Y., Elgavish Sh., et al. Early age decline in DNA repair capacity in the liver: in depth profile of differential gene expression. Aging. 2016;8(11):3131–3146. doi: 10.18632/aging.101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hong I.H., Lewis K., Iakova P., Jin J., Sullivan E., Jawanmardi N., et al. Age-associated change of C/EBP family proteins causes severe liver injury and acceleration of liver proliferation after CCl4 treatments. J. Biol. Chem. 2014;289(2):1106–1118. doi: 10.1074/jbc.M113.526780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hartwig A. Role of magnesium in genomic stability. Mutat. Res. 2001;475(1-2):113–121. doi: 10.1016/s0027-5107(01)00074-4. [DOI] [PubMed] [Google Scholar]
- 122.Seitz H.K., Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol. Chem. 2006;387(4):349–360. doi: 10.1515/BC.2006.047. [DOI] [PubMed] [Google Scholar]
- 123.Meier P., Seitz H.K. Age, alcohol metabolism and liver disease. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11(1):21–26. doi: 10.1097/MCO.0b013e3282f30564. [DOI] [PubMed] [Google Scholar]
- 124.Thurman R.G., II II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am. J. Physiol. 1998;275(4):G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 125.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- Noureddin M., Yates K.P., Vaughn I.A., Neuschwander-Tetri B.A., Sanyal A.J., McCullough A., et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. 2013;58(5):1644–1654. doi: 10.1002/hep.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barzilai N., Huffman D.M., Muzumdar R.H., Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61(6):1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hanna S., Harrison M.T., MacIntyre I., Fraser R. Effects of growth hormone on calcium and magnesium metabolism. Br. Med. J. 1961;2(5243):12–15. doi: 10.1136/bmj.2.5243.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Poynard T., Ratziu V., Charlotte F., Goodman Z., McHutchison J., Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J. Hepatol. 2001;34(5):730–739. doi: 10.1016/s0168-8278(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 130.Forrest E.H., Evans C.D.J., Stewart S., Phillips M., Oo Y.H., McAvoy N.C., et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54(8):1174–1179. doi: 10.1136/gut.2004.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mahrouf-Yorgov M., Collin de l’Hortet A., Cosson C., Slama A., Abdoun E., Guidotti J.E., et al. Increased susceptibility to liver fibrosis with age is correlated with an altered inflammatory response. Rejuvenation Res. 2011;14(4):353–363. doi: 10.1089/rej.2010.1146. [DOI] [PubMed] [Google Scholar]
- 132.Liang R.Y., Wu W., Huang J., Jiang S.P., Lin Y. Magnesium affects the cytokine secretion of CD4⁺ T lymphocytes in acute asthma. J. Asthma. 2012;49(10):1012–1015. doi: 10.3109/02770903.2012.739240. [DOI] [PubMed] [Google Scholar]
- 133.Zubiete-Franco I., Fernández-Tussy P., Barbier-Torres L., Simon J., Fernández-Ramos D., Lopitz-Otsoa F., et al. Deregulated Neddylation in liver fibrosis. Hepatology. 2017;65(2):694–709. doi: 10.1002/hep.28933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gramenzi A., Conti F., Cammà C., Grieco A., Picciotto A., Furlan C., et al. Hepatitis C in the elderly: A multicentre cross-sectional study by the Italian association for the study of the Liver. Dig. Liver Dis. 2012;44(8):674–680. doi: 10.1016/j.dld.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 135.Kainuma M., Furusyo N., Kajiwara E., Takahashi K., Nomura H., Tanabe Y., et al. Pegylated interferon α-2b plus ribavirin for older patients with chronic hepatitis C. World J. Gastroenterol. 2010;16(35):4400–4409. doi: 10.3748/wjg.v16.i35.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu S., Zhang H., Gu C., Yin J., He Y., Xie J., et al. Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: A meta-analysis. J. Natl. Cancer Inst. 2009;101(15):1066–1082. doi: 10.1093/jnci/djp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Randall H.B., Cao S., deVera M.E. Transplantation in elderly patients. Arch. Surg. 2003;138(10):1089–1092. doi: 10.1001/archsurg.138.10.1089. [DOI] [PubMed] [Google Scholar]
- 138.Dong J.Y., Xun P., He K., Qin L.Q. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care. 2011;34(9):2116–2122. doi: 10.2337/dc11-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li W., Zhu X., Song Y., Fan L., Wu L., Kabagambe E.K., et al. Intakes of magnesium, calcium and risk of fatty liver disease and prediabetes. Public Health Nutr. Cult. 2018;21(11):2088–2095. doi: 10.1017/S1368980018000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martin H., Uring-Lambert B., Adrian M., Lahlou A., Bonet A., Demougeot C., et al. Effects of long-term dietary intake of magnesium on oxidative stress, apoptosis and ageing in rat liver. Magnes. Res. 2008;21(2):124–130. [PubMed] [Google Scholar]
- 141.Weglicki W.B., Mak I.T., Kramer J.H., Dickens B.F., Cassidy M.M., Stafford R.E., et al. Role of free radicals and substance P in magnesium deficiency. Cardiovasc. Res. 1996;31(5):677–682. [PubMed] [Google Scholar]
- 142.Durlach J., Bac P., Durlach V., Rayssiguier Y., Bara M., Guiet-Bara A. Magnesium status and ageing: an update. Magnes. Res. 1998;11(1):25–42. [PubMed] [Google Scholar]
- 143.Schwalfenberg G.K., Genuis S.J. The importance of magnesium in clinical healthcare. Scientifica (Cairo). 2017. 2017:4179326. doi: 10.1155/2017/4179326. [DOI] [PMC free article] [PubMed] [Google Scholar]