Abstract
The last decade has seen tremendous advances in the prevention and treatment of recurrent hyperkalemia. In this narrative review, we aim to highlight contemporary data on key areas in the epidemiology and management of hyperkalemia. Focusing on drug-induced hyperkalemia (the implications of renin-angiotensin-aldosterone system inhibitors [RAASi] discontinuation and the role of mineralocorticoid receptor antagonists), newer concurrent therapies that modify potassium handling (sodium-glucose transporter 2 inhibitors [SGLT2i]), the introduction of new treatment agents (oral potassium binding agents), and the controversial role of dietary potassium restriction, we apply recent research findings and review the evidence in a case-based format.
Keywords: dietary potassium, diuretics, hyperkalemia, novel potassium binders, renin-angiotensin-aldosterone inhibitors (RAAS inhibitors), sodium-glucose transporter 2 inhibitors (SGLT2 inhibitors)
The kidneys play the pivotal role in maintaining potassium homeostasis and eliminating excess potassium ingested from diet or released from cells. Hyperkalemia is therefore a commonly encountered problem in chronic kidney disease (CKD), particularly in patients with reduced kidney function who are taking medications that alter the kidneys’ ability to eliminate excess potassium. These medications include renin-angiotensin-aldosterone system inhibitors (RAASi), including angiotensin converting enzyme inhibitors (ACEi), and angiotensin II receptor blockers (ARBs); and mineralocorticoid receptor antagonists (MRAs). Unfortunately, patients who often have the most to gain from these therapies, such as those with CKD, diabetic kidney disease (DKD), and heart failure, are also the patients at greatest risk of developing hyperkalemia from RAASi or MRA use. Management of potassium balance is rapidly evolving with new and emerging medications that prevent or directly treat hyperkalemia such as sodium-glucose transporter 2 inhibitors (SGLT2i) and novel gastrointestinal potassium exchangers such as patiromer and sodium zirconium cyclosilicate (SZC). Therefore, clinicians caring for patients with CKD have multiple options at their disposal to control hyperkalemia, without having to revert to potentially unhealthy restrictive dietary changes or removal of RAASi, which have proven long-term benefits.
In this narrative review, we focus on the management of recurrent, ambulatory, hyperkalemia, particularly in the context of RAASi use, and evaluate recent evidence on the emerging therapeutics available when faced with hyperkalemia in patients with CKD, along with their advantages and pitfalls summarized in Figure 1. Using a case-based approach, with fictional scenarios typical of the types of patients or consultations nephrologists would encounter in their clinical practice, we focus specifically on the implications of RAASi discontinuation in the face of hyperkalemia, the newer MRAs, the role of SGLTs, oral potassium binders, and the role of dietary potassium restriction. Of note, this review focuses on the management of milder forms of hyperkalemia and not severe, life-threatening hyperkalemia, which can be encountered with acute kidney injury in acutely ill patients. For this, we refer the reader to recent reviews done on the topic.1,2
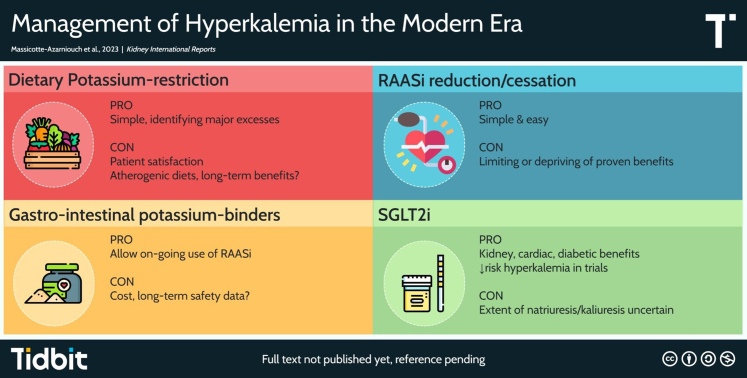
Figure 1.
Summary of pros and cons of various management options for outpatient hyperkalemia. RAASi, renin-angiotensin-aldosterone system inhibitors; SGLT2i, sodium-glucose transporter 2 inhibitors.
Case 1
A 63-year-old male with hypertension and presumed diabetic nephropathy with proteinuric CKD (serum creatinine 180 μmol/l, estimated glomerular filtration rate [eGFR] 36 ml/min, urine albumin-to-creatinine ratio 60 mg/mmol) is followed by his primary care physician for blood pressure and diabetes management. Medications include amlodipine 10 mg daily, metformin 500 mg twice daily, and gliclazide 30 mg daily. There is no history of cardiovascular disease or heart failure. Kidney function has slowly worsened over the last few years. Recent blood pressure readings were 140 to 150/85 to 95 mm Hg and subsequently lisinopril 10 mg daily was initiated. On repeat blood testing 3 weeks later, serum potassium was 5.9 mmol/l and then 5.8 mmol/l on repeat testing a few days later with no significant change in eGFR. The patient’s primary care physician discontinues the lisinopril and sends a referral to nephrology for further management of hypertension and hyperkalemia.
How Common is Hyperkalemia After Initiation of RAASi?
Hyperkalemia is typically defined as a serum potassium measurement >5.0 to 5.5 mmol/l, depending on the laboratory doing the test, the equipment used, and the population distribution of potassium values used to determine the reference range. Pseudohyperkalemia occurs most often because of blood drawing techniques (fist clenching, use of a tourniquet) which may release potassium from cells causing a false elevation.3,4 Other rarer causes of pseudohyperkalemia include thrombocytosis (release from platelets after clotting has occurred, thereby causing elevated serum potassium measurement) and severe leukocytosis (release during blood processing because of cell fragility).5,6 True hyperkalemia may occur when potassium shifts or gets released outside of cells because of tissue injury or trauma, tumor lysis syndrome, insulin deficiency in diabetes, metabolic acidosis from inorganic acids, and to a lesser extent from severe respiratory acidosis.7, 8, 9, 10 True hyperkalemia may also occur when there is excess total body potassium, which is more likely to develop among individuals in whom there is malfunction of the major players in potassium homeostasis, namely CKD, heart failure, DKD, any combination of these, and the use of RAASi. By decreasing glomerular filtration pressure and the production or effect of aldosterone on the principal cell, RAASi medications affect the kidneys’ ability to eliminate excess potassium. With the use of RAASi, the reported frequency of hyperkalemia will vary based on the type of study (clinical trial, observational study) and based on patient comorbidities. A review of clinical trials up to 2008 evaluating RAASi medications in patients with hypertension, CKD, or heart failure found that ≤2% of patients without CKD or heart failure developed a serum potassium >5.5 mmol/l after RAASi monotherapy initiation. In patients with CKD or heart failure, this proportion increased to 5% to 10%; however, the absolute increases in serum potassium post-RAASi were generally mild, ranging between 0.1 and 0.3 mmol/l.11 In the PARADIGM-HF trial, which examined patients with ejection fraction ≤40%, the angiotensin-receptor-neprilysin inhibitor, sicabutril-valsartan, led to hyperkalemia (>5.5 mmol/l) in 16% of patients.12 A meta-analysis of clinical trials of MRA use on top of RAASi in patients with proteinuric CKD found that approximately 5% of patients developed hyperkalemia.13 With the new nonsteroidal MRA, finerenone, the FIGARO and FIDELIO trials, where eGFR cutoffs for inclusion were 25 ml/min, showed that hyperkalemia occurred in 11% to 16% of patients with proteinuric DKD when added to RAASi.14,15 Observational studies have demonstrated similar findings for patients with stage 3 CKD, which constitute the bulk of patients included in RAASi clinical trials, where approximately 10% who are prescribed RAASi develop hyperkalemia; in patients with CKD stages 4 or 5, this increases to 15% to 25% and these numbers may be even higher in patients who have concurrent diabetes and heart failure.16, 17, 18 Therefore, anywhere from 5% to 25% of patients with CKD, depending on their CKD stage and comorbidities, will develop hyperkalemia after initiation of a RAASi. Major society guidelines recommend checking serum potassium within 1 to 4 weeks after initiation or dose increase of RAASi medications.19,20 It is not unexpected that the patient in the clinical vignette with proteinuric CKD developed hyperkalemia after initiation of lisinopril.
What are the Management Options for this Patient?
Stopping or Reducing Lisinopril
The most common intervention when faced with hyperkalemia is exactly what was done in the vignette, stopping the offending agent. Although probably the simplest solution, it is unlikely to be the best long-term option. First, a phone call to ensure the patient is feeling well and to review any potential excess dietary sources of potassium would certainly be appropriate (we will discuss dietary management of hyperkalemia in more detail with case 3 below). Holding the ACEi and rechecking potassium in a few days after dietary counseling might permit resumption and ongoing use of ACEi. Second, mild elevations of potassium in otherwise well individuals are unlikely to be of clinical consequence. It is well demonstrated that RAASi are underutilized in patients for whom it is indicated, often because of a fear of hyperkalemia.21,22 The most feared consequence of hyperkalemia, severe cardiac arrythmias, will only occur at levels much greater than what is generally encountered in outpatients prescribed RAASi medications. Cardiac conduction delays with prolongation or the PR interval and widening of the QRS typically begin once serum potassium is between 6.5 and 8 mmol/l and it is usually only at levels above 8 mmol/l that the dreaded “sine-wave” pattern, ventricular fibrillation or asystole occur.23,24 Although these cutoffs are not perfectly sensitive for the electrocardiograph changes described25, otherwise well outpatients who do not have severe hyperkalemia are unlikely to sustain a cardiac arrhythmia unless an acute event further increases serum potassium. Such hyperkalemia should be intervened in, and being cognizant that the potassium level in itself is unlikely to be immediately dangerous in an otherwise well individual. There is time to institute appropriate measures rather than immediately withdrawing the RAASi. Severe hyperkalemia (typically defined as potassium >6.5 mmol/l)26 is unlikely to develop after RAASi medication initiation alone unless there is a concurrent acute medical illness. A prospective observational study of 4661 patients with CKD who received a RAASi medication found that, within 90 days of RAASi prescription, only 0.3% developed potassium >6 mmol/l.16 In addition, mild levels of hyperkalemia are not necessarily causally associated with adverse outcomes. Observational studies demonstrate a U-shaped curve of the relation between serum potassium levels and adverse outcomes in patients with CKD. At levels below 4 mmol/l or >5.5 mmol/l, there begins to be a significantly increased risk for adverse outcomes such as cardiovascular events or death.27,28 Given that severe cardiac abnormalities do not occur at mild levels of hyperkalemia, much of the association with adverse outcomes from potassium level >5.5 mmol/l could be explained by a confounding variable and less likely causally related to the hyperkalemia itself, particularly for potassium levels <6.5 mmol/l in asymptomatic outpatients. The medical condition leading to the development of hyperkalemia, or warranting initiation of a medication causing hyperkalemia, is more likely to explain the association with adverse outcomes (confounding by indication). A serum potassium level of 5.8 mmol/l leaves less leeway should something occur further affecting the kidneys’ ability to excrete potassium, such as volume depletion or a congestive heart failure exacerbation or should there be ingestion of a large amount of potassium. Third, studies demonstrate that removal or reduction of RAASi in response to hyperkalemia may have deleterious consequences. Approximately 50% of patients who develop hyperkalemia after initiation of RAASi will have it discontinued.17,18,29 A recent, large population-based cohort study of adults in the provinces of Manitoba and Ontario, Canada, who developed hyperkalemia (≥5.5 mmol/l) after receiving a prescription for a RAASi found that 16% of individuals had it discontinued where the mean (SD) potassium level was 5.9 (0.5) mmol/l. In this study, RAASi discontinuation was associated with a significantly higher risk of all-cause mortality (hazard ratios [HRs] 1.32 and 1.47, respectively), cardiovascular mortality (HRs 1.28 and 1.32, respectively) and dialysis initiation (HRs 1.65 and 1.11, respectively) in both regions.30 Another retrospective cohort study found an increased risk for adverse outcomes (mortality and cardiovascular events) when RAASi was stopped after patients experienced a decrease in eGFR.31 Even the use of suboptimal doses of RAASi (<50% guideline recommended dose) is associated with a higher risk of adverse cardiovascular outcomes and mortality compared to receiving >50% of the recommended RAASi dose.32 Reduction or cessation of RAASi, whatever the reason, may carry certain risks by depriving patients of their proven benefits. It should be acknowledged that residual unmeasured confounding may also be playing a role in these findings. For example, a physician’s clinical judgment when stopping a RAASi in someone who develops hyperkalemia is likely based on factors which are impossible to fully capture in an observational study. The STOP-ACEi trial tested in a randomized controlled setting whether discontinuation of RAASi (ACEi or ARB) would stabilize or preserve kidney function in patients with CKD stage 4 to 5 who had progressive deterioration in kidney function. At 3 years follow-up, the change in eGFR, occurrence of end-stage kidney disease (ESKD), cardiovascular events and death were similar in both groups. Although these findings could indicate that stopping ACEi or ARB, when faced for example with hyperkalemia, is not deleterious in the long-term, there was a nonstatistically significant greater risk for ESKD and a greater number of cardiovascular events in the discontinuation group compared to the continuation group.33 Therefore, one must consider that continuing the RAASi, and thereby facilitating long-term use, potentially improves outcomes by appropriately treating the condition for which it was prescribed and providing benefits which have been consistently demonstrated in large randomized controlled trials. In fact, the latest Kidney Disease Improving Global Outcomes guidelines recommend that hyperkalemia associated with RAASi use be managed through methods other than dose reduction or cessation.19 Therefore, discontinuation of the RAASi in the clinical vignette, although probably the simplest, is unlikely to be the best long-term option for the patient.
Initiation of an SGLT2i
SGLT2i seem to play a supporting role in mitigating RAASi-associated hyperkalemia in CKD. A meta-analysis of 6 randomized controlled trials (comprising nearly 50,000 patients) examining SGLT2i use in individuals with diabetes at high risk for cardiovascular disease or with CKD found that SGLT2i significantly reduced the risk for developing hyperkalemia >6 mmol/l (HR 0.84, 95% confidence interval 0.76–0.93), even in patients who were being treated with RAASi at baseline.34 In addition, in the recent FIDELIO trial examining the nonsteroidal mineralocorticoid antagonist, finerenone, in patients with DKD, use of an SGLT2i concomitant with finerenone reduced hyperkalemia (>5.5 mmol/l) compared to placebo (HR 0.45, 95% confidence interval 0.27–0.75), although only 4.6% of study participants were on an SGLT2i.35 The ability for SGLT2i to mitigate RAASi-induced hyperkalemia is consistent with its perceived diuretic effect as will be discussed further below. In addition, in multiple studies, SGLT2i have demonstrated a preservation of renal function in patients with DKD, improved cardiovascular outcomes, and decreased mortality36,37, along with improvements in diabetic and blood pressure control.38, 39, 40, 41 Therefore, regardless of HbA1c level, an SGLT2i would be an attractive option to allow use of RAASi and at optimal doses by mitigating risks for hyperkalemia and providing benefits for the same treatment indications as RAASi.
Initiation of a Potassium Wasting Diuretic
Loop and thiazide diuretics have an established role in preventing or controlling RAASi-related hyperkalemia and are available commercially as combination pills, often with hydrochlorothiazide or a thiazide-like diuretic. Use of diuretics concurrent with ACEi decrease the risk of developing hyperkalemia42, and initiation of a new diuretic or increase of the dose is a management option often chosen by clinicians.43 Even in advanced CKD, thiazide-like diuretics may help manage hyperkalemia. A study from the 1970s demonstrated that metolazone use in 14 patients with stage 5 CKD produced a 33% increase in urinary potassium excretion with doses ranging from 20 to 150 mg per day.44 More recently, chlorthalidone was studied for the treatment of hypertension in patients with stage 4 CKD and proved to be effective in control of blood pressure.45 Changes in serum potassium were not reported in that study; however, hypokalemia was more common in those treated with chlorthalidone (10 events vs. none in the placebo arm) suggesting that it likely influenced urinary potassium excretion. Although the efficacy of diuretic add-on for the treatment of RAASi-induced hyperkalemia has not been evaluated in a randomized controlled setting that we are aware of, reviews of trials and published data on the effects of diuretics for decreasing serum potassium have shown a decrease of approximately 0.3 to 0.6 mmol/l can be expected depending on the dose and type of diuretics (loop vs. thiazide vs. thiazide-like)46,47; and decades of clinician experience suggest that these agents can help mitigate and/or treat RAASi-induced hyperkalemia. In the clinical vignette, if the patient had signs of hypervolemia or if blood pressure were still above target, addition of a diuretic would certainly be a valid option, and one that clinicians commonly revert to. However, the lack of proven benefit on hard outcomes such as cardiovascular events and mortality, along with possible metabolic or electrolyte derangements, make them a less attractive option than SGLT2i. Therefore, it may not be the best option in this case.
Case 2
A 56-year-old woman has mild CKD (creatinine 130 μmol/l, eGFR 42 ml/min, urine albumin-to-creatinine ratio 10 mg/mmol) and chronic heart failure from ischemic cardiomyopathy (left ventricular ejection fraction 35%). She is not diabetic. Medications include metoprolol 50 mg twice daily, perindopril 8 mg daily, spironolactone 50 mg daily, and furosemide 40 mg daily. Potassium measurements have mostly been ranging from 4.5 to 5.5 mmol/l; however, over the last 4 months, they have ranged between 5.5 and 6.0 mmol/l after the doses of perindopril and spironolactone were increased to their current levels. The patient is clinically doing well but the most recent potassium readings are 5.8 and 6.0 mmol/l, prompting a reduction of perindopril and spironolactone to 2 mg and 12.5 mg daily, respectively. A referral is sent to nephrology for further advice on management of hyperkalemia.
What Other Options Would be Available for Managing This Patient’s Hyperkalemia?
Initiation of SGLT2i
This patient does not have diabetes nor significant proteinuria, which of themselves would be indications for an SGLT2i. The EMPA-KIDNEY trial, which examined the use of empagliflozin in patients with proteinuric and nonproteinuric CKD, demonstrated a significant reduction in progression of kidney disease or death from cardiovascular causes, although this effect was not seen in the subgroup of patients with low-level or no proteinuria.48 More studies are needed to clarify SGLT2i’s benefit in minimally or nonproteinuric CKD. The patient’s reduced cardiac ejection fraction (heart failure with reduced ejection fraction) though would provide an appropriate evidence-based indication for the initiation of dapagliflozin or empagliflozin. The DAPA-HF and EMPEROR-Reduced trials, totaling over 8000 patients with heart failure with reduced ejection fraction, randomized participants to SGLT2i or placebo on top of standard-of-care therapy. Both studies found a 25% to 30% relative risk reduction in the composite outcome of cardiovascular death or hospitalization for heart failure, with the effect mostly driven by reductions in heart failure.49,50 This also supports the probable diuretic effect of SGLT2i.51 In a placebo-controlled crossover study of empagliflozin 10 mg daily for 14 days in patients with type 2 diabetes and stable heart failure, there was a significant increase in fractional excretion of sodium and decrease in blood volume with empagloflozin.52 Such natriuresis could increase distal delivery of sodium in the nephron and promote potassium excretion at the level of the principal cell through the effect of aldosterone, which may also be increased from SGLT2i use.53 Although no studies have specifically examined the role of SGLT2i for treating hyperkalemia, these mechanisms along with the decreased risk for hyperkalemia seen in randomized controlled trials could support the use of SGLT2i in the patient from the clinical vignette. Therefore, addition of an SGLT2i in the present case would be an interesting option because it would provide benefit for managing heart failure and could help control potassium as well. If the patient is not demonstrating signs of volume overload, reassessment could be given to the furosemide dose because of the diuretic effect of SGLT2i. Alternatively, SGLT2i combined with loop-diuretics may have a synergistic effect for urinary sodium excretion, perhaps up to 4 times greater than with SGLT2i monotherapy.52 As clinicians become more familiar with these agents and their indications outside of diabetes, they may become a valuable substitute or add-on to diuretics for chronic volume management, and providing cardiovascular benefits, mortality benefits and potentially treating hyperkalemia.
Gastrointestinal Cation Exchangers
Sodium polystyrene sulfonate (SPS) has been approved by the US Food and Drug Administration for the treatment of hyperkalemia as a gastrointestinal cation exchange resin for over 6 decades. Yet, surprisingly, empirical data on its efficacy and safety remain sparse. A retrospective study and a small, randomized placebo-controlled trial, both demonstrated a potassium lowering effect of about 1 mmol/l with SPS.54 However, there have been growing concerns over risks for serious gastrointestinal adverse events55,56, including bowel necrosis reported with SPS even without sorbitol57, 58, 59, raising concerns about long-term exposure to SPS for the management or prevention of hyperkalemia.
Newer cation exchangers, notably patiromer and SZC, appear to be effective in the prevention and management of hyperkalemia in patients with CKD, heart failure and/or on RAASi. The AMETHYST-DN trial demonstrated that patiromer twice daily for 4 weeks could decrease serum potassium by 0.35 to 1.00 mmol/l in patients with stage 3 to 4 diabetic CKD who were treated with RAASi and developed hyperkalemia (5–6 mmol/l).60 The OPAL-HK trial demonstrated a similar effect from patiromer on patients with stage 3 to 4 CKD (57% with diabetes and 42% with heart failure) receiving RAASi and who had hyperkalemia (5.1–6.5 mmol/l); there was approximately 1 mmol/l decrease in serum potassium after 4 weeks of patiromer use.61 SZC also has trial data demonstrating efficacy in acutely reducing potassium levels in patients with hyperkalemia and in maintaining normokalemia with chronic use, allowing continued use, an increase in dose, or new initiation of RAASi medication.62
The addition of either patiromer or SZC would be a reasonable option to manage the hyperkalemia of the patient in the clinical vignette. In fact, the described patient is representative of the type of patient included in the DIAMOND trial. This recent randomized placebo-controlled trial examined the use of patiromer for managing hyperkalemia in patients with heart failure and reduced ejection fraction. Patients either had 2 readings of serum potassium >5 mmol/l while receiving RAASi or a history of dose reduction of RAASi because of hyperkalemia in the past 12 months. In the run-in phase, 84.6% of 1195 enrolled participants achieved optimization of RAASi (≥50% recommended dose of ACEi/ARB and target dose 50 mg spironolactone or eplerenone). These participants were then randomized to continue patiromer or placebo. Patiromer demonstrated a 37% reduction in risk for hyperkalemia (>5.5 mmol/l) and 38% reduction of risk for having to reduce MRA dose.63 With this emerging evidence on efficacy for managing hyperkalemia, and the lack of reported serious gastrointestinal side effects, these new potassium binders are bound to gain popularity for managing chronic hyperkalemia and for allowing optimal use of RAASi. They have certain side effects to be made aware of though. Patiromer may affect absorption of other medications and lead to hypomagnesemia in nearly 10% of patients.60 SZC may cause edema from increased sodium absorption, but this is mostly seen with the use of the 10 or 15 g doses.64 Both can cause mild gastrointestinal side effects; however, there have been no cases yet of serious gastrointestinal complications such as bowel necrosis. Furthermore, cost of these agents is a consideration (10 g SCZ priced at $25 Canadian)65 because they are not covered on many private or government drug plans (for example, in Ontario, Canada).
Case 3
A 65-year-old male has ESKD because of diabetic nephropathy. He receives in-center hemodialysis 3 times weekly via an arterio-venous fistula and has urine output ranging between 250 and 500 ml per day. His predialysis serum potassium is consistently around 6 mmol/l on routine testing (pre-72-hour interval dialysis session).
What are Some Options for Managing Hyperkalemia in Such a Patient?
Dietary Recommendations
In patients with kidney failure, counseling for reduction of high potassium containing foods is a common, and usually the first, method for managing recurring hyperkalemia. The degree to which such recommendations are effective and beneficial for the patient remains unclear. Logically, if someone with a drastically limited ability to eliminate excess potassium from their body ingests too much potassium, it would make sense that they would be at greater risk for hyperkalemia, and potentially cardiac arrythmia if severe enough. A retrospective study from Japan found an association between urinary potassium excretion on 24-hour urine collections, used as a proxy for dietary potassium intake, and the development of hyperkalemia among patients with CKD.66 It would certainly make sense for a patient such as the one in the vignette who has demonstrated recurring hyperkalemia to have their diet reviewed by a dietician and be counseled to limit excess intake of high potassium foods. A blanket recommendation to limit potassium intake in patients with kidney failure is probably not warranted. The DIET-HD study, a prospective observational study of over 8000 individuals on maintenance hemodialysis, examined the impact of dietary potassium intake on mortality. Surprisingly, increases in dietary potassium intake of 1 g/d were not associated with an increase in serum potassium concentration, the prevalence of hyperkalemia (≥6 mmol/l), or mortality.67 A similar lack of association between dietary potassium intake and hyperkalemia was shown in a cross-sectional study in Brazil which included nondialysis dependent CKD.68 An important caveat of these 2 studies is that they were performed in Europeans and Brazilians, respectively, where dietary and lifestyle patterns may be quite different from in North America. Furthermore, the sources of dietary potassium in the DIET-HD study were mostly from fruits, vegetables, and fiber-containing foods, which are heart-healthy and contain alkali, which may reduce acidosis and promote intracellular shifting of potassium, decreasing the risk of hyperkalemia. A smaller study by Noori et al.69 also examined the impact of dietary potassium intake on mortality among patients on hemodialysis in California, USA. They found an increase in mortality with higher potassium intake, where the sources of potassium were mostly from meats, processed food, and fast food. These conflicting results are likely explained by the additional benefits of healthier potassium-containing foods and their potential association with healthier lifestyles. Regarding pharmacologic sources of potassium, the run-in phase of a trial on 191 patients with stage 3b to 4 CKD examined the results of 2 weeks of 40 mmol per day potassium chloride supplementation. This increased serum potassium levels by a mean of 0.4 mmol/l and 89% of individuals remained normokalemic.70 Although not a dietary intervention study, it challenges the dogma that increased potassium intake will necessarily lead to hyperkalemia in CKD. Therefore, it seems unclear to what extent dietary potassium intake contributes to hyperkalemia and adverse outcomes in patients with advanced CKD and ESKD. Unfortunately, potassium-restricted diets are difficult to implement for patients, are poorly adhered to, and lead to the loss of potential potassium-rich dietary benefits. In fact, patients on hemodialysis often have what would be considered atherogenic diets71,72, possibly from low fruit, vegetable, and fiber intake which come with restricting high potassium foods.73 Concerns arise when recommending overly restrictive potassium diets to individuals with advanced CKD and ESKD when they are already at such high risk for cardiovascular events. In fact, the recent Kidney Disease Improving Global Outcomes controversies conference on potassium homeostasis and management of dyskalemia acknowledged the uncertainty about general potassium restriction in CKD and potential risks with depriving patients of benefits which come from high potassium containing diets.26 It is possible that other methods proven to control hyperkalemia in patients with kidney failure would be better suited to allow for healthier diets.
Lowering of Dialysate Potassium
Along with dietary recommendations, this is an intervention often used to prevent hyperkalemia in patients on hemodialysis but may carry certain risks, notably from rapid decrease in serum potential and transient hypokalemia. A large study of over 55,000 patients in the Dialysis Outcomes and Practice Patterns Study cohort found no difference in arrhythmia hospitalization, sudden death, or all-cause mortality between the use of the 2 most common dialysate potassium concentrations, 3 mEq/l compared to 2 mEq/l. However, use of dialysate potassium 1 to 1.5 mEq/l showed a nonstatistically significant greater risk for arrhythmia compared to 2 to 2.5 mEq/l.74 A study of 52 patients on hemodialysis who underwent Holter monitoring from the first session of the week to the second session demonstrated that QT prolongation, ventricular premature contractions, and ST depressions could occur, mostly in the 12 hours after the first dialysis session of the week. Such electrical abnormalities were more common with dialysate potassium concentrations of 2 mmol/l versus 3 mmol/l and with greater dialysate-serum potassium gradient.75 A prospective observational study of 624 patients on hemodialysis from the Modification of Diet in Renal Disease cohort found that the use of a dialysate potassium of 1 mmol/l, compared to 2 mmol/l, was associated with an increased risk for all-cause mortality, particularly among those who had a predialysis potassium level >5 mmol/l.76 Therefore, if the patient in the vignette had a dialysate potassium concentration of ≥3 mmol/l, it would be reasonable to decrease it to the next lower level; however, lowering the dialysate concentration to <2 mmol/l may have adverse effects because of a greater dialysate-serum gradient leading to a drastic and rapid drop in serum potassium, which could predispose to arrhythmias. Other ways of managing and preventing recurring hyperkalemia may be more appropriate.
Laxatives
The gastrointestinal tract plays an important role in the elimination of potassium in patients with kidney failure. The colon undergoes adaptive changes allowing for greater excretion of potassium in stool compared to individuals with intact kidney function, possibly through increased expression of potassium secretory channels on the apical large intestine epithelial cell.77, 78, 79 A large retrospective cohort study of over 36,000 US veterans transitioning to dialysis found that the use of any type of laxative in the last year before the initiation of dialysis significantly decreased the risk for developing hyperkalemia (>5.5 mmol/l).80 A small trial demonstrated that the use of bisacodyl in patients with advanced CKD and on dialysis was able to produce modest but significant reductions in serum potassium (0.4 mmol/l), which was not seen with lactulose.81 It is thought that bisacodyl may enhance the ability of colonic epithelial cells to excrete potassium through cAMP activation of apical potassium channels.82 Laxatives, including bisacodyl, could be a potential option for managing hyperkalemia in the patient presented in the above scenario; however, there have been rare case reports of bisacodyl-induced ischemic colitis83, 84, 85, possibly from increased intracolonic pressure because of its promotility effect.
Gastrointestinal Cation Exchangers
As mentioned above, SPS may reduce serum potassium. A recent small, randomized crossover trial of 51 patients on regular hemodialysis with predialysis potassium between 5.0 and 6.4 mmol/l compared 15 g SPS 3 times daily on nondialysis days to patiromer 16.8 g daily on nondialysis days. Potassium levels were lower with SPS (mean weekly potassium 4.55 vs. 5.0 mmol/l) and there was less hyperkalemia observed with SPS compared to patiromer.86 There were no serious gastrointestinal adverse events but tolerability and compliance were lower with SPS. In addition, use of SPS may be associated with rare, but potentially life-threatening gastrointestinal effects, making it a less attractive option for managing chronic hyperkalemia. In the DIALYZE randomized placebo-controlled trial, patients on maintenance hemodialysis with recurring hyperkalemia (>5.4 mmol/l) were treated with SZC 5 g daily titrated up to 15 g daily to achieve potassium levels of 4 to 5 mmol/l or with placebo. Forty-one percent of those receiving SZC responded to treatment (serum potassium between 4–5 mmol/l at 8 weeks) compared to only 1% with placebo.87 There was in addition, less rescue therapy for hyperkalemia used in the SZC group and there were similar rates of serious adverse events. This could be a beneficial intervention for the above patient by not only improving predialysis potassium values, but also by potentially allowing a more potassium-liberalized and overall healthier diet in the long-term.
Conclusion and Future Directions
Hyperkalemia is a frequent occurrence in patients with CKD, particularly among the many who are prescribed RAASi medications. The implications of various management strategies for hyperkalemia in these patients are important to understand such that patients may benefit from long-term use of RAASi medications. Most hyperkalemia which develops with RAASi is mild and probably not causally associated with harm. Severe and truly life-threatening hyperkalemia is unlikely to develop unless in the context of acute medical illness causing acute kidney injury. Therefore, instead of clinicians fearing these mostly mild and isolated episodes of hyperkalemia with RAASi use in patients who have a strong indication for them, focus should be put on providing proper education and sick-day recommendations to their patients to hold RAASi on days of acute illness when there is a limited ability to ensure adequate intake of food and water. Dietary recommendations to educate about excessive intake of high potassium containing foods should be done in such a way as to avoid being overly restrictive, by taking an individualized approach, and by identifying certain individual foods which may be taken in excess and limiting those. Given the importance many patients place on being able to control certain aspects of their treatment through dietary choices and the frequency with which dietary recommendations are made in patients with CKD, proper randomized controlled trials examining the efficacy and long-term outcomes of potassium-restricted diets in patients with advanced CKD and kidney failure are needed. The efficacy of newer potassium binding agents in allowing patients with advanced CKD and kidney failure to benefit from liberalized potassium diets should also be examined. Finally, the SGLT2i medications, which have taken by storm the fields of nephrology, cardiology, and endocrinology, should find a place in the management of RAASi-induced hyperkalemia. Data from randomized trials suggest that they reduce RAASi-induced hyperkalemia, but further studies are needed to confirm this effect. Both RAASi and SGLT2i often share similar indications in patients with CKD, heart failure, and diabetes, such that combination pills of ACEi or ARB with SGLT2i may be a convenient way of providing therapies with proven benefits on hard outcomes, and limiting hyperkalemia risks.
Disclosure
DMA and MC report no conflicting interests. GLH reports grant funding from CIHR and KFoC; MMS reports consulting fees from Astrazeneca and Otsuka, honoraria from Astrazeneca, Bayer, and GlaxoSmithKline.
References
- 1.Dépret F., Peacock W.F., Liu K.D., Rafique Z., Rossignol P., Legrand M. Management of hyperkalemia in the acutely ill patient. Ann Intensive Care. 2019;9:32. doi: 10.1186/s13613-019-0509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemoine L., Le Bastard Q., Batard E., Montassier E. An evidence-based narrative review of the emergency department management of acute hyperkalemia. J Emerg Med. 2021;60:599–606. doi: 10.1016/j.jemermed.2020.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Don B.R., Sebastian A., Cheitlin M., Christiansen M., Schambelan M. Pseudohyperkalemia caused by fist clenching during phlebotomy. N Engl J Med. 1990;322:1290–1292. doi: 10.1056/nejm199005033221806. [DOI] [PubMed] [Google Scholar]
- 4.Wiederkehr M.R., Moe O.W. Factitious hyperkalemia. Am J Kidney Dis. 2000;36:1049–1053. doi: 10.1053/ajkd.2000.19084. [DOI] [PubMed] [Google Scholar]
- 5.Graber M., Subramani K., Corish D., Schwab A. Thrombocytosis elevates serum potassium. Am J Kidney Dis. 1988;12:116–120. doi: 10.1016/s0272-6386(88)80005-2. [DOI] [PubMed] [Google Scholar]
- 6.Kellerman P.S., Thornbery J.M. Pseudohyperkalemia due to pneumatic tube transport in a leukemic patient. Am J Kidney Dis. 2005;46:746–748. doi: 10.1053/j.ajkd.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Sever M.S., Erek E., Vanholder R., et al. Serum potassium in the crush syndrome victims of the Marmara disaster. Clin Nephrol. 2003;59:326–333. doi: 10.5414/cnp59326. [DOI] [PubMed] [Google Scholar]
- 8.Howard S.C., Jones D.P., Pui C.H. The tumor lysis syndrome. N Engl J Med. 2011;364:1844–1854. doi: 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viberti G.C. Glucose-induced hyperkalaemia: a hazard for diabetics? Lancet. 1978;1:690–691. doi: 10.1016/s0140-6736(78)90801-2. [DOI] [PubMed] [Google Scholar]
- 10.Adrogué H.J., Madias N.E. Changes in plasma potassium concentration during acute acid-base disturbances. Am J Med. 1981;71:456–467. doi: 10.1016/0002-9343(81)90182-0. [DOI] [PubMed] [Google Scholar]
- 11.Weir M.R., Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol. 2010;5:531–548. doi: 10.2215/cjn.07821109. [DOI] [PubMed] [Google Scholar]
- 12.McMurray J.J., Packer M., Desai A.S., et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 13.Chung E.Y., Ruospo M., Natale P., et al. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2020;10:CD007004. doi: 10.1002/14651858.CD007004.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitt B., Filippatos G., Agarwal R., et al. Cardiovascular events with finerenone in kidney disease and Type 2 diabetes. N Engl J Med. 2021;385:2252–2263. doi: 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 15.Bakris G.L., Agarwal R., Anker S.D., et al. Effect of finerenone on chronic kidney disease outcomes in Type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 16.Garlo K.G., Bates D.W., Seger D.L., Fiskio J.M., Charytan D.M. Association of changes in creatinine and potassium levels after initiation of renin angiotensin aldosterone system inhibitors with emergency department visits, hospitalizations, and mortality in individuals with chronic kidney disease. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riccio E., Capuano I., Buonanno P., et al. RAAS inhibitor prescription and hyperkalemia event in patients with chronic kidney disease: a single-center retrospective study. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.824095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jun M., Jardine M.J., Perkovic V., et al. Hyperkalemia and renin-angiotensin aldosterone system inhibitor therapy in chronic kidney disease: a general practice-based, observational study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99:S1–S87. doi: 10.1016/j.kint.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Winkelmayer W.C., Fischer M.A., Schneeweiss S., Wang P.S., Levin R., Avorn J. Underuse of ACE inhibitors and angiotensin II receptor blockers in elderly patients with diabetes. Am J Kidney Dis. 2005;46:1080–1087. doi: 10.1053/j.ajkd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Yildirim T., Arici M., Piskinpasa S., et al. Major barriers against renin-angiotensin-aldosterone system blocker use in chronic kidney disease stages 3-5 in clinical practice: a safety concern? Ren Fail. 2012;34:1095–1099. doi: 10.3109/0886022x.2012.717478. [DOI] [PubMed] [Google Scholar]
- 23.Mattu A., Brady W.J., Robinson D.A. Electrocardiographic manifestations of hyperkalemia. Am J Emerg Med. 2000;18:721–729. doi: 10.1053/ajem.2000.7344. [DOI] [PubMed] [Google Scholar]
- 24.Campese V.M., Adenuga G. Electrophysiological and clinical consequences of hyperkalemia. Kidney Int Suppl (2011) 2016;6:16–19. doi: 10.1016/j.kisu.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Vea A., Bardají A., Garcia C., Oliver J.A. Severe hyperkalemia with minimal electrocardiographic manifestations: a report of seven cases. J Electrocardiol. 1999;32:45–49. doi: 10.1016/s0022-0736(99)90020-1. [DOI] [PubMed] [Google Scholar]
- 26.Clase C.M., Carrero J.J., Ellison D.H., et al. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020;97:42–61. doi: 10.1016/j.kint.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Korgaonkar S., Tilea A., Gillespie B.W., et al. Serum potassium and outcomes in CKD: insights from the RRI-CKD cohort study. Clin J Am Soc Nephrol. 2010;5:762–769. doi: 10.2215/cjn.05850809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovesdy C.P., Matsushita K., Sang Y., et al. Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta-analysis. Eur Heart J. 2018;39:1535–1542. doi: 10.1093/eurheartj/ehy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epstein M. Hyperkalemia constitutes a constraint for implementing renin-angiotensin-aldosterone inhibition: the widening gap between mandated treatment guidelines and the real-world clinical arena. Kidney Int Suppl (2011) 2016;6:20–28. doi: 10.1016/j.kisu.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leon S.J., Whitlock R., Rigatto C., et al. Hyperkalemia-related discontinuation of renin-angiotensin-aldosterone system inhibitors and clinical outcomes in CKD: a population-based cohort study. Am J Kidney Dis. 2022;80:164–173.e1. doi: 10.1053/j.ajkd.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Qiao Y., Shin J.I., Chen T.K., et al. Association between renin-angiotensin system blockade discontinuation and all-cause mortality among persons with low estimated glomerular filtration rate. JAMA Intern Med. 2020;180:718–726. doi: 10.1001/jamainternmed.2020.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linde C., Bakhai A., Furuland H., et al. Real-world associations of renin-angiotensin-aldosterone system inhibitor dose, hyperkalemia, and adverse clinical outcomes in a cohort of patients with new-onset chronic kidney disease or heart failure in the United Kingdom. J Am Heart Assoc. 2019;8 doi: 10.1161/jaha.119.012655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhandari S., Mehta S., Khwaja A., et al. Renin-angiotensin system inhibition in advanced chronic kidney disease. N Engl J Med. 2022;387:2021–2032. doi: 10.1056/NEJMoa2210639. [DOI] [PubMed] [Google Scholar]
- 34.Neuen B.L., Oshima M., Agarwal R., et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation. 2022;145:1460–1470. doi: 10.1161/circulationaha.121.057736. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal R., Joseph A., Anker S.D., et al. Hyperkalemia risk with finerenone: results from the FIDELIO-DKD trial. J Am Soc Nephrol. 2022;33:225–237. doi: 10.1681/asn.2021070942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer S.C., Tendal B., Mustafa R.A., et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. doi: 10.1136/bmj.m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salah H.M., Al’Aref S.J., Khan M.S., et al. Effect of sodium-glucose cotransporter 2 inhibitors on cardiovascular and kidney outcomes-Systematic review and meta-analysis of randomized placebo-controlled trials. Am Heart J. 2021;232:10–22. doi: 10.1016/j.ahj.2020.10.064. [DOI] [PubMed] [Google Scholar]
- 38.Vasilakou D., Karagiannis T., Athanasiadou E., et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–274. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]
- 39.Kario K., Okada K., Kato M., et al. 24-hour blood pressure-lowering effect of an SGLT-2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension: results from the randomized, placebo-controlled SACRA study. Circulation. 2018;139:2089–2097. doi: 10.1161/circulationaha.118.037076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tikkanen I., Narko K., Zeller C., et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–428. doi: 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- 41.Ferdinand K.C., Izzo J.L., Lee J., et al. Antihyperglycemic and blood pressure effects of empagliflozin in black patients with type 2 diabetes mellitus and hypertension. Circulation. 2019;139:2098–2109. doi: 10.1161/circulationaha.118.036568. [DOI] [PubMed] [Google Scholar]
- 42.Reardon L.C., Macpherson D.S. Hyperkalemia in outpatients using angiotensin-converting enzyme inhibitors. How much should we worry? Arch Intern Med. 1998;158:26–32. doi: 10.1001/archinte.158.1.26. [DOI] [PubMed] [Google Scholar]
- 43.Hundemer G.L., Talarico R., Tangri N., et al. Ambulatory treatments for RAAS inhibitor-related hyperkalemia and the 1-year risk of recurrence. Clin J Am Soc Nephrol. 2021;16:365–373. doi: 10.2215/cjn.12990820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dargie H.J., Allison M.E., Kennedy A.C., Gray M.J. High dosage metolazone in chronic renal failure. Br Med J. 1972;4:196–198. doi: 10.1136/bmj.4.5834.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agarwal R., Sinha A.D., Cramer A.E., et al. Chlorthalidone for hypertension in advanced chronic kidney disease. N Engl J Med. 2021;385:2507–2519. doi: 10.1056/NEJMoa2110730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgan D.B., Davidson C. Hypokalaemia and diuretics: an analysis of publications. Br Med J. 1980;280:905–908. doi: 10.1136/bmj.280.6218.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ernst M.E., Carter B.L., Zheng S., Grimm R.H., Jr. Meta-analysis of dose-response characteristics of hydrochlorothiazide and chlorthalidone: effects on systolic blood pressure and potassium. Am J Hypertens. 2010;23:440–446. doi: 10.1038/ajh.2010.1. [DOI] [PubMed] [Google Scholar]
- 48.Herrington W.G., Staplin N., Wanner C., et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388:117–127. doi: 10.1056/NEJMoa2204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McMurray J.J.V., Solomon S.D., Inzucchi S.E., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 50.Packer M., Anker S.D., Butler J., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 51.Tang J., Ye L., Yan Q., Zhang X., Wang L. Effects of sodium-glucose cotransporter 2 inhibitors on water and sodium metabolism. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.800490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griffin M., Rao V.S., Ivey-Miranda J., et al. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation. 2020;142:1028–1039. doi: 10.1161/circulationaha.120.045691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawler P.R., Liu H., Frankfurter C., et al. Changes in cardiovascular biomarkers associated with the sodium-glucose cotransporter 2 (SGLT2) inhibitor ertugliflozin in patients with chronic kidney disease and type 2 diabetes. Diabetes Care. 2021;44:e45–e47. doi: 10.2337/dc20-2265. [DOI] [PubMed] [Google Scholar]
- 54.Lepage L., Dufour A.C., Doiron J., et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;10:2136–2142. doi: 10.2215/cjn.03640415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laureati P., Xu Y., Trevisan M., et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant. 2020;35:1518–1526. doi: 10.1093/ndt/gfz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noel J.A., Bota S.E., Petrcich W., et al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med. 2019;179:1025–1033. doi: 10.1001/jamainternmed.2019.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goutorbe P., Montcriol A., Lacroix G., Bordes J., Meaudre E., Souraud J.B. Intestinal necrosis associated with orally administered calcium polystyrene sulfonate without sorbitol. Ann Pharmacother. 2011;45:e13. doi: 10.1345/aph.1M547. [DOI] [PubMed] [Google Scholar]
- 58.Rugolotto S., Gruber M., Solano P.D., Chini L., Gobbo S., Pecori S. Necrotizing enterocolitis in a 850 gram infant receiving sorbitol-free sodium polystyrene sulfonate (Kayexalate): clinical and histopathologic findings. J Perinatol. 2007;27:247–249. doi: 10.1038/sj.jp.7211677. [DOI] [PubMed] [Google Scholar]
- 59.Joo M., Bae W.K., Kim N.H., Han S.R. Colonic mucosal necrosis following administration of calcium polystryrene sulfonate (kalimate) in a uremic patient. J Korean Med Sci. 2009;24:1207–1211. doi: 10.3346/jkms.2009.24.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bakris G.L., Pitt B., Weir M.R., et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314:151–161. doi: 10.1001/jama.2015.7446. [DOI] [PubMed] [Google Scholar]
- 61.Weir M.R., Bakris G.L., Bushinsky D.A., et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 62.Spinowitz B.S., Fishbane S., Pergola P.E., et al. Sodium zirconium cyclosilicate among individuals with hyperkalemia: a 12-month Phase 3 study. Clin J Am Soc Nephrol. 2019;14:798–809. doi: 10.2215/cjn.12651018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Butler J., Anker S.D., Lund L.H., et al. Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial. Eur Heart J. 2022;43:4362–4373. doi: 10.1093/eurheartj/ehac401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kosiborod M., Rasmussen H.S., Lavin P., et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312:2223–2233. doi: 10.1001/jama.2014.15688. [DOI] [PubMed] [Google Scholar]
- 65.Canada’s Drugs and Health Technology Agency Sodium zirconium cyclosilicate. AstraZeneca Canada Inc. https://www.cadth.ca/sodium-zirconium-cyclosilicate
- 66.Ogata S., Akashi Y., Kato S., et al. Association between dietary potassium intake estimated from multiple 24-hour urine collections and serum potassium in patients with CKD. Kidney Int Rep. 2023;8:584–595. doi: 10.1016/j.ekir.2022.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bernier-Jean A., Wong G., Saglimbene V., et al. Dietary potassium intake and all-cause mortality in adults treated with hemodialysis. Clin J Am Soc Nephrol. 2021;16:1851–1861. doi: 10.2215/cjn.08360621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramos C.I., González-Ortiz A., Espinosa-Cuevas A., Avesani C.M., Carrero J.J., Cuppari L. Does dietary potassium intake associate with hyperkalemia in patients with chronic kidney disease? Nephrol Dial Transplant. 2021;36:2049–2057. doi: 10.1093/ndt/gfaa232. [DOI] [PubMed] [Google Scholar]
- 69.Noori N., Kalantar-Zadeh K., Kovesdy C.P., et al. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. 2010;56:338–347. doi: 10.1053/j.ajkd.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gritter M., Wouda R.D., Yeung S.M.H., et al. Effects of short-term potassium chloride supplementation in patients with CKD. J Am Soc Nephrol. 2022;33:1779–1789. doi: 10.1681/asn.2022020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khoueiry G., Waked A., Goldman M., et al. Dietary intake in hemodialysis patients does not reflect a heart healthy diet. J Ren Nutr. 2011;21:438–447. doi: 10.1053/j.jrn.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 72.St-Jules D.E., Goldfarb D.S., Sevick M.A. Nutrient non-equivalence: does restricting high-potassium plant foods help to prevent hyperkalemia in hemodialysis patients? J Ren Nutr. 2016;26:282–287. doi: 10.1053/j.jrn.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clegg D.J., Headley S.A., Germain M.J. Impact of dietary potassium restrictions in CKD on clinical outcomes: benefits of a plant-based diet. Kidney Med. 2020;2:476–487. doi: 10.1016/j.xkme.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karaboyas A., Zee J., Brunelli S.M., et al. Dialysate potassium, serum potassium, mortality, and arrhythmia events in hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2017;69:266–277. doi: 10.1053/j.ajkd.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vareesangthip K., Yincharoen P., Winijkul A., Chanchairujira T. Cardiac arrhythmia during early-week and mid-week dialysis in hemodialysis patients. Ther Apher Dial. 2021;25:890–898. doi: 10.1111/1744-9987.13622. [DOI] [PubMed] [Google Scholar]
- 76.Ferrey A., You A.S., Kovesdy C.P., et al. Dialysate potassium and mortality in a prospective hemodialysis cohort. Am J Nephrol. 2018;47:415–423. doi: 10.1159/000489961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sandle G.I., Gaiger E., Tapster S., Goodship T.H. Evidence for large intestinal control of potassium homoeostasis in uraemic patients undergoing long-term dialysis. Clin Sci (Lond) 1987;73:247–252. doi: 10.1042/cs0730247. [DOI] [PubMed] [Google Scholar]
- 78.Sandle G.I., Gaiger E., Tapster S., Goodship T.H. Enhanced rectal potassium secretion in chronic renal insufficiency: evidence for large intestinal potassium adaptation in man. Clin Sci (Lond) 1986;71:393–401. doi: 10.1042/cs0710393. [DOI] [PubMed] [Google Scholar]
- 79.Mathialahan T., Maclennan K.A., Sandle L.N., Verbeke C., Sandle G.I. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol. 2005;206:46–51. doi: 10.1002/path.1750. [DOI] [PubMed] [Google Scholar]
- 80.Sumida K., Dashputre A.A., Potukuchi P.K., et al. Laxative use and risk of dyskalemia in patients with advanced CKD transitioning to dialysis. J Am Soc Nephrol. 2021;32:950–959. doi: 10.1681/asn.2020081120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mathialahan T., Sandle G.I. Dietary potassium and laxatives as regulators of colonic potassium secretion in end-stage renal disease. Nephrol Dial Transplant. 2003;18:341–347. doi: 10.1093/ndt/18.2.341. [DOI] [PubMed] [Google Scholar]
- 82.Perry M.D., Sandle G.I. Regulation of colonic apical potassium (BK) channels by cAMP and somatostatin. Am J Physiol Gastrointest Liver Physiol. 2009;297:G159–G167. doi: 10.1152/ajpgi.00132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ajani S., Hurt R.T., Teeters D.A., Bellmore L.R. Ischaemic colitis associated with oral contraceptive and Bisacodyl use. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr-12-2011-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lopez Morra H.A., Fine S.N., Dickstein G. Colonic ischemia with laxative use in young adults. Am J Gastroenterol. 2005;100:2134–2136. doi: 10.1111/j.1572-0241.2005.50395_8.x. [DOI] [PubMed] [Google Scholar]
- 85.Baudet J.S., Castro V., Redondo I. Recurrent ischemic colitis induced by colonoscopy bowel lavage. Am J Gastroenterol. 2010;105:700–701. doi: 10.1038/ajg.2009.637. [DOI] [PubMed] [Google Scholar]
- 86.Jaques D.A., Stucker F., Ernandez T., et al. Comparative efficacy of patiromer and sodium polystyrene sulfonate on potassium levels in chronic haemodialysis patients: a randomized crossover trial. Clin Kidney J. 2022;15:1908–1914. doi: 10.1093/ckj/sfac129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fishbane S., Ford M., Fukagawa M., et al. A Phase 3b, Randomized, Double-Blind, Placebo-Controlled Study of Sodium Zirconium Cyclosilicate for Reducing the Incidence of predialysis hyperkalemia. J Am Soc Nephrol. 2019;30:1723–1733. doi: 10.1681/asn.2019050450. [DOI] [PMC free article] [PubMed] [Google Scholar]