Abstract
Primary liver cancer is a significant health problem worldwide. Hepatocellular carcinoma (HCC) is the main pathological type of primary liver cancer, accounting for 75%−85% of cases. In recent years, radiotherapy has become an emerging treatment for HCC and is effective for various stages of HCC. However, radiosensitivity of liver cancer cells has a significant effect on the efficacy of radiotherapy and is regulated by various factors. How to increase radiosensitivity and improve the therapeutic effects of radiotherapy require further exploration. This review summarizes the recent research progress on the mechanisms affecting sensitivity to radiotherapy, including epigenetics, transportation and metabolism, regulated cell death pathways, the microenvironment, and redox status, as well as the effect of nanoparticles on the radiosensitivity of liver cancer. It is expected to provide more effective strategies and methods for clinical treatment of liver cancer by radiotherapy.
Keywords: Hepatocellular carcinoma, radiosensitivity, epigenetics, non-coding RNA, cell death, metabolism, tumor microenvironment, reactive oxygen species, nanoparticle
Introduction
Liver cancer is the fourth most frequently occurring malignant tumor and the second most common cause of tumor-related mortality in China (1,2). The advent of advanced radiotherapy technology has ushered in the era of precision radiotherapy. External beam radiotherapy (EBRT), including intensity-modulated radiation therapy (IMRT), image-guided radiotherapy, stereotactic body radiation therapy (SBRT), and selective internal radiation therapy (SIRT) that combine radiotherapy with vascular intervention, have achieved accurate treatment of liver cancer and other cancers (3). Recent clinical research and meta-analyses have demonstrated that early-stage liver cancer can be treated curatively by EBRT. EBRT can also be used as a comprehensive treatment for middle- and late-stage liver cancers including unresectable liver cancer, portal vein thrombosis complicating hepatic cancer, vascular infiltration, peripheral invasion, and multiple liver cancer nodules. This helps to control tumor progression and metastasis, and reduces tumor staging, providing new treatment options for patients with liver cancer. Several countries have already incorporated EBRT into their medical guidelines, with SBRT considered to be a potential substitute for radiofrequency ablation and surgery to cure early liver cancer (4,5). SIRT has also shown efficacy as an alternative treatment option to transcatheter arterial chemoembolization (TACE) in some clinical studies (6-10). SIRT provides promising outcomes for patients with certain unresectable liver cancers. The role of radiotherapy in liver cancer treatment has been significantly elevated (11).
Investigation of the radiosensitivity of hepatocellular carcinoma (HCC) cells is currently a prominent topic in the field of radiotherapy. The unresponsiveness of HCC cells to radiotherapy is a major factor against its recommendation for HCC treatment (12). However, recently, studies on radiation tolerance of HCC cells have been increasing. Tang et al. was among the first to investigate radiation tolerance of HCC cells at the molecular level in 2004 (13). Since then, many studies have demonstrated that the sensitivity of HCC cells to radiation is strongly linked to epigenetic regulation, cellular death, metabolism, redox status, and tumor microenvironment (TME), which affect the effectiveness of radiotherapy. This review summarizes the current research on radiosensitivity mechanisms, covering their fundamental outcomes, and presenting a perspective on future research directions.
Epigenetic modification of HCC cells and sensitivity to radiotherapy
Epigenetic modification regulates gene expression through chemical modifications that affect DNA and proteins on chromosomes, changing gene expression by affecting gene transcription, splicing, translation, nucleosome assembly, and chromatin structural stability at multiple levels, thereby affecting the physiological and pathological processes and genetic phenotype of cells (14). Numerous studies have confirmed the close link between epigenetic regulation of liver cancer cells and their oncological properties, such as invasion and migration, and this link is under constant refinement in terms of sensitivity to radiotherapy (15,16). Recent studies have primarily focused on histone/chromatin modifications and non-coding RNAs, aiming to refine knowledge about HCC radiotherapy sensitivity and epigenetic regulation (17). RNA methylation, histone and chromatin modifications, non-coding RNAs, and gene shearing affect cellular sensitivity to irradiation, primarily through mechanisms such as DNA repair and the cell cycle.
RNA methylation
RNA methylation (18) is a chemical modification that selectively adds methyl adenine to RNA through catalysis by methyltransferases. This process has a close relationship with tumorigenesis and cancer development. Although limited research exists on the relationship between RNA methylation and radiotherapy sensitivity in HCC, preliminary studies suggest that RNA affects the sensitivity of HCC cells to radiotherapy through N6-methyladenosine (m6A) methylation. Qu et al. found that inhibition of demethylase alpha-ketoglutarate-dependent dioxygenase alkB homolog 5 (ALKBH5) expression increases the radiosensitivity of HCC cells. Specifically, m6A modification of Toll-interleukin 1 receptor domain-containing adaptor protein (TIRAP) mRNA activates the TIRAP/nuclear factor-kappa B pathway, inducing C-C motif chemokine ligand 5 (CCL5) overexpression and further modulation of the CCL5-inducible chemokine receptor 5 (CCR5) axis to activate irradiated hepatic stellate cells, ultimately reducing the sensitivity of HCC to radiotherapy. This process is mediated by ALKBH5, and blocking the ALKBH5-CCR5 axis increases the sensitivity of HCC cells to radiotherapy (19).
Histone and chromatin modifications
Acetylation
Acetylation is a chemical modification process that attaches acetyl groups to proteins, providing crucial functions in organisms such as regulating transcription, the cell cycle, and chromatin structure and functions (20). Recent studies indicate that acetylation may also be closely related to sensitivity to radiotherapy in HCC. Inhibition of histone deacetylases (HDACs) increases the sensitivity of HCC cells to radiotherapy. Jin et al. found that expression of the oncogene MIR22HG and its derivative miR-22-5p is upregulated in HCC cells after irradiation. Further analyses revealed that histone acetylation is significantly enhanced in the MIR22HG promoter region and HDAC2 activity is reduced in HCC tissues. Subsequent in vitro experiments demonstrated that increasing histone acetylation levels upregulate MIR22HG and miR-22-5p expression, increase irradiation lethality of HCC cells, and significantly reduce cell proliferation, migration, and invasion (21). These results suggest the involvement of histone acetylation in regulating gene transcription and enhancing oncogene expression, thereby increasing the sensitivity of HCC cells to radiotherapy. Tsai et al. found that downregulation of HDAC4 gene expression forms a complex with DNA repair protein Rad51 and ubiquitin-binding enzyme 9 to reduce homologous recombination repair of DNA double-strand breaks and protein kinase B activation, leading to increased apoptosis and sensitivity to radiotherapy in HCC cells (22). Additionally, Choi et al. found that HDAC inhibitor panobinostat increases sensitivity to radiotherapy in prostate, non-small cell lung, and bladder cancers, and may have the same effect on HCC. Panobinostat downregulates expression of anti-apoptotic protein Mcl-1 and enhances proton beam irradiation-induced DNA damage, reactive oxygen species (ROS) production, and sensitivity of HCC cells to proton beam radiotherapy (23).
Ubiquitination
Ubiquitination is a post-translational modification process by which a ubiquitin protein is covalently bound to a target protein through enzymes. The process participates in regulating the stability, activity, subcellular localization, interaction, and other biological functions of the target protein (24). Zhang et al. found that carbamoyl-phosphate synthase 1 (CPS-1), a critical enzyme of the urea cycle, is downregulated in HCC tissues, and its presence is associated with a poor prognosis. Silencing CPS-1 may promote HCC progression and radioresistance via ubiquitin-proteasome system-mediated c-Myc gene stabilization. In vivo experiments have demonstrated that CPS1 depletion accelerates HCC progression and induces irradiation tolerance in HCC (25).
Inhibiting ubiquitin-conjugating enzyme E2T is negatively associated with the effectiveness of radiotherapy in HCC patients. In vitro experiments have shown that ubiquitin-conjugating enzyme E2T interacts with monoubiquitinated histone variant H2AX/γH2AX after irradiation, promoting phosphorylation of cell cycle checkpoint kinase 1, causing its release from chromatin to cytoplasm, and facilitating its degradation, thereby reducing the radiation tolerance of HCC cells (26).
mRNA splicing
mRNA splicing is a transcriptional process that fragments exons, generating various mRNA and protein variants (27). This mechanism plays a critical role in regulating gene expression and functions, including radiosensitivity of HCC. Wen et al. (28) reported that PRMT5-ISO5, a splice variant of protein arginine methyltransferase 5 (PRMT5) precursor mRNA that lacks exon 3 and parts of exon 4, is overexpressed in HCC patients who undergo SBRT. PRMT5-ISO5 inhibits the malignancy of HCC cells and improves poor prognosis of HCC patients. Additionally, radiation-induced downregulation of serine and arginine-rich splicing factor 3 increases exogenous expression of PRMT5-ISO5, amplifying HCC cell susceptibility to radiotherapy.
Non-coding RNA (ncRNA)
NcRNAs, including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), affect oncogenesis, tumor development, prognosis, and the effectiveness of antitumor therapy (29). Mounting evidence suggests that ncRNAs regulate the response of HCC to radiotherapy through gene expression and regulation of signal transduction pathways. Targeted ncRNA therapy alters the oncological properties and radiosensitivity of HCC cells, reducing their proliferative, invasive and migratory potentials, regulating the cell cycle and signaling, and indirectly regulating autophagy, apoptosis and necrosis (6,30). Thus, incorporating ncRNA therapy may be complementary to radiotherapy, offering a new avenue to assess pretreatment efficacy and the prognosis of HCC patients (Table 1).
Table 1. HCC radiosensitivity-associated non-coding RNA types and mechanisms involved.
| Non-coding RNA | Expression level | Key mechanisms/pathways | Effect on sensitivity to radiotherapy |
| HCC, hepatocellular carcinoma; HIF-1α, hypoxia inducible factor-1α; HDAC2, histone deacetylase 2; CHD5, chromodomain helicase DNA binding protein 5; PEX5, peroxisome biogenesis factor 5; CDK1, cyclin dependent kinase 1; FBXW7, F-box and WD repeat domain containing 7; SETDB1, SET domain bifurcated histone lysine methyltransferase 1; Bmi-1, B-cell-specific moloney leukemia virus insertion site 1; EphA2, ephrin receptor A2; GABARAP, GABA type a receptor-associated protein; RAD18, ubiquitin protein ligase; WEE1, WEE1 G2 checkpoint kinase. | |||
| miR-138-5p (31) | Increase | Downward revision of HIF-1ɑ | Enhance |
| miR-101-3p (32) | Increase | Trichothecene regulates miR-101-3p/WEE1 axis | Enhance |
| miR-122 (33) | Increase | Inhibition of cell cycle protein G1 expression | Enhance |
| miR-22-5p (21) | Increase | Inhibition of HDAC2 activity increases histone acetylation | Enhance |
| miR-301b-3p (34) | Increase | SLC16A1-AS1 regulation miR-301b-3p/CHD5 axis | Enhance |
| miR-31-5p (35) | Increase | Activation of Wnt/β-catenin signaling pathway by PEX5 | Enhance |
| miR-1271-5p (36) | Increase | Targeting CDK1 | Enhance |
| miR-106a (37) | Decrease | Upgraded FBXW7 | Enhance |
| miR-621 (38) | Increase | Targeted inhibition of SETDB1 and activation of the p53 signaling pathway | Enhance |
| miR-146a-5p (39) | Increase | DNA repair pathway induced by replication protein A3 | Enhance |
| miR-203 (40) | Increase | Targeting Bmi-1 | Enhance |
| miR-26b (40) | Increase | Targeting EphA2 | Enhance |
| miR-20a (41) | Increase | Via PTEN/PI3K/Akt signaling pathway | Diminish |
| LncR-NEAT1 (42) | Increase | Induction of autophagy by GABARAP | Enhance |
| lncR-GAS5 (43) | Increase | Via miR-144-5p/ATF2 | Enhance |
| lncR-ROR (44) | Increase | Action of RAD18 via microRNA-145 | Diminish |
| lncR-KCNQ1OT1 (45) | Decrease | Inhibition of miR-146a-5p/ACER3 | Enhance |
| lncR-NEAT1-2 (46) | Decrease | Via miR-101-3p/WEE1 | Enhance |
| circR-LARP1B (47) | Increase | Via miR-578/IGF1R | Diminish |
| circ-ZNF292 (48) | Increase | Via the Wnt/β-catenin signaling pathway | Enhance |
MiRNA
MiRNAs are a group of small non-coding RNAs that bind to target mRNAs to regulate their translation or degradation, thereby affecting gene expression (49-52). Several studies have demonstrated the importance of miRNAs in regulating the sensitivity and tolerance of HCC cells to radiotherapy. These mechanisms focus on regulating the structural domains of HCC proteins, DNA damage repair, cell cycle arrest, and cancer-related gene pathways.
Recent studies have demonstrated the role of miR-106a and miR-621 in regulating sensitivity to radiotherapy through modulation of protein domain expression. MiR-106a targets and inhibits expression of F-box and WD repeat domain-containing 7, influencing the migration and invasion of HCC cells (37). Similarly, Shao et al. reported that miR-621 downregulates SET domain bifurcated histone lysine methyltransferase 1 expression, which is upregulated in HCC and activates the p53 signaling pathway, subsequently repressing the tumorigenic properties of HCC cells and enhancing radiosensitivity (38).
MiRNAs modify the sensitizing effect of radiotherapy on HCC cells by regulation of multiple signaling pathways. Pei et al. showed that miR-301b-3p is targeted and regulated by SLC16A1-AS1, and through regulation of chromodomain helicase DNA-binding protein 5, it decreases the malignant behavior of HCC cells and increases sensitivity to radiotherapy (34). MiR-26b negatively regulates the expression of proto-oncogene ephrin type-A receptor 2, which reduces the invasive and migratory abilities of cancer cells and decreases tolerance to irradiation (53). Overexpression of miR-203 decreases expression of B-cell-specific moloney leukemia virus insertion site 1 (Bmi-1) protein to increase the sensitizing effect of radiotherapy. Bmi-1 is aberrantly expressed in numerous human malignancies including breast, colorectal, and esophageal squamous cancers and HCC (40). Furthermore, Zhang et al. found that miR-20a activates the PTEN/PI3K/Akt signaling pathway in HCC, driving cellular metabolism, proliferation, survival, growth, and angiogenesis. Modulation of miR-20a leads to cellular radioresistance (41).
LncRNA
LncRNAs are RNA molecules over 200 nucleotides in length and are a class of biological process regulators rich in regulatory and functional units. Numerous studies have confirmed the major role of lncRNAs in tumor development (54,55). Nuclear enriched abundant transcript-1 (NEAT1) is a long non-coding RNA associated with maintenance of stem cell properties and enhances resistance to 5-fluorouracil and cisplatin in HCC cells. Recent research has shown that excessive accumulation of NEAT1 in HCC cells promotes γ-aminobutyric acid receptor-related protein expression, which triggers autophagosome-lysosome fusion, resulting in increased cellular autophagy and enhanced radiosensitivity of HCC cells (42).
Specific lncRNAs may serve as molecular sponges, binding to miRNAs and inhibiting their capacity to silence target genes to regulate the radiosensitivity of HCC cells. Linc-ROR functions as a molecular sponge for miR-145, which increases the level of miR-145 and promotes translation of target gene ubiquitin ligase RAD18, thereby improving the DNA repair capacity of HCC cells. This ultimately improves their overall resilience to DNA damage and enhances the tolerance of these cells to irradiation (44). Overexpression of lncRNA GAS5 which promotes cell growth arrest, modulates the expression of activating transcription factor 2 (ATF2) by acting as a molecular sponge for miR-144-5p, thereby competing with it to regulate ATF2 expression and improve radiotherapy sensitivity of HCC (43). In the same manner, LncRNA NEAT1_2 inhibits expression of WEE1 by serving as a molecular sponge, which competitively binds to miR-101-3p and changes the sensitivity of HCC to radiotherapy (46).
CircRNA
CircRNA is a stable cyclic ribonucleic acid molecule produced by reverse shear events rather than a linear form of RNA (56,57). CircRNAs play a significant role in regulating the sensitivity and tolerance of HCC cells to radiotherapy. Circ-LARP1B enhances the malignant behavior and radiotherapy tolerance of HCC cells by reducing the inhibitory effect of miR-578 on insulin-like growth factor 1 receptor expression through competitive binding (47). Additionally, circ-ZNF292 inhibits the proliferation of hypoxic HCC cells and increases the efficacy of radiotherapy by activating the Wnt/β-catenin pathway (48).
Regulated cell death (RCD) and radiosensitivity of HCC cells
RCD is a process that involves signal transduction pathways or cascades triggered by external factors or intracellular disturbances. It is of great importance to maintain tissue morphology and functions, and can be affected by changes in cell cycle regulation. Induction of RCD in HCC cells has become a current topic of interest because of its potential effect on the oncological properties and radiotherapy sensitivity of HCC cells (Table 2).
Table 2. Modes and main mechanisms of cell death associated with radiosensitivity in HCC.
| Inducing factors | Expression level | Main mechanisms | Mode of death |
| HCC, hepatocellular carcinoma; phospho-CDC2, phosphorylated cyclin-dependent kinase 2; DNA-PKcs, DNA-dependent protein kinase; L02, 2-phenyl-imidazo (4, 5f) (1,10) phenanthroline; GSTM3, glutathione S-transferase M3; Stattic, signal transduction and transcription activator 3 inhibitor; p27kip1, inhibitor of cyclin-dependent kinase; SOCS2, suppressor of cytokine signaling 2; COMMD10, copper metabolism gene MURR1 structural domain 10; CLTRN, amino acid transport regulator collectrin; ADAM9, a disintegrin and a metalloprotease 9; HIF-1α, hypoxia inducible factor-1α; SLC7A11, solute carrier family 7 member 11; GPX4, glutathione peroxidase 4; FTH1, ferritin heavy chain 1. | |||
| Costunolide (58) | Increase | Promote DNA damage, chromosomal aberrations, G2/M phase block | Apoptosis |
| Tetrandrine (59) | Increase | Activate apoptotic gene Bax, increase Caspase-3 expression, promote cyclin B1, inhibit phospho-CDC2 expression and reduce the number of HCC cells with radiotherapy-mediated G2 phase block | Apoptosis |
| DNA-PKcs (60) | Increase | Increase the number of apoptotic cells entering subG1 phase 72 h after radiotherapy | Apoptosis |
| PIP (61) | Increase | Induce p53 overexpression, anti-apoptotic protein Bcl-2 degradation and increase Caspase-3 expression | Apoptosis |
| GSTM3 (62) | Decrease | Decrease Bcl-21 expression and increase Bax, p21, p27 and p53 expression | Apoptosis |
| Stattic (63) | Increase | Inhibition of radiotherapy-mediated STAT activation in HCC cells | Apoptosis |
| SSd (64) | Increase | Enhance radiation-induced G0/G1 blockade, reduce G2/M phase cell numbers under hypoxic conditions, upregulate p53 and Bax expression, and downregulate Bcl-2 expression | Apoptosis and autophagy |
| SOCS2 (65) | Increase | Suppression of SLC7A11 with GPX4 | Ferroptosis |
| COMMD10 (66) | Increase | Inhibit HIF-1α ubiquitin degradation, impair COMMD10 binding to HIF-1α, activate the HIF-1α/CP positive feedback loop and promote SLC7A11 transcription, disrupting Cu-Fe homeostasis in HCC | Ferroptosis |
| CLTRN (67) | Increase | GPX4, SLC7A11 and FTH1 expressions are down-regulated | Ferroptosis |
| ADAM9 (68) | Decrease | Inhibition of Nrf2 pathway | Autophagy |
| Mesima (69) | Increase | Reduce the number of cells entering G2/M phase and maintain a state of DNA damage | Apoptosis |
HCC cell cycle, apoptosis and sensitivity to radiotherapy
The cell cycle and apoptosis are critical biological processes closely linked to tumorigenesis, development, and radiosensitivity. Apoptosis is an ordered and genetically programmed form of cell death that is essential for normal development and tissue homeostasis. The cell cycle consisting of five phases [G0 (stationary phase), G1 (prophase), S (DNA synthesis), G2 (prometaphase), and M (mitosis)] plays a vital role in cell division (70). Abnormalities in cell cycle regulation and apoptosis can lead to increased radiosensitivity of HCC cells.
The expression and function of cell cycle proteins, related kinases, and phosphatases play a crucial role in determining the radiosensitivity of tumor cells. After radiotherapy, depletion of DNA-dependent protein kinase catalytic subunit increases the number of apoptotic cells entering sub-G1 phase. This depletion increases the radiosensitivity of HCC cells. Liu et al. found that costunolide treatment arrests HCC cells in the mitotic phase and promotes the expression of various molecules, including phosphorylated CDK1 and cell cycle protein B1. Such treatment achieved a 1.9-fold increase in the sensitization effect of radiotherapy (58). Guan et al. found that overexpression of cyclin-dependent kinase inhibitor p27kip1 arrests HepG2 cells in G0/G1 phase, thereby increasing their sensitivity to 60Co γ-radiation (71).
Apoptosis-related genes, including p53, Bax, Bcl-2, and caspase-3, significantly affect the radiosensitivity of tumor cells. HCC cells that develop tolerance to irradiation show reduced expression of glutathione S-transferase Mu 3, a probable oncogenic factor. Substantial overexpression of glutathione S-transferase Mu 3 decreases the expression of anti-apoptotic protein Bcl-2, while increasing the expression of proapoptotic genes, including Bax, p21, p27, and p53. This, in turn, inhibits tumor growth and improves sensitivity to radiotherapy (62). Stattic, a potent STAT3 inhibitor, neutralizes activation of the proto-oncogene STAT in HCC cells during radiotherapy. Consequently, this promotes apoptosis and weakens malignant behavior, resulting in improved sensitivity to radiotherapy (63). Similarly, Liu et al. found that the combination of 2-phenyl-imidazo (4, 5f) (1,10) phenanthroline, and radiotherapy triggers overproduction of pro-apoptotic p53. This, in turn, initiates degradation of anti-apoptotic protein Bcl-2, leading to the formation of pores in mitochondrial membranes that allows cytochrome c leakage. As a result, the mitochondrial apoptotic pathway activates in cells, leading to overexpression of pro-apoptotic protein caspase-3. Consequently, this induces mitochondrion-dependent apoptosis and improves sensitivity to radiotherapy (61).
Cell cycle blockade induces apoptosis of tumor cells, which in turn increases sensitivity to radiotherapy (72). Yan et al. found that low concentrations of dynein protect normal liver cells from radiotherapy at doses below 12 Gy and high concentrations enhance sensitivity to radiotherapy. In vitro experiments demonstrated that 5 µmol/L genistein promotes DNA damage and chromosomal aberrations, and causes G2/M phase arrest and apoptosis, significantly enhancing radiosensitivity (73). Jeong et al. performed cellular assays and showed that the tropical basidiomycete fungus Phellinus linteus (Mesima) decreases the number of cells in G2/M phase and induces early apoptosis, while maintaining DNA damage after radiotherapy. These results suggest that Mesima reduces the cell survival rate after radiotherapy and enhances the effect of radiotherapy (69). Tetrandrine promotes expression of cyclin B1 and inhibits expression of phosphorylation-classical type 2 DCs, thereby reducing the number of HCC cells arrested in G2 phase during radiotherapy. Moreover, tetrandrine activates the apoptotic gene Bax, making it antagonistic to Bcl-2 and increasing the expression of apoptotic protein caspase-3. This mechanism of action contributes to sensitization to radiotherapy (59). Saikosaponin-d (SSd) enhances radiation-induced G0/G1 blockade and reduces the number of G2/M phase cells under hypoxic conditions. SSd alone or in combination with radiotherapy upregulates the expression of pro-apoptotic proteins p53 and Bax, and downregulates the expression of anti-apoptotic protein Bcl-2, increasing sensitivity of HCC cells to radiotherapy (74).
Ferroptosis and sensitivity to radiotherapy in HCC cells
Ferroptosis is a novel form of regulated cell death triggered by iron overload and lipid peroxidation that inhibits cancer progression. Recent studies indicate that radiotherapy induces ferroptosis in cells, suggesting its potential use in cancer treatment (75,76). Ferroptosis proteins, such as glutathione peroxidase 4 (GPX4), solute carrier family 7 member 11 (SLC7A11/xCT), and ferritin heavy chain 1, are closely associated with HCC development (77-79).
Ferroptosis of HCC cells may be associated with sensitivity to radiotherapy. Chen et al. found that radiotherapy induces overexpression of suppressor of cytokine signaling 2, which in turn inhibits SLC7A11 and GPX4, and promotes ferroptosis in HCC cells. Follow-up experiments demonstrated that knockdown of GPX4 and SLC7A11 expression effectively reduces the irradiation tolerance of HCC cells, further suggesting that radiotherapy-mediated overexpression of suppressor of cytokine signaling 2 increases sensitivity to radiotherapy by regulating ferroptosis in HCC cells (65). Feng and Liu found that radiation mediates ferroptosis in HCC cells through the AdipoR1-Nrf2-xCT pathway, and that treatment with ferroptosis inhibitor ferrostatin-1 significantly ameliorates the radiation-induced decrease in cell activity (80). Yuan et al. found that expression of GPX4, SLC7A11, and ferritin heavy chain 1, important proteins for ferroptosis, is significantly down-regulated in HCC cells overexpressing the amino acid transport regulator collectrin, and their expression is further reduced by irradiation, suggesting that collectrin enhances the sensitivity of HCC cells to radiotherapy by promoting ferroptosis (67).
Ionizing radiation reduces the expression of copper metabolism MURR1 domain 10, which causes an imbalance in Cu-Fe homeostasis, induces ferroptosis, and decreases tolerance to irradiation. Copper metabolism MURR1 domain 10 directly regulates intracellular Cu accumulation, indirectly activates the HIF-1α/CP positive feedback loop, promotes SLC7A11 transcription by inhibiting ubiquitin-mediated degradation of HIF-1α, and decreases the probability of binding between the two, reducing intracellular Fe2+ levels (66).
Autophagy and sensitivity to radiotherapy in HCC cells
Autophagy is the process by which a cell degrades some or all of its intracellular components through its lysosomes to maintain cellular metabolic homeostasis and obtain nutrients (81). In HCC cells, abnormal enhancement or inhibition of the autophagic process may lead to the development and progression of HCC (82). Autophagy in HCC cells regulates the cellular stress response and affects the sensitivity of HCC cells to radiotherapy.
A disintegrin and a metalloprotease 9 (ADAM9) is overexpressed in human HCC cells and induces malignant behavior in tumor cells, EMT, and ROS production. Zhu et al. found that an increase in ADAM9 expression in HCC cells following irradiation increases irradiation tolerance, which promotes Nrf2-mediated autophagy and lowers radiotherapy sensitivity in cells (68). Additionally, Tian et al. reported that SSd suppresses HCC cell growth and enhances their radiosensitivity via autophagy induction (64). These findings suggest that inhibiting the autophagic process in HCC cells represents a promising therapeutic approach for HCC treatment combined with radiotherapy.
Metabolism and sensitivity to radiotherapy in HCC cells
Dysregulation of metabolic pathways is closely associated with radiotherapy sensitivity in HCC cells. These metabolic changes are a significant factor in the development and progression of HCC, and a major mechanism underlying radiotherapy resistance in cancerous cells. Disruptions in glucose and lipid metabolism, along with excess lactic acid and ketone body production, are hallmarks of the abnormal metabolism in HCC cells (83,84). These metabolic changes provide cells with the necessary energy and nutrients, while increasing their radiotherapy tolerance. Moreover, radiotherapy alters the metabolic pathways of HCC cells, potentially causing imbalances in the acid-base balance and changes in glucose metabolism. Such dysregulation of metabolic pathways exacerbates sensitivity to radiotherapy in cancer cells (85,86). The management of metabolic abnormalities in HCC cells and improvement of their sensitivity to radiotherapy represent major challenges and areas of focus in current HCC research.
Glucose metabolism
Gildin, an actin-binding protein containing the convoluted helix domain 88A, is upregulated in HCC cells and is closely associated with the tumor size, progression, and prognosis. Yu et al. showed that silencing gildin in HCC cells by shRNA significantly increases sensitivity to radiotherapy and reduces the capacity to take up glucose and undergo glycolysis. Further research revealed that inhibition of glycolysis by gildin-shRNA improves sensitivity to radiotherapy by suppressing the PI3K-Akt signaling pathway in HCC cells (87). Furthermore, osthole, a Chinese herbal extract, induces apoptosis, inhibits HCC progression, and increases sensitivity to radiotherapy in HCC cells by weakening glycolysis through inhibition of the AMPK/mTOR pathway (88).
Lipid metabolism
Adiponectin is a bioactive protein produced and secreted by adipocytes, and its role has been widely studied in chronic diseases. Among the three lipocalin receptors, AdipoR1 is closely linked to malignancy development, progression, proliferation and apoptosis. Liu et al. demonstrated that silencing AdipoR1 arrests irradiated HCC cells in G2/M phase and modulates the expression of caspase-3, Bax, and Bcl-2 to promote apoptosis and increase sensitivity to radiotherapy. Moreover, in vivo experiments showed that low AdipoR1 expression improves post-radiation anemia and mitigates liver function abnormalities in rats (89). Additionally, Feng and Liu found that radiotherapy increases AdipoR1 expression and activates the Nrf2/xCT pathway, resulting in a reduction in radiation-induced ferroptosis and an improvement in irradiation tolerance in HCC cells (80).
TME and sensitivity to radiotherapy in HCC cells
TME is complex and is comprised of various components, such as immune cells, tumor-associated fibroblasts, extracellular matrix, and cytokines, which interact with each other. Various microenvironments, including immune microenvironment, TME that lack nutrients, microenvironments in inflammatory diseases, and physical microenvironments in tumors, are being extensively researched on the basis of TME components (90,91). The changes that occur in the TME of HCC cells following radiotherapy are currently a widely discussed topic, emphasizing the importance of avoiding its restructuring and reducing the sensitivity of tumors to radiotherapy (91). In clinical practice, PD-1 inhibitors and granulocyte-macrophage colony-stimulating factor in combination with radiotherapy have shown progress in treating digestive tract tumors (92,93). This provides new possibilities and ideas for future combined treatment of HCC.
Immune microenvironment
The tumor immune microenvironment encompasses a complex network of cells and molecules, including immune cells, proinflammatory factors, and cytokines, which are integral to tumorigenesis, development, and therapeutic responses (94,95). The immune microenvironment of HCC significantly affects the efficacy of radiotherapy (96).
The immune microenvironment might enable HCC cells to evade host immune surveillance and attack by suppressing the host immune response. Such a mechanism may promote increased tolerance of HCC cells to radiotherapy, leading to reduced treatment efficacy. Macrophages are classified into two main subtypes, namely antitumoral M1 and protumoral M2 phenotypes. Zhuang et al. showed that low concentrations of CCL5 (5−10 ng/mL) induce polarization of THP-1 M0 macrophages to the M2 phenotype. By blocking the CCL5-CCR5 axis through CCR5 antagonists, they demonstrated that more macrophages repolarize to the M1 type through the STAT3-SOCS3 signaling pathway. This approach also reduces the number of M2-polarized macrophages, promotes apoptosis, and significantly improves radiosensitivity of hepatoma cells (97). Furthermore, the immune microenvironment has the potential to improve the efficacy of radiotherapy by augmenting the host immune response. Huang and colleagues found that the Wnt/β-catenin inhibitor ICG-001 combined with radiotherapy facilitates CD8+ T cell infiltration and IFN-γ production, while decreasing the number of regulatory T cells and promoting a better immune microenvironment for tumor cells. Additionally, ICG-001 increases the radiation-induced DNA damage response in HCC cells by suppressing the p53 pathway and activating the cGAS/STING pathway to achieve radiosensitization. Subsequent in vivo investigations have demonstrated that this therapeutic approach might induce immune memory and block tumor recurrence (98).
Hypoxic microenvironments
Hypoxia is prevalent in the HCC microenvironment, leading to distinct biological changes in cancerous cells. These alterations include changes in cell metabolism and regulation of gene expression, affecting the sensitivity of hepatocellular cells to radiotherapy (99-102).
Hypoxia-inducible factors (HIFs) in HCC cells under hypoxia regulate processes such as metabolic transformation and cell survival, thereby increasing the sensitivity of HCC cells to radiotherapy. HIF-1 plays a major role in the adaptive response to hypoxia by regulating cell proliferation, apoptosis, angiogenesis, pH homeostasis, and anaerobic glycolysis to induce tumor tolerance to radiotherapy (100).
Hypoxic environments provoke epigenetic modifications contributing to elevation of resistance to radiotherapy in HCC cells. Bai et al. demonstrated a negative feedback loop between miR-138-5p and histone methyltransferase enhancer of zeste homolog 2 expression. Overexpression of miR-138-5p in HCC cells suppresses the expression of enhancer of zeste homolog 2 and HIF-1α, mitigating the effects of the hypoxic environment on EMT and malignant behavior, while enhancing sensitivity to radiotherapy in cells (31).
Cancer stem cells (CSCs) and sensitivity to radiotherapy
CSCs are a subpopulation of cells capable of self-renewal and differentiation in various directions. These cells are highly tumorigenic and recurring, and display considerable resistance to conventional therapies, including radiotherapy and chemotherapy (103).
The specific markers and transcription factors expressed by HCC stem cells play a major role in the regulation of radioresistance. PRRX1 is a novel EMT inducer that is resistant to classical EMT inducers. Downregulation of PRRX1 expression induces CSC properties in HCC cells, impedes tumor spheroidy, and decreases their tolerance to 5-fluorouracil and irradiation (104).
CSCs enhance radioresistance through cell cycle regulation. 14-3-3ζ is a regulatory protein with oncogenic properties that is associated with apoptosis and the cell cycle. Inhibition of its expression may increase the sensitivity of HCC cell lines to cisplatin. Similar to chemoresistance, Lee et al. found that silencing 14-3-3ζ inhibits the stemness and activity of hepatic CSCs and enhances radiation-induced apoptosis and sensitivity to radiotherapy (105).
Nanoparticles and radiosensitivity
Nanoparticles, which are small substances measuring 1−100 nanometers, are commonly employed in medical imaging and tumor therapy because of their unique physicochemical properties. Studies have shown that nanotechnology affects tumor prevention, detection, and treatment (106,107). Regarding HCC, nanoparticles have been studied for their implications in radiosensitivity. The resistance mechanism to radiotherapy involving nanoparticles is multifaceted and involves various molecular mechanisms and signaling pathways.
The use of nanoparticles has an inherent radiosensitizing effect. Nanoparticles absorb radiation, generate electrons, and promote the formation of ROS from surrounding oxygen and water molecules, leading to DNA double-strand breaks, and increase apoptosis of tumor cells. This results in enhanced radiosensitization of tumor cells. Ankur Sood et al. found that alpha-ketoglutarate-decorated iron oxide-gold core-shell nanoparticles have a significant effect on ROS generation and DNA fragmentation. This leads to radiosensitization of tumor cells to γ-radiation (108).
Nanoparticles enhance tolerance of HCC cells to radiotherapy by upregulating DNA repair mechanisms, cell cycle regulation, and apoptosis. Nano-silver and nano-gold were investigated by Zheng et al. and found to upregulate Bax and caspase-3 expression and downregulate Bcl-2, catalase, and superoxide dismutase (SOD) expression as well as total glutathione. They increase DNA damage and apoptosis, reduce the activity of HCC cells, and significantly enhance radiosensitivity (109). Xie et al. reported that nanoparticles loaded with lupeol block DNA repair by inhibiting activation of the PI3K/Akt pathway and suppressing MAPK phosphorylation in the Raf/MEK/ERK signaling pathway, thereby bolstering the sensitivity of HCC to radiotherapy (110). Gadolinium-containing nanoparticle AGuIX has high permeability and long intrahepatic elimination times, and induces apoptosis of HCC cells, thereby improving radiosensitivity. Therefore, AGuIX can be deployed as an MRI contrast agent and radiosensitizer (111). Moreover, Cur@Hb derived from hemoglobin-curcumin nanoparticles reduces the toxicity of curcumin, improves its water solubility, and significantly increases the radiosensitivity ratio of HCC cells to 1.510. Furthermore, Cur@Hb significantly inhibits the proliferation, migration, and angiogenesis of HCC cells, arrests HCC cells in G2/M phase, and induces apoptosis (112).
Redox state of HCC cells and sensitivity to radiotherapy
The redox state is the balance of redox reactions in intracellular and extracellular microenvironments, which includes oxidative stress and antioxidant systems. Oxidative stress, which results from intracellular oxidative metabolism such as ROS, causes oxidative damage to cellular DNA, proteins and lipids, and ultimately affects cell growth and metabolic functions. Conversely, the antioxidant system includes a range of intracellular enzymatic and non-enzymatic antioxidants, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and reduced glutathione (GSH), which scavenge ROS and protect cells from oxidative damage. Recent studies have suggested that the redox state of HCC cells is related to sensitivity to radiotherapy (113).
Excessive accumulation of intracellular ROS enhances the cellular stress response, which triggers increased sensitivity of HCC cells to injury by radiotherapy (114,115). As described above, HDAC inhibitors panobinostat (23), ADAM9 (68), and nanoparticles containing redox agents (108) induce apoptosis and necrosis, while increasing the sensitivity of HCC cells to radiotherapy.
Research has identified a correlation between the antioxidant capacity of HCC cells and radiotherapy tolerance. Therefore, reducing the antioxidant capacity of HCC cells may improve their response to radiotherapy. Overexpression of γ-glutamylcysteine synthetase heavy chain, a GSH synthase, decreases radioresistance of HCC cells (116). Sun and colleagues found that exposing HCC cells to isoliquiritigenin (ISL), a natural antioxidant, induces Keap1 expression and inhibits Nrf2, resulting in an excess of ROS and a defective antioxidant defense system that disrupts redox homeostasis. As a result of ISL treatment, irradiated HCC cells exhibited a decreased clonogenic capacity and significantly increased DNA damage and apoptosis compared with the control group, indicating that ISL-induced redox disturbance increases sensitivity of HCC cells to radiotherapy (117).
Discussion
The large number of liver cancer patients in China accounts for nearly half of the global cases, which means that effective treatment of liver cancer is currently the biggest challenge (118-120). The use of precision radiotherapy technology to treat liver cancer represents a significant paradigm shift on the basis of technological innovation. This new approach overcomes the limitations of traditional radiotherapy for liver cancer treatment and facilitates novel treatment strategies for inoperable liver cancer patients. Beyond effective control of tumor progression and reduction of tumor staging, precision radiotherapy offers a curative potential to patients, while retaining the advantages of non-invasiveness, convenience, and efficacy that contrast surgical treatment options. It is thus an increasingly viable alternative treatment to traditional modes.
Surgical resection is widely regarded as the most effective treatment for liver cancer. However, while a mature technology, laparoscopic surgery is associated with significant drawbacks, including serious intraoperative and postoperative complications, a poor overall condition, and reduced liver functions. Radiofrequency ablation therapy, another first-line treatment, has issues such as high costs, technical intricacy, and increased toxic reactions. Some liver cancer patients cannot undergo surgical resection because of the tumor location near the Glisson system and diaphragm, previous local treatments leading to adhesions between the tumor and surrounding tissues or organs, and underlying diseases that limit treatment options. Radiofrequency ablation also necessitates technical assistance, which can lead to complications such as bile duct fistula, pleural effusion, diaphragm injury, and local lung collapse. These issues limit the indications for these invasive treatment methods, thus making it difficult for late-stage tumors, poor liver functions, severe liver cirrhosis, and underlying diseases, such as stroke and coronary heart disease, to benefit. Radiotherapy offers an alternative, non-invasive method that mitigates these risks. It is appropriate to treat liver cancer of all stages. SBRT is recommended as a first-class treatment for small liver cancers because of its equivalent survival benefits to surgery and ablation. For advanced liver cancer, radiotherapy combined with TACE improves local tumor control and prolongs survival. For patients with portal vein tumor thrombus, preoperative neoadjuvant radiotherapy or postoperative adjuvant radiotherapy significantly prolong survival. At the end of HCC radiotherapy, the tumor size is mostly stable, but significant tumor shrinkage can occur at 3−9 months after treatment, making it difficult to determine the curative effect and distinguish relapse. Hence, research related to radiosensitization and improving radiotherapy efficacy is essential.
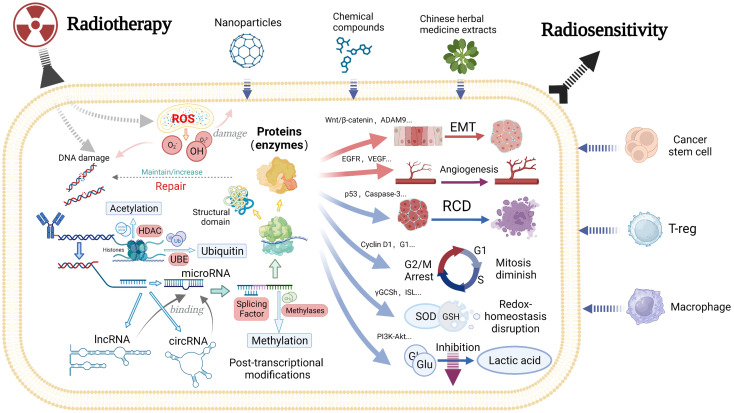
Studies have mainly focused on exploring the factors and mechanisms that enhance sensitivity to radiotherapy in HCC through the biological effects of radiotherapy. The direct biological effect of radiotherapy is induction of DNA damage, which promotes HCC cell death. Additionally, radiotherapy elicits an indirect biological effect by increasing ROS production and radiation tolerance of HCC cells. Regulation of epigenetics, cell death, and intracellular homeostasis induces cell death and inhibits various malignant behaviors, such as spheroid formation, invasion and migration of HCC cells. These cellular effects influence the biological behavior of tumors, thereby enhancing sensitivity to radiotherapy (Figure 1).
Figure 1.
A schematic diagram of mechanisms that affect radiosensitivity of HCC. HCC, hepatocellular carcinoma; ROS, reactive oxygen species; HDAC, histone deacetylase; UBE, ubiquitin-conjugating enzyme; EMT, epithelial mesenchymal transition; RCD, regulated cell death; ADAM9, A disintegrin and a metalloprotease; EGFR, epidermal growth factor receptor; VEGF, vascular endothelial growth factor; ISL, isoliquiritigenin; SOD, superoxide dismutase; GSH, glutathione; Glu, glucose; T-reg, regulatory T cell.
Several studies have shown that manipulating target genes affects radiosensitivity as well as drug sensitivity to some chemotherapeutic and targeted agents in HCC cells. This provides new treatment options for combination therapy of HCC. Future research may explore pathways that affect tumor sensitivity to radiotherapy, including genes and their downstream products that influence resistance to targeted therapy and immunotherapy in other cancers. Studies have investigated the combined application of Chinese medicine extracts, compound derivatives, and molecular materials such as nanoparticles with radiotherapy. Local application of small molecule drugs loaded in nanoparticles and combined with radiotherapy appears feasible. Chinese doctors express their desire to learn from international advanced technology, while also wanting to use traditional Chinese medicine with modern advanced technology to achieve more precise cancer suppression with fewer side effects and less systemic effects for Chinese HCC patients. The combination of particles with various contents and radiotherapy techniques integrated with genetic sequencing results of HCC patients may be a novel approach to enhance the accuracy and effectiveness of radiotherapy. This approach may promote the integration of microscopic intervention with internal and external radiotherapy, which presents a promising opportunity for interventional and radiotherapy departments to work collaboratively.
Conclusions
Radiotherapy for liver cancer is receiving increasing attention and recognition. The advantages of radiotherapy, surgical resection, and local ablation are combined in a complementary manner. Various radiotherapies, such as adjuvant radiotherapy after liver cancer resection, conversion treatment of TACE, and targeted immunotherapy used in combination with radiotherapy, have been employed to increase surgical opportunities and preoperative new adjuvant radiotherapy to reduce tumors, accurately define tumor margins, and reduce intraoperative bleeding and tumor blood supply. These cases indicate that radiotherapy has become a significant approach in treating liver cancer. The combined use of radiotherapy with other liver cancer treatments offers more benefits to patients. We should explore the mechanism of radiotherapy sensitization, which promotes the efficacy of radiotherapy and enhances its ability to support surgical treatment. This would benefit more patients and doctors by offering updated and optimal solutions for liver cancer treatment and improving prognosis.
Acknowledgements
This work was supported by the Science and Technology Plan Project of Guangzhou (No. 202102010171); National Natural Science Foundation Cultivation Project of The Third Affiliated Hospital of Sun Yat-sen University (No. 2020GZRPYMS11); Natural Science Foundation of Guangdong Province (No. 2018A030313641) and CSCO-Roche Joint Cancer Research Fund (No. Y-Roche2019/2-0041).
Contributor Information
Zhicheng Yao, Email: dengmeih@mail.sysu.edu.cn.
Meihai Deng, Email: dengmeih@mail.sysu.edu.cn.
References
- 1.Chen W, Zheng R, Baade PD, et al Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Zhou M, Wang H, Zeng X, et al Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394:1145–58. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricke J, Klümpen HJ, Amthauer H, et al Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71:1164–74. doi: 10.1016/j.jhep.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Shirono T, Koga H, Niizeki T, et al Usefulness of a novel transarterial chemoinfusion plus external-beam radiation therapy for advanced hepatocellular carcinoma with tumor thrombi in the inferior vena cava and right atrium: Case study. Cancer Rep (Hoboken) 2021;5:e1539. doi: 10.1002/cnr2.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rim CH, Park S, Shin IS, et al Is the concurrent use of sorafenib and external radiotherapy feasible for advanced hepatocellular carcinoma. A meta-analysis. Cancers (Basel) 2021;13:2912. doi: 10.3390/cancers13122912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu CH, Chen CY, Yeh CT, et al Radiosensitization of hepatocellular carcinoma through targeting radio-associated microRNA. Int J Mol Sci. 2020;21:1859. doi: 10.3390/ijms21051859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogel A, Cervantes A, Chau I, et al Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv238–55. doi: 10.1093/annonc/mdy308. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Luna LE, Yang JD, Sanchez W, et al Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36:714–23. doi: 10.1007/s00270-012-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salem R, Lewandowski RJ, Kulik L, et al Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497–507.e2. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salem R, Lewandowski RJ, Mulcahy MF, et al Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Sangro B, Carpanese L, Cianni R, et al Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: A European evaluation. Hepatology. 2011;54:868–78. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 12.Sangro B, Iñarrairaegui M, Bilbao JI Radioembolization for hepatocellular carcinoma. J Hepatol. 2012;56:464–73. doi: 10.1016/j.jhep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Tang WY, Chau SPY, Tsang WP, et al The role of Raf-1 in radiation resistance of human hepatocellular carcinoma Hep G2 cells. Oncol Rep. 2004;12:1349–54. doi: 10.3892/or.12.6.1349. [DOI] [PubMed] [Google Scholar]
- 14.Sun L, Zhang H, Gao P Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell. 2022;13:877–919. doi: 10.1007/s13238-021-00846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagaraju GP, Dariya B, Kasa P, et al Epigenetics in hepatocellular carcinoma. Semin Cancer Biol. 2022;86:622–32. doi: 10.1016/j.semcancer.2021.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Wilson CL, Mann DA, Borthwick LA Epigenetic reprogramming in liver fibrosis and cancer. Adv Drug Deliv Rev. 2017;121:124–32. doi: 10.1016/j.addr.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy T, Mann DA Epigenetics in liver disease: from biology to therapeutics. Gut. 2016;65:1895–905. doi: 10.1136/gutjnl-2015-311292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang B, Wang JQ, Tan Y, et al RNA methylation and cancer treatment. Pharmacol Res. 2021;174:105937. doi: 10.1016/j.phrs.2021.105937. [DOI] [PubMed] [Google Scholar]
- 19.Qu J, Yan H, Hou Y, et al RNA demethylase ALKBH5 in cancer: from mechanisms to therapeutic potential. J Hematol Oncol. 2022;15:8. doi: 10.1186/s13045-022-01224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shvedunova M, Akhtar A Modulation of cellular processes by histone and non-histone protein acetylation. Nat Rev Mol Cell Biol. 2022;23:329–49. doi: 10.1038/s41580-021-00441-y. [DOI] [PubMed] [Google Scholar]
- 21.Jin Q, Hu H, Yan S, et al lncRNA MIR22HG-derived miR-22-5p enhances the radiosensitivity of hepatocellular carcinoma by increasing histone acetylation through the inhibition of HDAC2 activity. Front Oncol. 2021;11:572585. doi: 10.3389/fonc.2021.572585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai CL, Liu WL, Hsu FM, et al Targeting histone deacetylase 4/ubiquitin‐conjugating enzyme 9 impairs DNA repair for radiosensitization of hepatocellular carcinoma cells in mice. Hepatology. 2018;67:586–99. doi: 10.1002/hep.29328. [DOI] [PubMed] [Google Scholar]
- 23.Choi C, Lee GH, Son A, et al Downregulation of Mcl-1 by panobinostat potentiates proton beam therapy in hepatocellular carcinoma cells. Cells. 2021;10:554. doi: 10.3390/cells10030554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swatek KN, Komander D Ubiquitin modifications. Cell Res. 2016;26:399–422. doi: 10.1038/cr.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Hu Y, Wu Z, et al Deficiency of carbamoyl phosphate synthetase 1 engenders radioresistance in hepatocellular carcinoma via deubiquitinating c-Myc. Int J Radiat Oncol Biol Phys. 2023;115:1244–56. doi: 10.1016/j.ijrobp.2022.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Zhu Z, Li W, et al UBE2T-regulated H2AX monoubiquitination induces hepatocellular carcinoma radioresistance by facilitating CHK1 activation. J Exp Clin Cancer Res. 2020;39:222. doi: 10.1186/s13046-020-01734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang E, Aifantis I RNA splicing and cancer. Trends Cancer. 2020;6:631–44. doi: 10.1016/j.trecan.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Wen C, Tian Z, Li L, et al SRSF3 and HNRNPH1 regulate radiation-induced alternative splicing of protein arginine methyltransferase 5 in hepatocellular carcinoma. Int J Mol Sci. 2022;23:14832. doi: 10.3390/ijms232314832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan H, Bu P Non-coding RNA in cancer. Essays Biochem. 2021;65:625–39. doi: 10.1042/EBC20200032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo J, Jin K, Tang T, et al A new biomarker to enhance the radiosensitivity of hepatocellular cancer: miRNAs. Future Oncol. 2022;18:3217–28. doi: 10.2217/fon-2022-0136. [DOI] [PubMed] [Google Scholar]
- 31.Bai B, Liu Y, Fu XM, et al Dysregulation of EZH2/miR-138-5p axis contributes to radiosensitivity in hepatocellular carcinoma cell by downregulating hypoxia-inducible factor 1 alpha (HIF-1α) Oxid Med Cell Longev. 2022;2022:7608712. doi: 10.1155/2022/7608712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun L, Cai H, Zhou T, et al Verbascoside enhances radiosensitivity of hepatocellular carcinoma cells through regulating miR-101-3p/Wee1 axis. Drug Dev Res. 2022;83:891–9. doi: 10.1002/ddr.21914. [DOI] [PubMed] [Google Scholar]
- 33.Xu G, Bu S, Wang X, et al MiR-122 radiosensitize hepatocellular carcinoma cells by suppressing cyclin G1. Int J Radiat Biol. 2022;98:11–7. doi: 10.1080/09553002.2021.1987561. [DOI] [PubMed] [Google Scholar]
- 34.Pei S, Chen Z, Tan H, et al SLC16A1-AS1 enhances radiosensitivity and represses cell proliferation and invasion by regulating the miR-301b-3p/CHD5 axis in hepatocellular carcinoma. Environ Sci Pollut Res Int. 2020;27:42778–90. doi: 10.1007/s11356-020-09998-1. [DOI] [PubMed] [Google Scholar]
- 35.Wen J, Xiong K, Aili A, et al PEX5, a novel target of microRNA-31-5p, increases radioresistance in hepatocellular carcinoma by activating Wnt/β-catenin signaling and homologous recombination. Theranostics. 2020;10:5322–40. doi: 10.7150/thno.42371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu HM, Tan HY, Lin Y, et al MicroRNA-1271-5p inhibits cell proliferation and enhances radiosensitivity by targeting CDK1 in hepatocellular carcinoma. J Biochem. 2020;167:513–24. doi: 10.1093/jb/mvz114. [DOI] [PubMed] [Google Scholar]
- 37.Deng P, Wu Y Knockdown of miR-106a suppresses migration and invasion and enhances radiosensitivity of hepatocellular carcinoma cells by upregulating FBXW7. Int J Clin Exp Pathol. 2019;12:1184–93. [PMC free article] [PubMed] [Google Scholar]
- 38.Shao Y, Song X, Jiang W, et al MicroRNA-621 acts as a tumor radiosensitizer by directly targeting SETDB1 in hepatocellular carcinoma. Mol Ther. 2019;27:355–64. doi: 10.1016/j.ymthe.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo J, Si ZZ, Li T, et al MicroRNA-146a-5p enhances radiosensitivity in hepatocellular carcinoma through replication protein A3-induced activation of the DNA repair pathway. Am J Physiol Cell Physiol. 2019;316:C299–311. doi: 10.1152/ajpcell.00189.2018. [DOI] [PubMed] [Google Scholar]
- 40.Shao Y, Zhang D, Li X, et al MicroRNA-203 increases cell radiosensitivity via directly targeting Bmi-1 in hepatocellular carcinoma. Mol Pharm. 2018;15:3205–15. doi: 10.1021/acs.molpharmaceut.8b00302. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Zheng L, Ding Y, et al MiR-20a induces cell radioresistance by activating the PTEN/PI3K/Akt signaling pathway in hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2015;92:1132–40. doi: 10.1016/j.ijrobp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Sakaguchi H, Tsuchiya H, Kitagawa Y, et al NEAT1 confers radioresistance to hepatocellular carcinoma cells by inducing autophagy through GABARAP. Int J Mol Sci. 2022;23:711. doi: 10.3390/ijms23020711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu C, Liang Y, Jin Y, et al LncRNA GAS5 enhances radiosensitivity of hepatocellular carcinoma and restricts tumor growth and metastasis by miR-144-5p/ATF2. Am J Transl Res. 2021;13:10896–07. [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Shen Z, Zhi Y, et al Long non-coding RNA ROR promotes radioresistance in hepatocelluar carcinoma cells by acting as a ceRNA for microRNA-145 to regulate RAD18 expression. Arch Biochem Biophys. 2018;645:117–25. doi: 10.1016/j.abb.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Huang B, Chen Z, et al Knockdown of LINC00473 enhances radiosensitivity in hepatocellular carcinoma via regulating the miR-345-5p/FOXP1 axis. Onco Targets Ther. 2020;13:173–83. doi: 10.2147/OTT.S240113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, Zhang N Downregulation of lncRNA NEAT1_2 radiosensitizes hepatocellular carcinoma cells through regulation of miR-101-3p/WEE1 axis. Cell Biol Int. 2019;43:44–55. doi: 10.1002/cbin.11077. [DOI] [PubMed] [Google Scholar]
- 47.Zhu S, Chen Y, Ye H, et al Circ-LARP1B knockdown restrains the tumorigenicity and enhances radiosensitivity by regulating miR-578/IGF1R axis in hepatocellular carcinoma. Ann Hepatol. 2022;27:100678. doi: 10.1016/j.aohep.2022.100678. [DOI] [PubMed] [Google Scholar]
- 48.Yang W, Liu Y, Gao R, et al Knockdown of cZNF292 suppressed hypoxic human hepatoma SMMC7721 cell proliferation, vasculogenic mimicry, and radioresistance. Cell Signal. 2019;60:122–35. doi: 10.1016/j.cellsig.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 49.He B, Zhao Z, Cai Q, et al miRNA-based biomarkers, therapies, and resistance in Cancer. Int J Biol Sci. 2020;16:2628–47. doi: 10.7150/ijbs.47203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill M, Tran N miRNA interplay: mechanisms and consequences in cancer. Dis Model Mech. 2021;14:dmm047662. doi: 10.1242/dmm.047662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu J, Liu WF, Zhang XY, et al. Synthetic miR-26a mimics delivered by tumor exosomes repress hepatocellular carcinoma through downregulating lymphoid enhancer factor 1. Hepatol Int 2023. [Epub ahead of print]
- 52.Koustas E, Trifylli EM, Sarantis P, et al An insight into the arising role of microRNAs in hepatocellular carcinoma: future diagnostic and therapeutic approaches. Int J Mol Sci. 2023;24:7168. doi: 10.3390/ijms24087168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin Q, Li XJ, Cao PG miR-26b enhances radiosensitivity of hepatocellular carcinoma cells by targeting EphA2. Iran J Basic Med Sci. 2016;19:851–7. [PMC free article] [PubMed] [Google Scholar]
- 54.Bhan A, Soleimani M, Mandal SS Long non-coding RNA (LncRNA) and cancer: a new paradigm. Cancer Res. 2017;77:3965–81. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan YT, Lin JF, Li T, et al LncRNA-mediated posttranslational modifications and reprogramming of energy metabolism in cancer. Cancer Commun (Lond) 2020;41:109–20. doi: 10.1002/cac2.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen L, Shan G CircRNA in cancer: Fundamental mechanism and clinical potential. Cancer Lett. 2021;505:49–57. doi: 10.1016/j.canlet.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Kristensen LS, Jakobsen T, Hager H, et al The emerging roles of circRNAs in cancer and oncology. Nat Rev Clin Oncol. 2022;19:188–206. doi: 10.1038/s41571-021-00585-y. [DOI] [PubMed] [Google Scholar]
- 58.Liu CY, Chang HS, Chen IS, et al Costunolide causes mitotic arrest and enhances radiosensitivity in human hepatocellular carcinoma cells. Radiat Oncol. 2011;6:56. doi: 10.1186/1748-717X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao XM, Hu WX, Wu ZF, et al Tetrandrine enhances radiosensitization in human hepatocellular carcinoma cell lines. Radiat Res. 2018;190:385–95. doi: 10.1667/RR14981.1. [DOI] [PubMed] [Google Scholar]
- 60.Choi C, Son A, Lee GH, et al Targeting DNA-dependent protein kinase sensitizes hepatocellular carcinoma cells to proton beam irradiation through apoptosis induction. PLoS One. 2019;14:e0218049. doi: 10.1371/journal.pone.0218049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu HM, Wu Q, Cao JQ, et al A phenanthroline derivative enhances radiosensitivity of hepatocellular carcinoma cells by inducing mitochondria-dependent apoptosis. Eur J Pharmacol 2019;843: 285-91.
- 62.Sun Y, Wang Y, Yin Y, et al GSTM3 reverses the resistance of hepatoma cells to radiation by regulating the expression of cell cycle/apoptosis-related molecules. Oncol Lett. 2014;8:1435–40. doi: 10.3892/ol.2014.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu G, Zhu L, Wang Y, et al Stattic enhances radiosensitivity and reduces radio-induced migration and invasion in HCC cell lines through an apoptosis pathway. Biomed Res Int. 2017;2017:1832494. doi: 10.1155/2017/1832494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tian YD, Lin S, Yang PT, et al Saikosaponin-d increases the radiosensitivity of hepatoma cells by adjusting cell autophagy. J Cancer. 2019;10:4947–53. doi: 10.7150/jca.30286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Q, Zheng W, Guan J, et al SOCS2-enhanced ubiquitination of SLC7A11 promotes ferroptosis and radiosensitization in hepatocellular carcinoma. Cell Death Differ. 2023;30:137–51. doi: 10.1038/s41418-022-01051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang M, Wu X, Hu J, et al COMMD10 inhibits HIF1α/CP loop to enhance ferroptosis and radiosensitivity by disrupting Cu-Fe balance in hepatocellular carcinoma. J Hepatol. 2022;76:1138–50. doi: 10.1016/j.jhep.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 67.Yuan Y, Cao W, Zhou H, et al CLTRN, regulated by NRF1/RAN/DLD protein complex, enhances radiation sensitivity of hepatocellular carcinoma cells through ferroptosis pathway. Int J Radiat Oncol Biol Phys. 2021;110:859–71. doi: 10.1016/j.ijrobp.2020.12.062. [DOI] [PubMed] [Google Scholar]
- 68.Zhu L, Zhao Y, Yu L, et al Overexpression of ADAM9 decreases radiosensitivity of hepatocellular carcinoma cell by activating autophagy. Bioengineered. 2021;12:5516–28. doi: 10.1080/21655979.2021.1965694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeong YK, Oh JY, Yoo JK, et al The biofunctional effects of mesima as a radiosensitizer for hepatocellular carcinoma. Int J Mol Sci. 2020;21:871. doi: 10.3390/ijms21030871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Evan GI, Vousden KH Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–8. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 71.Guan XX, Chen LB, Ding GX, et al Transfection of p27kip1 enhances radiosensitivity induced by 60Co gamma-irradiation in hepatocellular carcinoma HepG2 cell line. World J Gastroenterol. 2004;10:3103–6. doi: 10.3748/wjg.v10.i21.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu G, Bu S, Wang X, et al Silencing the expression of cyclin G1 enhances the radiosensitivity of hepatocellular carcinoma in vitro and in vivo by inducing apoptosis. Radiat Res. 2021;195:378–84. doi: 10.1667/RADE-20-00180.1. [DOI] [PubMed] [Google Scholar]
- 73.Yan H, Jiang J, Du A, et al Genistein enhances radiosensitivity of human hepatocellular carcinoma cells by inducing G2/M arrest and apoptosis. Radiat Res. 2020;193:286–300. doi: 10.1667/RR15380.1. [DOI] [PubMed] [Google Scholar]
- 74.Wang BF, Dai ZJ, Wang XJ, et al Saikosaponin-d increases the radiosensitivity of smmc-7721 hepatocellular carcinoma cells by adjusting the g0/g1 and g2/m checkpoints of the cell cycle. BMC Complement Altern Med. 2013;13:263. doi: 10.1186/1472-6882-13-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lei G, Zhang Y, Koppula P, et al The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146–62. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lang X, Green MD, Wang W, et al Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9:1673–85. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun X, Ou Z, Chen R, et al Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–84. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jennis M, Kung CP, Basu S, et al An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev. 2016;30:918–30. doi: 10.1101/gad.275891.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim DH, Kim WD, Kim SK, et al TGF-β1-mediated repression of SLC7A11 drives vulnerability to GPX4 inhibition in hepatocellular carcinoma cells. Cell Death Dis. 2020;11:406. doi: 10.1038/s41419-020-2618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng H, Liu Y, Gan Y, et al AdipoR1 regulates ionizing radiation-induced ferroptosis in HCC cells through Nrf2/xCT pathway. Oxid Med Cell Longev. 2022;2022:8091464. doi: 10.1155/2022/8091464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li X, He S, Ma B Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19:12. doi: 10.1186/s12943-020-1138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li YJ, Lei YH, Yao N, et al Autophagy and multidrug resistance in cancer. Chin J Cancer. 2017;36:52. doi: 10.1186/s40880-017-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Du D, Liu C, Qin M, et al Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm Sin B. 2022;12:558–80. doi: 10.1016/j.apsb.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bechmann LP, Hannivoort RA, Gerken G, et al The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952–64. doi: 10.1016/j.jhep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 85.Cheng C, Geng F, Cheng X, et al Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun (Lond) 2018;38:27. doi: 10.1186/s40880-018-0301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu L, Zhu X, Wu Y Effects of glucose metabolism, lipid metabolism, and glutamine metabolism on tumor microenvironment and clinical implications. Biomolecules. 2022;12:580. doi: 10.3390/biom12040580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu L, Sun Y, Li J, et al Silencing the Girdin gene enhances radio-sensitivity of hepatocellular carcinoma via suppression of glycolytic metabolism. J Exp Clin Cancer Res. 2017;36:110. doi: 10.1186/s13046-017-0580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang H, Xue J, Xie T, et al Osthole increases the radiosensitivity of hepatoma cells by inhibiting GSK-3β/AMPK/mTOR pathway-controlled glycolysis. Naunyn Schmiedebergs Arch Pharmacol. 2023;396:683–92. doi: 10.1007/s00210-022-02347-8. [DOI] [PubMed] [Google Scholar]
- 89.Liu Y, Qi M, Liu L, et al Blocking Adipor1 enhances radiation sensitivity in hepatoma carcinoma cells. Arch Biochem Biophys. 2022;718:109152. doi: 10.1016/j.abb.2022.109152. [DOI] [PubMed] [Google Scholar]
- 90.Wu J, Chan YT, Lu Y, et al. The tumor microenvironment in the postsurgical liver: Mechanisms and potential targets of postoperative recurrence in human hepatocellular carcinoma. Med Res Rev 2023. [Epub ahead of print]
- 91.Wang J, Han Y, Li Y, et al Targeting tumor physical microenvironment for improved radiotherapy. Small Methods. 2022;6:e2200570. doi: 10.1002/smtd.202200570. [DOI] [PubMed] [Google Scholar]
- 92.Xu H, Hong Z, Xu M, et al PRaG therapy of refractory metastatic gastric cancer: A case report. Front Immunol. 2022;13:926740. doi: 10.3389/fimmu.2022.926740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao X, Kong Y, Zhang L Anti-PD-1 immunotherapy combined with stereotactic body radiation therapy and GM-CSF as salvage therapy in a PD-L1-negative patient with refractory metastatic esophageal squamous cell carcinoma: A case report and literature review. Front Oncol. 2020;10:1625. doi: 10.3389/fonc.2020.01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu T, Dai Y Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–8. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 95.Binnewies M, Roberts EW, Kersten K, et al Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–50. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Charpentier M, Spada S, Van Nest SJ, et al Radiation therapy-induced remodeling of the tumor immune microenvironment. Semin Cancer Biol. 2022;86:737–47. doi: 10.1016/j.semcancer.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 97.Zhuang Y, Zhao X, Yuan B, et al Blocking the CCL5-CCR5 axis using maraviroc promotes M1 polarization of macrophages cocultured with irradiated hepatoma cells. J Hepatocell Carcinoma. 2021;8:599–611. doi: 10.2147/JHC.S300165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang Y, Sheng H, Xiao Y, et al Wnt/β-catenin inhibitor ICG-001 enhances the antitumor efficacy of radiotherapy by increasing radiation-induced DNA damage and improving tumor immune microenvironment in hepatocellular carcinoma. Radiother Oncol. 2021;162:34–44. doi: 10.1016/j.radonc.2021.06.034. [DOI] [PubMed] [Google Scholar]
- 99.Kabakov AE, Yakimova AO Hypoxia-induced cancer cell responses driving radioresistance of hypoxic tumors: Approaches to targeting and radiosensitizing. Cancers (Basel) 2021;13:1102. doi: 10.3390/cancers13051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harada H. Hypoxia-inducible factor 1-mediated characteristic features of cancer cells for tumor radioresistance. J Radiat Res 2016;57 Suppl 1(Suppl 1):i99-105.
- 101.Yuen VW, Wong CC Hypoxia-inducible factors and innate immunity in liver cancer. J Clin Invest. 2020;130:5052–62. doi: 10.1172/JCI137553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bao MH, Wong CC Hypoxia, metabolic reprogramming, and drug resistance in liver cancer. Cells. 2021;10:1715. doi: 10.3390/cells10071715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun JH, Luo Q, Liu LL, et al Liver cancer stem cell markers: Progression and therapeutic implications. World J Gastroenterol. 2016;22:3547–57. doi: 10.3748/wjg.v22.i13.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hirata H, Sugimachi K, Takahashi Y, et al. Downregulation of PRRX1 confers cancer stem cell-like properties and predicts poor prognosis in hepatocellular carcinoma. Ann Surg Oncol 2015;22 Suppl 3:S1402-9.
- 105.Lee YK, Hur W, Lee SW, et al Knockdown of 14-3-3ζ enhances radiosensitivity and radio-induced apoptosis in CD133(+) liver cancer stem cells. Exp Mol Med. 2014;46:e77. doi: 10.1038/emm.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chakraborty E, Sarkar D Emerging therapies for hepatocellular carcinoma (HCC) Cancers (Basel) 2022;14:2798. doi: 10.3390/cancers14112798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu T, Song Y, Huang Z, et al Photothermal photodynamic therapy and enhanced radiotherapy of targeting copolymer-coated liquid metal nanoparticles on liver cancer. Colloids Surf B Biointerfaces. 2021;207:112023. doi: 10.1016/j.colsurfb.2021.112023. [DOI] [PubMed] [Google Scholar]
- 108.Sood A, Dev A, Sardoiwala MN, et al Alpha-ketoglutarate decorated iron oxide-gold core-shell nanoparticles for active mitochondrial targeting and radiosensitization enhancement in hepatocellular carcinoma. Mater Sci Eng C Mater Biol Appl. 2021;129:112394. doi: 10.1016/j.msec.2021.112394. [DOI] [PubMed] [Google Scholar]
- 109.Zheng Q, Yang H, Wei J, et al The role and mechanisms of nanoparticles to enhance radiosensitivity in hepatocellular cell. Biomed Pharmacother. 2013;67:569–75. doi: 10.1016/j.biopha.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 110.Xie Y, Liu C, Zhou S, et al Lupeol-Loaded Nanoparticles Enhance the Radiosensitivity of Hepatocellular Carcinoma by Inhibiting the Hyperactivation in Raf/Mitogen-activated protein kinase and phospatidylinositol-3 Kinase/mTOR pathways. J Biomed Nanotechnol. 2021;17:2247–58. doi: 10.1166/jbn.2021.3194. [DOI] [PubMed] [Google Scholar]
- 111.Hu P, Fu Z, Liu G, et al Gadolinium-based nanoparticles for theranostic MRI-guided radiosensitization in hepatocellular carcinoma. Front Bioeng Biotechnol. 2019;7:368. doi: 10.3389/fbioe.2019.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gao R, Gu Y, Yang Y, et al Robust radiosensitization of hemoglobin-curcumin nanoparticles suppresses hypoxic hepatocellular carcinoma. J Nanobiotechnology. 2022;20:115. doi: 10.1186/s12951-022-01316-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harris IS, DeNicola GM The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 2020;30:440–51. doi: 10.1016/j.tcb.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 114.Huang G, Pan ST ROS-mediated therapeutic strategy in chemo-/radiotherapy of head and neck cancer. Oxid Med Cell Longev. 2020;2020:5047987. doi: 10.1155/2020/5047987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Srinivas US, Tan BWQ, Vellayappan BA, et al ROS and the DNA damage response in cancer. Redox Biol. 2019;25:101084. doi: 10.1016/j.redox.2018.101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin LC, Chen CF, Ho CT, et al γ-glutamylcysteine synthetase (γ-GCS) as a target for overcoming chemo- and radio-resistance of human hepatocellular carcinoma cells. Life Sci. 2018;198:25–31. doi: 10.1016/j.lfs.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 117.Sun C, Wang ZH, Liu XX, et al Disturbance of redox status enhances radiosensitivity of hepatocellular carcinoma. Am J Cancer Res. 2015;5:1368–81. [PMC free article] [PubMed] [Google Scholar]
- 118.Li Z, Chen G, Cai Z, et al Profiling of hepatocellular carcinoma neoantigens reveals immune microenvironment and clonal evolution related patterns. Chin J Cancer Res. 2021;33:364–78. doi: 10.21147/j.issn.1000-9604.2021.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cao M, Ding C, Xia C, et al Attributable deaths of liver cancer in China. Chin J Cancer Res. 2021;33:480–9. doi: 10.21147/j.issn.1000-9604.2021.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Q, Cao M, Lei L, et al Burden of liver cancer: From epidemiology to prevention. Chin J Cancer Res. 2022;34:554–66. doi: 10.21147/j.issn.1000-9604.2022.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]