ABSTRACT
This post-hoc analysis compared the receptor-binding domain (RBD)-specific and pseudovirus neutralizing antibodies against the wild-type SARS-CoV-2 strain elicited by one or two doses (56-d interval) of Ad5-nCoV vaccine regimen (NCT04341389 and NCT04566770). Both trials had low-dose and high-dose groups. Propensity score matching was used to adjust the baseline between one- and two-dose regimens. To predict the decrease in antibody titers 1 y after vaccination, half-lives of RBD-binding antibodies and pseudovirus neutralizing antibodies were computed. We obtained 34 and 29 pairs of participants in the low- and high-dose groups based on the propensity score matching. The two-dose regimen of Ad5-nCoV increased the peaking level of neutralizing antibodies compared to the one-dose regimen at day 28, but the responses of the neutralizing antibodies were not consistent with those of the RBD antibodies. Half-lives of the RBD-binding antibodies in the two-dose Ad5-nCoV regimen (202–209 days) were longer than those in the one-dose regimen (136–137 d); half-lives of the pseudovirus neutralizing antibody in the one-dose Ad5-nCoV regimen (177 d) were longer than those in the two-dose regimen (116–131 d). The predicted positive rates of RBD-binding antibodies in the one-dose regimen (34.1%–38.3%) would be lower than those in the two-dose Ad5-nCoV regimen (67.0%–84.0%), while the positive rates of pseudovirus neutralizing antibodies in the one-dose regimen (65.4%–66.7%) would be higher than those in the two-dose regimen (48.3%–58.0%). The two-dose Ad5-nCoV regimen with a 56-d interval had no effect on the persistence of neutralizing antibodies but slowed decay trend of RBD-binding antibodies.
KEYWORDS: Ad5-nCoV, persistence of immunity, RBD-binding antibody, pseudovirus neutralizing antibody, power-law model, exponential decay model
Introduction
COVID-19 vaccines are the most effective weapon against the SARS-CoV-2 associated infections, severe diseases, and deaths.1–3 Although many COVID-19 vaccines induce a quick protective response within a short time, antibody responses and vaccine effectiveness have been observed to decrease over time.4,5 BNT162b2 and mRNA-1273 elicited excellent short-term neutralizing antibody responses and protective effectiveness.6,7 But these mRNA vaccines induced high serum neutralizing antibodies that waned by 3–6 months, with a half-life of 60 d.8,9 Conversely, Ad26.COV2.S generated lower initial neutralizing antibody titers than BNT162b2 and mRNA-1273,10 but these neutralizing antibody responses and clinical effectiveness were fairly durable for at least 8 months.11,12 Different COVID-19 vaccines could have different profiles for antibody persistency, which needs to be explored.
Ad5-nCoV, an adenoviral vector vaccine based on a replication-defective human adenovirus type 5 encoding the full spike of SARS-CoV-2, was developed by CanSino in China.13 In the phase 2 trial of one-dose Ad5-nCoV, in the 1 × 1011 and 5 × 1010 viral particles dose groups, significant neutralizing antibody responses to pseudovirus SARS-CoV-2 were produced by both doses of vaccine, with GMTs of 61.4 (95% CI 53.0, 71.0) and 55.3 (95% CI 45.3, 67.5).14 Besides, in the two-dose Ad5-nCoV regimen study, in participants aged 18 to 55, the second vaccination of Ad5-nCoV elicited higher pseudovirus neutralizing antibodies (76.8; 95% CI 52.4, 112.7) at day 28 than the first vaccination (45.1; 95% CI 24.8, 81.8).15 In an efficacy trial of one-dose Ad5-nCoV, protection against symptomatic, PCR-confirmed, COVID-19 infection at 28 d or more postvaccination was reported to be 57.5% (95% CI 39.7%, 70.0%).16 On May 19, 2022, the World Health Organization (WHO) issued an emergency use listing (EUL) for Ad5-nCoV by intramuscular injection,17 which was approved by more than 10 countries later.18 However, previous studies about Ad5-nCoV presented above only reported antibody responses at day 28 after one-dose or two-dose vaccination; the antibody persistency induced by one or two doses of Ad5-nCoV is still unknown.14,15
In this study, the antibody persistency in one- and two-dose regimens of Ad5-nCoV were reported and whether two-dose Ad5-nCoV vaccines with the 56-d interval could yield benefits of a longer duration of antibody responses than the one-dose regimen were explored.
Materials and methods
Study design, participants and procedures
These two randomized, open-label, placebo-controlled trials evaluating the safety and immunogenicity of the one-dose and two-dose regimens of Ad5-nCoV vaccine were conducted from April 2020 to December 2020 and from September 2020 to July 2021, respectively, in China.14,15 Two trial protocols and informed consents were approved by the institutional review board of the Jiangsu Provincial Center of Disease Control and Prevention. Written informed consent was obtained from all participants before screening. Detailed study designs have been reported previously. In brief, 253, 129 and 126 healthy adults aged 18 y and older were recruited to receive a single injection of the Ad5-nCoV vaccine of high-dose (1 × 1011 viral particles per dose), low-dose (5 × 1010 viral particles per dose), or placebo (only the vaccination excipients without viral particles) in the one-dose regimen trial (ClinicalTrials.gov number: NCT04341389, Supplementary Figure S1a). Blood samples were taken from participants at day 0 immediately before vaccination, at day 28, and month 6 post vaccination. While in the two-dose regimen trial (ClinicalTrials.gov number: NCT04566770, Supplementary Figure S1b), 100, 120, and 60 participants aged 18 y and older were randomly assigned to receive high-dose Ad5-nCoV (1 × 1011 viral particles per dose), or low-dose Ad5-nCoV (5 × 1010 viral particles per dose), or placebo, with a 56-d interval. For the second injection, the same dosage as for the first injection was given to each group. Blood samples were taken from participants at day 0 immediately before the first dose, at day 28 and month 6 after the second dose. We used participants in the vaccine groups in these two trials aged 18 y and above for the analysis (as described in Figure 1).
Figure 1.
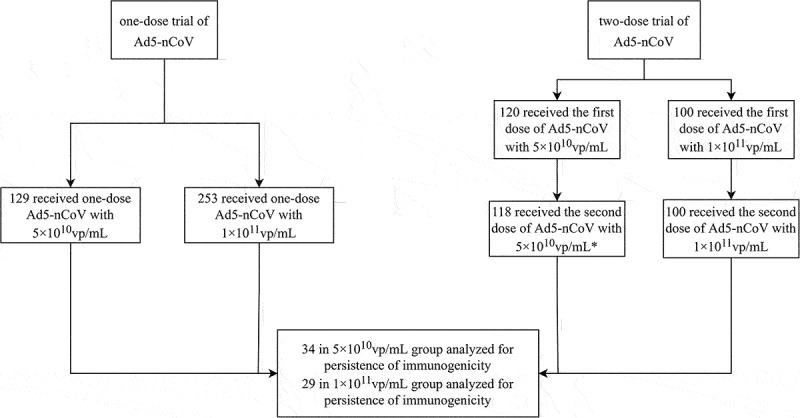
Trial profile.
vp=viral particles. *2 did not receive the second vaccination at day 56.
The detailed methods of assays have been reported previously.19 The receptor-binding domain (RBD)-specific antibodies against the wild-type SARS-CoV-2 strain were measured by ELISA kits (Beijing Wantai BioPharm, Beijing, China) and pseudovirus neutralization antibodies against the wild-type SARS-CoV-2 strain were measured by neutralizing antibodies responding to pseudovirus (a vesicular stomatitis virus pseudovirus system expressing the spike glycoprotein).20 Neutralizing antibodies against the vaccine vector Ad5 were measured by serum neutralizing assay.21 The detection limits for the RBD-specific antibody test, neutralizing antibody test to pseudovirus, and neutralizing antibody test to vaccine vector Ad5 were 1:40, 1:10, and 1:12, respectively. Undetectable antibody titers were assigned values of one-half the limits of detection.
Outcomes
We compared the vaccine elicited antibody titers (RBD-binding antibodies and pseudovirus neutralizing antibodies) in participants receiving one-dose or two-dose regimens of Ad5-nCoV at day 28 and month 6 after the vaccination. The half-life of antibodies were also assessed in one- and two-dose Ad5-nCoV regimens to predict positive rates (proportion of participants whose measured value was higher than the detection limits) and GMTs of antibodies 1 y later.
Statistical analyses and models
Multiple linear regression analysis was performed to evaluate the multivariate associations between independent variables in baseline (age, sex, BMI, and levels of preexisting adenovirus type-5 neutralizing antibody) and dependent variables (RBD-binding antibodies and pseudovirus neutralizing antibodies). An analysis of covariance was used to evaluate the consistency of the measurement of the RBD-binding or pseudovirus neutralizing antibody responses in two different laboratories (assays in the one-dose regimen trial were conducted by National Institutes for Food and Drug Control and assays in the two-dose regimen trial were conducted by Vazyme Biotech Co., Ltd). Propensity score matching would be applied in these two Ad5-nCoV regimens in order to visualize the differences in antibody titers when baseline characteristics varied between the one- and two-dose regimens and had an impact on antibody titers.
Levels of RBD-binding and pseudovirus neutralizing antibodies were presented as GMTs with 95% CIs, and fold decreases were calculated. Log-transformed antibody titers were tested for normality and homogeneity of variance using a Shapiro-Wilk and Levene’s test, respectively. Comparisons were done between two groups by using Student’s t-test for data with a normal distribution and homogeneous variance; otherwise, data were analyzed using the nonparametric Wilcoxon rank-sum test. Categorical data were analyzed using the Chi-square test or Fisher’s exact test. Except for the part about decay models, subsequent analyses were based on the participants after propensity score matching. Statistical analysis other than model fitting was performed using SPSS (Version 26).
In order to estimate the antibody half-lives to predict positive rates and GMTs (positive participants) 1 y later, decay models were fitted based on data collected on day 28 and month 6 after the last Ad5-nCoV vaccination. The use of decay models was supported by prior results from the persistence of mRNA-1273 vaccination for the COVID-19 study, which demonstrated that the power-law model and exponential decay models, respectively, are the best matches for binding and neutralizing antibodies.9 Statistical analyses were done using the lme4 package in R 4.0.4 to compute models and 95% confidence intervals of decay rates.
Exponential decay model
The exponential decay model was assumed a steady decay rate over time. Exponential model was given by the following:9,22
| (1.1) |
The half-life of exponential model was calculated by following formula:9,22
| (1.2) |
Power law model
The power law model was assumed decay rates decreased over time. Power law model in one-dose Ad5-nCoV trial was given by the following:9,23
| (2.1.1) |
The half-life of power law model in one-dose trial was calculated as:
| (2.1.2) |
Study day was offset by 56 d to account for the two-dose regimen and the power law model in the two-dose Ad5-nCoV trial was given by the following:9
| (2.2.1) |
And the half-life of power law model in two-dose trial was calculated following the formula below:
| (2.2.2) |
and were the intercept and the decay rate, representing fixed effect. c was an arbitrary small constant.
was the point estimate of decay rate. was the day when titers decrease to half compared with the starting value. The 95% confidence intervals of half-life were computed by 1000 bootstrap simulations.
Results
Population characteristics
This post-hoc analysis for the durability of the vaccine-elicited humoral immune response was limited to participants aged 18 and older receiving the one-dose or two-dose regimen of Ad5-nCoV. As described in the Supplementary Tables S1 and S2, multiple linear regression revealed that antibody titers were influenced by age and preexisting Ad5 neutralizing antibodies. And there were marked differences in age distribution between one-dose and two-dose regimens: in the low-dose groups, 116 (89.9%) participants aged 18–55 were in the one-dose regimen, while 20 (16.9%) were in the two-dose regimen; in the high-dose groups, 223 (88.1%) participants aged 18–55 were in the one-dose regimen, while nobody aged 18–55 y was in the two-dose regimen. Furthermore, regardless of the vaccine dosage, the antibody titers were comparable between laboratories from the two regimens when age and preexisting Ad5 neutralizing antibody titers were set as covariables using analysis of covariance (p = .303 and .471 for the low-dose groups, and p = .103 and .319 for the high-dose groups).
We obtained 34 and 29 pairs of participants in the low- and high-dose groups by propensity score matching in order to visualize the difference in antibody titers between participants in the one- and two-dose Ad5-nCoV regimens since age and preexisting adenovirus type-5 neutralizing antibody titers were different between these two regimens (Supplementary Table S2). The demographic characteristics of the matched participants were similar between the trials. The mean ages of the participants in the low-dose groups were 51.3 (SD 8.2) and 51.4 (SD 8.0) y in one- and two-dose regimens, respectively; in the high-dose groups, they were 60.9 and 60.5 y (As described in Table 1). Both the RBD-binding and pseudovirus neutralizing antibody titers at day 28 following the first immunization were comparable between matching groups with the same vaccine dosages from the two trials (p = .464 and .320 for the low-dose groups, and p = .507 and .652 for the high-dose groups).
Table 1.
Baseline characteristics in the one- and two-dose trials of Ad5-nCoV after propensity score matching.
| Low-dose group |
High-dose group |
|||||
|---|---|---|---|---|---|---|
| One-dose trial | Two-dose trial | p value | One-dose trial | Two-dose trial | p value | |
| N | 34 | 34 | 29 | 29 | ||
| Age, years | 51.3 (8.2) | 51.4 (8.0) | .964 | 60.9 (5.6) | 60.5 (4.2) | .770 |
| Sex | ||||||
| Male | 16 (47.1%) | 13 (38.2%) | .462 | 16 (55.2%) | 18 (62.1%) | .594 |
| Body-mass index, kg/m2 | 24.0 (2.5) | 24.5 (3.4) | .514 | 23.8 (2.5) | 24.0 (2.7) | .799 |
| Pre-existing adenovirus type-5 neutralizing antibody | ||||||
| ≤1:200, titer | 12 (35.3%) | 14 (41.2%) | .618 | 13 (44.8%) | 11 (37.9%) | .524 |
| >1:200, titer | 22 (64.7%) | 20 (58.8%) | 16 (55.2%) | 18 (65.5%) | ||
Data are mean (SD), number of participants (%) and p value. SD = standard deviation. BMI = body-mass index. N = the number of participants after propensity score matching.
Antibody responses through 6 months after the last vaccination
At day 28 after the last vaccination, no difference in RBD-binding antibodies was found between one-dose and two-dose Ad5-nCoV regimens both for the low- or high-dose group. At month 6, the two-dose regimen of Ad5-nCoV (low-dose group: 285.3; 95% CI 179.1, 454.3; high-dose group: 178.0; 95% CI 126.7, 250.1) showed significantly higher RBD-binding antibodies in terms of GMTs than did the one-dose regimen of Ad5-nCoV (low-dose group: 59.5; 95% CI 41.4, 85.5; high-dose group: 57.7; 95% CI 39.4, 84.6, Table 2 and Supplementary Figure S2).
Table 2.
RBD-binding and pseudovirus neutralizing antibody titers of participants after propensity score matching.
| Time point | Low-dose group |
High-dose group |
||||||
|---|---|---|---|---|---|---|---|---|
| n | One-dose trial | Two-dose trial | p value | n | One-dose trial | Two-dose trial | p value | |
| RBD-binding antibody | ||||||||
| Day 0 | 34 | 21.5 (18.6, 24.9) |
20.7 (19.3, 22.3) |
.654 | 29 | 21.4 (18.7, 24.5) |
20.0 (20.0, 20.0) |
.326 |
| 28 days after the last dose | 34 | 456.9 (285.7, 730.7) |
604.7 (411.0, 890.0) |
.352 | 29 | 491.3 (320.2, 753.8) |
422.3 (289.4, 616.1) |
.589 |
| 6 months after the last dose | 34 | 59.5 (41.4, 85.5) |
285.3 (179.1, 454.3) |
<.001 | 29 | 57.7 (39.4, 84.6) |
178.0 (126.7, 250.1) |
<.001 |
| Pseudovirus neutralizing antibody | ||||||||
| Day 0 | 34 | 6.0 (4.9, 7.3) |
5.6 (5.0, 6.3) |
.538 | 29 | 5.5 (4.9, 6.2) |
5.4 (4.9, 6.0) |
.750 |
| 28 days after the last dose | 34 | 55.2 (34.8, 87.4) |
96.4 (70.3, 132.0) |
.026 | 29 | 46.3 (28.6, 75.1) |
104.9 (77.0, 143.0) |
.005 |
| 6 months after the last dose | 34 | 25.1 (17.6, 35.9) |
32.2 (20.7, 50.1) |
.376 | 29 | 25.7 (17.1, 38.7) |
41.6 (29.6, 58.4) |
.069 |
Antibody titers were compared at timepoints of before and after the last vaccination. Data are GMT (95% CI) and p value. n = the number of participants after propensity score matching. Bold indicates p < 0.05.
However, pseudovirus neutralizing antibodies post-vaccination showed different trends from RBD-binding antibodies. At day 28 after the last Ad5-nCoV shot, the GMTs of pseudovirus neutralizing antibodies in the two-dose regimen (low-dose group: 96.4; 95% CI 70.3, 132.0; high-dose group: 104.9; 95% CI 77.0, 143.0) were higher than those in the one-dose regimen (low-dose group: 55.2; 95% CI 34.8, 87.4; high-dose group: 46.3; 95% CI 28.6, 75.1). At month 6 after the last vaccination, pseudovirus neutralizing antibodies were not significantly different between the one-dose and two-dose regimens of Ad5-nCoV (Table 2 and Supplementary Figure S2).
The reduction in the one-dose regimen of RBD-binding antibody titers for low-dose groups was 7.7 folds (95% CI 5.8, 10.3) between peak responses at day 28 and month 6, while in the two-dose regimen there was a gradual decline of a 2.1-fold (95% CI 1.5, 3.0) decrease. In the high-dose group, similar decreases in RBD antibodies were observed as in the low-dose group: in the one- and two-dose regimens, RBD-binding antibodies decreased by 8.5 (95% CI 6.0, 12.0) and 2.4 folds (95% CI 1.8, 3.1), respectively. Both the one- and two-dose Ad5-nCoV regimens demonstrated a gradual decline in pseudovirus neutralizing antibodies from day 28 to month 6 following the last vaccination. These declines were 2.2 (95% CI 1.5, 3.2) and 3.0 (95% CI 2.2, 4.0) folds in the low-dose groups in one- and two-dose Ad5-nCoV regimens and 1.8 (95% CI 1.2, 2.8) and 2.5 (95% CI 1.9, 3.4) folds in the high-dose groups, respectively.
Modeling the persistence of antibody responses
In the low-dose groups, by using power-law models, the estimated half-life of RBD-binding antibodies for participants was 137 d (95% CI 129, 149) in one-dose Ad5-nCoV regimen and shorter than 202 d (95% CI 168, 250) in the two-dose Ad5-nCoV regimen. Likewise, the high-dose group showed similar decay trends as the low-dose group (one-dose regimen: 136; 95% CI 129, 145; two-dose regimen: 209; 95% CI 174, 256). While the estimated half-lives of pseudovirus neutralizing antibodies calculated by exponential decay models were 177 d (95% CI 143, 251) in the one-dose regimen, which was longer than 116 d (95% CI 104, 131) in the two-dose regimen (p < .001); the high-dose group showed similar half-lives of pseudovirus neutralizing antibodies as the low-dose group (one-dose regimen: 177 95% CI 143, 232; two-dose-regimen: 131; 95% CI 116, 151, as described in Table 3).
Table 3.
Estimates of antibody half-life.
| Dose group | N | Regimen | Decay rate | Half-time |
|---|---|---|---|---|
| Power-law model for RBD-binding antibody | ||||
| Low-dose group | 126 | one-dose | −1.1594 (−1.2193, −1.0922) |
137 (129, 149) |
| 118 | two-dose | −0.8782 (−0.9982, −0.7614) |
202 (168, 250) |
|
| High-dose group | 243 | one-dose | −1.1696 (−1.2181, −1.1167) |
136 (129, 145) |
| 100 | two-dose | −0.8567 (−0.9766, −0.7479) |
209 (174, 256) |
|
| Exponential decay model for pseudovirus neutralizing antibody | ||||
| Low-dose group | 126 | one-dose | −0.0017 (−0.0021, −0.0012) |
177 (143, 251) |
| 118 | two-dose | −0.0026 (−0.0029, −0.0023) |
116 (104, 131) |
|
| High-dose group | 243 | one-dose | −0.0017 (−0.0021, −0.0013) |
177 (143, 232) |
| 100 | two-dose | −0.0023 (−0.0026, −0.0020) |
131 (116, 151) |
|
Half-life in power law models estimated at month 6 after the last vaccination in one- and two-dose Ad5-nCoV regimens. Decay rate in the table is the β in the power-law or exponential decay model. N = the number of participants aged 18 y above in one-dose and two-dose trials of Ad5-nCoV.
As shown in Table 4, 1 y following vaccination, the positive rates and GMTs for RBD-binding antibodies with low dosage in the one-dose and two-dose Ad5-nCoV regimens were estimated to be 34.1% (95% CI 32.5%, 39.7%) with a GMT of 98.9 (95% CI 77.6, 125.9), and 67.0% (95% CI 66.1%, 72.9%) with a GMT of 164.7 (95% CI 134.9, 204.2), respectively. In the high-dose groups, positive rates of RBD-binding antibodies would be 38.3% (95% CI 35.8%, 39.5%) and 84.0% (95% CI 82.0, 85.0) in the one-dose and two-dose Ad5-nCoV regimens. It suggested that positive rate and GMT for RBD-binding antibodies in the two-dose Ad5-nCoV regimen were higher than those in the one-dose regimen (low-dose group: 67.0% vs. 34.1%, p < .001; high-dose group: 84.0% vs. 38.3%, p < .001). The positive rates and GMTs for pseudovirus neutralizing antibodies with low dosage in one- and two-dose regimens were 66.7% (95% CI 58.7%, 72.2%) with a GMT of 27.7 (95% CI 23.4, 33.1), and 48.3% (95% CI 44.9%, 52.5%) with a GMT of 24.2 (95% CI 20.4, 28.8), respectively. In the high-dose groups, the positive rate in the one-dose regimen would be 65.4% (95% CI 57.6%, 71.6%), with a GMT of 31.8 (95% CI 28.2, 36.3); in the two-dose regimen, the positive rate would be 58.0% (95% CI 54.0%, 69.0%), with a GMT of 26.4 (95% CI 21.9, 32.4). And findings showed that the positive rate for pseudovirus neutralizing antibodies with a low dosage in the two-dose Ad5-nCoV regimen was lower than it was in the one-dose regimen (low-dose group: 48.31% vs. 66.67%, p = .004; high-dose group: 58.0% vs. 65.4%, p = .194).
Table 4.
Predictions for antibodies 1 y after vaccination.
| Dose group | N | Regimen | Responder | Positive rate (%) | GMT |
|---|---|---|---|---|---|
| Power-law model for RBD-binding antibody | |||||
| Low-dose | 126 | one-dose | 43 (41, 50) |
34.1 (32.5, 39.7) |
98.9 (77.6, 125.9) |
| 118 | two-dose | 79 (78, 86) |
67.0 (66.1, 72.9) |
164.7 (134.9, 204.2) |
|
| High-dose | 243 | one-dose | 93 (87, 96) |
38.3 (35.8, 39.5) |
103.7 (89.1, 120.2) |
| 100 | two-dose | 84 (82, 85) |
84.0 (82.0, 85.0) |
120.1 (102.3, 141.3) |
|
| Exponential decay model for pseudovirus neutralizing antibody | |||||
| Low-dose | 126 | one-dose | 84 (74, 91) |
66. 7 (58.7, 72.2) |
27.7 (23.4, 33.1) |
| 118 | two-dose | 57 (53, 62) |
48.3 (44.9, 52.5) |
24.2 (20.4, 28.8) |
|
| High-dose | 243 | one-dose | 159 (140, 174) |
65.4 (57.6, 71.6) |
31.8 (28.2, 36.3) |
| 100 | two-dose | 58 (54, 69) |
58.0 (54.0, 69.0) |
26.4 (21.9, 32.4) |
|
Positive rates in the power-law model were estimated based on titers at month 6 of the one- and two-dose regimen of Ad5-nCoV. N = the number of participants aged 18 y and older in the one-dose and two-dose Ad5-nCoV. Data are number of responders, positive rates (%) and GMT (95% CI) of positive participants. GMT = geometric mean titer. 95% CI = 95% confidence interval. Responders were participants whose measured value was higher than the detection limits.
Discussion
In this study, we discovered that the two-dose Ad5-nCoV regimen produced higher peaked pseudovirus neutralizing antibodies than the one-dose regimen, but it had no effect on the peaking levels of RBD-binding antibody titers. Compared with one-dose Ad5-nCoV, although the decay trend of pseudoviruses was not slowed by the two-dose regimen, it did slow the decline of RBD-binding antibodies. We speculate that the phenomenon that there is no difference in pseudovirus-neutralizing antibodies between the two-dose immunization group and the one-dose immunization group at 6 months after immunization may be due to the small sample size obtained after propensity score matching, resulting in “false negative” results.
The half-lives of RBD antibodies (approximately 7 months in two-dose Ad5-nCoV regimen and 5 months in one-dose regimen) and pseudovirus-neutralizing antibodies of Ad5-nCoV (approximately 4 months and 6 months in two-dose and one-dose Ad5-nCoV regimen) were longer than those of two-dose mRNA-1273, whose estimated half-lives for binding antibodies and pseudovirus neutralizing antibodies were 109 d (3.9 months) and 69 d (2.5 months), respectively. These findings were also supported by a comparison of the durability of the Ad26.COV2.S and mRNA COVID-19 vaccines, where Ad26.COV2.S exhibited a greater durability.4 It suggested that antibodies elicited by adenovirus-vectored COVID-19 vaccines may be more durable than mRNA COVID-19 vaccines. However, in our study, antibody titers 12 months after vaccination exhibited a greater reduction relative to peak antibody titers, and neutralizing antibody positivity was reduced by half by 1 y after vaccination. Among these positive participants, the antibody titers of some participants were only higher than the detection limit, and such critical antibody titers may not be enough to prevent breakthrough infection with COVID-19. Therefore, from the perspective of antibody titers, homologous immunization by two doses of Ad5-nCoV vaccine with a 56-d interval has limited benefit compared with one dose of Ad5-nCoV vaccine. In addition, the level of antibodies produced by high doses of this vaccine is similar to that of low doses, which means that if you consider increasing the dose of this vaccine to induce a stronger level of antibody immunity, it will also have little effect, and low doses of vaccine are sufficient to induce immune levels.
The vaccine-elicited immune responses against SARS-CoV-2 may also be compromised by preexisting neutralizing antibodies against adenovirus type-5 vaccine vectors. In the study of two-dose regimen of Ad5-nCoV, the first dose increased anti-Ad5 neutralizing antibodies by 2.4–7.5 times before the second vaccination.15 The high levels of anti-vectored antibodies induced by the first vaccination had an impact on the two-dose regimen of adenovirus-vectored COVID-19 vaccines. However, ChAdOx1 nCoV-19 demonstrated that a prime-boost interval of over 12 weeks provided higher protective efficacy than one of about 6 weeks.24 This confirmed that a longer interval between homologous vaccine doses can improve the negative effect of the response to the vectors. In order to minimize the negative effect of preexisting anti-Ad5 antibodies, a wider prime-boost interval for homologous or heterologous prime-boost regimens needs to be considered. The immune persistence and booster effects of Ad5-nCoV were studied (NCT04568811), and it was discovered that when boosted at 6 months, neutralizing antibody titers increased more than 7 times compared to the peak level following the first dose.25 However, according to our findings, the peak neutralizing antibody titers only increased by 1.7–2.3 times after the booster immunizations with an interval of 56 d, which was much lower than the 7-fold peak neutralizing antibody titers increased by the booster immunizations with an interval of 6 months. Therefore, boosting homologous immunization with Ad5-nCoV every 6 months is recommended.
Our study had some limitations. Firstly, we just recorded antibody titers within 6 months after the last immunization, decay models need to be validated with real data of longer time points to verify their accuracy. Secondly, we did not measure neutralizing antibodies to live virus because neutralization assays against live virus can only be done in specialist laboratories under category 3 biological safety conditions. Pseudovirus neutralizing antibodies, however, typically correlate with live virus neutralizing antibodies, so they can be used in place of those in the absence of the P3 laboratory.26–29 Thirdly, because immunological surrogate endpoints of COVID-19 vaccines have not been established, the predicted antibody titers at future time points may not be directly related to the vaccine efficacy. Fourth, the Omicron strain, which is currently the most prevalent epidemic in the world, has a number of spike mutations, is highly transmissible, and is partially immune to partial neutralizing antibodies. Some investigations indicated that SARS-CoV-2 variants of concerns (VOCs), particularly Omicron, decreased the antibody responses generated by COVID-19 vaccinations.30–32 However, responses to these VOCs were not assessed in our study.
Conclusion
The results of this study have extended our knowledge of the antibody persistency of the Ad5-vectored COVID-19 vaccines (Ad5-nCoV) in one-dose and two-dose regimens. Given the titers and model predictions, the two-dose Ad5-nCoV regimen did not bring a significant benefit over than the one-dose regimen; other strategies of boosting, such as extending the interval time between two Ad5-nCoV injections require further study.
Supplementary Material
Acknowledgments
We thank the China National Institutes for Food and Drug Control and the Vazyme Biotech Co., Ltd. in China, for their independent serologic assays.
Funding Statement
This work was supported by the National Science Foundation for Excellent Young Scholars of China (grant number 82222062 to Jing-xin Li); the General Program of the National Natural Science Foundation of China (grant number 82173584 to Jing-xin Li); Jiangsu Provincial Science Foundation for Distinguished Young Scholars (grant number BK20220064 to Jing-xin Li) and the Key Program of Jiangsu Provincial Science and Technology Plan (grant number BE2021738 to Feng-cai Zhu).
Author contributions
Jia-Lu Feng and Wen-Juan Wang drafted the manuscript. Jing-Xin Li and Feng-Cai Zhu contributed to critical review and revising of the report. Jia-Lu Feng, Hui Zheng and Lai-Run Jin were responsible for statistical analysis. Wen-Juan Wang, Peng-Fei Jin, Xin Xia, Xiao-Yin Zhang and Zhuo-Pei Li participated in the site work, including the follow-up, and data collection. All authors contributed to drafting/critically revising the manuscript for intellectual content, provided final approval of the version to be published.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data reported in this study are not publicly shared. The data that support the findings of this study are available from the authors under reasonable request and with permission of the Jiangsu Provincial Center for Disease Control and Prevention. Individuals wishing to access data should submit requests for access to the corresponding author (jszfc@vip.sina.com).
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2230760.
References
- 1.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–8. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knoll MD, Wonodi C.. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397(10269):72–4. doi: 10.1016/s0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, Groome MJ, Huppert A, O’Brien KL, Smith PG, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–44. doi: 10.1016/s0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collier AY, Yu J, McMahan K, Liu J, Chandrashekar A, Maron JS, Atyeo C, Martinez DR, Ansel JL, Aguayo R, et al. Differential kinetics of immune responses elicited by COVID-19 vaccines. N Engl J Med. 2021;385(21):2010–2. doi: 10.1056/NEJMc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Mateus J, Coelho CH, Dan JM, Moderbacher CR, Gálvez RI, Cortes FH, Grifoni A, Tarke A, Chang J, et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell. 2022;185(14):2434–51.e17. doi: 10.1016/j.cell.2022.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skowronski DM, De Serres, G.. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2021;384:1577. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- 7.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80–2. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doria-Rose N, Suthar MS, Makowski M, O’Connell S, McDermott AB, Flach B, Ledgerwood JE, Mascola JR, Graham BS, Lin BC, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384(23):2259–61. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephenson KE, Le Gars M, Sadoff J, de Groot AM, Heerwegh D, Truyers C, Atyeo C, Loos C, Chandrashekar A, McMahan K, et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. Jama. 2021;325(15):1535–44. doi: 10.1001/jama.2021.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polinski JM, Weckstein AR, Batech M, Kabelac C, Kamath T, Harvey R, Jain S, Rassen JA, Khan N, Schneeweiss S. Durability of the single-dose Ad26.COV2.S vaccine in the prevention of COVID-19 infections and hospitalizations in the US before and during the delta variant surge. JAMA Netw Open. 2022;5(3):e222959. doi: 10.1001/jamanetworkopen.2022.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barouch DH, Stephenson KE, Sadoff J, Yu J, Chang A, Gebre M, McMahan K, Liu J, Chandrashekar A, Patel S, et al. Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. N Engl J Med. 2021;385(10):951–3. doi: 10.1056/NEJMc2108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu S, Huang J, Zhang Z, Wu J, Zhang J, Hu H, Zhu T, Zhang J, Luo L, Fan P, et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCov) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect Dis. 2021;21(12):1654–64. doi: 10.1016/s1473-3099(21)00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, Li JX, Yang BF, Wang L, Wang WJ, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–88. doi: 10.1016/s0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu F, Jin P, Zhu T, Wang W, Ye H, Pan H, Hou L, Li J, Wang X, Wu S, et al. Safety and immunogenicity of a recombinant adenovirus type-5–vectored coronavirus disease 2019 (COVID-19) vaccine with a homologous prime-boost regimen in healthy participants aged ≥6 years: a randomized, double-blind, placebo-controlled, phase 2b trial. Clin Infect Dis. 2022;75(1):e783–e91. doi: 10.1093/cid/ciab845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halperin SA, Ye L, MacKinnon-Cameron D, Smith B, Cahn PE, Ruiz-Palacios GM, Ikram A, Lanas F, Lourdes Guerrero M, Muñoz Navarro SR, et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet. 2022;399(10321):237–48. doi: 10.1016/s0140-6736(21)02753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO validates 11th vaccine for COVID-19. [accessed 2022 Dec 28]. https://www.who.int/news/item/19-05-2022-who-validates-11th-vaccine-for-covid-19.
- 18.11 vaccines granted emergency use listing (EUL) by WHO. [accessed 2022 Dec 28]. https://covid19.trackvaccines.org/agency/who/.
- 19.Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, Wu SP, Wang BS, Wang Z, Wang L, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–54. doi: 10.1016/s0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nie J, Li Q, Wu J, Zhao C, Hao H, Liu H, Zhang L, Nie L, Qin H, Wang M, et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg Microbes Infect. 2020;9(1):680–6. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprangers MC, Lakhai W, Koudstaal W, Verhoeven M, Koel BF, Vogels R, Goudsmit J, Havenga MJ, Kostense S. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol. 2003;41(11):5046–52. doi: 10.1128/jcm.41.11.5046-5052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antia A, Ahmed H, Handel A, Carlson NE, Amanna IJ, Antia R, Slifka M. Heterogeneity and longevity of antibody memory to viruses and vaccines. PLoS Biol. 2018;16(8):e2006601. doi: 10.1371/journal.pbio.2006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin JC, Petrecz ML, Stek JE, Simon JK, Goveia MG, Klopfer SO. Using the power law model to predict the long-term persistence and duration of detectable hepatitis a antibody after receipt of hepatitis a vaccine (VAQTA™). Vaccine. 2021;39(20):2764–71. doi: 10.1016/j.vaccine.2021.03.052. [DOI] [PubMed] [Google Scholar]
- 24.Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–91. doi: 10.1016/s0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Interim recommendations for use of the CanSinoBIO Ad5-nCoV-S [recombinant] vaccine (Convidecia™) against COVID-19. [accessed 2023 May 30]. https://apps.who.int/iris/bitstream/handle/10665/354409/WHO-2019-nCoV-vaccines-SAGE-recommendation-Ad5-nCoV-S-2022.1-chi.pdf.
- 26.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–11. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt F, Weisblum Y, Muecksch F, Hoffmann HH, Michailidis E, Lorenzi JCC, Mendoza P, Rutkowska M, Bednarski E, Gaebler C, et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J Exp Med. 2020;217(11). doi: 10.1084/jem.20201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sholukh AM, Fiore-Gartland A, Ford ES, Hou Y, Tse LV, Lempp FA, Kaiser H, Saint Germain R, Bossard E, Kee JJ, et al., Evaluation of SARS-CoV-2 neutralization assays for antibody monitoring in natural infection and vaccine trials. medRxiv. 2020. doi: 10.1101/2020.12.07.20245431. [DOI] [Google Scholar]
- 29.Li J, Hou L, Guo X, Jin P, Wu S, Zhu J, Pan H, Wang X, Song Z, Wan J, et al. Heterologous AD5-nCOV plus CoronaVac versus homologous CoronaVac vaccination: a randomized phase 4 trial. Nat Med. 2022;28(2):401–9. doi: 10.1038/s41591-021-01677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A, Huang W, Li Q, Wang P, An R, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602(7898):657–63. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann M, Arora P, Groß R, Seidel A, Hörnich BF, Hahn AS, Krüger N, Graichen L, Hofmann-Winkler H, Kempf A, et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184(9):2384–93.e12. doi: 10.1016/j.cell.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M, Lou F, Fan H. SARS-CoV-2 variant Omicron: currently the most complete “escapee” from neutralization by antibodies and vaccines. Signal Transduct Target Ther. 2022;7(1):28. doi: 10.1038/s41392-022-00880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data reported in this study are not publicly shared. The data that support the findings of this study are available from the authors under reasonable request and with permission of the Jiangsu Provincial Center for Disease Control and Prevention. Individuals wishing to access data should submit requests for access to the corresponding author (jszfc@vip.sina.com).


