Abstract
Background
Compared to room temperature (RT, 22–24°C) storage, refrigeration of platelet concentrates (PC) may provide advantages due to lower risks of bacterial growth and increased responsiveness of platelets. However, storage at cold temperature (CT, 2–6°C) may also strongly influence the plasmatic composition of PC. This study analysed the content of plasma in apheresis-derived platelet concentrates (APC).
Materials and methods
APC were stored under blood bank conditions at CT or RT. On days 0 and 6, samples were drawn for analysis. Coagulation parameters comprised global coagulation assays, single factors or inhibitors. The distribution pattern of von Willebrand multimers was investigated by immunoblotting. Thrombin generation was assessed with a fluorescence assay. Immunological and clinical chemistry parameters were determined on automated analysers.
Results
After storage at CT, coagulation factors V, VII, IX or protein S activity are partially reduced, but less compromised than under RT. There was a large reduction in Factor VIII levels and this was similar at both temperatures. In contrast to RT, von Willebrand Factor (vWF) activity was remarkably decreased at CT, and this was accompanied by the shift from high molecular to low molecular weight multimers. Thrombin generation showed improved preservation at CT. Other plasma proteins like immunoglobulins were stable at both conditions.
Discussion
Refrigeration mediates a bivalent effect on plasmatic coagulation in APC. At CT, the partial reduction of labile coagulation factors is less emphasised. However, CT does not prevent Factor VIII depletion, but induces an additional loss of vWF activity by multimer cleavage. Preserved thrombin generation may indicate a higher hemostatic capacity for cold storage.
Keywords: platelet concentrate, cold storage, coagulation, von Willebrand Factor, thrombin generation
INTRODUCTION
Platelet concentrates (PC) are used for the treatment or prophylaxis of hemorrhage caused by thrombocytopenia or platelet dysfunction1. One disadvantage is that stored platelets develop functional deterioration, a process known as storage lesion, and this reduces the efficacy of transfusions. Furthermore, PC are commonly stored at room temperature (RT, 20–24°C) after which they are associated with the risk of bacterial growth2. Consequently, the shelf life of PC is usually limited to 4–5 days, depending on manufacturing conditions and country-specific regulations3.
Up until the 1980s, PC were also stored at a cold temperature (CT, 2–6°C). This offered the opportunity to reduce the risk of bacterial growth and to prolong the storage period4. Although low temperatures induce clustering of glycoprotein Ib (GPIb) on the platelet surface, desialylation and the subsequent rapid clearance of platelets after re-transfusion in vivo5,6, cold storage of PC is now once again the focus of scientific interest. A number of studies have demonstrated that cold-stored platelets show a higher reactivity, attenuated inhibitory signaling, and improved aggregation responses7–12. Therefore, refrigeration of PC may be an option to treat patients with acute hemorrhage more effectively than with RT-stored PC13,14. In this regard, initial results of current clinical trials with patients undergoing cardiothoracic surgery revealed a reduction of periprocedural blood loss15.
In addition to platelets, the liquid milieu of PC also contains hemostatically active components, derived from autologous plasma or released from platelets and potentially contributing to the procoagulatory effect of PC16. In apheresis-derived PC (APC) stored at RT, the composition of the liquid phase has been characterised by Weiss et al.17, indicating that the process of any possible deterioration of coagulation factors varies. The largest reductions were observed for Factor VIII (by 37%), for Factor V (by 20%), and for the inhibitor protein S (by 76%), during a storage period of 7 days. Other factors were not affected to any relevant degree or displayed only slight deviations.
However, the variation in storage temperature may exert a great influence on the content of the liquid milieu in APC. Under refrigeration, the decline of plasmatic coagulation factors may be reduced due to suppressed enzymatic processes, and pre-activation of platelets may induce the release of hemostatic components from granules.
In this study, therefore, we analysed the plasmatic components in APC stored at CT in comparison to storage at RT for 6 days. The study addressed coagulation factors, the von Willebrand Factor (vWF) with its multimeric structure, coagulation inhibitors, thrombin generation, and essential plasma proteins.
MATERIALS AND METHODS
Collection of apheresis-derived platelet concentrates and sampling
Apheresis-derived platelet concentrates were obtained from informed healthy voluntary donors without any drug intake. Our studies with human platelets and the informed consent procedure were approved by our local ethics committee at the University of Wuerzburg (approval n. 101/15). The participants provided their written informed consent to participate in this study. The study was performed in accordance with our institutional guidelines and the Declaration of Helsinki.
Nine A PC pairs (2.5×1011 platelets in 250 mL of plasma) were collected using Trima Accel devices with version 11.3 software and the Trima Accel LRS Platelet, Plasma Set (Terumo BCT, Lakewood, CO, USA). The ratio of inlet blood volume to anticoagulant (ACD-A) was 10:1. After preparation, APC were stored either at RT or at CT for 6 days according to blood bank conditions on a standard agitator: (i) on day 0, 2–3 hours (h) after finalised apheresis, directly before placement at different temperatures; and (ii) on day 6, samples from APC were collected in polypropylene tubes for further analysis with sampled volume of approximately 15 mL at each time point.
Coagulation assays and plasma proteins
The tubes were centrifuged at 2,500 × g for 10 min and the obtained plasma was frozen at ≤20°C until further analysis, except for direct measurement of prothrombin time, International Normalised Ratio (INR), Factor I, Factor XIII, d-dimer, vWF activity (vWF:Ac; using an assay based on binding of vWF to recombinant glycoprotein Ib without the presence of ristocetin), vWF antigen (vWF:Ag), antithrombin, lactate dehydrogenase, total protein, albumin, immunoglobulins, and complement factors (Table I).
Table I.
Technical und methodological details
| Parameter | Reagent | Reagent manufacturer | Technical device |
|---|---|---|---|
| PT, INR, factor II, factor V, factor VII, factor X | Innovin | Siemens Healthineeers, Marburg, Germany | 1 |
| aPTT, factor IX, factor XI, factor XII | Actin FS | Siemens Healthineeers, Marburg, Germany | 1 |
| Factor I | Testthrombin | Siemens Healthineeers, Marburg, Germany | 1 |
| Factor VII (chromogenic) | FVIII chromogenic assay | Siemens Healthineeers, Marburg, Germany | 1 |
| Factor XII | Berichrom Faktor XIII | Siemens Healthineeers, Marburg, Germany | 1 |
| D-dimer | Innovance D-Dimer | Siemens Healthineeers, Marburg, Germany | 1 |
| vWF: Ac | Innovance vWF Ac | Siemens Healthineeers, Marburg, Germany | 1 |
| vWF: Ag | vWF Ag | Siemens Healthineeers, Marburg, Germany | 1 |
| Antithrombin | Innovance Antithrombin | Siemens Healthineeers, Marburg, Germany | 1 |
| Protein S activity | Protein S Ac | Siemens Healthineeers, Marburg, Germany | 1 |
| Protein S free | Innovance freePSAg | Siemens Healthineeers, Marburg, Germany | 1 |
| Protein C activity | Berichrom Protein C | Siemens Healthineeers, Marburg, Germany | 1 |
| Protein C antigen | Asserachrom Protein C | Stago, Asnières sur Seine, France | 2 |
| Protein S antigen | Protein S antigen | Corgenix, Broomfield, CO, USA | 2 |
| Lactate dehydrogenase, total protein, albumin, IgG, IgA, IgM, C3c, C4 | cobas c system reagents | Roche/Hitachi, Mannheim, Germany | 3 |
The assays were performed on the (1) Atellica COAG 360 system (Siemens Healthineers, Marburg, Germany), (2) semi-automatically as enzyme immunoassay on the Hydro flex system (Tecan Deutschland GmbH, Crailsheim, Germany) in combination with the photometrical system Sunrise and the Magellan software for calculations (Tecan Deutschland GmbH, Crailsheim, Germany) or (3) on the cobas c system (Roche/Hitachi, Mannheim, Germany). PT: prothrombin time; INR: international normalized ratio; aPTT: activated partial thromboplastin time; vWF:Ag: von Willebrand factor antigen; vWF:Ac: von Willebrand factor activity
Measurement of thrombin generation
Thrombin generation was measured by a fluorescence-based assay (Technoclone GmbH, Vienna, Austria) using reagents containing tissue factor and high (Technothrombin TGA RC High [Technoclone]) or low (Technothrombin TGA RC Low [Technoclone]) phospholipid concentrations. APC represents platelet-rich plasma, which is initially generated during apheresis by centrifugation (removing red blood cells and leukocytes). The second centrifugation was performed with 2,500 × g for 10 min for the preparation of platelet-poor plasma. The upper half of plasma in the tube was used to ultimately achieve low residual platelet counts: below the detection limit of 1,000 platelets/μL in the blood cell count (hematology analyser KX-21N, Sysmex Deutschland GmbH, Norderstedt, Germany).
A total of 40 μL of platelet-poor plasma samples from APC were loaded together with 10 μL of TGA RCL or TGA RCH reagent in each well of a 96-well black plate. The reaction was started by the addition of 50 μL fluorogenic thrombin substrate. Fluorescence signals, representing generated thrombin, were measured every minute for the next 60 min using the Varioscan device from Fisher Scientific GmbH (Schwerte, Germany). Parameters were recorded as Area Under the Curve (AUC, nmol thrombinxmin), as thrombin peak height (nM), and as lag phase time (min) according to the manufacturer’s instructions.
Immunoblotting and multimer analysis of von Willebrand factor
Immunoblotting was performed (with samples from 4 APC pairs) according to previous studies (see Budde et al.18). In brief, 40 μL plasma samples were loaded in each lane onto a 2.25% sodium dodecyl sulfate (SDS)-agarose gel. Gel electrophoresis was performed with a Multiphore II electrophoresis system (Amersham Biosciences [now GE Healthcare, Piscataway, NJ, USA]) overnight for 16–20 h. After transfer on nitrocellulose membranes, von Willebrand multimers were stained with the primary polyclonal rabbit anti-human vWF antibody (Agilent Technologies, Singapore, Singapore) and the secondary alkaline phosphatase-conjugated Af finiPure donkey anti-rabbit IgG antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). For visualisation, the 5-Bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (BCIP/NBT) substrate was used. The analysis of multimer distribution was performed with Chemidoc MP imaging system (Bio-Rad Laboratories Inc., Hercules, CA, USA) and corresponding Image Lab Software Version 6.0.
Statistical analysis
Descriptive data were calculated with GraphPad PRISM 7 (GraphPad Software, San Diego, CA, USA). Data distribution analysis was performed with Shapiro-Wilk test. Differences of variances between groups were analysed by one-way analysis of variance (ANOVA) followed by post-hoc Tukey-Kramer-Test.
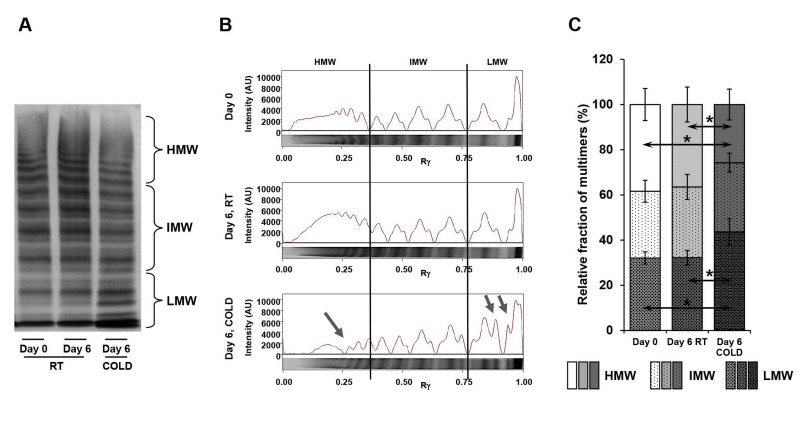
The comparison of band intensities for multimer fractions (Figure 1) was performed with the Kolmogorov-Smirnov test and rank analysis with the two-tailed Mann-Whitney test. p<0.05 was considered statistically significant; p<0.1 as tendency.
Figure 1.
von Willebrand Factor (vWF) multimer analysis in apheresis-derived platelet concentrates (APC) stored under room temperature (RT) and cold temperature (CT)
Samples of APC from day 0 or day 6, either stored at RT or CT, were separated on an SDS-agarose gel and blotted on a nitrocellulose membrane. von Willebrand multimers were stained with a primary polyclonal rabbit anti-human von Willebrand factor antibody and a secondary alkaline phosphatase-conjugated donkey anti-rabbit IgG antibody. A representative immunoblot is shown (A) with indicated multimer sizes (HMW: high molecular weight; IMW: intermediate molecular weight; LMW: low molecular weight). The densitometric pattern, given in arbitrary units (AU; Rγ: lane position), shows the distribution of different multimer sizes (B). Cold storage induces a relative shift from HMW to LMW multimers, indicated by blue arrows. The shift is also calculated in %, as relative fraction of multimer band intensities (C). Comparison of band intensities for multimer fractions was performed with Kolmogorov-Smirnov test and rank analysis with the two-tailed Mann-Whitney test. n=4; *p<0.05, compared as indicated.
RESULTS
The global coagulation assays showed deviations under storage of APC at both temperatures: RT and CT (Table II). Prothrombin time decreased from 99.6±16.5 to 74.2±14.3% at CT, and there was a greater decrease (to 60.0±9.9%) at RT. The values for activated partial thromboplastin time (aPTT) were similarly prolonged from 25.2±2.2 to 33.5±4.7 seconds (s) at RT and 31.7±2.7 s at CT. A general activation of coagulation was not observed, indicated by low d-dimer levels and stable fibrinogen concentrations. The single coagulation factor analysis revealed unchanged values within the reference ranges for Factors II, X, XI, XII and XIII throughout storage regardless of storage conditions (Table II). However, Factor VIII showed the most substantial reduction, from an initial 143±45.3 IU/dL to 69.1±22.6 IU/dL at RT, and to 56.3±22.8 IU/dL at CT. Factor V dropped from 88.7±23.3 IU/dL to 50.7±14.8 IU/dL at RT and, less remarkably, to 68.1±20.8 IU/dL at CT. There was also a greater reduction in Factor VII levels (initial levels 97.4±20.0 IU/dL) under storage at RT (to 65.4±13.3 IU/dL) than under cold storage (to 77±18.4 IU/dL). There was only a slight decrease in Factor IX levels at RT.
Table II.
Coagulation assays
| Parameter | Unit | Reference range | Day 0 | Day 6 RT | Day 6 CT |
|---|---|---|---|---|---|
| PT | % | 80–126 | 99.6±16.5 | 60±9.9* | 74.2±14.3° |
| INR | - | 0.85–1.18 | 1.02±0.07 | 1.3±0.1* | 1.2±0.1°# |
| aPTT | s | 21–31 | 25.2±2.2 | 33.5±4.7* | 31.7±2.7° |
| D-dimer | mg/L | 0–0.5 | 0.27±0.14 | 0.31±0.14 | 0.32±0.16 |
| Factor I | g/L | 1.9–3.9 | 2.4±0.54 | 2.4±0.51 | 2.4±0.54 |
| Factor II | IU/dL | 77–126 | 92.3±13.3 | 86±11.4 | 87.3±12.5 |
| Factor V | IU/dL | 66–149 | 88.7±23.3 | 50.7±14.8* | 68.1±20.8§ |
| Factor VII | IU/dL | 56–157 | 97.4±20.0 | 65.4±13.3* | 77±18.4§ |
| Factor VII | IU/dL | 87–210 | 143±45.3 | 69.1±22.6* | 56.3±22.8° |
| Factor IX | IU/dL | 78–150 | 93.4±14,4 | 78.8±9.8* | 82.3±12.1 |
| Factor X | IU/dL | 65–135 | 91.8±13.5 | 78.9±11.1 | 87.4±13.3 |
| Factor XI | IU/dL | 83–154 | 94.3±25.1 | 94.3±28.3 | 90.6±24.1 |
| Factor XII | IU/dL | 53–150 | 96.0±26.5 | 108.8±30.5 | 90.4±27.6 |
| Factor XII | IU/dL | 86–150 | 106.8±20.0 | 111±18.5 | 111.4±18.4 |
| vWF: Ag | IU/dL | 60–192 | 134.8±32.6 | 147.3±35.3 | 121.6±36.8 |
| vWF: Ac | IU/dL | 50–200 | 130.7±30.1 | 134.8±32.5 | 75.6±29.7°# |
| Antithrombin | % | 83–115 | 96.6±7.4 | 95.9±6.9 | 98.4±9.1 |
| Protein S antigen | % | 60–150 | 89.2±17.7 | 93±18.3 | 92±17.1 |
| Protein S activity | % | m 81–130 f 68–130 |
90.8±14.0 | 16.6±4.2* | 55.8±17.9° |
| Protein S free | % | m 74–136 f 57–126 |
93.9±17.2 | 86.6±13.6 | 90.2±16.4 |
| Protein C antigen | % | 70–140 | 86.2±19.2 | 88.7±23.9 | 85.4±21.8 |
| Protein C activity | % | 63–143 | 106.8±27.2 | 103.4±25.9 | 107.6±26.5 |
For each parameter mean ± SD is given. n=9;
p<0.05, day 0 compared with day 6 stored at RT;
p<0.05, day 0 compared with day 6 stored at CT;
p<0.05, day 6 stored at RT with day 6 stored at CT;
p<0.1 (as tendency), day 0 compared with day 6 stored at CT.
PT: prothrombin time; INR: international normalised ratio; aPTT: activated partial thromboplastin time; vWF:Ag: von Willebrand factor antigen; vWF:Ac: von Willebrand factor activity; Ig: immunoglobulin; C:complement factor; m: male; f: female.
Levels of vWF antigen maintained the reference ranges under both storage temperatures (Table II). vWF:Ac was also stable at RT, but not at CT, with a large reduction from 130.7±30.1 IU/dL to 75.6±29.7 IU/dL. Concomitantly, the multimer analysis revealed a loss of high molecular weight multimers (HMW) and an accumulation of low molecular weight multimers (LMW) under cold storage, but not at RT (Figure 1A). The fraction of HMW multimers decreased from 38.4%±7.1% to 25.7%±6.8%, whereas the fraction of LMW multimers increased from 32.1%±2.7% to 43.7%±5.9% (Figure 1B).
The inhibitors of plasmatic coagulation antithrombin and protein C showed normal values, and remained unchanged during storage of APC at RT and at CT (Table II). The levels of protein S antigen (total and free protein S) were also unaffected by storage conditions, whereas the initial protein S activity of 90.8±14.0% was diminished to 55.8±17.9% after 6 days at CT, and was markedly reduced to 16.6±4.2% at RT.
Determination of thrombin generation was performed using two reagents containing different concentrations of phospholipids (Figures 2 and 3). With low phospholipid content, thrombin generation showed a tendency towards higher values, from an initial 4,100.8±646.0 to 4,564.3±272.3 nmol × min under cold storage of APC, whereas levels remained stable under RT with 3,814.3±351.8 nmol × min (Figure 2B). The results for maximal thrombin peaks were similarly improved at CT (Figure 2C) while there was no difference in lag phase time (Figure 2D).
Figure 2.
Thrombin generation in apheresis-derived platelet concentrates (APC) induced with low phospholipid concentrations
Samples from APC on day 0 or day 6, either stored at RT or CT, were analysed for thrombin generation with the RCL thrombin generation reagent containing low concentrations of phospholipids. The mean trace of thrombin generation is shown for each sample type (A). The box-and-whisker plots illustrate the values for thrombin generation as area under the curve (AU: nmol thrombin×min) (B), for thrombin peak height (nM) (C), and for lag phase time (min) (D). n=9; mean±Standard Deviation; #p<0.1 (as tendency), compared as indicated.
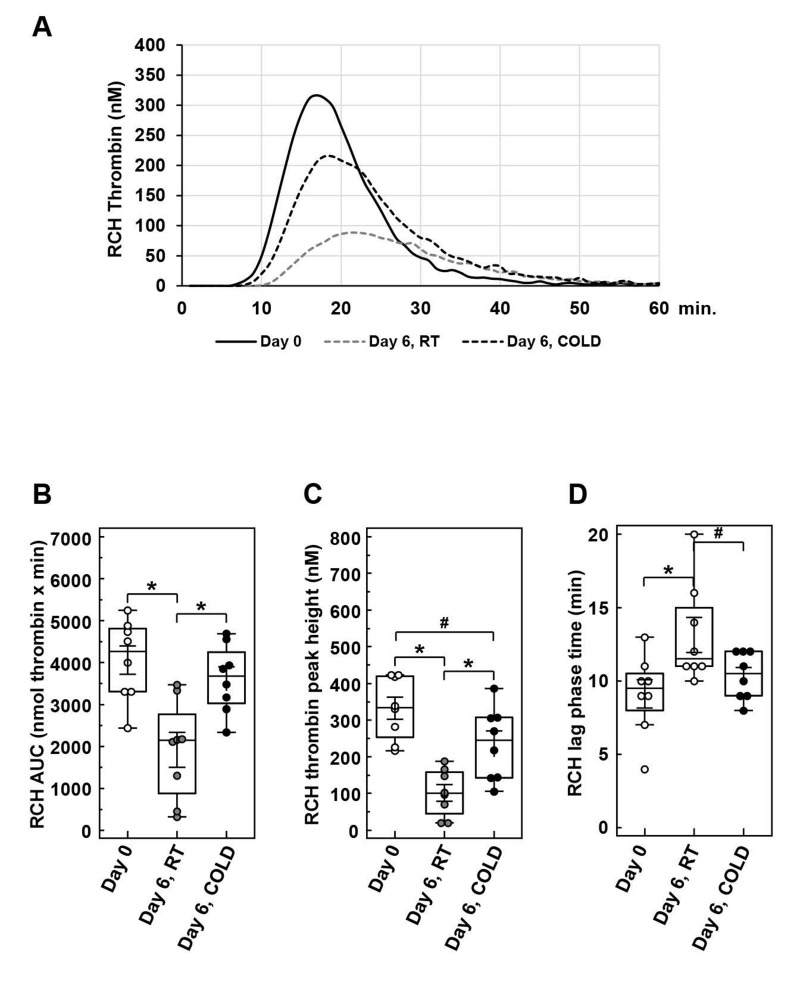
Figure 3.
Thrombin generation in apheresis-derived platelet concentrates (APC) induced with high phospholipid concentrations
Samples from APC on day 0 or day 6, either stored at room temperature (RT) or cold temperature (CT), were analysed for thrombin generation with the RCH thrombin generation reagent containing high concentrations of phospholipids. The mean trace of thrombin generation is shown for each sample type (A). The box-and-whisker plots illustrate the values for thrombin generation as area under the curve (AU: nmol thrombin x minute) (B), for thrombin peak height (nM) (C), and for lag phase time (minutes) (D). n=9; mean±Standard Deviation; *p<0.05, #p<0.1 (as tendency), compared as indicated.
In the presence of high phospholipid levels, thrombin generation in cold-stored APC with 3,616.1±305.2 nmol × min was comparable to day 0 with 4,055.3±365.1 nmol × min, but remarkably decreased in RT-stored A PC with 1,919.0±444.4 nmol × min (Figure 3B). The peak thrombin values were considerably reduced under storage at RT, but not under cold storage (Figure 3C). In addition, lag phase times were increased after storage at RT (Figure 3D).
The content of plasma proteins in APC, like total protein, albumin, immunoglobulins or complement factors, were within reference ranges and were unchanged irrespective of the storage temperature (Table III). There was no increase in lactate dehydrogenase (a typical marker for cell destruction) throughout storage.
Table III.
Plasma proteins
| Parameter | Unit | Reference range | Day 0 | Day 6 RT | Day 6 CT |
|---|---|---|---|---|---|
| total protein | g/dL | 6.6–8.7 | 5.6±0.3 | 5.7±0.3 | 5.6±0.2 |
| albumin | g/dL | 3.5–5.2 | 3.7±0.2 | 3.7±0.2 | 3.6±0.2 |
| IgG | mg/dL | 690–1,600 | 758.7±183.4 | 782.9±201.8 | 764.8±190.3 |
| IgA | mg/dL | m 88–410 f 70–370 |
187.7±98.1 | 190.1±100.8 | 187.8±97.1 |
| IgM | mg/dL | m 34–210 f 40–240 |
76.8±29.3 | 75.2±29.5 | 74.7±29.7 |
| C3c | mg/dL | 75–140 | 81.9±9.1 | 90.7±12.2 | 83±10.2 |
| C4 | mg/dL | 10–34 | 15.5±4.4 | 17.1±5.0 | 16.0±4.3 |
| lactate dehydrogenase | U/L | ≤250 | 139.7±33.3 | 138.7±17.1 | 140.2±13.5 |
For each parameter mean±Standard Deviation is given. n=9. Ig: immunoglobulin; C: complement factor; m: male; f: female.
DISCUSSION
Optimisation of ex vivo storage conditions is an ongoing challenge in transfusion medicine in order to maintain the biological integrity and effectiveness of blood components like PC19. This study analysed the influence of storage temperature (RT or CT) on the composition of plasma in APC.
Storage at RT for 6 days resulted in a marked depletion of Factor VIII and in a partial reduction in Factors V, VII and IX. There is less degradation under refrigeration, except in the case of Factor VIII, possibly suggesting specific clearance mechanisms during storage, e.g., by the involvement of differently cold-affected enzymes. The instability of Factor VIII in stored or thawed plasma is generally well-known20–22. Despite the reduction, residual activities of these factors were usually maintained in sufficient ranges above 50 IU/dL at RT and activity was even higher at CT for Factors V, VII and IX, contributing only to minor deviations of PT, INR or aPTT.
These results were similar to those obtained in the study by Weiss et al. with APC stored at RT for up to 7 days; but the differences in baseline values of coagulation factors were smaller, possibly attributable to different sensitivities of the methods used17. Although not significant, we also found a tendency for an increase in Factor XII at RT, although this was not observed under cold storage.
The vWF plays a special role in coagulation, forming large multimers and interacting with platelets and the endothelial surface as a result of conformational changes induced by shear stress in the bloodstream23. Under RT, vWF:Ag and vWF:Ac were stable for several days, again confirming the results of previous investigations17,24. Therefore, additional degradation of vWF cannot be ruled out, since vWF is also stored in platelets and is also potentially shed during storage. In contrast, cold storage of APC results in a decrease in vWF:Ac by almost 50% in relation to baseline values, whereas vWF:Ag remains unchanged. This functional degradation of vWF is caused by the loss of HMW and the accumulation of LMW multimers. The reduction in vWF:Ac with similar shifts from HMW towards LMW multimers has also been observed in experiments with stored whole blood on crushed ice, and this was quickly seen within 3–6 h24. It had been speculated that the presence of platelets is responsible for cleavage due to the stability of vWF and its multimeric form in platelet-free plasma for several hours at CT, but not in platelet-rich plasma, showing diminished vWF:Ag and vWF:RCo after 3 h on ice. In another study, it was assumed that leukocyte-derived proteases would cause vWF depletion, since leukocyte filtration prevented the loss of vWF in whole blood under refrigeration for several days. However, this filtration step concomitantly led to a 90% reduction in platelets25. The choice of anticoagulants may also possibly play a role for vWF stability under different experimental storage conditions24.
In this study, APC were manufactured as standardised leukocyte-depleted products with residual leukocyte counts less than 106 per unit, suggesting that platelets rather than leukocytes are involved in vWF cleavage. In general, vWF multimers are prone to deviations in the milieu of high platelet counts26,27. It has been reported that CT induces the binding of vWF to platelet GPIb28,29, possibly contributing to conformational changes and protease-driven cleavage of vWF multimers. The role of accumulating LMW multimers under CT remains speculative. They may be considered as byproducts that are not essential for transfusion safety or for hemostatic function. However, in cases of massive transfusion, it can be assumed that the lower proportion of HMW multimers may contribute to limited hemostatic efficacy.
The analysis of inhibitors revealed that antithrombin and protein C remain stable throughout storage at either temperature. Similar to stored plasma without presence of platelets, the activity of protein S is widely labile at RT and much better preserved at CT30,31. Values for free protein S were not affected by storage regardless of storage temperatures. Reported decline of free protein S in plasma may be caused by differences in methodology, as previously discussed32,33.
In addition to classical assays addressing the activity of coagulation factors, the measurement of thrombin generation is a method to analyse the global procoagulant capacity of platelet-free or platelet-rich-plasma32, which is also used for analysis and therapeutical monitoring of coagulation disorders34,35. The parameters of thrombin potential induced with both tissue factor-containing reagents. But since their phospholipid content differs, they showed better preserved values in cold-stored APC, potentially ref lecting an elevated hemostatic capacity compared to RT-stored APC, despite affected vWF multimers.
It has to be considered that pre-analytical procedures and the composition of reagents may strongly influence the results of thrombin generation assays36. Therefore, it was important to treat samples from both APC types in exactly the same way during pre-analytical procedures and to achieve a large reduction in platelet count. Further studies of thrombin generation using different reagents or enforced centrifugation steps may throw extra light on the role of cold storage in hemostatic capacity in APC.
This study has some limitations, and it should be remembered that the results presented here refer to APC. It would also be of interest to analyse the plasmatic components in PC derived from whole blood, being another product often used in clinical practice. In this regard, the manufacturing comprises several steps of processing whole blood with transient storage periods, possibly influencing the content of plasmatic factors in a different manner. Similar to APC, the storage of buffy-coat PC at RT for several days also led to the decrease in Factors V, VII and VIII32. In buffy-coat PC with reduced plasma content, the hemostatic capacity is generally affected due to the large substitution of plasma with additive solutions. For PC with 30% plasma, 70% additive solution resulted in coagulation factor levels that were initially in the range of 20–30%. These showed a similar further decrease during storage for 21 days at both RT and under cold storage, except for protein S for which values at CT were unchanged.
Furthermore, the sample collection for analysis was performed after 6 days of storage. In platelet-free plasma, first reductions of Factor VIII or Factor V occur within 3 h at RT37. Therefore, for practical considerations, it would be of interest to investigate the onset of deviations in more detail, e.g., by measuring coagulation factors and VWF at further time points during early storage, together with changes in platelet responsiveness. In this way, the storage conditions associated with maximal hemostatic capacity could be defined more precisely.
This study focused on the direct comparison of RT- and CT-stored platelet concentrates. Since in most countries PC are currently stored for 4–5 days at RT, we selected the 6-day time point as a rational and initial approach for the investigation of prolonged storage time at CT. The analysis of PC with extended periods at CT, e.g., for 10 or 14 days, would also be an interesting issue for further studies, including different storage media and manufacturing methods.
In addition to coagulation factors, it is important to address non-hemostatic components of plasma in PC, that can potentially contribute to adverse transfusion-related complications16. There was no difference in levels of immunoglobulins or complement factors under both temperatures. This study did not investigate the shedding of bioactive molecules, but it has previously been reported that the content of α-granules, including more than 80 proteins and cytokines, was not enhanced in the refrigerated plasma-reduced PC16.
CONCLUSIONS
In summary, refrigeration mediates a bivalent effect on plasmatic components in APC. The partial reduction in Factors V, VII and IX is less emphasised at cold storage compared to RT storage. However, refrigeration does not prevent Factor VIII depletion, but induces an additional loss of vWF activity by multimer cleavage. Improved thrombin generation may point to an advantage of cold storage for APC, but the hemostatic effect of temperature-dependent changes remains to be clarified in further experimental and clinical trials.
ACKNOWLEDGEMENTS
The Authors wish to thank the colleagues at the Institute of Clinical Transfusion Medicine and Hemotherapy for donation management and the staff of the Central Laboratory for their support in multimer analysis.
Footnotes
AUTHORSHIP CONTRIBUTIONS
AKob, MB and JK designed the research; AP, JS and SK are responsible for data acquisition; AKob, AP, PK, MN and JK analysed and interpreted the data; AKob, AKoe, MB and JK drafted and revised the paper.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Gottschall J, Wu Y, Triulzi D, Kleinman S, Strauss R, Zimrin AB, et al. The epidemiology of platelet transfusions: an analysis of platelet use at 12 US hospitals. Transfusion. 2020;60:46–53. doi: 10.1111/trf.15637. [DOI] [PubMed] [Google Scholar]
- 2.Cauwenberghs S, van Pampus E, Curvers J, Akkerman JW, Heemskerk JW. Hemostatic and signaling functions of transfused platelets. Transfus Med Rev. 2007;21:287–294. doi: 10.1016/j.tmrv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Hegde S, Akbar H, Zheng Y, Cancelas JA. Towards increasing shelf life and haemostatic potency of stored platelet concentrates. Curr Opin Hematol. 2018;25:500–508. doi: 10.1097/MOH.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stubbs JR, Tran SA, Emery RL, Hammel SA, Haugen AL, Zielinski MD, et al. Cold platelets for trauma-associated bleeding: regulatory approval, accreditation approval, and practice implementation-just the “tip of the iceberg”. Transfusion. 2017;57:2836–2844. doi: 10.1111/trf.14303. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmeister KM, Felbinger TW, Falet H, Denis CV, Bergmeier W, Mayadas TN, et al. The clearance mechanism of chilled blood platelets. Cell. 2003;112:87–97. doi: 10.1016/s0092-8674(02)01253-9. [DOI] [PubMed] [Google Scholar]
- 6.Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability--deleterious effect of refrigerated storage. N Engl J Med. 1969;280:1094–1098. doi: 10.1056/NEJM196905152802004. [DOI] [PubMed] [Google Scholar]
- 7.Getz TM. Physiology of cold-stored platelets. Transfus Apher Sci. 2019;58:12–15. doi: 10.1016/j.transci.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Getz TM, Montgomery RK, Bynum JA, Aden JK, Pidcoke HF, Cap AP. Storage of platelets at 4 degrees C in platelet additive solutions prevents aggregate formation and preserves platelet functional responses. Transfusion. 2016;56:1320–1328. doi: 10.1111/trf.13511. [DOI] [PubMed] [Google Scholar]
- 9.Koessler J, Klingler P, Niklaus M, Weber K, Koessler A, Boeck M, et al. The impact of cold storage on adenosine diphosphate-mediated platelet responsiveness. TH Open. 2020;4:e163–e172. doi: 10.1055/s-0040-1714254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pidcoke HF, McFaul SJ, Ramasubramanian AK, Parida BK, Mora AG, Fedyk CG, et al. Primary hemostatic capacity of whole blood: a comprehensive analysis of pathogen reduction and refrigeration effects over time. Transfusion. 2013;53(Suppl 1):137S–149S. doi: 10.1111/trf.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandgren P, Shanwell A, Gulliksson H. Storage of buffy coat-derived platelets in additive solutions: in vitro effects of storage at 4 degrees C. Transfusion. 2006;46:828–834. doi: 10.1111/j.1537-2995.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 12.Sandgren P, Hansson M, Gulliksson H, Shanwell A. Storage of buffy-coatderived platelets in additive solutions at 4 degrees C and 22 degrees C: flow cytometry analysis of platelet glycoprotein expression. Vox Sang. 2007;93:27–36. doi: 10.1111/j.1423-0410.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- 13.Strandenes GK, Kristoffersen EK, Bjerkvig CK, Fosse TK, Hervig T, R Haaverstad R, et al. [abstract]. Cold-stored apheresis platelets in treatment of postoperative bleeding in cardiothoracic surgery. Transfusion. 2016;56(Issue S4):16A. [Google Scholar]
- 14.Krachey E, Viele K, Spinella PC, Steiner ME, Zantek ND, Lewis RJ. The design of an adaptive clinical trial to evaluate the efficacy of platelets stored at low temperature in surgical patients. J Trauma Acute Care Surg. 2018;84:S41–S46. doi: 10.1097/TA.0000000000001876. [DOI] [PubMed] [Google Scholar]
- 15.Strandenes G, Sivertsen J, Bjerkvig CK, Fosse TK, Cap AP, Del Junco DJ, et al. A Pilot Trial of Platelets Stored Cold versus at Room Temperature for Complex Cardiothoracic Surgery. Anesthesiology. 2020;133:1173–1183. doi: 10.1097/ALN.0000000000003550. [DOI] [PubMed] [Google Scholar]
- 16.Johnson L, Tan S, Jenkins E, Wood B, Marks DC. Characterization of biologic response modifiers in the supernatant of conventional, refrigerated, and cryopreserved platelets. Transfusion. 2018;58:927–937. doi: 10.1111/trf.14475. [DOI] [PubMed] [Google Scholar]
- 17.Weiss DR, Franke D, Strasser EF, Ringwald J, Zimmermann R, Eckstein R. von Willebrand factor, clotting factors, and clotting inhibitors in apheresis platelet concentrates. Transfusion. 2014;54:633–639. doi: 10.1111/trf.12304. [DOI] [PubMed] [Google Scholar]
- 18.Budde U, Drewke E, Mainusch K, Schneppenheim R. Laboratory diagnosis of congenital von Willebrand disease. Semin Thromb Hemost. 2002;28:173–190. doi: 10.1055/s-2002-27820. [DOI] [PubMed] [Google Scholar]
- 19.Quach ME, Chen W, Li R. Mechanisms of platelet clearance and translation to improve platelet storage. Blood. 2018;131:1512–1521. doi: 10.1182/blood-2017-08-743229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bostrom F, Sjodahl M, Wehlin L, Egberg N, Lundahl J. Coagulation parameters in apheresis and leukodepleted whole-blood plasma during storage. Transfusion. 2007;47:460–463. doi: 10.1111/j.1537-2995.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 21.Backholer L, Green L, Huish S, Platton S, Wiltshire M, Doughty H, et al. A paired comparison of thawed and liquid plasma. Transfusion. 2017;57:881–889. doi: 10.1111/trf.13915. [DOI] [PubMed] [Google Scholar]
- 22.Cardigan R, Green L. Thawed and liquid plasma--what do we know? Vox Sang. 2015;109:1–10. doi: 10.1111/vox.12251. [DOI] [PubMed] [Google Scholar]
- 23.Huck V, Schneider MF, Gorzelanny C, Schneider SW. The various states of von Willebrand factor and their function in physiology and pathophysiology. Thromb Haemost. 2014;111:598–609. doi: 10.1160/TH13-09-0800. [DOI] [PubMed] [Google Scholar]
- 24.Bohm M, Taschner S, Kretzschmar E, Gerlach R, Favaloro EJ, Scharrer I. Cold storage of citrated whole blood induces drastic time-dependent losses in factor VIII and von Willebrand factor: potential for misdiagnosis of haemophilia and von Willebrand disease. Blood Coagul Fibrinolysis. 2006;17:39–45. doi: 10.1097/01.mbc.0000198990.16598.85. [DOI] [PubMed] [Google Scholar]
- 25.Farrugia A, Street A, Douglas S, Raines G, Aumann H, Whyte G, et al. Stabilization of von Willebrand factor in banked blood by leucocyte depletion. Transfus Med. 1993;3:51–57. doi: 10.1111/j.1365-3148.1993.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 26.Budde U, Scharf RE, Franke P, Hartmann-Budde K, Dent J, Ruggeri ZM. Elevated platelet count as a cause of abnormal von Willebrand factor multimer distribution in plasma. Blood. 1993;82:1749–1757. [PubMed] [Google Scholar]
- 27.Budde U, van Genderen PJ. Acquired von Willebrand disease in patients with high platelet counts. Semin Thromb Hemost. 1997;23:425–431. doi: 10.1055/s-2007-996119. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, Druzak SA, Wang Y, Josephson CD, Hoffmeister KM, Ware J, et al. Refrigeration-Induced Binding of von Willebrand Factor Facilitates Fast Clearance of Refrigerated Platelets. Arterioscler Thromb Vasc Biol. 2017;37:2271–2279. doi: 10.1161/ATVBAHA.117.310062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W, Voos KM, Josephson CD, Li R. Short-Acting Anti-VWF (von Willebrand Factor) Aptamer Improves the Recovery, Survival, and Hemostatic Functions of Refrigerated Platelets. Arterioscler Thromb Vasc Biol. 2019;39:2028–2037. doi: 10.1161/ATVBAHA.119.312439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thiele T, Kellner S, Hron G, Wasner C, Nauck M, Zimmermann K, et al. Storage of thawed plasma for a liquid plasma bank: impact of temperature and methylene blue pathogen inactivation. Transfusion. 2012;52:529–536. doi: 10.1111/j.1537-2995.2011.03317.x. [DOI] [PubMed] [Google Scholar]
- 31.von Heymann C, Keller MK, Spies C, Schuster M, Meinck K, Sander M, et al. Activity of clotting factors in fresh-frozen plasma during storage at 4 degrees C over 6 days. Transfusion. 2009;49:913–920. doi: 10.1111/j.1537-2995.2008.02063.x. [DOI] [PubMed] [Google Scholar]
- 32.Cookson P, Lawrie A, Green L, Dent E, Proffitt S, Bashir S, et al. Thrombin generation and coagulation factor content of thawed plasma and platelet concentrates. Vox Sang. 2015;108:160–168. doi: 10.1111/vox.12206. [DOI] [PubMed] [Google Scholar]
- 33.Cardigan R, Lawrie AS, Mackie IJ, Williamson LM. The quality of fresh-frozen plasma produced from whole blood stored at 4 degrees C overnight. Transfusion. 2005;45:1342–1348. doi: 10.1111/j.1537-2995.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 34.Binder NB, Depasse F, Mueller J, Wissel T, Schwers S, Germer M, et al. Clinical use of thrombin generation assays. Journal of thrombosis and haemostasis: JTH. 2021;19:2918–2929. doi: 10.1111/jth.15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tripodi A. Thrombin Generation Assay and Its Application in the Clinical Laboratory. Clin Chem. 2016;62:699–707. doi: 10.1373/clinchem.2015.248625. [DOI] [PubMed] [Google Scholar]
- 36.Kintigh J, Monagle P, Ignjatovic V. A review of commercially available thrombin generation assays. Res Pract Thromb Haemost. 2018;2:42–48. doi: 10.1002/rth2.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Runkel S, Haubelt H, Hitzler W, Hellstern P. The quality of plasma collected by automated apheresis and of recovered plasma from leukodepleted whole blood. Transfusion. 2005;45:427–432. doi: 10.1111/j.1537-2995.2005.04276.x. [DOI] [PubMed] [Google Scholar]