Abstract
Objectives
The importance of blood cell markers in frailty has been studied. However, research on haemoglobin-to-red blood cell distribution width ratio (HRR) and frailty in older persons is still limited. We investigated the association between HRR and frailty in older adults.
Design
Cross-sectional population-based study.
Setting
Community-dwelling older adults older than 65 years were recruited from September 2021 to December 2021.
Participants
A total of 1296 community-dwelling older adults (age ≥65 years) in Wuhan were included in the study.
Main outcome measures
The main outcome was the presence of frailty. The Fried Frailty Phenotype Scale was used to evaluate the frailty status of the participants. Multivariable logistic regression analysis was performed to determine the relationship between HRR and frailty.
Results
A total of 1296 (564 men) older adults were included in this cross-sectional study. Their mean age was 70.89±4.85 years. Receiver operating characteristic curve analysis showed that HRR is a good predictor of frailty in older people, the area under the curve (AUC) was 0.802 (95% CI: 0.755 to 0.849), and the highest sensitivity was 84.5% and the specificity was 61.9% with the optimal critical values 9.97 (p<0.001). Multiple logistic regression analysis indicated that lower HRR (<9.97) (OR: 3.419, 1.679 to 6.964, p=0.001) is independently associated with frailty in older people, even after adjusting confounding factors.
Conclusion
Lower HRR is closely associated with an increased risk of frailty in older people. Lower HRR may be an independent risk factor for frailty in community-dwelling older adults.
Keywords: GERIATRIC MEDICINE, EPIDEMIOLOGY, GENERAL MEDICINE (see Internal Medicine)
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Frailty was diagnosed by following Fried’s frailty phenotype.
This cross-sectional analysis was performed in a medium-volume population.
Some variables were self-reported, but the best available measures were used.
The study reflects the situation of older adults in Wuhan, China, and the generalisability needs to be further verified.
This was a cross-sectional study that cannot assess the cause–effect relationship.
Introduction
As life expectancy increases, human societies are ageing globally, in both developed and developing countries.1 By 2050, the proportion of people aged over 60 years is projected to increase from 11% to 22%, and the number of people aged over 60 years will increase from 605 million to 2.1 billion, including 425 million people aged over 80 years in the world.2 Frailty becomes an emerging global public health burden, with the rapid growth of the global ageing population. Frailty is considered to be a complex age-related clinical condition characterised by a decline in the physiological function of multiple organs, with a resultant increased vulnerability to stressors.3 It is related to adverse health-related events, including increased mortality, hospitalisation, falls and fractures, cognitive decline, disability and admission to long-term care.4 Therefore, early identifying modifiable risk factors of frailty is becoming increasingly crucial for delaying and reversing frailty and its associated adverse events in older persons.5
As an important part of complete blood count (CBC), haemoglobin (Hb) is usually used as an indicator of the degree of anaemia. However, previous studies showed that low Hb reflects to a decline in physiological function including decreased immune response, malnutrition and low resistance to external invasion.6 Meanwhile, there are several studies indicated that Hb is related to frailty in older persons.7–10 Red blood cell distribution width (RDW) is a simple parameter of CBC, which reflects the degree of heterogeneity of the erythrocyte volume, and is traditionally used for the differential diagnosis of anaemia.11 However, with the deepening of the study, it was found to be related to the prognosis of many diseases. Increased RDW reflects dysregulation of erythrocyte homeostasis, which may be attributed to various underlying metabolic abnormalities such as shortened telomere length, oxidative stress, inflammation, malnutrition, dyslipidaemia, hypertension, erythrocyte fragmentation and altered erythropoietin function.11
Inflammation has been identified as a potential cause of frailty.12 Inflammation in response to elevated RDW may be highly correlated with frailty. Hou et al13 indicated that RDW is significantly associated with the risk of frailty in older patients with coronary heart disease (CHD). In addition, studies showed that increased RDW is associated with frailty both in older inpatients and in community-dwelling older people.14 15 However, it is still controversial whether RDW alone can predict frailty.16 17 The Hb-to-RDW ratio (HRR) is a cheap, rapid and readily available novel prognostic, which combines the prognostic information of Hb and RDW and reflects a more comprehensive health status.16 18 Recently, Qu et al16 found that lower HRR is independently related to the risk of frailty in older patients with CHD. They verified that HRR may be a more useful biomarker compared with RDW or Hb alone.16
Studies have shown a significant association of HRR with frailty in specific populations (patients with CHD).16 However, research on HRR and frailty in general older persons is still limited, and the significance of evaluating frailty is not yet clear. In the present study, we investigated the relationship between HRR and frailty in community-dwelling older adults.
Material and Methods
Patient and public involvement
The source population was the community-dwelling adults older than 65 years living in communities in Wuhan. The study population consisted of a random sample of older people from each community. Inclusion criteria were the community-dwelling adults older than 65 years living in communities in Wuhan. Exclusion criteria were malignant disease or advanced organic diseases, haematologic diseases, acute stage of disease and participants missing the key parameters.
Participants and sociodemographic characteristics
In this study, we recruited 1296 community-dwelling adults older than 65 years living in communities in Wuhan between September 2021 and December 2021. Sociodemographic characteristics, including age, gender, education years, marital status, smoking history, alcohol consumption and comorbidities, including hypertension, diabetes, CHD, hyperlipidaemia and cerebrovascular disease, were recorded. The body mass index (BMI), waistline, blood pressure and pulse rate were measured by two professional clinicians.
Peripheral blood parameters
Blood samples were collected, and full blood count was measured by an automated haematology analyzer (Mindray, BC-7500, China). Other related biochemical indicators were detected by an automatic biochemical analyzer (Beckman, AU680, American). HRR=Hb (g/L)/RDW (%).
Fried’s frailty phenotype
According to Fried’s frailty phenotype, we evaluated frailty in five criteria, as follows: (1) Weight loss: unintentional weight loss >5% or a loss of more than 4.5 kg in the past 1 year. (2) Physical weakness: a dynamometer was used for participants for three trials, and the maximum value was recorded. Low grip strength was defined according to the standards proposed by Fried et al.19 (3) Slowness: slowness was defined as when the time required to walk 4.6 m was more than 7 s for men (height ≤173 cm) and women (height ≤159 cm) or more than 6 s for men (height >173 cm) and women (height >159 cm). (4) Physical activity: low physical activity was defined as <383 kcal/week for men and <270 kcal/week for women. (5) Exhaustion: exhaustion was assessed by the following two questions from the center for epidemiological-Depression Scale (CES-D). ‘In the last week, I felt that everything I did was an effort’ and ‘Could not get going in the last week’. If the participant responded ‘yes’ to either of these questions, the participant was considered exhausted. Participants with >3 indicators were defined as frail, 1–2 as prefrail, and none as robust.
Patient and public involvement statement
Patients and/or the public were not involved in the design, conduct, reporting, or dissemination of this research.
Statistical analysis
Continuous and categorical variables were expressed as the mean SD and numbers with percentages, respectively. The baseline characteristics of the groups were compared using a one-way analysis of variance and χ2 test. The predictive value of HRR for frailty was assessed by receiver operating characteristic curve (ROC) analysis. The prognostic value of lower HRR on frailty was assessed using the logistic regression model. Variables were selected as candidates for the multivariate analysis when p<0.1 in the univariate analysis. After adjustment for confounding factors including age, gender, marital status, education years, living alone, BMI, diabetes, RBC, albumin, triglyceride, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C), the independent risk factors for frailty in older adults were assessed. Kendall’s tau-b correlation analysis was used to assessing the correlation between lower Hb, lower RDW, lower HRR and frailty in older adults. The p value<0.05 was considered statistically significant. All statistical analyses were conducted using SPSS (V.26.0, IBM Corporation).
Results
Characteristics of the study population
A total of 1296 (564 men) older adults were included in our study. Their mean age was 70.89±4.85 years. Of the 1296 participants, 582 (44.9%) had hypertension, 183 (14.1%) were patients with diabetes, 98 (7.6%) had cardiac diseases and 43 (3.3%) had cranial vascular disease. The proportions of individuals with a habit of smoking and drinking were 11.1% and 11.7%, respectively. And 149 (11.5%) older adults were living alone. According to Fried’s frailty phenotype, there were 55.09% (714) in the robust group, 36.81% (477) in the prefrail group and 8.10% (105) in the frail group. The baseline characteristics of the three groups were shown in table 1.
Table 1.
Baseline characteristics of the study population stratified by frailty
| Characteristics | Robust (n=714) |
Prefrail (n=477) |
Frailty (n=105) |
P value |
| Age, years (SD) | 69.81±3.89 | 71.71±5.34 | 70.89±4.85 | <0.001 |
| Male gender, n (%) | 347 (48.60) | 184 (38.57) | 33 (31.43) | <0.001 |
| Marital status | ||||
| Married, n (%) | 622 (87.11) | 408 (85.53) | 77 (73.33) | 0.001 |
| Other*, n (%) | 92 (12.89) | 69 (14.47) | 28 (26.67) | |
| Education years | ||||
| 0–12, n (%) | 466 (65.27) | 337 (70.65) | 85 (80.95) | 0.002 |
| >12, n (%) | 248 (24.73) | 140 (29.35) | 20 (19.05) | |
| Alone living, n (%) | 74 (10.36) | 53 (11.11) | 22 (20.95) | 0.006 |
| Smoking, n (%) | 90 (12.61) | 44 (9.22) | 10 (9.52) | 0.165 |
| Drinking, n (%) | 92 (12.89) | 50 (10.48) | 10 (9.52) | 0.345 |
| Hypertension, n (%) | 314 (43.98) | 212 (44.44) | 56 (53.33) | 0.192 |
| Diabetes mellitus, n (%) | 81 (11.34) | 82 (17.19) | 20 (19.05) | 0.006 |
| Cardiac diseases, n (%) | 46 (6.44) | 41 (8.60) | 11 (10.48) | 0.194 |
| Cranial vascular disease, n (%) | 24 (2.94) | 15 (3.14) | 4 (3.80) | 0.938 |
| BMI, kg/m2 (SD) | 24.69±3.05 | 24.32±3.14 | 23.83±3.51 | 0.011 |
| Waist circumference, cm (SD) | 86.99±8.66 | 86.32±8.57 | 85.42±9.46 | 0.143 |
| SBP, mm Hg (SD) | 141.61±18.53 | 142.73±17.74 | 143.01±19.37 | 0.516 |
| DBP, mm Hg (SD) | 83.77±10.44 | 82.87±9.75 | 80.44±10.91 | 0.006 |
| Heart rate, beats/min (SD) | 79.9±13.4 | 79.8±13.2 | 81.1±13.0 | 0.659 |
| WBC, 109 /L (SD) | 6.39±1.51 | 6.36±1.56 | 6.29±1.69 | 0.781 |
| Neutrophils, 109 /L (SD) | 3.89±1.22 | 3.86±1.26 | 3.96±1.29 | 0.699 |
| Lymphocytes, 109 /L (SD) | 1.95±0.58 | 1.96±0.64 | 1.76±0.59 | 0.007 |
| Eosinophils, 109 /L (SD) | 0.14±0.12 | 0.14±0.12 | 0.15±0.18 | 0.628 |
| PLT, 109 /L (SD) | 212.4±52.2 | 216.3±52.0 | 207.9±66.1 | 0.251 |
| RBC, 109 /L (SD) | 4.73±0.41 | 4.51±0.42 | 4.41±0.65 | <0.001 |
| Haemoglobin, g/L (SD) | 145.51±12.15 | 137.29±11.79 | 129.55±13.73 | <0.001 |
| Anaemia, n (%) | 6 (0.84%) | 17 (3.56%) | 21 (20%) | <0.001 |
| RDW, % (SD) | 12.89±0.55 | 13.15±0.69 | 13.88±1.39 | <0.001 |
| HRR | 11.31±1.02 | 10.47±1.05 | 9.43±1.41 | <0.001 |
| FBG, mmol/L (SD) | 6.28±1.59 | 6.37±1.90 | 6.56±2.13 | 0.278 |
| Albumin, g/dL (SD) | 46.23±2.19 | 45.86±2.47 | 45.13±2.90 | <0.001 |
| Globulin, g/dL (SD) | 30.26±3.44 | 30.64±3.65 | 30.55±5.25 | 0.194 |
| Triglyceride, mmol/L (SD) | 1.49±0.85 | 1.46±0.90 | 1.44±0.85 | 0.883 |
| Total cholesterol, mmol/L (SD) | 5.17±1.04 | 5.19±1.15 | 4.96±1.05 | 0.122 |
| HDL-C, mmol/L (SD) | 1.50±0.39 | 1.54±0.42 | 1.54±0.38 | 0.333 |
| LDL-C, mmol/L (SD) | 3.03±0.82 | 3.02±0.90 | 2.82±0.88 | 0.060 |
*Including separated, divorced, never married or widowed.
BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; HRR, haemoglobin-to-RDW ratio; LDL-C, low-density lipoprotein cholesterol; PLT, platelet count; RBC, red blood cell; RDW, red blood cell distribution width; SBP, systolic blood pressure; WBC, white blood cell.
ROC curve analysis
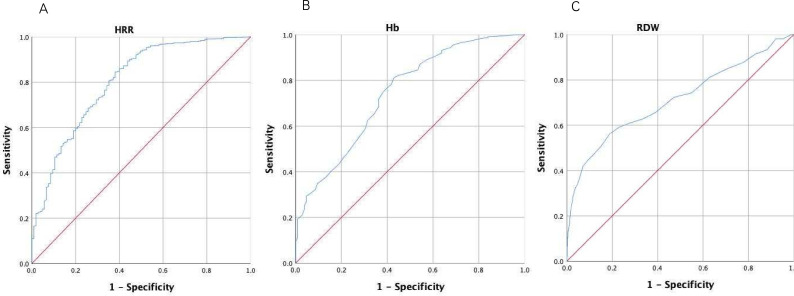
The predictive value of HRR for frailty in older adults was assessed by ROC analysis. The AUC for HRR in the frailty older adults was 0.802 (95% CI: 0.755 to 0.849), the highest sensitivity was 84.5% and the specificity was 61.9% with the optimal critical value of 9.97 (figure 1). The AUC for Hb was 0.742 (95% CI: 0.691 to 0.793), the highest sensitivity was 81% and the specificity was 57.1% with the optimal critical value of 131.5 (figure 1). The AUC for RDW was 0.712 (95% CI: 0.651 to 0.772), the highest sensitivity was 56.2% and the specificity was 81.1% with the optimal critical values of 13.45 (figure 1). Compared with Hb and RDW alone, HRR was a more strong prognostic biomarker for frailty.
Figure 1.
ROC curve for HRR (A), Hb (B) and RDW (C). (A) ROC curve indicated that the best intercept value for HRR was 9.97 (sensitivity 84.5%, specificity 61.9%, AUC=0.802, p<0.001). (B) ROC curve indicated that the best intercept value for Hb was 131.5 (sensitivity 81%, specificity 57.1%, AUC=0.742, p<0.001). (C) ROC curve indicated that the best intercept value for RDW was 13.45 (sensitivity 56.2%, specificity 81.1%, AUC=0.712, p<0.001). Hb, haemoglobin; HRR, Hb-to-RDW; RDW, red blood cell distribution width; ROC, receiver operating characteristic.
Differences in clinical characteristics of the study population stratified by HRR
According to ROC analysis, the optimal critical value of HRR was 9.97. Participants were grouped according to the optimal critical values of HRR, as follows: 1046 (80.71%) in the normal HRR group and 250 (19.29%) in the lower HRR group (table 2). Compared with the normal HRR group, the lower HRR group had a higher lymphocytes count (p=0.002) and RDW (p<0.001), but lower Hb (p<0.001), RBC (p<0.001) and albumin (p<0.001). Compared with the normal HRR group, the lower HRR group was more likely to have frailty (p<0.001).
Table 2.
Baseline characteristics of the study population stratified by HRR
| Characteristics | Normal HRR (n=1046) | Lower HRR (n=250) | P value |
| Age, years (SD) | 70.50±4.64 | 72.50±5.40 | <0.001 |
| Male gender, n (%) | 501 (47.9) | 63 (25.2) | <0.001 |
| Marital status | |||
| Married, n (%) | 901 (86.14) | 206 (82.4) | 0.133 |
| Other*, n (%) | 145 (13.86) | 44 (17.6) | |
| Education years | |||
| 0–12, n (%) | 691 (66.06) | 197 (78.8) | <0.001 |
| >12, n (%) | 355 (33.94) | 53 (21.2) | |
| Alone living, n (%) | 114 (10.90) | 35 (14.0) | 0.167 |
| Smoking, n (%) | 129 (12.33) | 15 (6.0) | 0.004 |
| Drinking, n (%) | 136 (13.0) | 16 (6.4) | 0.004 |
| Hypertension, n (%) | 456 (43.59) | 126 (50.4) | 0.052 |
| Diabetes mellitus, n (%) | 140 (13.38) | 43 (17.2) | 0.120 |
| Cardiac diseases, n (%) | 82 (7.84) | 16 (6.4) | 0.439 |
| Cranial vascular disease, n (%) | 32 (3.06) | 11 (4.4) | 0.288 |
| BMI, kg/m2 (SD) | 24.56±3.07 | 24.15±3.36 | 0.026 |
| Waist circumference, cm (SD) | 86.90±8.51 | 85.45±9.37 | 0.143 |
| SBP, mm Hg (SD) | 142.3±18.3 | 141.3±18.5 | 0.611 |
| DBP, mm Hg (SD) | 83.7±10.2 | 80.9±10.1 | 0.004 |
| Heart rate, beats/min (SD) | 80.2±13.4 | 79.0±12.7 | 0.362 |
| WBC, 109 /L (SD) | 6.39±1.49 | 6.28±1.75 | 0.556 |
| Neutrophils, 109 /L (SD) | 3.89±1.19 | 3.86±1.43 | 0.441 |
| Lymphocytes, 109 /L (SD) | 1.96±0.59 | 1.86±0.69 | 0.002 |
| Eosinophils, 109 /L (SD) | 0.14±0.11 | 0.15±0.17 | 0.621 |
| PLT, 109 /L (SD) | 212.3±50.8 | 218.3±62.8 | 0.264 |
| RBC, 109 /L (SD) | 4.71±0.38 | 4.27±0.56 | <0.001 |
| Haemoglobin, g/L (SD) | 145.08±10.98 | 124.90±8.46 | <0.001 |
| RDW, % (IQR) | 12.89±0.51 | 13.79±1.09 | <0.001 |
| FBG, mmol/L (SD) | 6.36±1.77 | 6.24±1.70 | 0.166 |
| Albumin, g/dL (SD) | 46.18±2.27 | 45.30±2.69 | <0.001 |
| Globulin, g/dL (SD) | 30.35±3.45 | 30.72±4.58 | 0.708 |
| Triglyceride, mmol/L (SD) | 1.48±0.87 | 1.42±0.83 | 0.755 |
| Total cholesterol, mmol/L (SD) | 5.17±1.07 | 5.10±1.27 | 0.041 |
| HDL-C, mmol/L (SD) | 1.50±0.39 | 1.58±0.42 | 0.539 |
| LDL-C, mmol/L (SD) | 3.03±0.85 | 2.91±0.87 | 0.019 |
| Frailty status | |||
| Robust, n (%) | 669 (63.96) | 45 (18.0) | <0.001 |
| Prefrail, n (%) | 337 (32.22) | 140 (56.0) | |
| Frailty, n (%) | 40 (3.82) | 65 (26.0) |
Normal HRR=HRR above 9.97, Lower HRR: HRR below 9.97 (optimal cutoff value of the ROC curve).
*Including separated, divorced, never married or widowed.
BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; HRR, haemoglobin-to-RDW ratio; LDL-C, low-density lipoprotein cholesterol; PLT, platelets count; RBC, red blood cell; RDW, red blood cell distribution width; ROC, receiver operating characteristic; SBP, systolic blood pressure; WBC, white blood cell.
Logistic regression analysis
Among 1296 older adults, 105 (8.10%) were considered frail. Multiple logistic regression analysis was conducted to assess the associations of the lower HRR with frailty. Unadjusted model 1 showed that lower Hb (OR: 2.129, 1.133 to 4.001, p=0.019) and lower HRR (OR: 3.285, 1.676 to 6.440, p=0.001) were risk factors related to frailty, whereas lower RDW (OR: 0.310, 0.193 to 0.497, p<0.001) was a protective factor. After adjustment for confounding factors (including age, gender, marital status, education years, living alone, BMI, diabetes, RBC, albumin, triglyceride, HDL-C and LDL-C), there was a significant association of lower HRR (OR: 3.419, 1.679 to 6.964, p=0.001) and lower RDW (OR: 0.285, 0.170 to 0.477, p<0.001) with frailty (table 3). Lower HRR was independently related to frailty in older adults.
Table 3.
Multiple logistic regression analysis of blood parameters and frailty in older adults
| Model 1 OR (95% CI) |
P value | Model 2 OR (95% CI) |
P value | |
| Categorical variable | ||||
| Lower Hb | 2.129 (1.133 to 4.001) | 0.019 | 1.163 (0.562 to 2.409) | 0.684 |
| Lower RDW | 0.310 (0.193 to 0.497) | <0.001 | 0.285 (0.170 to 0.477) | <0.001 |
| Lower HRR | 3.285 (1.676 to 6.440) | 0.001 | 3.419 (1.679 to 6.964) | 0.001 |
Lower Hb: <131.5 g/L, lower RDW: <13.45%, lower HRR: <9.97 (optimal cutoff value of the ROC curve). Model 1, unadjusted; model 2, adjusted for age, gender, marital status, education years, alone living, BMI, diabetes, RBC, albumin, triglyceride, HDL-C and LDL-C.
BMI, body mass index; Hb, haemoglobin; HDL-C, high-density lipoprotein cholesterol; HRR, haemoglobin-to-RDW ratio; LDL-C, low-density lipoprotein cholesterol; RDW, red blood cell distribution width; ROC, receiver operating characteristic.
Correlation analysis
Correlation analysis indicated that there was an obvious positive correlation between RDW (Kendall’s tau-b= 0.173, p<0.001) and frailty. Nevertheless, HRR (Kendall’s tau-b=−0.239, p<0.001) and Hb (Kendall’s tau-b=−0.194, p<0.001) were a negative correlation with frailty (table 4).
Table 4.
Correlation analysis of Hb, RDW, HRR and frailty in older adults
| Kendall’s tau-b* | P values | |
| Hb | −0.194 | <0.001 |
| RDW | 0.173 | <0.001 |
| HRR | −0.239 | <0.001 |
*Correlation is significant at the 0.01 level (2-tailed).
Hb, haemoglobin; HRR, haemoglobin-to-RDW ratio; RDW, red blood cell distribution width.
Discussion
Frailty, as a geriatric syndrome, has attracted more and more scientific attention in the background of continuously increasing global population ageing.20 In this cross-sectional study including 1296 community-dwelling older adults, we found that lower HRR is independently related to frailty in older people, even after adjusting confounding factors (p=0.001). Multiple logistic regression analysis showed that lower HRR is associated with a threefold more likelihood or odds of frailty (OR=3.419, 95% CI 1.679 to 6.964). ROC analysis showed that the AUC of HRR was 0.802, the highest sensitivity was 84.5% and the specificity was 61.9% with the optimal critical value of 9.97. The results of the present study confirmed that HRR was also significantly associated with frailty in general older people, not only in patients with CHD in previous studies.
HRR is a cost-effective, common and accessible laboratory parameter for clinicians. As a novel inflammatory factor, Qu et al16 found that HRR is significantly associated with frailty in older patients with CHD. In their study the AUC of HRR in the frailty patients was exceed Hb and RDW alone, and after adjusting confounding factors lower HRR was a risk factor for frailty in older patients with CHD. These findings are consistent with our results. Now the pathophysiological mechanism has not been fully understood. We try to provide a possible explanation for the association between HRR and frailty in older adults.
A decrease in HRR may be due to low Hb, high RDW or both. As we all know, low Hb indicates a condition of anaemia, which is one of the acknowledged risk factors for hospitalisation, morbidity and mortality in older people.21 Anaemia decreases the oxygen-carrying capacity, leading to tissue hypoxia and even organ failure, especially in older patients, increasing the risk of frailty.22 Besides, anaemia can cut down submaximal and maximal aerobic capacity, leading to several adverse outcomes including loss of muscle strength, cognitive decline and development of frailty.10 In addition, chronic conditions and comorbidities lead to a low grade of inflammation-reducing Hb level,23 also known as chronic disease anaemia, which is the most common type of anaemia in older adults.22 And a state of chronic inflammation has been suggested as contributor to frailty.24 Furthermore, anaemia caused by malnutrition is also a significant health-affecting factor among older adults.22
Anaemia, reaching a prevalence of 17% in older people, is a threat to healthy ageing.25 Pires Corona et al8 found that lower Hb and anaemia were related to frailty in Brazilian older adults. In their study, anaemia was related to low physical activity, weakness (weaker) and walking more slowly. Another study from Spain indicated that anaemia is independently associated with frailty in older people.26 Moreover, Xu et al9 found that Hb is closely associated with frailty in older patients in the hospital. A systematic review and meta-analysis including 19 studies indicated that older adults with anaemia have more than a twofold increased odds of frailty.27 Silva et al28 suggested that lower Hb levels should be considered a significant component of frailty in older persons. Similarly, another meta-analysis including 32 934 robust participants and 6864 frail participants found that Hb is a useful biomarker of frailty.7 However, there were no significant association between lower Hb and frailty after adjusting confounding factors in our study. The discrepancy in results may be due to the definition of lower Hb being determined by the optimal cutoff value of the ROC curve in this research. Therefore, further studies are needed to confirm the relationship between Hb and frailty in older people.
An increasing RDW also can lead to low HRR. Studies have proved that increased RDW is related to inflammation. Inflammatory cytokines reduce erythropoietin gene expression and erythropoietin receptor expression, which leads to the release of immature erythrocytes and the heterogeneity of the erythrocyte volume increasing.29 30 In addition, metabolic abnormalities including shortened telomere length, oxidative stress and malnutrition may also contribute to increased RDW.11 31 What’s more, others have suggested that RDW may be a potential biomarker for biological ageing.30 Study has indicated that a high RDW was related to a high sarcopenia risk.32 Sarcopenia, which plays a key role in frailty, is a progressive loss of skeletal muscle mass and strength.28
RDW is a biomarker of inflammation, oxidative stress, poor nutritional status, ageing and sarcopenia, and all of these could be underlying reasons for the development of frailty. A study that enrolled 3635 community-dwelling older men indicated that participants with a high RDW are more likely to have functional limitations and frailty.30 Li et al15 indicated that increased RDW may be closely related to frailty through inflammation. Hou et al13 proved that frailty is closely associated with RDW in older patients with CHD. Another study including 2932 community-dwelling older adults found RDW is independently associated with high frailty risk even after adjusting for potential confounding factors.14
Increased RDW combined with anaemia is more likely to lead to decreased HRR. Elevated RDW suggests chronic inflammation, malnutrition and ageing.11 Anaemia is the cause of reduced tissue oxygenation and the consequent increase in fatigue, weakness and functional impairment.33 Also, anaemia may affect muscle mass and strength loss through inflammatory pathways.33 Therefore, decreased HRR may be associated with sarcopenia, slowness, weakness, inflammation, malnutrition and weight loss in frailty patients.
To sum up, a large number of researchers have verified the association between frailty and a low Hb and a high RDW among older persons. However, both RDW and Hb are susceptible to many other disease conditions and sub-health states; HRR may provide a more powerful parameter than a single parameter alone. Moreover, ROC analysis showed that the AUC and highest sensitivity of HRR are higher than RDW and Hb. It may be a more reliable and effective marker than Hb and RDW alone.
This inexpensive and common laboratory parameter may provide useful information to identify the risk of frailty in older adults. Furthermore, the use of HRR may help clinicians to identify people at high risk of frailty and take effective measures to reduce the occurrence and development of frailty, reduce the rate of disability and mortality related to frailty in the elderly, and reduce the waste of medical resources, and promote healthy ageing.
There were also some limitations of our present study. First, because cross-sectional studies measure the outcome and the exposures in the study participants at the same time, it is difficult to assess the cause–effect relationship. Second, our participants are limited to local participants; these findings need to be validated in different populations around the world. What is more, we are unable to investigate the temporal relation between outcomes and risk factors. In addition, despite the inevitable selection bias and information bias in cross-sectional studies, we improved this problem through more rational statistical methods and interviewer training. Finally, we did not assess iron, folic and vitamin B12, which may affect RDW and Hb levels.
Conclusion
In conclusion, a low HRR is independently associated with higher frailty risk in community-dwelling older adults. And this relationship is not affected by confounding factors. However, the causal relationship and the specific mechanism between HRR and frailty are unclear. Evidence is needed from prospective studies to verify these conclusions in the future.
Supplementary Material
Acknowledgments
The authors thank all the volunteers that participated in this study.
Footnotes
Contributors: Conceptualisation: ZX, MZ; data curation: MZ, CW, YX; formal analysis: MZ, CW; visualisation: XY, YH; writing—original draft: MZ; writing—review and editing: ZX, MZ, CW, XY, YH, YX; all authors read and approved the final manuscript. Guarantor: ZX
Funding: This study was supported by the National Key Research and Development Program of China (2018YFC2002000).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Medical Ethics Committee of Liyuan Hospital, Tongji Medical College, Huazhong University of Science and Technology (IRB ID: [2020] IEC (A016)). Participants gave informed consent to participate in the study before taking part.
References
- 1.Kameda M, Teruya T, Yanagida M, et al. Frailty markers comprise blood metabolites involved in antioxidation, cognition, and mobility. Proc Natl Acad Sci U S A 2020;117:9483–9. 10.1073/pnas.1920795117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travers J, Romero-Ortuno R, Bailey J, et al. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract 2019;69:e61–9. 10.3399/bjgp18X700241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dent E, Martin FC, Bergman H, et al. Management of frailty: opportunities, challenges, and future directions. Lancet 2019;394:1376–86. 10.1016/S0140-6736(19)31785-4 [DOI] [PubMed] [Google Scholar]
- 4.Coelho-Junior HJ, Marzetti E, Picca A, et al. Protein intake and frailty: a matter of quantity, quality, and timing. Nutrients 2020;12:2915. 10.3390/nu12102915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward RE, Orkaby AR, Chen J, et al. Association between diet quality and frailty prevalence in the physicians' health study. J Am Geriatr Soc 2020;68:770–6. 10.1111/jgs.16286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhai Z, Gao J, Zhu Z, et al. The ratio of the hemoglobin to red cell distribution width combined with the ratio of platelets to lymphocytes can predict the survival of patients with gastric cancer liver metastasis. Biomed Res Int 2021;2021:8729869. 10.1155/2021/8729869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mailliez A, Guilbaud A, Puisieux F, et al. Circulating biomarkers characterizing physical frailty: CRP, hemoglobin, albumin, 25OHD and free testosterone as best biomarkers. Results of a meta-analysis. Exp Gerontol 2020;139:111014. 10.1016/j.exger.2020.111014 [DOI] [PubMed] [Google Scholar]
- 8.Pires Corona L, Drumond Andrade FC, de Oliveira Duarte YA, et al. The relationship between anemia, hemoglobin concentration and frailty in Brazilian older adults. J Nutr Health Aging 2015;19:935–40. 10.1007/s12603-015-0502-3 [DOI] [PubMed] [Google Scholar]
- 9.Xu L, Zhang J, Shen S, et al. Clinical frailty scale and biomarkers for assessing frailty in elder inpatients in China. J Nutr Health Aging 2021;25:77–83. 10.1007/s12603-020-1455-8 [DOI] [PubMed] [Google Scholar]
- 10.Ruan Y, Guo Y, Kowal P, et al. Association between anemia and frailty in 13,175 community-dwelling adults aged 50 years and older in China. BMC Geriatr 2019;19:327. 10.1186/s12877-019-1342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86–105. 10.3109/10408363.2014.992064 [DOI] [PubMed] [Google Scholar]
- 12.Dent E, Lien C, Lim WS, et al. The Asia-Pacific clinical practice guidelines for the management of frailty. J Am Med Dir Assoc 2017;18:564–75. 10.1016/j.jamda.2017.04.018 [DOI] [PubMed] [Google Scholar]
- 13.Hou P, Xue H-P, Mao X-E, et al. Inflammation markers are associated with frailty in elderly patients with coronary heart disease. Aging (Albany NY) 2018;10:2636–45. 10.18632/aging.101575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C-M, Chao C-T, Chen S-I, et al. Elevated red cell distribution width is independently associated with a higher frailty risk among 2,932 community-dwelling older adults. Front Med (Lausanne) 2020;7:470. 10.3389/fmed.2020.00470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Chen X, Han B. Red blood cell distribution width is associated with frailty in older Inpatients in China: sex differences in a cross-sectional study. Exp Gerontol 2021;150:111392. 10.1016/j.exger.2021.111392 [DOI] [PubMed] [Google Scholar]
- 16.Qu J, Zhou T, Xue M, et al. Correlation analysis of hemoglobin-to-red blood cell distribution width ratio and frailty in elderly patients with coronary heart disease. Front Cardiovasc Med 2021;8:728800. 10.3389/fcvm.2021.728800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su Y-C, Wen S-C, Li C-C, et al. Low hemoglobin-to-red cell distribution width ratio is associated with disease progression and poor prognosis in upper tract urothelial carcinoma. Biomedicines 2021;9:672. 10.3390/biomedicines9060672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun P, Zhang F, Chen C, et al. The ratio of hemoglobin to red cell distribution width as a novel prognostic parameter in esophageal squamous cell carcinoma: a retrospective study from Southern China. Oncotarget 2016;7:42650–60. 10.18632/oncotarget.9516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 20.Kojima G, Taniguchi Y, Iliffe S, et al. Transitions between frailty states among community-dwelling older people: a systematic review and meta-analysis. Ageing Res Rev 2019;50:81–8. 10.1016/j.arr.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 21.Lee CT, Chen MZ, Yip CYC, et al. Prevalence of anemia and its association with Frailty, physical function and cognition in community-dwelling older adults: findings from the HOPE study. J Nutr Health Aging 2021;25:679–87. 10.1007/s12603-021-1625-3 [DOI] [PubMed] [Google Scholar]
- 22.Röhrig G. Anemia in the frail, elderly patient. Clin Interv Aging 2016;11:319–26. 10.2147/CIA.S90727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinmeyer Z, Delpierre C, Soriano G, et al. Hemoglobin concentration; a pathway to frailty. BMC Geriatr 2020;20:202. 10.1186/s12877-020-01597-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson D, Jackson T, Sapey E, et al. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev 2017;36:1–10. 10.1016/j.arr.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 25.Stauder R, Valent P, Theurl I. Anemia at older age: etiologies, clinical implications, and management. Blood 2018;131:505–14. 10.1182/blood-2017-07-746446 [DOI] [PubMed] [Google Scholar]
- 26.Esquinas-Requena JL, García-Nogueras I, Hernández-Zegarra P, et al. Anemia and frailty in older adults from Spain. The FRADEA study. Rev Esp Geriatr Gerontol 2021;56:129–35. 10.1016/j.regg.2021.01.010 [DOI] [PubMed] [Google Scholar]
- 27.Palmer K, Vetrano DL, Marengoni A, et al. The relationship between anaemia and frailty: a systematic review and meta-analysis of observational studies. J Nutr Health Aging 2018;22:965–74. 10.1007/s12603-018-1049-x [DOI] [PubMed] [Google Scholar]
- 28.Silva JC, Moraes Z de, Silva C, et al. Understanding red blood cell parameters in the context of the frailty phenotype: interpretations of the FIBRA (frailty in Brazilian seniors) study. Arch Gerontol Geriatr 2014;59:636–41. 10.1016/j.archger.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 29.Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion 2005;20:83–90. 10.1191/0267659105pf793oa [DOI] [PubMed] [Google Scholar]
- 30.Kim KM, Lui L-Y, Browner WS, et al. Association between variation in red cell size and multiple aging-related outcomes. J Gerontol A Biol Sci Med Sci 2021;76:1288–94. 10.1093/gerona/glaa217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic Hematopoiesis. Antioxid Redox Signal 2008;10:1923–40. 10.1089/ars.2008.2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J, Im J-S, Choi CH, et al. The association between red blood cell distribution width and sarcopenia in U.S. Sci Rep 2018;8. 10.1038/s41598-018-29855-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Picca A, Coelho-Junior HJ, Calvani R, et al. Biomarkers shared by frailty and Sarcopenia in older adults: a systematic review and meta-analysis. Ageing Res Rev 2022;73:101530. 10.1016/j.arr.2021.101530 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.